Exploring the Potential of Broadband Complementary Metal Oxide Semiconductor Micro-Coil Nuclear Magnetic Resonance for Environmental Research
Abstract
:1. Introduction
2. Results and Discussion
2.1. Exploring Heteronuclei
2.2. Limits of Detection for Heteronuclei
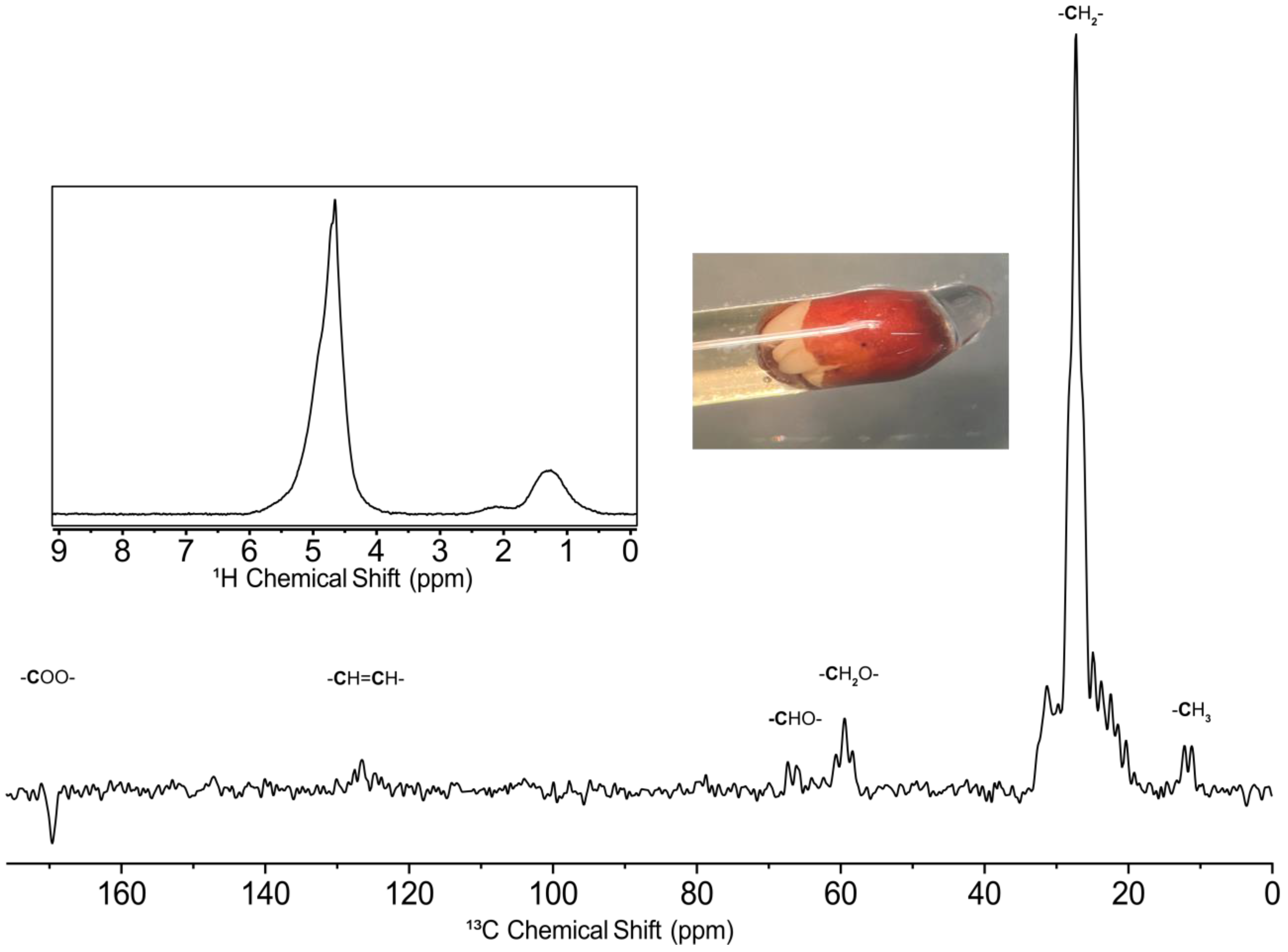
2.3. 13C Analysis of a Broccoli Seed
2.4. SSFP Compared to Standard 13C NMR
2.5. 1H Analysis of a D. magna Egg
2.6. 19F Contaminant Tracking in a D. magna Egg
3. Materials and Methods
4. Conclusions and Outlook
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Maggio, R.M.; Calvo, N.L.; Vignaduzzo, S.E.; Kaufman, T.S. Pharmaceutical Impurities and Degradation Products: Uses and Applications of NMR Techniques. J. Pharm. Biomed. Anal. 2014, 101, 102–122. [Google Scholar] [CrossRef] [PubMed]
- Spraul, M.; Schütz, B.; Humpfer, E.; Mörtter, M.; Schäfer, H.; Koswig, S.; Rinke, P. Mixture Analysis by NMR as Applied to Fruit Juice Quality Control. Magn. Reson. Chem. 2009, 47, S130–S137. [Google Scholar] [CrossRef] [PubMed]
- Anaraki, M.T.; Lysak, D.H.; Soong, R.; Simpson, M.J.; Spraul, M.; Bermel, W.; Heumann, H.; Gundy, M.; Boenisch, H.; Simpson, A.J. NMR Assignment of the: In Vivo Daphnia Magna Metabolome. Analyst 2020, 145, 5787–5800. [Google Scholar] [CrossRef]
- Kovacs, H.; Moskau, D.; Spraul, M. Cryogenically Cooled Probes—A Leap in NMR Technology. Prog. Nucl. Magn. Reson. Spectrosc. 2005, 46, 131–155. [Google Scholar] [CrossRef]
- Styles, P.; Soffe, N.F.; Scott, C.A.; Cragg, D.A.; Row, F.; White, D.J.; White, P.C.J. A High-Resolution NMR Probe in Which the Coil and Preamplifier Are Cooled with Liquid Helium. J. Magn. Reson. 2011, 213, 347–354. [Google Scholar] [CrossRef]
- Wikus, P.; Frantz, W.; Kümmerle, R.; Vonlanthen, P. Commercial Gigahertz-Class NMR Magnets. Supercond. Sci. Technol. 2022, 35, 033001. [Google Scholar] [CrossRef]
- Eills, J.; Budker, D.; Cavagnero, S.; Chekmenev, E.Y.; Elliott, S.J.; Jannin, S.; Lesage, A.; Matysik, J.; Meersmann, T.; Prisner, T.; et al. Spin Hyperpolarization in Modern Magnetic Resonance. Chem. Rev. 2023, 123, 1417–1551. [Google Scholar] [CrossRef] [PubMed]
- Webb, A.G. Microcoil Nuclear Magnetic Resonance Spectroscopy. In NMR Spectroscopy in Pharmaceutical Analysis; Elsevier: Amsterdam, The Netherlands, 2008; Volume 38, pp. 83–130. [Google Scholar] [CrossRef]
- Fugariu, I.; Soong, R.; Lane, D.; Fey, M.; Maas, W.; Vincent, F.; Beck, A.; Schmidig, D.; Treanor, B.; Simpson, A.J. Towards Single Egg Toxicity Screening Using Microcoil NMR. Analyst 2017, 142, 4812–4824. [Google Scholar] [CrossRef]
- Olson, D.L.; Lacey, M.E.; Sweedler, J.V. Microcoils Significantly Boost NMR Mass Sensitivity and Provide New Detection Opportunities.: The Nanoliter Niche. Anal. Chem. 1998, 70, 257A–264A. [Google Scholar] [CrossRef]
- van Bentum, P.J.M.; Janssen, J.W.G.; Kentgens, A.P.M.; Bart, J.; Gardeniers, J.G.E. Stripline Probes for Nuclear Magnetic Resonance. J. Magn. Reson. 2007, 189, 104–113. [Google Scholar] [CrossRef]
- Grisi, M.; Vincent, F.; Volpe, B.; Guidetti, R.; Harris, N.; Beck, A.; Boero, G. NMR Spectroscopy of Single Sub-NL Ova with Inductive Ultra-Compact Single-Chip Probes. Sci. Rep. 2017, 7, srep44670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bastawrous, M.; Gruschke, O.; Soong, R.; Jenne, A.; Gross, D.; Busse, F.; Nashman, B.; Lacerda, A.; Simpson, A.J. Comparing the Potential of Helmholtz and Planar NMR Microcoils for Analysis of Intact Biological Samples. Anal. Chem. 2022, 94, 8523–8532. [Google Scholar] [CrossRef] [PubMed]
- Moxley-Paquette, V.; Lane, D.; Soong, R.; Ning, P.; Bastawrous, M.; Wu, B.; Pedram, M.Z.; Haque Talukder, M.A.; Ghafar-Zadeh, E.; Zverev, D.; et al. 5-Axis CNC Micromilling for Rapid, Cheap, and Background-Free NMR Microcoils. Anal. Chem. 2020, 92, 15454–15462. [Google Scholar] [CrossRef]
- Persoone, G.; Baudo, R.; Cotman, M.; Blaise, C.; Thompson, K.C.; Moreira-Santos, M.; Vollat, B.; Törökne, A.; Han, T. Review on the Acute Daphnia Magna Toxicity Test? Evaluation of the Sensitivity and the Precision of Assays Performed with Organisms from Laboratory Cultures or Hatched from Dormant Eggs. Knowl. Manag. Aquat. Ecosyst. 2009, 393, 01. [Google Scholar] [CrossRef] [Green Version]
- De Coen, W.M.; Janssen, C.R. The Use of Biomarkers in Daphnia Magna Toxicity Testing. Hydrobiologia 1998, 367, 199–209. [Google Scholar] [CrossRef]
- Nasser, F.; Lynch, I. Updating Traditional Regulatory Tests for Use with Novel Materials: Nanomaterial Toxicity Testing with Daphnia Magna. Saf. Sci. 2019, 118, 497–504. [Google Scholar] [CrossRef]
- Edison, A.S.; Hall, R.D.; Junot, C.; Karp, P.D.; Kurland, I.J.; Mistrik, R.; Reed, L.K.; Saito, K.; Salek, R.M.; Steinbeck, C.; et al. The Time Is Right to Focus on Model Organism Metabolomes. Metabolites 2016, 6, 8. [Google Scholar] [CrossRef] [Green Version]
- Doma, S. Ephippia of Daphnia Magna Straus—A Technique for Their Mass Production and Quick Revival. Hydrobiologia 1979, 67, 183–188. [Google Scholar] [CrossRef]
- De Meester, L.; De Jager, H. Hatching of Daphnia Sexual Eggs. II. The Effect of Age and a Second Stimulus. Freshw. Biol. 1993, 30, 227–233. [Google Scholar] [CrossRef]
- Poynton, H.C.; Varshavsky, J.R.; Chang, B.; Cavigiolio, G.; Chan, S.; Holman, P.S.; Loguinov, A.V.; Bauer, D.J.; Komachi, K.; Theil, E.C.; et al. Daphnia Magna Ecotoxicogenomics Provides Mechanistic Insights into Metal Toxicity. Environ. Sci. Technol. 2007, 41, 1044–1050. [Google Scholar] [CrossRef]
- Cuenca Cambronero, M.; Marshall, H.; De Meester, L.; Davidson, T.A.; Beckerman, A.P.; Orsini, L. Predictability of the Impact of Multiple Stressors on the Keystone Species Daphnia. Sci. Rep. 2018, 8, 17572. [Google Scholar] [CrossRef] [Green Version]
- Lysak, D.H.; Kock, F.V.C.; Mamone, S.; Soong, R.; Glöggler, S.; Simpson, A.J. In Vivo Singlet State Filtered Nuclear Magnetic Resonance: Towards Monitoring Toxic Responses inside Living Organisms. Chem. Sci. 2023, 14, 1413–1418. [Google Scholar] [CrossRef]
- Krewski, D.; Acosta, D.; Andersen, M.; Anderson, H.; Bailar, J.C.; Boekelheide, K.; Brent, R.; Charnley, G.; Cheung, V.G.; Green, S.; et al. Staff of Committee on Toxicity Test. Toxicity Testing in the 21st Century: A Vision and a Strategy. J. Toxicol. Environ. Health Part B 2010, 13, 51–138. [Google Scholar] [CrossRef]
- Barata, C.; Alañon, P.; Gutierrez-Alonso, S.; Riva, M.C.; Fernández, C.; Tarazona, J.V. A Daphnia Magna Feeding Bioassay as a Cost Effective and Ecological Relevant Sublethal Toxicity Test for Environmental Risk Assessment of Toxic Effluents. Sci. Total Environ. 2008, 405, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Grisi, M. Broadband Single-Chip Transceivers for Compact NMR Probes; EPFL: Lausanne, Switzerland, 2017; Volume 7755. [Google Scholar]
- Grisi, M.; Conley, G.M.; Rodriguez, K.J.; Riva, E.; Egli, L.; Moritz, W.; Lichtenberg, J.; Brugger, J.; Boero, G. NMR Microsystem for Label-Free Characterization of 3D Nanoliter Microtissues. Sci. Rep. 2020, 10, 18306. [Google Scholar] [CrossRef] [PubMed]
- Grisi, M.; Gualco, G.; Boero, G. A Broadband Single-Chip Transceiver for Multi-Nuclear NMR Probes. Rev. Sci. Instrum. 2015, 86, 044703. [Google Scholar] [CrossRef] [PubMed]
- Anders, J.; Dreyer, F.; Krüger, D.; Schwartz, I.; Plenio, M.B.; Jelezko, F. Progress in Miniaturization and Low-Field Nuclear Magnetic Resonance. J. Magn. Reson. 2021, 322, 106860. [Google Scholar] [CrossRef]
- Grisi, M.; Conley, G.M. Cmos-Based Sensors as New Standard for Micro-Nmr: Magnetic Resonance at the Embryo Scale. eMagRes 2020, 9, 259–266. [Google Scholar] [CrossRef]
- Anders, J.; Korvink, J.G. Micro and Nano Scale NMR; Wiley-VCH: Weinheim, Germany, 2018. [Google Scholar]
- Anders, J.; Chiaramonte, G.; SanGiorgio, P.; Boero, G. A Single-Chip Array of NMR Receivers. J. Magn. Reson. 2009, 201, 239–249. [Google Scholar] [CrossRef]
- Yang, Q.; Zhao, J.; Dreyer, F.; Krüger, D.; Anders, J. A Portable NMR Platform with Arbitrary Phase Control and Temperature Compensation. Magn. Reson. 2022, 3, 77–90. [Google Scholar] [CrossRef]
- Sivelli, G.; Conley, G.M.; Herrera, C.; Marable, K.; Rodriguez, K.J.; Bollwein, H.; Sudano, M.J.; Brugger, J.; Simpson, A.J.; Boero, G.; et al. NMR Spectroscopy of a Single Mammalian Early Stage Embryo. J. Magn. Reson. 2022, 335, 107142. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Song, W.; Son, D.-Y.; Ono, L.K.; Qi, Y. Lithium-Ion Batteries: Outlook on Present, Future, and Hybridized Technologies. J. Mater. Chem. A 2019, 7, 2942–2964. [Google Scholar] [CrossRef]
- Mrozik, W.; Ali Rajaeifar, M.; Heidrich, O.; Christensen, P. Environmental Impacts, Pollution Sources and Pathways of Spent Lithium-Ion Batteries. Energy Environ. Sci. 2021, 14, 6099–6121. [Google Scholar] [CrossRef]
- Stamp, A.; Lang, D.J.; Wäger, P.A. Environmental Impacts of a Transition toward E-Mobility: The Present and Future Role of Lithium Carbonate Production. J. Clean. Prod. 2012, 23, 104–112. [Google Scholar] [CrossRef]
- Brown, T.R.; Ugurbil, K.; Shulman, R.G. 31P Nuclear Magnetic Resonance Measurements of ATPase Kinetics in Aerobic Escherichia Coli Cells. Proc. Natl. Acad. Sci. USA 1977, 74, 5551–5553. [Google Scholar] [CrossRef] [Green Version]
- de Graaf, R.A.; van Kranenburg, A.; Nicolay, K. In Vivo 31P-NMR Diffusion Spectroscopy of ATP and Phosphocreatine in Rat Skeletal Muscle. Biophys. J. 2000, 78, 1657–1664. [Google Scholar] [CrossRef] [Green Version]
- Sundareshwar, P.V.; Morris, J.T.; Pellechia, P.J.; Cohen, H.J.; Porter, D.E.; Jones, B.C. Occurrence and Ecological Implications of Pyrophosphate in Estuaries. Limnol. Oceanogr. 2001, 46, 1570–1577. [Google Scholar] [CrossRef]
- Clark, L.L.; Ingall, E.D.; Benner, R. Marine Organic Phosphorus Cycling; Novel Insights from Nuclear Magnetic Resonance. Am. J. Sci. 1999, 299, 724–737. [Google Scholar] [CrossRef] [Green Version]
- Turner, B.L.; Newman, S. Phosphorus Cycling in Wetland Soils. J. Environ. Qual. 2005, 34, 1921–1929. [Google Scholar] [CrossRef] [Green Version]
- Galván-Arzate, S.; Santamaría, A. Thallium Toxicity. Toxicol. Lett. 1998, 99, 1–13. [Google Scholar] [CrossRef]
- Weaver, C.D.; Harden, D.; Dworetzky, S.I.; Robertson, B.; Knox, R.J. A Thallium-Sensitive, Fluorescence-Based Assay for Detecting and Characterizing Potassium Channel Modulators in Mammalian Cells. SLAS Discov. 2004, 9, 671–677. [Google Scholar] [CrossRef] [Green Version]
- Douglas, K.T.; Bunni, M.A.; Baindur, S.R. Thallium in Biochemistry. Int. J. Biochem. 1990, 22, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.K.; Becker, E.D.; Cabral de Menezes, S.M.; Goodfellow, R.; Granger, P. NMR Nomenclature. Nuclear Spin Properties and Conventions for Chemical Shifts. Pure Appl. Chem. 2001, 73, 1795–1818. [Google Scholar] [CrossRef]
- Ashbrook, S.E. Recent Advances in Solid-State NMR Spectroscopy of Quadrupolar Nuclei. Phys. Chem. Chem. Phys. 2009, 11, 6892–6905. [Google Scholar] [CrossRef] [PubMed]
- Anaraki, M.T.; Lysak, D.H.; Downey, K.; Kock, F.V.C.; You, X.; Majumdar, R.D.; Barison, A.; Lião, L.M.; Ferreira, A.G.; Decker, V.; et al. NMR Spectroscopy of Wastewater: A Review, Case Study, and Future Potential. Prog. Nucl. Magn. Reson. Spectrosc. 2021, 126–127, 121–180. [Google Scholar] [CrossRef]
- Subramanian, R.; Lam, M.M.; Webb, A.G. RF Microcoil Design for Practical NMR of Mass-Limited Samples. J. Magn. Reson. 1998, 133, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Bieri, O.; Scheffler, K. Fundamentals of Balanced Steady State Free Precession MRI. J. Magn. Reson. Imaging 2013, 38, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Murphy, D.J. Plant Storage Lipids. In Encyclopedia of Life Sciences; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2016; pp. 1–7. [Google Scholar] [CrossRef]
- Moraes, T.B.; Kock, F.V.C.; Salome, K.S.; Barison, A.; Simpson, A.; Colnago, L.A. Steady-State Free Precession Sequences for High and Low Field NMR Spectroscopy in Solution: Challenges and Opportunities. J. Magn. Reson. Open 2023, 14–15, 100090. [Google Scholar] [CrossRef]
- Mobarhan, Y.L.; Struppe, J.; Fortier-McGill, B.; Simpson, A.J. Effective Combined Water and Sideband Suppression for Low-Speed Tissue and in Vivo MAS NMR. Anal. Bioanal. Chem. 2017, 409, 5043–5055. [Google Scholar] [CrossRef]
- Taipale, S.J.; Kainz, M.J.; Brett, M.T. Diet-Switching Experiments Show Rapid Accumulation and Preferential Retention of Highly Unsaturated Fatty Acids in Daphnia. Oikos 2011, 120, 1674–1682. [Google Scholar] [CrossRef]
- Martin-Creuzburg, D.; von Elert, E. Good Food versus Bad Food: The Role of Sterols and Polyunsaturated Fatty Acids in Determining Growth and Reproduction of Daphnia Magna. Aquat. Ecol. 2009, 43, 943–950. [Google Scholar] [CrossRef] [Green Version]
- Masclaux, H.; Bec, A.; Kainz, M.J.; Perrière, F.; Desvilettes, C.; Bourdier, G. Accumulation of Polyunsaturated Fatty Acids by Cladocerans: Effects of Taxonomy, Temperature and Food. Freshw. Biol. 2012, 57, 696–703. [Google Scholar] [CrossRef]
- Becker, C.; Boersma, M. Differential Effects of Phosphorus and Fatty Acids on Daphnia Magna Growth and Reproduction. Limnol. Oceanogr. 2005, 50, 388–397. [Google Scholar] [CrossRef] [Green Version]
- Hakumäki, J.M.; Poptani, H.; Sandmair, A.-M.; Ylä-Herttuala, S.; Kauppinen, R.A. 1H MRS Detects Polyunsaturated Fatty Acid Accumulation during Gene Therapy of Glioma: Implications for the in Vivo Detection of Apoptosis. Nat. Med. 1999, 5, 1323–1327. [Google Scholar] [CrossRef] [PubMed]
- Glüge, J.; Scheringer, M.; Cousins, I.T.; DeWitt, J.C.; Goldenman, G.; Herzke, D.; Lohmann, R.; Ng, C.A.; Trier, X.; Wang, Z. An Overview of the Uses of Per- and Polyfluoroalkyl Substances (PFAS). Environ. Sci. Process. Impacts 2020, 22, 2345–2373. [Google Scholar] [CrossRef] [PubMed]
- Brunn, H.; Arnold, G.; Körner, W.; Rippen, G.; Steinhäuser, K.G.; Valentin, I. PFAS: Forever Chemicals—Persistent, Bioaccumulative and Mobile. Reviewing the Status and the Need for Their Phase out and Remediation of Contaminated Sites. Environ. Sci. Eur. 2023, 35, 20. [Google Scholar] [CrossRef]
- Podder, A.; Sadmani, A.H.M.A.; Reinhart, D.; Chang, N.-B.; Goel, R. Per and Poly-Fluoroalkyl Substances (PFAS) as a Contaminant of Emerging Concern in Surface Water: A Transboundary Review of Their Occurrences and Toxicity Effects. J. Hazard. Mater. 2021, 419, 126361. [Google Scholar] [CrossRef]
- Young, C.J.; Furdui, V.I.; Franklin, J.; Koerner, R.M.; Muir, D.C.G.; Mabury, S.A. Perfluorinated Acids in Arctic Snow: New Evidence for Atmospheric Formation. Environ. Sci. Technol. 2007, 41, 3455–3461. [Google Scholar] [CrossRef]
- Miner, K.R.; Clifford, H.; Taruscio, T.; Potocki, M.; Solomon, G.; Ritari, M.; Napper, I.E.; Gajurel, A.P.; Mayewski, P.A. Deposition of PFAS ‘Forever Chemicals’ on Mt. Everest. Sci. Total Environ. 2021, 759, 144421. [Google Scholar] [CrossRef]
- Jian, J.-M.; Chen, D.; Han, F.-J.; Guo, Y.; Zeng, L.; Lu, X.; Wang, F. A Short Review on Human Exposure to and Tissue Distribution of Per- and Polyfluoroalkyl Substances (PFASs). Sci. Total Environ. 2018, 636, 1058–1069. [Google Scholar] [CrossRef]
- Murphy, C.D. Microbial Degradation of Fluorinated Drugs: Biochemical Pathways, Impacts on the Environment and Potential Applications. Appl. Microbiol. Biotechnol. 2016, 100, 2617–2627. [Google Scholar] [CrossRef] [PubMed]
- Shirzadi, A.; Simpson, M.J.; Xu, Y.; Simpson, A.J. Application of Saturation Transfer Double Difference NMR to Elucidate the Mechanistic Interactions of Pesticides with Humic Acid. Environ. Sci. Technol. 2008, 42, 1084–1090. [Google Scholar] [CrossRef]
- Buchholz, C.R.; Pomerantz, W.C.K. 19F NMR Viewed through Two Different Lenses: Ligand-Observed and Protein-Observed 19F NMR Applications for Fragment-Based Drug Discovery. RSC Chem. Biol. 2021, 2, 1312–1330. [Google Scholar] [CrossRef] [PubMed]
- Jan Hendriks, A. Modelling Non-Equilibrium Concentrations of Microcontaminants in Organisms: Comparative Kinetics as a Function of Species Size and Octanol-Water Partitioning. Chemosphere 1995, 30, 265–292. [Google Scholar] [CrossRef] [PubMed]
- Shirzadi, A.; Simpson, M.J.; Kumar, R.; Baer, A.J.; Xu, Y.; Simpson, A.J. Molecular Interactions of Pesticides at the Soil−Water Interface. Environ. Sci. Technol. 2008, 42, 5514–5520. [Google Scholar] [CrossRef]
- Carr, H.Y. Steady-State Free Precession in Nuclear Magnetic Resonance. Phys. Rev. 1958, 112, 1693–1701. [Google Scholar] [CrossRef]
- Wolf, T.; Jaroszewicz, M.J.; Frydman, L. Steady-State Free Precession and Solid-State NMR: How, When, and Why. J. Phys. Chem. C 2021, 125, 1544–1556. [Google Scholar] [CrossRef]





| Nucleus | Limit of Detection (pmol) | Lineshape (Hz) |
|---|---|---|
| 1H | 15 | 4 |
| 7Li | 72 | 3 |
| 11B | 313 | 100 |
| 19F | 19 | 10 |
| 23Na | 253 | 19 |
| 27Al | 296 | 21 |
| 31P | 454 | 25 |
| 55Mn | 272 | 50 |
| 59Co | 251 | 189 |
| 205Tl | 172 | 31 |
| Nucleus | 1H | 7Li | 11B | 19F | 23Na | 27Al | 31P | 55Mn | 59Co | 205Tl |
|---|---|---|---|---|---|---|---|---|---|---|
| TD | 32,768 | 32,768 | 16,384 | 32,768 | 16,384 | 16,384 | 32,768 | 16,384 | 8192 | 16,384 |
| NS | 32 | 256 | 3072 | 1 | 256 | 98,304 | 256 | 15,360 | 72,704 | 32,768 |
| D1 (s) | 3 | 5 | 1.5 | 3 | 1 | 0.03 | 3 | 0.03 | 0.11 | 0.03 |
| Line Broadening (Hz) | 1 | 1 | 25 | 5 | 5 | 15 | 10 | 15 | 25 | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lysak, D.H.; Grisi, M.; Marable, K.; Conley, G.M.; Michal, C.A.; Moxley-Paquette, V.; Wolff, W.W.; Downey, K.; Kock, F.V.C.; Costa, P.M.; et al. Exploring the Potential of Broadband Complementary Metal Oxide Semiconductor Micro-Coil Nuclear Magnetic Resonance for Environmental Research. Molecules 2023, 28, 5080. https://doi.org/10.3390/molecules28135080
Lysak DH, Grisi M, Marable K, Conley GM, Michal CA, Moxley-Paquette V, Wolff WW, Downey K, Kock FVC, Costa PM, et al. Exploring the Potential of Broadband Complementary Metal Oxide Semiconductor Micro-Coil Nuclear Magnetic Resonance for Environmental Research. Molecules. 2023; 28(13):5080. https://doi.org/10.3390/molecules28135080
Chicago/Turabian StyleLysak, Daniel H., Marco Grisi, Kathryn Marable, Gaurasundar M. Conley, Carl A. Michal, Vincent Moxley-Paquette, William W. Wolff, Katelyn Downey, Flavio V. C. Kock, Peter M. Costa, and et al. 2023. "Exploring the Potential of Broadband Complementary Metal Oxide Semiconductor Micro-Coil Nuclear Magnetic Resonance for Environmental Research" Molecules 28, no. 13: 5080. https://doi.org/10.3390/molecules28135080






