The Impact of Copper Ions on the Activity of Antibiotic Drugs
Abstract
:1. Introduction
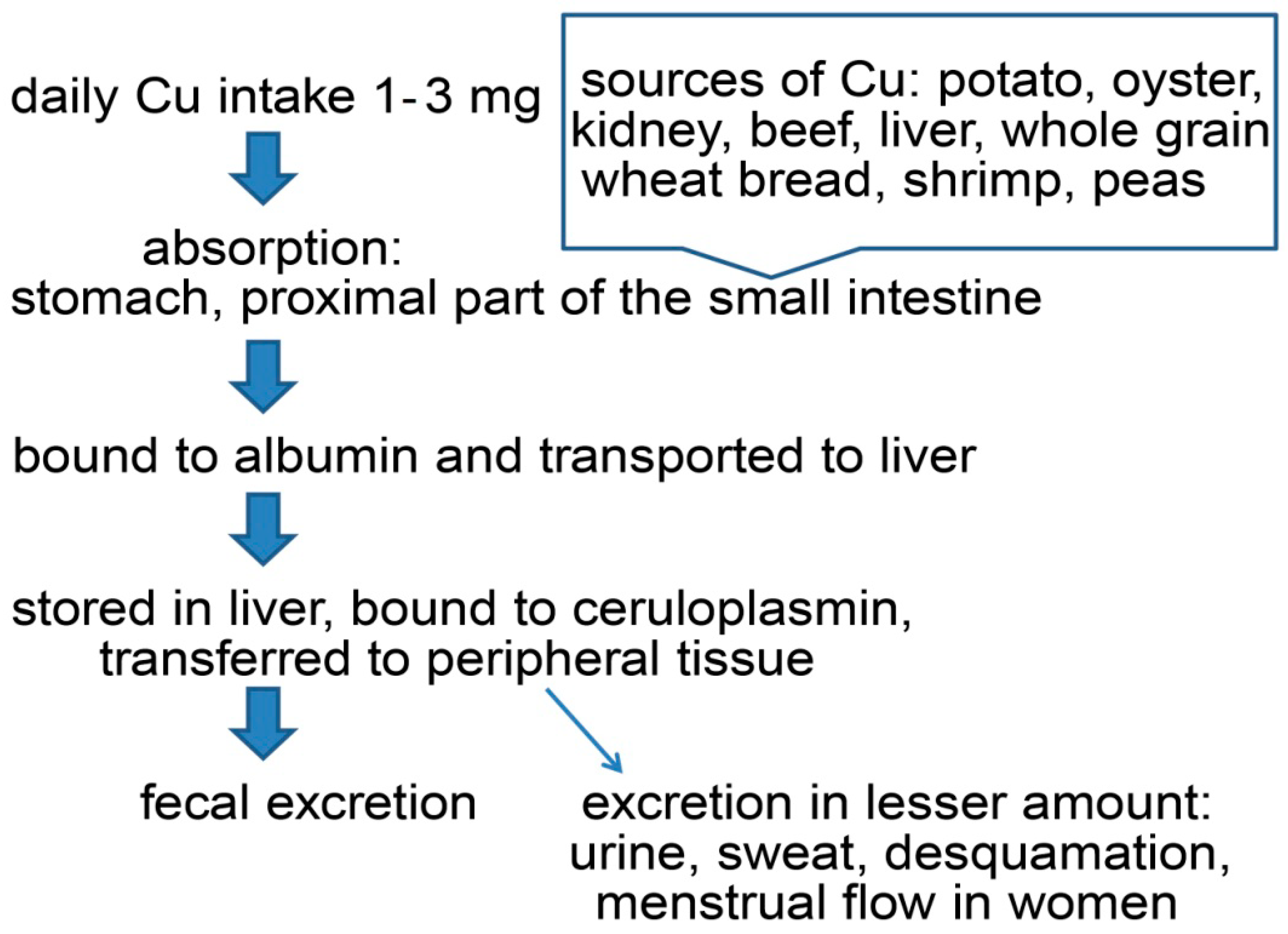
2. Copper’s Role in the Human Organism
Copper Pathway in Human Organism
3. Copper as an Antimicrobial Agent
The Effects of Elevated Copper on Bacteria
4. Copper-Dependent Compounds
Mechanism of Cu-Dependent Compounds
5. Copper Interactions with Antibiotics
5.1. Copper Interactions with Penicillins and Cephalosporins
5.2. Copper Interactions with Carbapenems
5.3. Copper Interactions with Tetracyclines
5.4. Copper Interactions with Fluoroquinolones
5.5. Copper Interactions with Aminoglycosides
5.6. Copper Interactions with Other Antibiotics and Antibiotic Groups
6. The Effects of Copper on Bacterial Resistance
7. Future Perspectives
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Dalecki, A.G.; Crawford, C.L.; Wolschendorf, F. Copper and Antibiotics: Discovery, Modes of Action, and Opportunities for Medicinal Applications. Adv. Microb. Physiol. 2017, 70, 193–260. [Google Scholar] [PubMed]
- Lewis, K. Platforms for antibiotic discovery. Nat. Rev. Drug Discov. 2013, 12, 371–387. [Google Scholar] [CrossRef] [PubMed]
- Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Crawford, C.L.; Dalecki, A.G.; Perez, M.D.; Schaaf, K.; Wolschendorf, F.; Kutsch, O. A copper-dependent compound restores ampicillin sensitivity in multidrug-resistant Staphylococcus aureus. Sci. Rep. 2020, 10, 8955. [Google Scholar] [CrossRef] [PubMed]
- Božić, B.; Korać, J.; Stanković, D.M.; Stanić, M.; Romanović, M.; Pristov, J.B.; Spasić, S.; Popović-Bijelić, A.; Spasojević, I.; Bajčetić, M. Coordination and redox interactions of β-lactam antibiotics with Cu2+ in physiological settings and the impact on antibacterial activity. Free Radic. Biol. Med. 2018, 129, 279–285. [Google Scholar] [CrossRef] [Green Version]
- Ince, A.T.; Kayadibi, H.; Soylu, A.; Ovunç, O.; Gültepe, M.; Toros, A.B.; Yaşar, B.; Kendir, T.; Abut, E. Serum copper, ceruloplasmin and 24-h urine copper evaluations in celiac patients. Dig. Dis. Sci. 2008, 53, 1564–1572. [Google Scholar] [CrossRef]
- Iakovidis, I.; Delimaris, I.; Piperakis, S.M. Copper and Its Complexes in Medicine: A Biochemical Approach. Mol. Biol. Int. 2011, 2011, 594529. [Google Scholar] [CrossRef] [Green Version]
- Twomey, P.J.; Reynolds, T.M.; Wierzbicki, A.S.; Viljoen, A. The relationship between serum copper and ceruloplasmin in routine clinical practice. Int. J. Clin. Pract. 2008, 62, 485–487. [Google Scholar] [CrossRef]
- Schulpis, K.H.; Karakonstantakis, T.; Gavrili, S.; Costalos, C.; Romac, E.; Papassotiriou, I. Serum copper is decreased in premature newborns and increased in newborns with hemolytic jaundice. Clin. Chem. 2004, 50, 1253–1256. [Google Scholar] [CrossRef]
- Yoshikawa, S.; Muramoto, K.; Shinzawa-Itoh, K. Reaction mechanism of mammalian mitochondrial cytochrome c oxidase. Adv. Exp. Med. Biol. 2012, 748, 215–236. [Google Scholar]
- Smith-Mungo, L.I.; Kagan, H.M. Lysyl oxidase: Properties, regulation and multiple functions in biology. Matrix Biol. 1998, 16, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Valentine, J.S.; Doucette, P.A.; Zittin Potter, S. Copper-zinc superoxide dismutase and amyotrophic lateral sclerosis. Annu. Rev. Biochem. 2005, 74, 563–593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shepard, E.M.; Dooley, D.M. Inhibition and oxygen activation in copper amine oxidases. Acc. Chem. Res. 2015, 48, 1218–1226. [Google Scholar] [CrossRef] [PubMed]
- Akyilmaz, E.; Yorganci, E.; Asav, E. Do copper ions activate tyrosinase enzyme? A biosensor model for the solution. Bioelectrochemistry 2010, 78, 155–160. [Google Scholar] [CrossRef]
- Lowe, J.; Taveira-da-Silva, R.; Hilário-Souza, E. Dissecting copper homeostasis in diabetes mellitus. IUBMB Life 2017, 69, 255–262. [Google Scholar] [CrossRef] [Green Version]
- Moraes, M.L.; Ramalho, D.M.; Delogo, K.N.; Miranda, P.F.; Mesquita, E.D.; de Melo Guedes de Oliveira, H.M.; Netto, A.R.; Dos Anjos, M.J.; Kritski, A.L.; de Oliveira, M.M. Association of serum levels of iron, copper, and zink, and inflammatory markers with bacteriological sputum conversion during tuberculosis treatment. Biol. Trace Elem. Res. 2014, 160, 176–184. [Google Scholar] [CrossRef]
- Bost, M.; Houdart, S.; Oberli, M.; Kalonji, E.; Huneau, J.F.; Margaritis, I. Dietary copper and human health: Current evidence and unresolved issues. J. Trace Elem. Med. Biol. 2016, 35, 107–115. [Google Scholar] [CrossRef]
- Georgieff, M.K. Nutrition and the developing brain: Nutrient priorities and measurement. Am. J. Clin. Nutr. 2007, 85, 614S–620S. [Google Scholar]
- Pourahmad, J.; O’Brien, P.J. A comparison of hepatocyte cytotoxic mechanisms for Cu2+ and Cd2+. Toxicology 2000, 143, 263–273. [Google Scholar] [CrossRef]
- Bonda, D.J.; Liu, G.; Men, P.; Perry, G.; Smith, M.A.; Zhu, X. Nanoparticle Delivery of Transition-Metal Chelators to the Brain: Oxidative Stress will Never See it Coming! CNS Neurol Disord Drug Targets. CNS Neurol. Disord. Drug Targets 2012, 11, 81–85. [Google Scholar] [CrossRef]
- Viktorinova, A. Current insights on the role of iron and copper dyshomeostasis in the pathogenesis of bilirubin neurotoxicity. Life Sci. 2017, 191, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Grass, G.; Rensing, C.; Solioz, M. Metallic copper as an antimicrobial surface. Appl. Environ. Microbiol. 2011, 77, 1541–1547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemire, J.A.; Harrison, J.J.; Turner, R.J. Antimicrobial activity of metals: Mechanisms, molecular targets and applications. Nat. Rev. Microbiol. 2013, 11, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Mozafari, M.R.; Sarabanou, T.; Karamouzian, F.M.; Fatemeh, M.; Babak, R.; Bikash, B. Antimicrobial Applications of Nanoliposome Encapsulated Silver Nanoparticles: A Potential Strategy to Overcome Bacterial Resistance. Curr. Nanosci. 2021, 17, 26–40. [Google Scholar] [CrossRef]
- Usman, M.S.; El Zowalaty, M.E.; Shameli, K.; Zainuddin, N.; Salama, M.; Ibrahim, N.A. Synthesis, characterization, and antimicrobial properties of copper nanoparticles. Int. J. Nanomed. 2013, 8, 4467–4479. [Google Scholar]
- Gaetke, L.M.; Chow, C.K. Copper toxicity, oxidative stress, and antioxidant nutrients. Toxicology 2003, 189, 147–163. [Google Scholar] [CrossRef]
- Teitzel, G.M.; Geddie, A.; De Long, S.K.; Kirisits, M.J.; Whiteley, M.; Parsek, M.R. Survival and growth in the presence of elevated copper: Transcriptional profiling of copper-stressed Pseudomonas aeruginosa. J. Bacteriol. 2006, 188, 7242–7256. [Google Scholar] [CrossRef] [Green Version]
- Buettner, G.R. The pecking order of free radicals and antioxidants: Lipid peroxidation, alpha-tocopherol, and ascorbate. Arch. Biochem. Biophys. 1993, 300, 535–543. [Google Scholar] [CrossRef]
- Harrison, J.J.; Tremaroli, V.; Stan, M.A.; Chan, C.S.; Vacchi-Suzzi, C.; Heyne, B.J.; Parsek, M.R.; Ceri, H.; Turner, R.J. Chromosomal antioxidant genes have metal ion-specific roles as determinants of bacterial metal tolerance. Environ. Microbiol. 2009, 11, 2491–2509. [Google Scholar] [CrossRef]
- Macomber, L.; Imlay, J.A. The iron-sulfur clusters of dehydratases are primary intracellular targets of copper toxicity. Proc. Natl. Acad. Sci. USA 2009, 106, 8344–8349. [Google Scholar] [CrossRef] [Green Version]
- Vincent, M.; Duval, R.E.; Hartemann, P.; Engels-Deutsch, M. Contact killing and antimicrobial properties of copper. J. Appl. Microbiol. 2018, 124, 1032–1046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Djoko, K.Y.; Goytia, M.M.; Donnelly, P.S.; Schembri, M.A.; Shafer, W.M.; McEwan, A.G. Copper(II)-Bis(Thiosemicarbazonato) Complexes as Antibacterial Agents: Insights into Their Mode of Action and Potential as Therapeutics. Antimicrob. Agents Chemother. 2015, 59, 6444–6453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haeili, M.; Moore, C.; Davis, C.J.; Cochran, J.B.; Shah, S.; Shrestha, T.B.; Zhang, Y.; Bossmann, S.H.; Benjamin, W.H.; Kutsch, O.; et al. Copper complexation screen reveals compounds with potent antibiotic properties against methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2014, 58, 3727–3736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalecki, A.G.; Haeili, M.; Shah, S.; Speer, A.; Niederweis, M.; Kutsch, O.; Wolschendorf, F. Disulfiram and Copper Ions Kill Mycobacterium tuberculosis in a Synergistic Manner. Antimicrob. Agents Chemother. 2015, 59, 4835–4844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, S.; Dalecki, A.G.; Malalasekera, A.P.; Crawford, C.L.; Michalek, S.M.; Kutsch, O.; Sun, J.; Bossmann, S.H.; Wolschendorf, F. 8-Hydroxyquinolines Are Boosting Agents of Copper-Related Toxicity in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2016, 60, 5765–5776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ventura, R.F.; Galdino, A.C.M.; Viganor, L.; Schuenck, R.P.; Devereux, M.; McCann, M.; Santos, A.L.S.; Nunes, A.P.F. Antimicrobial action of 1,10-phenanthroline-based compounds on carbapenemase-producing Acinetobacter baumannii clinical strains: Efficacy against planktonic- and biofilm-growing cells. Braz. J. Microbiol. 2020, 51, 1703–1710. [Google Scholar] [CrossRef]
- Crawford, C.L.; Dalecki, A.G.; Naramore, W.T.; Hoff, J.; Hargett, A.A.; Renfrow, M.B.; Zhang, M.; Kalubowilage, M.; Bossmann, S.H.; Queern, S.L.; et al. Pyrazolopyrimidinones, a novel class of copper-dependent bacterial antibiotics against multi-drug resistant S. aureus. Metallomics 2019, 11, 784–798. [Google Scholar] [CrossRef]
- Chiem, K.; Fuentes, B.A.; Lin, D.L.; Tran, T.; Jackson, A.; Ramirez, M.S.; Tolmasky, M.E. Inhibition of aminoglycoside 6′-N-acetyltransferase type Ib-mediated amikacin resistance in Klebsiella pneumoniae by zinc and copper pyrithione. Antimicrob. Agents Chemother. 2015, 59, 5851–5853. [Google Scholar] [CrossRef] [Green Version]
- Djoko, K.Y.; Paterson, B.M.; Donnelly, P.S.; McEwan, A.G. Antimicrobial effects of copper(II) bis(thiosemicarbazonato) complexes provide new insight into their biochemical mode of action. Metallomics 2014, 6, 854–863. [Google Scholar] [CrossRef] [Green Version]
- Reeder, N.L.; Kaplan, J.; Xu, J.; Youngquist, R.S.; Wallace, J.; Hu, P.; Juhlin, K.D.; Schwartz, J.R.; Grant, R.A.; Fieno, A.; et al. Zinc pyrithione inhibits yeast growth through copper influx and inactivation of iron-sulfur proteins. Antimicrob. Agents Chemother. 2011, 55, 5753–5760. [Google Scholar] [CrossRef] [Green Version]
- Ng, N.S.; Leverett, P.; Hibbs, D.E.; Yang, Q.; Bulanadi, J.C.; Wu, M.J.; Aldrich-Wright, J.R. The antimicrobial properties of some copper(II) and platinum(II) 1,10-phenanthroline complexes. Dalton Trans. 2013, 42, 3196–3209. [Google Scholar] [CrossRef] [PubMed]
- Galdino, A.C.M.; Viganor, L.; Pereira, M.M.; Devereux, M.; McCann, M.; Branquinha, M.H.; Molphy, Z.; O’Carroll, S.; Bain, C.; Menounou, G.; et al. Copper(II) and silver(I)-1,10-phenanthroline-5,6-dione complexes interact with double-stranded DNA: Further evidence of their apparent multi-modal activity towards Pseudomonas aeruginosa. J. Biol. Inorg. Chem. 2022, 27, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Deegan, C.; Coyle, B.; McCann, M.; Devereux, M.; Egan, D.A. In vitro anti-tumour effect of 1,10-phenanthroline-5,6-dione (phendione), [Cu(phendione)3](ClO4)2.4H2O and [Ag(phendione)2]ClO4 using human epithelial cell lines. Chem. Biol. Interact. 2006, 164, 115–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baym, M.; Stone, L.K.; Kishony, R. Multidrug evolutionary strategies to reverse antibiotic resistance. Science 2016, 351, aad3292. [Google Scholar] [CrossRef] [Green Version]
- Lima, L.M.; Silva, B.N.M.D.; Barbosa, G.; Barreiro, E.J. β-lactam antibiotics: An overview from a medicinal chemistry perspective. Eur. J. Med. Chem. 2020, 208, 112829. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, M.; Verdino, A.; Soriente, A.; Marabotti, A. The Odd Couple(s): An Overview of Beta-Lactam Antibiotics Bearing More Than One Pharmacophoric Group. Int. J. Mol. Sci. 2021, 22, 617. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.L. The penicillins: A review and update. J. Midwifery Womens Health. 2002, 47, 426–434. [Google Scholar] [CrossRef]
- Kong, K.F.; Schneper, L.; Mathee, K. Beta-lactam antibiotics: From antibiosis to resistance and bacteriology. APMIS 2010, 118, 1–36. [Google Scholar] [CrossRef] [Green Version]
- Abraham, E.P.; Newton, G.G.F. The structure of cephalosporin C. Biochem. J. 1961, 79, 377–393. [Google Scholar] [CrossRef] [Green Version]
- Möhler, J.S.; Kolmar, T.; Synnatschke, K.; Hergert, M.; Wilson, L.A.; Ramu, S.; Elliott, A.G.; Blaskovich, M.A.; Sidjabat, H.E.; Paterson, D.L.; et al. Enhancement of antibiotic-activity through complexation with metal ions—Combined ITC, NMR, enzymatic and biological studies. J. Inorg. Biochem. 2017, 167, 134–141. [Google Scholar] [CrossRef] [Green Version]
- Cressman, W.A.; Sugita, E.T.; Doluisio, J.T.; Niebergall, P.J. Complexation of penicillins and penicilloic acids by cupric ion. J. Pharm. Pharmacol. 1966, 18, 801–808. [Google Scholar] [CrossRef] [PubMed]
- Niebergall, P.J.; Hussar, D.A.; Cressman, W.A.; Sugita, E.T.; Doluisio, J.T. Metal binding tendencies of various antibiotics. J. Pharm. Pharmacol. 1966, 18, 729–738. [Google Scholar] [CrossRef] [PubMed]
- Lapshin, S.V.; Alekseev, V.G. Copper(II) complexation with ampicillin, amoxicillin, and cephalexin. Russ. J. Inorg. Chem. 2009, 54, 1066–1069. [Google Scholar] [CrossRef]
- Guo, Y.; Tsang, D.C.W.; Zhang, X.; Yang, X. Cu(II)-catalyzed degradation of ampicillin: Effect of pH and dissolved oxygen. Environ. Sci. Pollut. Res. Int. 2018, 25, 4279–4288. [Google Scholar] [CrossRef]
- Chohan, Z.H.; Supuran, C.T.; Scozzafava, A. Metalloantibiotics: Synthesis and antibacterial activity of cobalt(II), copper(II), nickel(II) and zinc(II) complexes of kefzol. J. Enzyme Inhib. Med. Chem. 2004, 19, 79–84. [Google Scholar] [CrossRef] [Green Version]
- El-Gamel, N.E.A. Metal chelates of ampicillin versus amoxicillin: Synthesis, structural investigation, and biological studies. J. Coord. Chem. 2010, 63, 534–543. [Google Scholar] [CrossRef]
- Auda, S.H.; Mrestani, Y.; Fetouh, M.I.; Neubert, R.H. Characterization and activity of cephalosporin metal complexes. Pharmazie 2008, 63, 555–561. [Google Scholar]
- Anacona, J.R.; Rodrigues, I. Synthesis and antibacterial activity of cephalexin metal complexes. J. Coord. Chem. 2004, 57, 1263–1269. [Google Scholar] [CrossRef]
- Anacona, J.R.; Acosta, F. Synthesis and antibacterial activity of cephradine metal complexes. J. Coord. Chem. 2005, 59, 621–627. [Google Scholar] [CrossRef]
- Anacona, J.R.; Osorio, I. Synthesis and antibacterial activity of copper(II) complexes with sulphathiazole and cephalosporin ligands. Transit. Met. Chem. 2008, 33, 517–521. [Google Scholar] [CrossRef]
- Ali, A.E. Synthesis, spectral, thermal and antimicrobial studies of some new tri metallic biologically active ceftriaxone complexes. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2011, 78, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Auda, S.H.; Knütter, I.; Bretschneider, B.; Brandsch, M.; Mrestani, Y.; Große, C.; Neubert, R.H. Effect of Different Metal Ions on the Biological Properties of Cefadroxil. Pharmaceuticals 2009, 2, 184–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, B.K.; Bhojak, N.; Mishra, P.; Garg, B.S. Copper(II) complexes with bioactive carboxyamide: Synthesis, characterization and biological activity. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2008, 70, 758–765. [Google Scholar] [CrossRef] [PubMed]
- Chohan, Z.H.; Pervez, H.; Khan, K.M.; Rauf, A.; Supuran, C.T. Binding of Transition Metal Ions [Cobalt, Copper, Nickel and Zinc] with Furanyl-, Thiophenyl-, Pyrrolyl-, Salicylyland Pyridyl-Derived Cephalexins as Potent Antibacterial Agents. J. Enzyme Inhib. Med. Chem. 2004, 19, 51–56. [Google Scholar] [CrossRef]
- Djoko, K.Y.; Achard, M.E.S.; Phan, M.D.; Lo, A.W.; Miraula, M.; Prombhul, S.; Hancock, S.J.; Peters, K.M.; Sidjabat, H.E.; Harris, P.N.; et al. Copper Ions and Coordination Complexes as Novel Carbapenem Adjuvants. Antimicrob. Agents Chemother. 2018, 62, e02280-17. [Google Scholar] [CrossRef] [Green Version]
- Aurilio, C.; Sansone, P.; Barbarisi, M.; Pota, V.; Giaccari, L.G.; Coppolino, F.; Barbarisi, A.; Passavanti, M.B.; Pace, M.C. Mechanisms of Action of Carbapenem Resistance. Antibiotics 2022, 11, 421. [Google Scholar] [CrossRef]
- Zhao, Y.; Tan, Y.; Guo, Y.; Gu, X.; Wang, X.; Zhang, Y. Interactions of tetracycline with Cd (II), Cu (II) and Pb (II) and their cosorption behavior in soils. Environ. Pollut. 2013, 180, 206–213. [Google Scholar] [CrossRef]
- Chopra, I.; Roberts, M. Tetracycline antibiotics: Mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol. Mol. Biol. Rev. 2001, 65, 232–260. [Google Scholar] [CrossRef] [Green Version]
- Poole, K. At the Nexus of Antibiotics and Metals: The Impact of Cu and Zn on Antibiotic Activity and Resistance. Trends Microbiol. 2017, 25, 820–832. [Google Scholar] [CrossRef]
- Tong, F.; Zhao, Y.; Gu, X.; Gu, C.; Lee, C.C. Joint toxicity of tetracycline with copper(II) and cadmium(II) to Vibrio fischeri: Effect of complexation reaction. Ecotoxicology 2015, 24, 346–355. [Google Scholar] [CrossRef]
- Feio, M.J.; Sousa, I.; Ferreira, M.; Cunha-Silva, L.; Saraiva, R.G.; Queirós, C.; Alexandre, J.G.; Claro, V.; Mendes, A.; Ortiz, R.; et al. Fluoroquinolone-metal complexes: A route to counteract bacterial resistance? J. Inorg. Biochem. 2014, 138, 129–143. [Google Scholar] [CrossRef] [PubMed]
- Sousa, I.; Claro, V.; Pereira, J.L.; Amaral, A.L.; Cunha-Silva, L.; de Castro, B.; Feio, M.J.; Pereira, E.; Gameiro, P. Synthesis, characterization and antibacterial studies of a copper(II) levofloxacin ternary complex. J. Inorg. Biochem. 2012, 110, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, R.; Lopes, S.; Ferreira, M.; Novais, F.; Pereira, E.; Feio, M.J.; Gameiro, P. Solution and biological behaviour of enrofloxacin metalloantibiotics: A route to counteract bacterial resistance? J. Inorg. Biochem. 2010, 104, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, P.; Sousa, I.; Cunha-Silva, L.; Ferreira, M.; de Castro, B.; Pereira, E.F.; Feio, M.J.; Gameiro, P. Synthesis, characterization and antibacterial studies of a copper(II) lomefloxacin ternary complex. J. Inorg. Biochem. 2014, 131, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Efthimiadou, E.K.; Katsarou, M.E.; Karaliota, A.; Psomas, G. Copper(II) complexes with sparfloxacin and nitrogen-donor heterocyclic ligands: Structure-activity relationship. J. Inorg. Biochem. 2008, 102, 910–920. [Google Scholar] [CrossRef]
- Patel, M.N.; PArmar, P.A.; Gandhi, D.S. Square pyramidal copper(II) complexes with forth generation fluoroquinolone and neutral bidentate ligand: Structure, antibacterial, SOD mimic and DNA-interaction studies. Bioorg. Med. Chem. 2010, 18, 1227–1235. [Google Scholar] [CrossRef]
- Kotra, L.P.; Haddad, J.; Mobashery, S. Aminoglycosides: Perspectives on mechanisms of action and resistance and strategies to counter resistance. Antimicrob. Agents Chemother. 2000, 44, 3249–3256. [Google Scholar] [CrossRef] [Green Version]
- Lesniak, W.; Harris, W.R.; Kravitz, J.Y.; Schacht, J.; Pecoraro, V.L. Solution chemistry of copper(II)-gentamicin complexes: Relevance to metal-related aminoglycoside toxicity. Inorg. Chem. 2003, 42, 1420–1429. [Google Scholar] [CrossRef]
- Toth, M.; Frase, H.; Antunes, N.T.; Smith, C.A.; Vakulenko, S.B. Crystal structure and kinetic mechanism of aminoglycoside phosphotransferase-2″-IVa. Protein Sci. 2010, 19, 1565–1576. [Google Scholar] [CrossRef] [Green Version]
- Szczepanik, W.; Kaczmarek, P.; Jezowska-Bojczuk, M. Oxidative activity of copper(II) complexes with aminoglycoside antibiotics as implication to the toxicity of these drugs. Bioinorg. Chem. Appl. 2004, 2, 55–68. [Google Scholar] [CrossRef] [Green Version]
- Song, B.B.; Sha, S.H.; Schacht, J. Iron chelators protect from aminoglycoside-induced cochleo- and vestibulo-toxicity. Free Radic. Biol. Med. 1998, 25, 189–195. [Google Scholar] [CrossRef]
- Szczepanik, W.; Dworniczek, E.; Ciesiołka, J.; Wrzesiński, J.; Skala, J.; Jezowska-Bojczuk, M. In vitro oxidative activity of cupric complexes of kanamycin A in comparison to in vivo bactericidal efficacy. J. Inorg. Biochem. 2003, 94, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Abu-el-wafa, S.M.; El-ries, M.A.; Abou-attia, F.M.; Issa, R.M. Coordination chemical studies of some polymeric transition metal complexes with neomycin and their biological activity uses. Indirect determination of neomycin by atomic absorption spectroscopy (AAS). Anal. Lett. 1989, 22, 2703–2716. [Google Scholar] [CrossRef]
- Miljkovic, V.; Arsic, B.; Bojanic, Z.; Nikolic, G.; Nikolic, L.; Kalicanin, B.; Savic, V. Interactions of metronidazole with other medicines: A brief review. Pharmazie 2014, 69, 571–577. [Google Scholar] [PubMed]
- Palmer, J.H.; Wub, J.S.; Upmacis, R.K. Coordination of metronidazole to Cu(II): Structural characterization of a mononuclear square-planar compound. J. Mol. Struct. 2015, 1091, 177–182. [Google Scholar] [CrossRef]
- Galván-Tejada, N.; Bernès, S.; Castillo-Blum, S.E.; Nöth, H.; Vicente, R.; Barba-Behrens, N. Supramolecular structures of metronidazole and its copper(II), cobalt(II) and zinc(II) coordination compounds. J. Inorg. Biochem. 2002, 91, 339–348. [Google Scholar] [CrossRef]
- Rafique, B.; Shafique, K.; Hamid, S.; Kalsoom, S.; Hashim, M.; Mirza, B.; Jafri, L.; Iqbal, M. Novel copper complexes of metronidazole and metronidazole benzoate: Synthesis, characterization, biological and computational studies. J. Biomol. Struct. Dyn. 2022, 40, 5446–5461. [Google Scholar] [CrossRef]
- Wijesekara, P.N.K.; Kumbukgolla, W.W.; Jayaweera, J.A.A.S.; Rawat, D. Review on Usage of Vancomycin in Livestock and Humans: Maintaining Its Efficacy, Prevention of Resistance and Alternative Therapy. Vet. Sci. 2017, 4, 6. [Google Scholar] [CrossRef] [Green Version]
- Swiatek, M.; Valensin, D.; Migliorini, C.; Gaggelli, E.; Valensin, G.; Jezowska-Bojczuk, M. Unusual binding ability of vancomycin towards Cu2+ ions. Dalton Trans. 2005, 23, 3808–3813. [Google Scholar] [CrossRef]
- Hanson, J.C.; Camerman, N.; Camerman, A. Structure of a copper-isoniazid complex. J. Med. Chem. 1981, 24, 1369–1371. [Google Scholar] [CrossRef]
- Sakurai, H.; Shimomura, S.; Ishizu, K. Green and purple copper (II)-chloramphenicol complexes in methanol: Evidence for the coordination of deprotonated amide nitrogen. J. Antibiot. 1980, 33, 458–459. [Google Scholar] [CrossRef] [PubMed]
- Jezowska-Bojczuk, M.; Lesniak, W.; Szczepanik, W.; Gatner, K.; Jezierski, A.; Smoluch, M.; Bal, W. Copper(II)-lincomycin: Complexation pattern and oxidative activity. J. Inorg. Biochem. 2001, 84, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Hamdan, I.I. Comparative in vitro investigations of the interaction between some macrolides and Cu(II), Zn(II) and Fe(II). Pharmazie 2003, 58, 223–224. [Google Scholar]
- Anedda, E.; Farrell, M.L.; Morris, D.; Burgess, C.M. Evaluating the impact of heavy metals on antimicrobial resistance in the primary food production environment: A scoping review. Environ. Pollut. 2023, 320, 121035. [Google Scholar] [CrossRef]
- Yamamoto, K.; Ishihama, A. Characterization of copper-inducible promoters regulated by CpxA/CpxR in Escherichia coli. Biosci. Biotechnol. Biochem. 2006, 70, 1688–1695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, Z.; Zuo, L.; Li, C.; Tian, Y.; Wang, H. Copper Ions Facilitate the Conjugative Transfer of SXT/R391 Integrative and Conjugative Element Across Bacterial Genera. Front. Microbiol. 2021, 11, 616792. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.; Liu, H.; Yang, M.; Zhao, C.; He, Z.G. A copper-responsive global repressor regulates expression of diverse membrane-associated transporters and bacterial drug resistance in Mycobacteria. J. Biol. Chem. 2012, 287, 39721–39731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ladomersky, E.; Petris, M.J. Copper tolerance and virulence in bacteria. Metallomics 2015, 7, 957–964. [Google Scholar] [CrossRef] [Green Version]
- Chatterjee, S.; Kumari, S.; Rath, S.; Priyadarshanee, M.; Das, S. Diversity, structure and regulation of microbial metallothionein: Metal resistance and possible applications in sequestration of toxic metals. Metallomics 2020, 12, 1637–1655. [Google Scholar] [CrossRef]
| Cu-Dependent Compounds | Microorganisms |
|---|---|
| N4-methyl-thiosemicarbazones | Methicillin-resistant Staphylococcus aureus (MRSA), M. tuberculosis, N. gonorrhoeae, S. pneumoniae, H. influenza [32,33] |
| Disulfiram | M. tuberculosis [34] |
| Thiocarlide | MRSA [33] |
| 8-hydroxyquinoline | M. tuberculosis, C. Neoformans, L. monocytogenes [1,35] |
| 1,10,phenanthroline | A. baumannii, P. aeruginosa [36] |
| Neocuproine | MRSA, M. gallisepticum, P. denitrificans [1,33] |
| P yrazolopyrimidinone | S. aureus [37] |
| P yrithione | K. pneumoniae [38] |
| Antimicrobial Activity of Selected Antibiotics in the Presence of Copper | |||||||
|---|---|---|---|---|---|---|---|
| Antibiotic | E. coli | S. aureus | K. pneumoniae | P. mirabilis | S. enteriditis | S. sonnei | B. subtilis |
| Cephalexin | ↑ [5,57,58] | ↑ [57,58] No changes [5] | No changes [58] | ↑ [58] | ↑ [58] | ||
| Cephadroxil | No changes [62] | No changes [62] | |||||
| Cefradine | ↑ [57,59] | ↓ [57,59] | ↓ [59] | ↓ [59] | ↓ [59] | ||
| Cefazolin | ↑ [57] No changes [60] | No changes [57,60] | No changes [60] | No changes [60] | No changes [60] | ||
| Cefaclor | ↑ [5] | No changes [5] | |||||
| Ceftriaxone | ↓ [5,57,60,61] | ↓ [5,57,60,61] | ↓ [60] | ↓ [60] | ↓ [60] ↑ [57] | ||
| Ceftazidime | No changes [5] | ↓ [5] | |||||
| Cefepime | ↑ [60] | ↑ [60] | ↓ [60] | No changes [60] | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Božić Cvijan, B.; Korać Jačić, J.; Bajčetić, M. The Impact of Copper Ions on the Activity of Antibiotic Drugs. Molecules 2023, 28, 5133. https://doi.org/10.3390/molecules28135133
Božić Cvijan B, Korać Jačić J, Bajčetić M. The Impact of Copper Ions on the Activity of Antibiotic Drugs. Molecules. 2023; 28(13):5133. https://doi.org/10.3390/molecules28135133
Chicago/Turabian StyleBožić Cvijan, Bojana, Jelena Korać Jačić, and Milica Bajčetić. 2023. "The Impact of Copper Ions on the Activity of Antibiotic Drugs" Molecules 28, no. 13: 5133. https://doi.org/10.3390/molecules28135133
APA StyleBožić Cvijan, B., Korać Jačić, J., & Bajčetić, M. (2023). The Impact of Copper Ions on the Activity of Antibiotic Drugs. Molecules, 28(13), 5133. https://doi.org/10.3390/molecules28135133





