Phytochemical Analysis, In Vitro Biological Activities, and Computer-Aided Analysis of Potentilla nepalensis Hook Compounds as Potential Melanoma Inhibitors Based on Molecular Docking, MD Simulations, and ADMET
Abstract
:1. Introduction
2. Results
2.1. Quantification of Total Phenolic and Flavonoid Content
2.2. In Vitro Antioxidant Activity
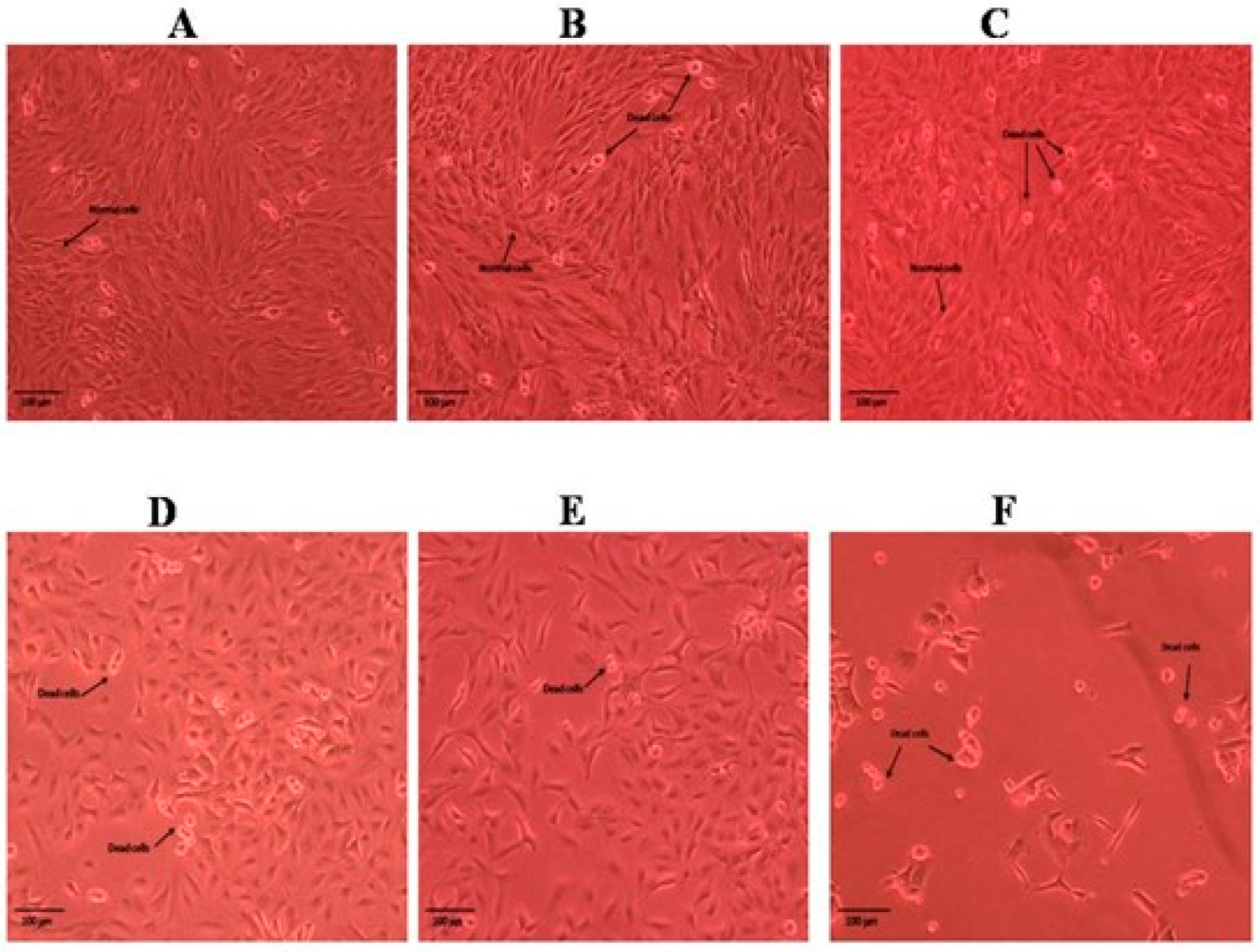
2.3. Cytotoxic Activity of Roots and Shoots of P. nepalensis
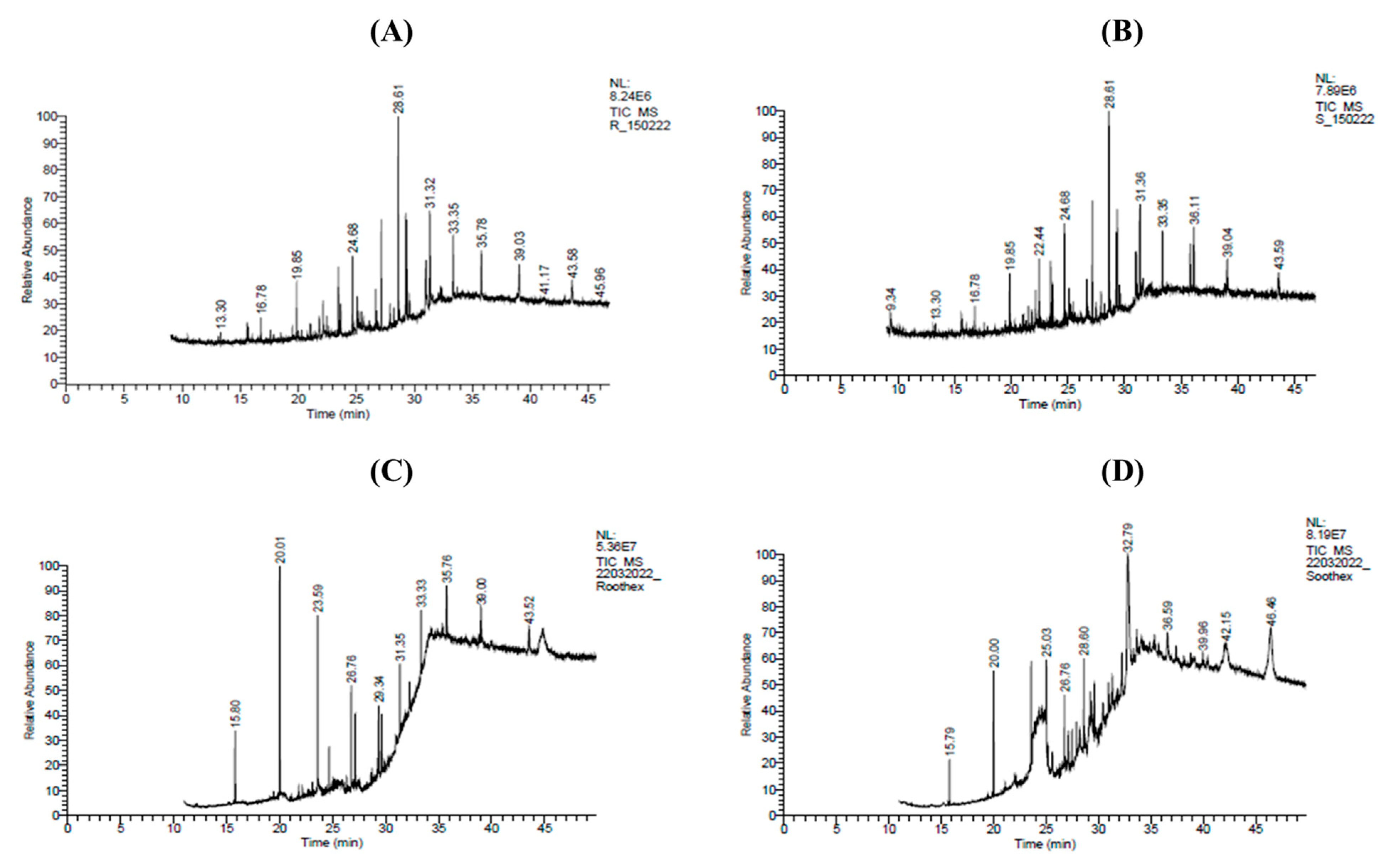
2.4. Identification of Major Phytocompounds of Methanolic and n-Hexane Extracts of Roots and Shoots of P. nepalensis via GC-MS Profiling
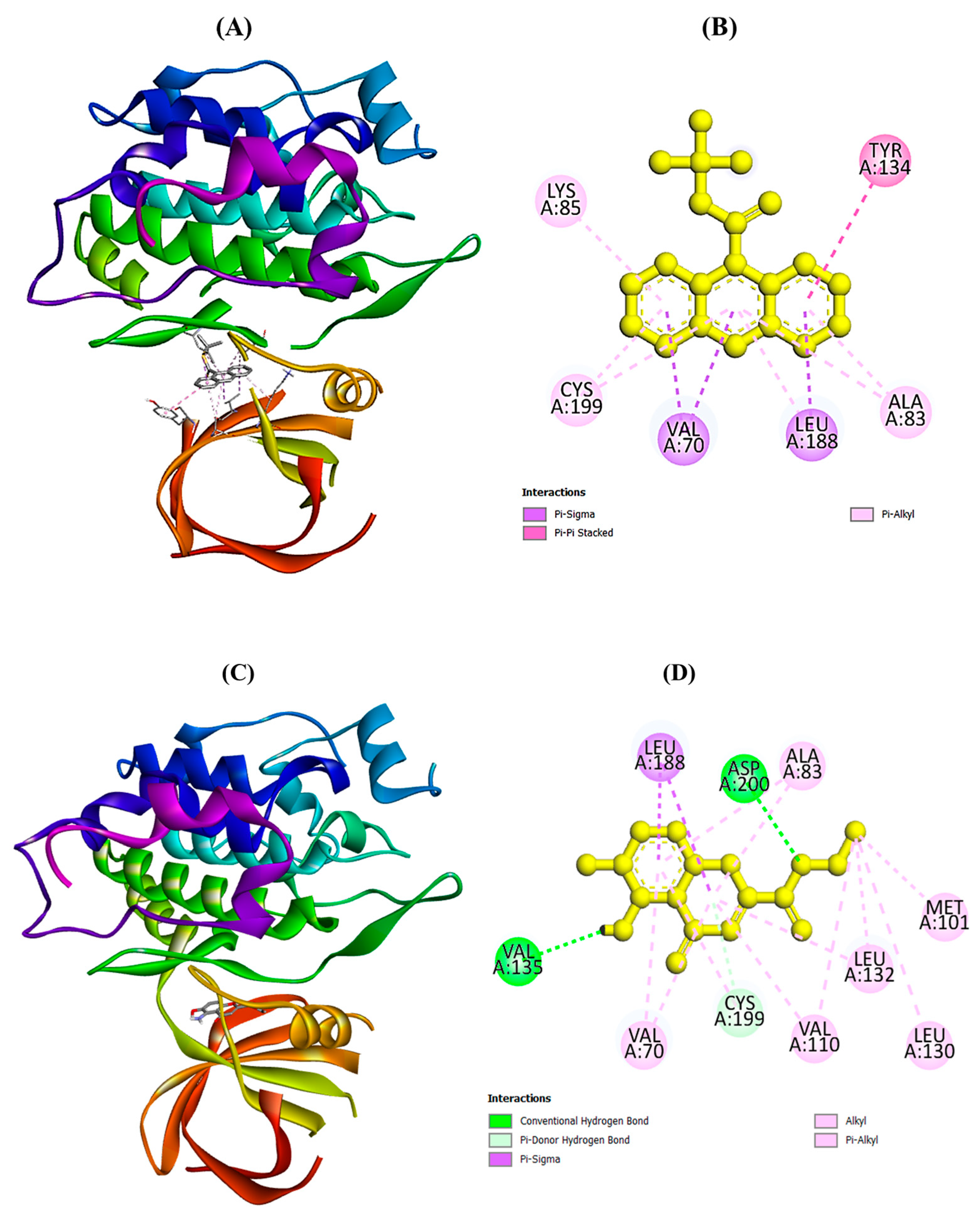
2.5. Molecular Docking Analysis
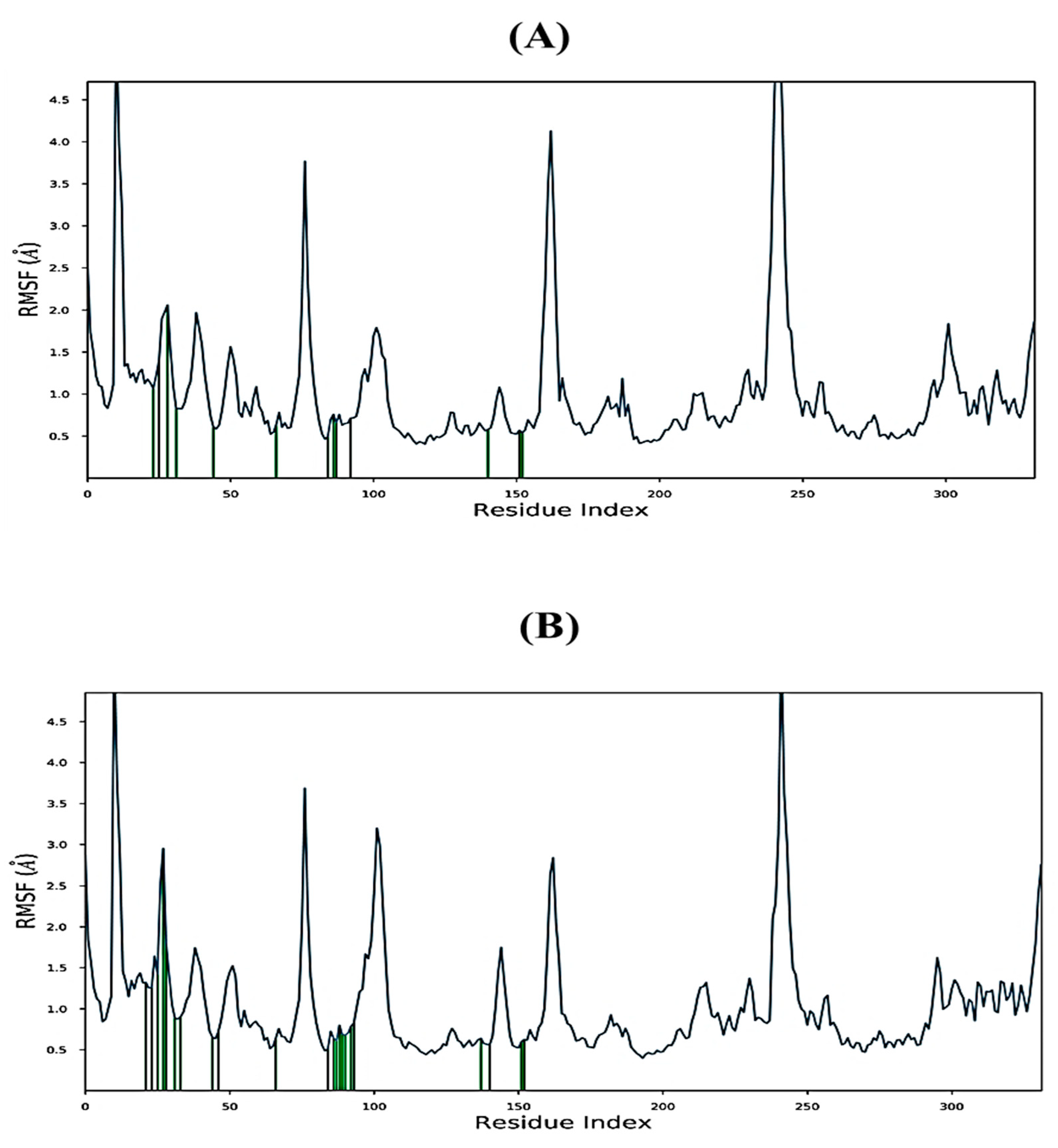
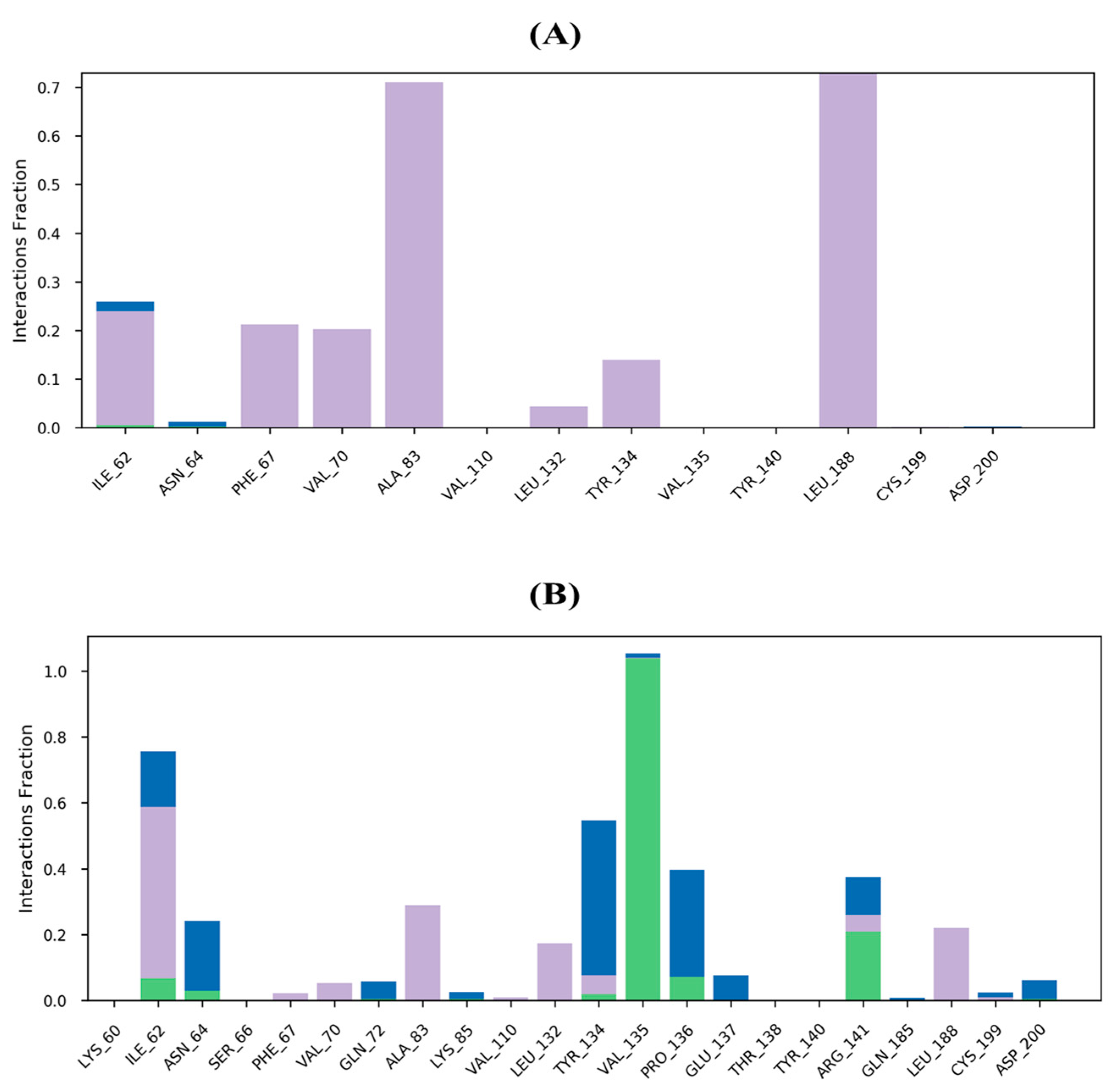
2.6. MD Simulations Study
2.7. Assessment of Drug Likeness and Toxicity Prediction
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Collection and Identification of Plant Material
4.3. Preparation of Extracts of Roots and Shoots of P. nepalensis
4.4. Spectrophotometric Quantification of Total Phenolic (TPC) and Flavonoid Content (TFC)
4.5. In Vitro Antioxidant Potential Using DPPH Radical Scavenging Assay
4.6. In Vitro Cytotoxic Activity of P. nepalensis Roots and Shoots
4.7. GC-MS Profiling of Root and Shoot Extracts of P. nepalensis to Identify Major Phytocompounds
4.8. Ligand Preparation
4.9. Retrieval and Preparation of Target Proteins
4.10. Molecular Docking
4.11. Molecular Dynamics Simulations
4.12. Evaluation of Drug-Likeness and ADME/Toxicity Properties
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumari, S.; Seth, A.; Sharma, S.; Attri, C. A holistic overview of different species of Potentilla a medicinally important plant along with their pharmaceutical significance: A review. J. Herb Med. 2021, 29, 100460. [Google Scholar] [CrossRef]
- Anand, U.; Jacobo-Herrera, N.; Altemimi, A.; Lakhssassi, N. A comprehensive review on medicinal plants as antimicrobial therapeutics: Potential avenues of biocompatible drug discovery. Metabolites 2019, 9, 258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehta, P.; Bisht, K.; Sekar, K.C. Diversity of threatened medicinal plants of Indian Himalayan Region. Plant Biosyst. 2021, 155, 1121–1132. [Google Scholar] [CrossRef]
- Tomczyk, M.; Latté, K.P. Potentilla—A review of its phytochemical and pharmacological profile. J. Ethnopharmacol. 2009, 122, 184–204. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.; Jain, V.K.; Sharma, S. Rosaceae of Solan District of Himachal Pradesh. IJSRST 2018, 4, 1580–1589. [Google Scholar]
- Eddy, K.; Chen, S. Overcoming immune evasion in melanoma. Int. J. Mol. Sci. 2020, 21, 8984. [Google Scholar] [CrossRef]
- Feng, S.; Zhou, Y.; Huang, H.; Lin, Y.; Zeng, Y.; Han, S.; Huang, K.; Liu, Q.; Zhu, W.; Yuan, Z.; et al. Nobiletin Induces Ferroptosis in Human Skin Melanoma Cells Through the GSK3β-Mediated Keap1/Nrf2/HO-1 Signalling Pathway. Front. Gen. 2022, 13, 865073. [Google Scholar] [CrossRef]
- Leiter, U.; Keim, U.; Garbe, C. Epidemiology of skin cancer: Update 2019. In Sunlight, Vitamin D and Skin Cancer; Springer: Cham, Switzerland, 2020; pp. 123–139. [Google Scholar]
- Scala, J.; Vojvodic, A.; Vojvodic, P.; Vlaskovic-Jovicevic, T.; Peric-Hajzler, Z.; Matovic, D.; Dimitrijevic, S.; Vojvodic, J.; Sijan, G.; Stepic, N.; et al. New Trends in Cutaneous Melanoma Surgery. Open Access Maced. J. Med. Sci. 2019, 7, 3090–3092. [Google Scholar] [CrossRef] [Green Version]
- Swayden, M.; Chhouri, H.; Anouar, Y.; Grumolato, L. Tolerant/persister cancer cells and the path to resistance to targeted therapy. Cells 2020, 9, 2601. [Google Scholar] [CrossRef]
- Matias, M.; Pinho, J.O.; Penetra, M.J.; Campos, G.; Reis, C.P.; Gaspar, M.M. The challenging melanoma landscape: From early drug discovery to clinical approval. Cells 2021, 10, 3088. [Google Scholar] [CrossRef]
- Theeuwes, W.F.; Gosker, H.R.; Langen, R.C.J.; Verhees, K.J.P.; Pansters, N.A.M.; Schols, A.M.W.J.; Remels, A.H.V. Inactivation of glycogen synthase kinase-3β (GSK-3β) enhances skeletal muscle oxidative metabolism. Biochim. Biophys. Acta BBA 2017, 1863, 3075–3086. [Google Scholar] [CrossRef] [PubMed]
- Domoto, T.; Uehara, M.; Bolidong, D.; Minamoto, T. Glycogen synthase kinase 3β in cancer biology and treatment. Cells 2020, 9, 1388. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Du, S.; Lei, T.; Xie, X.; Wang, Y. Glycogen Synthase Kinase 3β in Tumorigenesis and Oncotherapy (Review). Oncol. Rep 2020, 44, 2373–2385. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Liu, C.; Li, Z.; Gai, C.; Ding, D.; Chen, W.; Hao, F.; Li, W. Regulation of GSK3β/Nrf2 signaling pathway modulated erastin-induced ferroptosis in breast cancer. Mol. Cell. Biochem. 2020, 473, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ouyang, S.; Li, B.; Wu, H.; Wang, F. GSK-3β manipulates ferroptosis sensitivity by dominating iron homeostasis. Cell Death Disc. 2021, 7, 334. [Google Scholar] [CrossRef] [PubMed]
- John, J.K.; Paraiso, K.H.; Rebecca, V.W.; Cantini, L.P.; Abel, E.V.; Pagano, N.; Meggers, E.; Mathew, R.; Krepler, C.; Izumi, V.; et al. GSK3β inhibition blocks melanoma cell/host interactions by downregulating N-cadherin expression and decreasing FAK phosphorylation. J. Invest. Dermat. 2012, 132, 2818–2827. [Google Scholar] [CrossRef] [Green Version]
- Kubic, J.D.; Mascarenhas, J.B.; Iizuka, T.; Wolfgeher, D.; Lang, D. GSK-3 promotes cell survival, growth, and PAX3 levels in human melanoma cells. Mol. Can. Res. 2012, 10, 1065–1076. [Google Scholar] [CrossRef] [Green Version]
- Sanghani, H.V.; Ganatra, S.H.; Pande, R. Molecular—Docking studies of potent anticancer agent. J. Comput. Sci. Syst. Biol. 2012, 5, 12–15. [Google Scholar] [CrossRef]
- Taghizadeh, M.S.; Niazi, A.; Moghadam, A.; Afsharifar, A. Experimental, molecular docking and molecular dynamic studies of natural products targeting overexpressed receptors in breast cancer. PLoS ONE 2022, 17, 0267961. [Google Scholar] [CrossRef]
- Kandasamy, S.; Sahu, S.K.; Kandasamy, K. In Silico studies on fungal metabolite against skin cancer protein (4, 5-Diarylisoxazole HSP90 Chaperone). Int. Sch. Res. Notices 2012, 2012, 626214. [Google Scholar]
- Ajayi, G.O.; Olagunju, J.A.; Ademuyiwa, O.; Martins, O.C. Gas chromatography-mass spectrometry analysis and phytochemical screening of ethanolic root extract of Plumbago zeylanica, Linn. J. Med. Plants Res. 2011, 5, 1756–1761. [Google Scholar]
- Musthafa, K.S.; Sahu, S.K.; Ravi, A.V.; Kathiresan, K. Anti-quorum sensing potential of the mangrove Rhizophora annamalayana. World J. Microbiol. Biot. 2013, 29, 1851–1858. [Google Scholar] [CrossRef] [PubMed]
- Patil, A.; Jadhav, V. GC-MS analysis of bioactive components from methanol leaf extract of Toddalia asiatica (L.). Int. J. Pharm. Sci. Rev. Res. 2014, 29, 18–20. [Google Scholar]
- Ferdosi, M.F.; Javaid, A.; Khan, I.H.; Khan, S.; Shad, N. Analysis of n-butanol flower extract of Cassia fistula through GC-MS and identification of antimicrobial compounds. Pak. J. Phytopathol. 2021, 33, 103–107. [Google Scholar] [CrossRef]
- Yakubu, O.E.; Udeh, C.S.; Asuk, A.A.; Moses, A.A.; Richard, B. Comparative determination of antioxidant activities and phytochemicals from fractions of ethanol extract of Senna occidentalis using GC-MS. J. Emerg. Technol. Innov. Res. 2021, 8, 195–211. [Google Scholar]
- Qureshi, M.Z.; Javed, S.; Javaid, A.; Al-Taie, A.H. Identification of antimicrobial compounds from n-hexane stem extract of Kochia indica by GC-MS analysis. MYCOPATH 2020, 16, 51–55. [Google Scholar]
- Abdel-Lateff, A.; Fisch, K.M.; Wright, A.D.; König, G.M. A new antioxidant isobenzofuranone derivative from the algicolous marine fungus Epicoccum sp. Planta Med. 2003, 69, 831–834. [Google Scholar]
- Ingole, S.N. Phytochemical analysis of leaf extract of Ocimum americanum L.(Lamiaceae) by GCMS method. WSN 2016, 37, 76–87. [Google Scholar]
- Selvamangai, G.; Bhaskar, A. GC–MS analysis of phytocomponents in the methanolic extract of Eupatorium triplinerve. Asian Pac. J. Trop. Biomed. 2012, 2, S1329–S1332. [Google Scholar] [CrossRef]
- Cottaz, A.; Bouarab, L.; De Clercq, J.; Oulahal, N.; Degraeve, P.; Joly, C. Potential of incorporation of antimicrobial plant phenolics into polyolefin-based food contact materials to produce active packaging by melt-blending: Proof of concept with isobutyl-4-hydroxybenzoate. Front. Chem. 2019, 7, 148. [Google Scholar] [CrossRef]
- Akpuaka, A.; Ekwenchi, M.M.; Dashak, D.A.; Dildar, A. Biological activities of characterized isolates of n-hexane extract of Azadirachta indica A. Juss (Neem) leaves. Nat. Sci. 2013, 11, 141–147. [Google Scholar]
- Kumaresan, S.; Senthilkumar, V.; Stephen, A.; Balakumar, B.S. GC-MS analysis and pass-assisted prediction of biological activity spectra of extract of Phomopsis sp. isolated from Andrographis paniculata. World J. Pharm. Res. 2015, 4, 1035–1053. [Google Scholar]
- Khan, M.S.; Yusufzai, S.K.; Ying, L.Y.; Zulnashriq, W. GC-MS based chemical profiling and evaluation of antioxidant potential of leaves and stems of Alternanthera sessilis red from Sabah, Malaysia. Int. J. Pharm. Sci. 2018, 10, 4–9. [Google Scholar] [CrossRef]
- Bagheri, G.; Ayatollahi, S.A.; Ramírez-Alarcón, K.; Fernández, M.; Salehi, B.; Forman, K.; Martorell, M.; Moghadam, M.H.; Sharifi-Rad, J. Phytochemical screening of Alstonia scholaris leaf and bark extracts and their antimicrobial activities. Cell. Mol. Biol. 2020, 66, 270–279. [Google Scholar] [CrossRef]
- Konappa, N.; Udayashankar, A.C.; Krishnamurthy, S.; Pradeep, C.K.; Chowdappa, S.; Jogaiah, S. GC–MS analysis of phytoconstituents from Amomum nilgiricum and molecular docking interactions of bioactive serverogenin acetate with target proteins. Sci. Rep. 2020, 10, 16438. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Nau, W.M. A conformational flexibility scale for amino acids in peptides. Angew. Chem. Int. Ed. 2003, 42, 2269–2272. [Google Scholar] [CrossRef]
- Muhamad, N.; Muhmed, S.A.; Yusoff, M.M.; Gimbun, J. Influence of solvent polarity and conditions on extraction of antioxidant, flavonoids and phenolic content from Averrhoa bilimbi. J. Food Eng. 2014, 4, 255–260. [Google Scholar]
- Tomczyk, M.; Pleszczyńska, M.; Wiater, A. Variation in total polyphenolics contents of aerial parts of Potentilla species and their anticariogenic activity. Molecules 2010, 15, 4639–4651. [Google Scholar] [CrossRef]
- Dolkar, P.; Dolkar, D.; Angmo, S.; Kumar, B.; Stobdan, T. Variability in phenolics, flavonoids and antioxidants in seabuckthorn (Hippophae rhamnoides L.) seed from nine trans-Himalayan natural population. J. Berry Res. 2017, 7, 109–116. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, G.; Zhao, P.; Zhou, Q.; Zhao, X. The abundance of certain metabolites responds to drought stress in the highly drought tolerant plant Caragana korshinskii. Acta Physiol. Plant 2017, 39, 116. [Google Scholar] [CrossRef]
- Kumar, V.; Sharma, N.; Sourirajan, A.; Khosla, P.K.; Dev, K. Correlation of biological activities of bark and leaves of Terminalia arjuna collected from different geographical regions of Himachal Pradesh, India. Biochem. Sys. Ecol. 2023, 106, 104563. [Google Scholar] [CrossRef]
- Sharma, S.; Kumar, V.; Attri, C.; Sourirajan, A.; Dev, K. Comparison of phytochemicals, antioxidant, and antimicrobial activities of in vitro propagated and wild grown Potentilla Nepalensis, an endemic medicinal plant from North Western Himalayas. J. Herbs Spices Med. Plants 2022, 28, 324–336. [Google Scholar] [CrossRef]
- Taruscio, T.G.; Barney, D.L.; Exon, J. Content and profile of flavanoid and phenolic acid compounds in conjunction with the antioxidant capacity for a variety of northwest Vaccinium berries. J. Agric. Food Chem. 2004, 52, 3169–3176. [Google Scholar] [CrossRef]
- Kumar, V.; Sharma, N.; Sourirajan, A.; Khosla, P.K.; Dev, K. Comparative evaluation of antimicrobial and antioxidant potential of ethanolic extract and its fractions of bark and leaves of Terminalia arjuna from north-western Himalayas, India. J. Tradit. Complement. Med. 2018, 8, 100–106. [Google Scholar] [CrossRef]
- Kumar, V.; Chandel, S.R.; Guleria, S.; Sharma, N.; Sourirajan, A.; Khosla, P.K.; Dev, K. Comparative analysis of phytochemicals, antimicrobial and antioxidant activity of different species of Terminalia from Himachal Pradesh, India. Vegetos 2021, 34, 528–539. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Meth. Enzymol. 1999, 299, 152–178. [Google Scholar]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Barros, L.; Ferreira, M.J.; Queiros, B.; Ferreira, I.C.; Baptista, P. Total phenols, ascorbic acid, β-carotene and lycopene in Portuguese wild edible mushrooms and their antioxidant activities. Food Chem. 2007, 103, 413–419. [Google Scholar] [CrossRef]
- Sreelekha, T. Evaluation of antioxidant, antitumor and immunomodulatory properties of polysaccharide isolated from fruit rind of Punica granatum. Mol. Med. Rep. 2011, 5, 489–496. [Google Scholar] [CrossRef] [Green Version]
- Liang, S.H.; Chen, J.M.; Normandin, M.D.; Chang, J.S.; Chang, G.C.; Taylor, C.K.; Trapa, P.; Plummer, M.S.; Para, K.S.; Conn, E.L.; et al. Discovery of a highly selective glycogen synthase kinase-3 inhibitor (PF-04802367) that modulates tau phosphorylation in the brain: Translation for PET neuroimaging. Angew. Chem. Int. Ed. 2016, 55, 9601–9605. [Google Scholar] [CrossRef] [Green Version]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comp. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banks, J.L.; Beard, H.S.; Cao, Y.; Cho, A.E.; Damm, W.; Farid, R.; Felts, A.K.; Halgren, T.A.; Mainz, D.T.; Maple, J.R.; et al. Integrated modeling program, applied chemical theory (IMPACT). J. Comput. Chem. 2005, 26, 1752–1780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shivakumar, D.; Williams, J.; Wu, Y.; Damm, W.; Shelley, J.; Sherman, W. Prediction of absolute solvation free energies using molecular dynamics free energy perturbation and the OPLS force field. J. Chem. Theory Comput. 2010, 6, 1509–1519. [Google Scholar] [CrossRef]
- Patel, C.N.; Georrge, J.J.; Modi, K.M.; Narechania, M.B.; Patel, D.P.; Gonzalez, F.J.; Pandya, H.A. Pharmacophore-based virtual screening of catechol-o-methyltransferase (COMT) inhibitors to combat Alzheimer’s disease. J. Biomol. Struct. Dyn. 2018, 36, 3938–3957. [Google Scholar] [CrossRef] [PubMed]
- Patel, C.N.; Kumar, S.P.; Modi, K.M.; Soni, M.N.; Modi, N.R.; Pandya, H.A. Cardiotonic steroids as potential Na+/K+-ATPase inhibitors–a computational study. J. Recept. Signal Transduct. 2019, 39, 226–234. [Google Scholar] [CrossRef]
- Reddy, S.V.G.; Reddy, K.T.; Kumari, V.V.; Basha, S.H. Molecular docking and dynamic simulation studies evidenced plausible immunotherapeutic anticancer property by Withaferin A targeting indoleamine 2, 3-dioxygenase. J. Biomol. Struct. Dyn. 2015, 33, 2695–2709. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Maxwell, D.S.; Tirado-Rives, J. Development and testing of the OPLS all-atom force field on conformational energetics and properties of organic liquids. J. Am. Chem. Soc. 1996, 118, 11225–11236. [Google Scholar] [CrossRef]
- Kaminski, G.A.; Friesner, R.A.; Tirado-Rives, J.; Jorgensen, W.L. Evaluation and reparametrization of the OPLS-AA force field for proteins via comparison with accurate quantum chemical calculations on peptides. J. Phys. Chem. B 2001, 105, 6474–6487. [Google Scholar] [CrossRef]
- Panchal, U.; Modi, K.; Dey, S.; Prajapati, U.; Patel, C.; Jain, V.K. A resorcinarene-based “turn-off” fluorescence sensor for 4-nitrotoluene: Insights from fluorescence and 1H NMR titration with computational approach. J. Lumin. 2017, 184, 74–82. [Google Scholar] [CrossRef]
- Mall, S.; Srivastava, R.; Sharma, N.; Patel, C.N.; Rolta, R.; Sourirajan, A.; Dev, K.; Ghosh, A.; Kumar, V. Antihypertensive activity of phytocompounds from selected medicinal plants via inhibition of angiotensin-converting enzyme (ACE) protein: An in-silico approach. Nat. Prod. Res. 2022, 36, 4526–4529. [Google Scholar] [CrossRef]
- Patel, C.N.; Goswami, D.; Jaiswal, D.G.; Parmar, R.M.; Solanki, H.A.; Pandya, H.A. Pinpointing the potential hits for hindering interaction of SARS-CoV-2 S-protein with ACE2 from the pool of antiviral phytochemicals utilizing molecular docking and molecular dynamics (MD) simulations. J. Mol. Graph. Model. 2021, 105, 107874. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, P.; Eckert, A.O.; Schrey, A.K.; Preissner, R. ProTox-II: A webserver for the prediction of toxicity of chemicals. Nucleic Acids Res. 2018, 46, W257–W263. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Plant Part | Solvent Used | TPC (mg g−1 GAE) | TFC (mg g−1 RE) | DPPH Activity IC50 (µg mL−1) |
|---|---|---|---|---|
| Roots | Methanol | 21.21 ± 0.54 | 4.24 ± 0.17 | 23.5 ± 0.92 |
| Shoots | Methanol | 15.68 ± 2.79 | 2.58 ± 0.1 | 12.83 ± 0.35 |
| Roots | n-Hexane | 0.90 ± 0.19 | 0.06 ± 0.03 | 65.69 ± 0.77 |
| Shoots | n-Hexane | 1.59 ± 0.13 | 0.49 ± 0.06 | 74.93 ± 1.01 |
| Ascorbic Acid | Methanol | - | - | 5.86 ± 0.13 |
| RT (min) | Area% | Name of Phytocompound | Molecular Formula | Bioactivity Reported | References |
|---|---|---|---|---|---|
| 28.61 | 12.64 | Tetradecanoic acid, 10,13-dimethyl-, methyl ester | C17H34O2 | - | - |
| 31.32 | 10.58 | Heptadecanoic acid, 16-methyl-, methyl ester | C19H38O2 | Effective against skin cancer | [21] |
| 27.15 | 6.44 | 1,1,1,5,7,7,7-Heptamethyl-3,3-bis (trimethyl siloxy) tetrasiloxane | C13H40O5Si6 | Anti-quorum sensing | [22,23] |
| 29.26 | 6.34 | Phthalic acid, butyl hept-4-yl ester | C19H28O4 | - | - |
| 29.35 | 5.87 | 1,1,1,3,5,5,7,7,7-Nonamethyl-3-(trimethyl siloxy) tetrasiloxane | C12H36O4Si5 | - | - |
| 24.68 | 4.95 | Norepinephrine, (R)-, 4TMS derivative | C20H43NO3Si4 | - | - |
| 31.02 | 4.63 | 2-Isopropyl-6-phenylnicotinonitrile | C15H14N2 | - | - |
| 33.35 | 4.56 | Cyclononasiloxane, octadecamethyl | C18H54O9Si9 | antifungal, antibacterial and/or antiviral properties | [24,25] |
| 39.03 | 4.23 | Oxalic acid, 2TMS derivative | C8H18O4Si2 | - | - |
| 23.47 | 3.8 | 2-Tetradecanol | C14H30O | Ingredient in cosmetics such as cold creams for its emollient properties | [26] |
| RT (min) | Area% | Name of Phytocompound | Molecular Formula | Bioactivity Reported | Reference |
|---|---|---|---|---|---|
| 28.61 | 11.82 | Hexadecanoic acid, methyl ester | C17H34O2 | Antimicrobial | [27] |
| 31.36 | 8.58 | 1,1,1,5,7,7,7-Heptamethyl-3,3-bis(trimethylsiloxy)tetrasiloxane | C13H40O5Si6 | Anti-quorum sensing | [22,23] |
| 29.35 | 5.54 | Oxalic acid, 2TMS derivative | C8H18O4Si2 | - | - |
| 24.68 | 5.4 | Cyclooctasiloxane, hexadecamethyl- | C16H48O8Si8 | - | - |
| 36.11 | 5.11 | 3-Ethyl-7-hydroxyphthalide | C10H10O3 | Antioxidant | [28] |
| 29.26 | 4.7 | Phthalic acid, butyl hex-3-yl ester | C18H26O4 | Antimicrobial, insecticidal activity | [29] |
| 33.35 | 4.36 | 1,1,1,3,5,5,7,7,7-Nonamethyl-3-(trimethylsiloxy)tetrasiloxane | C12H36O4Si5 | - | - |
| 31.02 | 4.09 | 1-Hexyl-1-nitrocyclohexane | C12H23NO2 | Antimicrobial and anti-inflammatory | [30] |
| 22.44 | 3.71 | Isobutyl 4-hydroxybenzoate | C11H14O3 | Antimicrobial activity | [31] |
| 39.04 | 3.55 | Octanoic acid, 5-(acetyloxy)-, methyl ester | C11H20O4 | - | - |
| RT (min) | Area% | Name of Phytocompound | Molecular Formula | Bioactivity Reported | Reference |
|---|---|---|---|---|---|
| 44.85 | 22.9 | Trichloromethyl 9-anthracenecarbodithioate | C16H9Cl3S2 | - | - |
| 20.01 | 17.71 | Heptane, 3,3-dimethyl- | C9H20 | - | - |
| 23.59 | 12.28 | Hexadecane | C16H34 | Antifungal, Antibacterial, antioxidant activity | [32] |
| 39 | 7.01 | 1,1,1,5,7,7,7-Heptamethyl-3, 3-bis(trimethylsiloxy)tetrasiloxane | C13H40O5Si6 | Anti-quorum sensing | [22,23] |
| 15.8 | 5.57 | Dodecane | C12H26 | Antifungal activity | [32] |
| 26.76 | 4.99 | Eicosane | C20H42 | Antimicrobial activity against clinical pathogens | [33] |
| 29.34 | 4.79 | 1,1,1,3,5,5,7,7,7-Nonamethyl-3-(trimethylsiloxy) tetrasiloxane | C12H36O4Si5 | - | [34] |
| 33.33 | 4.68 | Propanoic acid, 2-oxo-3-(trimethylsilyl)-, trimethylsilyl ester | C9H20O3Si2 | - | - |
| 43.52 | 4.3 | 1,1,1,3,5,7,7,7-Octamethyl-3,5-bis(trimethylsiloxy) tetrasiloxane | C14H42O5Si6 | - | [35] |
| 11 | 2 | 2,4,6,9-Dehydroadamantane | C10H12 | - | - |
| RT (min) | Area% | Name of Phytocompound | Molecular Formula | Bioactivity Reported | Reference |
|---|---|---|---|---|---|
| 25.03 | 23.19 | Benzene, 1,3,5-tri-tert-butyl- | C18H30 | - | - |
| 32.79 | 23.19 | 1,1,1,3,5,5,5-Heptamethyltrisiloxane | C7H22O2Si3 | Anti-inflammatory and antimicrobial properties | [36] |
| 46.46 | 17.32 | 4H-1-Benzopyran-2-carboxylic acid, 5-amino-6-hydroxy-4-oxo-, ethyl ester | C12H11NO5 | - | - |
| 42.15 | 10.13 | 1-Propene, 3-methoxy- | C4H8O | - | - |
| 29.24 | 8.05 | Phthalic acid, butyl hept-4-yl ester | C19H28O4 | - | [29] |
| 20 | 3.14 | Heptane, 3,3-dimethyl- | C9H20 | - | - |
| 36.59 | 2.71 | N- (Methyl sulfonyl)-N, O-bis (trimethyl silyl) hydroxylamine | C7H21NO3SSi2 | - | - |
| 28.6 | 2.3 | Dodecanoic acid, 2-methyl- | C13H26O2 | - | - |
| 38.8 | 2.27 | 2-Acetyl-3-ethylpyrazine | C8H10N2O | - | - |
| 26.76 | 1.87 | Eicosane | C20H42 | Antimicrobial activity against food-borne pathogens | [33] |
| Sr. No. | Phytocompounds | PubChem ID | PDB ID: 5K5N | |
|---|---|---|---|---|
| Binding Energy (Kcal/mol) | Interacting Amino Acids | |||
| 1 | Trichloromethyl 9-anthracenecarbodithioate | 613595 | −8.9 | Val A:70, Ala A:83, Lys A:85, Tyr A:134, Leu A:188, Cys A:199 |
| 2 | 4H-1-Benzopyran-2-carboxylic acid, 5-amino-6-hydroxy-4-oxo-, ethyl ester | 619354 | −7.4 | Val A:70, Ala A:83, Met A:101, Val A:110, Leu A:130, Leu A:132, Val A:135, Leu A:188, Cys A:199, Asp A:200 |
| 3 | Benzene, 1,3,5-tri-tert-butyl- | 15089 | −7.1 | Ile A:62, Phe A:67, Val A:70, Leu A:132, Cys A:199 |
| 4 | Heptane, 3,3-dimethyl- | 520991 | −4.6 | Val A:70, Ala A:83, Lys A:85, A:132, Tyr A:134, Leu A:188, Cys A:199 |
| 5 | Hexadecane | 11006 | −5.1 | Ile A:62, Val A:70, Ala A:83, Lys A:85, Leu A:132, Tyr A:134, Leu A:188, Cys A:199 |
| 6 | 1,1,1,3,5,5,5-Heptamethyltrisiloxane | 6327366 | −3.6 | - |
| 7 | 1-Propene, 3-methoxy- | 69392 | −2.8 | Tyr A71, Ile A: 84 |
| 8 | Encorafenib (drug) | 50922675 | −8.6 | Gly A:65, Phe A:67, Lys A:183, Asp A:200 |
| Compound | cLogP | nrot | MW | HBD | HBA | Lipinski Rule | Hepato-Toxicity | Immuno-Genicity | Carcino-Genicity | Cyto- Toxicity | LD50 (mg/kg) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Trichloromethyl 9-anthracenecarbodithioate | 3.29 | 3 | 371.73 | 0 | 0 | Yes | No | No | Yes | No | 493 mg/kg (Class IV) |
| 4H-1-Benzopyran-2-carboxylic acid, 5-amino-6-hydroxy-4-oxo-, ethyl ester | 1.89 | 3 | 249.22 | 2 | 5 | Yes | No | No | No | No | 100 mg/kg (Class III) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, S.; Kumar, V.; Yaseen, M.; S. Abouzied, A.; Arshad, A.; Bhat, M.A.; Naglah, A.M.; Patel, C.N.; Sivakumar, P.K.; Sourirajan, A.; et al. Phytochemical Analysis, In Vitro Biological Activities, and Computer-Aided Analysis of Potentilla nepalensis Hook Compounds as Potential Melanoma Inhibitors Based on Molecular Docking, MD Simulations, and ADMET. Molecules 2023, 28, 5108. https://doi.org/10.3390/molecules28135108
Sharma S, Kumar V, Yaseen M, S. Abouzied A, Arshad A, Bhat MA, Naglah AM, Patel CN, Sivakumar PK, Sourirajan A, et al. Phytochemical Analysis, In Vitro Biological Activities, and Computer-Aided Analysis of Potentilla nepalensis Hook Compounds as Potential Melanoma Inhibitors Based on Molecular Docking, MD Simulations, and ADMET. Molecules. 2023; 28(13):5108. https://doi.org/10.3390/molecules28135108
Chicago/Turabian StyleSharma, Subhash, Vikas Kumar, Muhammad Yaseen, Amr S. Abouzied, Abgeena Arshad, Mashooq Ahmad Bhat, Ahmed M. Naglah, Chirag N. Patel, Prasanth Kumar Sivakumar, Anuradha Sourirajan, and et al. 2023. "Phytochemical Analysis, In Vitro Biological Activities, and Computer-Aided Analysis of Potentilla nepalensis Hook Compounds as Potential Melanoma Inhibitors Based on Molecular Docking, MD Simulations, and ADMET" Molecules 28, no. 13: 5108. https://doi.org/10.3390/molecules28135108







