Icariin Promotes Osteogenic Differentiation in a Cell Model with NF1 Gene Knockout by Activating the cAMP/PKA/CREB Pathway
Abstract
1. Introduction
2. Results
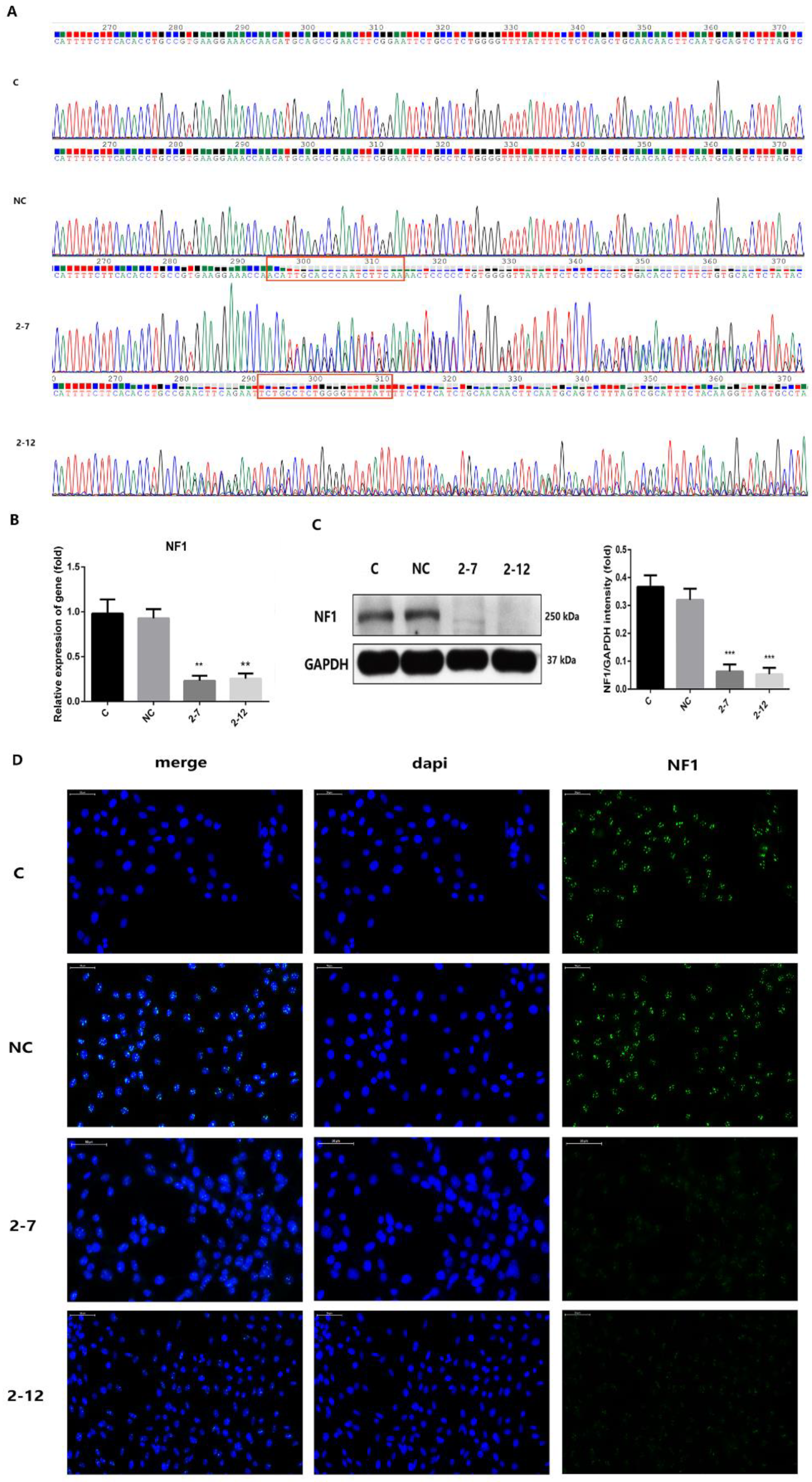
2.1. Two Lines of C2C12 Cells with NF1 Gene Knockout Were Successfully Constructed
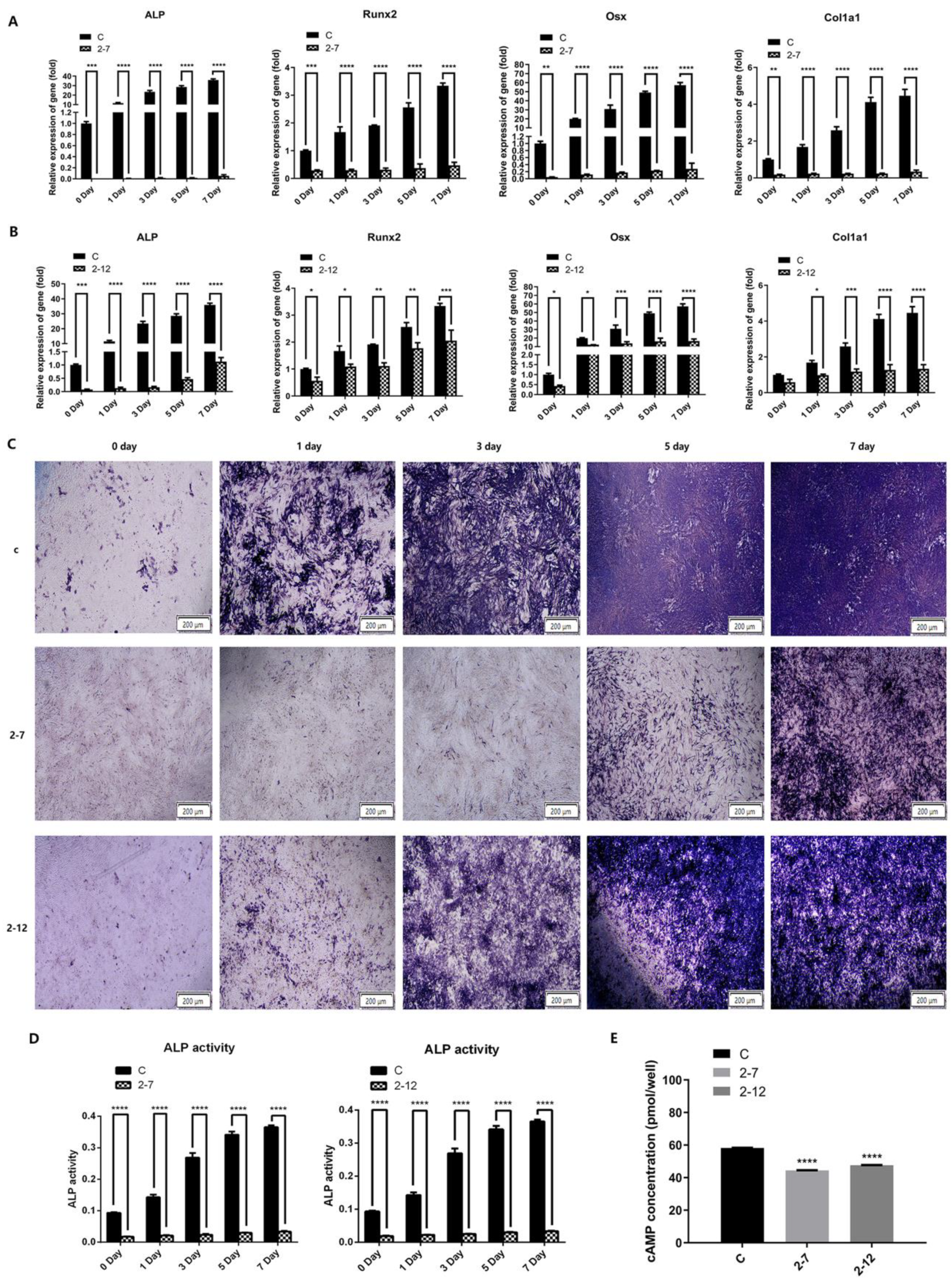
2.2. The Osteogenic Differentiation and the Intracellular cAMP Level of the C2C12 Cells with NF1 Gene Knockout Were Significantly Reduced
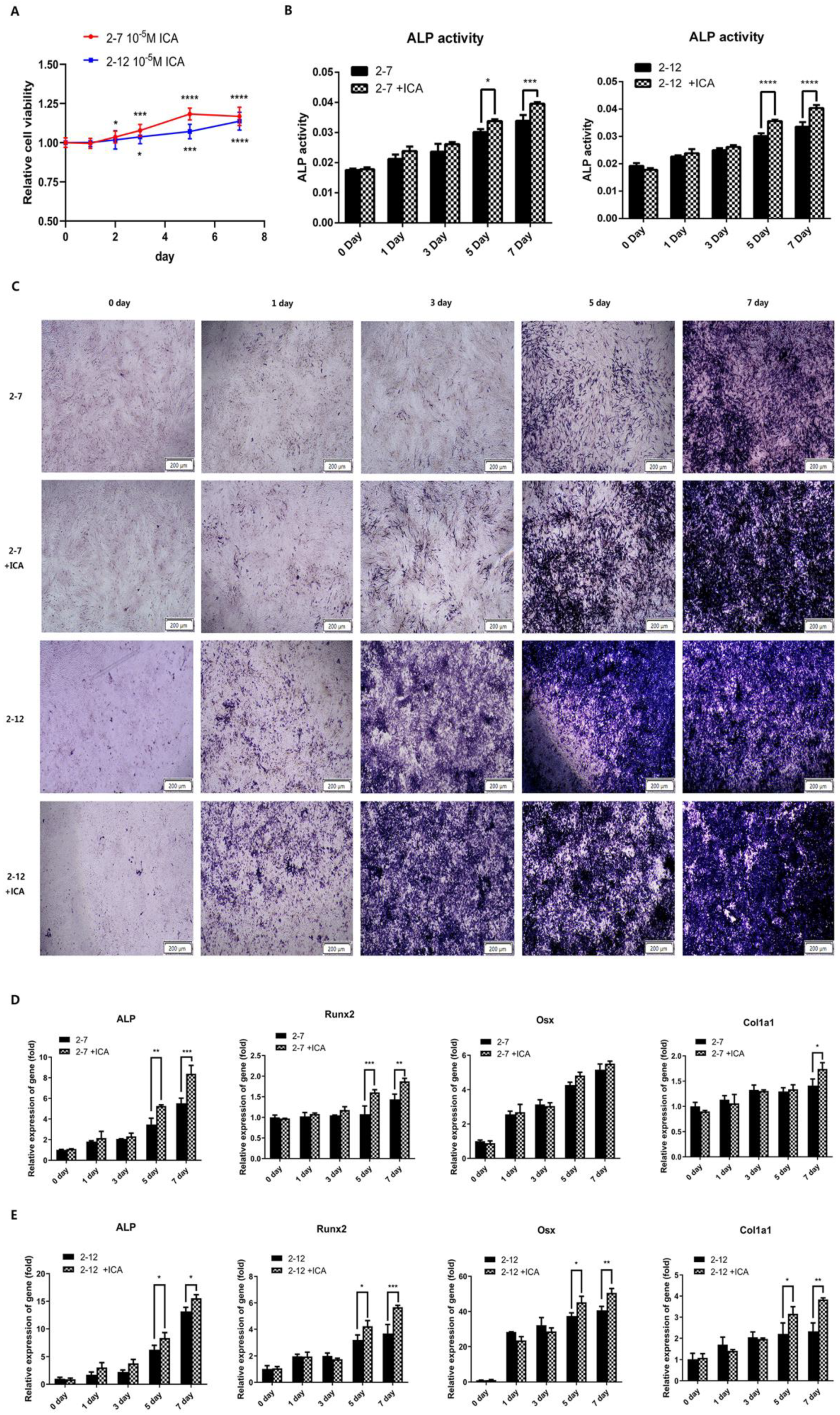
2.3. Icariin Improved the Osteogenic Differentiation of the NF1 Gene Knockout Cell Models at the Later Stage of the Osteogenic Induction Process
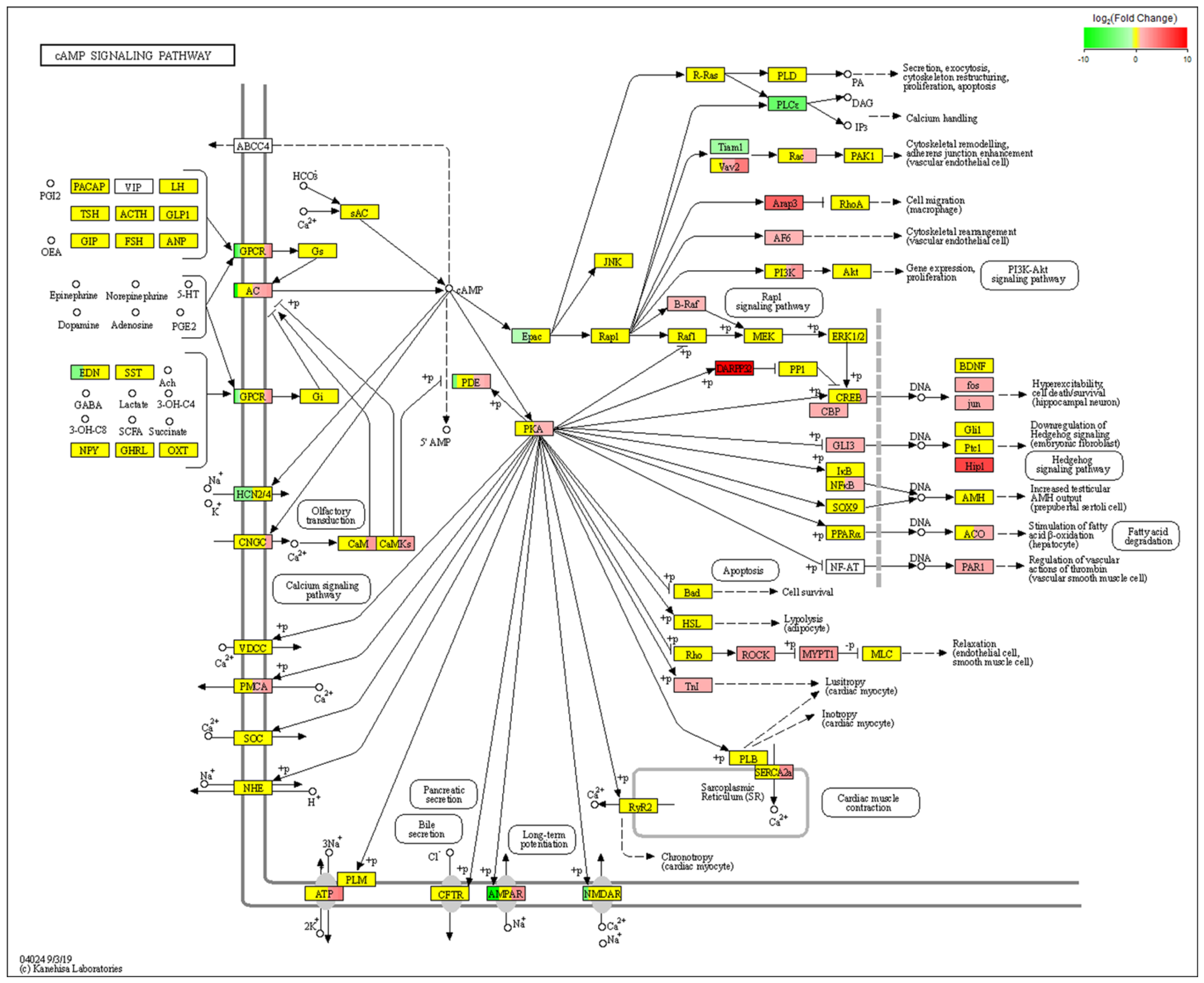
2.4. RNA-Seq Analysis of Differentially Expressed Genes (DEGs) before and after Icariin Treatment during the Induction of Osteogenesis in the NF1 Cell Model Suggested cAMP Pathway Activation
2.5. Icariin Activated the cAMP/PKA/CREB Pathway in the NF1 Cell Model
2.6. A PKA Inhibitor (H89) Inhibited Icariin-Induced Osteogenesis by Blocking the cAMP/PKA/CREB Signaling Pathway in the NF1 Cell Model
3. Discussion
4. Materials and Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Ge, L.-L.; Xing, M.-Y.; Zhang, H.-B.; Wang, Z.-C. Neurofibroma Development in Neurofibromatosis Type 1: Insights from Cellular Origin and Schwann Cell Lineage Development. Cancers 2022, 14, 4513. [Google Scholar] [CrossRef]
- Elefteriou, F.; Kolanczyk, M.; Schindeler, A.; Viskochil, D.H.; Hock, J.M.; Schorry, E.K.; Crawford, A.H.; Friedman, J.M.; Little, D.; Peltonen, J.; et al. Skeletal abnormalities in neurofibromatosis type 1: Approaches to therapeutic options. Am. J. Med. Genet. A 2009, 149A, 2327–2338. [Google Scholar] [CrossRef] [PubMed]
- de la Croix Ndong, J.; Stevens, D.M.; Vignaux, G.; Uppuganti, S.; Perrien, D.S.; Yang, X.; Nyman, J.S.; Harth, E.; Elefteriou, F. Combined MEK inhibition and BMP2 treatment promotes osteoblast differentiation and bone healing in Nf1Osx -/- mice. J. Bone Miner. Res. 2015, 30, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Kaspiris, A.; Savvidou, O.D.; Vasiliadis, E.S.; Hadjimichael, A.C.; Melissaridou, D.; Iliopoulou-Kosmadaki, S.; Iliopoulos, I.D.; Papadimitriou, E.; Chronopoulos, E. Current Aspects on the Pathophysiology of Bone Metabolic Defects during Progression of Scoliosis in Neurofibromatosis Type 1. J. Clin. Med. 2022, 11, 444. [Google Scholar] [CrossRef] [PubMed]
- Kolanczyk, M.; Kossler, N.; Kühnisch, J.; Lavitas, L.; Stricker, S.; Wilkening, U.; Manjubala, I.; Fratzl, P.; Spörle, R.; Herrmann, B.G.; et al. Multiple roles for neurofibromin in skeletal development and growth. Hum. Mol. Genet. 2007, 16, 874–886. [Google Scholar] [CrossRef]
- Moramarco, A.; Mallone, F.; Sacchetti, M.; Lucchino, L.; Miraglia, E.; Roberti, V.; Lambiase, A.; Giustini, S. Hyperpigmented spots at fundus examination: A new ocular sign in Neurofibromatosis Type I. Orphanet. J. Rare Dis. 2021, 16, 147. [Google Scholar] [CrossRef]
- Ratner, N.; Miller, S.J. A RASopathy gene commonly mutated in cancer: The neurofibromatosis type 1 tumour suppressor. Nat. Rev. Cancer 2015, 15, 290–301. [Google Scholar] [CrossRef]
- Machado Almeida, P.; Lago Solis, B.; Stickley, L.; Feidler, A.; Nagoshi, E. Neurofibromin 1 in mushroom body neurons mediates circadian wake drive through activating cAMP-PKA signaling. Nat. Commun. 2021, 12, 5758. [Google Scholar] [CrossRef]
- Abramowicz, A.; Gos, M. Neurofibromin in neurofibromatosis type 1—Mutations in NF1gene as a cause of disease. Dev. Period Med. 2014, 18, 297–306. [Google Scholar]
- Petramala, L.; Giustini, S.; Zinnamosca, L.; Marinelli, C.; Colangelo, L.; Cilenti, G.; Formicuccia, M.C.; D’Erasmo, E.; Calvieri, S.; Letizia, C. Bone mineral metabolism in patients with neurofibromatosis type 1 (von Recklingausen disease). Arch Dermatol. Res. 2012, 304, 325–331. [Google Scholar] [CrossRef]
- Fowlkes, J.L.; Thrailkill, K.M.; Bunn, R.C. RASopathies: The musculoskeletal consequences and their etiology and pathogenesis. Bone 2021, 152, 116060. [Google Scholar] [CrossRef]
- Tucker, T.; Schnabel, C.; Hartmann, M.; Friedrich, R.E.; Frieling, I.; Kruse, H.P.; Mautner, V.F.; Friedman, J.M. Bone health and fracture rate in individuals with neurofibromatosis 1 (NF1). J. Med. Genet. 2009, 46, 259–265. [Google Scholar] [CrossRef]
- Stevenson, D.A.; Schwarz, E.L.; Viskochil, D.H.; Moyer-Mileur, L.J.; Murray, M.; Firth, S.D.; D’Astous, J.L.; Carey, J.C.; Pasquali, M. Evidence of increased bone resorption in neurofibromatosis type 1 using urinary pyridinium crosslink analysis. Pediatr. Res. 2008, 63, 697–701. [Google Scholar] [CrossRef]
- Lee, D.Y.; Cho, T.-J.; Lee, H.R.; Lee, K.; Moon, H.J.; Park, M.S.; Yoo, W.J.; Chung, C.Y.; Choi, I.H. Disturbed osteoblastic differentiation of fibrous hamartoma cell from congenital pseudarthrosis of the tibia associated with neurofibromatosis type I. Clin. Orthop. Surg. 2011, 3, 230–237. [Google Scholar] [CrossRef]
- Kuorilehto, T.; Nissinen, M.; Koivunen, J.; Benson, M.D.; Peltonen, J. NF1 tumor suppressor protein and mRNA in skeletal tissues of developing and adult normal mouse and NF1-deficient embryos. J. Bone Miner. Res. 2004, 19, 983–989. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Estwick, S.A.; Chen, S.; Yu, M.; Ming, W.; Nebesio, T.D.; Li, Y.; Yuan, J.; Kapur, R.; Ingram, D.; et al. Neurofibromin plays a critical role in modulating osteoblast differentiation of mesenchymal stem/progenitor cells. Hum. Mol. Genet. 2006, 15, 2837–2845. [Google Scholar] [CrossRef] [PubMed]
- Elefteriou, F.; Benson, M.D.; Sowa, H.; Starbuck, M.; Liu, X.; Ron, D.; Parada, L.F.; Karsenty, G. ATF4 mediation of NF1 functions in osteoblast reveals a nutritional basis for congenital skeletal dysplasiae. Cell Metab. 2006, 4, 441–451. [Google Scholar] [CrossRef]
- Liang, W.; Lin, M.; Li, X.; Li, C.; Gao, B.; Gan, H.; Yang, Z.; Lin, X.; Liao, L.; Yang, M. Icariin promotes bone formation via the BMP-2/Smad4 signal transduction pathway in the hFOB 1.19 human osteoblastic cell line. Int. J. Mol. Med. 2012, 30, 889–895. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, J.; Fong, C.; Yao, X.; Yang, M. Herba epimedii flavonoids suppress osteoclastic differentiation and bone resorption by inducing G2/M arrest and apoptosis. Biochimie 2012, 94, 2514–2522. [Google Scholar] [CrossRef]
- Shi, W.; Gao, Y.; Wang, Y.; Zhou, J.; Wei, Z.; Ma, X.; Ma, H.; Xian, C.J.; Wang, J.; Chen, K. The flavonol glycoside icariin promotes bone formation in growing rats by activating the cAMP signaling pathway in primary cilia of osteoblasts. J. Biol. Chem. 2017, 292, 20883–20896. [Google Scholar] [CrossRef] [PubMed]
- Kuorilehto, T.; Pöyhönen, M.; Bloigu, R.; Heikkinen, J.; Väänänen, K.; Peltonen, J. Decreased bone mineral density and content in neurofibromatosis type 1: Lowest local values are located in the load-carrying parts of the body. Osteoporos. Int. 2005, 16, 928–936. [Google Scholar] [CrossRef] [PubMed]
- Yaffe, D.; Saxel, O. Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature 1977, 270, 725–727. [Google Scholar] [CrossRef] [PubMed]
- Won, G.W.; Sung, M.; Lee, Y.; Lee, Y.H. MST2 kinase regulates osteoblast differentiation by phosphorylating and inhibiting Runx2 in C2C12 cells. Biochem. Biophys. Res. Commun. 2019, 512, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Soundharrajan, I.; Kim, D.H.; Srisesharam, S.; Kuppusamy, P.; Sivanesan, R.; Choi, K.C. Limonene promotes osteoblast differentiation and 2-deoxy-d-glucose uptake through p38MAPK and Akt signaling pathways in C2C12 skeletal muscle cells. Phytomedicine 2018, 45, 41–48. [Google Scholar] [CrossRef]
- Hidaka, Y.; Chiba-Ohkuma, R.; Karakida, T.; Onuma, K.; Yamamoto, R.; Fujii-Abe, K.; Saito, M.M.; Yamakoshi, Y.; Kawahara, H. Combined Effect of Midazolam and Bone Morphogenetic Protein-2 for Differentiation Induction from C2C12 Myoblast Cells to Osteoblasts. Pharmaceutics 2020, 12, 218. [Google Scholar] [CrossRef]
- Chen, M.; Cui, Y.; Li, H.; Luan, J.; Zhou, X.; Han, J. Icariin Promotes the Osteogenic Action of BMP2 by Activating the cAMP Signaling Pathway. Molecules 2019, 24, 3875. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.-J.; Cao, L.-G.; Wu, T.; Wang, D.-X.; Jin, D.; Jiang, S.; Zhang, Z.-Y.; Bi, L.; Pei, G.-X. The dose-effect of icariin on the proliferation and osteogenic differentiation of human bone mesenchymal stem cells. Molecules 2011, 16, 10123–10133. [Google Scholar] [CrossRef]
- Luo, G.; Xu, B.; Wang, W.; Wu, Y.; Li, M. Study of the osteogenesis effect of icariside II and icaritin on canine bone marrow mesenchymal stem cells. J. Bone Miner. Metab. 2018, 36, 668–678. [Google Scholar] [CrossRef]
- Zhao, J.; Ohba, S.; Shinkai, M.; Chung, U.-I.; Nagamune, T. Icariin induces osteogenic differentiation in vitro in a BMP- and Runx2-dependent manner. Biochem. Biophys. Res. Commun. 2008, 369, 444–448. [Google Scholar] [CrossRef]
- Zhang, D.; Fong, C.; Jia, Z.; Cui, L.; Yao, X.; Yang, M. Icariin Stimulates Differentiation and Suppresses Adipocytic Transdifferentiation of Primary Osteoblasts Through Estrogen Receptor-Mediated Pathway. Calcif. Tissue Int. 2016, 99, 187–198. [Google Scholar] [CrossRef]
- Wu, Z.; Ou, L.; Wang, C.; Yang, L.; Wang, P.; Liu, H.; Xiong, Y.; Sun, K.; Zhang, R.; Zhu, X. Icaritin induces MC3T3-E1 subclone14 cell differentiation through estrogen receptor-mediated ERK1/2 and p38 signaling activation. Biomed. Pharmacother. 2017, 94, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-Q.; Yang, Q.-X.; Cheng, M.-C.; Xiao, H.-B. Synergistic inhibitory effect of Icariside II with Icaritin from Herba Epimedii on pre-osteoclastic RAW264.7 cell growth. Phytomedicine 2014, 21, 1633–1637. [Google Scholar] [CrossRef]
- Yong, E.-L.; Cheong, W.F.; Huang, Z.; Thu, W.P.P.; Cazenave-Gassiot, A.; Seng, K.Y.; Logan, S. Randomized, double-blind, placebo-controlled trial to examine the safety, pharmacokinetics and effects of Epimedium prenylflavonoids, on bone specific alkaline phosphatase and the osteoclast adaptor protein TRAF6 in post-menopausal women. Phytomedicine 2021, 91, 153680. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, X.; Li, K.-F.; Li, D.-X.; Xiao, Y.-M.; Fan, Y.-J.; Zhang, X.-D. Icariin promotes extracellular matrix synthesis and gene expression of chondrocytes in vitro. Phytother. Res. 2012, 26, 1385–1392. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Yuan, T.; Zhang, X.; Xiao, Y.; Wang, R.; Fan, Y.; Zhang, X. Icariin: A potential promoting compound for cartilage tissue engineering. Osteoarthr. Cartil. 2012, 20, 1647–1656. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.; He, J.; Zheng, H.; Chen, C.; Lan, S. Icariin Prevents Diabetes-Induced Bone Loss in Rats by Reducing Blood Glucose and Suppressing Bone Turnover. Molecules 2019, 24, 1871. [Google Scholar] [CrossRef]
- Li, G.-W.; Xu, Z.; Chang, S.-X.; Nian, H.; Wang, X.-Y.; Qin, L.-D. Icariin prevents ovariectomy-induced bone loss and lowers marrow adipogenesis. Menopause 2014, 21, 1007–1016. [Google Scholar] [CrossRef]
- Feng, R.; Feng, L.; Yuan, Z.; Wang, D.; Wang, F.; Tan, B.; Han, S.; Li, T.; Li, D.; Han, Y. Icariin protects against glucocorticoid-induced osteoporosis in vitro and prevents glucocorticoid-induced osteocyte apoptosis in vivo. Cell Biochem. Biophys. 2013, 67, 189–197. [Google Scholar] [CrossRef]
- Li, X.-F.; Xu, H.; Zhao, Y.-J.; Tang, D.-Z.; Xu, G.-H.; Holz, J.; Wang, J.; Cheng, S.-D.; Shi, Q.; Wang, Y.-J. Icariin Augments Bone Formation and Reverses the Phenotypes of Osteoprotegerin-Deficient Mice through the Activation of Wnt/β-Catenin-BMP Signaling. Evid. Based Complement Alternat. Med. 2013, 2013, 652317. [Google Scholar] [CrossRef]
- Izawa, I.; Tamaki, N.; Saya, H. Phosphorylation of neurofibromatosis type 1 gene product (neurofibromin) by cAMP-dependent protein kinase. FEBS Lett. 1996, 382, 53–59. [Google Scholar] [CrossRef]
- The, I.; Hannigan, G.E.; Cowley, G.S.; Reginald, S.; Zhong, Y.; Gusella, J.F.; Hariharan, I.K.; Bernards, A. Rescue of a Drosophila NF1 mutant phenotype by protein kinase A. Science 1997, 276, 791–794. [Google Scholar] [CrossRef]
- Siddappa, R.; Martens, A.; Doorn, J.; Leusink, A.; Olivo, C.; Licht, R.; van Rijn, L.; Gaspar, C.; Fodde, R.; Janssen, F.; et al. cAMP/PKA pathway activation in human mesenchymal stem cells in vitro results in robust bone formation in vivo. Proc. Natl. Acad. Sci. USA 2008, 105, 7281–7286. [Google Scholar] [CrossRef]
- Chen, B.; Lin, T.; Yang, X.; Li, Y.; Xie, D.; Cui, H. Intermittent parathyroid hormone (1-34) application regulates cAMP-response element binding protein activity to promote the proliferation and osteogenic differentiation of bone mesenchymal stromal cells, via the cAMP/PKA signaling pathway. Exp. Ther. Med. 2016, 11, 2399–2406. [Google Scholar] [CrossRef] [PubMed]
- Nakao, Y.; Koike, T.; Ohta, Y.; Manaka, T.; Imai, Y.; Takaoka, K. Parathyroid hormone enhances bone morphogenetic protein activity by increasing intracellular 3′,5′-cyclic adenosine monophosphate accumulation in osteoblastic MC3T3-E1 cells. Bone 2009, 44, 872–877. [Google Scholar] [CrossRef]
- Gupta, A.; Anderson, H.; Buo, A.M.; Moorer, M.C.; Ren, M.; Stains, J.P. Communication of cAMP by connexin43 gap junctions regulates osteoblast signaling and gene expression. Cell Signal 2016, 28, 1048–1057. [Google Scholar] [CrossRef]
- Doorn, J.; Siddappa, R.; van Blitterswijk, C.A.; de Boer, J. Forskolin enhances in vivo bone formation by human mesenchymal stromal cells. Tissue Eng. Part A 2012, 18, 558–567. [Google Scholar] [CrossRef]
- Tsutsumimoto, T.; Wakabayashi, S.; Kinoshita, T.; Horiuchi, H.; Takaoka, K. A phosphodiesterase inhibitor, pentoxifylline, enhances the bone morphogenetic protein-4 (BMP-4)-dependent differentiation of osteoprogenitor cells. Bone 2002, 31, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.H.; Duong, P.; Moran, J.J.; Junaidi, N.; Svaren, J. Polycomb repression regulates Schwann cell proliferation and axon regeneration after nerve injury. Glia 2018, 66, 2487–2502. [Google Scholar] [CrossRef]
- Stornetta, R.L.; Zhu, J.J. Ras and Rap signaling in synaptic plasticity and mental disorders. Neuroscientist 2011, 17, 54–78. [Google Scholar] [CrossRef]
- Dasgupta, B.; Dugan, L.L.; Gutmann, D.H. The neurofibromatosis 1 gene product neurofibromin regulates pituitary adenylate cyclase-activating polypeptide-mediated signaling in astrocytes. J. Neurosci. 2003, 23, 8949–8954. [Google Scholar] [CrossRef] [PubMed]



| Gene Id | Gene Name | MeanTPM (2−7 ICA) | MeanTPM (2−7 C) | log2FoldChange | p Value | q Value | Result |
|---|---|---|---|---|---|---|---|
| ENSMUSG00000101859 | Gm29233 | 7.683309 | 0.0001 | 16.22944016 | 4.74 × 10−9 | 2.18 × 10−8 | up |
| ENSMUSG00000023078 | Cxcl13 | 7.085217333 | 0.0001 | 16.11252449 | 1.21 × 10−14 | 8.37 × 10−14 | up |
| ENSMUSG00000055874 | Foxi3 | 6.177744333 | 0.0001 | 15.91479255 | 1.27 × 10−14 | 8.73 × 1014 | up |
| ENSMUSG00000048368 | Omd | 6.120898667 | 0.0001 | 15.90145586 | 5.23 × 10−16 | 3.92 × 10−15 | up |
| ENSMUSG00000036960 | Clca2 | 5.547391 | 0.0001 | 15.75952179 | 5.21 × 10−18 | 4.34 × 10−17 | up |
| ENSMUSG00000084932 | Gm15156 | 4.823441667 | 0.0001 | 15.5577753 | 1.21 × 10−8 | 5.36 × 10−08 | up |
| ENSMUSG00000077285 | Gm26394 | 3.750423 | 0.0001 | 15.1947657 | 0.00138128 | 0.003356227 | up |
| ENSMUSG00000098698 | Mir6960 | 3.651845 | 0.0001 | 15.15633791 | 0.02583538 | 0.048832188 | up |
| ENSMUSG00000062773 | Tex101 | 3.636343 | 0.0001 | 15.15020067 | 1.55 × 10−11 | 8.75 × 10−11 | up |
| ENSMUSG00000041552 | Ptchd1 | 3.509552667 | 0.0001 | 15.09899953 | 1.68 × 10−14 | 1.15 × 10−13 | up |
| ENSMUSG00000000730 | Dnmt3l | 0.0001 | 11.46687133 | −16.80711229 | 8.03 × 10−18 | 6.61 × 10−17 | down |
| ENSMUSG00000078302 | Foxd1 | 0.0001 | 4.958846333 | −15.5977169 | 1.45 × 10−18 | 1.25 × 10−17 | down |
| ENSMUSG00000036938 | Try5 | 0.0001 | 3.806281333 | −15.21609458 | 2.33 × 10−12 | 1.38 × 10−11 | down |
| ENSMUSG00000112148 | Lilrb4a | 0.0001 | 3.492889 | −15.09213318 | 4.60 × 10−15 | 3.24 × 10−14 | down |
| ENSMUSG00000000732 | Icosl | 0.0001 | 3.242253 | −14.98470905 | 5.86 × 10−17 | 4.64 × 10−16 | down |
| ENSMUSG00000035513 | Ntng2 | 0.0001 | 3.095218333 | −14.91775356 | 3.13 × 10−14 | 2.10 × 10−13 | down |
| ENSMUSG00000021464 | Ror2 | 0.0001 | 2.735676667 | −14.73961011 | 1.46 × 10−18 | 1.26 × 10−17 | down |
| ENSMUSG00000031548 | Sfrp1 | 0.0001 | 2.401819667 | −14.55184021 | 3.84 × 10−18 | 3.22 × 10−17 | down |
| ENSMUSG00000050578 | Mmp13 | 0.0001 | 2.337899333 | −14.51292519 | 1.01 × 10−15 | 7.45 × 10−15 | down |
| ENSMUSG00000040867 | Begain | 0.0001 | 2.319114333 | −14.50128633 | 3.46 × 10−15 | 2.46 × 10−14 | down |
| Name | Sequence |
|---|---|
| NF1 | 3′→5′: GGGTCGGGCTTCAATGGTAA 5′→3′: TCCCACATGCTTTAGGCACT |
| Name | Sequence |
|---|---|
| NF1 | 3′→5′: TTCGGATAAGCCCTCACAACAAC 5′→3′: CAGCATCAATCTTAGGCCACCA |
| Alp | 3′→5′: GGCTCTGCCTTTATTCCCTAGT 5′→3′: AAATAAGGTGCTTTGGGAATCTGT |
| Runx2 | 3′→5′: GCCGGGAATGATGAGAACTA 5′→3′: GGTGAAACTCTTGCCTCGTC |
| Osx | 3′→5′: AGGCCTTTGCCAGTGCCTA 5′→3′:GCCAGATGGAAGCTGTGAAGA |
| Col1a1 | 3′→5′: GACATGTTCAGCTTTGTGGACCTC 5′→3′:GGGACCCTTAGGCCATTGTGTA |
| Gapdh | 3′→5′: CATCCCAGAGCTGAACG 5′→3′: CTGGTCCTCAGTGTAGCC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, M.; Lu, L.; Cheng, D.; Zhang, J.; Liu, X.; Zhang, J.; Zhang, T. Icariin Promotes Osteogenic Differentiation in a Cell Model with NF1 Gene Knockout by Activating the cAMP/PKA/CREB Pathway. Molecules 2023, 28, 5128. https://doi.org/10.3390/molecules28135128
Chen M, Lu L, Cheng D, Zhang J, Liu X, Zhang J, Zhang T. Icariin Promotes Osteogenic Differentiation in a Cell Model with NF1 Gene Knockout by Activating the cAMP/PKA/CREB Pathway. Molecules. 2023; 28(13):5128. https://doi.org/10.3390/molecules28135128
Chicago/Turabian StyleChen, Meng, Lianhua Lu, Dong Cheng, Jing Zhang, Xinyong Liu, Jianli Zhang, and Tianliang Zhang. 2023. "Icariin Promotes Osteogenic Differentiation in a Cell Model with NF1 Gene Knockout by Activating the cAMP/PKA/CREB Pathway" Molecules 28, no. 13: 5128. https://doi.org/10.3390/molecules28135128
APA StyleChen, M., Lu, L., Cheng, D., Zhang, J., Liu, X., Zhang, J., & Zhang, T. (2023). Icariin Promotes Osteogenic Differentiation in a Cell Model with NF1 Gene Knockout by Activating the cAMP/PKA/CREB Pathway. Molecules, 28(13), 5128. https://doi.org/10.3390/molecules28135128






