Salicylic Acid Release from Syndiotactic Polystyrene Staple Fibers
Abstract
:1. Introduction
2. Results and Discussion
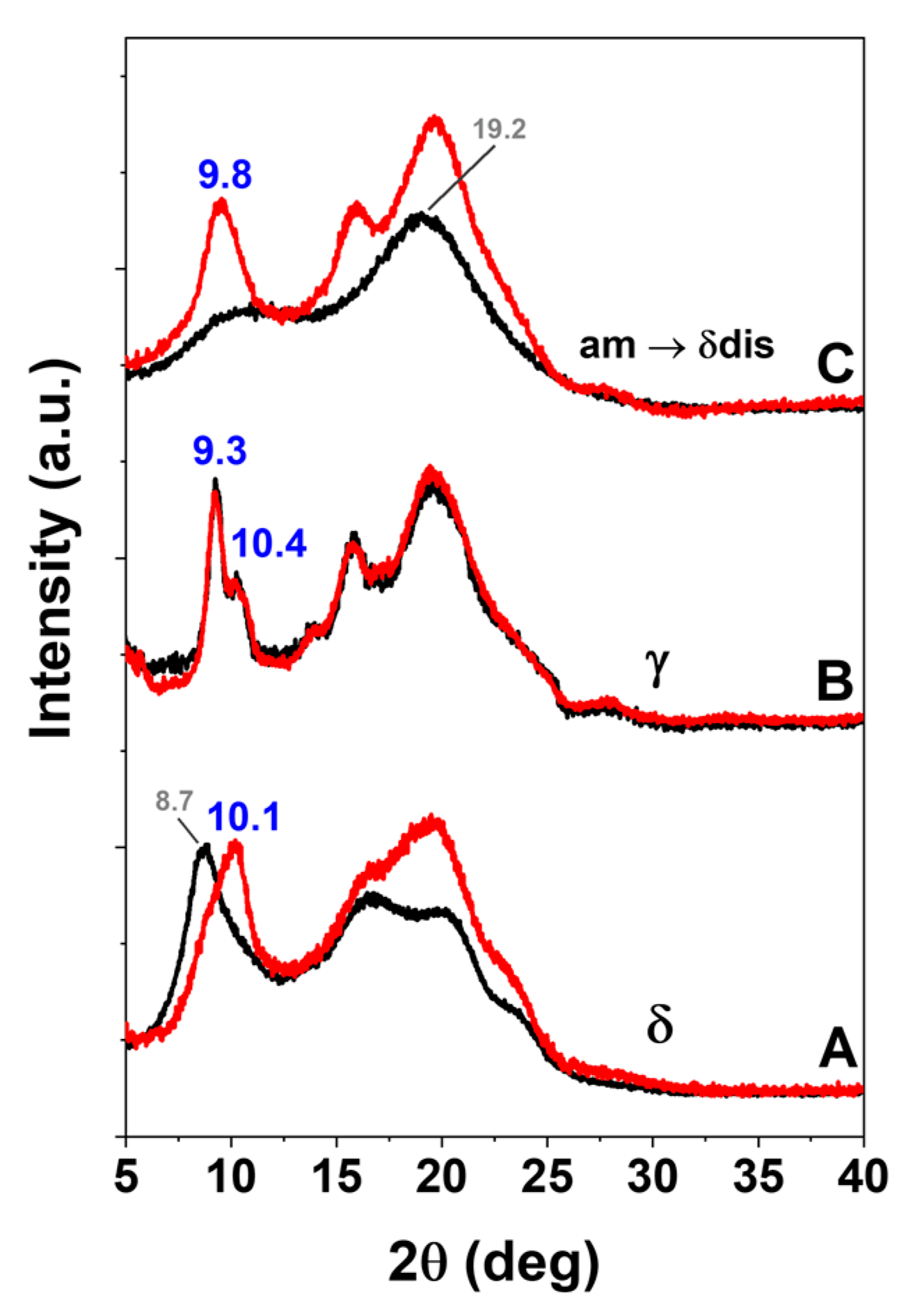
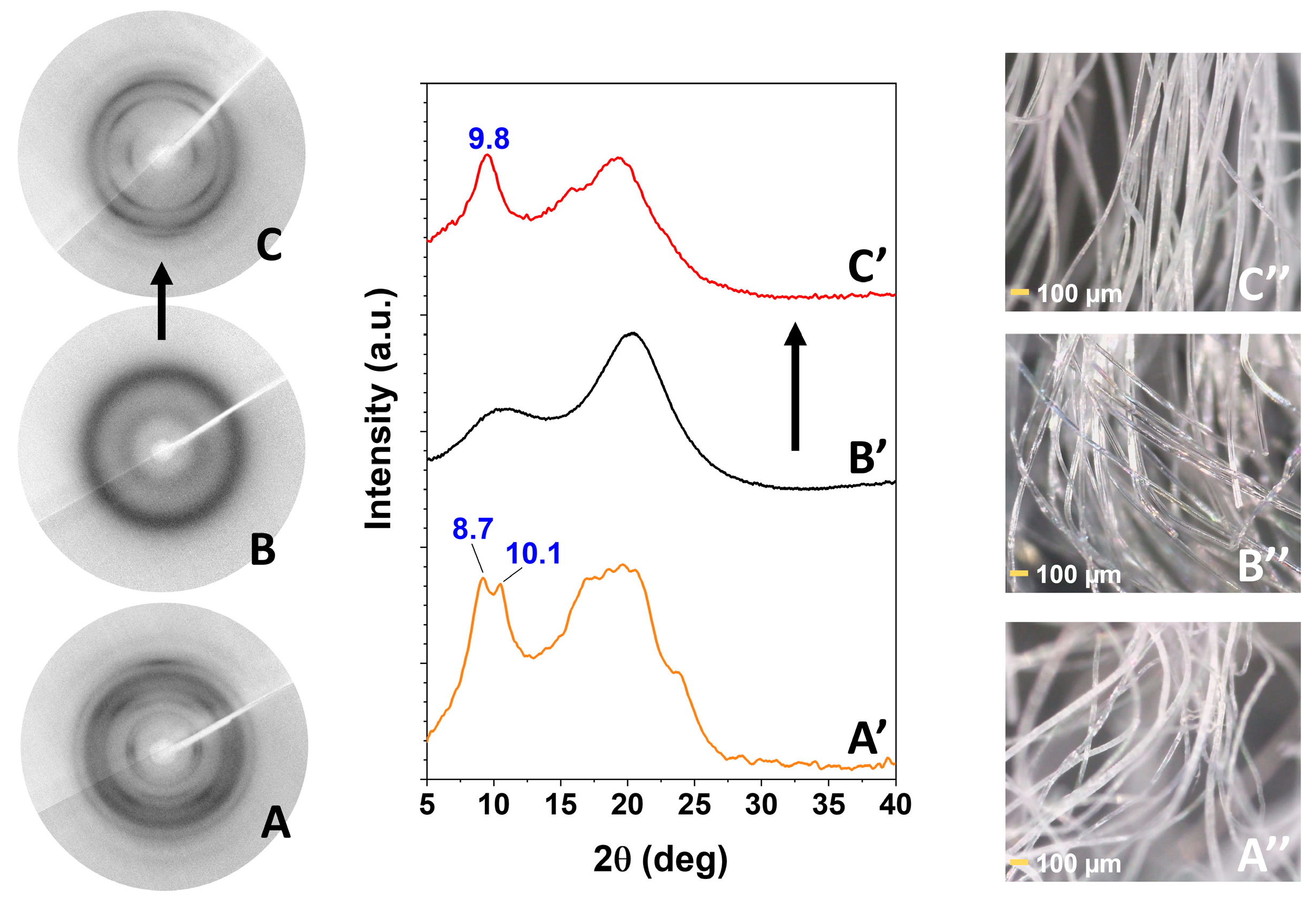
2.1. Information on sPS/SA CC Form from Axially Oriented Films
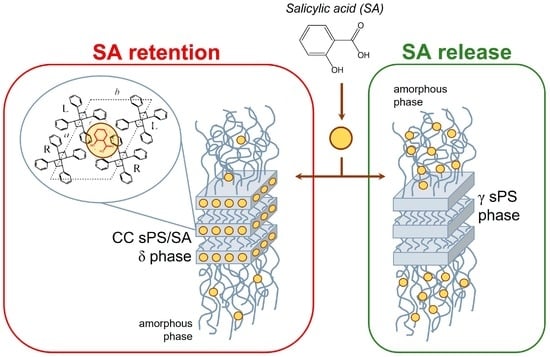
2.2. sPS Fibers with SA Guest
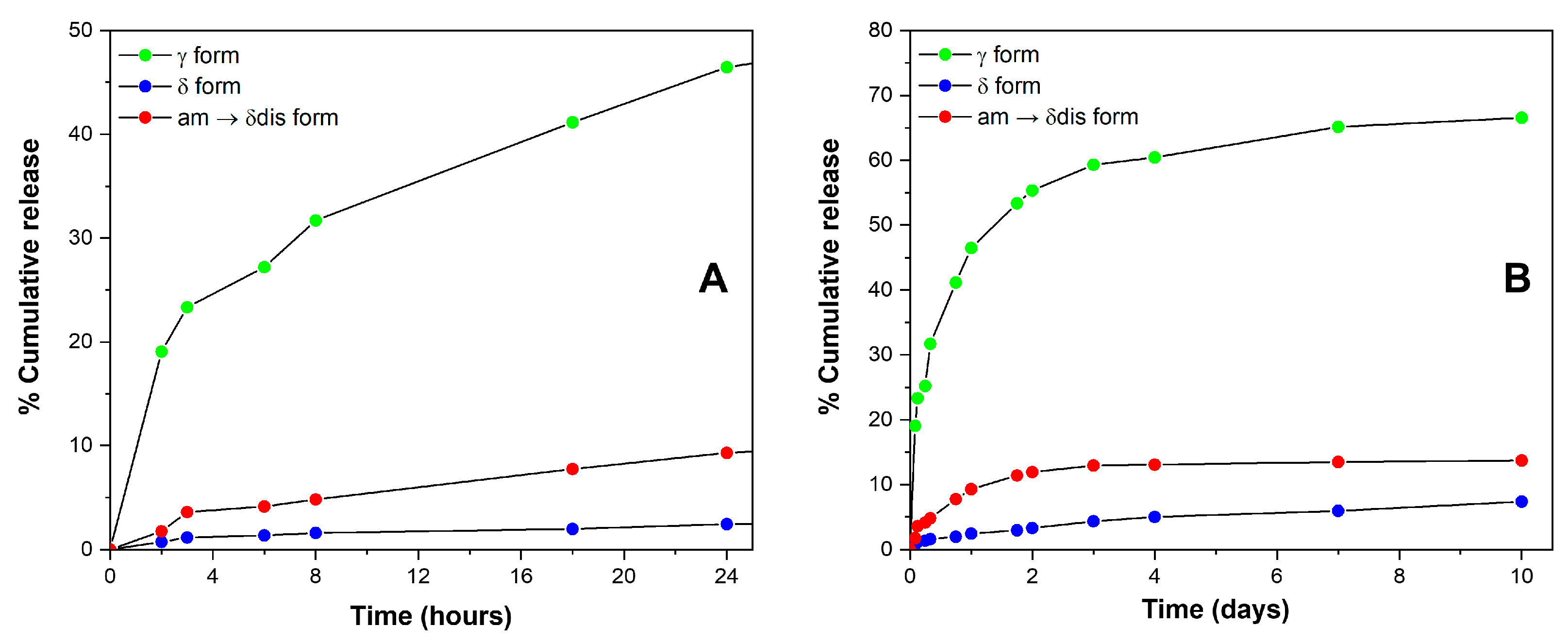
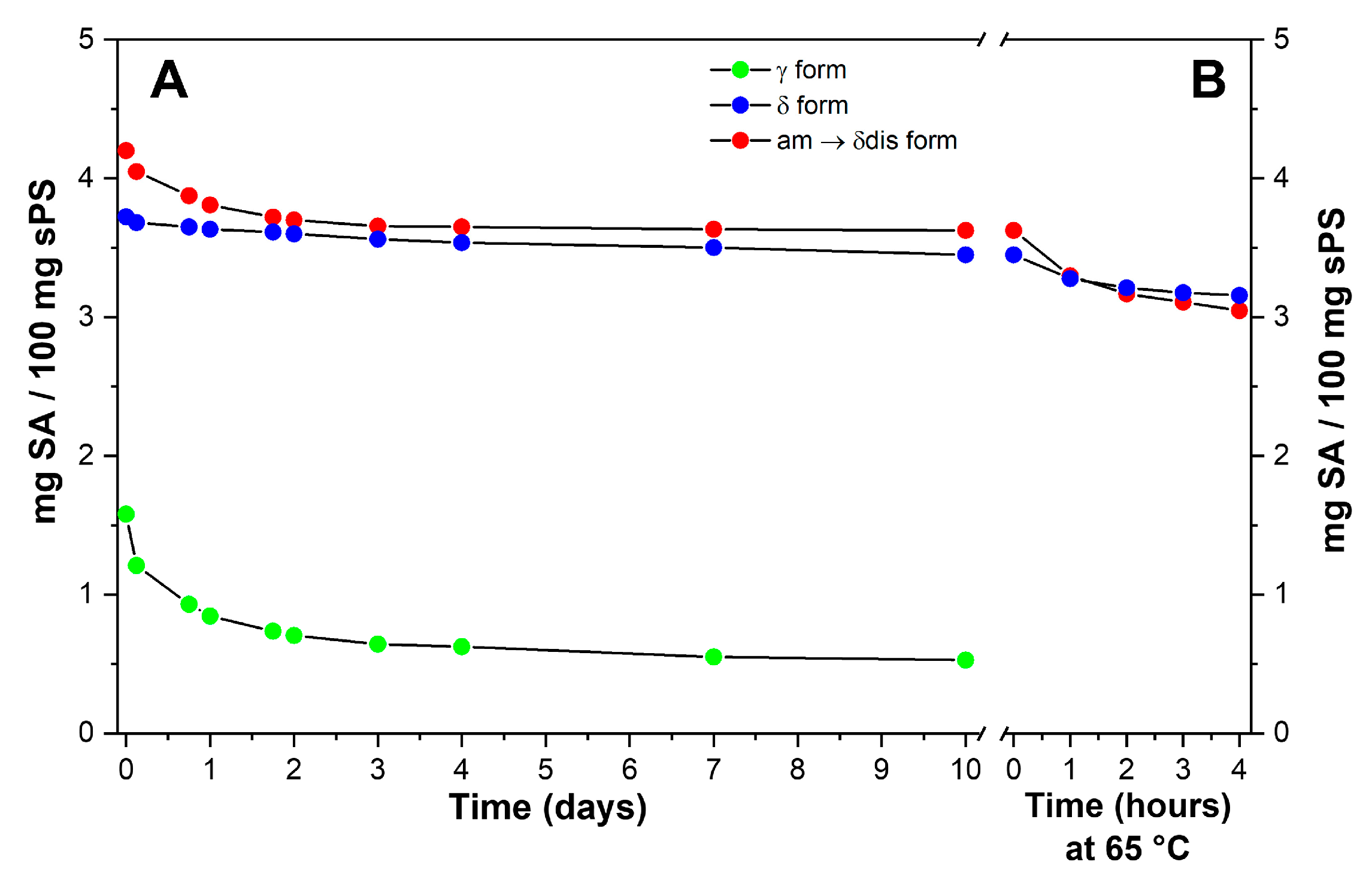
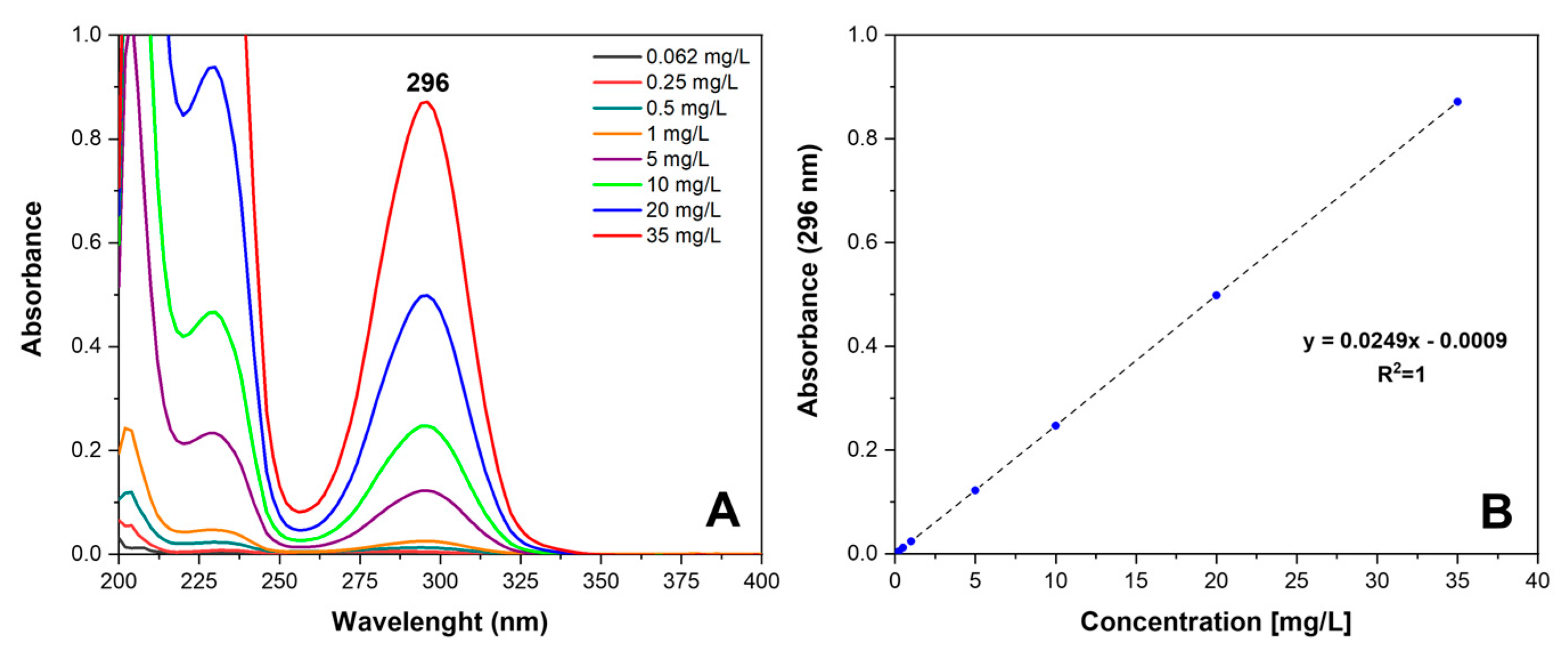
2.3. SA Release and Retention in sPS Staple Fibers
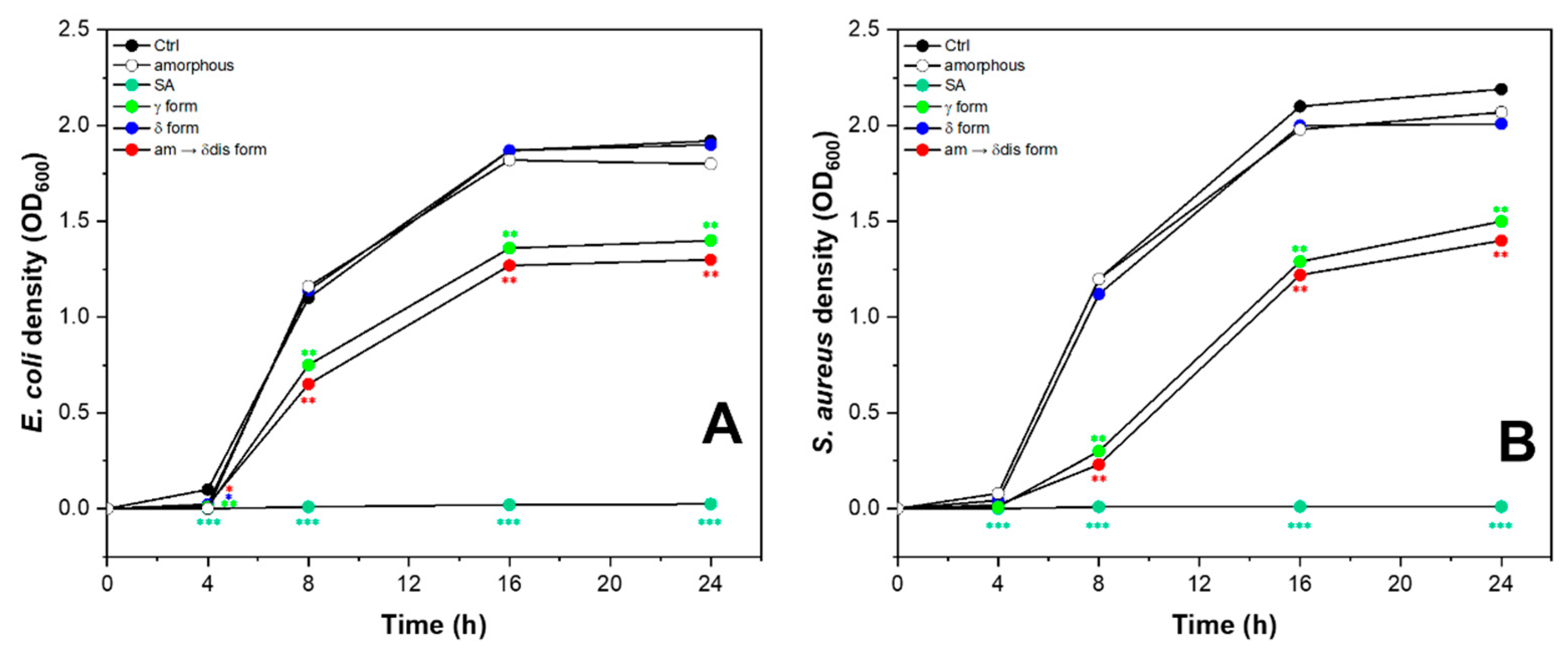
2.4. Antimicrobial Activity of sPS Staple Fibers
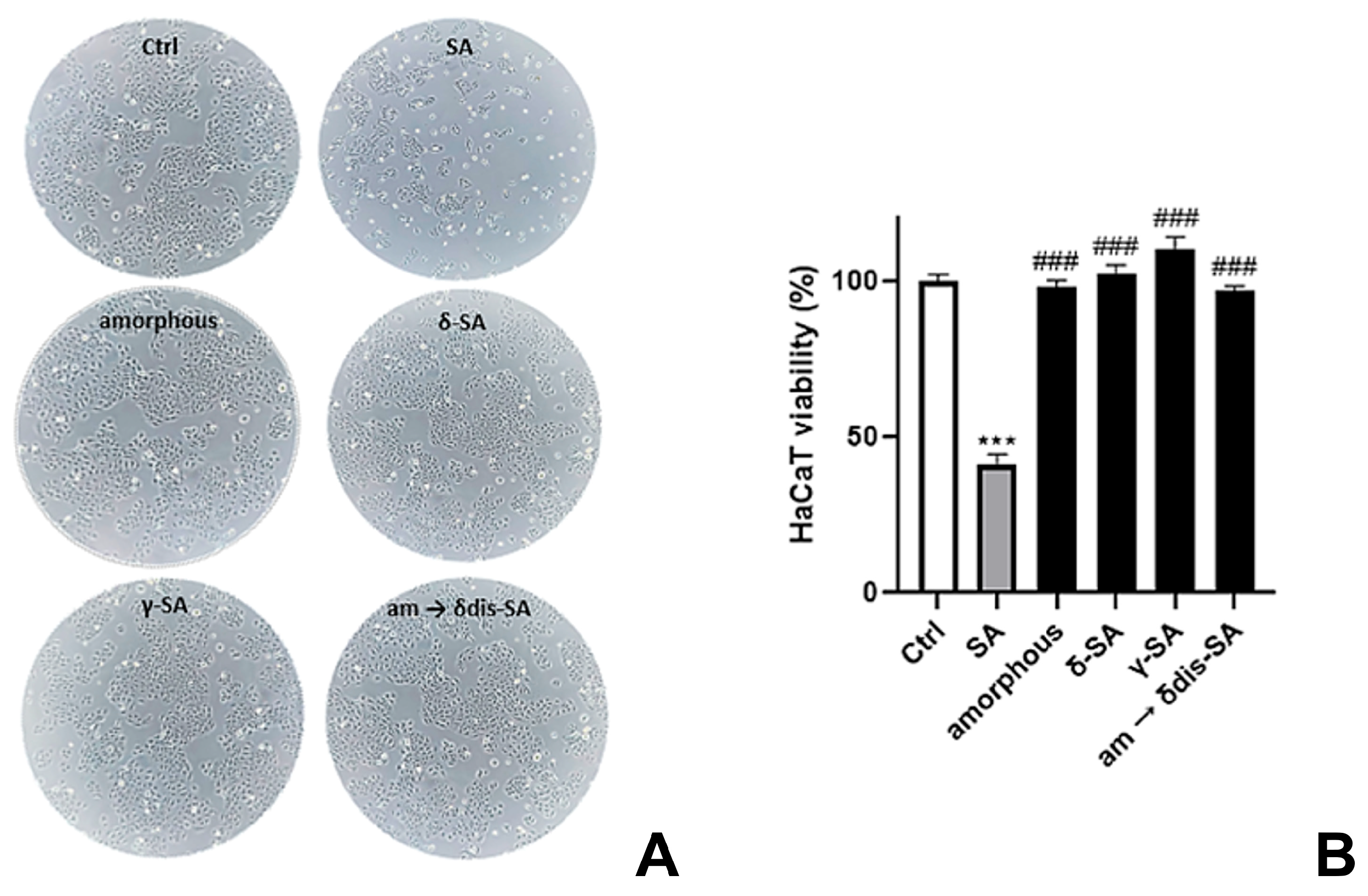
2.5. Skin Safety Evaluation of sPS Staple Fibers
3. Materials and Methods
3.1. Materials
3.2. Preparation of Films and Fibers
3.3. Structural Characterizations
3.4. Drug Release Studies
3.5. Investigation of Antimicrobial Activity
3.6. Cell Cultures and Determination of Cell Viability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- De Rosa, C.; Guerra, G.; Petraccone, V.; Pirozzi, B. Crystal Structure of the Emptied Clathrate Form (δe Form) of Syndiotactic Polystyrene. Macromolecules 1997, 30, 4147–4152. [Google Scholar] [CrossRef]
- Uda, Y.; Kaneko, F.; Kawaguchi, T. Selective Guest Uptake from Solvent Mixtures in the Clathrate Phase of Syndiotactic Polystyrene. Macromol. Rapid Commun. 2004, 25, 1900–1904. [Google Scholar] [CrossRef]
- Shaiju, P.; Bhoje Gowd, E. Structural Phase Transitions of Syndiotactic Polystyrene upon the Guest Extraction Process. Macromol. Symp. 2016, 359, 104–110. [Google Scholar] [CrossRef]
- Petraccone, V.; Ruiz de Ballesteros, O.; Tarallo, O.; Rizzo, P.; Guerra, G. Nanoporous Polymer Crystals with Cavities and Channels. Chem. Mater. 2008, 20, 3663–3668. [Google Scholar] [CrossRef]
- Tarallo, O.; Petraccone, V.; Albunia, A.; Daniel, C.; Guerra, G. Monoclinic and Triclinic δ-Clathrates of Syndiotactic Polystyrene. Macromolecules 2010, 43, 8549–8558. [Google Scholar] [CrossRef]
- Guerra, G.; Daniel, C.; Rizzo, P.; Tarallo, O. Advanced Materials Based on Polymer Cocrystalline Forms. J. Polym. Sci. B Polym. Phys. 2012, 50, 305–322. [Google Scholar] [CrossRef]
- Nagendra, B.; Cozzolino, A.; Daniel, C.; Rizzo, P.; Guerra, G.; Auriemma, F.; De Rosa, C.; D’Alterio, M.C.; Tarallo, O.; Nuzzo, A. Two Nanoporous Crystalline Forms of Poly(2,6-Dimethyl-1,4-Phenylene)Oxide and Related Co-Crystalline Forms. Macromolecules 2019, 52, 9646–9656. [Google Scholar] [CrossRef]
- Alentiev, A.Y.; Levin, I.S.; Buzin, M.I.; Belov, N.A.; Nikiforov, R.Y.; Chirkov, S.V.; Blagodatskikh, I.V.; Kechekyan, A.S.; Kechekyan, P.A.; Bekeshev, V.G.; et al. Gas Transport Parameters, Density and Free Volume of Nanocrystalline Poly-2,6-Dimethylphenylene Oxide. Polymer 2021, 226, 123804. [Google Scholar] [CrossRef]
- Alentiev, A.Y.; Levin, I.S.; Belov, N.A.; Nikiforov, R.Y.; Chirkov, S.V.; Bezgin, D.A.; Ryzhikh, V.E.; Kostina, J.V.; Shantarovich, V.P.; Grunin, L.Y. Features of the Gas-Permeable Crystalline Phase of Poly-2,6-Dimethylphenylene Oxide. Polymers 2022, 14, 120. [Google Scholar] [CrossRef]
- Auriemma, F.; Daniel, C.; Golla, M.; Nagendra, B.; Rizzo, P.; Tarallo, O.; Guerra, G. Polymorphism of Poly(2,6-Dimethyl-1,4-Phenylene) Oxide (PPO): Co-Crystalline and Nanoporous-Crystalline Phases. Polymer 2022, 258, 125290. [Google Scholar] [CrossRef]
- Albunia, A.R.; Rizzo, P.; Ianniello, G.; Rufolo, C.; Guerra, G. Syndiotactic Polystyrene Films with a Cocrystalline Phase Including Carvacrol Guest Molecules. J. Polym. Sci. Part B Polym. Phys. 2014, 52, 657–665. [Google Scholar] [CrossRef]
- Rizzo, P.; Albunia, A.R.; Milano, G.; Vendiito, V.; Guerra, G.; Mensitieri, G.; Di Maio, L. Crystalline Orientation and Molecular Transport Properties in Nanoporous Syndiotactic Polystyrene Films. Macromol. Symp. 2002, 185, 65–75. [Google Scholar] [CrossRef]
- Rizzo, P.; Cozzolino, A.; Guerra, G. Chemical Stabilization of Hexanal Molecules by Inclusion as Guests of Nanoporous-Crystalline Syndiotactic Polystyrene Crystals. Macromolecules 2019, 52, 2255–2264. [Google Scholar] [CrossRef]
- Rizzo, P.; Montefusco, T.; Guerra, G. Chiral Optical Films Based on Achiral Chromophore Guests. J. Am. Chem. Soc. 2011, 133, 9872–9877. [Google Scholar] [CrossRef]
- Cozzolino, A.; Pappalardo, S.; Rizzo, P.; Guerra, G. Linear Hydrogen Bonded Aggregates of Carboxylic Acids in Crystalline Channels of Syndiotactic Polystyrene. Polymer 2022, 262, 125484. [Google Scholar] [CrossRef]
- Cozzolino, A.; Monaco, G.; Rizzo, P.; Guerra, G. Segregation of Benzoic Acid in Polymer Crystalline Cavities. Polymers 2022, 15, 177. [Google Scholar] [CrossRef]
- Daniel, C.; Antico, P.; Yamaguchi, H.; Kogure, M.; Guerra, G. Microporous-Crystalline Microfibers by Eco-Friendly Guests: An Efficient Tool for Sorption of Volatile Organic Pollutants. Microporous Mesoporous Mater. 2016, 232, 205–210. [Google Scholar] [CrossRef]
- Daniel, C.; Antico, P.; Guerra, G. Etched Fibers of Syndiotactic Polystyrene with Nanoporous-Crystalline Phases. Macromolecules 2018, 51, 6138–6148. [Google Scholar] [CrossRef]
- Cozzolino, A.; Rizzo, P.; Gallo, C.; Bianchi, R.; Daniel, C.; Guerra, G. Axially Oriented Guest Induced Crystallization in Syndiotactic Polystyrene Unstretched Fibers. Polymer 2021, 228, 123908. [Google Scholar] [CrossRef]
- Cozzolino, A.; Daniel, C.; Guerra, G.; Rizzo, P.; Nicolais, L. Active Yarns and Textiles for the Stabilization and Controlled Release of Active Compounds. U.S. Patent 20210324151A1, 21 October 2020. [Google Scholar]
- Saleem, M.; Fariduddin, Q.; Castroverde, C.D.M. Salicylic Acid: A Key Regulator of Redox Signalling and Plant Immunity. Plant Physiol. Biochem. 2021, 168, 381–397. [Google Scholar] [CrossRef]
- Amborabé, B.-E.; Fleurat-Lessard, P.; Chollet, J.-F.; Roblin, G. Antifungal Effects of Salicylic Acid and Other Benzoic Acid Derivatives towards Eutypa Lata: Structure–Activity Relationship. Plant Physiol. Biochem. 2002, 40, 1051–1060. [Google Scholar] [CrossRef]
- Price, C.T.D.; Lee, I.R.; Gustafson, J.E. The Effects of Salicylate on Bacteria. Int. J. Biochem. Cell Biol. 2000, 32, 1029–1043. [Google Scholar] [CrossRef] [PubMed]
- Laezza, A.; Pepe, A.; Henry, A.; Duca, L.; Bochicchio, B. Polysaccharide-Enriched Electrospun Nanofibers for Salicylic Acid Controlled Release. ACS Appl. Eng. Mater. 2023, 1, 508–518. [Google Scholar] [CrossRef]
- Sorzabal-Bellido, I.; Diaz-Fernandez, Y.A.; Susarrey-Arce, A.; Skelton, A.A.; McBride, F.; Beckett, A.J.; Prior, I.A.; Raval, R. Exploiting Covalent, H-Bonding, and π–π Interactions to Design Antibacterial PDMS Interfaces That Load and Release Salicylic Acid. ACS Appl. Bio Mater. 2019, 2, 4801–4811. [Google Scholar] [CrossRef] [PubMed]
- Nowatzki, P.J.; Koepsel, R.R.; Stoodley, P.; Min, K.; Harper, A.; Murata, H.; Donfack, J.; Hortelano, E.R.; Ehrlich, G.D.; Russell, A.J. Salicylic Acid-Releasing Polyurethane Acrylate Polymers as Anti-Biofilm Urological Catheter Coatings. Acta Biomater. 2012, 8, 1869–1880. [Google Scholar] [CrossRef] [PubMed]
- Grigorev, S.V.; Illarionova, K.V.; Konarev, A.V.; Shelenga, T.V. Differences in Metabolites of White and Naturally Colored Cotton: Implications for Biofunctional and Aseptic Textiles. J. Nat. Fibers 2022, 19, 7060–7072. [Google Scholar] [CrossRef]
- Kantouch, A.; El-Sayed, A.A.; Salama, M.; El-Kheir, A.A.; Mowafi, S. Salicylic Acid and Some of Its Derivatives as Antibacterial Agents for Viscose Fabric. Int. J. Biol. Macromol. 2013, 62, 603–607. [Google Scholar] [CrossRef]
- Biagiotti, G.; Salvatore, A.; Toniolo, G.; Caselli, L.; Di Vito, M.; Cacaci, M.; Contiero, L.; Gori, T.; Maggini, M.; Sanguinetti, M.; et al. Metal-Free Antibacterial Additives Based on Graphene Materials and Salicylic Acid: From the Bench to Fabric Applications. ACS Appl. Mater. Interfaces 2021, 13, 26288–26298. [Google Scholar] [CrossRef]
- Acocella, M.R.; Rizzo, P.; Daniel, C.; Tarallo, O.; Guerra, G. Nanoporous Triclinic δ Modification of Syndiotactic Polystyrene. Polymer 2015, 63, 230–236. [Google Scholar] [CrossRef]
- Humbert, B.; Alnot, M.; Quilès, F. Infrared and Raman Spectroscopical Studies of Salicylic and Salicylate Derivatives in Aqueous Solution. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 1998, 54, 465–476. [Google Scholar] [CrossRef]
- Suresh, S.; Gunasekaran, S.; Srinivasan, S. Spectroscopic (FT-IR, FT-Raman, NMR and UV–Visible) and Quantum Chemical Studies of Molecular Geometry, Frontier Molecular Orbital, NLO, NBO and Thermodynamic Properties of Salicylic Acid. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 132, 130–141. [Google Scholar] [CrossRef]
- Boczar, M.; Boda, Ł.; Wójcik, M.J. Theoretical Modeling of Infrared Spectra of Hydrogen-Bonded Crystals of Salicylic Acid. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2006, 64, 757–760. [Google Scholar] [CrossRef]
- Tashiro, K.; Ueno, Y.; Yoshioka, A.; Kobayashi, M. Molecular Mechanism of Solvent-Induced Crystallization of Syndiotactic Polystyrene Glass. 1. Time-Resolved Measurements of Infrared/Raman Spectra and X-Ray Diffraction. Macromolecules 2001, 34, 310–315. [Google Scholar] [CrossRef]
- Musto, P.; Rizzo, P.; Guerra, G. Host−Guest Interactions and Crystalline Structure Evolution in Clathrate Phases Formed by Syndiotactic Polystyrene and 1,2-Dichloroethane: A Two-Dimensional FTIR Spectroscopy Investigation. Macromolecules 2005, 38, 6079–6089. [Google Scholar] [CrossRef]
- Torres, F.J.; Civalleri, B.; Meyer, A.; Musto, P.; Albunia, A.R.; Rizzo, P.; Guerra, G. Normal Vibrational Analysis of the Syndiotactic Polystyrene s(2/1)2 Helix. J. Phys. Chem. B 2009, 113, 5059–5071. [Google Scholar] [CrossRef]
- Saini, P.; Arora, M.; Kumar, M.N.V.R. Poly(Lactic Acid) Blends in Biomedical Applications. Adv. Drug Deliv. Rev. 2016, 107, 47–59. [Google Scholar] [CrossRef]
- Croll, T.I.; O’Connor, A.J.; Stevens, G.W.; Cooper-White, J.J. Controllable Surface Modification of Poly(Lactic-co-Glycolic Acid) (PLGA) by Hydrolysis or Aminolysis I: Physical, Chemical, and Theoretical Aspects. Biomacromolecules 2004, 5, 463–473. [Google Scholar] [CrossRef]
- Ciarfaglia, N.; Laezza, A.; Lods, L.; Lonjon, A.; Dandurand, J.; Pepe, A.; Bochicchio, B. Thermal and Dynamic Mechanical Behavior of Poly(Lactic Acid) (PLA)-based Electrospun Scaffolds for Tissue Engineering. J. Appl. Polym. Sci. 2021, 138, 51313. [Google Scholar] [CrossRef]
- Ciarfaglia, N.; Pepe, A.; Piccirillo, G.; Laezza, A.; Daum, R.; Schenke-Layland, K.; Bochicchio, B. Nanocellulose and Elastin Act as Plasticizers of Electrospun Bioinspired Scaffolds. ACS Appl. Polym. Mater. 2020, 2, 4836–4847. [Google Scholar] [CrossRef]
- Tyler, B.; Gullotti, D.; Mangraviti, A.; Utsuki, T.; Brem, H. Polylactic Acid (PLA) Controlled Delivery Carriers for Biomedical Applications. Adv. Drug Deliv. Rev. 2016, 107, 163–175. [Google Scholar] [CrossRef]
- Lin, A.N.; Nakatsui, T. Salicylic Acid Revisited. Int. J. Dermatol. 1998, 37, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Yeoh, S.C.; Goh, C.F. Topical Delivery of Salicylates. Drug Deliv. Transl. Res. 2022, 12, 981–1001. [Google Scholar] [CrossRef] [PubMed]
- Arif, T. Salicylic Acid as a Peeling Agent: A Comprehensive Review. Clin. Cosmet. Investig. Dermatol. 2015, 8, 455–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Decker, A.; Graber, E.M. Over-the-Counter Acne Treatments: A Review. J. Clin. Aesthet. Dermatol. 2012, 5, 32–40. [Google Scholar]
- Cozzolino, A.; Botta, C.; Daniel, C.; Rizzo, P. Thymol and Carvacrol: Phenolic Monoterpenes Extracted from the Essential Oil of Thymus Vulgaris as Natural Antimicrobial Guests of Nanoporous Crystalline Syndiotactic Polystyrene Fibers. Macromol. Symp. 2023, 408, 2200064. [Google Scholar] [CrossRef]
- Kang, S.G.; Lee, K.E.; Singh, M.; Vinayagam, R. Salicylic-Zinc Nanocomposites with Enhanced Antibacterial Activity. Coatings 2023, 13, 941. [Google Scholar] [CrossRef]
- Frickmann, H.; Hahn, A.; Berlec, S.; Ulrich, J.; Jansson, M.; Schwarz, N.G.; Warnke, P.; Podbielski, A. On the Etiological Relevance of Escherichia Coli and Staphylococcus Aureus in Superficial and Deep Infections—A Hypothesis-Forming, Retrospective Assessment. Eur. J. Microbiol. Immunol. 2019, 9, 124–130. [Google Scholar] [CrossRef]
- Jain, A.K.; Singh, D.; Dubey, K.; Maurya, R.; Mittal, S.; Pandey, A.K. Models and Methods for In Vitro Toxicity. In In Vitro Toxicology; Elsevier: Amsterdam, The Netherlands, 2018; pp. 45–65. ISBN 978-0-12-804667-8. [Google Scholar]
- Colombo, I.; Sangiovanni, E.; Maggio, R.; Mattozzi, C.; Zava, S.; Corbett, Y.; Fumagalli, M.; Carlino, C.; Corsetto, P.A.; Scaccabarozzi, D.; et al. HaCaT Cells as a Reliable In Vitro Differentiation Model to Dissect the Inflammatory/Repair Response of Human Keratinocytes. Mediat. Inflamm. 2017, 2017, 7435621. [Google Scholar] [CrossRef] [Green Version]
- Micallef, L.; Belaubre, F.; Pinon, A.; Jayat-Vignoles, C.; Delage, C.; Charveron, M.; Simon, A. Effects of Extracellular Calcium on the Growth-Differentiation Switch in Immortalized Keratinocyte HaCaT Cells Compared with Normal Human Keratinocytes. Exp. Dermatol. 2009, 18, 143–151. [Google Scholar] [CrossRef]


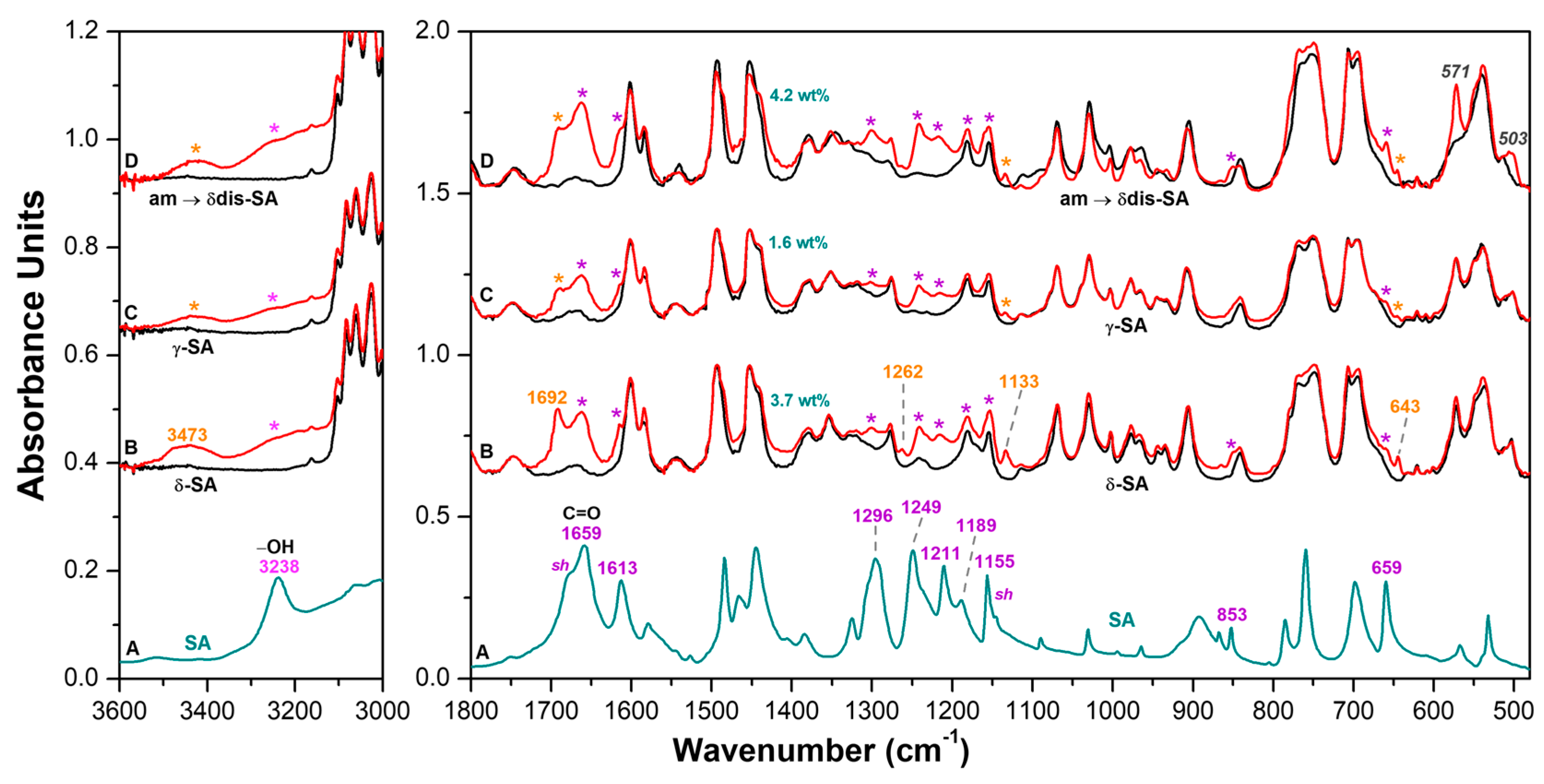

| SA in Aqueous Solution [31] | SA in KBr Pellet [32,33] | Vibrational Assignment [32,33] | SA in CC δ sPS | SA in KBr Pellet (Present Work) |
|---|---|---|---|---|
| 643 ⊥ | ||||
| 659 [32] | β OCO + β OCC + τ CCCC [32] | 659 //w | 659 | |
| 801calc/805exp [33] | γ (COOH) + γ (OH)mon + γ (CH)rings [33] | 800 // | 785 | |
| 852 | 852 [32] | 851 ⊥ | 853 | |
| 861 | 864 //w | 865 | ||
| 1133 ⊥ | 1145 sh | |||
| 1148 | 1155 [32] | ν CC + β HOC + β HCC [32] | 1152 ⊥ | 1155 |
| 1191 [32] | β HOC + β CCH [32] | 1181 ⊥ | 1189 | |
| 1214 ⊥ | 1211 | |||
| 1246 | 1248 [32] | β HOC [32] | 1241 ⊥ | 1249 |
| 1262 ⊥ | ||||
| 1326 | 1324 [32] | ν CC + β HOC + β HCC [32] | 1319 ⊥w | 1326 |
| 1409 ⊥w | 1406 | |||
| 1441 [32] | ν CC + β HCC [32] | 1441 ⊥w | 1445 | |
| 1488 | 1485 ⊥w | 1485 | ||
| 1610 | 1611 [32] | ν CC [32] | 1614 ⊥ | 1613 |
| 1660 | 1667calc/1658exp [33] | ν (C=O) + ν (CC)rings [33] | 1661 ⊥ | 1659 |
| 1692 ⊥ | 1679 sh | |||
| 3239exp [33] | ν (OH)phen [33] | 3219 ⊥ | 3238 | |
| 3473 ⊥ | ||||
| 3505 [32] | ν OH [32] | 3522 ⊥ | 3515 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Covelli, V.; Cozzolino, A.; Rizzo, P.; Rodriquez, M.; Vestuto, V.; Bertamino, A.; Daniel, C.; Guerra, G. Salicylic Acid Release from Syndiotactic Polystyrene Staple Fibers. Molecules 2023, 28, 5095. https://doi.org/10.3390/molecules28135095
Covelli V, Cozzolino A, Rizzo P, Rodriquez M, Vestuto V, Bertamino A, Daniel C, Guerra G. Salicylic Acid Release from Syndiotactic Polystyrene Staple Fibers. Molecules. 2023; 28(13):5095. https://doi.org/10.3390/molecules28135095
Chicago/Turabian StyleCovelli, Verdiana, Antonietta Cozzolino, Paola Rizzo, Manuela Rodriquez, Vincenzo Vestuto, Alessia Bertamino, Christophe Daniel, and Gaetano Guerra. 2023. "Salicylic Acid Release from Syndiotactic Polystyrene Staple Fibers" Molecules 28, no. 13: 5095. https://doi.org/10.3390/molecules28135095