Engineering Two-Dimensional Multilevel Supramolecular Assemblies from a Bifunctional Ligand on Au(111)
Abstract
:1. Introduction
2. Results and Discussion
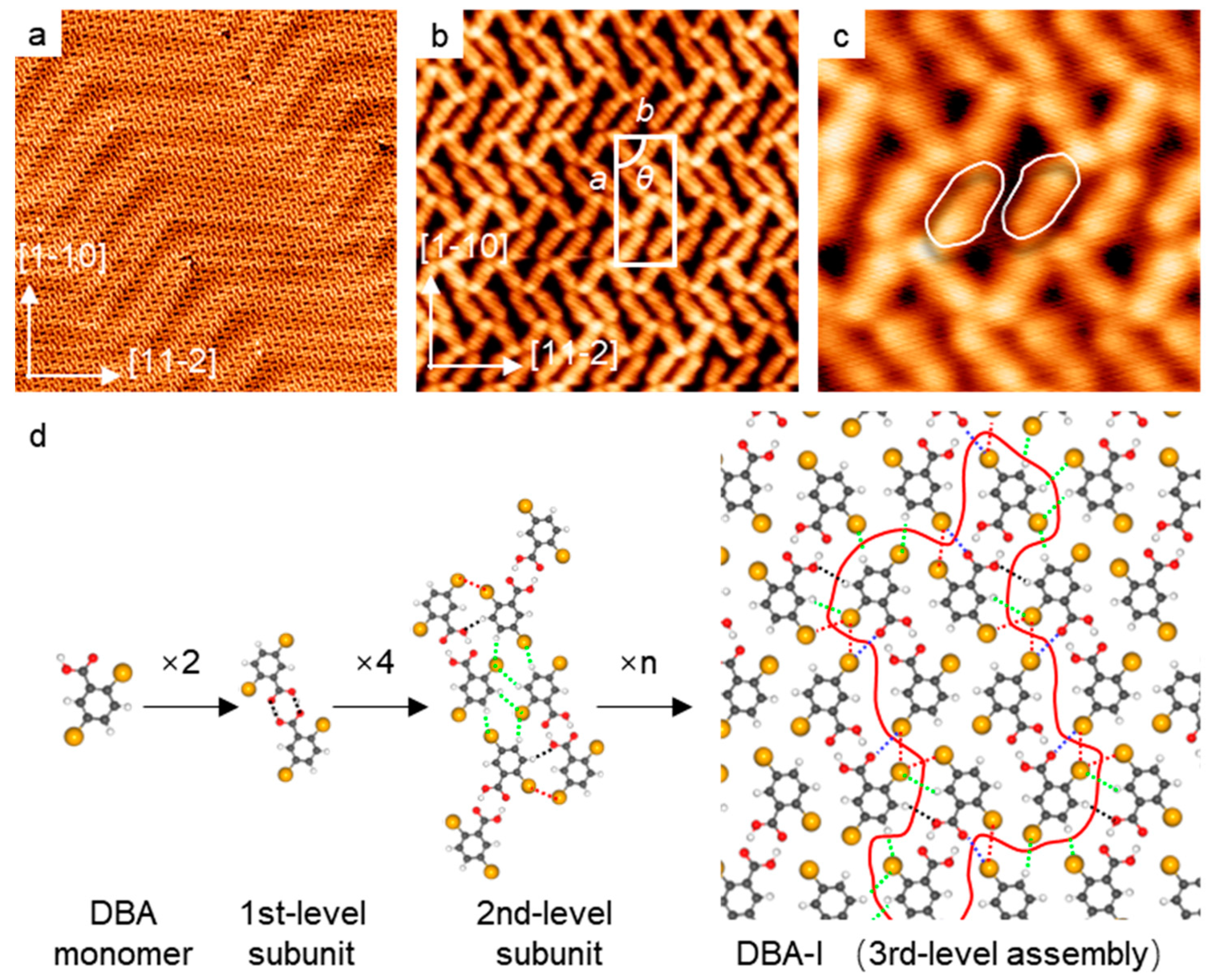
2.1. Self-Assembly of DBA on a Pristine Au(111)
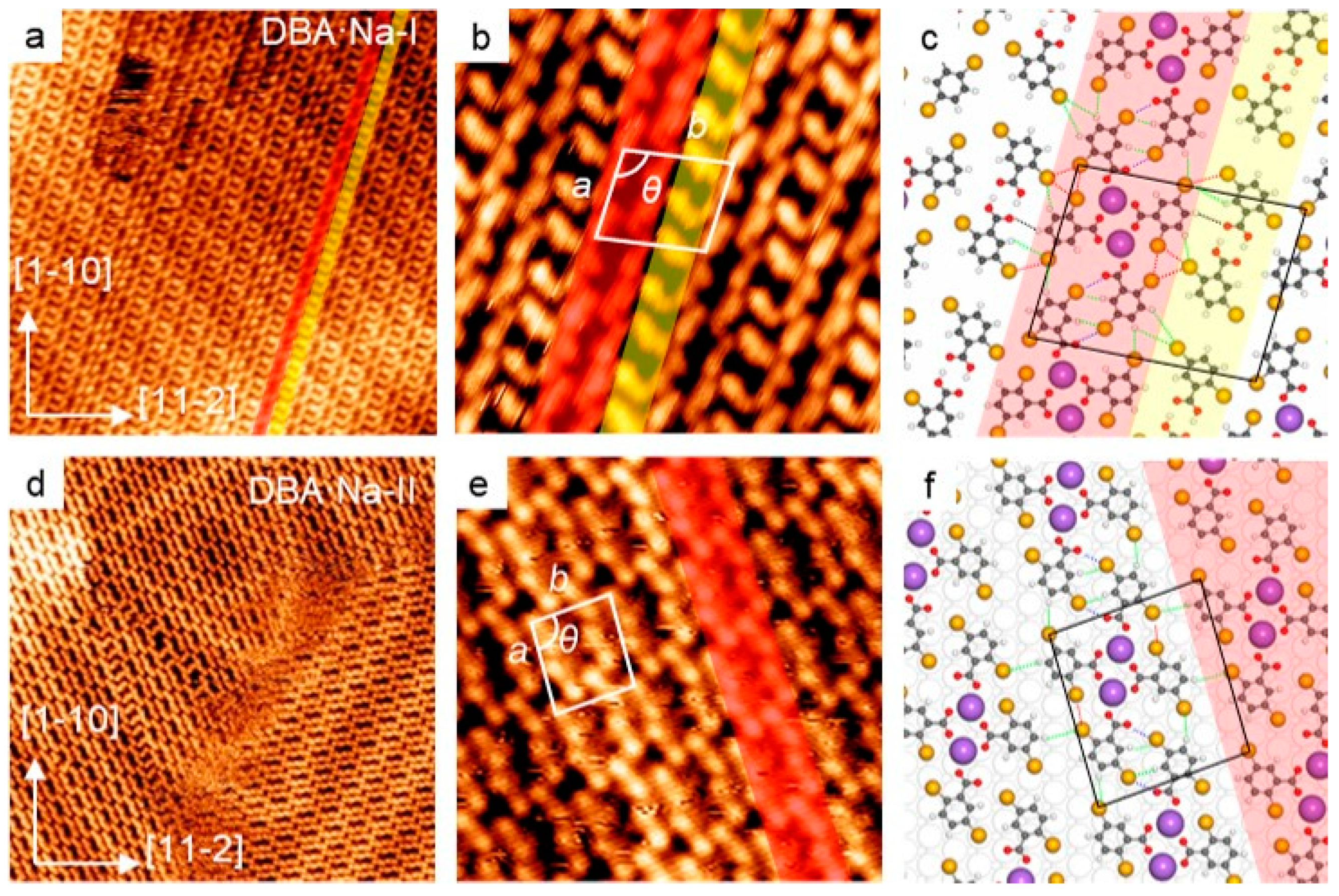
2.2. Self-Assembly of DBA in the Presence of NaCl
2.3. Size Effect of Alkali Coordination Centers
2.4. Reversible Structural Conversion of DBA·Cs-I and -II, and DFT Calculation
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- De Feyter, S.; De Schryver, F.C. Two-dimensional supramolecular self-assembly probed by scanning tunneling microscopy. Chem. Soc. Rev. 2003, 32, 139–150. [Google Scholar] [CrossRef]
- Barth, J.V.; Costantini, G.; Kern, K. Engineering atomic and molecular nanostructures at surfaces. Nature 2005, 437, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.; Stepanow, S.; Ruben, M.; Barth, J.V. Surface-Confined Supramolecular Coordination Chemistry. Top. Curr. Chem. 2009, 287, 1–44. [Google Scholar] [PubMed]
- Otero, R.; Gallego, J.M.; de Parga, A.L.V.; Martín, N.; Miranda, R. Molecular Self-Assembly at Solid Surfaces. Adv. Mater. 2011, 23, 5148–5176. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Gao, Z.A.; Lin, N. Self-assembly of metal–organic coordination structures on surfaces. Prog. Surf. Sci. 2016, 91, 101–135. [Google Scholar] [CrossRef]
- Goronzy, D.P.; Ebrahimi, M.; Rosei, F.; Arramel; Fang, Y.; De Feyter, S.; Tait, S.L.; Wang, C.; Beton, P.H.; Wee, A.T.S.; et al. Supramolecular Assemblies on Surfaces: Nanopatterning, Functionality, and Reactivity. ACS Nano 2018, 12, 7445–7481. [Google Scholar] [CrossRef]
- Xing, L.; Peng, Z.; Li, W.; Wu, K. On Controllability and Applicability of Surface Molecular Self-Assemblies. Acc. Chem. Res. 2019, 52, 1048–1058. [Google Scholar] [CrossRef]
- Yaghi, O.M.; O’Keeffe, M.; Ockwig, N.W.; Chae, H.K.; Eddaoudi, M.; Kim, J. Reticular synthesis and the design of new materials. Nature 2003, 423, 705. [Google Scholar] [CrossRef]
- Kalmutzki, M.J.; Hanikel, N.; Yaghi, O.M. Secondary building units as the turning point in the development of the reticular chemistry of MOFs. Sci. Adv. 2018, 4, eaat9180. [Google Scholar] [CrossRef] [Green Version]
- Spillmann, H.; Dmitriev, A.; Lin, N.; Messina, P.; Barth, J.V.; Kern, K. Hierarchical assembly of two-dimensional homochiral nanocavity arrays. J. Am. Chem. Soc. 2003, 125, 10725. [Google Scholar] [CrossRef]
- Cook, T.R.; Zheng, Y.-R.; Stang, P.J. Metal–Organic Frameworks and Self-Assembled Supramolecular Coordination Complexes: Comparing and Contrasting the Design, Synthesis, and Functionality of Metal–Organic Materials. Chem. Rev. 2013, 113, 734–777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Datta, S.; Saha, M.L.; Stang, P.J. Hierarchical Assemblies of Supramolecular Coordination Complexes. Acc. Chem. Res. 2018, 51, 2047–2063. [Google Scholar] [CrossRef] [PubMed]
- Dmitriev, A.; Lin, N.; Weckesser, J.; Barth, J.V.; Kern, K. Supramolecular Assemblies of Trimesic Acid on a Cu(100) Surface. J. Phys. Chem. B 2002, 106, 6907–6912. [Google Scholar] [CrossRef]
- Clair, S.; Pons, S.; Seitsonen, A.P.; Brune, H.; Kern, K.; Barth, J.V. STM Study of Terephthalic Acid Self-Assembly on Au(111): Hydrogen-Bonded Sheets on an Inhomogeneous Substrate. J. Phys. Chem. B 2004, 108, 14585–14590. [Google Scholar] [CrossRef] [Green Version]
- Ivasenko, O.; Perepichka, D.F. Mastering fundamentals of supramolecular design with carboxylic acids. Common lessons from X-ray crystallography and scanning tunneling microscopy. Chem. Soc. Rev. 2011, 40, 191–206. [Google Scholar] [CrossRef]
- Schwarz, D.; van Gastel, R.; Zandvliet, H.J.W.; Poelsema, B. In Situ Observation of a Deprotonation-Driven Phase Transformation: 4,4′-Biphenyldicarboxylic Acid on Au(111). J. Phys. Chem. C 2013, 117, 1020–1029. [Google Scholar] [CrossRef]
- Leasor, C.; Goshinsky, K.; Chen, K.-H.; Li, Z. Probing Molecular Nanostructures of Aromatic Terephthalic Acids Triggered by Intermolecular Hydrogen Bonds and Electrochemical Potential. Langmuir 2019, 35, 13259–13267. [Google Scholar] [CrossRef]
- Shi, J.; Li, Z.; Lin, T.; Shi, Z. Successive Deprotonation Steering the Structural Evolution of Supramolecular Assemblies on Ag(111). Molecules 2022, 27, 3876. [Google Scholar] [CrossRef]
- Chung, K.-H.; Kim, H.; Jang, W.J.; Yoon, J.K.; Kahng, S.-J.; Lee, J.; Han, S. Molecular Multistate Systems Formed in Two-Dimensional Porous Networks on Ag(111). J. Phys. Chem. C 2013, 117, 302–306. [Google Scholar] [CrossRef]
- Varadwaj, A.; Varadwaj, P.R.; Marques, H.M.; Yamashita, K. Halogen in materials design: Revealing the nature of hydrogen bonding and other non-covalent interactions in the polymorphic transformations of methylammonium lead tribromide perovskite. Mater. Today Chem. 2018, 9, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Tsukahara, N.; Yoshinobu, J. Substrate-Selective Intermolecular Interaction and the Molecular Self-Assemblies: 1,3,5-Tris(4-bromophenyl)benzene Molecules on the Ag(111) and Si(111) (√3 × √3)-Ag Surfaces. Langmuir 2022, 38, 8881–8889. [Google Scholar] [CrossRef] [PubMed]
- Fu, B.; Geng, J.; Yang, Z.; Zhang, Y.; Sun, S.; Ruan, Z.; Zhang, H.; Xiong, W.; Niu, G.; Zhang, Y.; et al. Coverage Modulated Transformation of the Supramolecular Assembly Structure of Brominated N-Heterocyclic Molecules on Au(111) Surfaces. J. Phys. Chem. C 2023, 127, 5833–5840. [Google Scholar] [CrossRef]
- Teyssandier, J.; Mali, K.S.; De Feyter, S. Halogen Bonding in Two-Dimensional Crystal Engineering. ChemistryOpen 2020, 9, 225–241. [Google Scholar] [CrossRef]
- Stepanow, S.; Ohmann, R.; Leroy, F.; Lin, N.; Strunskus, T.; Wöll, C.; Kern, K. Rational Design of Two-Dimensional Nanoscale Networks by Electrostatic Interactions at Surfaces. ACS Nano 2010, 4, 1813–1820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skomski, D.; Abb, S.; Tait, S.L. Robust Surface Nano-Architecture by Alkali–Carboxylate Ionic Bonding. J. Am. Chem. Soc. 2012, 134, 14165–14171. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, X.; Li, D.; Xu, W. Real-Space Evidence of Trimeric, Tetrameric, and Pentameric Uracil Clusters Induced by Alkali Metals. J. Phys. Chem. C 2020, 124, 5257–5262. [Google Scholar] [CrossRef]
- Kley, C.S.; Čechal, J.; Kumagai, T.; Schramm, F.; Ruben, M.; Stepanow, S.; Kern, K. Highly Adaptable Two-Dimensional Metal–Organic Coordination Networks on Metal Surfaces. J. Am. Chem. Soc. 2012, 134, 6072–6075. [Google Scholar] [CrossRef]
- Shan, H.; Zhou, L.; Ji, W.; Zhao, A. Flexible Alkali–Halogen Bonding in Two Dimensional Alkali-Metal Organic Frameworks. J. Phys. Chem. Lett. 2021, 12, 10808–10814. [Google Scholar] [CrossRef]
- Floris, A.; Comisso, A.; De Vita, A. Fine-tuning the electrostatic properties of an alkali-linked organic adlayer on a metal substrate. ACS Nano 2013, 7, 8059–8065. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, L.; Xie, L.; Kong, H.; Tan, Q.; Cai, L.; Sun, Q.; Xu, W. Solventless Formation of G-Quartet Complexes Based on Alkali and Alkaline Earth Salts on Au(111). ChemPhysChem 2015, 16, 2099–2105. [Google Scholar] [CrossRef]
- Zhou, K.; Liang, H.; Wang, M.; Xing, S.; Ding, H.; Song, Y.; Wang, Y.; Xu, Q.; He, J.-H.; Zhu, J.; et al. Fine-tuning of two-dimensional metal–organic nanostructures via alkali–pyridyl coordination. Nanoscale Adv. 2020, 2, 2170–2176. [Google Scholar] [CrossRef] [Green Version]
- Simonov, K.A.; Vinogradov, N.A.; Vinogradov, A.S.; Generalov, A.V.; Zagrebina, E.M.; Mårtensson, N.; Cafolla, A.A.; Carpy, T.; Cunniffe, J.P.; Preobrajenski, A.B. Effect of Substrate Chemistry on the Bottom-Up Fabrication of Graphene Nanoribbons: Combined Core-Level Spectroscopy and STM Study. J. Phys. Chem. C 2014, 118, 12532–12540. [Google Scholar] [CrossRef]
- Skomski, D.; Tait, S.L. Ordered and Robust Ionic Surface Networks from Weakly Interacting Carboxyl Building Blocks. J. Phys. Chem. C 2013, 117, 2959–2965. [Google Scholar] [CrossRef]
- Horcas, I.; Fernandez, R.; Gomez-Rodriguez, J.M.; Colchero, J.; Gomez-Herrero, J.; Baro, A.M. WSXM: A software for scanning probe microscopy and a tool for nanotechnology. Rev. Sci. Instrum. 2007, 78, 013705. [Google Scholar] [CrossRef] [Green Version]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 1993, 47, 558–561. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef] [PubMed]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [Green Version]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [Green Version]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. [Phys. Rev. Lett. 77, 3865 (1996)]. Phys. Rev. Lett. 1997, 78, 1396. [Google Scholar] [CrossRef] [Green Version]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dion, M.; Rydberg, H.; Schröder, E.; Langreth, D.C.; Lundqvist, B.I. Van der Waals Density Functional for General Geometries. Phys. Rev. Lett. 2004, 92, 246401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Auffinger, P.; Hays, F.A.; Westhof, E.; Ho, P.S. Halogen bonds in biological molecules. Proc. Natl. Acad. Sci. USA 2004, 101, 16789–16794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arunan, E.; Desiraju, G.; Klein, R.; Sadlej, J.; Scheiner, S.; Alkorta, I.; Clary, D.; Crabtree, R.; Duannenberg, J.; Hobza, P.; et al. Definition of the hydrogen bond (IUPAC Recommendations 2011). Pure Appl. Chem. 2010, 83, 1637–1641. [Google Scholar] [CrossRef]
- Bondi, A.V. van der Waals Volumes and Radii. J. Phys. Chem. C 1964, 68, 441–451. [Google Scholar] [CrossRef]

| Structure | DBA·Cs-I | DBA·Cs-II |
|---|---|---|
| No. of DBA | 12 | 8 |
| No. of Cs | 4 | 4 |
| Eb (eV) a | 11.30 | 11.23 |
| Eb per DBA (eV) | 0.94 | 1.40 |
| Eb per Cs (eV) | 2.82 | 2.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, R.; Song, Y.; Zhang, L.; Shi, Z. Engineering Two-Dimensional Multilevel Supramolecular Assemblies from a Bifunctional Ligand on Au(111). Molecules 2023, 28, 5116. https://doi.org/10.3390/molecules28135116
Tang R, Song Y, Zhang L, Shi Z. Engineering Two-Dimensional Multilevel Supramolecular Assemblies from a Bifunctional Ligand on Au(111). Molecules. 2023; 28(13):5116. https://doi.org/10.3390/molecules28135116
Chicago/Turabian StyleTang, Rongyu, Yang Song, Lizhi Zhang, and Ziliang Shi. 2023. "Engineering Two-Dimensional Multilevel Supramolecular Assemblies from a Bifunctional Ligand on Au(111)" Molecules 28, no. 13: 5116. https://doi.org/10.3390/molecules28135116





