In Vitro Inhibition of Xanthine Oxidase Purified from Arthritis Serum Patients by Nanocurcumin and Artemisinin Active Compounds
Abstract
:1. Introduction
2. Results and Discussion
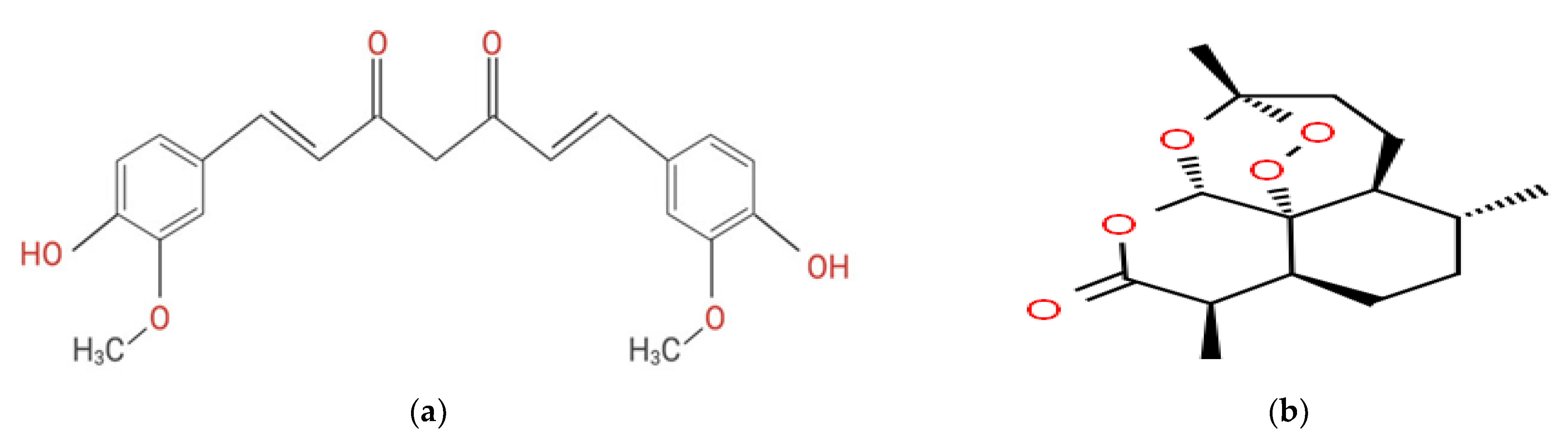
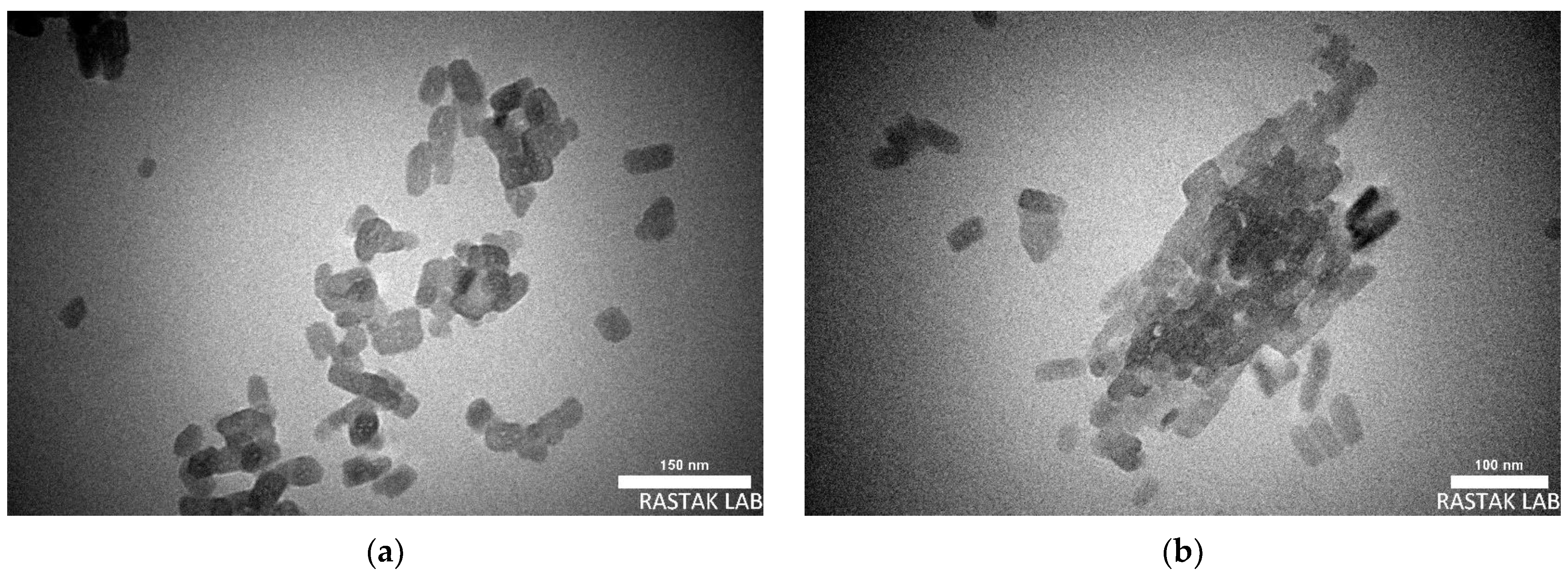
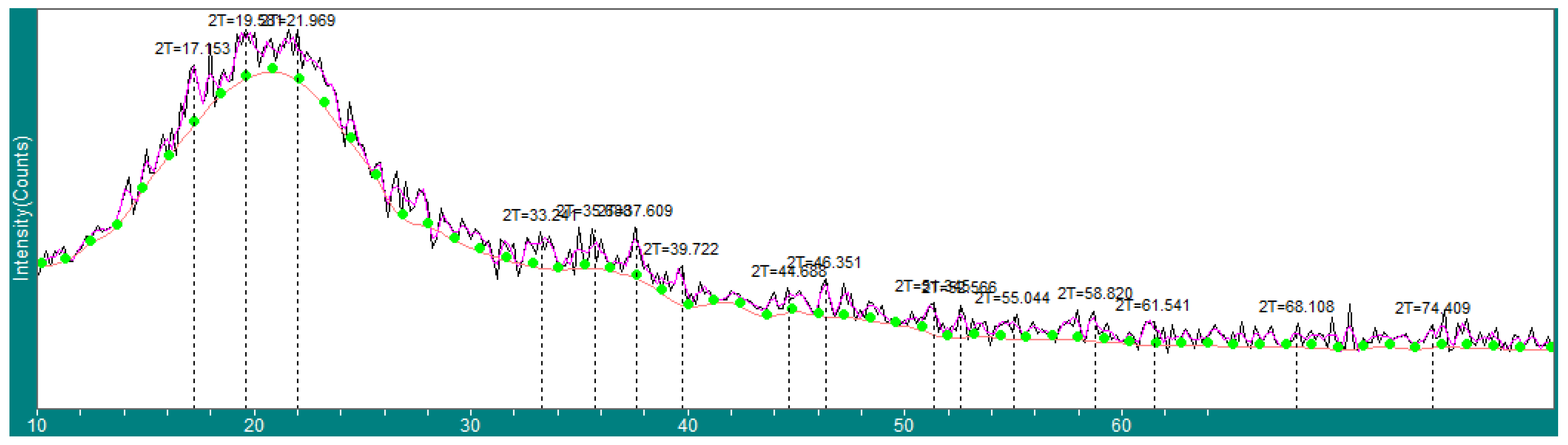
2.1. Nanocurcumin Characterizations
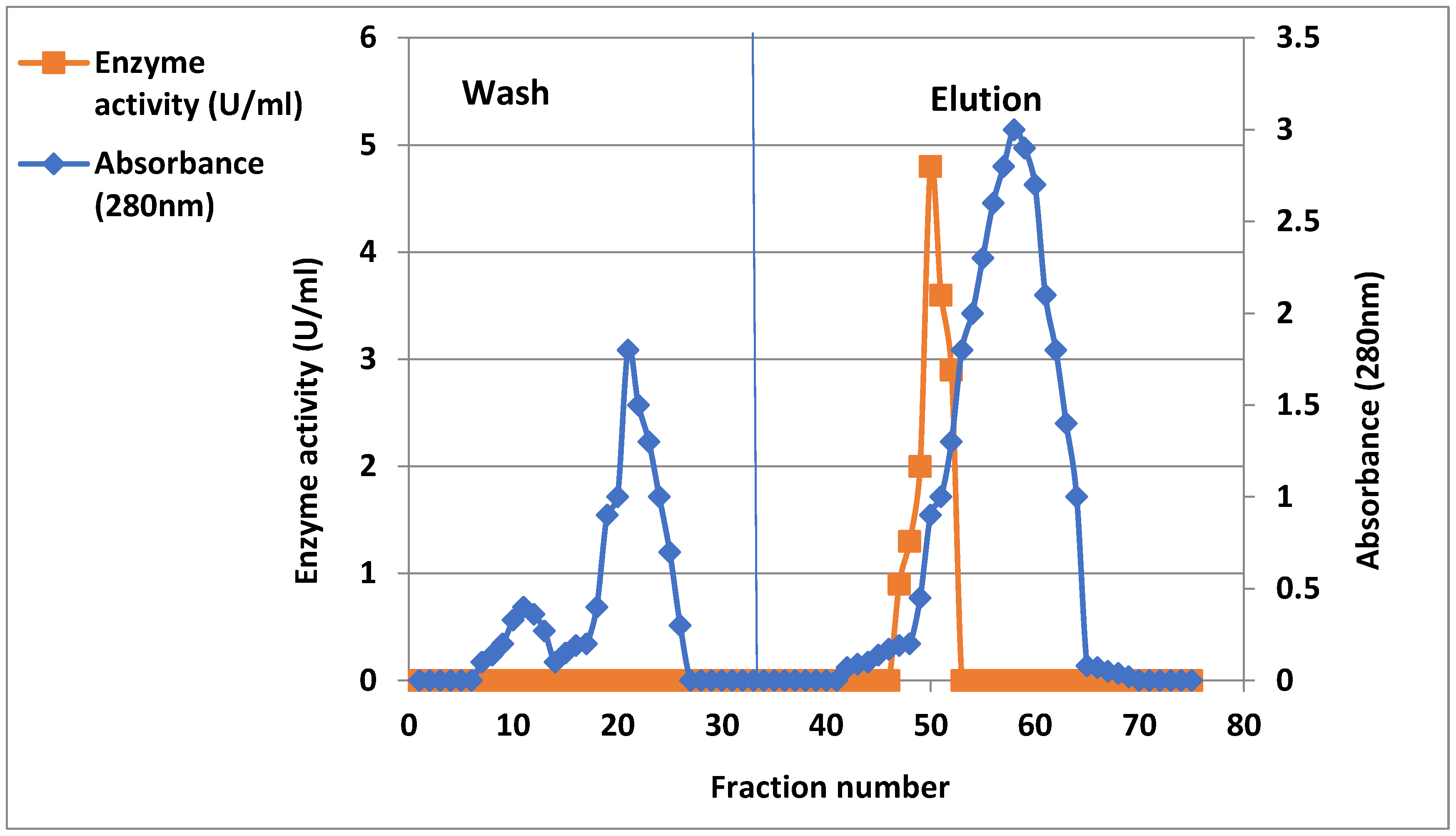
2.2. Purification of Xanthine Oxidase from Arthritis Patient’s Serum
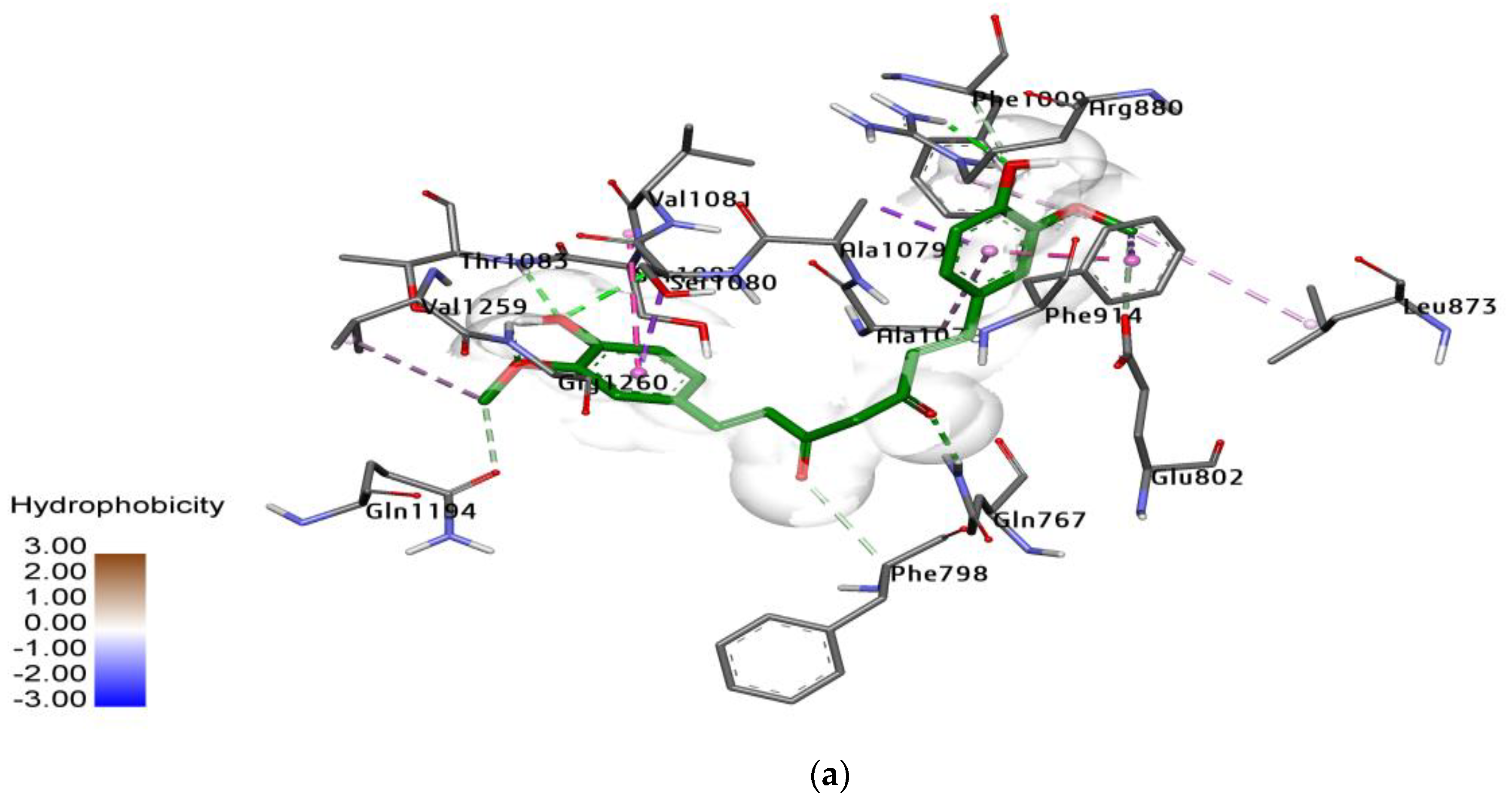
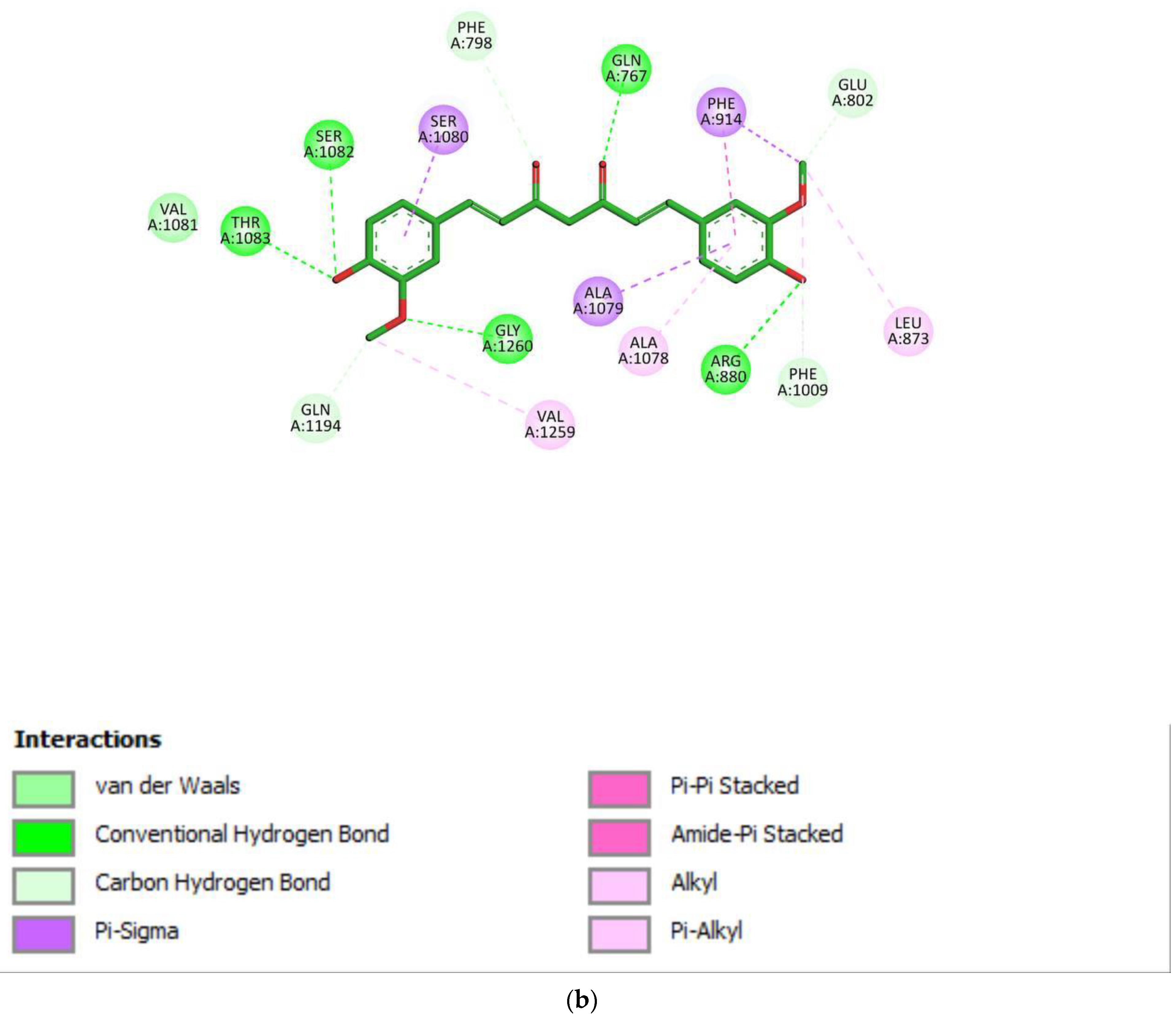
2.3. Docking Study for Enzyme Inhibitors
2.4. Xanthine Oxidase Inhibitory Activity of Nanocurcumin and Artemisinin
3. Materials and Methods
3.1. Chemicals
3.2. Characterization Instrumentations
3.3. Extraction and Purification of XO from Serum
3.4. Xanthine Oxidase Activity Assay
3.5. Molecular Docking of Curcumin and Artemisinin
3.6. Xanthine Oxidase Inhibitory Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Janczy-Cempa, E.; Mazuryk, O.; Kania, A.; Brindell, M. Significance of Specific Oxidoreductases in the Design of Hypoxia-Activated Prodrugs and Fluorescent Turn off–on Probes for Hypoxia Imaging. Cancers 2022, 14, 2686. [Google Scholar] [CrossRef]
- Eger, B.T.; Okamoto, K.; Enroth, C.; Sato, M.; Nishino, T.; Pai, E.F.; Nishino, T. Purification, crystallization and preliminary X-ray diffraction studies of xanthine dehydrogenase and xanthine oxidase isolated from bovine milk. Acta Crystallogr. Sect. D Biol. Crystallogr. 2000, 56, 1656–1658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parks, D.A.; Granger, D.N. Xanthine oxidase: Biochemistry, distribution, and physiology. Acta Physiol. Scand. Suppl. 1986, 548, 87–99. [Google Scholar] [PubMed]
- Bahadoran, Z.; Mirmiran, P.; Kashfi, K.; Ghasemi, A. Hyperuricemia-induced endothelial insulin resistance: The nitric oxide connection. Pflugers Arch. -Eur. J. Physiol. 2022, 474, 83–98. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.Y.; Baek, B.S.; Song, S.H.; Kim, M.S.; Huh, J.I.; Shim, K.H.; Kim, K.W.; Lee, K.H. Xanthine dehydrogenase/xanthine oxidase and oxidative stress. Age Omaha 1997, 20, 127–140. [Google Scholar] [CrossRef] [Green Version]
- Battelli, M.G.; Bolognesi, A.; Polito, L. Pathophysiology of circulating xanthine oxidoreductase: New emerging roles for a multi-tasking enzyme. Biochim. Biophys. Acta (BBA) 2014, 1842, 1502–1517. [Google Scholar] [CrossRef] [Green Version]
- Meneshian, A.; Bulkley, G.B. The physiology of endothelial xanthine oxidase: From urate catabolism to reperfusion injury to inflammatory signal transduction. Microcirculation 2002, 9, 161–175. [Google Scholar] [CrossRef]
- Martin, H.M.; Hancock, J.T.; Salisbury, V.; Harrison, R. Role of Xanthine oxidoreductase as an antimicrobial agent. Infect. Immun. 2004, 72, 4933–4939. [Google Scholar] [CrossRef] [Green Version]
- Mandava, K.; Batchu, U.R. Biochemical role of xanthine oxidoreductase and its natural inhibitors: An overview. Int. J. Pharm. Pharm. Sci. 2016, 8, 57–65. [Google Scholar] [CrossRef] [Green Version]
- Sureshbabu, A.; Ryter, S.W.; Choi, M.E. Oxidative stress and autophagy: Crucial modulators of kidney injury. Redox Biol. 2015, 4, 208–214. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.Q.; Hu, H.H.; Wang, Y.N.; Feng, Y.L.; Cao, G.; Zhao, Y.Y. Natural products for the prevention and treatment of kidney disease. Phytomedicine 2018, 50, 50–60. [Google Scholar] [CrossRef]
- Ayyappan, P.; Nampoothiri, S.V. Bioactive natural products as potent inhibitors of xanthine oxidase. Stud. Nat. Prod. Chem. 2020, 64, 391–416. [Google Scholar]
- Kumar, N.; Awasthi, A.; Kumari, A.; Sood, D.; Jain, P.; Singh, T.; Sharma, N.; Grover, A.; Chandra, R. Antitussive noscapine and antiviral drug conjugates as arsenal against COVID-19: A comprehensive chemoinformatics analysis. J. Biomol. Struct. Dyn. 2022, 40, 101–116. [Google Scholar] [CrossRef]
- Trivedi, M.K.; Gangwar, M.; Mondal, S.C.; Jana, S. Role of vital trace elements in Nanocurcumin-centered formulation: A novel approach to resuscitate the immune system. Biol. Trace Elem. Res. 2018, 182, 265–277. [Google Scholar] [CrossRef]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A review of Its’ effects on human health. Foods 2017, 6, 92. [Google Scholar] [CrossRef] [Green Version]
- de Almeida Alvarenga, L.; de Oliveira Leal, V.; Borges, N.A.; de Aguiar, A.S.; Faxén-Irving, G.; Stenvinkel, P.; Lindholm, B.; Mafra, D. Curcumin-a promising nutritional strategy for chronic kidney disease patients. J. Funct. Foods 2018, 40, 715–721. [Google Scholar] [CrossRef]
- Yallapu, M.M.; Nagesh, P.K.B.; Jaggi, M.; Chauhan, S.C. Therapeutic applications of curcumin and nanoformulations. AAPS J. 2015, 17, 1341–1356. [Google Scholar] [CrossRef] [Green Version]
- Strimpakos, A.S.; Sharma, R.A. Curcumin: Preventive and therapeutic properties in laboratory studies and clinical trials. Antioxid. Redox Signal. 2008, 10, 511–545. [Google Scholar] [CrossRef]
- Zhou, H.; Beevers, C.S.; Huang, S. The targets of curcumin. Curr. Drug Targets 2011, 12, 332–347. [Google Scholar] [CrossRef]
- Dende, C.; Meena, J.; Nagarajan, P.; Nagaraj, V.A.; Panda, A.K.; Padmanaban, G. Nanocurcumin is superior to native curcumin in preventing degenerative changes in experimental cerebral malaria. Sci. Rep. 2017, 7, 10062. [Google Scholar] [CrossRef] [Green Version]
- Hatamipour, M.; Sahebkar, A.H.; Alavizadeh, S.H.; Dorri, M.; Jaafari, M.R. Novel nanomicelle formulation to enhance bioavailability and stability of curcuminoids. Iran. J. Basic Med. Sci. 2019, 22, 282–289. [Google Scholar] [PubMed]
- Hosseini, A.; Rasaie, D.; Soleymani Asl, S.; Nili Ahmadabadi, A.; Ranjbar, A. Evaluation of the protective effects of curcumin and nanocurcumin against lung injury induced by sub-acute exposure to paraquat in rats. Toxin Rev. 2019, 40, 1233–1241. [Google Scholar] [CrossRef]
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2007, 2, 751–760. [Google Scholar] [CrossRef] [PubMed]
- White, N.J.; Hien, T.T.; Nosten, F.H. A Brief History of Qinghaosu. Trends Parasitol. 2015, 31, 607–610. [Google Scholar] [CrossRef] [Green Version]
- Chang, Z. The discovery of Qinghaosu (artemisinin) as an effective antimalarial drug: A unique China story. Sci. China Life Sci. 2016, 59, 81–88. [Google Scholar] [CrossRef] [Green Version]
- Vennerstrom, J.L.; Arbe-Barnes, S.; Brun, R.; Charman, S.A.; Chiu, F.C.; Chollet, J.; Dong, Y.; Dorn, A.; Hunziker, D.; Matile, H.; et al. Identification of an antimalarial synthetic trioxolane drug development candidate. Nature 2004, 430, 900–904. [Google Scholar] [CrossRef]
- Lalloo, D.G.; Shingadia, D.; Bell, D.J.; Beeching, N.J.; Whitty, C.J.M.; Chiodini, P.L. UK malaria treatment guidelines 2016. J. Infect. 2016, 72, 635–649. [Google Scholar] [CrossRef]
- Efferth, T.; Kaina, B. Toxicity of the antimalarial artemisinin and its dervatives. Crit. Rev. Toxicol. 2010, 40, 405–421. [Google Scholar] [CrossRef]
- Li, Y. Qinghaosu (artemisinin): Chemistry and pharmacology. Acta Pharmacol. Sin. 2012, 33, 1141–1146. [Google Scholar] [CrossRef] [Green Version]
- Jana, S.; Shekhawat, G.S.; Sharma, M. Chemometric evaluation of curcumin in different polymorphic forms and their antioxidant activities. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 171, 394–400. [Google Scholar] [CrossRef]
- Miazek, K.; Beton, K.; Śliwińska, A.; Brożek-Płuska, B. The Effect of β-Carotene, Tocopherols and Ascorbic Acid as Anti-Oxidant Molecules on Human and Animal In Vitro/In Vivo Studies: A Review of Research Design and Analytical Techniques Used. Biomolecules 2022, 12, 1087. [Google Scholar] [CrossRef]
- Zhang, H.; Hu, Y.; Xu, P.; Liang, W.; Zhou, J.; Liu, P.; Cheng, L.; Pu, J. Screening of potential xanthine oxidase inhibitors in Gnaphalium hypoleucum DC. by immobilized metal affinity chromatography and ultrafiltration-ultra performance liquid chromatography-mass spectrometry. Molecules 2016, 27, 12. [Google Scholar]
- Ibrahim, M.A.; Masoud, H.M.; Darwish, D.A.; Esa, S.S.; Zaahkouk, S.A. Purification and characterization of xanthine oxidase from liver of the water buffalo Bubalus bubalis. J. Appl. Pharm. Sci. 2015, 5, 063–068. [Google Scholar] [CrossRef]
- Damayanti, D.S.; Utomo, D.H.; Kusuma, C. Revealing the potency of Annona muricata leaves extract as FOXO1 inhibitor for diabetes mellitus treatment through computational study. Silico Pharmacol. 2017, 5, 3. [Google Scholar] [CrossRef] [Green Version]
- Sumirtanurdin, R.; Sungkar, S.; Hisprastin, Y.; Sidharta, K.D.; Nurhikmah, D.D. Molecular docking simulation studies of curcumin and its derivatives as cyclin-dependent kinase 2 inhibitors. Turk. J. Pharm. 2020, 17, 417–423. [Google Scholar] [CrossRef]
- Kostic, D.; Dimitrijevic, D.; Palic, R.; Stojanovic, G. Xanthine oxidase: Isolation, assay of activity and inhibition. J. Chem. 2015, 2015, 294858. [Google Scholar] [CrossRef] [Green Version]
- Kunnumakkara, A.B.; Hegde, M.; Parama, D.; Girisa, S.; Kumar, A.; Daimary, U.D.; Garodia, P.; Yenisetti, S.C.; Oommen, O.V.; Aggarwal, B.B. Role of Turmeric and Curcumin in Prevention and Treatment of Chronic Diseases: Lessons Learned from Clinical Trials. ACS Pharmacol. Transl. Sci. 2023, 6, 447–518. [Google Scholar] [CrossRef]
- Mehta, S.K.; Akmol, N. Natural xanthine oxidase inhibitors for management of gout: A review. J. Med. Health Sci. 2014, 3. [Google Scholar]
- Jadhao, S.; Bhise, N.; Khobragade, C. Isolation and purification of xanthine oxidase from Discarded fish liver notopterouskapirat: An easy source of enzyme. J. Food Technol. Food Chem. 2018, 1, 105. [Google Scholar]
- Machida, Y.; Nakanishi, T. Purification and properties of xanthine oxidase from Enterobacter cloacae. Agric. Biol. Chem. 1981, 45, 425–432. [Google Scholar] [CrossRef]
- Bradford, M. A rapid and sensitive method for the quantitation of microgram quantities of protein using the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, M.Z.; Ain, Q.; Zahid, S.; Zulfiqar, T.; Attique, A.; Bilal, M.; Alghamdi, H.A.; Yan, W.; Iqbal, M.N. Physiochemical features and structural analysis of xanthine oxidase as potential therapeutic target to prevent gout. J. Radiat. Res. Appl. Sci. 2020, 13, 616–628. [Google Scholar]
- Quy, T.N.; Xuan, T.D. Xanthine Oxidase Inhibitory Potential, Antioxidantand Antibacterial Activities of Cordyceps militaris (L.) Link Fruiting Body. Medicines 2019, 6, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Purification Step | Volume (mL) |
Enzyme Activity (U/mL) | Protein Concentration (mg/mL) |
Specific Activity (U/mg) |
Total Activity (U) | Purification (Folds) | Yield (%) |
|---|---|---|---|---|---|---|---|
| Crude enzyme | 50 | 1.4 | 0.3 | 4.6 | 70 | 1 | 100 |
| Ammonium sulfate precipitation 65% | 13 | 4 | 0.25 | 16 | 52 | 3.4 | 74.2 |
| DEAE-cellulose | 18 | 2.6 | 0.08 | 32.5 | 46.8 | 7 | 66.8 |
| Compound | Xanthine Oxidase Inhibition % | ||||
|---|---|---|---|---|---|
| 5 (µg/mL) | 10 (µg/mL) | 15 (µg/mL) | 20 (µg/mL) | 25 (µg/mL) | |
| Nano-Curcumin | 67.61 ± 1.52 | 84.01 ± 1.49 | 87.32 ± 2.08 | 89.01 ± 2.64 | 91.12 ± 2.34 |
| Artemisinin | 40.61 ± 1.15 | 50.32 ± 3.21 | 56.01 ± 2.31 | 59.21 ± 2.11 | 68.71 ± 2.56 |
| Allopurinol (Control) | 46.33 ± 6.50 | 55.12 ± 5.13 | 78.37 ± 3.15 | 83.29 ± 2.45 | 86.01 ± 4.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
AL-dulaimy, W.Y.M.; Hussein, A.A.; Mahdi, M.A.; Kadhom, M. In Vitro Inhibition of Xanthine Oxidase Purified from Arthritis Serum Patients by Nanocurcumin and Artemisinin Active Compounds. Molecules 2023, 28, 5124. https://doi.org/10.3390/molecules28135124
AL-dulaimy WYM, Hussein AA, Mahdi MA, Kadhom M. In Vitro Inhibition of Xanthine Oxidase Purified from Arthritis Serum Patients by Nanocurcumin and Artemisinin Active Compounds. Molecules. 2023; 28(13):5124. https://doi.org/10.3390/molecules28135124
Chicago/Turabian StyleAL-dulaimy, Waseem Yousif M., Asmaa A. Hussein, Mohammed Asaad Mahdi, and Mohammed Kadhom. 2023. "In Vitro Inhibition of Xanthine Oxidase Purified from Arthritis Serum Patients by Nanocurcumin and Artemisinin Active Compounds" Molecules 28, no. 13: 5124. https://doi.org/10.3390/molecules28135124
APA StyleAL-dulaimy, W. Y. M., Hussein, A. A., Mahdi, M. A., & Kadhom, M. (2023). In Vitro Inhibition of Xanthine Oxidase Purified from Arthritis Serum Patients by Nanocurcumin and Artemisinin Active Compounds. Molecules, 28(13), 5124. https://doi.org/10.3390/molecules28135124







