Construction of S-Scheme 2D/2D Crystalline Carbon Nitride/BiOIO3 van der Waals Heterojunction for Boosted Photocatalytic Degradation of Antibiotics
Abstract
:1. Introduction
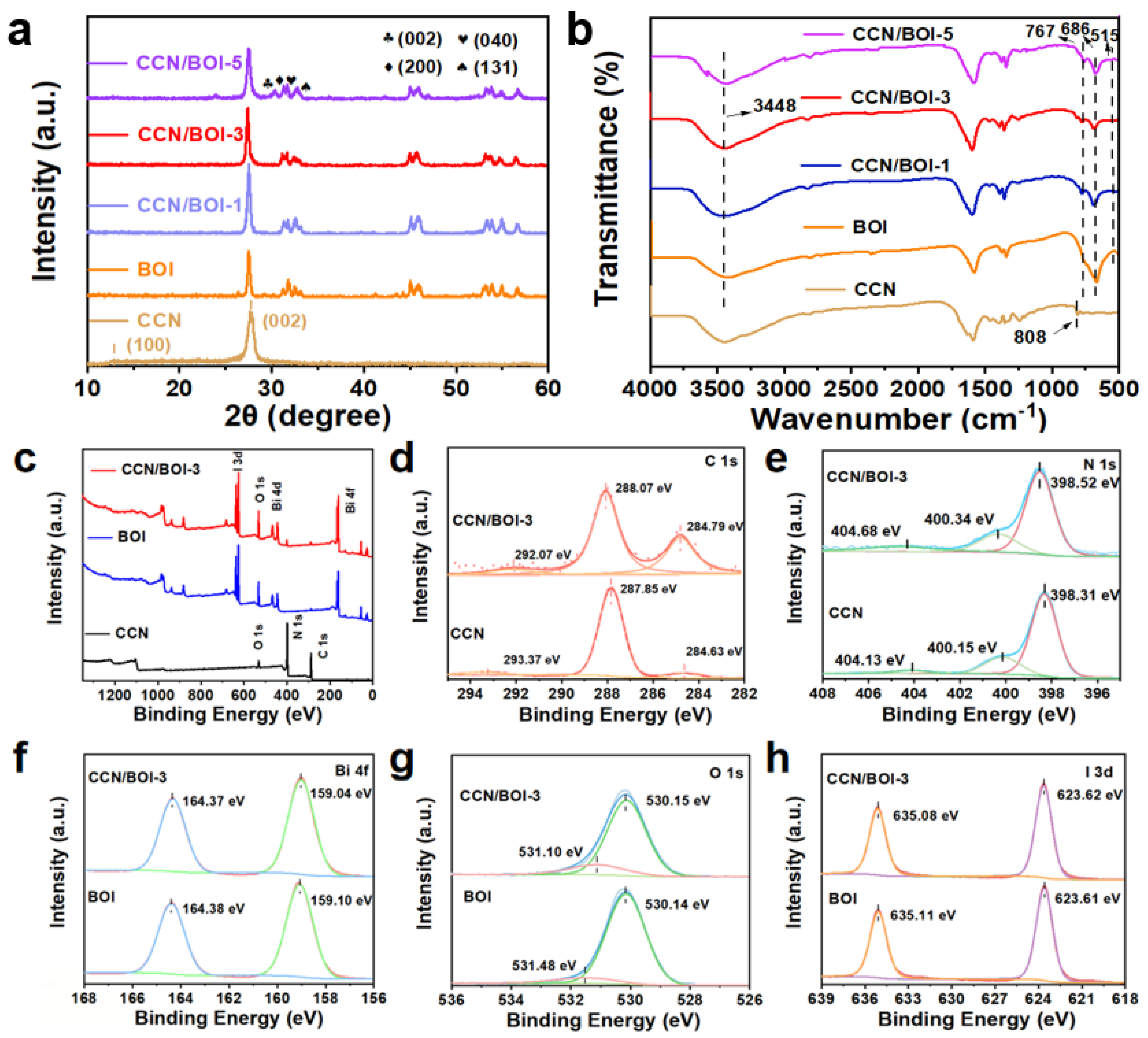
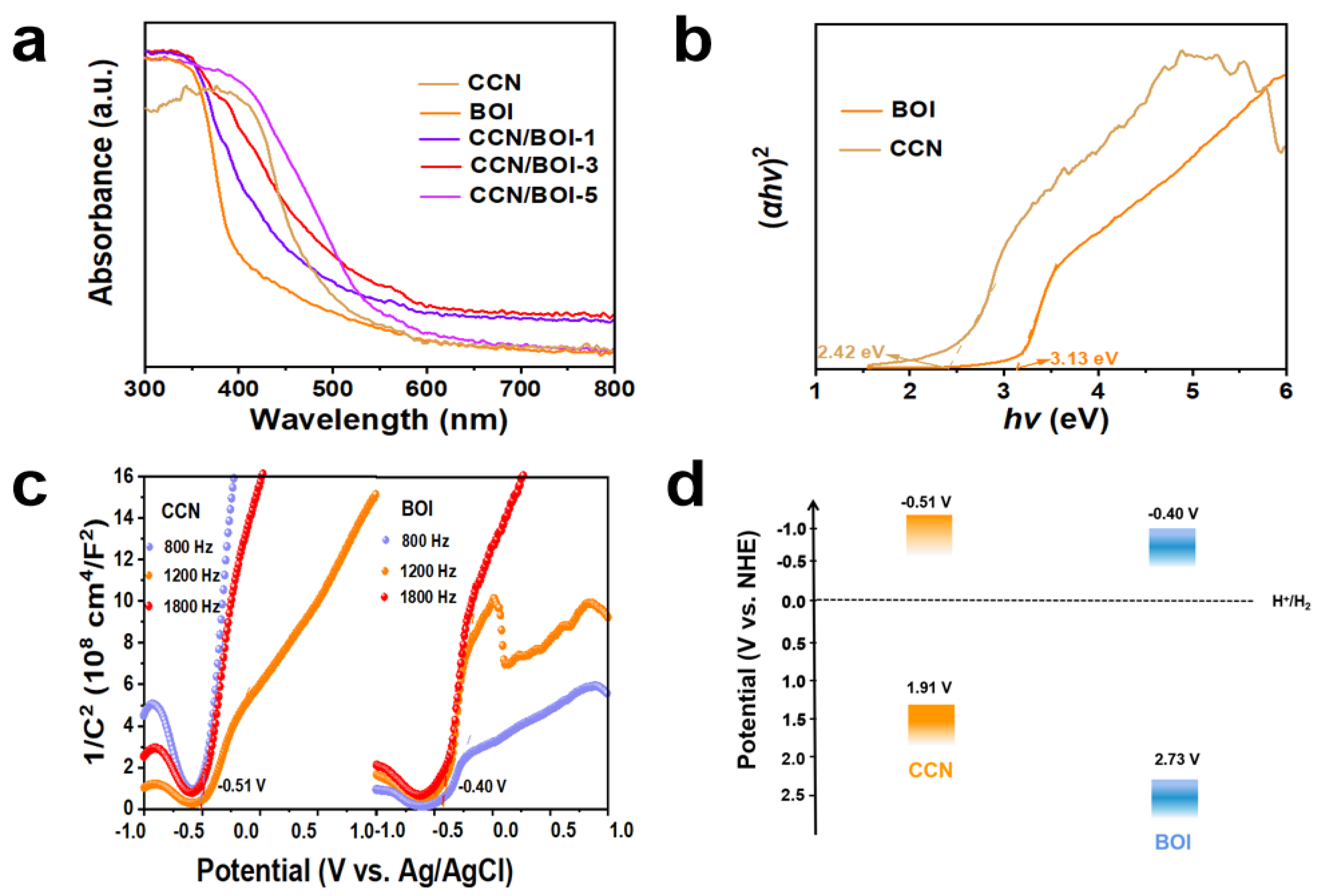
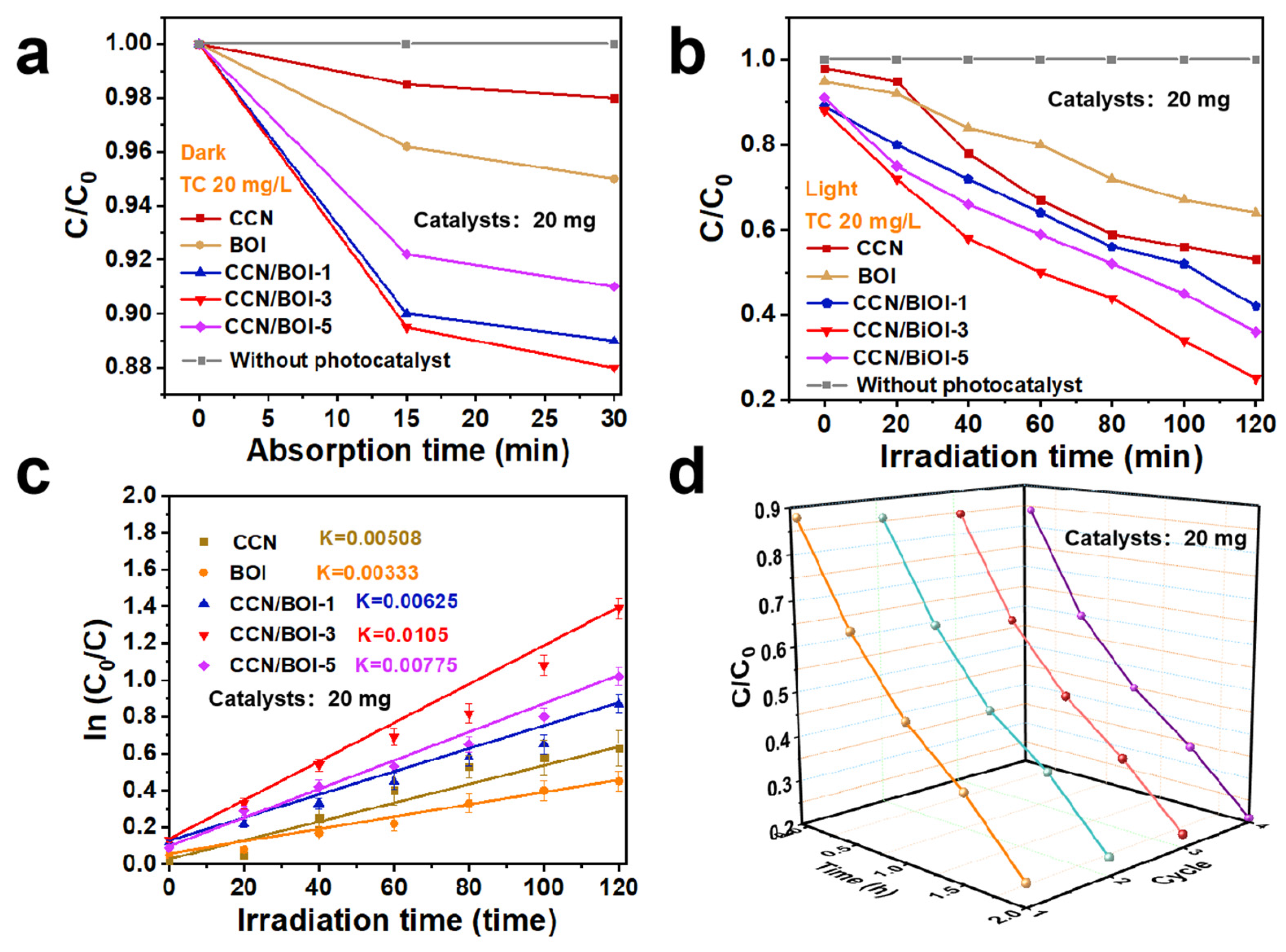
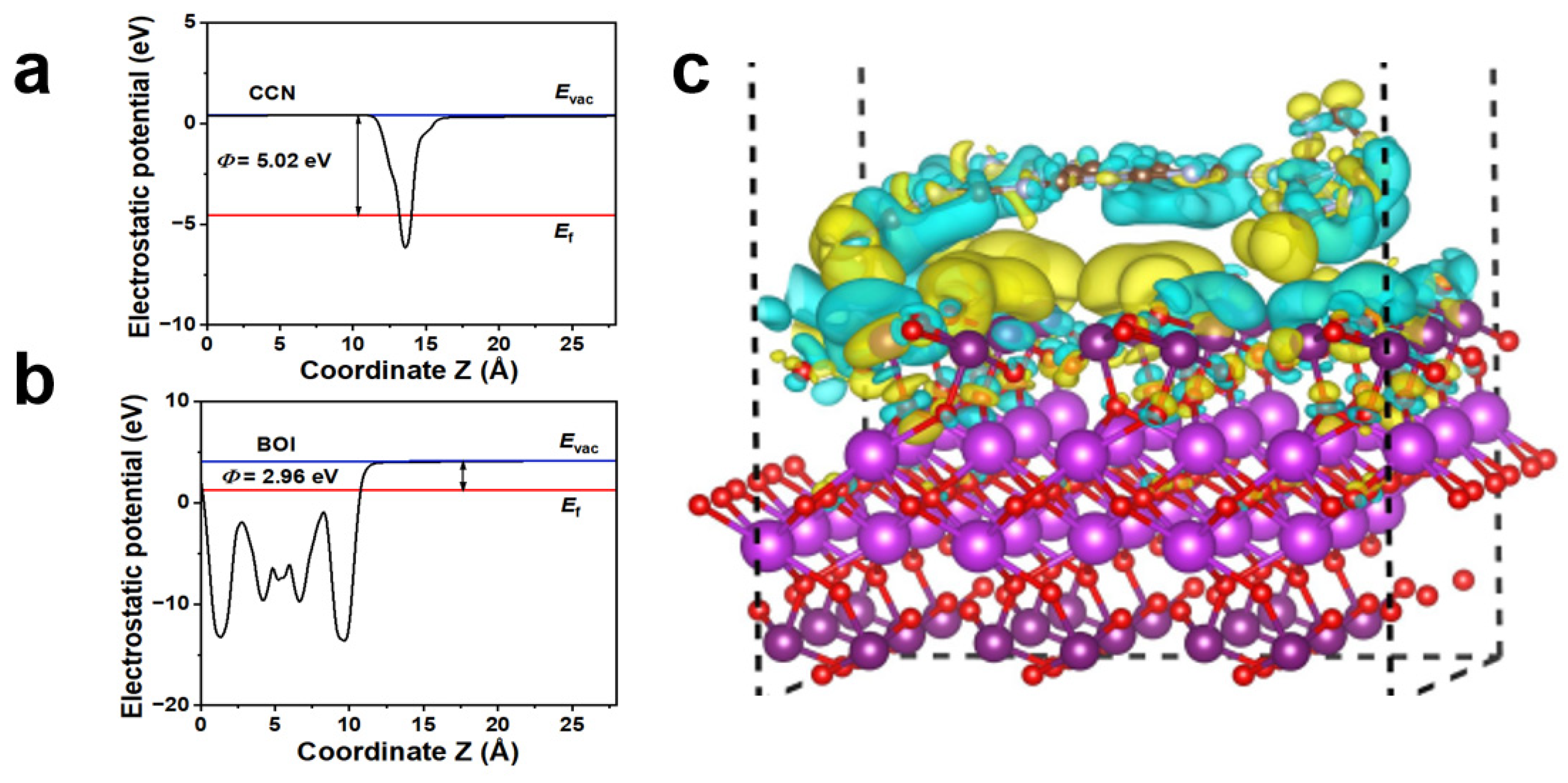
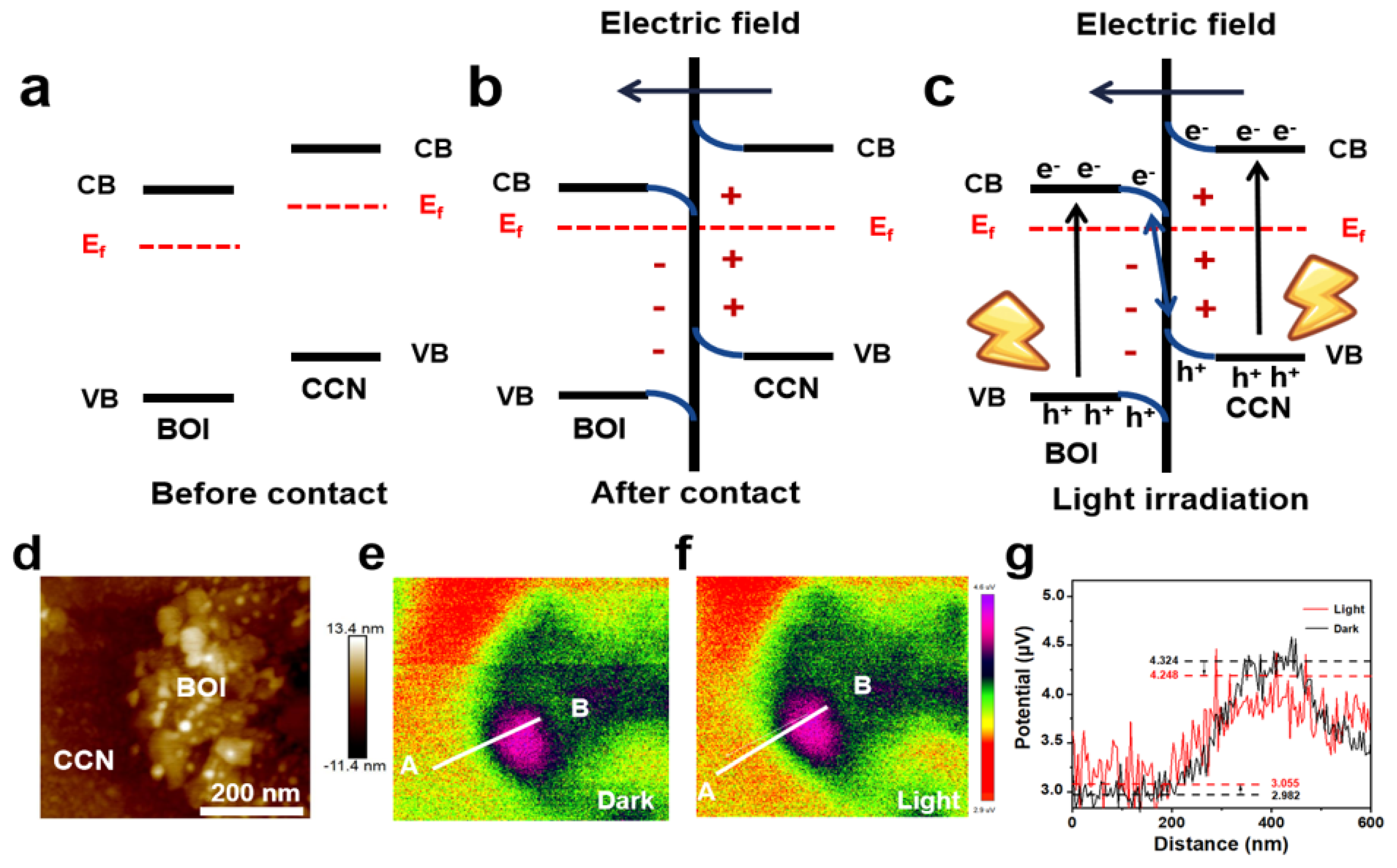
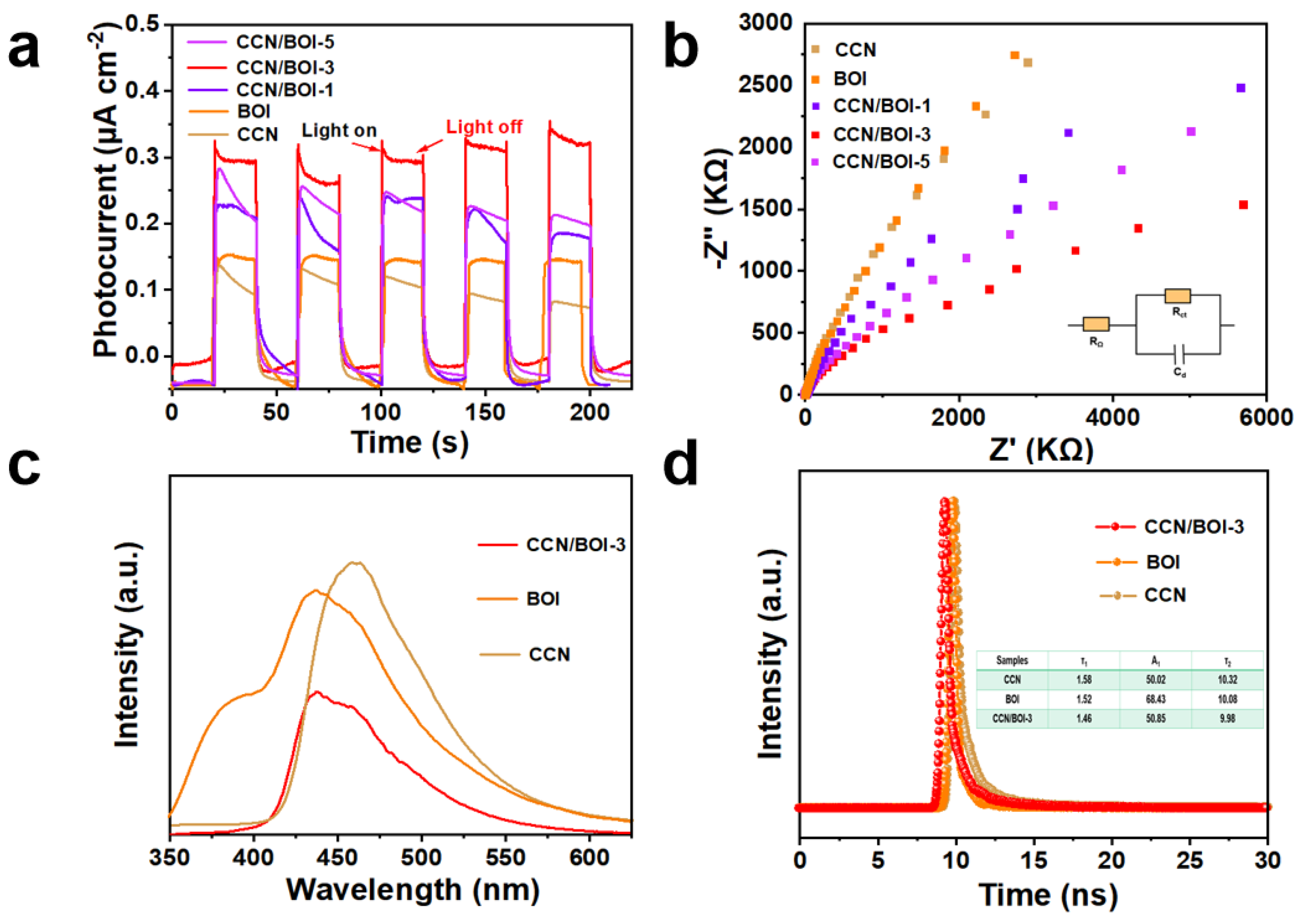
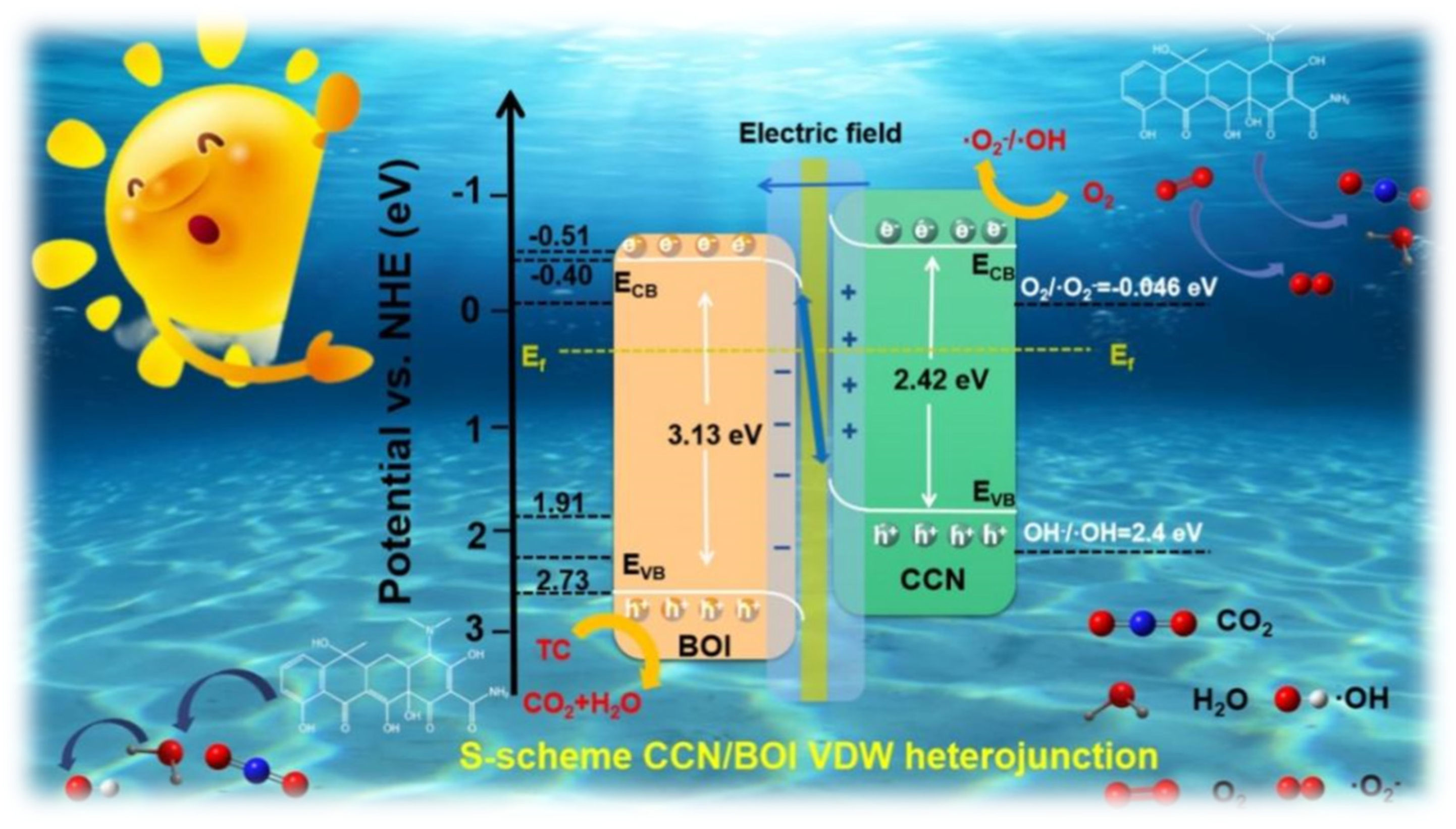
2. Results and Discussion
3. Materials and Methods
3.1. Chemical Reagents
3.2. Synthesis of Crystalline CN (CCN) Nanosheets
3.3. Synthesis of BiOIO3 (BOI) Nanosheets
3.4. Synthesis of CCN/BOI Heterojuntions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Wang, H.; Li, J.; Zhou, M.; Guan, Q.; Lu, Z.; Huo, P.; Yan, Y. Preparation and characterization of Ag2O/SWNTs photocatalysts and its photodegradation on tetracycline. J. Ind. Eng. Chem. 2015, 30, 64–70. [Google Scholar] [CrossRef]
- Delgado, N.; Bermeo, L.; Hoyos, D.A.; Peñuela, G.A.; Capparelli, A.; Marino, D.; Navarro, A.; Casas-Zapata, J.C. Occurrence and removal of pharmaceutical and personal care products using subsurface horizontal flow constructed wetlands. Water Res. 2020, 187, 116448. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Ma, T.; Zhang, T.; Zhang, Y.; Huang, H. Atomic-Level Charge Separation Strategies in Semiconductor-Based Photocatalysts. Adv. Mater. 2021, 33, e2005256. [Google Scholar] [CrossRef] [PubMed]
- Nemiwal, M.; Zhang, T.C.; Kumar, D. Recent progress in g-C3N4, TiO2 and ZnO based photocatalysts for dye degradation: Strategies to improve photocatalytic activity. Sci. Total Environ. 2021, 767, 144896. [Google Scholar] [CrossRef]
- Shen, H.; Wang, J.; Jiang, J.; Luo, B.; Mao, B.; Shi, W. All-solid-state Z-scheme system of RGO-Cu2O/Bi2O3 for tetracycline degradation under visible-light irradiation. Chem. Eng. J. 2017, 313, 508–517. [Google Scholar] [CrossRef]
- Nasir, J.A.; Rehman, Z.-U.; Shah, S.N.A.; Khan, A.; Butler, I.S.; Catlow, C.R.A. Recent developments and perspectives in CdS-based photocatalysts for water splitting. J. Mater. Chem. A 2020, 8, 20752–20780. [Google Scholar] [CrossRef]
- Gao, P.; Yang, Y.; Yin, Z.; Kang, F.; Fan, W.; Sheng, J.; Feng, L.; Liu, Y.; Du, Z.; Zhang, L. A critical review on bismuth oxyhalide based photocatalysis for pharmaceutical active compounds degradation: Modifications, reactive sites, and challenges. J. Hazard. Mater. 2021, 412, 125186. [Google Scholar] [CrossRef]
- Wu, Q.; Li, Z.; Hong, H. Adsorption of the quinolone antibiotic nalidixic acid onto montmorillonite and kaolinite. Appl. Clay Sci. 2012, 74, 66–73. [Google Scholar] [CrossRef]
- Shi, Y.; Li, L.; Xu, Z.; Guo, F.; Li, Y.; Shi, W. Synergistic coupling of piezoelectric and plasmonic effects regulates the Schottky barrier in Ag nanoparticles/ultrathin g-C3N4 nanosheets heterostructure to enhance the photocatalytic activity. Appl. Surf. Sci. 2023, 616, 156466. [Google Scholar] [CrossRef]
- Sun, H.; Guo, F.; Pan, J.; Huang, W.; Wang, K.; Shi, W. One-pot thermal polymerization route to prepare N-deficient modified g-C3N4 for the degradation of tetracycline by the synergistic effect of photocatalysis and persulfate-based advanced oxidation process. Chem. Eng. J. 2020, 406, 126844. [Google Scholar] [CrossRef]
- Guo, F.; Shi, C.; Sun, W.; Liu, Y.; Lin, X.; Shi, W. Pomelo biochar as an electron acceptor to modify graphitic carbon nitride for boosting visible-light-driven photocatalytic degradation of tetracycline. Chin. J. Chem. Eng. 2021, 48, 1–11. [Google Scholar] [CrossRef]
- Shi, Y.; Li, L.; Xu, Z.; Sun, H.; Guo, F.; Shi, W. One-step simple green method to prepare carbon-doped graphitic carbon nitride nanosheets for boosting visible-light photocatalytic degradation of tetracycline. J. Chem. Technol. Biotechnol. 2021, 96, 3122–3133. [Google Scholar] [CrossRef]
- Cao, D.; Wang, X.; Zhang, H.; Yang, D.; Yin, Z.; Liu, Z.; Lu, C.; Guo, F. Rational Design of Monolithic g-C3N4 with Floating Network Porous-like Sponge Monolithic Structure for Boosting Photocatalytic Degradation of Tetracycline under Simulated and Natural Sunlight Illumination. Molecules 2023, 28, 3989. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Chen, Z.; Huang, X.; Cao, L.; Cheng, X.; Shi, W.; Chen, L. Cu3P nanoparticles decorated hollow tubular carbon nitride as a superior photocatalyst for photodegradation of tetracycline under visible light. Sep. Purif. Technol. 2021, 275, 119223. [Google Scholar] [CrossRef]
- Shi, W.; Cao, L.; Shi, Y.; Chen, Z.; Cai, Y.; Guo, F.; Du, X. Environmentally friendly supermolecule self-assembly preparation of S-doped hollow porous tubular g-C3N4 for boosted photocatalytic H2 production. Ceram. Int. 2023, 49, 11989–11998. [Google Scholar] [CrossRef]
- Shi, W.; Liu, Y.; Sun, W.; Hong, Y.; Li, X.; Lin, X.; Guo, F.; Shi, J. Assembling g-C3N4 nanosheets on rod-like CoFe2O4 nanocrystals to boost photocatalytic degradation of ciprofloxacin with peroxymonosulfate activation. Mater. Today Commun. 2021, 29, 102871. [Google Scholar] [CrossRef]
- Yuan, H.; Shi, W.; Lu, J.; Wang, J.; Shi, Y.; Guo, F.; Kang, Z. Dual-channels separated mechanism of photo-generated charges over semiconductor photocatalyst for hydrogen evolution: Interfacial charge transfer and transport dynamics insight. Chem. Eng. J. 2023, 454, 140442. [Google Scholar] [CrossRef]
- Guo, F.; Chen, Z.; Huang, X.; Cao, L.; Cheng, X.; Shi, W.; Chen, L. Ternary Ni2P/Bi2MoO6/g-C3N4 composite with Z-scheme electron transfer path for enhanced removal broad-spectrum antibiotics by the synergistic effect of adsorption and photocatalysis. Chin. J. Chem. Eng. 2022, 44, 157. [Google Scholar] [CrossRef]
- Guo, F.; Huang, X.; Chen, Z.; Cao, L.; Cheng, X.; Chen, L.; Shi, W. Construction of Cu3P-ZnSnO3-g-C3N4 p-n-n heterojunction with multiple built-in electric fields for effectively boosting visible-light photocatalytic degradation of broad-spectrum antibiotics. Sep. Purif. Technol. 2021, 265, 118477. [Google Scholar] [CrossRef]
- Zhang, W.; Shi, W.; Sun, H.; Shi, Y.; Luo, H.; Jing, S.; Fan, Y.; Guo, F.; Lu, C. Fabrication of ternary CoO/g-C3N4/Co3O4 nanocomposite with p-n-p type heterojunction for boosted visible-light photocatalytic performance. J. Chem. Technol. Biotechnol. 2021, 96, 1854. [Google Scholar] [CrossRef]
- Zhu, X.; Guo, F.; Pan, J.; Sun, H.; Gao, L.; Deng, J.; Zhu, X.; Shi, W. Fabrication of visible-light-response face-contact ZnSnO3@g-C3N4 core–shell heterojunction for highly efficient photocatalytic degradation of tetracycline contaminant and mechanism insight. J. Mater. Sci. 2020, 56, 4366–4379. [Google Scholar] [CrossRef]
- Shi, W.; Cao, L.; Shi, Y.; Zhong, W.; Chen, Z.; Wei, Y.; Guo, F.; Chen, L.; Du, X. Boosted built-in electric field and active sites based on Ni-doped heptazine/triazine crystalline carbon nitride for achieving high-efficient photocatalytic H2 evolution. J. Mol. Struct. 2023, 1280, 135076. [Google Scholar] [CrossRef]
- Shi, W.; Yang, S.; Sun, H.; Wang, J.; Lin, X.; Guo, F.; Shi, J. Carbon dots anchored high-crystalline g-C3N4 as a metal-free composite photocatalyst for boosted photocatalytic degradation of tetracycline under visible light. J. Mater. Sci. 2020, 56, 2226–2240. [Google Scholar] [CrossRef]
- Xu, Z.; Shi, Y.; Li, L.; Sun, H.; Amin, S.; Guo, F.; Wen, H.; Shi, W. Fabrication of 2D/2D Z-scheme highly crystalline carbon nitride/δ-Bi2O3 heterojunction photocatalyst with enhanced photocatalytic degradation of tetracycline. J. Alloys Compd. 2021, 895, 162667. [Google Scholar] [CrossRef]
- Iqbal, W.; Qiu, B.; Zhu, Q.; Xing, M.; Zhang, J. Self-modified breaking hydrogen bonds to highly crystalline graphitic carbon nitrides nanosheets for drastically enhanced hydrogen production. Appl. Catal. B Environ. 2018, 232, 306–313. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, Y.; Zhang, L.; Cheng, B.; Yu, J.; Fan, J. Step-scheme CdS/TiO2 nanocomposite hollow microsphere with enhanced photocatalytic CO2 reduction activity. J. Mater. Sci. Technol. 2020, 56, 143–150. [Google Scholar] [CrossRef]
- Xie, Y.; Shang, X.; Liu, D.; Zhao, H.; Gu, Y.; Zhang, Z.; Wang, X. Non-noble metal thickness-tunable Bi2MoO6 nanosheets for highly efficient visible-light-driven nitrobenzene reduction into aniline. Appl. Catal. B Environ. 2019, 259, 118087. [Google Scholar] [CrossRef]
- Shi, W.; Li, M.; Huang, X.; Ren, H.; Yan, C.; Guo, F. Facile synthesis of 2D/2D Co3(PO4)2/g-C3N4 heterojunction for highly photocatalytic overall water splitting under visible light. Chem. Eng. J. 2020, 382, 122960. [Google Scholar] [CrossRef]
- Wang, L.; Xue, Y.; Yang, G.; Liu, X.; He, W.; Zhang, M.; Liu, Z. Electronic structure regulation of an S-scheme CuBi2O4/Sr0.5NaTaO3 heterojunction with efficient carrier spatial transfer. Sep. Purif. Technol. 2023, 317, 123856. [Google Scholar] [CrossRef]
- Chen, K.; Shi, Y.; Shu, P.; Luo, Z.; Shi, W.; Guo, F. Construction of core–shell FeS2@ZnIn2S4 hollow hierarchical structure S-scheme heterojunction for boosted photothermal-assisted photocatalytic H2 production. Chem. Eng. J. 2023, 454, 140053. [Google Scholar] [CrossRef]
- Lu, C.; Wang, J.; Cao, D.; Guo, F.; Hao, X.; Li, D.; Shi, W. Synthesis of magnetically recyclable g-C3N4/NiFe2O4 S-scheme heterojunction photocatalyst with promoted visible-light-response photo-Fenton degradation of tetracycline. Mater. Res. Bull. 2023, 158, 112064. [Google Scholar] [CrossRef]
- Shi, W.; Fu, Y.; Sun, H.; Sun, X.; Hao, C.; Guo, F.; Tang, Y. Construction of 0D/3D CoFe2O4/MIL-101(Fe) complement each other S-scheme heterojunction for effectively boosted photocatalytic degradation of tetracycline. Inorg. Chem. Commun. 2022, 146, 110140. [Google Scholar] [CrossRef]
- Wang, L.; Niu, M.; Liu, Y.; Xie, Y.; Ma, Z.; Zhang, M.; Hou, C. The Ovs surface defecting of an S-scheme g-C3N4/H2Ti3O7 nanoheterostructures with accelerated spatial charge transfer. J. Colloid Interface Sci. 2023, 645, 639. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Sun, W.; Liu, Y.; Li, X.; Lin, X.; Guo, F.; Hong, Y. Onion-ring-like g-C3N4 modified with Bi3TaO7 quantum dots: A novel 0D/3D S-scheme heterojunction for enhanced photocatalytic hydrogen production under visible light irradiation. Renew. Energy 2021, 182, 958–968. [Google Scholar] [CrossRef]
- Shi, Y.; Li, L.; Xu, Z.; Guo, F.; Shi, W. Construction of full solar-spectrum available S-scheme heterojunction for boosted photothermal-assisted photocatalytic H2 production. Chem. Eng. J. 2023, 459, 141549. [Google Scholar] [CrossRef]
- Wang, J.; Shi, Y.; Sun, H.; Shi, W.; Guo, F. Fabrication of Bi4Ti3O12/ZnIn2S4 S-scheme heterojunction for achieving efficient photocatalytic hydrogen production. J. Alloys Compd. 2023, 930, 167450. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Y.; Hao, J.; Ma, Z.; Lu, Y.; Zhang, M.; Hou, C. Construction of an S-scheme TiOF2/HTiOF3 heterostructures with abundant OVs and OH groups: Performance, kinetics and mechanism insight. J. Colloid Interface Sci. 2023, 640, 15. [Google Scholar] [CrossRef]
- Xu, Z.; Shi, W.; Shi, Y.; Sun, H.; Li, L.; Guo, F.; Wen, H. Carbon dots as solid-state electron mediator and electron acceptor in S-scheme heterojunction for boosted photocatalytic hydrogen evolution. Appl. Surf. Sci. 2022, 595, 153482. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, X.R.; Li, Y.F. Spin-orbit coupling-dominated catalytic activity of twodimensional bismuth toward CO2 electro-reduction: Not the thinner the better. J. Phys. Chem. Lett. 2019, 10, 4663–4667. [Google Scholar] [CrossRef]
- Wang, B.B.; Chen, D.Y.; Li, N.J.; Xu, Q.F.; Li, H.; He, J.H.; Lu, J.M. Z-scheme photocatalytic NO removal on a 2D/2D iodine doped BiOIO3/g-C3N4 under visiblelight irradiation. J. Colloid Interface Sci. 2020, 576, 426–434. [Google Scholar] [CrossRef]
- Wang, T.; He, S.F.; Zhang, Y.S.; Wang, J.W.; Pan, W.P. Photocatalytic removal of elemental mercury on TiO2-BiOIO3 heterostruc-tures: Mercury transformation, sulfur tolerance and SO2/SO3 conversion. Chem. Eng. J. 2020, 388, 124390. [Google Scholar] [CrossRef]
- Shifa, T.A.; Wang, F.; Liu, Y.; He, J. Heterostructures Based on 2D Materials: A Versatile Platform for Efficient Catalysis. Adv. Mater. 2018, 31, e1804828. [Google Scholar] [CrossRef]
- Lai, J.; Jiang, X.; Zhao, M.; Cui, S.; Yang, J.; Li, Y. Thickness-dependent layered BiOIO3 modified with carbon quantum dots for photodegradation of bisphenol A: Mechanism, pathways and DFT calculation. Appl. Catal. B Environ. 2021, 298, 120622. [Google Scholar] [CrossRef]
- Xing, W.N.; Tu, W.G.; Han, Z.H.; Hu, Y.D.; Meng, Q.Q.; Chen, G. Template-induced high-crystalline g-C3N4 nanosheets for enhanced photocatalytic H2 evolution. ACS Energy Lett. 2018, 3, 514–519. [Google Scholar] [CrossRef]
- Liu, G.; Xue, M.; Liu, Q.; Yang, H.; Zhou, Y. Facile synthesis of C-doped hollow spherical g-C3N4 from supramolecular self-assembly for enhanced photoredox water splitting. Int. J. Hydrogen Energy 2019, 44, 25671–25679. [Google Scholar] [CrossRef]
- Gao, H.L.; Yan, S.C.; Wang, J.J.; Huang, Y.A.; Wang, P.; Li, Z.S. Toward efficient solar hydrogen production by intercalated car-bon nitride photocatalyst. Phys. Chem. Chem. Phys. 2013, 15, 18077–18084. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Huang, H.W.; Zeng, C.; Du, X.; Zhang, Y.H. Achieving enhanced UV and visible light photocatalytic activity for ter-nary Ag/AgBr/BiOIO3: Decomposition for diverse industrial contaminants with distinct mechanisms and complete mineraliza-tion ability. ACS Sustain. Chem. Eng. 2017, 5, 7777–7791. [Google Scholar] [CrossRef]
- Su, L.X.; Liu, Z.Y.; Ye, Y.L.; Shen, C.L.; Lou, Q.; Shan, C.X. Heterostructured boron doped nanodiamonds@g-C3N4 nanocompo-sites with enhanced photocatalytic capability under visible light irradiation. Int. J. Hydrogen Energy 2019, 44, 19805–19815. [Google Scholar] [CrossRef]
- Jiang, R.R.; Wu, D.H.; Lu, G.H.; Yan, Z.H.; Liu, J.C. Modified 2D-2D ZnIn2S4/BiOCl van der Waals heterojunctions with CQDs: Accelerated charge transfer and enhanced photocatalytic activity under vis- and NIR-light. Chemosphere 2019, 227, 82–92. [Google Scholar] [CrossRef]
- Xie, J.; Cao, Y.L.; Hu, J.D.; Tang, Y.K.; Jia, D.Z. A solvent-free strategy to realize the substitution of I− for IO3− in a BiOIO3 photo-catalyst with an opposite charge transfer path. Green Chem. 2020, 22, 1424–1431. [Google Scholar] [CrossRef]
- Han, W.Y.; Li, D.G.; Zhang, M.Q.; Hu, X.M.; Duan, X.G.; Liu, S.M. Photocatalytic activation of peroxymonosulfate by sur-face-tailored carbon quantum dots. J. Hazard. Mater. 2020, 395, 122695. [Google Scholar] [CrossRef]
- Zhu, C.Z.; Wang, Y.T.; Jiang, Z.F.; Xu, F.C.; Xian, Q.M.; Sun, C. CeO2 nanocrystal-modified layered MoS2/g-C3N4 as 0D/2D ternary composite for visible-light photocatalytic hydrogen evolution: Interfacial consecutive multi-step electron transfer and enhanced H2O reactant adsorption. Appl. Catal. B-Environ. 2019, 259, 118072. [Google Scholar] [CrossRef]
- CBai, P.; Bi, J.C.; Wu, J.B.; Xu, Y.; Wohlrab, S.; Han, Y.D. In-situ solid-phase fabrication of Ag/AgX (X=Cl, Br, I)/g-C3N4 compo-sites for enhanced visible-light hydrogen evolution. Int. J. Hydrog. Energy 2019, 44, 21397–21405. [Google Scholar]
- Hu, X.; Guo, R.T.; Lin, Z.D.; Bi, Z.X.; Chen, X.; Wang, J.; Pan, W.G. Construction of Carbon Dot-Modified g-C3N4/BiOIO3 Z-Scheme Heterojunction for Boosting Photocatalytic CO2 Reduction under Full Spectrum Light. ACS Sustain. Chem. Eng. 2022, 10, 11143–11153. [Google Scholar] [CrossRef]
- Wu, Y.; Li, C.; Liu, W.; Li, H.; Gong, Y.; Niu, L.; Liu, X.; Sun, C.; Xu, S. Unexpected monoatomic catalytic-host synergetic OER/ORR by graphitic carbon nitride: Density functional theory. Nanoscale 2019, 11, 5064–5071. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wu, Y.; Li, L.; Gong, Y.; Niu, L.; Liu, X.; Wang, T.; Sun, C.; Li, C. Adjustable photocatalytic ability of monolayer g-C3N4 utilizing single–metal atom: Density functional theory. Appl. Surf. Sci. 2018, 457, 735–744. [Google Scholar] [CrossRef]
- Jiang, R.R.; Lu, G.H.; Yan, Z.H.; Wu, D.H.; Zhou, R.R.; Bao, X.H. Insights into a CQD-SnNb2O6/BiOCl Z-scheme system for the degradation of benzocaine: Influence factors, intermediate toxicity and photocatalytic mechanism. Chem. Eng. J. 2019, 374, 79–90. [Google Scholar] [CrossRef]
- Fang, L.J.; Li, Y.H.; Liu, P.F.; Wang, D.P.; Zeng, H.D.; Wang, X.L.; Yang, H.G. Facile Fabrication of Large-Aspect-Ratio g-C3N4 Nanosheets for Enhanced Photocatalytic Hydrogen Evolution. ACS Sustain. Chem. Eng. 2017, 5, 2039–2043. [Google Scholar] [CrossRef]
- Lu, S.; Li, C.; Li, H.H.; Zhao, Y.F.; Gong, Y.Y.; Niu, L.Y. The effects of nonmetal dopants on the electronic, optical and chemical performances of monolayer g-C3N4 by first-principles study. Appl. Surf. Sci. 2017, 392, 966–974. [Google Scholar] [CrossRef]
- Wang, B.; Cheng, L.Y.; Zhang, J.G.; Yu, Y.J.; Li, S.; Wageh, A.; Al-Ghamdi, A. S-Scheme 2D/2D Bi2MoO6/BiOI van der Waals heterojunction for CO2 photoreduction. Chin. J. Catal. 2022, 43, 1657–1666. [Google Scholar] [CrossRef]
- Li, G.; Lian, Z.; Wan, Z.; Liu, Z.; Qian, J.; Deng, Y.; Zhang, S.; Zhong, Q. Efficient photothermal-assisted photocatalytic NO removal on molecular cobalt phthalocyanine/Bi2WO6 Z-scheme heterojunctions by promoting charge transfer and oxygen activation. Appl. Catal. B Environ. 2022, 317, 121787. [Google Scholar] [CrossRef]
- Cheng, C.; He, B.; Fan, J.; Cheng, B.; Cao, S.; Yu, J. An Inorganic/Organic S-Scheme Heterojunction H2-Production Photocatalyst and its Charge Transfer Mechanism. Adv. Mater. 2021, 33, 2100317. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Wu, D.; Lu, G.; Yan, Z.; Liu, J.; Zhou, R.; Nkoom, M. Fabrication of Fe3O4 quantum dots modified BiOCl/BiVO4 p-n heterojunction to enhance photocatalytic activity for removing broad-spectrum antibiotics under visible light. J. Taiwan Inst. Chem. Eng. 2019, 96, 681–690. [Google Scholar] [CrossRef]
- Che, H.; Che, G.; Zhou, P.; Liu, C.; Dong, H.; Li, C.; Song, N.; Li, C. Nitrogen doped carbon ribbons modified g-C3N4 for markedly enhanced photocatalytic H2-production in visible to near-infrared region. Chem. Eng. J. 2020, 382, 122870. [Google Scholar] [CrossRef]
- Wang, J.B.; Liu, C.; Yang, S.; Lin, X.; Shi, W.L. Fabrication of a ternary heterostructure BiVO4 quantum dots/C60/g-C3N4 photo-catalyst with enhanced photocatalytic activity. J. Phys. Chem. Solids 2020, 136, 10924. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, K.; Li, Y.-L.; He, J.-Y.; Zhang, K.-S.; Liu, T.; Xu, W.; Huang, X.-J.; Kong, L.-T.; Liu, J.-H. Morphology-tunable WMoO nanowire catalysts for the extremely efficient elimination of tetracycline: Kinetics, mechanisms and intermediates. Nanoscale 2018, 11, 1047–1057. [Google Scholar] [CrossRef]
- Xie, Z.J.; Feng, Y.P.; Wang, F.L.; Chen, D.N.; Zhang, Q.X.; Zeng, Y.Q.; Lv, W.Y.; Liu, G.G. Construction of carbon dots modified MoO3/g-C3N4 Z-scheme photocatalyst with enhanced visible-light photocatalytic activity for the degradation of tetracycline. Appl. Catal. B-Environ. 2018, 229, 96–104. [Google Scholar] [CrossRef]
- Li, J.Z.; Ma, Y.; Ye, Z.F.; Zhou, M.J.; Wang, H.Q.; Ma, C.C.; Wang, D.D.; Huo, P.W.; Yan, Y.S. Fast electron transfer and enhanced visible light photo-catalytic activity using multi-dimensional components of carbon quantum-dots@3D daisy-like In2S3/single-wall carbon nanotubes. Appl. Catal. B-Environ. 2017, 204, 224–238. [Google Scholar] [CrossRef]
- Zhu, L.; Li, H.; Xia, P.; Liu, Z.; Xiong, D. Hierarchical ZnO Decorated with CeO2 Nanoparticles as the Direct Z-Scheme Heterojunction for Enhanced Photocatalytic Activity. ACS Appl. Mater. Interfaces 2018, 10, 39679–39687. [Google Scholar] [CrossRef]
- Guo, H.; Niu, C.G.; Zhang, L.; Wen, X.J.; Liang, C.; Zhang, X.G.; Guan, D.L.; Tang, N.; Zeng, G.M. Construction of direct Z-scheme AgI/Bi2Sn2O7 nanojunction system with enhanced photocatalytic activity: Accelerated interfacial charge transfer induced effi-cient Cr(VI) reduction, tetracycline degradation and escherichia coli inactivation. ACS Sustain. Chem. Eng. 2018, 6, 8003–8018. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, B.; Cheng, B.; Zhang, J.; Zhang, L.; Yu, J. In-situ preparation of TiO2/N-doped graphene hollow sphere pho-tocatalyst with enhanced photocatalytic CO2 reduction performance. Chin. J. Catal. 2021, 42, 1648–1658. [Google Scholar] [CrossRef]
- He, R.; Liu, H.; Liu, H.; Xu, D.; Zhang, L. S-scheme photocatalyst Bi2O3/TiO2 nanofiber with improved photocatalytic performance. J. Mater. Sci. Technol. 2020, 52, 145–151. [Google Scholar]
- Wang, L.; Hong, Y.; Liu, E.; Wang, Z.; Chen, J.; Yang, S.; Wang, J.; Lin, X.; Shi, J. Rapid polymerization synthesizing high-crystalline g-C3N4 towards boosting solar photocatalytic H2 generation. Int. J. Hydrogen Energy 2020, 45, 6425–6436. [Google Scholar] [CrossRef]
- Dong, X.Y.; Huang, X.Q.; Wang, D.B.; Lei, Y.; Han, J.C.; Liang, X.F.; Wei, Q.L. Constructing crystalline needle-mushroom-like/ amorphous nanosheet carbon nitride homojunction by molten salt method for photocatalytic degradation of tetracycline hydrochloride. J. Mater Sci-Mater El. 2022, 33, 6043–6058. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, J.Q.; Gong, J.; Wang, K.; Li, Y.; Wu, X.Y. Fabrication of Ag/carbon nitride photocatalysts and their enhanced photocatalytic performance for tetracycline degradation. Funct. Mater. Lett. 2020, 13, 2051033. [Google Scholar] [CrossRef]
- Chen, M.M.; Li, M.X.; Li, P.; Lee, S.L.J.; Tang, J.J.; Li, Q.; Lin, S.J. Enhanced visible light-driven photodegradation of tetracycline by salicylic acid-modified graphitic carbon nitride and toxicity assessment, Environ. Sci. Pollut. R. 2022, 29, 90768–90778. [Google Scholar] [CrossRef]
- Wang, B.; Cao, Q.T.; Li, G.M.; Zhang, J. Preparation of non-polluting Tb-doped mesoporous carbon nitride photocatalyst and study on the efficacy and mechanism of degradation of antibiotics in water. Environ. Sci. Pollut. R. 2022, 29, 36337–36350. [Google Scholar] [CrossRef]
- Wang, X.; Jing, H.X.; Gao, Y.L.; Xin, Y.Z.; Li, Q.L. CTAB assisted hydrothermal synthesis of 0D / 2D structure carbon quantum dots BiOIO3 composite for photocatalytic degradation of tetracycline. Inorg. Chem. Commun. 2022, 146, 110209. [Google Scholar] [CrossRef]
- Kuang, X.; Fu, M.; Kang, H.; Lu, P.; Bai, J.W.; Yang, Y.; Gao, S.X. A BiOIO3/BiOBr n-n heterojunction was constructed to enhance the photocatalytic degradation of TC. Opt. Mater. 2023, 138, 113690. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, X.; Cao, L.; Shi, Y.; Chen, Z.; Shi, W.; Du, X. Construction of S-Scheme 2D/2D Crystalline Carbon Nitride/BiOIO3 van der Waals Heterojunction for Boosted Photocatalytic Degradation of Antibiotics. Molecules 2023, 28, 5098. https://doi.org/10.3390/molecules28135098
Kong X, Cao L, Shi Y, Chen Z, Shi W, Du X. Construction of S-Scheme 2D/2D Crystalline Carbon Nitride/BiOIO3 van der Waals Heterojunction for Boosted Photocatalytic Degradation of Antibiotics. Molecules. 2023; 28(13):5098. https://doi.org/10.3390/molecules28135098
Chicago/Turabian StyleKong, Xiangyuan, Longwen Cao, Yuxing Shi, Zhouze Chen, Weilong Shi, and Xin Du. 2023. "Construction of S-Scheme 2D/2D Crystalline Carbon Nitride/BiOIO3 van der Waals Heterojunction for Boosted Photocatalytic Degradation of Antibiotics" Molecules 28, no. 13: 5098. https://doi.org/10.3390/molecules28135098




