Stannate-Based Materials as Anodes in Lithium-Ion and Sodium-Ion Batteries: A Review
Abstract
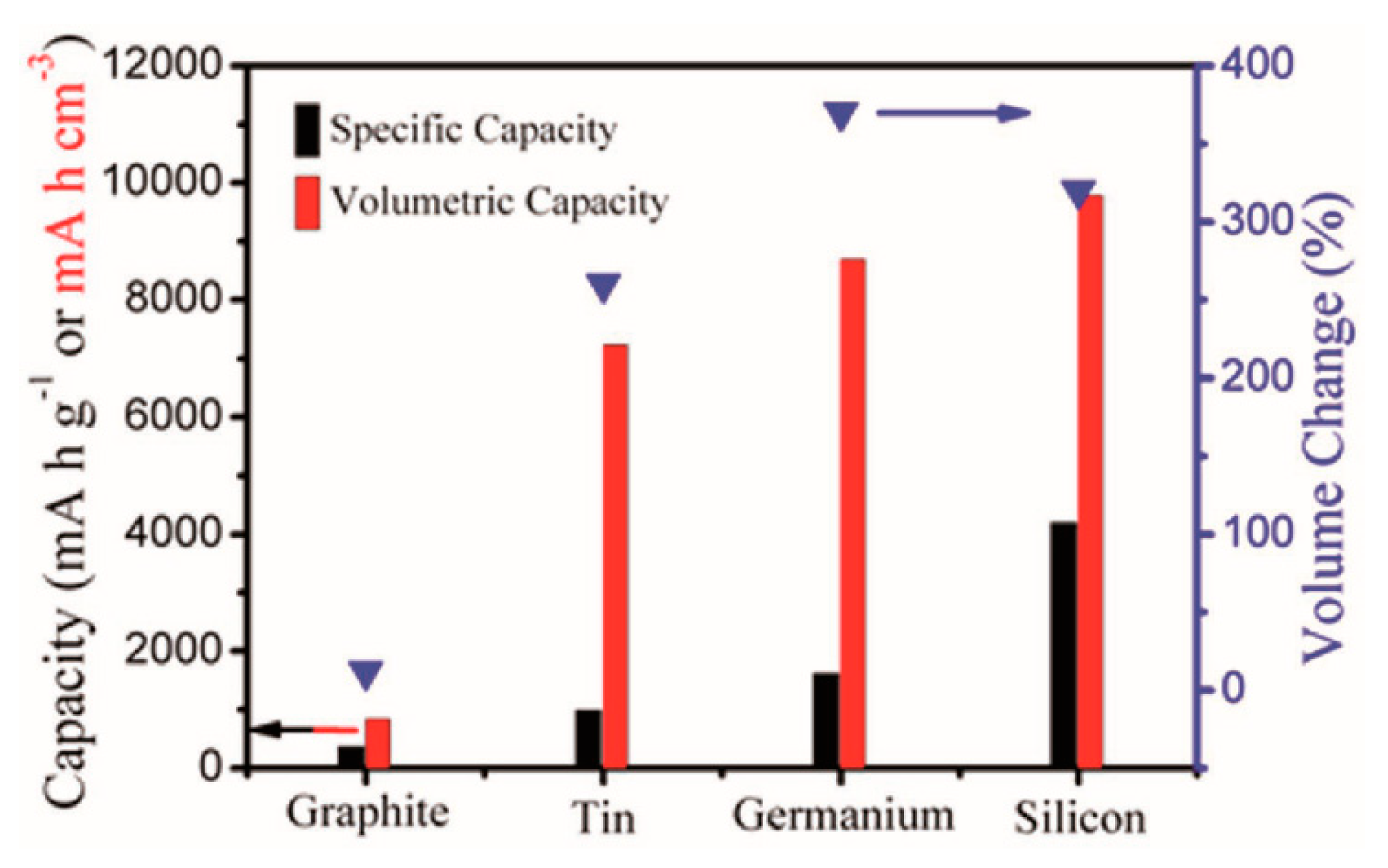
1. Introduction
2. M2SnO4-Based Anodes for LIBs
2.1. Lithium-Ion Storage
2.2. Design of M2SnO4-Based Anodes for LIBs
2.2.1. Nanostructures
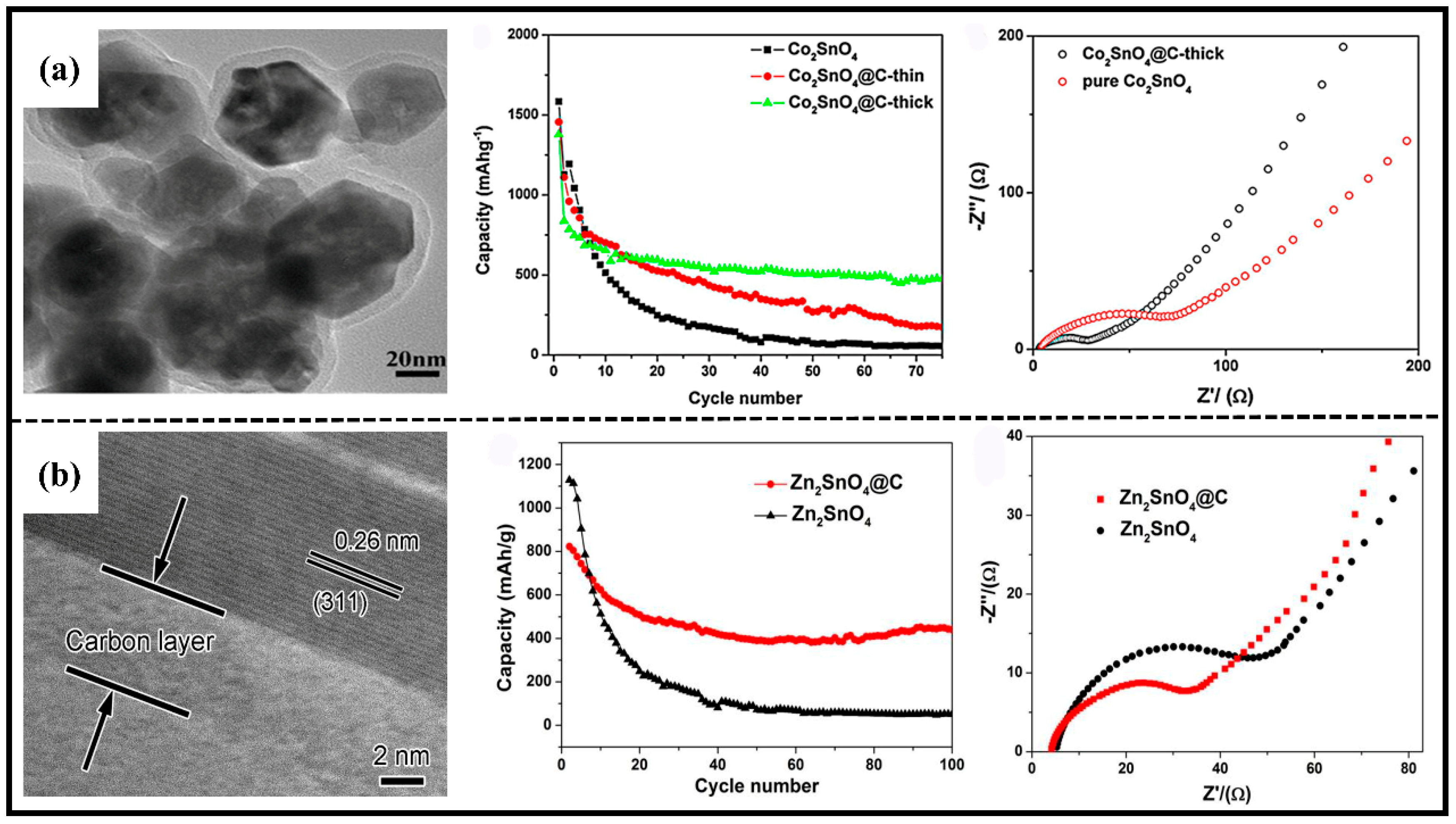
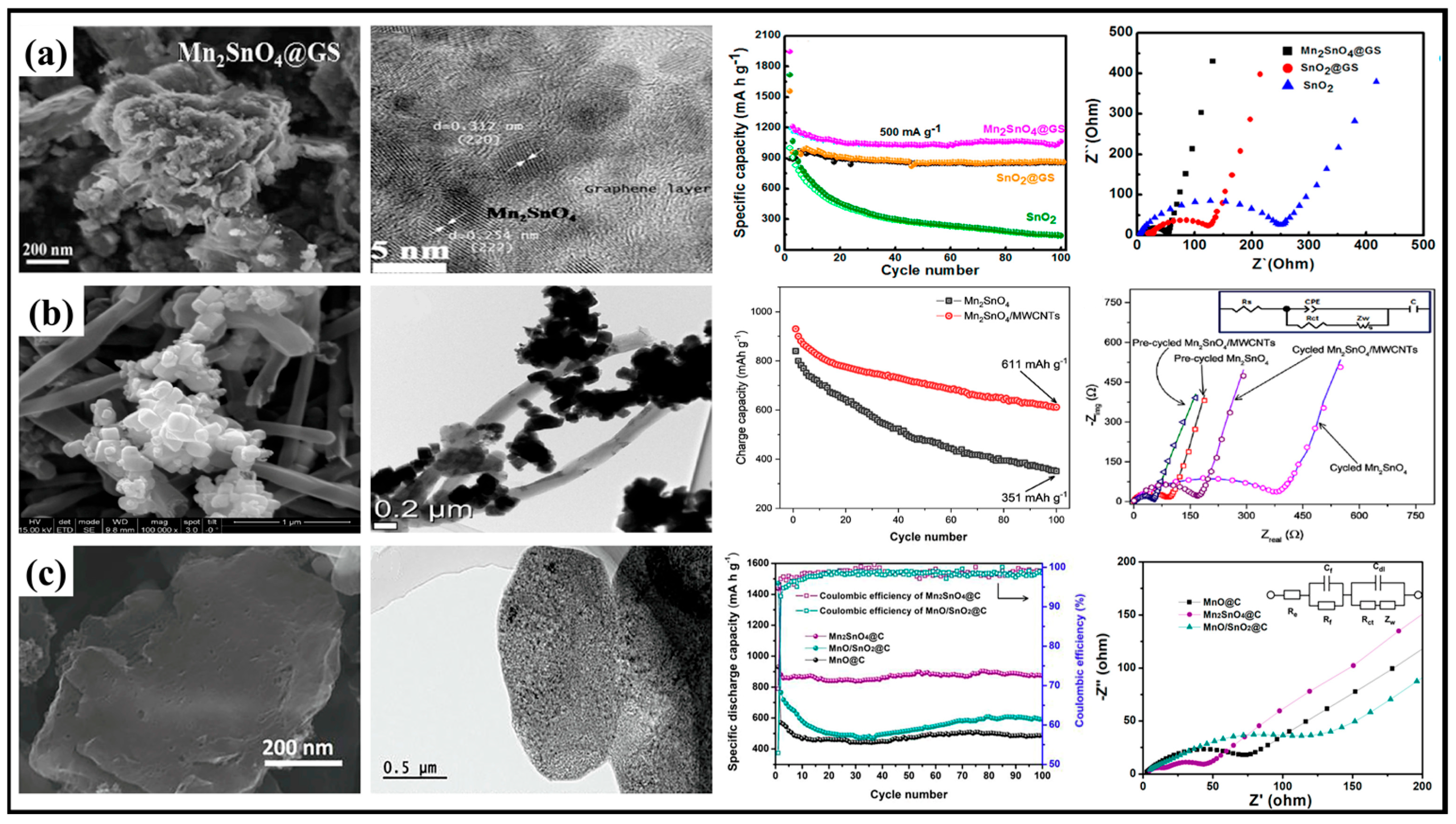
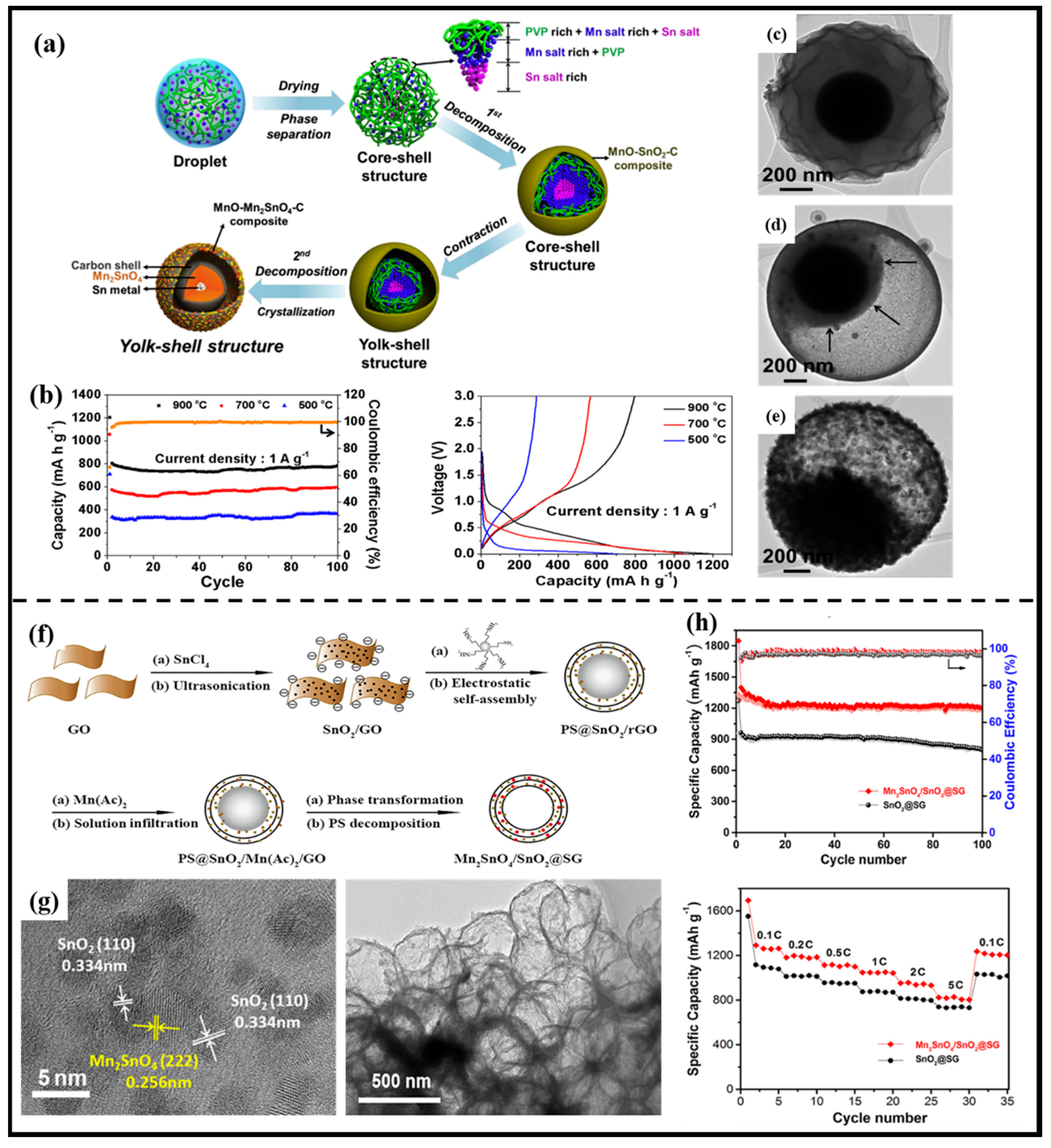
2.2.2. Composited with Carbon Materials
2.2.3. Heterogeneous Structures
2.2.4. Heteroatom Doping
3. M2SnO4-Based Anodes for SIBs
3.1. Sodium Ion Storage
3.2. Design of M2SnO4-Based Anodes for SIBs
3.2.1. Nanostructures
3.2.2. Composited with Carbon Materials
3.2.3. Heterogeneous Structures
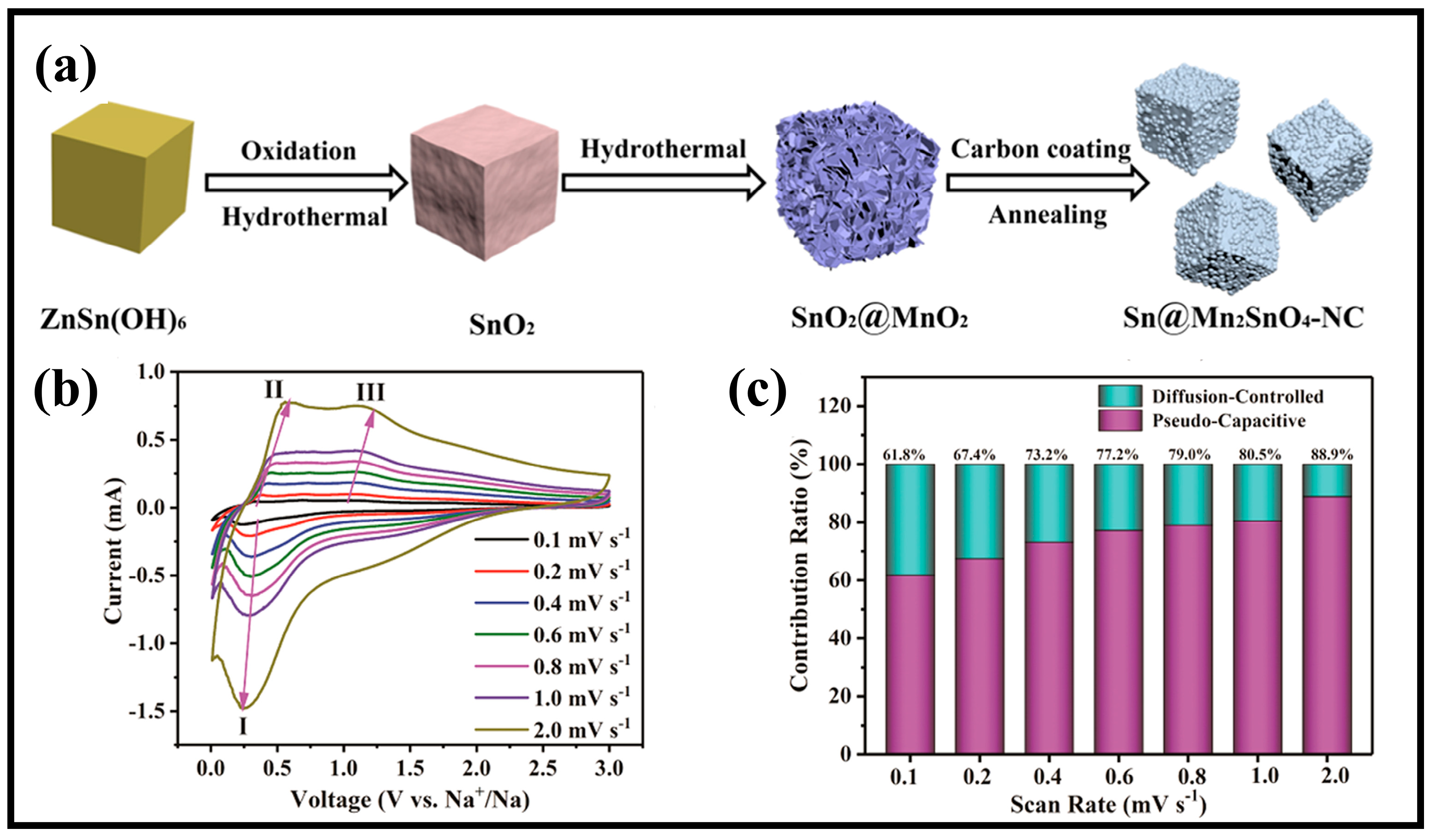
3.2.4. Heteroatom Doping
4. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, M.; Wang, H.; Luo, W.; Sherrell, P.C.; Chen, J.; Yang, J. Heterogeneous Single-Atom Catalysts for Electrochemical CO2 Reduction Reaction. Adv. Mater. 2020, 32, 2001848. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Y.; Ye, H.; Xia, X.; Jiao, X.; Li, G.; Mutahir, S.; Wang, L.; Mandler, D.; Lei, W.; Hao, Q. Hierarchical Electrodes of NiCo2S4 Nanosheets-Anchored Sulfur-Doped Co3O4 Nanoneedles with Advanced Performance for Battery-Supercapacitor Hybrid Devices. J. Mater. Chem. A 2019, 7, 3228–3237. [Google Scholar] [CrossRef]
- Feng, K.; Wang, F.; Yang, X.; Zhang, H.; Li, X.; Zhang, H. LiCr(MoO4)2: A New High Specific Capacity Cathode Material for Lithium Ion Batteries. J. Mater. Chem. A 2019, 7, 567–573. [Google Scholar] [CrossRef]
- Whittingham, M.S. Ultimate Limits to Intercalation Reactions for Lithium Batteries. Chem. Rev. 2014, 114, 11414–11443. [Google Scholar] [CrossRef]
- Chu, S.; Cui, Y.; Liu, N. The Path towards Sustainable Energy. Nat. Mater. 2017, 16, 16–22. [Google Scholar] [CrossRef]
- Wu, C.; Zhu, G.; Wang, Q.; Wu, M.; Zhang, H. Sn-Based Nanomaterials: From Composition and Structural Design to Their Electrochemical Performances for Li- and Na-Ion Batteries. Energy Storage Mater. 2021, 43, 430–462. [Google Scholar] [CrossRef]
- Li, W.-H.; Liang, H.-J.; Hou, X.-K.; Gu, Z.-Y.; Zhao, X.-X.; Guo, J.-Z.; Yang, X.; Wu, X.-L. Feasible Engineering of Cathode Electrolyte Interphase Enables the Profoundly Improved Electrochemical Properties in Dual-Ion Battery. J. Energy Chem. 2020, 50, 416–423. [Google Scholar] [CrossRef]
- Zhao, B.; Mattelaer, F.; Kint, J.; Werbrouck, A.; Henderick, L.; Minjauw, M.; Dendooven, J.; Detavernier, C. Atomic Layer Deposition of ZnO–SnO2 Composite Thin Film: The Influence of Structure, Composition and Crystallinity on Lithium-Ion Battery Performance. Electrochim. Acta 2019, 320, 134604. [Google Scholar] [CrossRef]
- Ying, H.; Han, W.-Q. Metallic Sn-Based Anode Materials: Application in High-Performance Lithium-Ion and Sodium-Ion Batteries. Adv. Sci. 2017, 4, 1700298. [Google Scholar] [CrossRef]
- Liu, P.; Zhu, K.; Xu, Y.; Bian, K.; Wang, J.; Tai, G.; Gao, Y.; Luo, H.; Lu, L.; Liu, J. Hierarchical Porous Intercalation-Type V2O3 as High-Performance Anode Materials for Li-Ion Batteries. Chem. Eur. J. 2017, 23, 7538–7544. [Google Scholar] [CrossRef]
- Schmuch, R.; Wagner, R.; Hörpel, G.; Placke, T.; Winter, M. Performance and Cost of Materials for Lithium-Based Rechargeable Automotive Batteries. Nat. Energy 2018, 3, 267–278. [Google Scholar] [CrossRef]
- Xin, F.; Whittingham, M.S. Challenges and Development of Tin-Based Anode with High Volumetric Capacity for Li-Ion Batteries. Electrochem. Energy Rev. 2020, 3, 643–655. [Google Scholar] [CrossRef]
- Rojo, T.; Hu, Y.-S.; Forsyth, M.; Li, X. Sodium-Ion Batteries. Adv. Energy Mater. 2018, 8, 1800880. [Google Scholar] [CrossRef]
- Vaalma, C.; Buchholz, D.; Weil, M.; Passerini, S. A Cost and Resource Analysis of Sodium-Ion Batteries. Nat. Rev. Mater. 2018, 3, 18013. [Google Scholar] [CrossRef]
- Kim, T.-H.; Shin, J.; Lee, K.-S.; Cho, E. Origin of the Superior Electrochemical Performance of Amorphous-Phase Conversion-Reaction-Based Electrode Materials for Na-Ion Batteries: Formation of a Bicontinuous Metal Network. ACS Appl. Mater. Interfaces 2020, 12, 22721–22729. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, H. Recent Developments of Phosphorus-Based Anodes for Sodium Ion Batteries. J. Mater. Chem. A 2018, 6, 24013–24030. [Google Scholar] [CrossRef]
- Hwang, J.-Y.; Myung, S.-T.; Sun, Y.-K. Sodium-Ion Batteries: Present and Future. Chem. Soc. Rev. 2017, 46, 3529–3614. [Google Scholar] [CrossRef]
- Abe, J.; Takahashi, K.; Kawase, K.; Kobayashi, Y.; Shiratori, S. Self-Standing Carbon Nanofiber and SnO2 Nanorod Composite as a High-Capacity and High-Rate-Capability Anode for Lithium-Ion Batteries. ACS Appl. Nano Mater. 2018, 1, 2982–2989. [Google Scholar] [CrossRef]
- Kim, H.; Kim, H.; Muhammad, S.; Um, J.H.; Sher Shah, M.S.A.; Yoo, P.J.; Yoon, W.-S. Catalytic Effect of Reduced Graphene Oxide on Facilitating Reversible Conversion Reaction in SnO2 for Next-Generation Li Rechargeable Batteries. J. Power Sources 2020, 446, 227321. [Google Scholar] [CrossRef]
- Gao, C.; Jiang, Z.; Wang, P.; Jensen, L.R.; Zhang, Y.; Yue, Y. Optimized Assembling of MOF/SnO2/Graphene Leads to Superior Anode for Lithium Ion Batteries. Nano Energy 2020, 74, 104868. [Google Scholar] [CrossRef]
- Bhattacharya, P.; Lee, J.H.; Kar, K.K.; Park, H.S. Carambola-Shaped SnO2 Wrapped in Carbon Nanotube Network for High Volumetric Capacity and Improved Rate and Cycle Stability of Lithium Ion Battery. Chem. Eng. J. 2019, 369, 422–431. [Google Scholar] [CrossRef]
- Zhang, R.; Upreti, S.; Stanley Whittingham, M. Tin-Iron Based Nano-Materials as Anodes for Li-Ion Batteries. J. Electrochem. Soc. 2011, 158, A1498. [Google Scholar] [CrossRef]
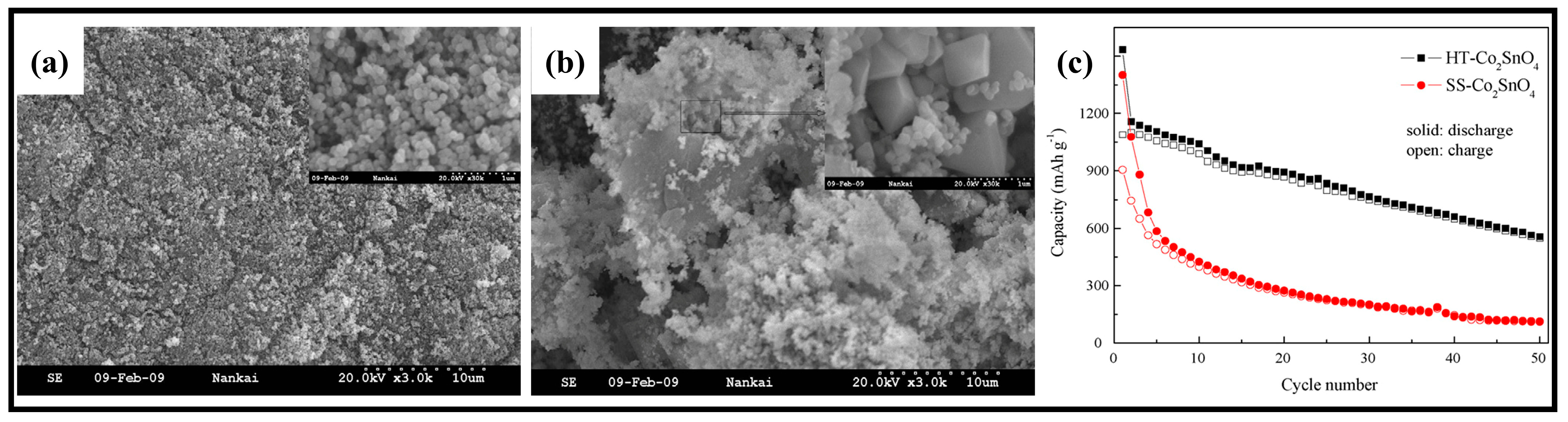
- Wang, G.; Gao, X.P.; Shen, P.W. Hydrothermal Synthesis of Co2SnO4 Nanocrystals as Anode Materials for Li-Ion Batteries. J. Power Sources 2009, 192, 719–723. [Google Scholar] [CrossRef]
- Kebede, M.A. Tin Oxide–Based Anodes for Both Lithium-Ion and Sodium-Ion Batteries. Curr. Opin. Electrochem. 2020, 21, 182–187. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, J.; Liu, S.; Du, Y. Effects of the Volume Changes and Elastic-Strain Energies on the Phase Transition in the Li-Sn Battery. J. Power Sources 2016, 330, 111–119. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, T.; Cao, G. Promises and Challenges of Tin-Based Compounds as Anode Materials for Lithium-Ion Batteries. Int. Mater. Rev. 2015, 60, 330–352. [Google Scholar] [CrossRef]
- Muñoz-Márquez, M.Á.; Saurel, D.; Gómez-Cámer, J.L.; Casas-Cabanas, M.; Castillo-Martínez, E.; Rojo, T. Na-Ion Batteries for Large Scale Applications: A Review on Anode Materials and Solid Electrolyte Interphase Formation. Adv. Energy Mater. 2017, 7, 1700463. [Google Scholar] [CrossRef]
- Che, H.; Chen, S.; Xie, Y.; Wang, H.; Amine, K.; Liao, X.-Z.; Ma, Z.-F. Electrolyte Design Strategies and Research Progress for Room-Temperature Sodium-Ion Batteries. Energy Environ. Sci. 2017, 10, 1075–1101. [Google Scholar] [CrossRef]
- Hu, R.; Zhang, H.; Lu, Z.; Liu, J.; Zeng, M.; Yang, L.; Yuan, B.; Zhu, M. Unveiling Critical Size of Coarsened Sn Nanograins for Achieving High Round-Trip Efficiency of Reversible Conversion Reaction in Lithiated SnO2 Nanocrystals. Nano Energy 2018, 45, 255–265. [Google Scholar] [CrossRef]
- Mueller, F.; Bresser, D.; Chakravadhanula, V.S.K.; Passerini, S. Fe-Doped SnO2 Nanoparticles as New High Capacity Anode Material for Secondary Lithium-Ion Batteries. J. Power Sources 2015, 299, 398–402. [Google Scholar] [CrossRef]
- Guo, J.; Zhu, H.; Sun, Y.; Tang, L.; Zhang, X. Flexible Foams of Graphene Entrapped SnO2–Co3O4 Nanocubes with Remarkably Large and Fast Lithium Storage. J. Mater. Chem. A 2016, 4, 16101–16107. [Google Scholar] [CrossRef]
- Reddy, M.V.; Subba Rao, G.V.; Chowdari, B.V.R. Metal Oxides and Oxysalts as Anode Materials for Li Ion Batteries. Chem. Rev. 2013, 113, 5364–5457. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Lin, X.; Liu, S.; Li, A.; Chen, X.; Zhou, J.; Ma, Z.; Song, H. Flake-like Carbon Coated Mn2SnO4 Nanoparticles as Anode Material for Lithium-Ion Batteries. Chem. Eng. J. 2019, 372, 269–276. [Google Scholar] [CrossRef]
- Zhuang, H.; Han, M.; Ma, W.; Ou, Y.; Jiang, Y.; Li, W.; Liu, X.; Zhao, B.; Zhang, J. Sandwich-Structured Graphene Hollow Spheres Limited Mn2SnO4/SnO2 Heterostructures as Anode Materials for High-Performance Lithium-Ion Batteries. J. Colloid Interface Sci. 2021, 586, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Muralidharan, N.; Nallathamby, K. CuCo2S4: Versatile Anode for High Capacity and High Rate for Lithium and Sodium Ion Battery Application. J. Phys. Chem. Solids 2021, 151, 109902. [Google Scholar] [CrossRef]
- Chen, J.S.; Li, C.M.; Zhou, W.W.; Yan, Q.Y.; Archer, L.A.; Lou, X.W. One-Pot Formation of SnO2 Hollow Nanospheres and α-Fe2O3@SnO2 Nanorattles with Large Void Space and Their Lithium Storage Properties. Nanoscale 2009, 1, 280–285. [Google Scholar] [CrossRef]
- Wan, S.; Liu, Q.; Cheng, M.; Chen, Y.; Chen, H. Binary-Metal Mn2SnO4 Nanoparticles and Sn Confined in a Cubic Frame with N-Doped Carbon for Enhanced Lithium and Sodium Storage. ACS Appl. Mater. Interfaces 2021, 13, 38278–38288. [Google Scholar] [CrossRef]
- Huang, B.; Pan, Z.; Su, X.; An, L. Tin-Based Materials as Versatile Anodes for Alkali (Earth)-Ion Batteries. J. Power Sources 2018, 395, 41–59. [Google Scholar] [CrossRef]
- Sun, S.; Liang, S. Morphological Zinc Stannate: Synthesis, Fundamental Properties and Applications. J. Mater. Chem. A 2017, 5, 20534–20560. [Google Scholar] [CrossRef]
- Chen, J.S.; Lou, X.W.D. SnO2-Based Nanomaterials: Synthesis and Application in Lithium-Ion Batteries. Small 2013, 9, 1877–1893. [Google Scholar] [CrossRef]
- Zhao, Q.; Ma, L.; Zhang, Q.; Wang, C.; Xu, X. SnO2-Based Nanomaterials: Synthesis and Application in Lithium-Ion Batteries and Supercapacitors. J. Nanomater. 2015, 2015, 850147. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, X.; Yan, B.; Li, D.; Lawes, S.; Sun, X. Significant Impact of 2D Graphene Nanosheets on Large Volume Change Tin-Based Anodes in Lithium-Ion Batteries: A Review. J. Power Sources 2015, 274, 869–884. [Google Scholar] [CrossRef]
- Liu, L.; Xie, F.; Lyu, J.; Zhao, T.; Li, T.; Choi, B.G. Tin-Based Anode Materials with Well-Designed Architectures for next-Generation Lithium-Ion Batteries. J. Power Sources 2016, 321, 11–35. [Google Scholar] [CrossRef]
- Zhao, M.; Zhao, Q.; Qiu, J.; Xue, H.; Pang, H. Tin-Based Nanomaterials for Electrochemical Energy Storage. RSC Adv. 2016, 6, 95449–95468. [Google Scholar] [CrossRef]
- Zhao, S.; Li, S.; Guo, T.; Zhang, S.; Wang, J.; Wu, Y.; Chen, Y. Advances in Sn-Based Catalysts for Electrochemical CO2 Reduction. Nano Micro Lett. 2019, 11, 62. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.-M.; Zhang, L.-J.; XiLi, D.-G.; Kang, J. Research Progress on Tin-Based Anode Materials for Sodium Ion Batteries. Rare Met. 2020, 39, 1005–1018. [Google Scholar] [CrossRef]
- Mou, H.; Xiao, W.; Miao, C.; Li, R.; Yu, L. Tin and Tin Compound Materials as Anodes in Lithium-Ion and Sodium-Ion Batteries: A Review. Front. Chem. 2020, 8, 141. [Google Scholar] [CrossRef]
- Dalapati, G.K.; Sharma, H.; Guchhait, A.; Chakrabarty, N.; Bamola, P.; Liu, Q.; Saianand, G.; Sai Krishna, A.M.; Mukhopadhyay, S.; Dey, A.; et al. Tin Oxide for Optoelectronic, Photovoltaic and Energy Storage Devices: A Review. J. Mater. Chem. A 2021, 9, 16621–16684. [Google Scholar] [CrossRef]
- Shinde, P.; Rout, C.S. Advances in Synthesis, Properties and Emerging Applications of Tin Sulfides and Its Heterostructures. Mater. Chem. Front. 2021, 5, 516–556. [Google Scholar] [CrossRef]
- Van Daele, K.; De Mot, B.; Pupo, M.; Daems, N.; Pant, D.; Kortlever, R.; Breugelmans, T. Sn-Based Electrocatalyst Stability: A Crucial Piece to the Puzzle for the Electrochemical CO2 Reduction toward Formic Acid. ACS Energy Lett. 2021, 6, 4317–4327. [Google Scholar] [CrossRef]
- Ansari, M.Z.; Ansari, S.A.; Kim, S.-H. Fundamentals and Recent Progress of Sn-Based Electrode Materials for Supercapacitors: A Comprehensive Review. J. Energy Storage 2022, 53, 105187. [Google Scholar] [CrossRef]
- Harish, S.; Sathyakam, P.U. A Review of Tin Selenide-Based Electrodes for Rechargeable Batteries and Supercapacitors. J. Energy Storage 2022, 52, 104966. [Google Scholar] [CrossRef]
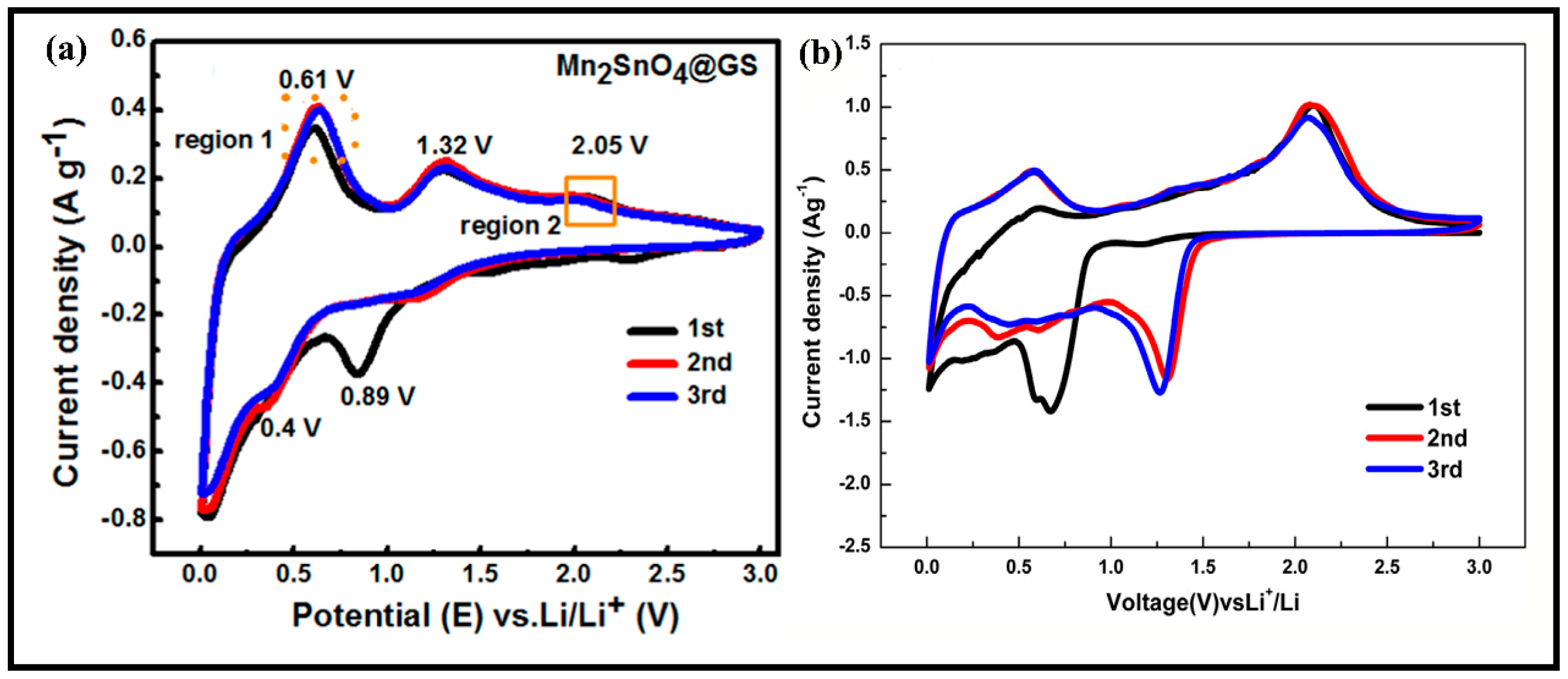
- Ur Rehman, W.; Xu, Y.; Sun, X.; Ullah, I.; Zhang, Y.; Li, L. Bouquet-Like Mn2SnO4 Nanocomposite Engineered with Graphene Sheets as an Advanced Lithium-Ion Battery Anode. ACS Appl. Mater. Interfaces 2018, 10, 17963–17972. [Google Scholar] [CrossRef]
- Zhang, R.; He, Y.; Xu, L. Controllable Synthesis of Hierarchical ZnSn(OH)6 and Zn2SnO4 Hollow Nanospheres and Their Applications as Anodes for Lithium Ion Batteries. J. Mater. Chem. A 2014, 2, 17979–17985. [Google Scholar] [CrossRef]
- Liang, K.; Cheang, T.; Wen, T.; Xie, X.; Zhou, X.; Zhao, Z.; Shen, C.; Jiang, N.; Xu, A. Facile Preparation of Porous Mn2SnO4/Sn/C Composite Cubes as High Performance Anode Material for Lithium-Ion Batteries. J. Phys. Chem. C 2016, 120, 3669–3676. [Google Scholar] [CrossRef]
- Zhong, K.; Xia, X.; Zhang, B.; Li, H.; Wang, Z.; Chen, L. MnO Powder as Anode Active Materials for Lithium Ion Batteries. J. Power Sources 2010, 195, 3300–3308. [Google Scholar] [CrossRef]
- Lim, Y.R.; Jung, C.S.; Im, H.S.; Park, K.; Park, J.; Cho, W.I.; Cha, E.H. Zn2GeO4 and Zn2SnO4 Nanowires for High-Capacity Lithium- and Sodium-Ion Batteries. J. Mater. Chem. A 2016, 4, 10691–10699. [Google Scholar] [CrossRef]
- Lei, S.; Tang, K.; Chen, C.; Jin, Y.; Zhou, L. Preparation of Mn2SnO4 Nanoparticles as the Anode Material for Lithium Secondary Battery. Mater. Res. Bull. 2009, 44, 393–397. [Google Scholar] [CrossRef]
- Kim, K.; Annamalai, A.; Park, S.H.; Kwon, T.H.; Pyeon, M.W.; Lee, M.-J. Preparation and Electrochemical Properties of Surface-Charge-Modified Zn2SnO4 Nanoparticles as Anodes for Lithium-Ion Batteries. Electrochim. Acta 2012, 76, 192–200. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.; Guo, X.; Yang, H. Construction of Macroporous Co2SnO4 with Hollow Skeletons as Anodes for Lithium-Ion Batteries. Gels 2022, 8, 257. [Google Scholar] [CrossRef]
- Qi, Y.; Du, N.; Zhang, H.; Wu, P.; Yang, D. Synthesis of Co2SnO4@C Core-Shell Nanostructures with Reversible Lithium Storage. J. Power Sources 2011, 196, 10234–10239. [Google Scholar] [CrossRef]
- Cherian, C.T.; Zheng, M.; Reddy, M.V.; Chowdari, B.V.R.; Sow, C.H. Zn2SnO4 Nanowires versus Nanoplates: Electrochemical Performance and Morphological Evolution during Li-Cycling. ACS Appl. Mater. Interfaces 2013, 5, 6054–6060. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Tian, X.; Gu, H.; Zhu, H.; Zhou, Y. Zn2SnO4@C Core–Shell Nanorods with Enhanced Anodic Performance for Lithium-Ion Batteries. J. Alloys Compd. 2015, 639, 239–243. [Google Scholar] [CrossRef]
- Yuvaraj, S.; Amaresh, S.; Lee, Y.S.; Selvan, R.K. Effect of Carbon Coating on the Electrochemical Properties of Co2SnO4 for Negative Electrodes in Li-Ion Batteries. RSC Adv. 2014, 4, 6407–6416. [Google Scholar] [CrossRef]
- Chen, C.; Ru, Q.; Hu, S.; An, B.; Song, X.; Hou, X. Co2SnO4 Nanocrystals Anchored on Graphene Sheets as High-Performance Electrodes for Lithium-Ion Batteries. Electrochim. Acta 2015, 151, 203–213. [Google Scholar] [CrossRef]
- Wang, B.-Y.; Wang, H.-Y.; Ma, Y.-L.; Zhao, X.-H.; Qi, W.; Jiang, Q.-C. Facile Synthesis of Fine Zn2SnO4 Nanoparticles/Graphene Composites with Superior Lithium Storage Performance. J. Power Sources 2015, 281, 341–349. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, J.; Xia, Z.; Shu, K. Facile Synthesis of Graphene Encapsulated Co2SnO4 Nanoparticles as Enhanced Anode Materials for Lithium-Ion Batteries. J. Nanosci. Nanotechnol. 2018, 18, 5679–5685. [Google Scholar] [CrossRef]
- Song, W.; Xie, J.; Hu, W.; Liu, S.; Cao, G.; Zhu, T.; Zhao, X. Facile Synthesis of Layered Zn2SnO4/Graphene Nanohybrid by a One-Pot Route and Its Application as High-Performance Anode for Li-Ion Batteries. J. Power Sources 2013, 229, 6–11. [Google Scholar] [CrossRef]
- Zhao, Y.; Huang, Y.; Sun, X.; Huang, H.; Wang, K.; Zong, M.; Wang, Q. Hollow Zn2SnO4 Boxes Wrapped with Flexible Graphene as Anode Materials for Lithium Batteries. Electrochim. Acta 2014, 120, 128–132. [Google Scholar] [CrossRef]
- Cui, L.; Li, X.; Yin, C.; Wang, J.; Li, S.; Zhang, Q.; Kang, S. Reduced Graphene Oxide Wrap Buffering Volume Expansion of Mn2SnO4 Anodes for Enhanced Stability in Lithium-Ion Batteries. Dalton Trans. 2019, 48, 504–511. [Google Scholar] [CrossRef]
- Zhang, J.; Liang, J.; Zhu, Y.; Wei, D.; Fan, L.; Qian, Y. Synthesis of Co2SnO4 Hollow Cubes Encapsulated in Graphene as High Capacity Anode Materials for Lithium-Ion Batteries. J. Mater. Chem. A 2014, 2, 2728–2734. [Google Scholar] [CrossRef]
- Wang, G.; Liu, Z.Y.; Liu, P. Co2SnO4-Multiwalled Carbon Nanotubes Composite as a Highly Reversible Anode Material for Lithium-Ion Batteries. Electrochim. Acta 2011, 56, 9515–9519. [Google Scholar] [CrossRef]
- Ali, G.; Mehboob, S.; Ahmad, M.; Akbar, M.; Kim, S.-O.; Ha, H.Y.; Chung, K.Y. High-Rate Lithium Storage and Kinetic Investigations of a Cubic Mn2SnO4@Carbon Nanotube Composite Anode. J. Alloys Compd. 2020, 823, 153789. [Google Scholar] [CrossRef]
- Huang, X.; Qi, X.; Boey, F.; Zhang, H. Graphene-Based Composites. Chem. Soc. Rev. 2012, 41, 666–686. [Google Scholar] [CrossRef]
- Schnorr, J.M.; Swager, T.M. Emerging Applications of Carbon Nanotubes. Chem. Mater. 2011, 23, 646–657. [Google Scholar] [CrossRef]
- Mishra, A.K.; Ramaprabhu, S. Magnetite Decorated Multiwalled Carbon Nanotube Based Supercapacitor for Arsenic Removal and Desalination of Seawater. J. Phys. Chem. C 2010, 114, 2583–2590. [Google Scholar] [CrossRef]
- Fan, Z.; Yan, J.; Zhi, L.; Zhang, Q.; Wei, T.; Feng, J.; Zhang, M.; Qian, W.; Wei, F. A Three-Dimensional Carbon Nanotube/Graphene Sandwich and Its Application as Electrode in Supercapacitors. Adv. Mater. 2010, 22, 3723–3728. [Google Scholar] [CrossRef]
- Hays, K.A.; Banek, N.A.; Wagner, M.J. High Performance Arsenic: Multiwall Carbon Nanotube Composite Anodes for Li-Ion Batteries. J. Electrochem. Soc. 2017, 164, A1635–A1643. [Google Scholar] [CrossRef]
- Xi, K.; Cao, S.; Peng, X.; Ducati, C.; Vasant Kumar, R.; Cheetham, A.K. Carbon with Hierarchical Pores from Carbonized Metal–Organic Frameworks for Lithium Sulphur Batteries. Chem. Commun. 2013, 49, 2192. [Google Scholar] [CrossRef]
- Wang, M.; Yang, H.; Zhou, X.; Shi, W.; Zhou, Z.; Cheng, P. Rational Design of SnO2@C Nanocomposites for Lithium Ion Batteries by Utilizing Adsorption Properties of MOFs. Chem. Commun. 2016, 52, 717–720. [Google Scholar] [CrossRef]
- Guan, B.Y.; Yu, X.Y.; Wu, H.B.; Lou, X.W.D. Complex Nanostructures from Materials Based on Metal-Organic Frameworks for Electrochemical Energy Storage and Conversion. Adv. Mater. 2017, 29, 1703614. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Wen, D.; Liang, Z.; Zou, R. Conductive Metal-Organic Frameworks for Electrochemical Energy Conversion and Storage. Coord. Chem. Rev. 2021, 446, 214119. [Google Scholar] [CrossRef]
- Shi, X.; Liu, S.; Tang, B.; Lin, X.; Li, A.; Chen, X.; Zhou, J.; Ma, Z.; Song, H. SnO2/TiO2 Nanocomposites Embedded in Porous Carbon as a Superior Anode Material for Lithium-Ion Batteries. Chem. Eng. J. 2017, 330, 453–461. [Google Scholar] [CrossRef]
- Li, A.; Song, H.; Bian, Z.; Shi, L.; Chen, X.; Zhou, J. ZnO Nanosheet/Squeezebox-like Porous Carbon Composites Synthesized by in Situ Pyrolysis of a Mixed-Ligand Metal–Organic Framework. J. Mater. Chem. A 2017, 5, 5934–5942. [Google Scholar] [CrossRef]
- Yue, H.; Shi, Z.; Wang, L.; Li, X.; Dong, H.; Yin, Y.; Yang, S. Rapid Calcination Synthesis of Zn2SnO4@C/Sn Composites for High-Performance Lithium Ion Battery Anodes. J. Alloys Compd. 2017, 723, 1018–1025. [Google Scholar] [CrossRef]
- Kennedy, T.; Bezuidenhout, M.; Palaniappan, K.; Stokes, K.; Brandon, M.; Ryan, K.M. Nanowire Heterostructures Comprising Germanium Stems and Silicon Branches as High-Capacity Li-Ion Anodes with Tunable Rate Capability. ACS Nano 2015, 9, 7456–7465. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhou, T.; Zhang, C.; Mao, J.; Liu, H.; Guo, Z. Boosted Charge Transfer in SnS/SnO2 Heterostructures: Toward High Rate Capability for Sodium-Ion Batteries. Angew. Chem. 2016, 128, 3469–3474. [Google Scholar] [CrossRef]
- Ju, H.; Hong, Y.; Cho, J.; Kang, Y. Strategy for Yolk-Shell Structured Metal Oxide-Carbon Composite Powders and Their Electrochemical Properties for Lithium-Ion Batteries. Carbon 2016, 100, 137–144. [Google Scholar] [CrossRef]
- Tian, J.; Yang, L.; Zha, L.; Wang, R.; Huang, S.; Xu, G.; Wei, T.; Li, H.; Cao, J.; Wei, X. Heterostructured Multi-Yolk-Shell SnO2/Mn2SnO4@C Nanoboxes for Stable and Highly Efficient Li/Na Storage. J. Power Sources 2021, 506, 230243. [Google Scholar] [CrossRef]
- Yuan, Y.; Chen, Z.; Yu, H.; Zhang, X.; Liu, T.; Xia, M.; Zheng, R.; Shui, M.; Shu, J. Heteroatom-Doped Carbon-Based Materials for Lithium and Sodium Ion Batteries. Energy Storage Mater. 2020, 32, 65–90. [Google Scholar] [CrossRef]
- Wang, H.-Y.; Wang, B.-Y.; Meng, J.-K.; Wang, J.-G.; Jiang, Q.-C. One-Step Synthesis of Co-Doped Zn2SnO4–Graphene–Carbon Nanocomposites with Improved Lithium Storage Performances. J. Mater. Chem. A 2015, 3, 1023–1030. [Google Scholar] [CrossRef]
- Suresh babu, G.N.; Kalaiselvi, N. Graphene Encapsulated Mn2SnO4/G Nanocubes as High-Capacity and Cycle-Stable Anode Material for Sodium Ion Batteries. J. Alloys Compd. 2021, 889, 161679. [Google Scholar] [CrossRef]
- Zou, G.; Hou, H.; Zhao, G.; Ge, P.; Yin, D.; Ji, X. N-Rich Carbon Coated CoSnO3 Derived from in Situ Construction of a Co-MOF with Enhanced Sodium Storage Performance. J. Mater. Chem. A 2018, 6, 4839–4847. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, W.; Zhou, C.; Yang, L.; Wang, H.; Gao, Q.; Zhu, M. N-Doped Carbon Encapsulated CoMoO4 Nanorods as Long-Cycle Life Anode for Sodium-Ion Batteries. J. Colloid Interface Sci. 2020, 576, 176–185. [Google Scholar] [CrossRef]
- Li, Z.; Ding, J.; Mitlin, D. Tin and Tin Compounds for Sodium Ion Battery Anodes: Phase Transformations and Performance. Acc. Chem. Res. 2015, 48, 1657–1665. [Google Scholar] [CrossRef]
- Ou, X.; Cao, L.; Liang, X.; Zheng, F.; Zheng, H.-S.; Yang, X.; Wang, J.-H.; Yang, C.; Liu, M. Fabrication of SnS2/Mn2SnS4/Carbon Heterostructures for Sodium-Ion Batteries with High Initial Coulombic Efficiency and Cycling Stability. ACS Nano 2019, 13, 3666–3676. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Fei, S.; Wu, C.; Xin, P.; Huang, S.; Selegård, L.; Uvdal, K.; Hu, Z. Integrated Design of Hierarchical CoSnO3@NC@MnO@NC Nanobox as Anode Material for Enhanced Lithium Storage Performance. ACS Appl. Mater. Interfaces 2020, 12, 19768–19777. [Google Scholar] [CrossRef]
- He, T.; Feng, J.; Ru, J.; Feng, Y.; Lian, R.; Yang, J. Constructing Heterointerface of Metal Atomic Layer and Amorphous Anode Material for High-Capacity and Fast Lithium Storage. ACS Nano 2019, 13, 830–838. [Google Scholar] [CrossRef] [PubMed]
- Denis, P.A. Heteroatom Codoped Graphene: The Importance of Nitrogen. ACS Omega 2022, 7, 45935–45961. [Google Scholar] [CrossRef]
- Li, Z.; Lin, J.; Li, B.; Yu, C.; Wang, H.; Li, Q. Construction of Heteroatom-Doped and Three-Dimensional Graphene Materials for the Applications in Supercapacitors: A Review. J. Energy Storage 2021, 44, 103437. [Google Scholar] [CrossRef]
- An, B.; Ru, Q.; Hu, S.; Song, X.; Li, J. Facile Synthesis and Electrochemical Performance of Co2SnO4/Co3O4 Nanocomposite for Lithium-Ion Batteries. Mater. Res. Bull. 2014, 60, 640–647. [Google Scholar] [CrossRef]
- An, B.; Ru, Q.; Hu, S.; Song, X.; Chen, C. Enhanced Electrochemical Performance of Nanomilling Co2SnO4/C Materials for Lithium Ion Batteries. Ionics 2015, 21, 2485–2493. [Google Scholar] [CrossRef]
- Wu, B.; Zhang, S.; Yao, F.; Zhang, F.; Xu, S. Synergistic Lithium Storage of a Multi-Component Co2SnO4/Co3O4/Al2O3/C Composite from a Single-Source Precursor. RSC Adv. 2015, 5, 69932–69938. [Google Scholar] [CrossRef]
- Miao, X.; Ge, X.; Wang, P.; Zhao, D.; Yin, L. Size-Tunable SnO2/Co2SnO4 Nanoparticles Loaded 3D Reduced Graphene Oxide Aerogel Architecture as Anodes for High Performance Lithium Ion Batteries. Electrochim. Acta 2020, 356, 136769. [Google Scholar] [CrossRef]
- Mani, V.; Babu, G.; Kalaiselvi, N. Li2MnSnO4/C Anode for High Capacity and High Rate Lithium Battery Applications. Electrochim. Acta 2014, 133, 347–353. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.; Liu, W.; Guo, X.; Yang, H. Sol–Gel Synthesis of Dictyophora-Shaped Hierarchically Porous Mn2SnO4/C Materials as Anodes for Li-Ion Batteries. New J. Chem. 2021, 45, 9538–9549. [Google Scholar] [CrossRef]
- Yuan, W.S.; Tian, Y.W.; Liu, G.Q. Synthesis and Electrochemical Properties of Pure Phase Zn2SnO4 and Composite Zn2SnO4/C. J. Alloys Compd. 2010, 506, 683–687. [Google Scholar] [CrossRef]
- Zhang, R.; He, Y.; Li, A.; Xu, L. Facile Synthesis of One-Dimensional Mn3O4/Zn2SnO4 Hybrid Composites and Their High Performance as Anodes for LIBs. Nanoscale 2014, 6, 14221–14226. [Google Scholar] [CrossRef]
- Gao, Z.; Zhang, X.; Hu, H.; Guo, D.; Zhao, H.; Yu, H. Influencing Factors of Low- and High-Temperature Behavior of Co-Doped Zn2SnO4–Graphene–Carbon Nanocomposite as Anode Material for Lithium-Ion Batteries. J. Electroanal. Chem. 2017, 791, 56–63. [Google Scholar] [CrossRef]
- Chen, H.; Zhou, J.; Guo, F.; Wang, Y.; Chen, Y.; Liang, Y.; Xu, Y.; Zhang, H. High-Performance Zn2SnO4 Anodes Enabled by MOF-Derived MnO Decoration and Carbon Confinement for Lithium-Ion Batteries. CrystEngComm 2021, 23, 2590–2598. [Google Scholar] [CrossRef]
- Kim, N.; Shim, J.-H.; Jae, W.; Song, J.; Kim, J. Zn2SnO4 Particles Coated with N-Doped Carbon as an Anode Material for Lithium and Sodium-Ion Batteries. J. Alloys Compd. 2019, 786, 346–355. [Google Scholar] [CrossRef]
- Song, J.; Lu, X.; Tian, Q.; Cui, L.; Chen, J.; Sui, Z. Dual-Stable Engineering Enables High-Performance Zn2SnO4-Based Lithium-Ion Battery Anode. J. Alloys Compd. 2022, 910, 164924. [Google Scholar] [CrossRef]


| Sr No. | Title of Paper | Reviewed Material | Year | Covers Stannate | Ref. |
|---|---|---|---|---|---|
| 1 | SnO2-Based Nanomaterials: Synthesis and Application in Lithium-Ion Batteries | SnO2-based nanomaterials | 2013 | No | [40] |
| 2 | SnO2-Based Nanomaterials: Synthesis and Application in Lithium-Ion Batteries and Supercapacitors | SnO2-based nanomaterials | 2015 | No | [41] |
| 3 | Significant impact of 2D graphene nanosheets on large volume change tin-based anodes in lithium-ion batteries: A review | Sn, SnO2, SnS2, MxSnOy (stannates) | 2015 | Section | [42] |
| 4 | Tin-based anode materials with well-designed architectures for next generation lithium-ion batteries | Sn-based multi-component intermetallics, SnO2, SnS2 | 2016 | No | [43] |
| 5 | Tin-based nanomaterials for electrochemical energy storage | Sn; Sn–M (M-Co, Ni, Cd, Zn, Fe), SnO2, SnS2, Sn4P3, SnF2 | 2016 | No | [44] |
| 6 | Morphological zinc stannate: synthesis, fundamental properties and applications | ZnSnO3 and Zn2SnO4 | 2017 | Only Zn-based | [39] |
| 7 | Metallic Sn-Based Anode Materials: Application in High-Performance Lithium-Ion and Sodium-Ion Batteries | Sn; Sn in carbon matrix, Sn alloy | 2017 | No | [9] |
| 8 | Tin-based materials as versatile anodes for alkali (earth)-ion batteries | SnO2, SnS2, Sn Alloy, MxSnOy | 2018 | Section | [38] |
| 9 | Advances in Sn-Based Catalysts for Electrochemical CO2 Reduction | SnO2, SnS2, tin alloy and its composites | 2019 | No | [45] |
| 10 | Tin oxide–based anodes for both lithium-ion and sodium-ion batteries | SnO2 and its composites | 2020 | No | [24] |
| 11 | Research progress on tin-based anode materials for sodium ion batteries | Sn, SnO2, SnSe, SnS and its composites | 2020 | No | [46] |
| 12 | Tin and Tin Compound Materials as Anodes in Lithium-Ion and Sodium-Ion Batteries: A Review | Tin alloy, SnO2, SnS2 and its composites | 2020 | No | [47] |
| 13 | Challenges and Development of Tin-Based Anode with High Volumetric Capacity for Li-Ion Batteries | Tin alloy and its composites | 2020 | No | [12] |
| 14 | Tin oxide for optoelectronic, photovoltaic and energy storage devices: a review | SnO2 and its composites | 2021 | No | [48] |
| 15 | Advances in Synthesis, Properties and Emerging Applications of Tin Sulfides and its Heterostructures | SnxSy and its composites | 2021 | No | [49] |
| 16 | Sn-Based Electrocatalyst Stability: A Crucial Piece to the Puzzle for the Electrochemical CO2 Reduction toward Formic Acid | Sn, SnO2 and its composites | 2021 | No | [50] |
| 17 | Sn-based nanomaterials: From composition and structural design to their electrochemical performances for Li- and Na-ion batteries | Sn, SnO2, SnS, SnS2 and its composites | 2021 | No | [6] |
| 18 | Fundamentals and recent progress of Sn-based electrode materials for supercapacitors: A comprehensive review | SnO2, SnSx and its composites | 2022 | No | [51] |
| 19 | A review of tin selenide-based electrodes for rechargeable batteries and supercapacitors | SnSe, SnSe2 and its composites | 2022 | No | [52] |
| Material | Synthesis Method | Morphology | ICE | Cycling Stability | Ref. |
|---|---|---|---|---|---|
| Co2SnO4 | hydrothermal reaction | nanoparticles | 71 | 556/50/0.03 | [23] |
| Co2SnO4@C | hydrothermal and heat treatment | core–shell nanostructures | - | 474/75/0.1 | [61] |
| Co2SnO4@MWCNTs | hydrothermal reaction | 3D network | 89 | 898/50/0.05 | [72] |
| Co2SnO4/Co3O4 | co-precipitation method | spherical and polyhedral | 74.4 | 702/50/0.1 | [101] |
| Co2SnO4@C | sonochemical and hydrothermal | cubic phase | 59 | 742/30/0.04 | [64] |
| Co2SnO4 HC@rGO | hydrothermal and heat treatment | hollow cubes | 69 | 1016/100/0.1 | [71] |
| Co2SnO4@C | co-precipitation process and high-energy ball milling | spherical particles | 58 | 573.8/100/0.2C | [102] |
| Co2SnO4/G | hydrothermal | nanoparticles | 69 | 1061/100/0.1 | [65] |
| Co2SnO4/Co3O4/Al2O3/C | co-precipitation | particle-like morphology | - | 1170/100/0.1 | [103] |
| Co2SnO4 NPs@rGO | hydrothermal | nanoparticles | 63.4 | 1037/200/0.2 | [67] |
| SnO2/Co2SnO4@rGOA | sol-gel and heat treatment | cubic phase | 70 | 588/1500/1 | [104] |
| Co2SnO4/C | sol–gel method combined with phase separation | hollow skeletons | 79.5 | 582/500/1 | [60] |
| Mn2SnO4 | hydrothermal and thermal decomposition | nanoparticles | 42 | - | [58] |
| Li2MnSnO4/C | oxalyl dihydrazide-assisted combustion method | spherical morphology | 69 | 610/100/0.1 | [105] |
| MnO/Mn2SnO4/C@ Sn/Mn2SnO4/C | spray pyrolysis | yolk–shell | 66 | 784/100/1 | [88] |
| Mn2SnO4/Sn/C | hydrothermal and heat treatment | porous cubes | 59.6 | 908/100/0.5 | [88] |
| Mn2SnO4@GS | hydrothermal and heat treatment | bouquet-like | 61 | 1070/200/0.4 | [53] |
| Mn2SnO4@rGO | hydrothermal and heat treatment | nanoparticles | - | 542/100/0.1 | [70] |
| Mn2SnO4@C | hydrothermal and heat treatment | flake-like | 69.2 | 986/100/0.1 | [33] |
| Mn2SnO4@MWCNTs | hydrothermal and heat treatment | cubic and nanotube | 72 | 611/100/0.1C | [73] |
| SnO2/Mn2SnO4@C | hydrothermal | multi-yolk–shell nanoboxes | 50.55 | 1293/100/0.2 | [89] |
| Sn@ Mn2SnO4-NC | hydrothermal and heat treatment | cubic frame | 73.88 | 823/600/1 | [37] |
| Mn2SnO4/C | sol–gel and heat treatment | Dictyophora-shaped hierarchically porous | - | 784/500/1 | [106] |
| Mn2SnO4/SnO2@SG | heat treatment | hollow spheres | 71.4 | 1180/100/0.1C | [34] |
| Zn2SnO4/C | hydrothermal and carbothermic reduction | nanoparticles | 61 | 563/40/- | [107] |
| Zn2SnO4 | vapor transport/hydrothermal | nanowires/nanoplates | 41 | 470/50/- | [62] |
| Zn2SnO4/G | in situ hydrothermal | layered | 54 | 688/50/0.2 | [68] |
| Mn3O4/Zn2SnO4 | hydrothermal | nanorod/nanoneedle | 59.6 | 529/50/0.5 | [108] |
| Zn2SnO4 | hydrothermal | hollow nanospheres | 66.2 | 602.5/60/0.1 | [54] |
| Zn2SnO4/G | co-precipitation and alkali etching method | hollow boxes | 62 | 678.2/45/0.3 | [69] |
| Zn2SnO4@C | hydrothermal and carbonization approach | core–shell nanorods | 53.6 | 495/100/0.1 | [63] |
| Zn2SnO4/G | hydrothermal | nanoparticles | 57.4 | 492/500/0.5 | [66] |
| Zn2SnO4–graphene–carbon | hydrothermal | nanoparticles | 62.3 | 461/200/0.2 | [91] |
| Zn2SnO4 | hydrothermal | nanowires | 52.5 | 983/100/0.1 | [57] |
| Zn2SnO4@C/Sn | calcination | large spheres | 92.4 | 1140/100/0.1 | [85] |
| Co–ZTO–G–C | hydrothermal and heat treatment | - | 62.3 | 695/50/0.1C | [109] |
| Zn2SnO4/N-doped carbon composite | hydrothermal and heat treatment | spherical shaped particles | 71.2 | 992.4/100/0.6 | [110] |
| LC@Zn2SnO4@MnO/C(MOF) | solvothermal method and high-temperature annealing treatment | porous micro/nanostructures | 65.9 | 1185.6/150/0.2 | [111] |
| Zn2SnO4@V@PC | carbonization | yolk–shell | 57.2 | 438/600/1 | [112] |
| Material | Synthesis Method | Morphology | ICE | Cycling Stability | Ref. |
|---|---|---|---|---|---|
| Zn2SnO4 | hydrothermal | nanowires | 52.5 | 306/100/0.1 | [57] |
| Zn2SnO4/NC | hydrothermal and heat treatment | spherical shaped particles | - | 324.4/100 | [110] |
| Mn2SnO4/G | hydrothermal and heat treatment | nanocubes | 45.6 | 106/1000/1 | [92] |
| SnO2/Mn2SnO4@C | hydrothermal and heat treatment | nanoboxes | 65.9 | 203/100/0.2 | [89] |
| Sn@ Mn2SnO4-NC | hydrothermal and heat treatment | cubic frame | 64 | 185.8/7000/2 | [37] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, Y.-K.; Li, Z.-W.; Zhang, S.-C.; Su, T.; Zhang, Z.-H.; Jiao, A.-J.; Fu, Z.-H. Stannate-Based Materials as Anodes in Lithium-Ion and Sodium-Ion Batteries: A Review. Molecules 2023, 28, 5037. https://doi.org/10.3390/molecules28135037
Duan Y-K, Li Z-W, Zhang S-C, Su T, Zhang Z-H, Jiao A-J, Fu Z-H. Stannate-Based Materials as Anodes in Lithium-Ion and Sodium-Ion Batteries: A Review. Molecules. 2023; 28(13):5037. https://doi.org/10.3390/molecules28135037
Chicago/Turabian StyleDuan, You-Kang, Zhi-Wei Li, Shi-Chun Zhang, Tong Su, Zhi-Hong Zhang, Ai-Jun Jiao, and Zhen-Hai Fu. 2023. "Stannate-Based Materials as Anodes in Lithium-Ion and Sodium-Ion Batteries: A Review" Molecules 28, no. 13: 5037. https://doi.org/10.3390/molecules28135037
APA StyleDuan, Y.-K., Li, Z.-W., Zhang, S.-C., Su, T., Zhang, Z.-H., Jiao, A.-J., & Fu, Z.-H. (2023). Stannate-Based Materials as Anodes in Lithium-Ion and Sodium-Ion Batteries: A Review. Molecules, 28(13), 5037. https://doi.org/10.3390/molecules28135037







