Recent Developments in Direct C–H Functionalization of Quinoxalin-2(1H)-Ones via Heterogeneous Catalysis Reactions
Abstract
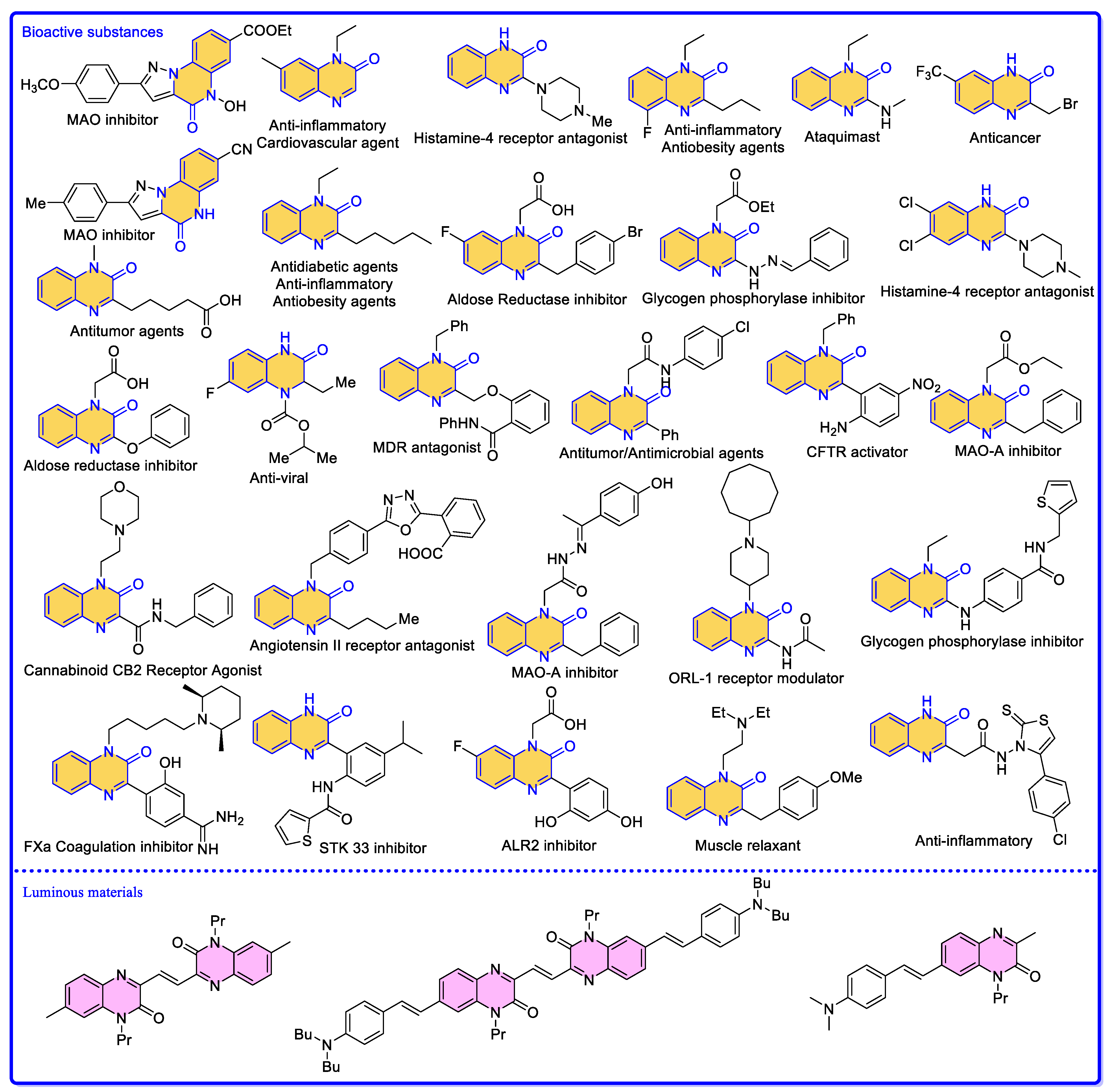
1. Introduction
2. Direct C–H Functionalization of Quinoxalin-2(1H)-Ones via Heterogeneous Catalysis
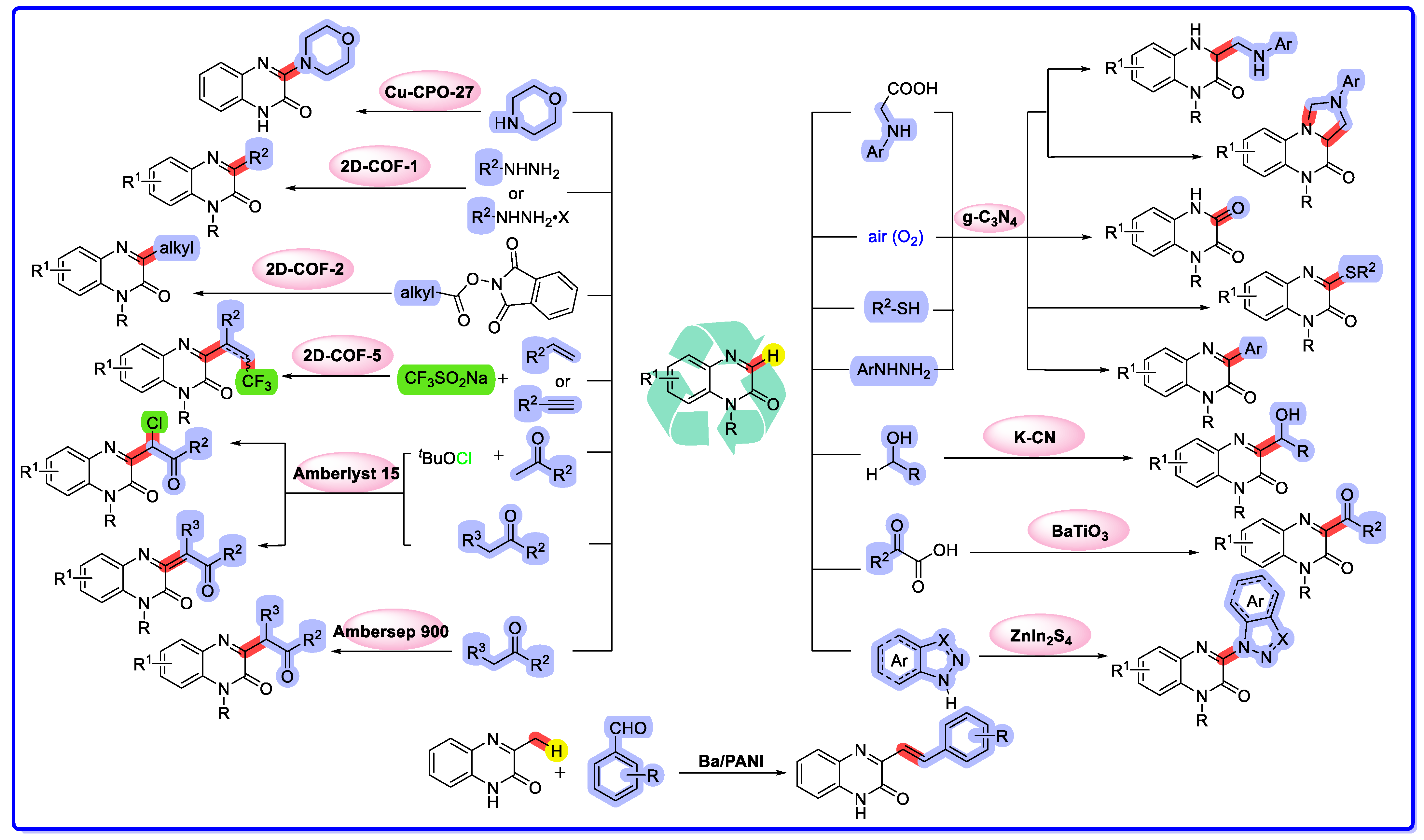
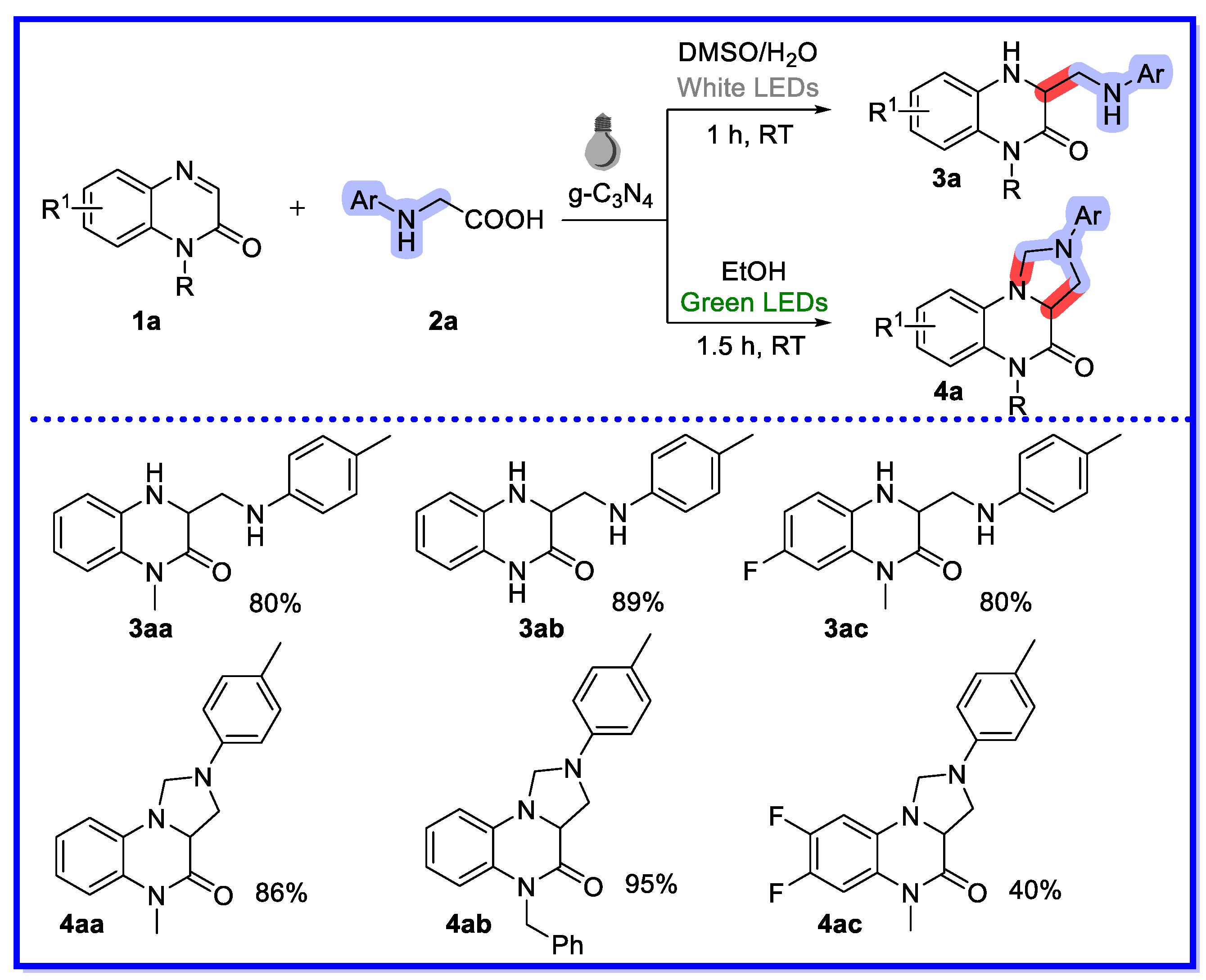
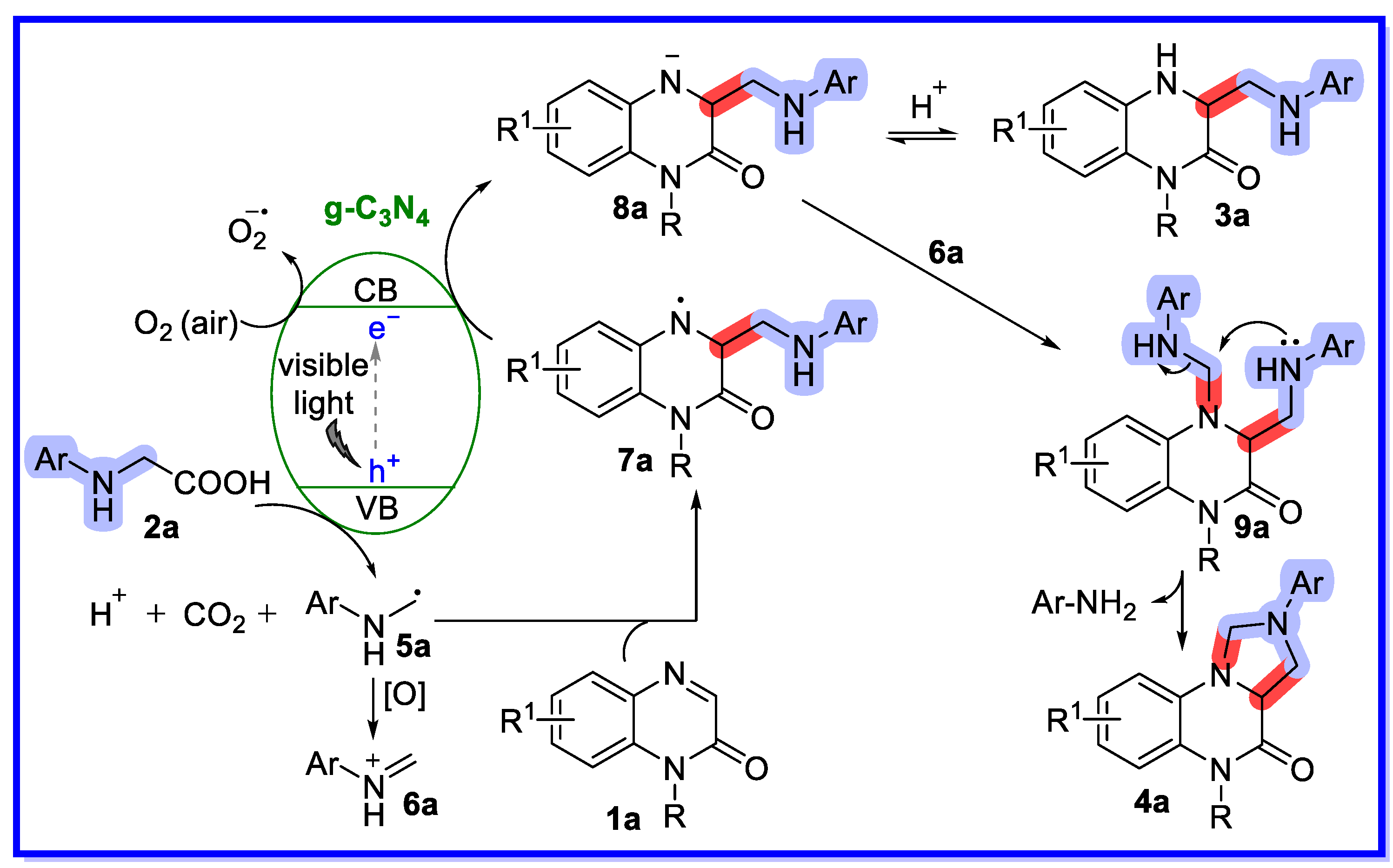
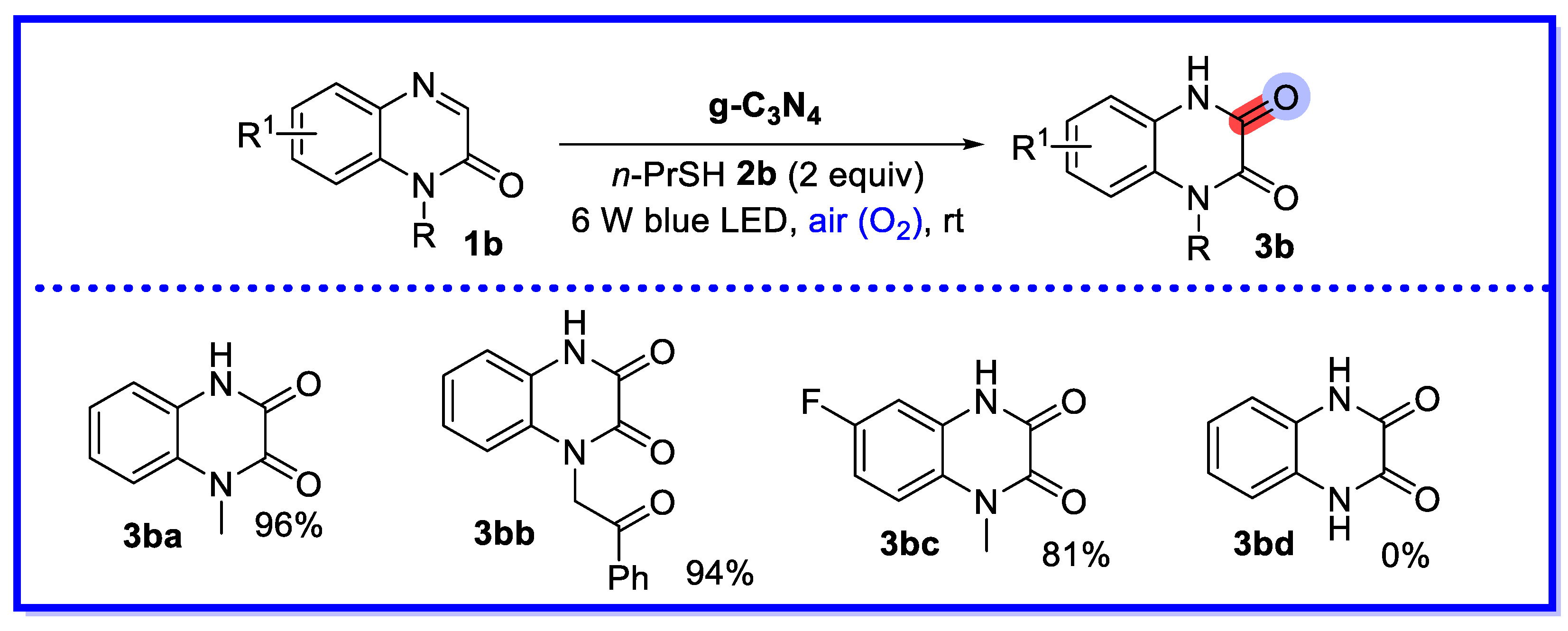
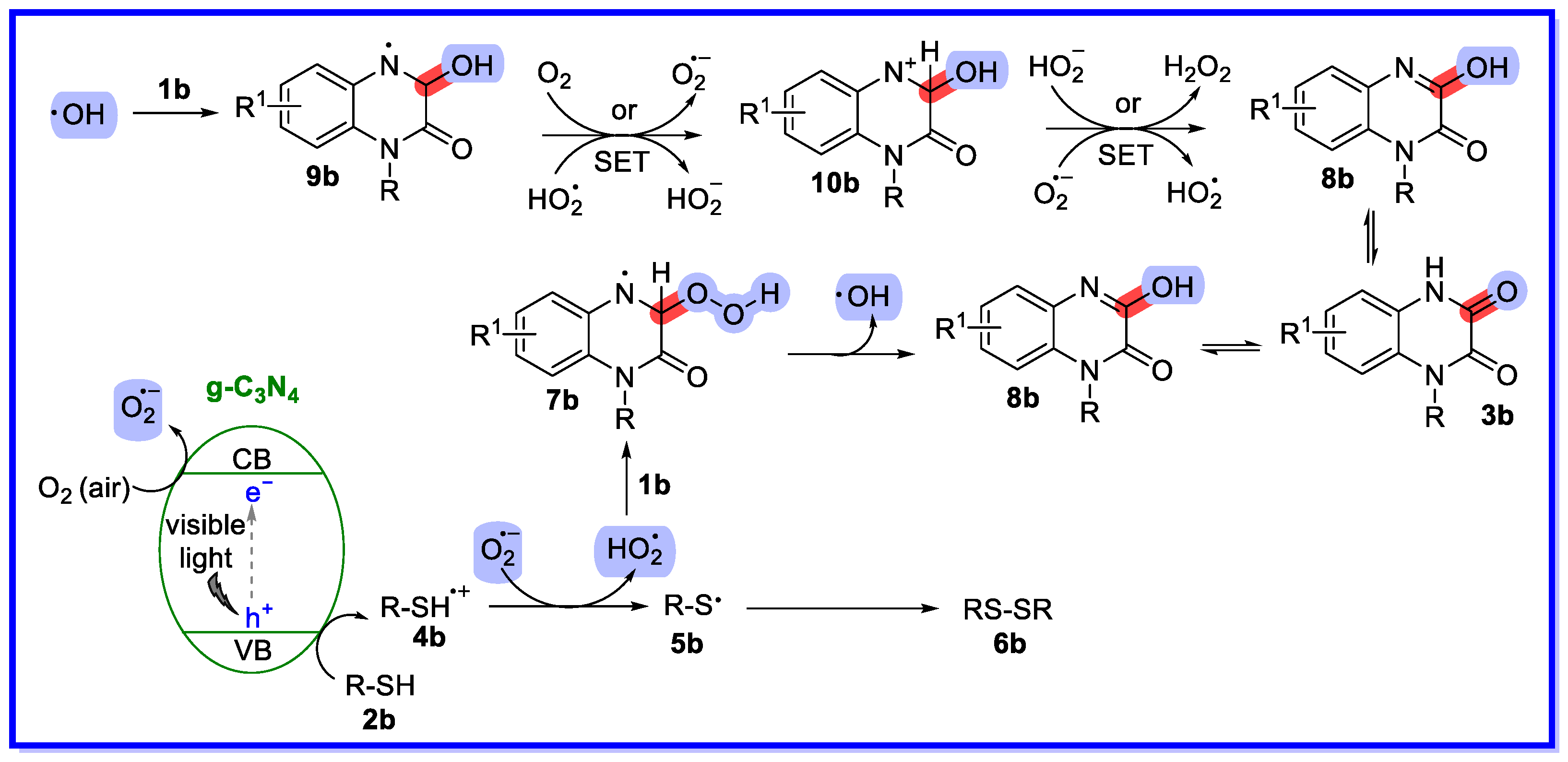
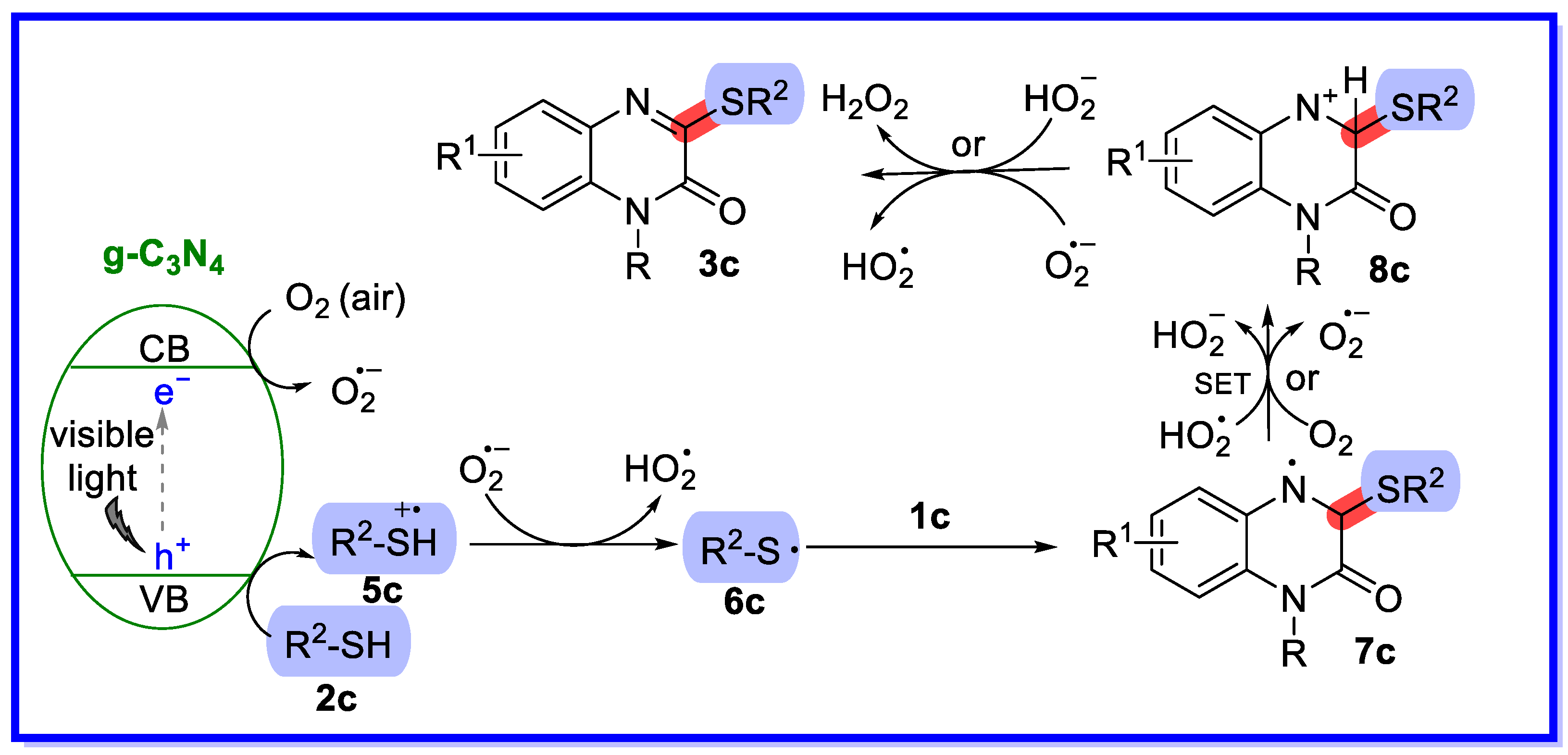
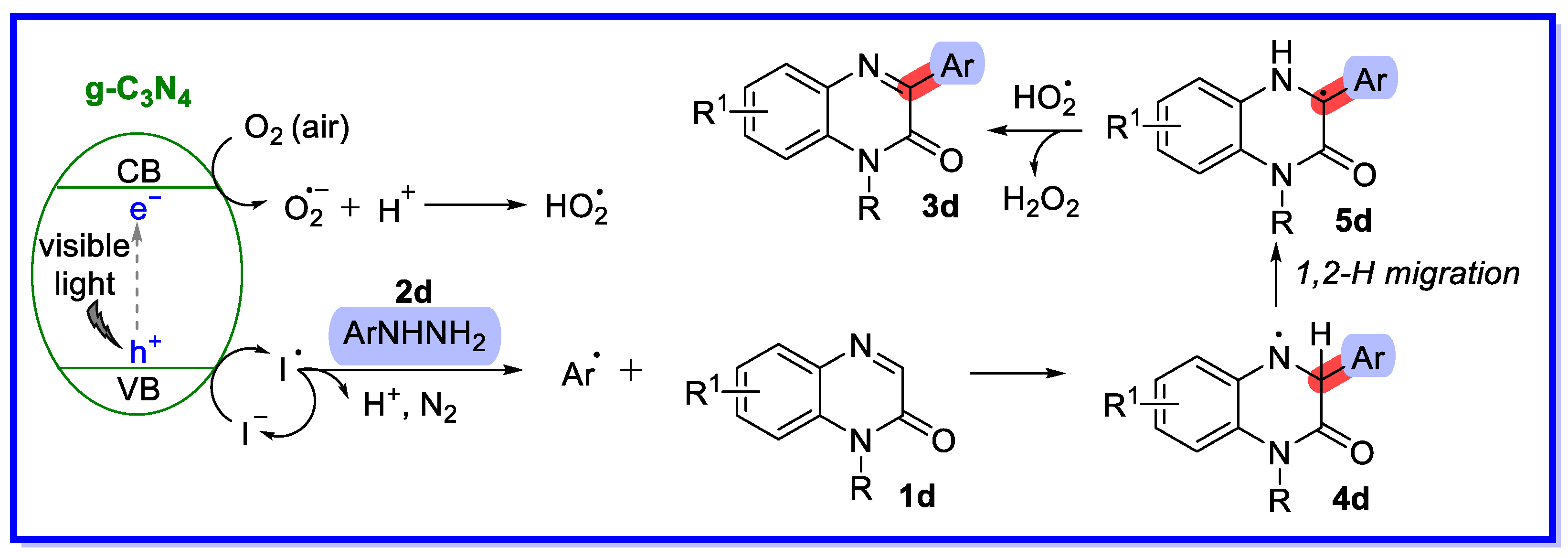
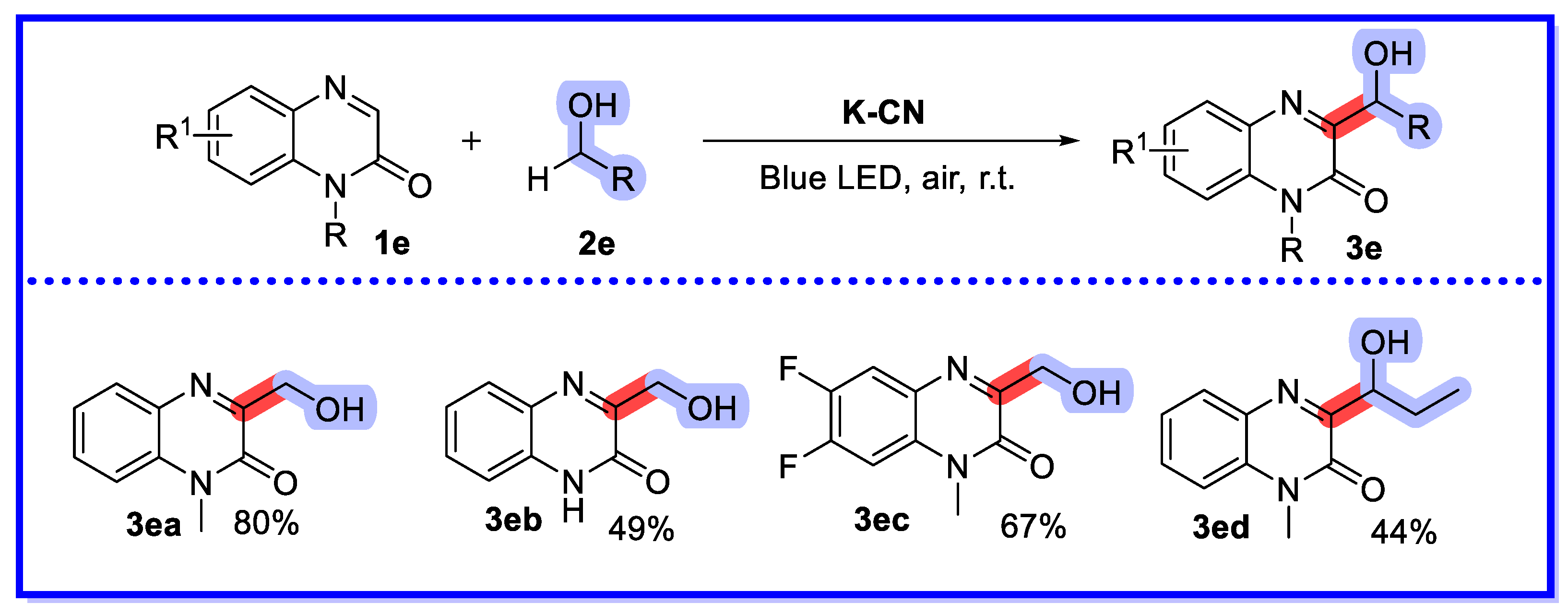
2.1. Graphitic Phase Carbon Nitride as a Heterogeneous Catalyst
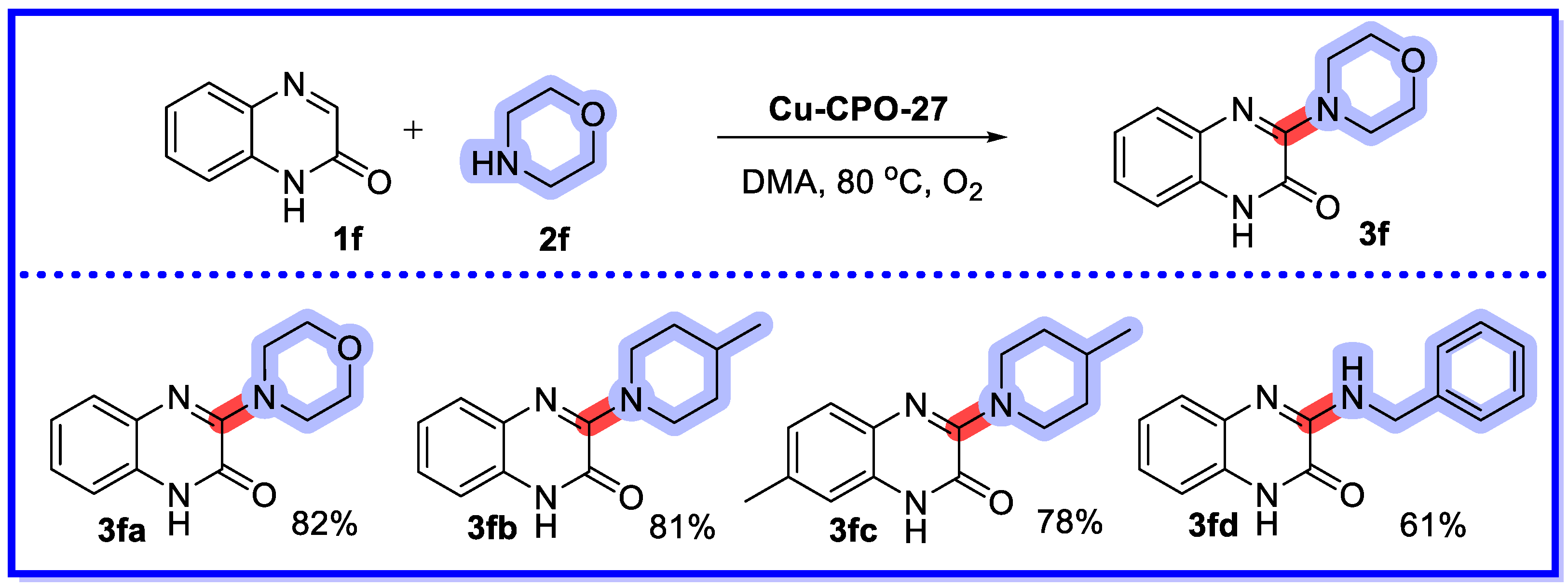
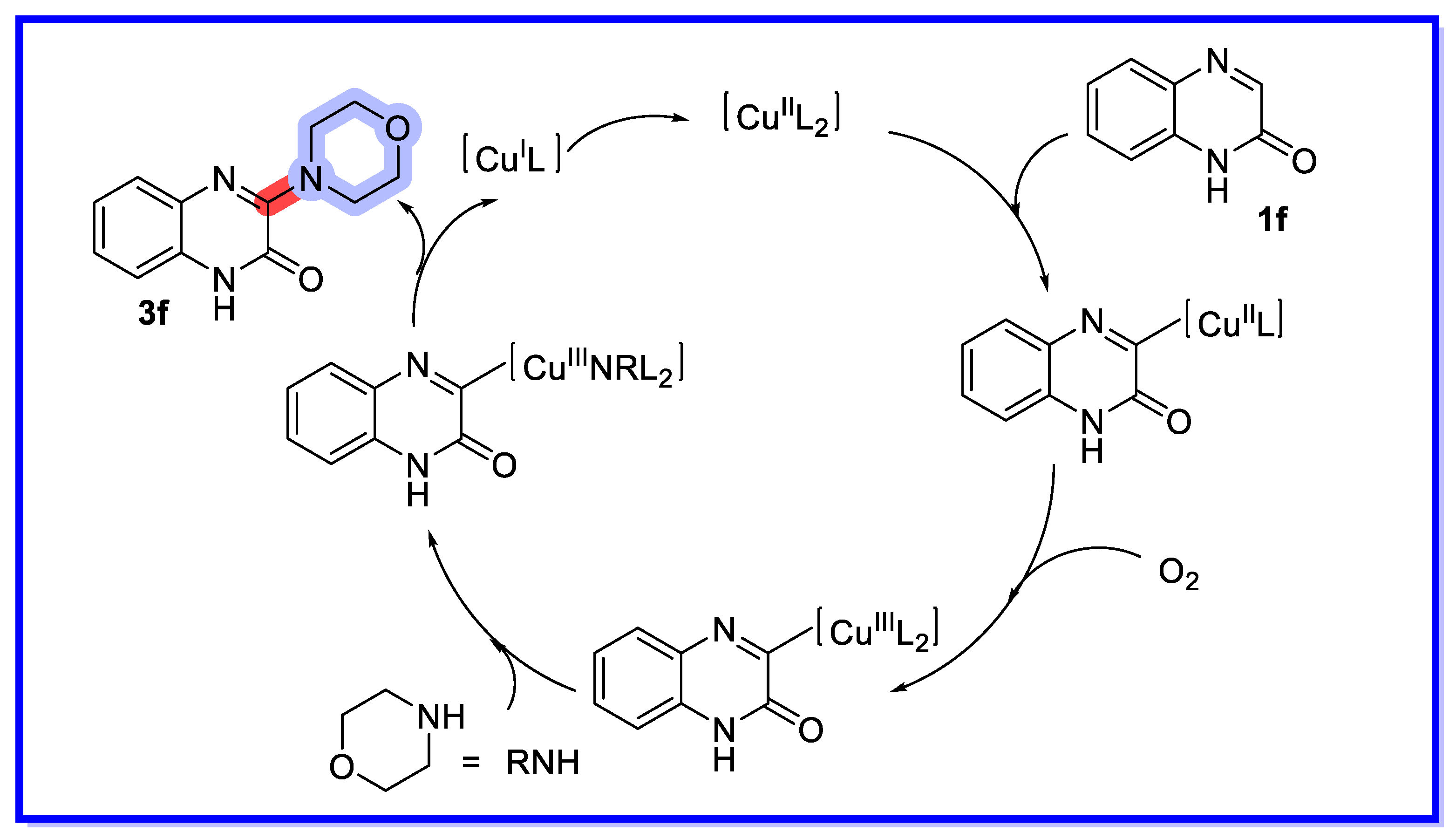
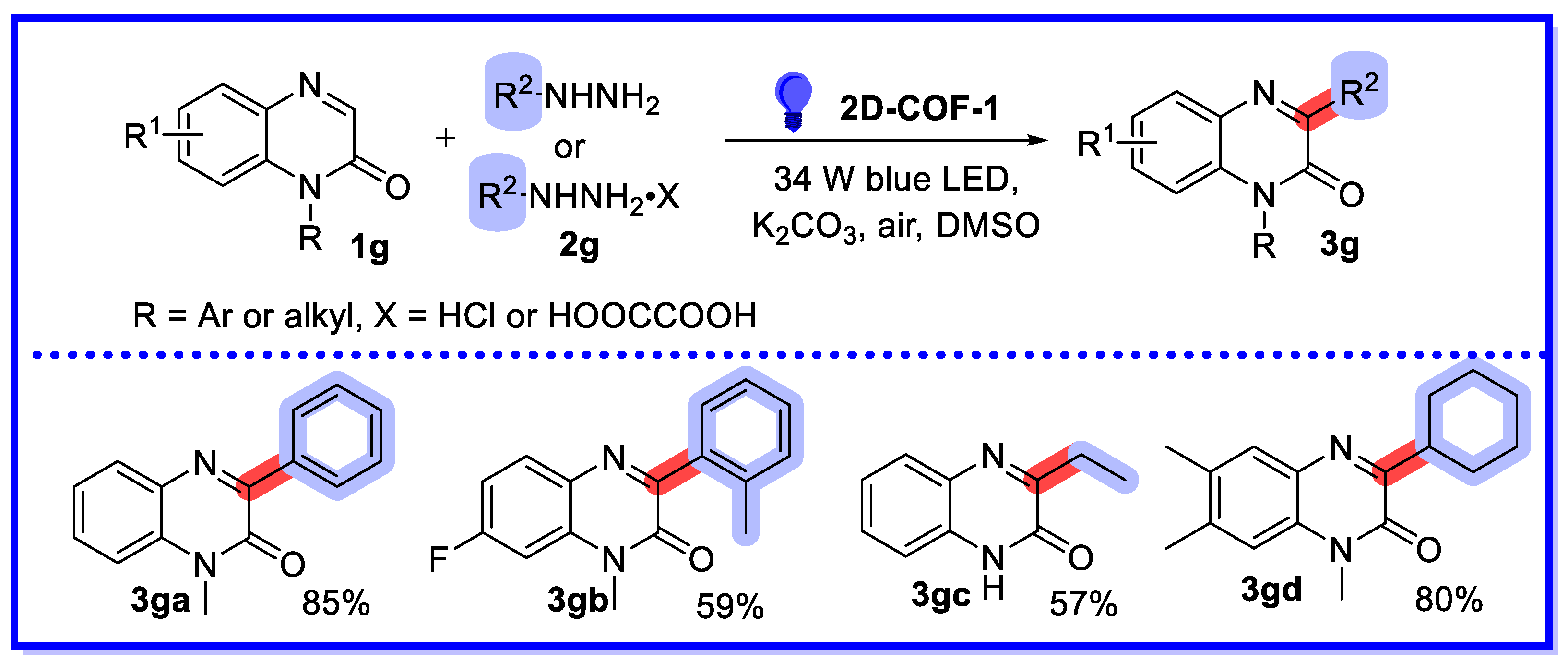
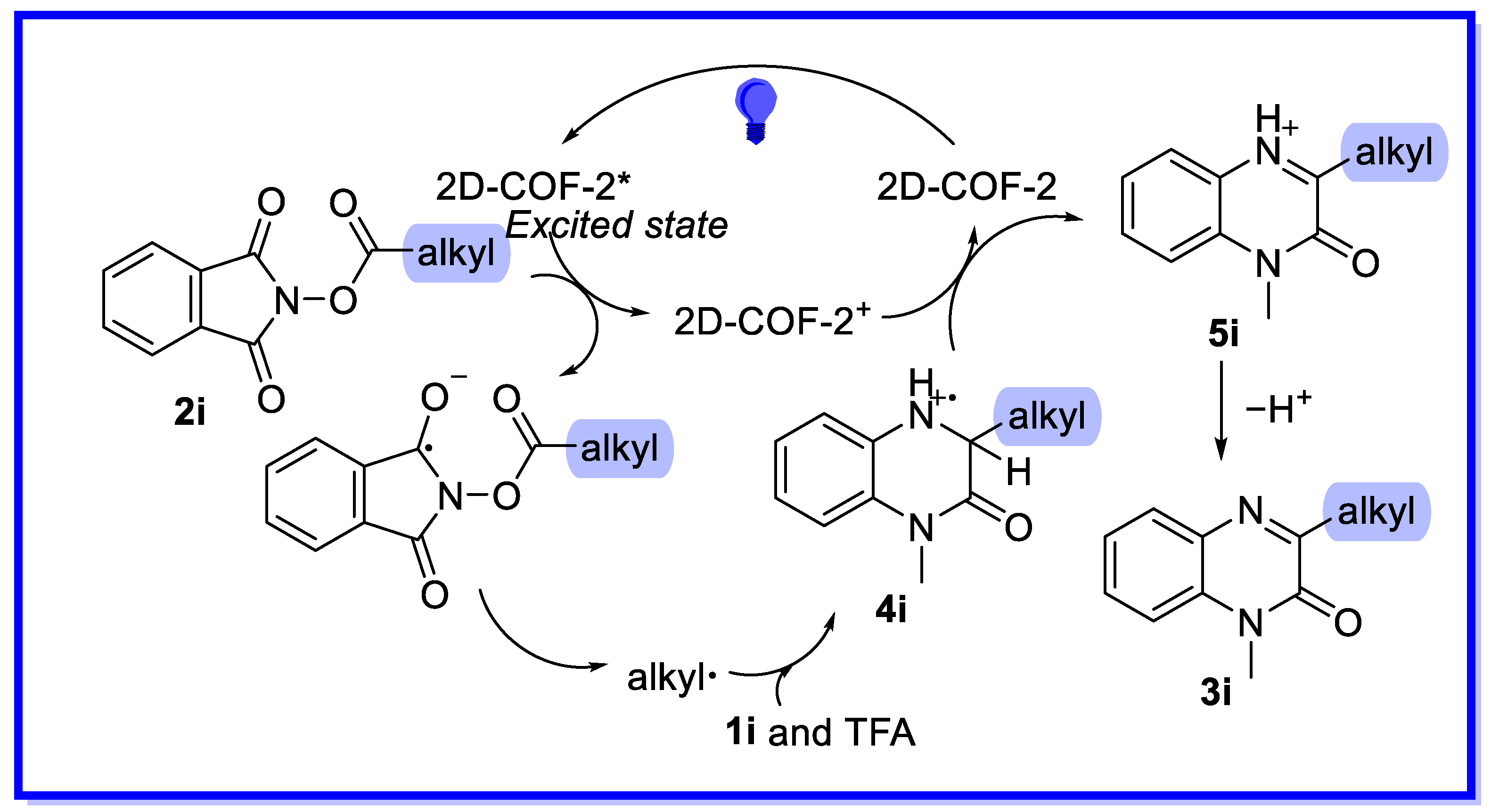
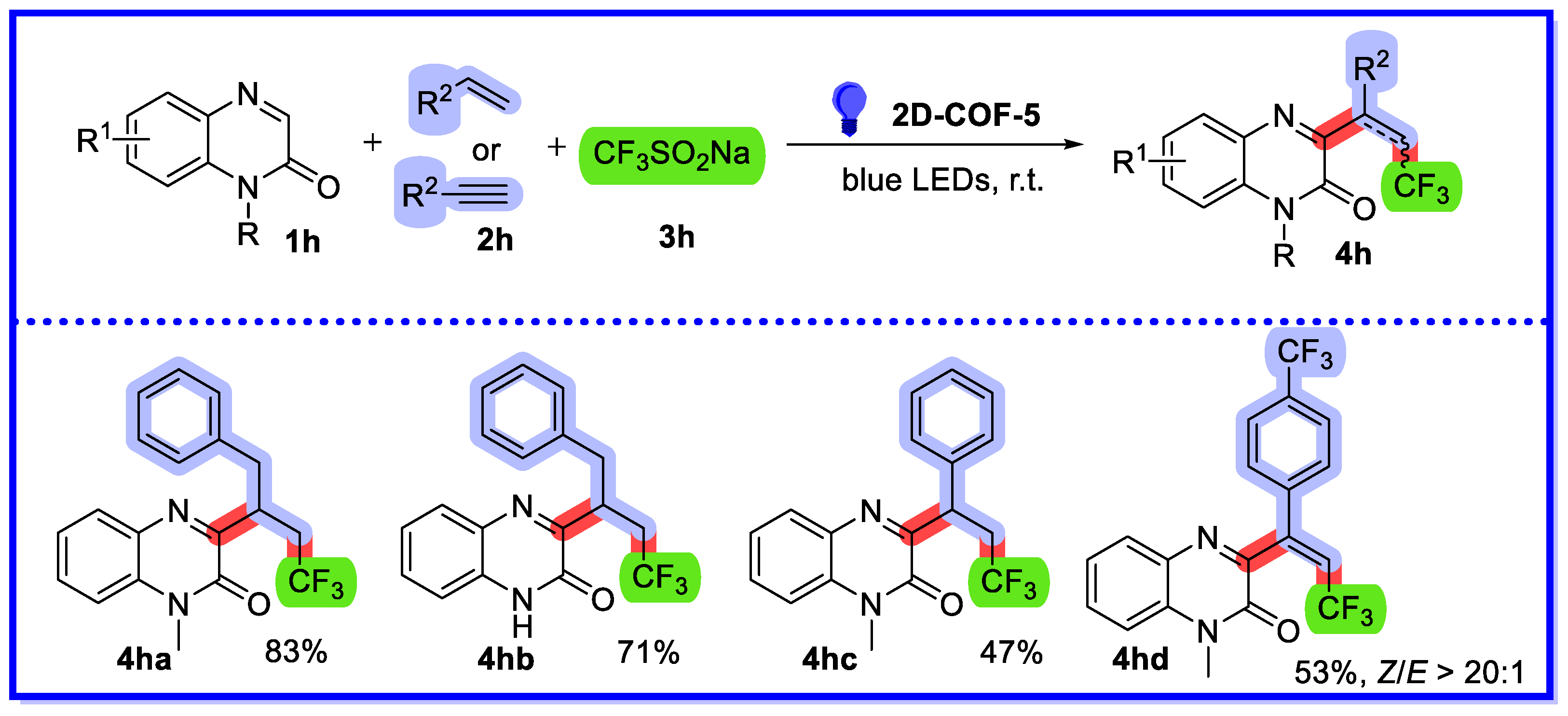
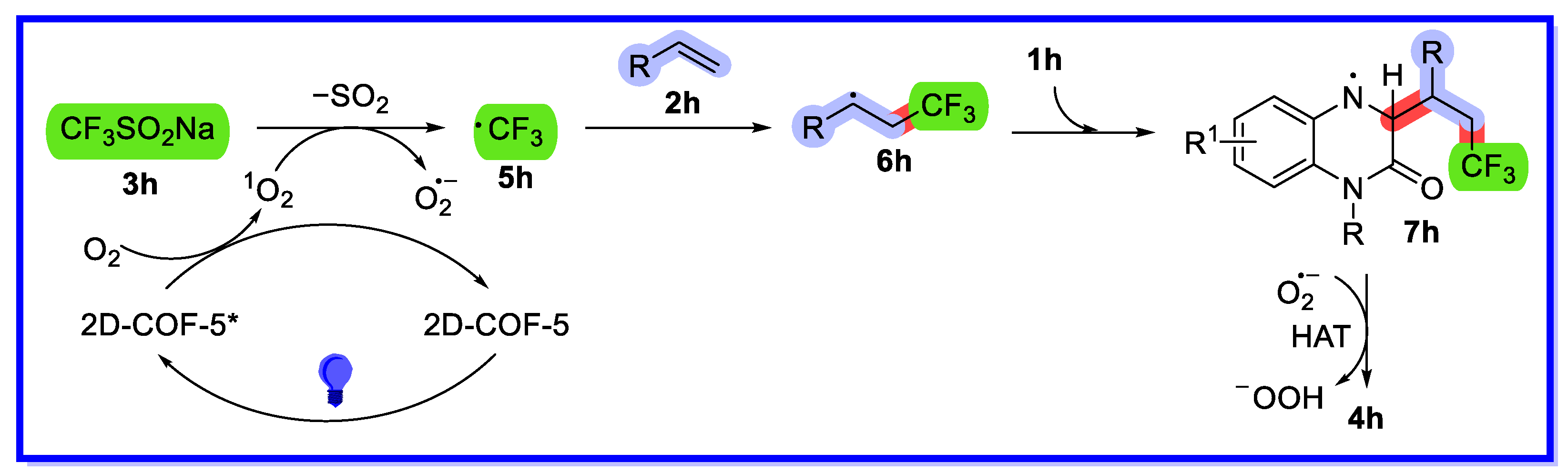
2.2. MOF/COF as Heterogeneous Catalysts
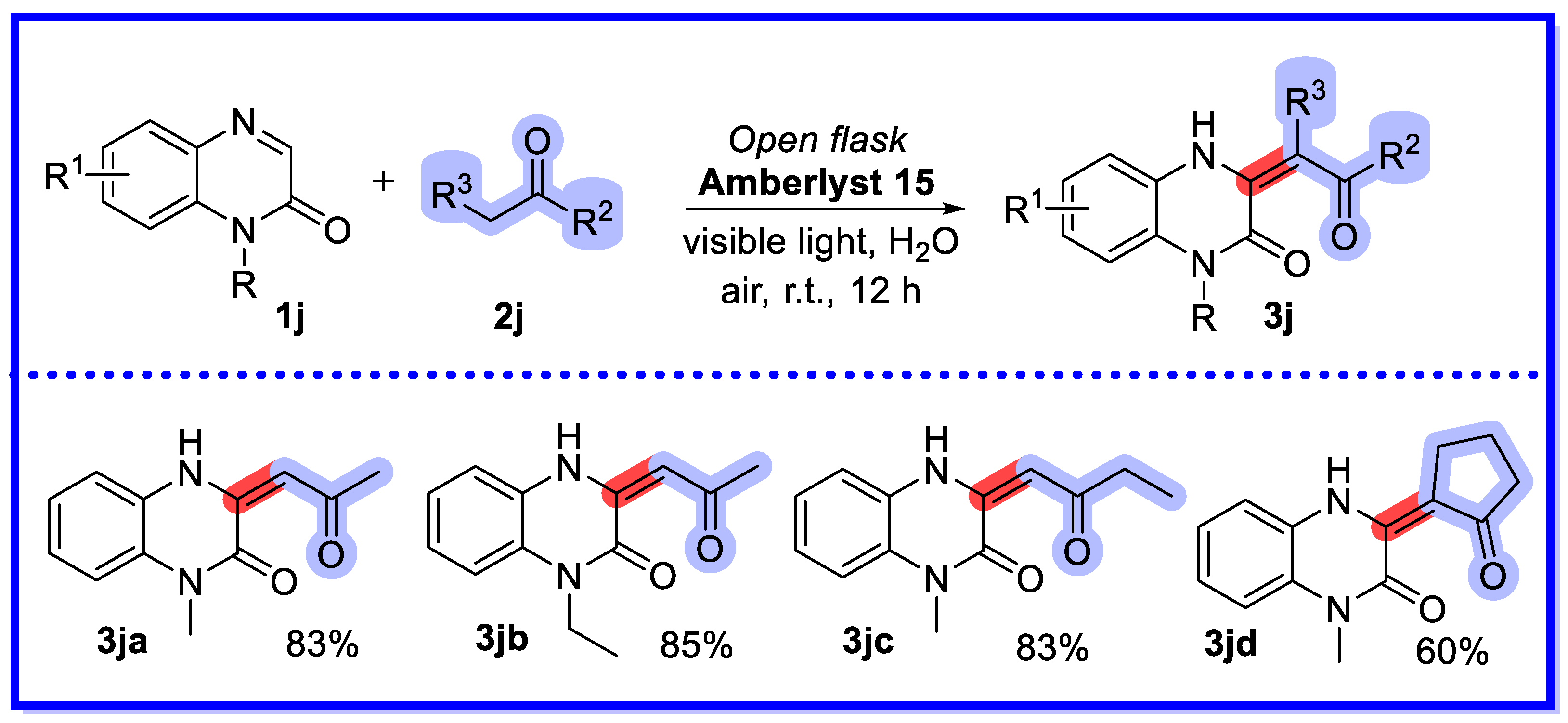
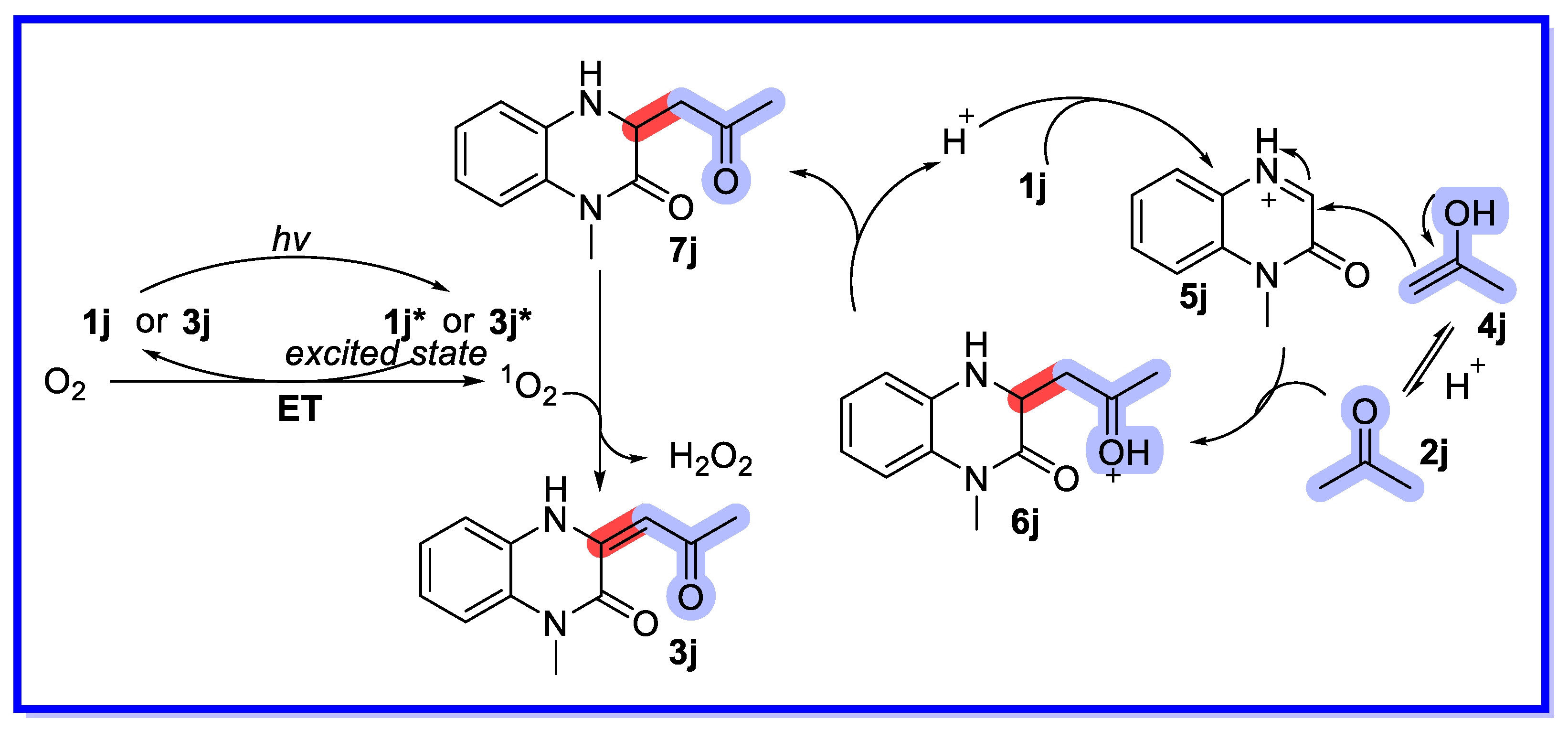
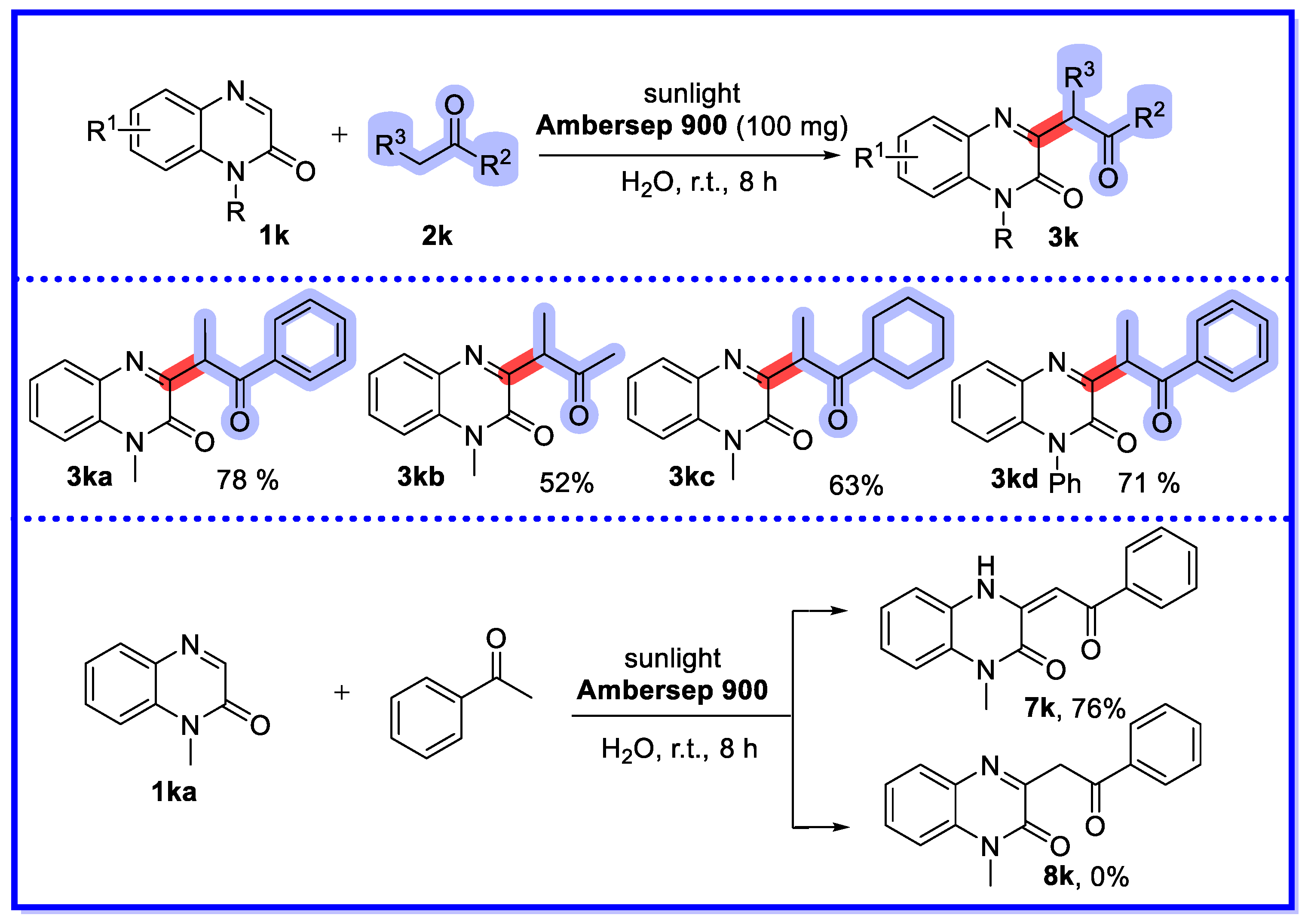
2.3. Ion-Exchange Resins as Heterogeneous Catalysts
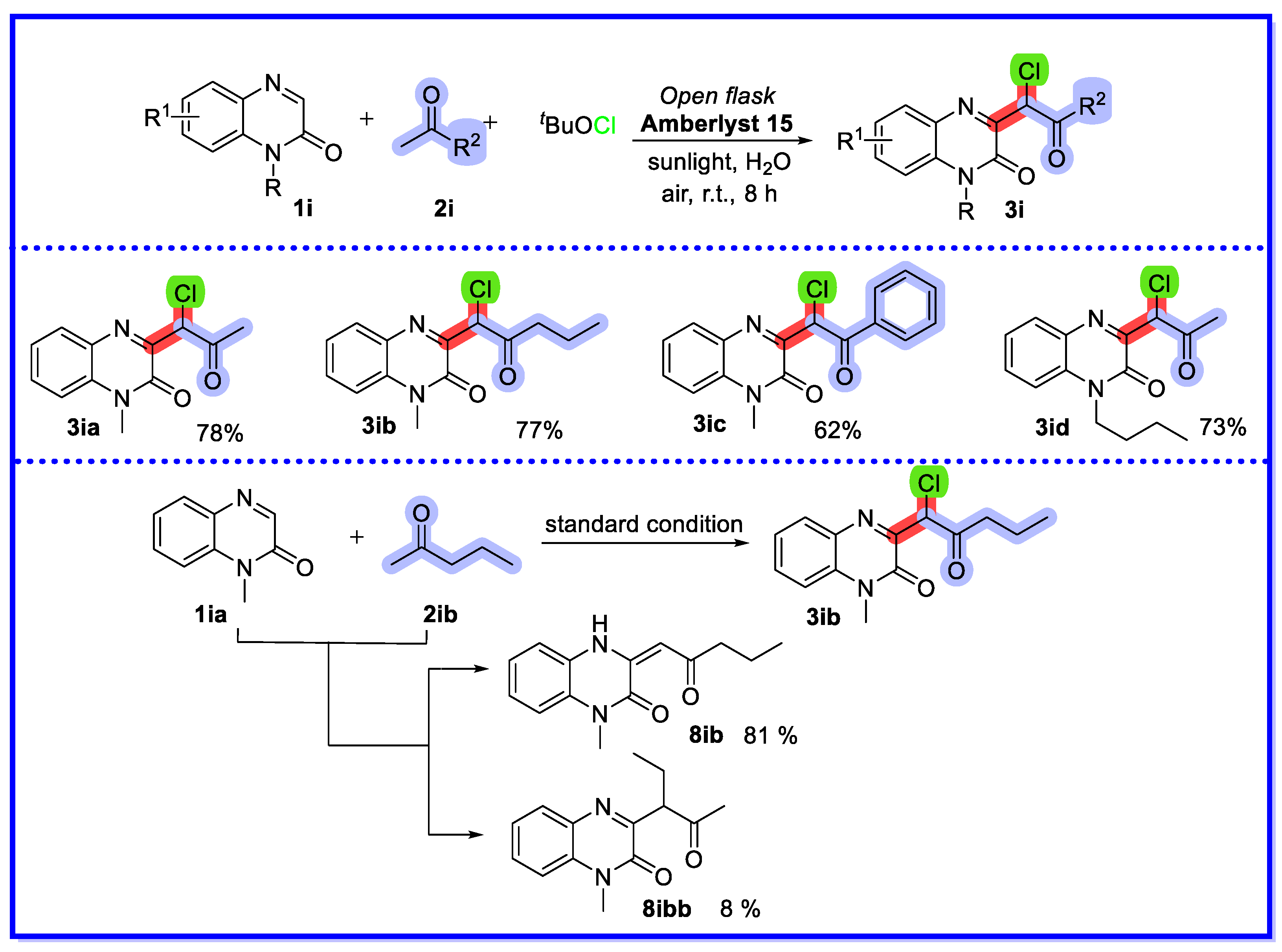
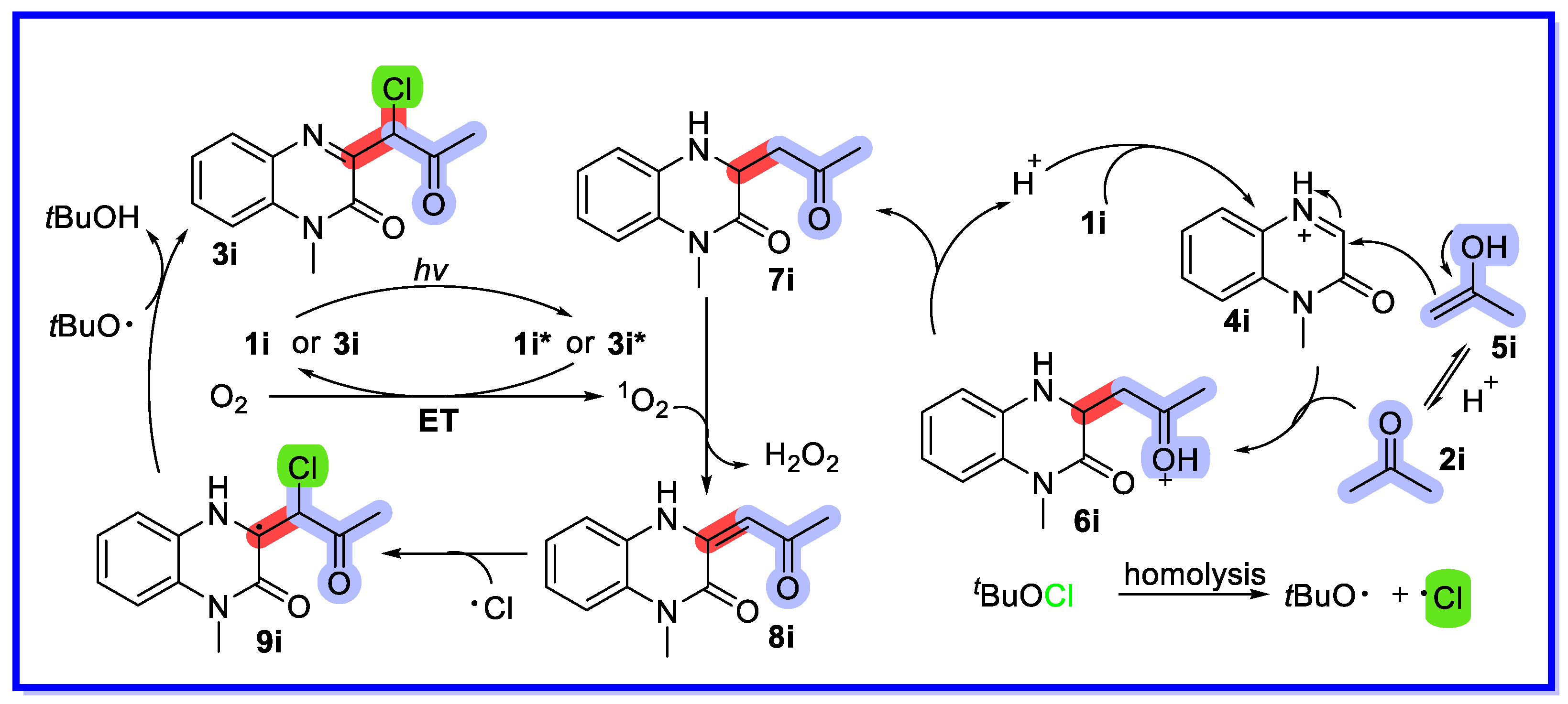
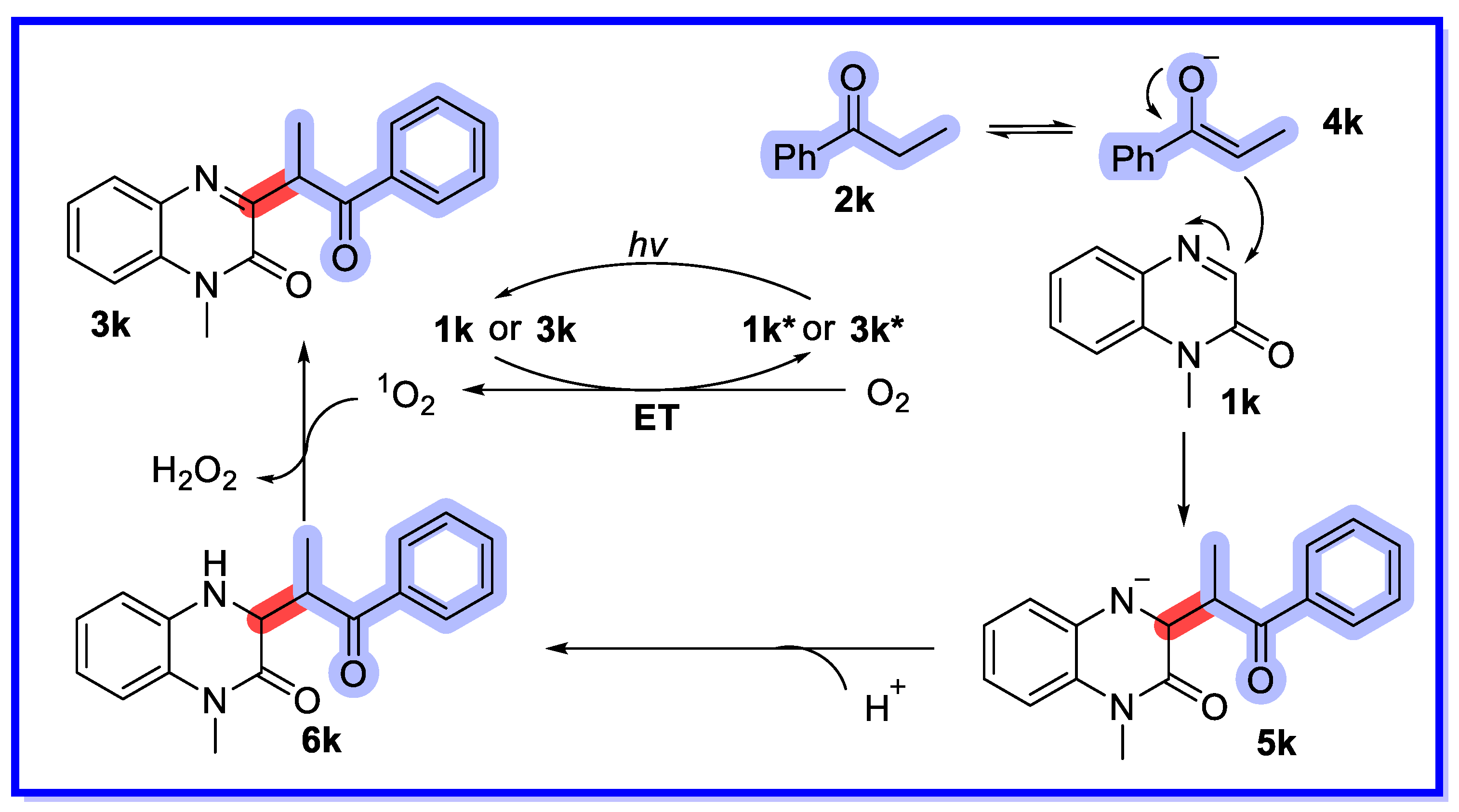
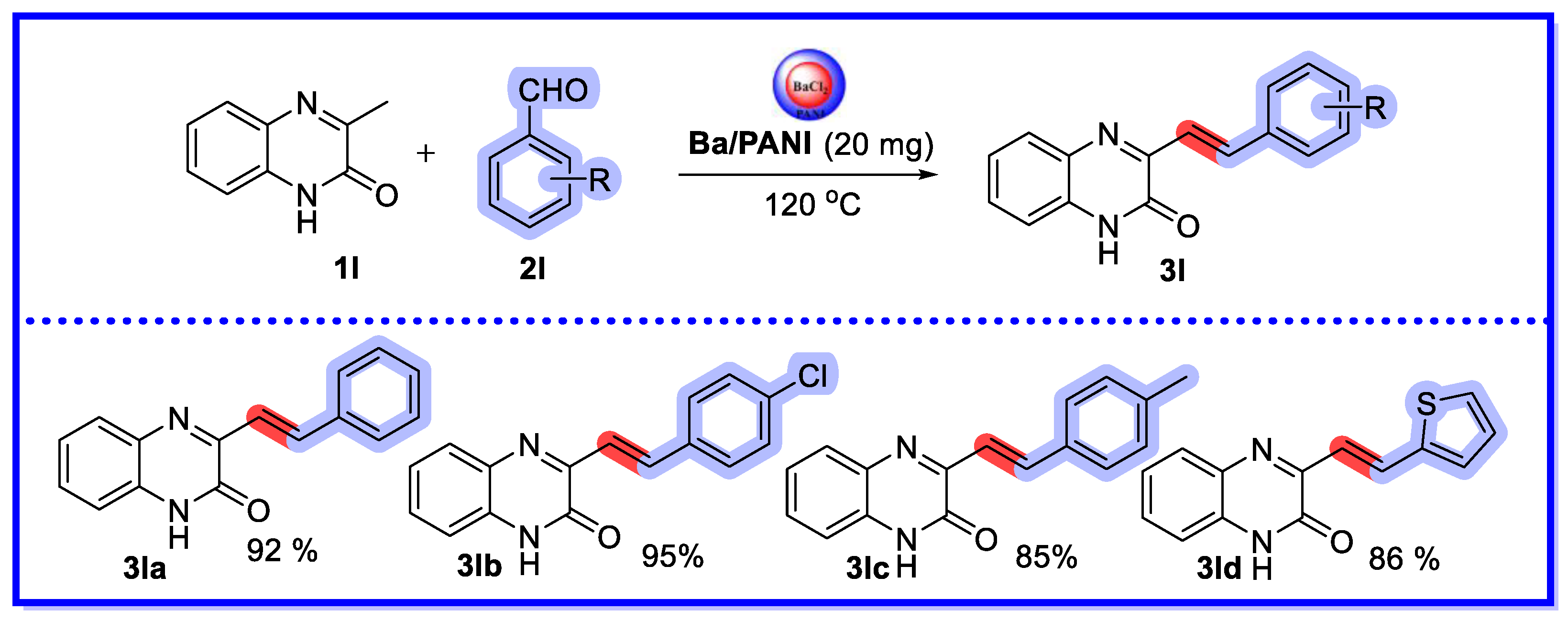
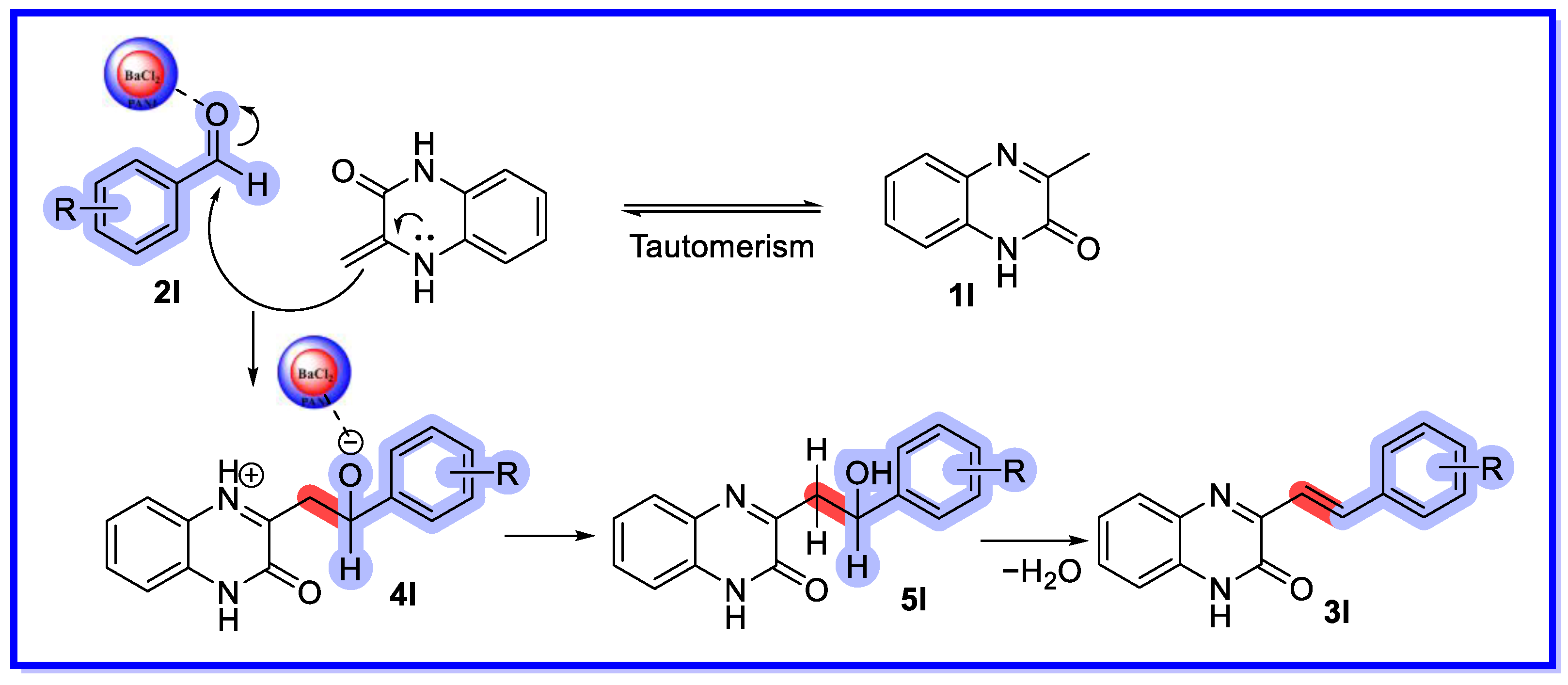
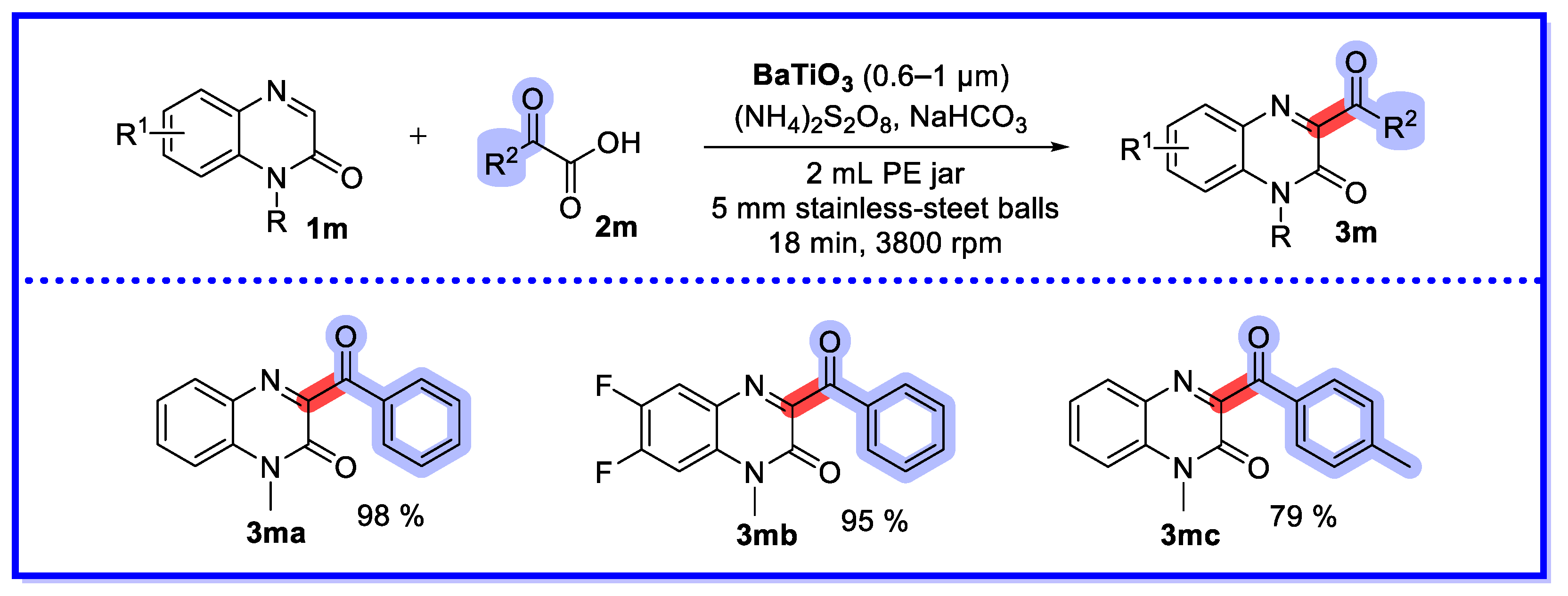
2.4. Other Heterogeneous Catalysts
3. Summary and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Schlögl, R. Heterogeneous Catalysis. Angew. Chem. Int. Ed. 2015, 54, 3465–3520. [Google Scholar] [CrossRef]
- Alsudairy, Z.; Brown, N.; Campbell, A.; Ambus, A.; Brown, B.; Smith-Petty, K.; Li, X. Covalent organic frameworks in heterogeneous catalysis: Recent advances and future perspective. Mater. Chem. Front. 2023, in press. [CrossRef]
- Shah, S.S.A.; Najam, T.; Bashir, M.S.; Peng, L.; Nazir, M.A.; Javed, M.S. Single-atom catalysts for next-generation rechargeable batteries and fuel cells. Energy Storage Mater. 2022, 45, 301–322. [Google Scholar] [CrossRef]
- Shen, Y.; Zhang, Q.; Sun, X.; Zhang, Y.; Cai, Q.; Deng, W.; Rao, S.; Wu, X.; Ye, Q. Conversion of wet microalgae to biodiesel with microalgae carbon based magnetic solid acid catalyst. Energy Convers. Manag. 2023, 286, 117022. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, H.; Fu, K.; Chen, X.; Qiu, M.; Fan, Y. Integration of solid acid catalyst and ceramic membrane to boost amine-based CO2 desorption. Energy 2023, 274, 127329. [Google Scholar] [CrossRef]
- Saetang, R.; Altamirano, M.; Romero-Zerón, L.B. Formulation of an Organic Base Catalyst with Animal Shells and Metal Oxide for Biodiesel Production. Energy Sources Part A Recovery Util. Environ. Eff. 2010, 32, 1690–1700. [Google Scholar] [CrossRef]
- Gao, M.; Wang, L.; Yang, Y.; Sun, Y.; Zhao, X.; Wan, Y. Metal and Metal Oxide Supported on Ordered Mesoporous Carbon as Heterogeneous Catalysts. ACS Catal. 2023, 13, 4060–4090. [Google Scholar] [CrossRef]
- Wang, S.; Wang, M.; Zhang, Y.; Wang, H.; Fei, H.; Liu, R.; Kong, H.; Gao, R.; Zhao, S.; Liu, T.; et al. Metal Oxide-Supported Metal Catalysts for Electrocatalytic Oxygen Reduction Reaction: Characterization Methods, Modulation Strategies, and Recent Progress. Small Methods 2023, e2201714. [Google Scholar] [CrossRef]
- Joshi, N.C.; Gururani, P.; Bhatnagar, P.; Kumar, V.; Vlaskin, M.S. Advances in Metal Oxide-based Nanocatalysts for Biodiesel Production: A Review. ChemBioEng Rev. 2023, 10, 258–271. [Google Scholar] [CrossRef]
- Shah, S.S.A.; Sufyan Javed, M.; Najam, T.; Molochas, C.; Khan, N.A.; Nazir, M.A.; Xu, M.; Tsiakaras, P.; Bao, S.-J. Metal oxides for the electrocatalytic reduction of carbon dioxide: Mechanism of active sites, composites, interface and defect engineering strategies. Coord. Chem. Rev. 2022, 471, 214716. [Google Scholar] [CrossRef]
- Malik, M.; Ibrahim, S.M.; Nazir, M.A.; Tahir, A.A.; Tufail, M.K.; Shah, S.S.A.; Anum, A.; Wattoo, M.A.; Rehman, A.u. Engineering of a Hybrid g-C3N4/ZnO-W/Cox Heterojunction Photocatalyst for the Removal of Methylene Blue Dye. Catalysts 2023, 13, 813. [Google Scholar] [CrossRef]
- Liu, T.; Wang, L.; Wu, K.; Wang, Q.; Yu, Z. Mono- and multinuclear pincer-type Ru(II) complex catalysts and their catalytic applications. Inorg. Chim. Acta 2023, 551, 121458. [Google Scholar] [CrossRef]
- Duangdee, B.; Rattanaphra, D.; Nuchdang, S.; Thanapimmetha, A.; Saisriyoot, M.; Srinophakun, P. Bifunctional mixed rare earth solid catalyst for biodiesel production from acid palm oil. J. Rare Earths 2023, 41, 240–249. [Google Scholar] [CrossRef]
- Li, C.; Wang, P.; He, M.; Yuan, X.; Fang, Z.; Li, Z. Rare earth-based nanomaterials in electrocatalysis. Coord. Chem. Rev. 2023, 489, 215204. [Google Scholar] [CrossRef]
- Song, S.; Sun, Y.; Yang, K.; Fo, Y.; Ji, X.; Su, H.; Li, Z.; Xu, C.; Huang, G.; Liu, J.; et al. Recent Progress in Metal-Molecular Sieve Catalysts for Propane Dehydrogenation. ACS Catal. 2023, 13, 6044–6067. [Google Scholar] [CrossRef]
- Sengupta, S.; Das, P.; Sharma, S.; Shukla, M.K.; Kumar, R.; Kumar Tonk, R.; Pandey, S.; Kumar, D. Role and Application of Biocatalysts in Cancer Drug Discovery. Catalysts 2023, 13, 250. [Google Scholar] [CrossRef]
- Feng, S.; Geng, Y.; Liu, H.; Li, H. Targeted Intermetallic Nanocatalysts for Sustainable Biomass and CO2 Valorization. ACS Catal. 2022, 12, 14999–15020. [Google Scholar] [CrossRef]
- Shah, S.S.A.; Najam, T.; Nazir, M.A.; Wu, Y.; Ali, H.; Rehman, A.U.; Rahman, M.M.; Imran, M.; Javed, M.S. Salt-assisted gas-liquid interfacial fluorine doping: Metal-free defect-induced electrocatalyst for oxygen reduction reaction. Mol. Catal. 2021, 514, 111878. [Google Scholar] [CrossRef]
- El-Hawash, S.A.M.; Habib, N.S.; Kassem, M.A. Synthesis of Some New Quinoxalines and 1,2,4-Triazolo[4,3-a]-quinoxalines for Evaluation of in vitro Antitumor and Antimicrobial Activities. Arch. Pharm. 2006, 339, 564–571. [Google Scholar] [CrossRef]
- Liu, R.; Huang, Z.; Murray, M.G.; Guo, X.; Liu, G. Quinoxalin-2(1H)-One Derivatives as Inhibitors Against Hepatitis C Virus. J. Med. Chem. 2011, 54, 5747–5768. [Google Scholar] [CrossRef]
- Qin, X.; Hao, X.; Han, H.; Zhu, S.; Yang, Y.; Wu, B.; Hussain, S.; Parveen, S.; Jing, C.; Ma, B.; et al. Design and Synthesis of Potent and Multifunctional Aldose Reductase Inhibitors Based on Quinoxalinones. J. Med. Chem. 2015, 58, 1254–1267. [Google Scholar] [CrossRef] [PubMed]
- Burganov, T.I.; Katsyuba, S.A.; Islamova, L.N.; Fazleeva, G.M.; Sharipova, S.M.; Kalinin, A.A.; Monari, A.; Assfeld, X. To what extent are the photophysical properties of quinoxaline- and quinoxalinone-based chromophores predictable? Dyes Pigm. 2019, 170, 107580. [Google Scholar] [CrossRef]
- Gerasimova, T.P.; Burganov, T.I.; Katsyuba, S.A.; Kalinin, A.A.; Islamova, L.N.; Fazleeva, G.M.; Ahmadeev, B.S.; Mustafina, A.R.; Monari, A.; Assfeld, X.; et al. Halochromic luminescent quinoxalinones as a basis for pH-sensing in organic and aqueous solutions. Dyes Pigm. 2021, 186, 108958. [Google Scholar] [CrossRef]
- Ke, Q.; Yan, G.; Yu, J.; Wu, X. Recent advances in the direct functionalization of quinoxalin-2(1H)-ones. Org. Biomol. Chem. 2019, 17, 5863–5881. [Google Scholar] [CrossRef]
- Sun, K.; Xiao, F.; Yu, B.; He, W.-M. Photo-/electrocatalytic functionalization of quinoxalin-2(1H)-ones. Chin. J. Catal. 2021, 42, 1921–1943. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, B.; Wu, M.; Lei, Y.-Z. Recent Developments in Direct C&H Functionalization of Quinoxalin-2(1H)-Ones via Multi-Component Tandem Reactions. Molecules 2023, 28, 2513. [Google Scholar]
- Azev, Y.A.; Kodess, M.I.; Ezhikova, M.A.; Ermakova, O.g.S.; Berseneva, V.S.; Bakulev, V.A. Reactions of quinoxalin-2-one with β-diketones: A new approach to 6a,7-dihydro-5H-pyrido[1,2-a]quinoxaline-6,8-diones. Mendeleev Commun. 2017, 27, 97–98. [Google Scholar] [CrossRef]
- Azev, Y.A.; Koptyaeva, O.S.; Mkrtchan, A.A.; Pospelova, T.A. Features of -C-C- coupling of quinoxaline-2-one with ethyl acetoacetate under acid catalysis. Chim. Techno Acta 2022, 9, 20229103. [Google Scholar] [CrossRef]
- Rusinov, G.L.; Slepukhin, P.A.; Charushin, V.N.; Dyachenko, O.A.; Kazheva, O.N.; Chekhlov, A.N.; Verbitsky, E.V.; Kodess, M.I.; Chupakhin, O.N. Chemistry of O- and C-adducts derived from 1,4-diazinium salts: The use of tetrahydropyrazines in the synthesis of condensed systems. Mendeleev Commun. 2006, 16, 26–29. [Google Scholar] [CrossRef]
- Utepova, I.A.; Chupakhin, O.N.; Trestsova, M.A.; Musikhina, A.A.; Kucheryavaya, D.A.; Charushin, V.N.; Rempel, A.A.; Kozhevnikova, N.S.; Valeeva, A.A.; Mikhaleva, A.I.; et al. Direct functionalization of the C–H bond in (hetero)arenes: Aerobic photoinduced oxidative coupling of azines with aromatic nucleophiles (SNH-reactions) in the presence of a CdS/TiO2 photocatalyst. Russ. Chem. Bull. 2016, 65, 445–450. [Google Scholar] [CrossRef]
- Dutysheva, E.A.; Utepova, I.A.; Trestsova, M.A.; Anisimov, A.S.; Charushin, V.N.; Chupakhin, O.N.; Margulis, B.A.; Guzhova, I.V.; Lazarev, V.F. Synthesis and approbation of new neuroprotective chemicals of pyrrolyl- and indolylazine classes in a cell model of Alzheimer’s disease. Eur. J. Med. Chem. 2021, 222, 113577. [Google Scholar] [CrossRef] [PubMed]
- Sonam; Shinde, V.N.; Rangan, K.; Kumar, A. Selectfluor-Mediated Regioselective C-3 Alkoxylation, Amination, Sulfenylation, and Selenylation of Quinoxalin-2(1H)-ones. J. Org. Chem. 2023, 88, 2344–2357. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Wang, P.; Dong, T.; Tsui, G.C.; Liao, S. Radical Fluorosulfonyl Heteroarylation of Unactivated Alkenes with Quinoxalin-2(1H)-ones and Related N-Heterocycles. Org. Lett. 2023, 25, 1088–1093. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, Y.; Yuan, S.; Yu, X.; Liu, L.; Li, J. Direct Alkylation of Quinoxalinones with Boracene-Based Alkylborate under Visible Light Irradiation. J. Org. Chem. 2023, 88, 6218–6226. [Google Scholar] [CrossRef]
- Li, Y.; Song, G.-T.; Tang, D.-Y.; Xu, Z.-G.; Chen, Z.-Z. Acid-Promoted Direct C–H Carbamoylation at the C-3 Position of Quinoxalin-2(1H)-ones with Isocyanide in Water. ACS Omega 2023, 8, 1577–1587. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Tang, Y.; Fan, L.; Lefebvre, Q.; Hou, H.; Rueping, M. Heterogeneous Visible-Light Photoredox Catalysis with Graphitic Carbon Nitride for α-Aminoalkyl Radical Additions, Allylations, and Heteroarylations. ACS Catal. 2018, 8, 9471–9476. [Google Scholar] [CrossRef]
- Savateev, A.; Ghosh, I.; König, B.; Antonietti, M. Photoredox Catalytic Organic Transformations using Heterogeneous Carbon Nitrides. Angew. Chem. Int. Ed. 2018, 57, 15936–15947. [Google Scholar] [CrossRef]
- Ghosh, I.; Khamrai, J.; Savateev, A.; Shlapakov, N.; Antonietti, M.; König, B. Organic semiconductor photocatalyst can bifunctionalize arenes and heteroarenes. Science 2019, 365, 360–366. [Google Scholar] [CrossRef]
- Xiao, Y.; Tian, G.; Li, W.; Xie, Y.; Jiang, B.; Tian, C.; Zhao, D.; Fu, H. Molecule Self-Assembly Synthesis of Porous Few-Layer Carbon Nitride for Highly Efficient Photoredox Catalysis. J. Am. Chem. Soc. 2019, 141, 2508–2515. [Google Scholar] [CrossRef]
- Geng, P.; Tang, Y.; Pan, G.; Wang, W.; Hu, J.; Cai, Y. A g-C3N4-based heterogeneous photocatalyst for visible light mediated aerobic benzylic C–H oxygenations. Green Chem. 2019, 21, 6116–6122. [Google Scholar] [CrossRef]
- Si, Y.-F.; Chen, X.-L.; Fu, X.-Y.; Sun, K.; Song, X.; Qu, L.-B.; Yu, B. Divergent g-C3N4-catalyzed Reactions of Quinoxalin-2(1H)-ones with N-Aryl Glycines under Visible Light: Solvent-Controlled Hydroaminomethylation and Annulation. ACS Sustain. Chem. Eng. 2020, 8, 10740–10746. [Google Scholar] [CrossRef]
- Xie, L.-Y.; Xie, Q.-X.; Chen, Y.-D.; Zhou, J.-Y.; Peng, S. Visible-Light-Induced Recyclable g-C3N4 Catalyzed C–H Hydroxylation of Quinoxalin-2(1H)-ones. Synthesis 2022, 55, 443–450. [Google Scholar] [CrossRef]
- Peng, S.; Hu, D.; Hu, J.-L.; Lin, Y.-W.; Tang, S.-S.; Tang, H.-S.; He, J.-Y.; Cao, Z.; He, W.-M. Metal-Free C3 Hydroxylation of Quinoxalin-2(1H)-ones in Water. Adv. Synth. Catal. 2019, 361, 5721–5726. [Google Scholar] [CrossRef]
- Peng, S.; Liu, J.; Yang, L.-H.; Xie, L.-Y. Sunlight Induced and Recyclable g-C3N4 Catalyzed C–H Sulfenylation of Quinoxalin-2(1H)-Ones. Molecules 2022, 27, 5044. [Google Scholar] [CrossRef]
- Zhou, J.; Zhou, P.; Zhao, T.; Ren, Q.; Li, J. (Thio)etherification of Quinoxalinones under Visible-Light Photoredox Catalysis. Adv. Synth. Catal. 2019, 361, 5371–5382. [Google Scholar] [CrossRef]
- Xie, L.-Y.; Chen, Y.-L.; Qin, L.; Wen, Y.; Xie, J.-W.; Tan, J.-X.; Huang, Y.; Cao, Z.; He, W.-M. Visible-light-promoted direct C–H/S–H cross-coupling of quinoxalin-2(1H)-ones with thiols leading to 3-sulfenylated quinoxalin-2(1H)-ones in air. Org. Chem. Front. 2019, 6, 3950–3955. [Google Scholar] [CrossRef]
- Song, H.-Y.; Jiang, J.; Wu, C.; Hou, J.-C.; Lu, Y.-H.; Wang, K.-L.; Yang, T.-B.; He, W.-M. Semi-heterogeneous g-C3N4/NaI dual catalytic C–C bond formation under visible light. Green Chem. 2023, 25, 3292–3296. [Google Scholar] [CrossRef]
- Paul, S.; Ha, J.H.; Park, G.E.; Lee, Y.R. Transition Metal-Free Iodosobenzene-Promoted Direct Oxidative 3-Arylation of Quinoxalin-2(H)-ones with Arylhydrazines. Adv. Synth. Catal. 2017, 359, 1515–1521. [Google Scholar] [CrossRef]
- Wu, S.-J.; Shi, Y.; Sun, K.; Yuan, X.-Y.; Tang, S.; Yu, B. Potassium doping carbon nitride: Dramatically enhanced photocatalytic properties for hydroxyalkylation of quinoxalin-2(1H)-ones with alcohol under air atmosphere. J. Catal. 2022, 415, 87–94. [Google Scholar] [CrossRef]
- Claes, B.; Boudewijns, T.; Muchez, L.; Hooyberghs, G.; Van der Eycken, E.V.; Vanderleyden, J.; Steenackers, H.P.; De Vos, D.E. Smart Metal–Organic Framework Coatings: Triggered Antibiofilm Compound Release. ACS Appl. Mater. Interfaces 2017, 9, 4440–4449. [Google Scholar] [CrossRef]
- Andrzejewski, M.; Katrusiak, A. Piezochromic Porous Metal–Organic Framework. J. Phys. Chem. Lett. 2017, 8, 279–284. [Google Scholar] [CrossRef]
- González Miera, G.; Bermejo Gómez, A.; Chupas, P.J.; Martín-Matute, B.; Chapman, K.W.; Platero-Prats, A.E. Topological Transformation of a Metal–Organic Framework Triggered by Ligand Exchange. Inorg. Chem. 2017, 56, 4576–4583. [Google Scholar] [CrossRef]
- Hu, L.; Lin, X.-M.; Mo, J.-T.; Lin, J.; Gan, H.-L.; Yang, X.-L.; Cai, Y.-P. Lead-Based Metal–Organic Framework with Stable Lithium Anodic Performance. Inorg. Chem. 2017, 56, 4289–4295. [Google Scholar] [CrossRef]
- Lemaire, P.C.; Zhao, J.; Williams, P.S.; Walls, H.J.; Shepherd, S.D.; Losego, M.D.; Peterson, G.W.; Parsons, G.N. Copper Benzenetricarboxylate Metal–Organic Framework Nucleation Mechanisms on Metal Oxide Powders and Thin Films formed by Atomic Layer Deposition. ACS Appl. Mater. Interfaces 2016, 8, 9514–9522. [Google Scholar] [CrossRef]
- Ding, S.-Y.; Wang, W. Covalent organic frameworks (COFs): From design to applications. Chem. Soc. Rev. 2013, 42, 548–568. [Google Scholar] [CrossRef]
- Lohse, M.; Bein, T. Covalent Organic Frameworks: Structures, Synthesis, and Applications. Adv. Funct. Mater. 2018, 28, 1705553. [Google Scholar] [CrossRef]
- Guan, X.; Chen, F.; Fang, Q.; Qiu, S. Design and applications of three dimensional covalent organic frameworks. Chem. Soc. Rev. 2020, 49, 1357–1384. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Xia, L.; Liu, X. Covalent organic frameworks (COFs): Perspectives of industrialization. CrystEngComm 2018, 20, 1613–1634. [Google Scholar] [CrossRef]
- Song, Y.; Sun, Q.; Aguila, B.; Ma, S. Opportunities of Covalent Organic Frameworks for Advanced Applications. Adv. Sci. 2019, 6, 1801410. [Google Scholar] [CrossRef] [PubMed]
- Yazdani, H.; Hooshmand, S.E.; Varma, R.S. Covalent organic frameworks and multicomponent reactions: An endearing give-and-take relationship. Org. Chem. Front. 2022, 9, 4178–4191. [Google Scholar] [CrossRef]
- Hoang, T.T.; To, T.A.; Cao, V.T.T.; Nguyen, A.T.; Nguyen, T.T.; Phan, N.T.S. Direct oxidative CH amination of quinoxalinones under copper-organic framework catalysis. Catal. Commun. 2017, 101, 20–25. [Google Scholar] [CrossRef]
- Li, Y.; Gao, M.; Wang, L.; Cui, X. Copper-catalysed oxidative amination of quinoxalin-2(1H)-ones with aliphatic amines. Org. Biomol. Chem. 2016, 14, 8428–8432. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Liu, S.; Bu, X.; Wang, Y.; Yu, J.; Yang, X. Covalent Organic Frameworks: A Sustainable Photocatalyst toward Visible-Light-Accelerated C3 Arylation and Alkylation of Quinoxalin-2(1H)-ones. Chem. Eur J. 2020, 26, 369–373. [Google Scholar] [CrossRef]
- Tian, M.; Wang, Y.; Bu, X.; Wang, Y.; Yang, X. An ultrastable olefin-linked covalent organic framework for photocatalytic decarboxylative alkylations under highly acidic conditions. Catal. Sci. Technol. 2021, 11, 4272–4279. [Google Scholar] [CrossRef]
- Wang, H.; Li, S.; Cui, Y.; Liu, M.; Bu, X.; Tian, H.; Yang, X. A covalent organic framework-catalyzed visible-light-induced three-component cascade synthesis of trifluoroalkyl and trifluoroalkenyl quinoxalin-2(1H)-one derivatives. New J. Chem. 2022, 46, 20412–20418. [Google Scholar] [CrossRef]
- Meng, N.; Lv, Y.; Liu, Q.; Liu, R.; Zhao, X.; Wei, W. Visible-light-induced three-component reaction of quinoxalin-2(1H)-ones, alkenes and CF3SO2Na leading to 3-trifluoroalkylated quinoxalin-2(1H)-ones. Chin. Chem. Lett. 2021, 32, 258–262. [Google Scholar] [CrossRef]
- Su, F.; Guo, Y. Advancements in solid acid catalysts for biodiesel production. Green Chem. 2014, 16, 2934–2957. [Google Scholar] [CrossRef]
- Russo, P.A.; Antunes, M.M.; Neves, P.; Wiper, P.V.; Fazio, E.; Neri, F.; Barreca, F.; Mafra, L.; Pillinger, M.; Pinna, N.; et al. Solid acids with SO3H groups and tunable surface properties: Versatile catalysts for biomass conversion. J. Mater. Chem. A 2014, 2, 11813–11824. [Google Scholar] [CrossRef]
- Chen, F.; Shi, L.; Yao, J.; Wang, Y.; Zhang, D.; Zhu, W.; Liu, Z. A highly efficient sulfonic acid resin for liquid-phase carbonylation of dimethoxymethane. Catal. Sci. Technol. 2018, 8, 580–590. [Google Scholar] [CrossRef]
- Xu, J.; He, L.; Liang, C.; Yue, X.; Ouyang, Y.; Zhang, P. Multicomponent Bifunctionalization of Methyl Ketones Enabled by Heterogeneous Catalysis and Solar Photocatalysis in Water. ACS Sustain. Chem. Eng. 2021, 9, 13663–13671. [Google Scholar] [CrossRef]
- Xu, J.; Huang, L.; He, L.; Ni, Z.; Shen, J.; Li, X.; Chen, K.; Li, W.; Zhang, P. A combination of heterogeneous catalysis and photocatalysis for the olefination of quinoxalin-2(1H)-ones with ketones in water: A green and efficient route to (Z)-enaminones. Green Chem. 2021, 23, 2123–2129. [Google Scholar] [CrossRef]
- He, L.; Liang, C.; Ouyang, Y.; Li, L.; Guo, Y.; Zhang, P.; Li, W. α-Functionalization of ketones promoted by sunlight and heterogeneous catalysis in the aqueous phase. Org. Biomol. Chem. 2022, 20, 790–795. [Google Scholar] [CrossRef] [PubMed]
- Devi, L.; Gupta, A.; Kapoor, K.K. Unexplored Potential of Polyaniline Embedded Barium Chloride Nanocomposite in the Synthesis of Styrylquinoxalin-2(1H)-Ones. Polycyclic Aromat. Compd. 2023, 43, 2104–2122. [Google Scholar] [CrossRef]
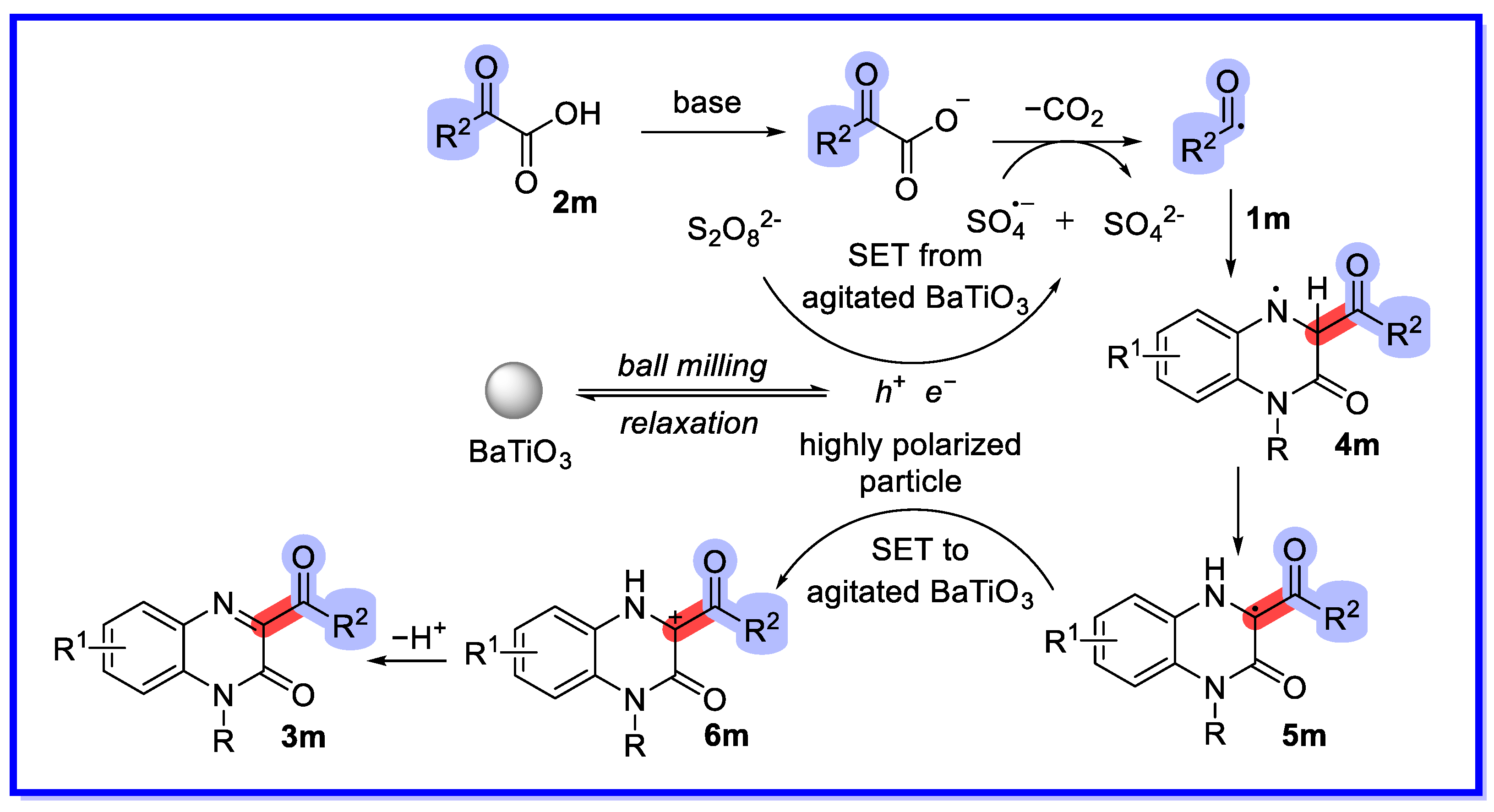
- He, Y.; Wang, G.; Hu, W.; Wei, D.; Jia, J.; Li, H.; Yuan, B. Piezocatalyzed Decarboxylative Acylation of Quinoxalin-2(1H)-ones Using Ball Milling. ACS Sustain. Chem. Eng. 2023, 11, 910–920. [Google Scholar] [CrossRef]
- Zeng, X.; Liu, C.; Wang, X.; Zhang, J.; Wang, X.; Hu, Y. Silver-catalyzed decarboxylative acylation of quinoxalin-2(1H)-ones with α-oxo-carboxylic acids. Org. Biomol. Chem. 2017, 15, 8929–8935. [Google Scholar] [CrossRef]
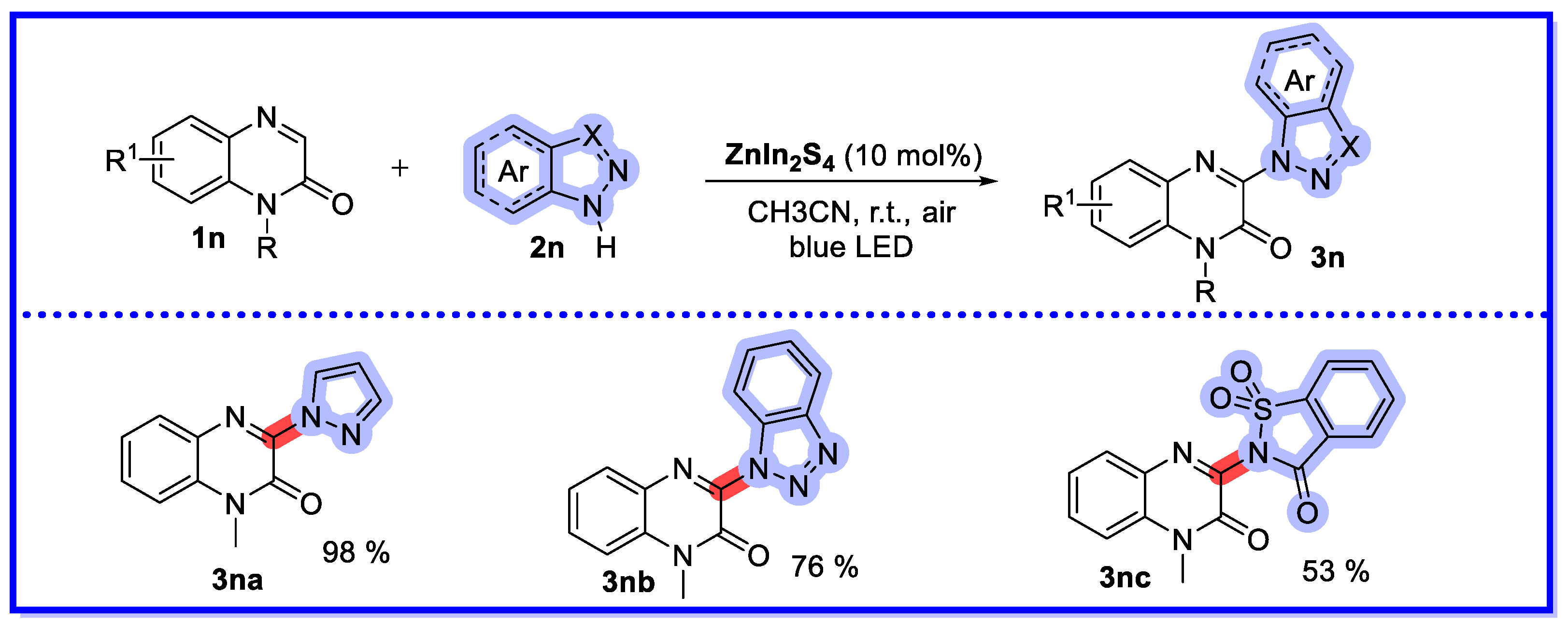
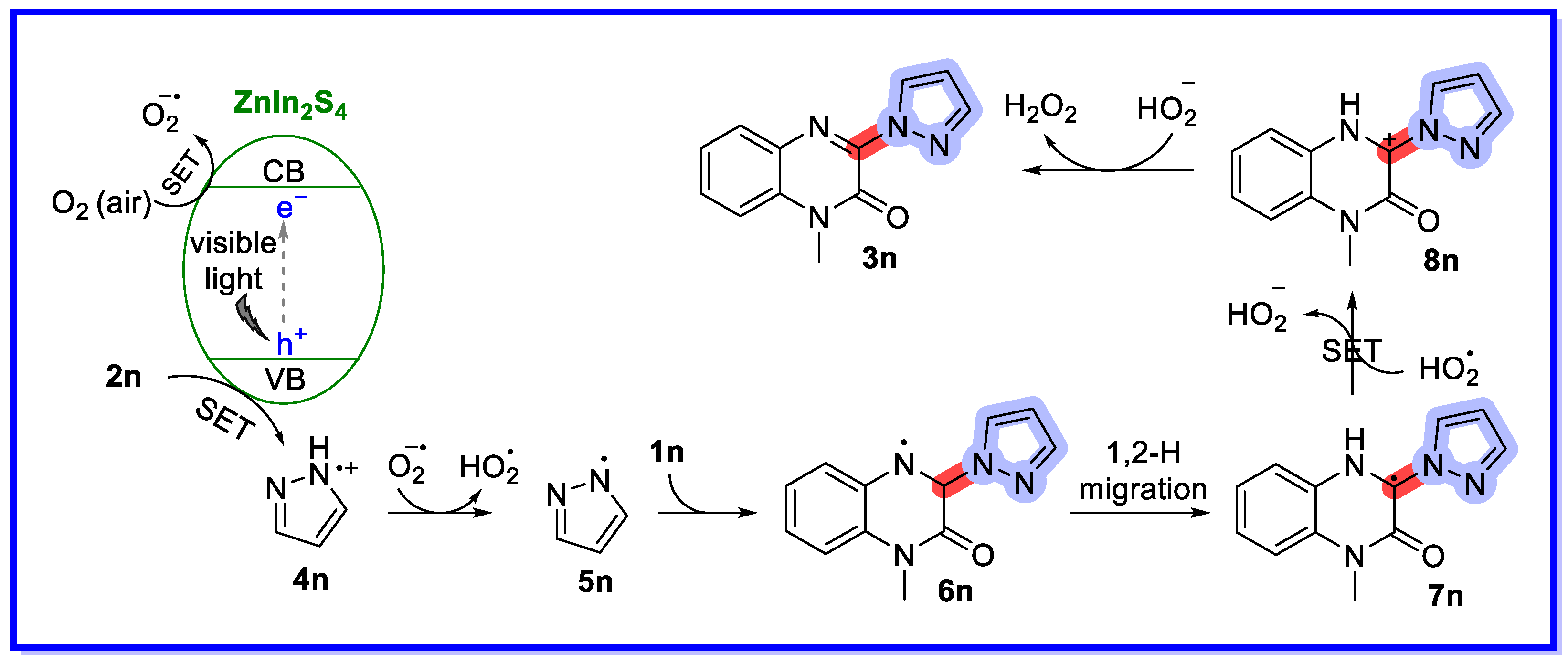
- Wang, R.-N.; Zeng, F.-L.; Chen, X.-L.; Zhu, H.-L.; Qu, L.-B.; Huang, X.-Q.; Tang, S.; Zhao, Y.-F.; Yu, B. Recyclable ZnIn2S4 Microspheres for Photocatalytic Azolation of N-Heterocycles. ACS Sustain. Chem. Eng. 2022, 10, 14212–14219. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Q.; Wang, H.; Wang, X.; Lei, Y. Recent Developments in Direct C–H Functionalization of Quinoxalin-2(1H)-Ones via Heterogeneous Catalysis Reactions. Molecules 2023, 28, 5030. https://doi.org/10.3390/molecules28135030
Yang Q, Wang H, Wang X, Lei Y. Recent Developments in Direct C–H Functionalization of Quinoxalin-2(1H)-Ones via Heterogeneous Catalysis Reactions. Molecules. 2023; 28(13):5030. https://doi.org/10.3390/molecules28135030
Chicago/Turabian StyleYang, Qiming, Hu Wang, Xiang Wang, and Yizhu Lei. 2023. "Recent Developments in Direct C–H Functionalization of Quinoxalin-2(1H)-Ones via Heterogeneous Catalysis Reactions" Molecules 28, no. 13: 5030. https://doi.org/10.3390/molecules28135030
APA StyleYang, Q., Wang, H., Wang, X., & Lei, Y. (2023). Recent Developments in Direct C–H Functionalization of Quinoxalin-2(1H)-Ones via Heterogeneous Catalysis Reactions. Molecules, 28(13), 5030. https://doi.org/10.3390/molecules28135030





