Determination of the Main Phase Transition Temperature of Phospholipids by Oscillatory Rheology
Abstract
:1. Introduction
2. Results and Discussion
2.1. Rheological Measurements
2.1.1. Rheological Experiments of Liposomal Dispersions
2.1.2. Rheological Experiments of Reverse-Typed Micelles
2.1.3. Rheological Experiments of Reverse-Type Phospholipid Complexes (RTPCs)
2.1.4. Evaluation of Oscillatory Measurements of Reverse-Type Phospholipid Complexes (RTPCs)
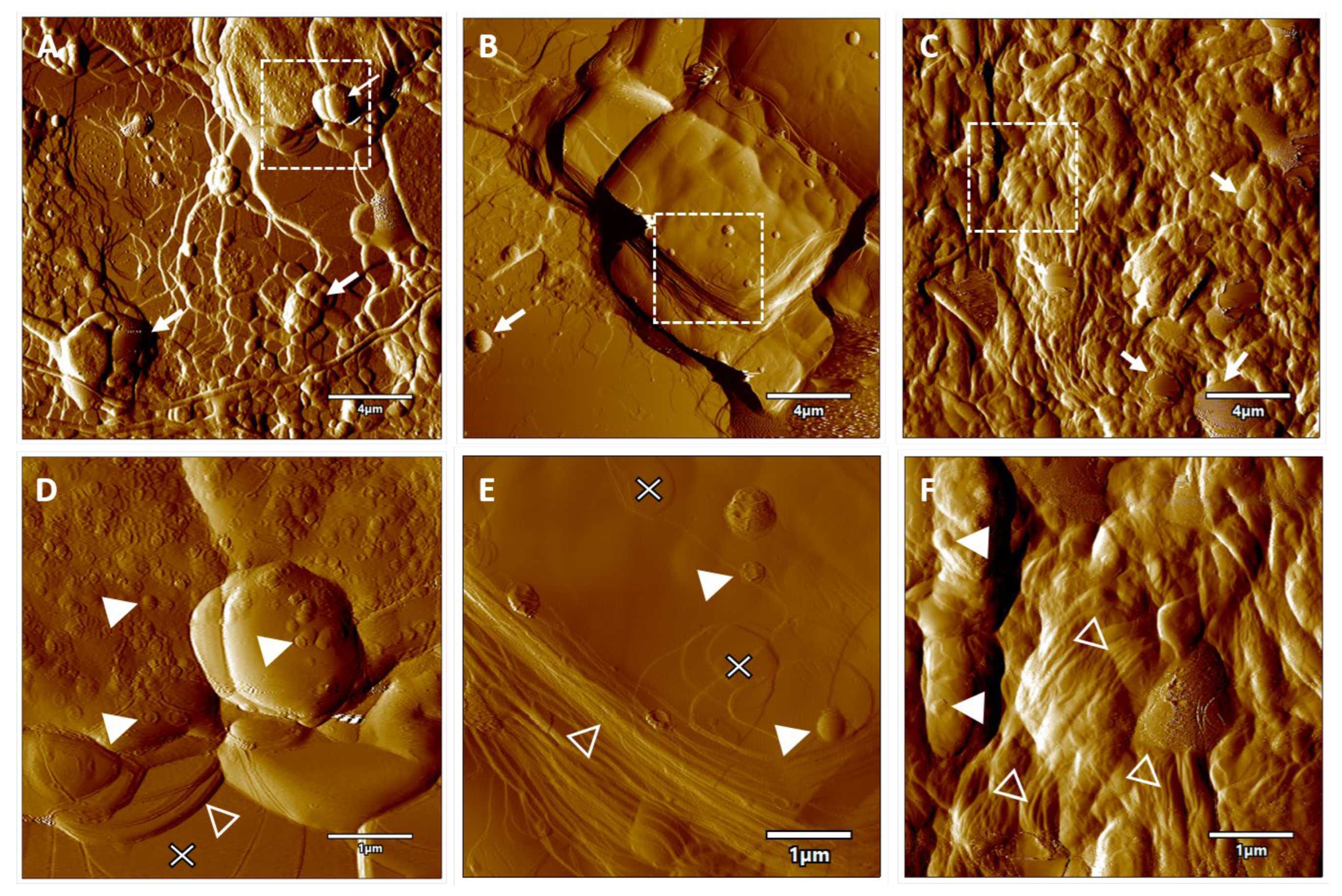
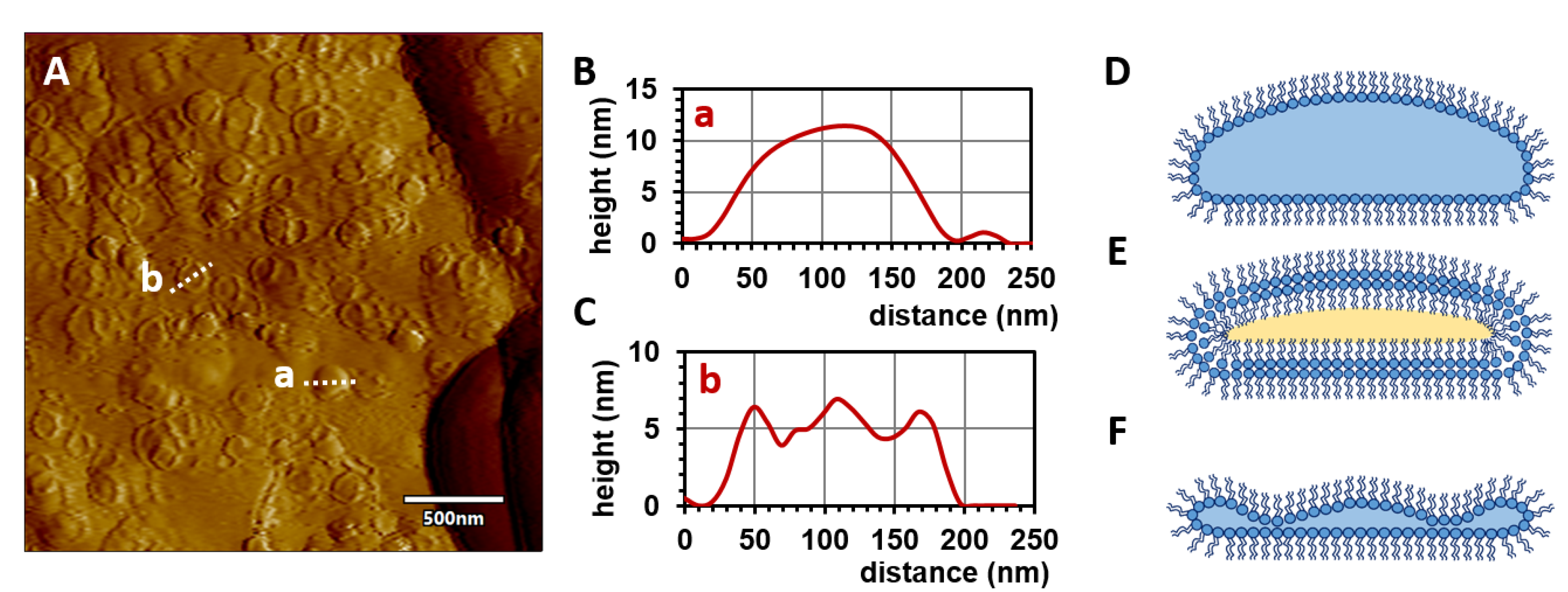
2.2. Microscopic Analysis
3. Materials and Methods
3.1. Materials
3.2. Preparation of Samples
3.2.1. Preparation of Liposomal Dispersions
3.2.2. Preparation of Reverse-Type Micelles
3.2.3. Preparation of Reverse-Type Phospholipid Complexes (RTPCs)
3.3. Rheological Experiments
3.4. Data Analysis for Rheological Measurements
3.5. Phase-Contrast Microscopy
3.6. Atomic Force Microscopy (AFM)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Kim, Y.-R.; Jung, S.; Ryu, H.; Yoo, Y.-E.; Kim, S.M.; Jeon, T.-J. Synthetic biomimetic membranes and their sensor applications. Sensors 2012, 12, 9530–9550. [Google Scholar] [CrossRef]
- Chen, J.; Cheng, D.; Li, J.; Wang, Y.; Guo, J.-X.; Chen, Z.-P.; Cai, B.-C.; Yang, T. Influence of lipid composition on the phase transition temperature of liposomes composed of both DPPC and HSPC. Drug Dev. Ind. Pharm. 2012, 39, 197–204. [Google Scholar] [CrossRef]
- Hald Albertsen, C.; Kulkarni, J.A.; Witzigmann, D.; Lind, M.; Petersson, K.; Simonsen, J.B. The role of lipid components in lipid nanoparticles for vaccines and gene therapy. Adv. Drug Deliv. Rev. 2022, 188, 114416. [Google Scholar] [CrossRef] [PubMed]
- Hajiaghapour Asr, M.; Dayani, F.; Saedi Segherloo, F.; Kamedi, A.; Neill, A.O.; MacLoughlin, R.; Doroudian, M. Lipid Nanoparticles as Promising Carriers for mRNA Vaccines for Viral Lung Infections. Pharmaceutics 2023, 15, 1127. [Google Scholar] [CrossRef]
- Mabrey, S.; Sturtevant, J.M. Investigation of phase transitions of lipids and lipid mixtures by sensitivity differential scanning calorimetry. Proc. Natl. Acad. Sci. USA 1976, 73, 3862–3866. [Google Scholar] [CrossRef] [Green Version]
- Biltonen, R.L.; Lichtenberg, D. The use of differential scanning calorimetry as a tool to characterize liposome preparations. Chem. Phys. Lipids 1993, 64, 129–142. [Google Scholar] [CrossRef]
- Sklar, L.A.; Hudson, B.S.; Simoni, R.D. Conjugated polyene fatty acids as fluorescent probes: Binding to bovine serum albumin. Biochem 1977, 16, 5100–5108. [Google Scholar] [CrossRef] [PubMed]
- Lentz, B.R.; Freire, E.; Biltonen, R.L. Fluorescence and calorimetric studies of phase transitions in phosphatidylcholine multilayers: Kinetics of the pretransition. Biochemistry 1978, 17, 4475–4480. [Google Scholar] [CrossRef] [PubMed]
- YashRoy, R.C. Determination of membrane lipid phase transition temperature from 13C-NMR intensities. J. Biochem. Bioph. Methods 1990, 20, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, P.M.; Strashko, V. A thermotropic phase transition in polyelectrolyte−surfactant complexes as characterized by deuterium NMR. Langmuir 1998, 14, 4758–4764. [Google Scholar] [CrossRef]
- Khalique Ahmed, M.; Choma, C.T.; Wong, P.T.T. High pressure ftir study of interaction of Melittin with dimyristoylphosphatidyl glycerol bilayers. Chem. Phys. Lipids 1992, 63, 139–148. [Google Scholar] [CrossRef] [PubMed]
- McMullen, T.P.W.; Lewis, R.N.A.H.; McElhaney, R.N. Differential scanning calorimetric and Fourier transform infrared spectroscopic studies of the effects of cholesterol on the thermotropic phase behavior and organization of a homologous series of linear saturated phosphatidylserine bilayer membranes. Biophys. J. 2000, 79, 2056–2065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leonenko, Z.V.; Finot, E.; Ma, H.; Dahms, T.E.S.; Cramb, D.T. Investigation of temperature-induced phase transitions in DOPC and DPPC phospholipid bilayers using temperature-controlled scanning force microscopy. Biophys. J. 2004, 86, 3783–3793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, A.F.; Yamada, R.; Gewirth, A.A.; Granick, S. Materials science of the gel to fluid phase transition in a supported phospholipid bilayer. Phys. Rev. Lett. 2002, 89, 246103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tokumasu, F.; Jin, A.J.; Feigenson, G.W.; Dvorak, J.A. Atomic Force Microscopy of nanometric liposome adsorption and nanoscopic membrane domain formation. Ultramicroscopy 2003, 97, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Charrier, A.; Thibaudau, F. Main phase transitions in supported lipid single-bilayer. Biophys. J. 2005, 89, 1094–1101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michel, N.; Fabiano, A.-S.; Polidori, A.; Jack, R.; Pucci, B. Determination of phase transition temperatures of lipids by light scattering. Chem. Phys. Lipids 2006, 139, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Duša, F.; Witos, J.; Ruokonen, S.-K.; Wiedmer, S.K. Determination of the main phase transition temperature of phospholipids by Nanoplasmonic sensing. Sci. Rep. 2018, 8, 14815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macosko, C.W. Rheology: Principles, Measurements, and Applications; Wiley-VCH: New York, NY, USA, 1994; pp. 117–126. [Google Scholar]
- Scartazzini, R.; Luisi, P.L. Organogels from lecithins. J. Phys. Chem. 1988, 92, 829–833. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing. 2022. Available online: https://www.R-project.org/ (accessed on 1 January 2023).
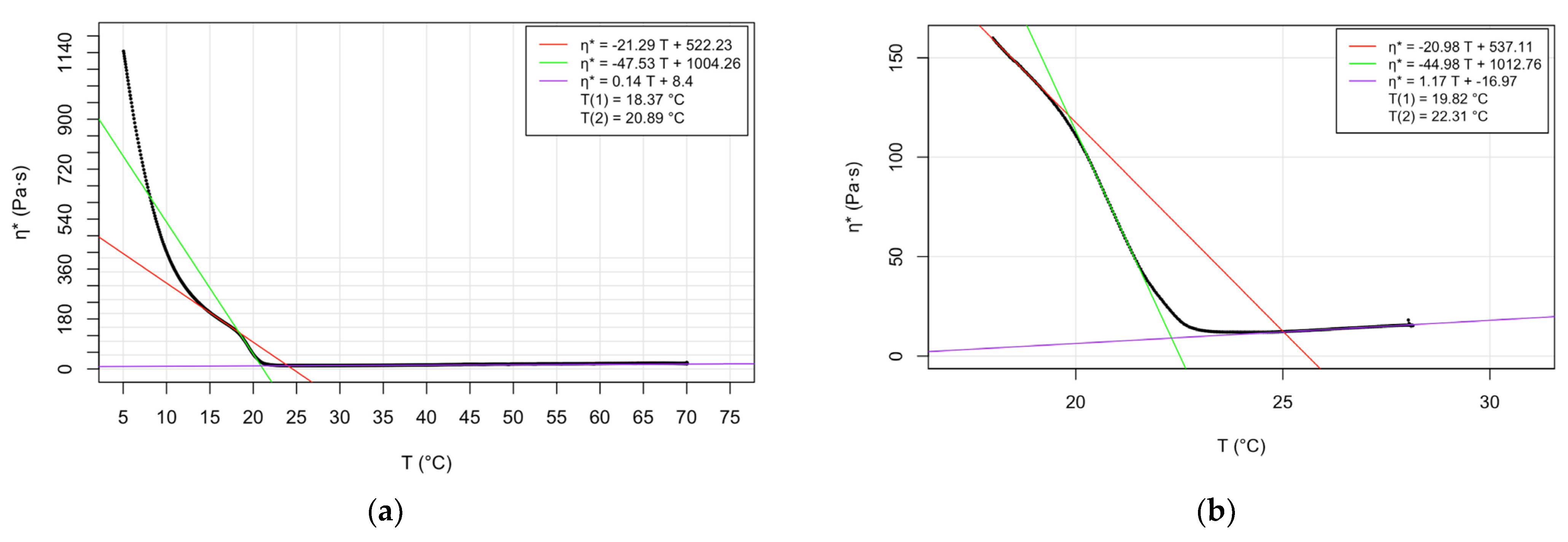
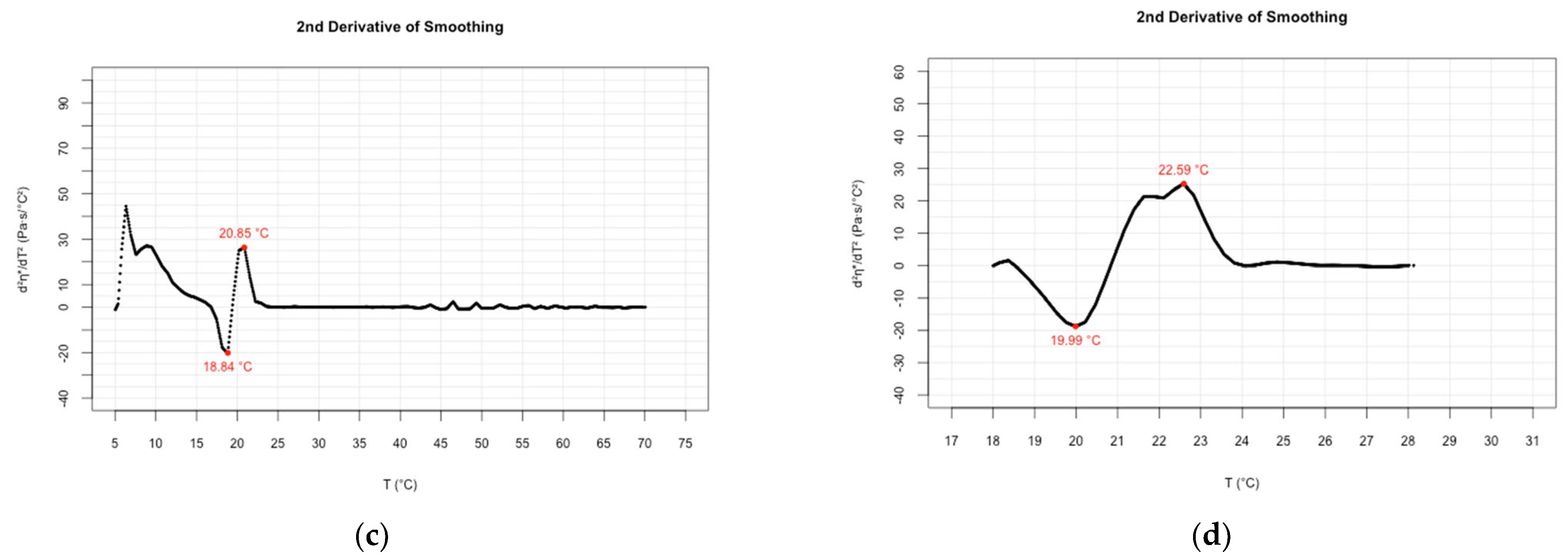
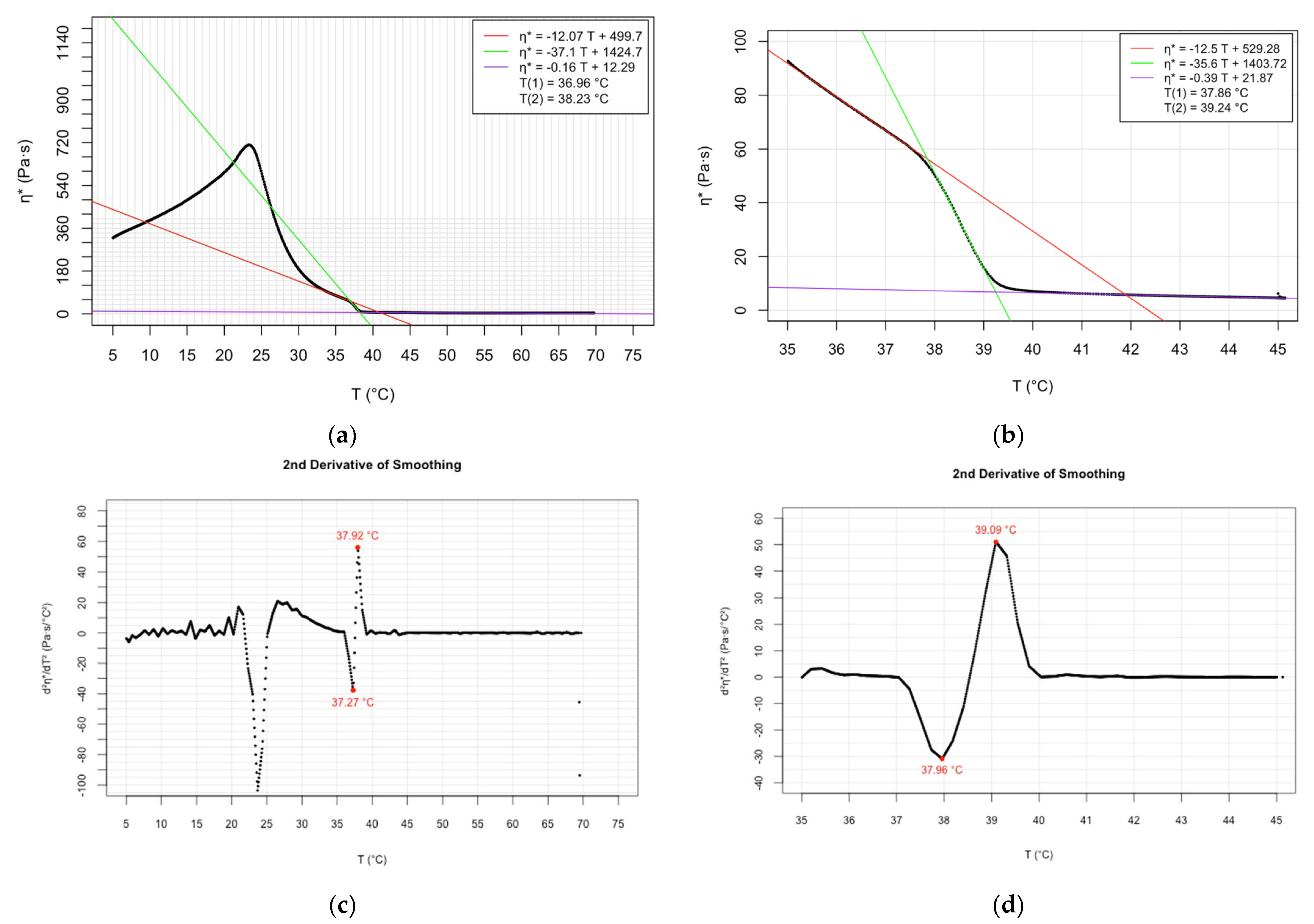
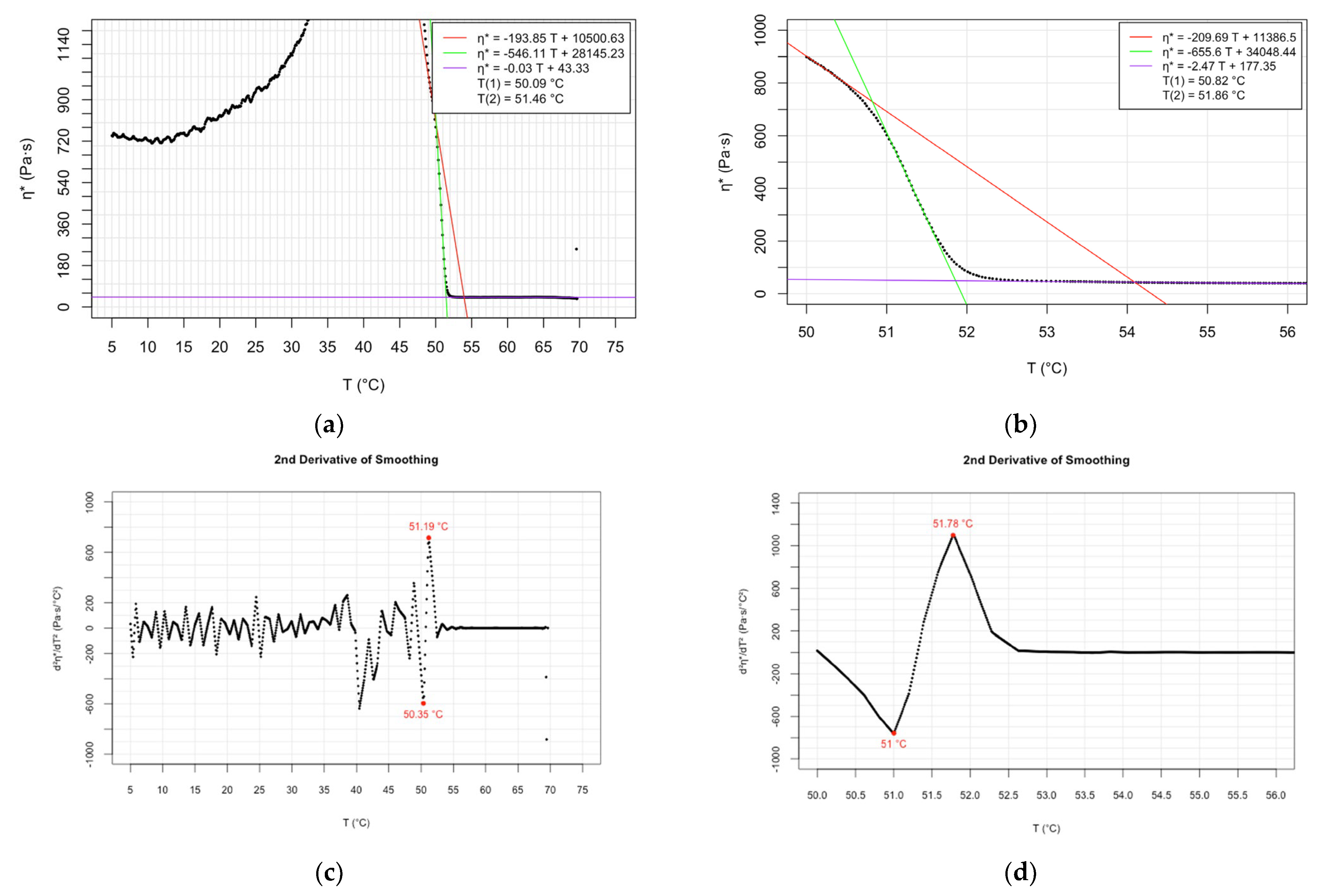

| Method | Pretransition Tc1 (°C) | Main Transition Tc2 (°C) | Upper Transition Tc3 (°C) | Reference |
|---|---|---|---|---|
| Differential scanning calorimetry | 14.2 | 23.9 | [5] | |
| Cis-parinaric fluorescence intensity | 10 | 23 | [7] | |
| Differential scanning calorimetry | 14.1 | 23.9 | [8] | |
| Fluorescence depolarization | 10; 12.4 | 23.8 | [8] | |
| Atomic force microscopy | 26–27.5 | [16] | ||
| Nanoplasmonic sensing | 16.8 | 20.7 | 22.5 | [18] |
| Oscillatory rheology (cooling speed 1 °C/min) | 19.82; 19.87 | 22.31; 22.33 | Present study | |
| Oscillatory rheology (cooling speed 3 °C/min) | 18.37; 18.59 | 20.89; 21.14 | Present study |
| Method | Pretransition Tc1 (°C) | Main Transition Tc2 (°C) | Upper Transition Tc3 (°C) | Reference |
|---|---|---|---|---|
| Differential scanning calorimetry | 35.3 | 41.4 | [5] | |
| Cis-parinaric fluorescence intensity | 32 | 42 | [7] | |
| Differential scanning calorimetry | 35.2 | 41.3 | [8] | |
| Fluorescence depolarization | 22.9; 29.8; 31.9 | 40.6 | [8] | |
| Atomic force microscopy | 42–52 | 53–60 | [13] | |
| Differential scanning calorimetry | 42.37 | [2] | ||
| Calcein release | 40 | [2] | ||
| Nanoplasmonic sensing | 34.9 | 39.1 | 41.0 | [18] |
| Oscillatory rheology (cooling speed 1 °C/min) | 37.86; 37.91 | 39.24; 39.3 | Present study | |
| Oscillatory rheology (cooling speed 3 °C/min) | 36.96; 37.0 | 38.23; 38.24 | Present study |
| Method | Pretransition Tc1 (°C) | Main Transition Tc2 (°C) | Upper Transition Tc3 (°C) | Reference |
|---|---|---|---|---|
| Differential scanning calorimetry | 51.5 | 54.9 | [5] | |
| Cis-parinaric fluorescence intensity | 49 | 54 | [7] | |
| Differential scanning calorimetry | 48.5 | 54.5 | [8] | |
| Fluorescence depolarization | 43.2; 45.6 | 53.7 | [8] | |
| Nanoplasmonic sensing | 46.7 | 51.7 | 55.5 | [18] |
| Oscillatory rheology (cooling speed 1 °C/min) | 50.82; 50.65 | 51.86; 51.57 | Present study | |
| Oscillatory rheology (cooling speed 3 °C/min) | 50.09; 50.21 | 51.46; 51.43 | Present study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Budai, L.; Budai, M.; Bozó, T.; Agócs, G.; Kellermayer, M.; Antal, I. Determination of the Main Phase Transition Temperature of Phospholipids by Oscillatory Rheology. Molecules 2023, 28, 5125. https://doi.org/10.3390/molecules28135125
Budai L, Budai M, Bozó T, Agócs G, Kellermayer M, Antal I. Determination of the Main Phase Transition Temperature of Phospholipids by Oscillatory Rheology. Molecules. 2023; 28(13):5125. https://doi.org/10.3390/molecules28135125
Chicago/Turabian StyleBudai, Lívia, Marianna Budai, Tamás Bozó, Gergely Agócs, Miklós Kellermayer, and István Antal. 2023. "Determination of the Main Phase Transition Temperature of Phospholipids by Oscillatory Rheology" Molecules 28, no. 13: 5125. https://doi.org/10.3390/molecules28135125
APA StyleBudai, L., Budai, M., Bozó, T., Agócs, G., Kellermayer, M., & Antal, I. (2023). Determination of the Main Phase Transition Temperature of Phospholipids by Oscillatory Rheology. Molecules, 28(13), 5125. https://doi.org/10.3390/molecules28135125







