Datura stramonium Flowers as a Potential Natural Resource of Bioactive Molecules: Identification of Anti-Inflammatory Agents and Molecular Docking Analysis
Abstract
:1. Introduction
2. Results and Discussion
2.1. Extraction Yields and Quantification of Total Phenolic, Flavonoid, and Tannin Contents
2.2. Antioxidant Properties of Crude Extracts
2.3. Antimicrobial Activity
2.4. Antiviral Activity
2.5. Nitric Oxide Inhibitory Activity and Cytotoxicity Effect
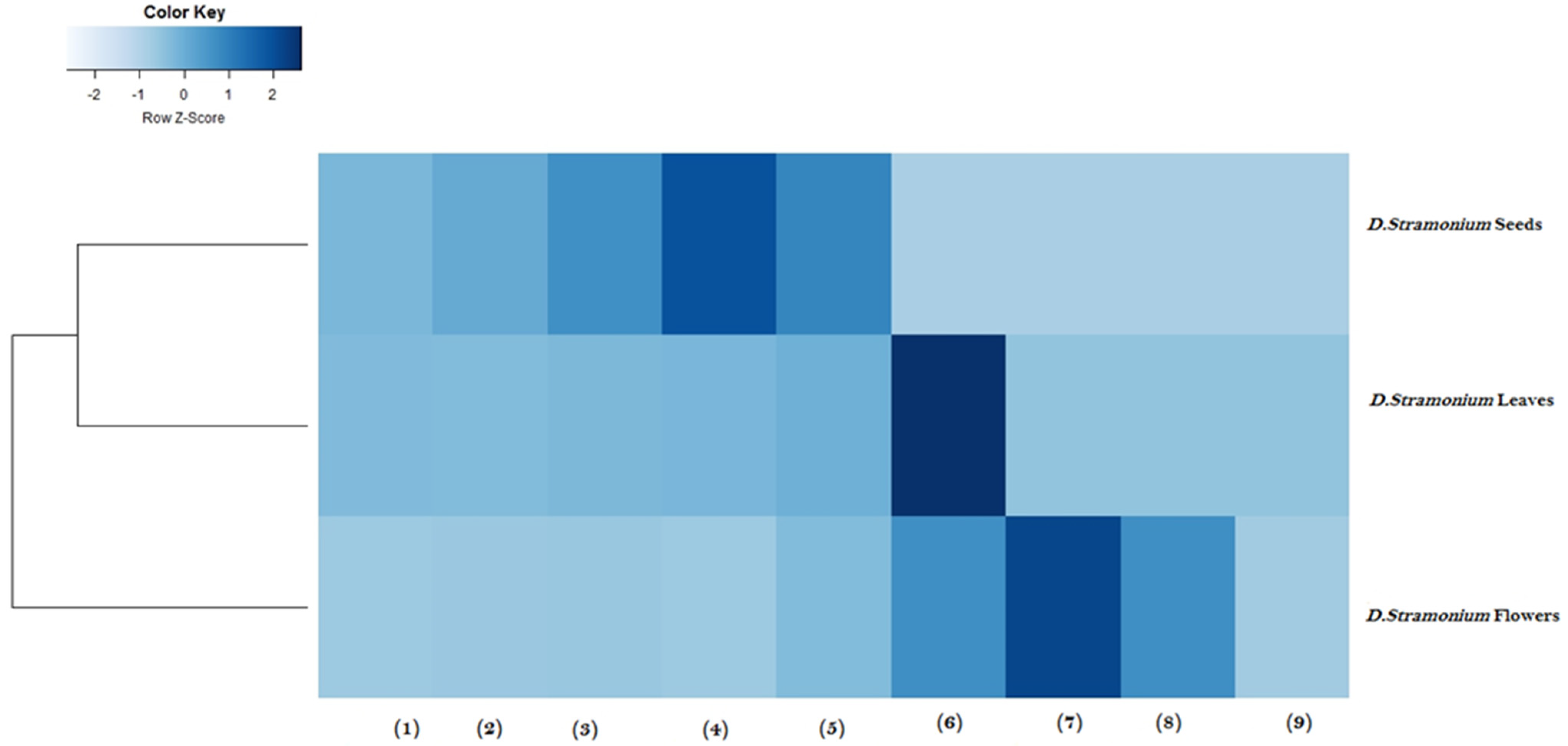
2.6. Heatmap Analysis
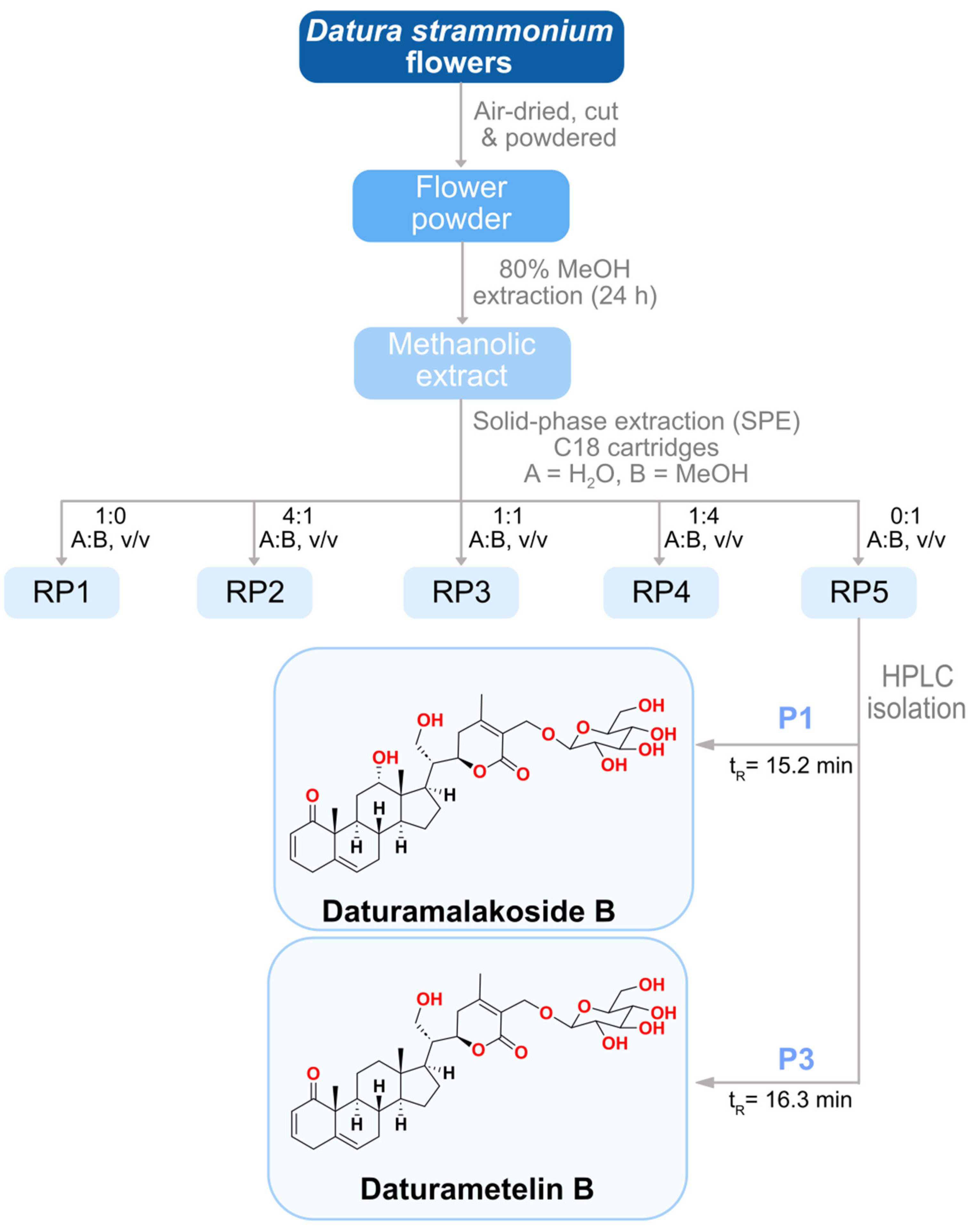
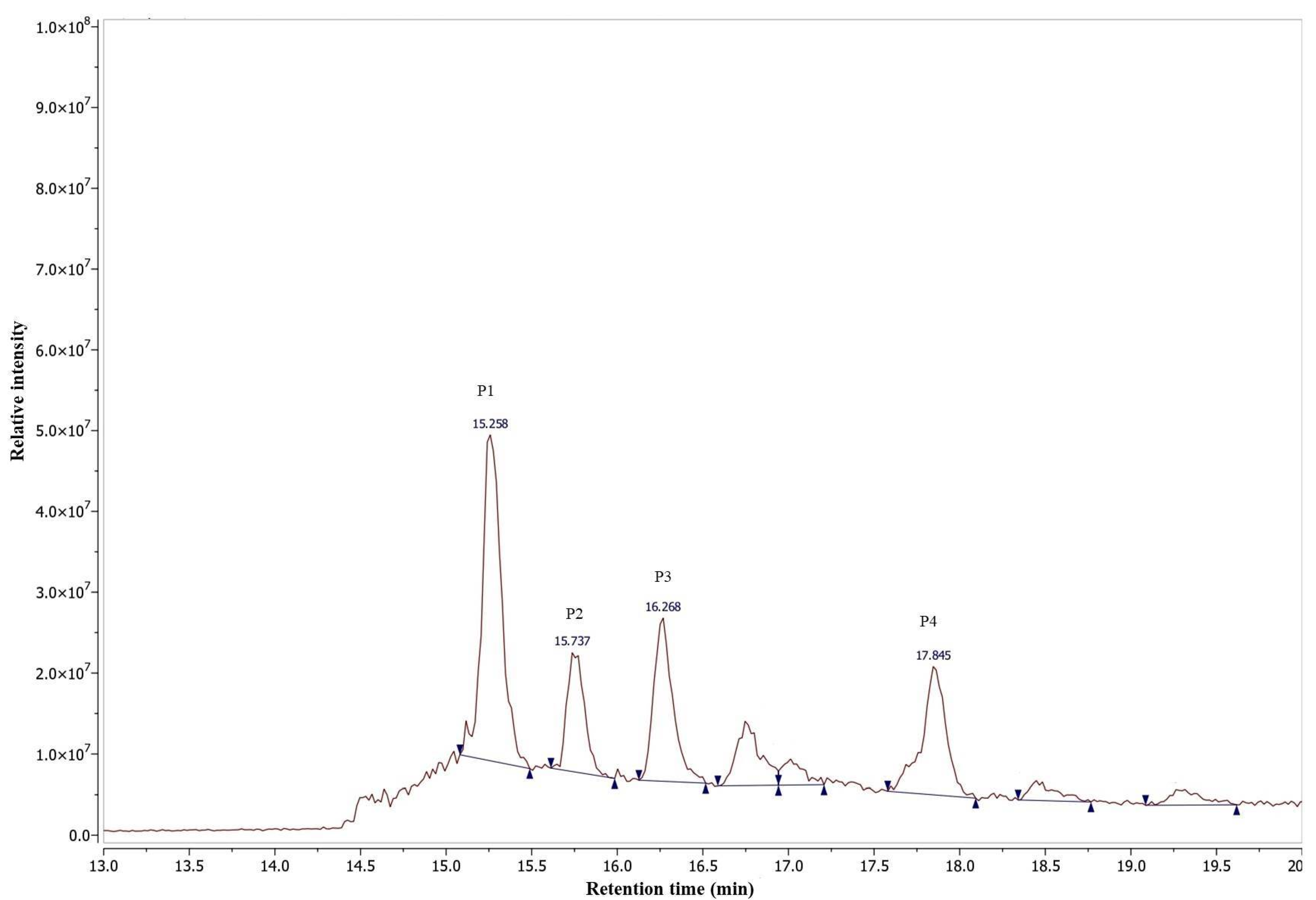
2.7. Purification and Characterization of Anti-Inflammatory Agents
2.8. Identification and Structure Elucidation of the Anti-Inflammatory Agents
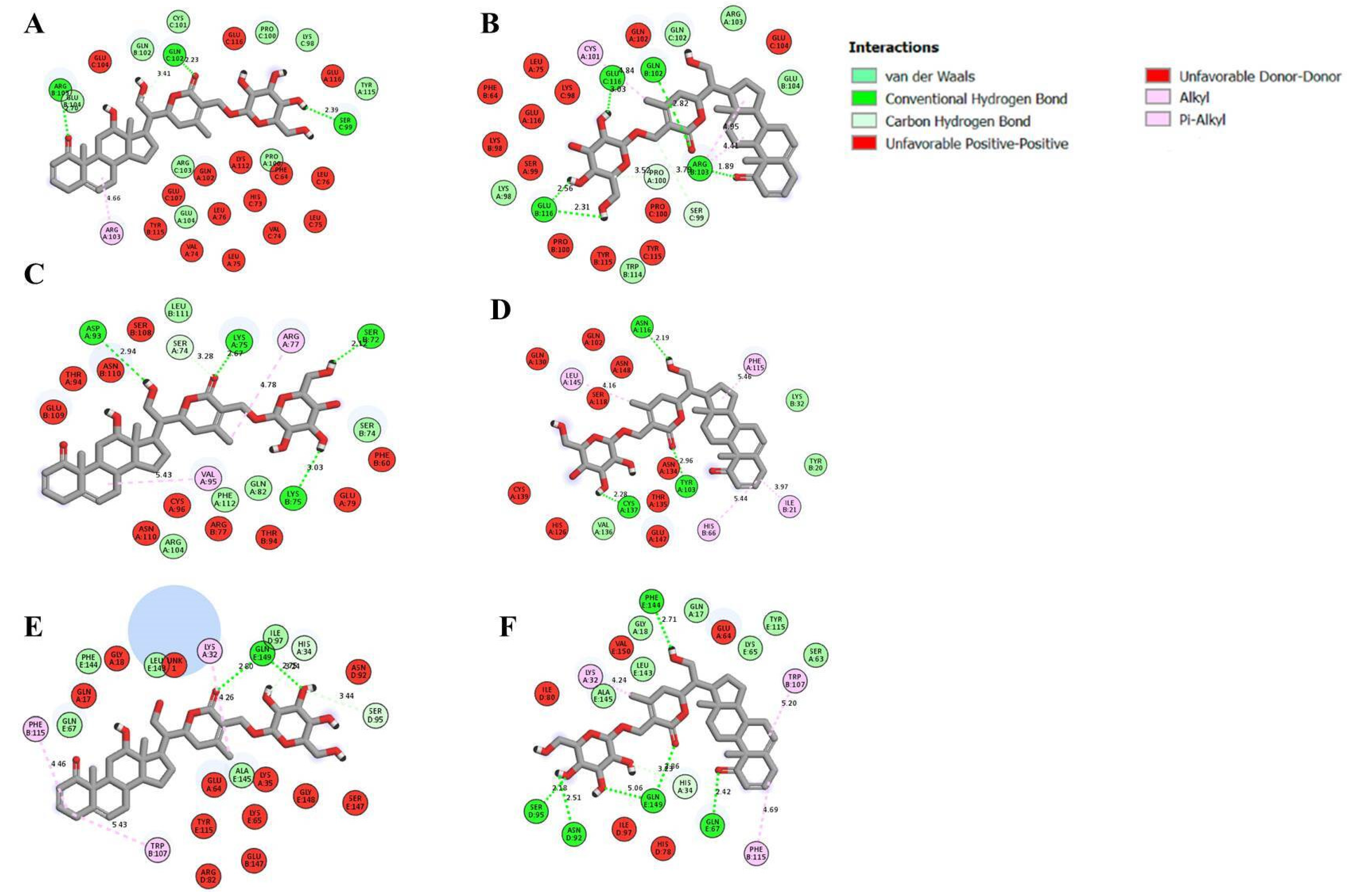
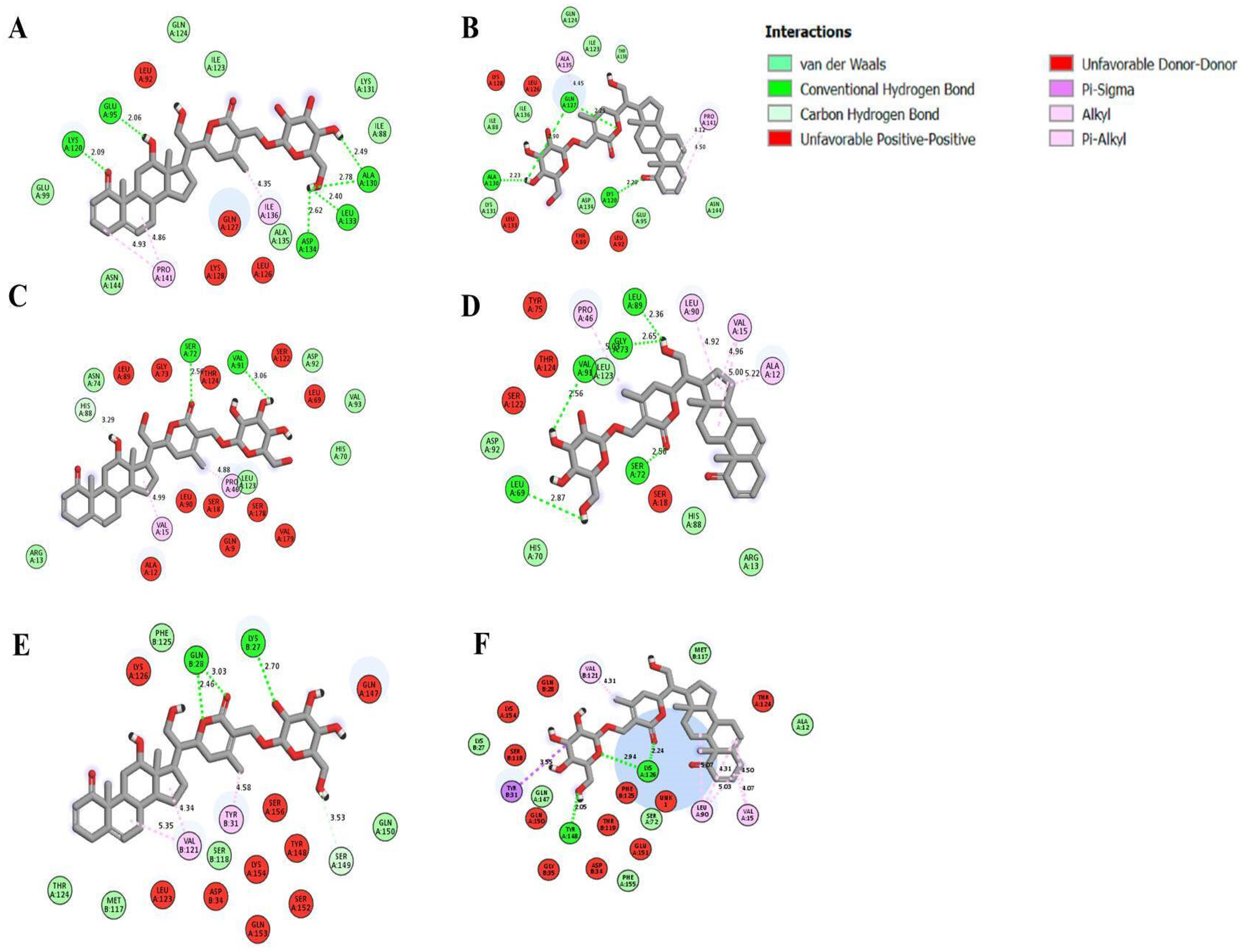
2.9. Molecular Docking
3. Material and Methods
3.1. Plant Material
3.2. Preparation of Crude Extracts
3.3. Determination of Total Phenolic Contents
3.4. Determination of Total Flavonoid Content
3.5. Determination of Condensed Tannin Content
3.6. Determination of Antioxidant Activity
3.6.1. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Radical Scavenging Assay
3.6.2. 2.2′-Azino-bis-3-ethylbenzthiazoline-6-sulphonic Acid (ABTS) Radical Scavenging Assay
3.6.3. Ferric Reducing Antioxidant Power (FRAP)
3.7. Antimicrobial Activity
3.8. Antiviral Activity
3.9. Nitrite Oxide “NO” Inhibitory Effect
3.10. Cytotoxicity Assay
3.11. Extraction and Isolation of the Active Compounds
3.12. Compound Characterization
3.13. Liquid Chromatography–Mass Spectrometry (HPLC/MS) Analysis
3.14. Molecular Docking
3.14.1. Preparation of Target Proteins and Ligands
3.14.2. Docking Simulation
3.15. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, A.; Pervaiz, A.; Ansari, B.; Ullah, R.; Shah, S.M.M.; Khan, H.; Jan, M.S.; Hussain, F.; Khan, I.K.; Albadrani, G.M.; et al. Phytochemical profiling, anti-inflammatory, anti-Oxidant and In-silico approach of Cornus macrophylla bioss (Bark). Molecules 2022, 27, 4081. [Google Scholar] [CrossRef]
- Anil, S.M.; Shalev, N.; Vinayaka, A.C.; Nadarajan, S.; Namdar, D.; Belausov, E.; Shoval, I.; Mani, K.A.; Mechrez, G.; Koltai, H. Cannabis compounds exhibit anti-infammatory activity in vitro in COVID-19-related infammation in lung epithelial cells and pro-infammatory activity in macrophages. Sci. Rep. 2021, 11, 1462–1514. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Wu, X.; Zheng, X.; Luo, S.; Xu, S.; Weng, J. Targeting inflammation and cytokine storm in COVID-19. Pharmacol. Res. 2020, 159, 105051. [Google Scholar] [CrossRef] [PubMed]
- Marmitt, D.J. Potential plants for inflammatory dysfunction in the SARS-CoV-2 infection. Inflammopharmacology 2022, 30, 749–773. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Li, Y.; Leung, E.L.-H.; Liu, X.; Liu, K.; Wang, Q.; Lan, Y.; Li, X.; Yu, H.; Cui, L.; et al. A review of therapeutic agents and Chinese herbal medicines against SARS-CoV-2 (COVID-19). Pharmacol. Res. 2020, 158, 104929. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, P.; Abbas, A.; Lehmann, C.; Rupasinghe, H.P.V. Antiviral and anti-inflammatory plant-derived bioactive compounds and their potential use in the treatment of COVID-19-related pathologies. J. Xenobiot. 2022, 12, 289–306. [Google Scholar] [CrossRef]
- Patel, V.K.; Shirbhate, E.; Patel, P.; Veerasamy, R.; Sharma, P.C.; Rajak, H. Corticosteroids for treatment of COVID-19: Effect, evidence, expectation and extent. Beni-Suef Univ. J. Basic Appl. Sci. 2021, 10, 78. [Google Scholar] [CrossRef]
- Liu, Y.X.; Zhou, Y.H.; Jiang, C.H.; Liu, J.; Chen, D.Q. Prevention, treatment and potential mechanism of herbal medicine for Corona viruses: A review. Bioengineered 2022, 13, 5480–5508. [Google Scholar] [CrossRef]
- Sharma, M.; Dhaliwal, I.; Rana, K.; Delta, A.K.; Kaushik, P. Phytochemistry, Pharmacology, and Toxicology of Datura Species—A Review. Antioxidants 2021, 10, 1291. [Google Scholar] [CrossRef]
- Gaire, B.P.; Subedi, L. A review on the pharmacological and toxicological aspects of Datura stramonium L. J. Integr. Med. 2013, 11, 73–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soni, P.; Siddiqui, A.A.; Dwivedi, J.; Soni, V. Pharmacological properties of Datura stramonium L. as a potential medicinal tree: An overview. Asian Pac. J. Trop. Biomed. 2012, 2, 1002–1008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choudhary, M.I.; Yousuf, S.; Rahman, A.U. Withanolides: Chemistry and antitumor activity. In Natural Products; Ramawat, K.G., Me’rillon, J.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 3465–3495. [Google Scholar] [CrossRef]
- Yang, B.Y.; Guo, R.; Li, T.; Wu, J.J.; Zhang, J.; Liu, Y.; Wang, Q.H.; Kuang, H.X. New anti-inflammatory withanolides from the leaves of Datura metel L. Steroids 2014, 87, 26–34. [Google Scholar] [CrossRef]
- Lee, D.; Yu, J.S.; Ha, J.W.; Lee, S.R.; Lee, B.S.; Kim, J.C.; Kim, J.K.; Kang, K.S.; Kim, K.H. Antitumor potential of withanolide glycosides from Ashwagandha (Withania somnifera) on apoptosis of human hepatocellular carcinoma cells and tube formation in human umbilical vein endothelial cells. Antioxidants 2022, 11, 1761. [Google Scholar] [CrossRef]
- White, P.T.; Subramanian, C.; Motiwala, H.F.; Cohen, M.S. Natural withanolides in the treatment of chronic diseases. Adv. Exp. Med. Biol. 2016, 928, 329–373. [Google Scholar] [CrossRef]
- Yang, B.Y.; Zhou, Y.Q.; Liu, Y.; Lu, Z.K.; Kuang, H.X. Withanolides as potential immunosuppressive agents against RAW264.7 cells from the Pericarps of Datura metel. Nat. Prod. Commun. 2017, 12, 1021–1024. [Google Scholar] [CrossRef] [Green Version]
- Ichikawa, H.; Takada, Y.; Shishodia, S.; Jayaprakasam, B.; Nair, M.G.; Aggarwal, B.B. Withanolides potentiate apoptosis, inhibit invasion, and abolish osteoclastogenesis through suppression of nuclear factor-KB (NF-KB) activation and NF-KB–regulated gene expression. Mol. Cancer. Ther. 2006, 5, 1434–1445. [Google Scholar] [CrossRef] [Green Version]
- Chandan, G.; Kumar, C.; Chibber, P.; Kumar, A.; Singh, G.; Satti, N.K.; Gulilat, H.; Saini, A.K.; Bishayee, A.; Saini, R.V. Evaluation of analgesic and anti-inflammatory activities and molecular docking analysis of steroidal lactones from Datura stramonium L. Phytomedicine 2021, 89, 153621. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, K.; Kumar, S.; Ojha, S. Antioxidant activity and FT-IR analysis of Datura innoxia and Datura metel leaf and seed methanolic extracts. Afr. J. Tradit. Complement. Altern. Med. 2016, 13, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Hmamou, A.; Eloutassi, N.; Alshawwa, S.Z.; Al Kamaly, O.; Kara, M.; Bendaoud, A.; El-Assri, E.M.; Tlemcani, S.; El Khomsi, M.; Lahkimi, A. Total phenolic content and antioxidant and antimicrobial activities of Papaver rhoeas L. organ extracts growing in Taounate region, Morocco. Molecules 2022, 27, 854. [Google Scholar] [CrossRef]
- Benabderrahim, M.A.; Sarikurkcu, C.; Elfalleh, W.; Ozer, M.S. Datura innoxia and Dipsacus laciniatus: Biological activity and phenolic composition. Biocatal. Agric. Biotechnol. 2019, 19, 101163. [Google Scholar] [CrossRef]
- Sreenivasa, S.; Vinay, K.; Mohan, N.R. Phytochemical analysis, antibacterial and antioxidant activity of leaf extract of Datura stramonium. Int. J. Sci. Res. 2012, 1, 83–86. [Google Scholar]
- Iqbal, S.; Sivaraj, C.; Gunasekaran, K. Antioxidant and anticancer activities of methanol extract of seeds of Datura stramonium L. Free Radic. Antioxid. 2017, 7, 184–189. [Google Scholar] [CrossRef]
- Prasathkumar, M.; Anisha, S.; Khusro, A.; Mohamed Essa, M.; Babu Chidambaram, S.; Walid Qoronfleh, M.; Sadhasivam, S.; Umar Khayam Sahibzada, M.; Alghamdi, S.; Almehmadi, M.; et al. Anti-Pathogenic, Anti-Diabetic, Anti-Inflammatory, Antioxidant, and Wound Healing Efficacy of Datura metel L. Leaves. Arab. J. Chem. 2022, 15, 104112. [Google Scholar] [CrossRef]
- Gachande, B.D.; Khillare, E.M. In-vitro evaluation of Datura species for potential antimicrobial activity. Biosci. Discov. 2013, 4, 78–81. [Google Scholar]
- Céspedes-Méndez, C.; Iturriaga-Vásquez, P.; Hormazábal, E. Secondary metabolites and biological profiles of Datura genus. J. Chil. Chem. Soc. 2021, 66, 5183–5189. [Google Scholar] [CrossRef]
- Konaté, K.; Hilou, A.; Mavoungou, J.F.; Lepengué, A.N.; Souza, A.; Barro, N.; Datté, J.Y.; M’batchi, B.; Nacoulma, O.G. Antimicrobial activity of polyphenol-rich fractions from Sida alba L. (Malvaceae) against cotrimoxazol- resistant bacteria strains. Ann. Clin. Microbiol. Antimicrob. 2012, 11, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouslamti, M.; El Barnossi, A.; Kara, M.; Alotaibi, B.S.; Al Kamaly, O.; Assouguem, A.; Lyoussi, B.; Benjelloun, A.S. Total Polyphenols Content, Antioxidant and Antimicrobial Activities of Leaves of Solanum elaeagnifolium Cav. from Morocco. Molecules 2022, 27, 4322. [Google Scholar] [CrossRef] [PubMed]
- Okwu, D.E.; Igara, E.C. Isolation, characterization and antibacterial activity of alkaloid from Datura metel Linn leaves. Afr. J. Pharm. Pharmacol. 2009, 3, 277–281. [Google Scholar] [CrossRef]
- Naz, R.; Ayub, H.; Nawaz, S.; Islam, Z.U.; Yasmin, T.; Bano, A.; Wakeel, A.; Zia, S.; Roberts, T.H. Antimicrobial Activity, Toxicity and Anti-Inflammatory Potential of Methanolic Extracts of Four Ethnomedicinal Plant Species from Punjab, Pakistan. BMC Complement. Altern. Med. 2017, 17, 302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amin, M.Z.; Afrin, M.; Meghla, N.S.; Nur, M.A.; Rahman, M.M.; Uddin, M.J. Assessment of Antibacterial, Anti-Inflammatory, and Cytotoxic Effects of Different Extracts of Gynura Procumbens Leaf. Curr. Ther. Res. Clin. Exp. 2021, 95, 100636. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane, G.; Sharma, G.; Sapkota, B.; Adhikari, M.; Ghimire, S.; Poudel, P.; Jung, H.-J. Screening of Antioxidant, Antibacterial, Anti-Adipogenic, and Anti-Inflammatory Activities of Five Selected Medicinal Plants of Nepal. J. Exp. Pharmacol. 2023, 15, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Lee, J.-B.; Atsumi, K.; Kanazashi, M.; Shibayama, T.; Okamoto, K.; Kawahara, T.; Hayashi, T. In Vitro and In Vivo Anti-Herpes Simplex Virus Activity of Monogalactosyl Diacylglyceride from Coccomyxa Sp. KJ (IPOD FERM BP-22254), a Green Microalga. PLoS ONE 2019, 14, e0219305. [Google Scholar] [CrossRef] [Green Version]
- Roy, S.; Mukherjee, S.; Pawar, S.; Chowdhary, A. Evaluation of in Vitro Antiviral Activity of Datura Metel Linn. against Rabies Virus. Pharmacogn. Res. 2016, 8, 265–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, W.; Kong, N.; Liu, M.-Z.; Han, T.; Xu, J.-F.; Liu, C. Anisodamine Potently Inhibits SARS-CoV-2 Infection in Vitro and Targets Its Main Protease. Biochem. Biophys. Res. Commun. 2022, 616, 8–13. [Google Scholar] [CrossRef]
- Alum, E.U.; Inya, J.E.; Ugwu, O.P.C.; Obeagu, E.I.; Aloke, C.; Aja, P.M.; Okpata, M.G.; John, E.C.; Orji, M.O.; Onyema, O. Ethanolic Leaf Extract of Datura Stramonium Attenuates Methotrexate-Induced Biochemical Alterations in Wistar Albino Rats. RPS Pharm. Pharmacol. Rep. 2023, 2, rqac011. [Google Scholar] [CrossRef]
- Nasir, B.; Khan, A.U.; Baig, M.W.; Althobaiti, Y.S.; Faheem, M.; Haq, I.U. Datura stramonium leaf extract exhibits anti-inflammatory activity in CCL4-Induced hepatic injury model by modulating oxidative stress markers and iNOS/Nrf2 expression. Biomed. Res. Int. 2022, 2022, 1382878. [Google Scholar] [CrossRef]
- Joshua, P.E.; Abonyi, U.C.; Eze, C.S.; Asomadu, R.O.; Obeta, J.N.; Nnamani, I.V.; Patrick, B.U.; Eze, K.C.; Obialor, O.F. Comparative effects of Datura stramonium leaf and seed extracts on membrane stabilization and platelet aggregation in-vitro. Afr. J. Biomed. Res. 2019, 22, 363–368. [Google Scholar]
- Darwish, A.G.G.; Samy, N.M.; Sugimoto, S.; Matsunami, K.; Otsuka, H. Principal component and heatmap analysis of the biological activities for some selected medicinal plants. Acad. J. Med. Plants 2018, 6, 101–113. [Google Scholar]
- Nthai, D.; Thibane, V.S.; Gololo, S.S. Comparative study of abiotic stress factors on GC-MS-detected phytoconstituents of Aloe greatheadii var: Davyana using heatmap and hierarchical clustering dendrogram. Biochem. Res. Int. 2022, 2022, 5365024. [Google Scholar] [CrossRef]
- Bellila, A.; Tremblay, C.; Pichette, A.; Marzouk, B.; Mshvildadze, V.; Lavoie, S.; Legault, J. Cytotoxic activity of withanolides isolated from Tunisian Datura metel L. Phytochemistry 2011, 72, 2031–2036. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.T.; Liu, Y.; Wang, S.Y.; Jiang, Y.K.; Li, X.M.; Naseem, A.; Guan, W.; Pan, J.; Kuang, H.X.; Yang, B.Y. Four new withanolides with anti-inflammatory activity from Datura inoxia Mill. leaves. Steroids 2022, 182, 109010. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.T.; Liu, X.; Kong, N.N.; Liu, S.J.; Xia, C.H. Two new withanolides from the halophyte Datura stramonium L. Nat. Prod. Res. 2013, 27, 1965–1970. [Google Scholar] [CrossRef] [PubMed]
- Logie, E.; Vanden Berghe, W. Tackling chronic inflammation with withanolide phytochemicals—A withaferin a perspective. Antioxidants 2020, 9, 1107. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Guan, W.; Yang, C.L.; Luo, Y.M.; Liu, Y.; Zhou, Y.Y.; Liu, L.N.; Yang, B.Y.; Kuang, H.X. Steroids with potential anti-inflammatory from the roots of Datura metel L. Can. J. Chem. 2019, 98, 57–113. [Google Scholar] [CrossRef]
- Tan, J.Y.; Liu, Y.; Cheng, Y.G.; Sun, Y.P.; Liu, Y.; Huang, J.; Guo, S.; Liu, G.Z.; Kuang, H.X.; Yang, B.Y. Daturmetesides A-E, five new ergostane-type C28 sterols from the leaves of Datura metel L. Steroids 2020, 156, 108583. [Google Scholar] [CrossRef]
- Kim, O.T.; Le, M.D.; Trinh, H.X.; Nong, H.V. In silico studies for the interaction of tumor necrosis factor-alpha (TNF-α) with different saponins from Vietnamese ginseng (Panax vietnamesis). Biophys. Physicobiol. 2016, 13, 173–180. [Google Scholar] [CrossRef] [Green Version]
- Choi, H.; Lee, Y.; Park, H.; Oh, D.S. Discovery of the inhibitors of tumor necrosis factor alpha with structure-based virtual screening. Bioorg. Med. Chem. Lett. 2010, 20, 6195–6198. [Google Scholar] [CrossRef]
- Hu, Z.; Qin, J.; Zhang, H.; Wang, D.; Hua, Y.; Ding, J.; Shan, L.; Jin, H.; Zhang, J.; Zhang, W. Japonicone A antagonizes the activity of TNF-alpha by directly targeting this cytokine and selectively disrupting its interaction with TNF receptor-1. Biochem. Pharmacol. 2012, 84, 1482–1491. [Google Scholar] [CrossRef]
- Cao, Y.; Li, Y.H.; Lv, D.Y.; Chen, X.F.; Chen, L.D.; Zhu, Z.Y.; Chai, Y.F.; Zhang, J.P. Identification of a ligand for tumor necrosis factor receptor from Chinese herbs by combination of surface plasmon resonance biosensor and UPLC-MS. Anal. Bioanal. Chem. 2016, 408, 5359–5367. [Google Scholar] [CrossRef]
- Saddala, M.S.; Huang, H. Identification of novel inhibitors for TNFα, TNFR1 and TNFα-TNFR1 complex using pharmacophore-based approaches. J. Transl. Med. 2019, 17, 215. [Google Scholar] [CrossRef]
- Ezaouine, A.; Salam, M.R.; Nouadi, B.; Anachad, O.; Messal, M.E.; Chegdani, F.; Bennis, F. In silico prediction of the bioactive profile and metabolites of Satureja nepeta in diseases related to the excessive production of interleukin-6. Bioinform. Biol. Insights 2022, 16, 1–9. [Google Scholar] [CrossRef]
- Wang, S.Q.; Shi, M.; Fang, L.; Xu, S.M.; Wang, C.; Yu, Z. Design of dual inhibitors of human TNF-α and IL-6 with potentials for the treatment of rheumatoid arthritis. Trop. J. Pharm. Res. 2019, 18, 2305–2312. [Google Scholar] [CrossRef]
- Sharma, S.; Malhotra, L.; Yadav, P.; Mishra, V.; Sharma, R.S.; Samath, E.A. Genistein: A novel inhibitor of IL-6/IL-6R interface of the Interleukin-6–mediated STAT3 dependent pathway of carcinogenesis. J. Mol. Struct. 2022, 1258, 132668. [Google Scholar] [CrossRef]
- Dewanto, V.; Wu, X.; Adom, K.K.; Liu, R.H. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J. Agric. Food. Chem. 2002, 50, 3010–3014. [Google Scholar] [CrossRef] [PubMed]
- Julkunen-Titto, R. Phenolic constituents in the leaves of northemwiliows methods for the analysis of certain phenolics. J. Agric. Food. Chem. 1985, 33, 213–217. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Khan, R.A.; Khan, M.R.; Sahreen, S.; Ahmed, M. Assessment of flavonoids contents and in vitro antioxidant activity of Launaea procumbens. Chem. Cent. J. 2012, 6, 43. [Google Scholar] [CrossRef] [Green Version]
- El Jemli, M.; Kamal, R.; Marmouzi, I.; Zerrouki, A.; Cherrah, Y.; Alaoui, K. Radical-scavenging activity and ferric reducing ability of Juniperus thurifera (L.), J. oxycedrus (L.), J. phoenicea (L.) and Tetraclinis articulata (L.). Ad. Pharmacol. Sci. 2016, 2016, 6392656. [Google Scholar] [CrossRef] [Green Version]
- Tabbene, O.; Karkouch, I.; Elkahoui, S.; Cosette, P.; Mangoni, M.L.; Jouenne, T.; Limam, F. A new antibacterial and antioxidant S07-2 compound produced by Bacillus subtilis B38. FEMS Microbiol. Lett. 2010, 303, 176–182. [Google Scholar] [CrossRef] [Green Version]
- Silva, A.C.O.; Santana, E.F.; Saraiva, A.M.; Coutinho, F.N.; Castro, R.H.A.; Pisciottano, M.N.C.; Amorim, E.L.C.; Albuquerque, U.P. Which Approach Is More Effective in the Selection of Plants with Antimicrobial Activity? Evid.-Based Complement. Altern. Med. 2013, 2013, 308980. [Google Scholar] [CrossRef] [Green Version]
- Bouslama, L.; Benzekri, R.; Nsaibia, S.; Papetti, A.; Limam, F. Identification of an antiviral compound isolated from Pistacia lentiscus. Arch. Microbiol. 2020, 202, 2569–2578. [Google Scholar] [CrossRef]
- Joo, T.; Sowndhararajan, K.; Hong, S.; Lee, J.; Park, S.Y.; Kim, S.; Jhoo, J.W. Inhibition of nitric oxide production in LPS-stimulated RAW 264.7 cells by stem bark of Ulmus pumila L. Saudi. J. Biol. Sci. 2014, 21, 427–435. [Google Scholar] [CrossRef] [Green Version]
- Essid, R.; Rahali, F.Z.; Msaada, K.; Sghair, I.; Hammami, M.; Bouratbine, A.; Aoun, K.; Limam, F. Antileishmanial and cytotoxic potential of essential oils frommedicinal plants in Northern Tunisia. Ind. Crops Prod. 2015, 77, 795–802. [Google Scholar] [CrossRef]
- Ranušová, P.; Matušíková, I.; Nemeček, P. Optimization of Plant Extract Purification Procedure for Rapid Screening Analysis of Sixteen Phenolics by Liquid Chromatography. Separations 2021, 8, 13. [Google Scholar] [CrossRef]
- Naismith, J.H.; Devine, T.Q.; Kohno, T.; Sprang, S.R. Structures of the extracellular domain of the type I tumor necrosis factor receptor. Structure 1996, 4, 1251–1262. [Google Scholar] [CrossRef] [Green Version]
- Eck, J.M.; Sprang, R.S. The Structure of tumor necrosis factor-cr at 2.6 A resolution. J. Biol. Chem. 1989, 264, 17595–17605. [Google Scholar] [CrossRef]
- Varghese, J.N.; Moritz, R.L.; Lou, M.Z.; Van Donkelaar, A.; Ji, H.; Ivancic, N.; Branson, K.M.; Hall, N.E.; Simpson, R.J. Simpson, Structure of the extracellular domains of the human interleukin-6 receptor-chain. Proc. Natl. Acad. Sci. USA 2002, 99, 15959–15964. [Google Scholar] [CrossRef] [PubMed]
- Somers, W.; Stahl, M.; Seehra, J.S. 1.9 Å crystal structure of interleukin 6: Implications for a novel mode of receptor dimerization and signaling. EMBO J. 1997, 16, 989–997. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Samples | Polyphenols (mg GAE/g DW) | Flavonoids (mg CE/g DW) | Flavonols (mg RE/g DW) | Condensed Tannin (mg CE/g DW) |
|---|---|---|---|---|
| Flower extract | 150.43 ± 3.08 (b) | 65.53 ± 2.22 (b) | 51.31 ± 0.68 (b) | 16.75 ± 1.16 (c) |
| Leaf extract | 133.05 ± 1.96 (b) | 79.20 ± 0.39 (a) | 76.21 ± 0.30 (a) | 74.37 ± 0.67 (b) |
| Seed extract | 219.65 ± 10.31 (a) | 84.79 ± 3.62 (a) | 53.53 ± 1.39 (b) | 91.12 ± 2.32 (a) |
| Samples | Antioxidant Activity | ||
|---|---|---|---|
| DPPH Radical Scavenging Activity IC50 (µg/mL) | ABTS Assay IC50 (µg/mL) | Reducing Power EC50 (µg/mL) | |
| Flower extract | 22.82 ± 2.01 (b) | 52.69 ± 1.55(b) | 41.75 ± 1.55 (b) |
| Leaf extract | 70.98 ± 0.56 (c) | 89.81 ± 1.31 (c) | 65.50 ± 3.92 (c) |
| Seed extract | 21.62 ± 1.72 (b) | 52.31 ± 0.69 (b) | 31.44 ± 1.25 (a) |
| BHT | 14.54 ± 0.30 (a) | - | - |
| Ascorbic acid | - | 28.23 ± 0.26 (a) | 30.75 ± 0.17 (a) |
| Microbial Strains | Antimicrobial Activity: MIC (µg/mL) | ||
|---|---|---|---|
| Leaf Extract | Flower Extract | Seed Extract | |
| Gram-positive bacteria | |||
| Staphylococcus aureus ATCC 6538 | 1000 | 500 | ≥2000 |
| Methicillin Resistant Staphylococcus aureus (MRSA) | ≥2000 | 1000 | ≥2000 |
| Bacillus cereus ATCC 14579 | ≥2000 | 500 | ≥2000 |
| Enterococcus feacalis ATCC 29212 | ≥2000 | 7.81 | ≥2000 |
| Gram-negative bacteria | |||
| Escherichia coli ATCC 25922 | ≥2000 | ≥2000 | ≥2000 |
| Salmonella enteritidis DMB 560 | ≥2000 | ≥2000 | ≥2000 |
| Klebsiella pneumonia CIP 104727 | ≥2000 | ≥2000 | ≥2000 |
| Pseudomonas aeruginosa ATCC 27853 | ≥2000 | ≥2000 | ≥2000 |
| Yeasts | |||
| Candida albicans ATCC 10231 | ≥2000 | ≥2000 | ≥2000 |
| Samples | NO Inhibition IC50 (µg/mL) | Cytotoxic Effect LC50 (µg/mL) | Selectivity Index (SI) |
|---|---|---|---|
| Leaf extract | 101.12 ± 6.59 (b) | 149.05 ± 0.43 (b) | 1.47 |
| Seed extract | 96.04 ± 7.32 (b) | 59.71 ± 0.44 (a) | 0.62 |
| Flower extract | 26.98 ± 0.42 (a) | 142.54 ± 4.15 (d) | 5.28 |
| L-NAME | 21.30 ± 0.24 (a) | 332.41 ± 1.7 (a) | 15.61 |
| RP5 fraction | 25.05 ± 0.32 (d) | 125.58 ± 0.60 (b) | 5.01 |
| P1 | 6.77 ± 0.03 (a) | 38.9 ± 0.02 (e) | 5.74 |
| P2 | 7.16 ± 0.02 (ab) | 29.83 ± 0.14 (f) | 4.16 |
| P3 | 7.55 ± 0.09 (b) | 55.65 ± 0.61 (c) | 7.37 |
| P4 | 14.48 ± 0.13 (c) | 48.92 ± 0.48 (d) | 3.37 |
| L-NAME | 21.30 ± 0.24 (d) | 332.41 ± 1.7 (a) | 15.61 |
| Receptors | Ligands | Binding Energy (kcal/mol) | Active Site Amino Acids | Interaction Type |
|---|---|---|---|---|
| TNF α | 12α-hydroxydaturametelin B (daturamalakoside B) | −10.70 | B/ARG.103, C/SER.99, C/GLN.102 | Hydrogen |
| A/ARG.103 | Alkyl | |||
| C/GLN.102 | Carbon–Hydrogen | |||
| A/PRO.100,A/GLU.104,A/TYR.115,B/GLN.102,B/GLU.104,C/LYS.98,C/PRO.100,C/CYS.101,C/ARG.103 | Van der Waals | |||
| Daturametelin B | −11.40 | B/GLN.102,B/ARG.103, B/GLU.116, C/GLU.116 | Hydrogen | |
| A/CYS.101,B/ARG.103 | Alkyl | |||
| A/PRO.100, C/SER.99 | Carbon–Hydrogen | |||
| A/LYS.98, A/AGR.103, B/GLU.104,B/TRP.114, C/GLN.102 | Van der Waals | |||
| TNFR1 | 12α-hydroxydaturametelin B (daturamalakoside B) | −10.30 | A/LYS.75, A/ASP.93, B/SER.72, B/LYS.75 | Hydrogen |
| A/ARG.77, A/VAL.95 | Alkyl | |||
| A/SER.74 | Carbon–Hydrogen | |||
| A/GLN.82,A/ARG.104, A/PHE.112, B/SER.74, B/LEU.111 | Van der Waals | |||
| Daturametelin B | −8.70 | ∅ | ||
| TNFα-TNFR1complex | 12α-hydroxydaturametelin B (daturamalakoside B) | −9.70 | E/GLN.149 | Hydrogen |
| B/TRP.107 | alkyl | |||
| B/LYS.32, B/PHE.115 | Pi-Alkyl | |||
| A/HIS.34, D/SER.95 | Carbon–Hydrogen | |||
| D/ILE.97, E/GLN.67, E/LEU.143, E/PHE.144, E/ALA.145 | Van der Waals | |||
| Daturametelin B | −9.60 | D/ASN.92, D/SER.95, E/GLN.67, E/PHE.144, E/GLN.149 | Hydrogen | |
| A/LYS.32, B/PHE.115 | Alkyl | |||
| B/TRP.107 | Pi-Alkyl | |||
| A/HIS.34 | Carbon–Hydrogen | |||
| A/GLN.17, A/GLY.18, A/SER.63, E/LYS.65, E/TYR.115,E/LEU.143, E/ALA.145 | Van der Waals |
| Receptors | Ligands | Binding Energy (kcal/mol) | Active Site Aminoacids | Interaction Type |
|---|---|---|---|---|
| IL-6 | 12α-hydroxydaturametelin B (daturamalakoside B) | −7.40 | A/GLU.95, A/LYS.120 | Hydrogen |
| A/PRO.141 | alkyl | |||
| A/GLU.99, A/ASN.144 | Van der Waals | |||
| Daturametelin B | −7.10 | A/LYS.120 | Hydrogen | |
| A/PRO.141 | Alkyl | |||
| A/GLU.95, A/ASN.144 | Van der Waals | |||
| IL-6R | 12α-hydroxydaturametelin B (daturamalakoside B) | −9.20 | A/SER.72, A/VAL.91 | Hydrogen |
| A/PRO.46 | Alkyl | |||
| A/ASP.92 | Van der Waals | |||
| Daturametelin B | −9.30 | A/LEU.69, A/SER.72, A/VAL.91 | Hydrogen | |
| A/PRO.46, A/LEU.90 | Alkyl | |||
| A/ASP.92, A/LEU.123 | Van der Waals | |||
| IL-6-IL-6R | 12α-hydroxydaturametelin B (daturamalakoside B) | −8.10 | B/LYS.27 | Hydrogen |
| B/TYR.31 | alkyl | |||
| A/SER.149 | Carbon–Hydrogen | |||
| A/GLN.150 | Van der Waals | |||
| Daturametelin B | −8.50 | A/TYR.148 | Hydrogen | |
| B/TYR.31 | Pi-Sigma | |||
| B/LYS.27 | Van der Waals |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Damergi, B.; Essid, R.; Fares, N.; Khadraoui, N.; Ageitos, L.; Ben Alaya, A.; Gharbi, D.; Abid, I.; Rashed Alothman, M.; Limam, F.; et al. Datura stramonium Flowers as a Potential Natural Resource of Bioactive Molecules: Identification of Anti-Inflammatory Agents and Molecular Docking Analysis. Molecules 2023, 28, 5195. https://doi.org/10.3390/molecules28135195
Damergi B, Essid R, Fares N, Khadraoui N, Ageitos L, Ben Alaya A, Gharbi D, Abid I, Rashed Alothman M, Limam F, et al. Datura stramonium Flowers as a Potential Natural Resource of Bioactive Molecules: Identification of Anti-Inflammatory Agents and Molecular Docking Analysis. Molecules. 2023; 28(13):5195. https://doi.org/10.3390/molecules28135195
Chicago/Turabian StyleDamergi, Bilel, Rym Essid, Nadia Fares, Nadine Khadraoui, Lucía Ageitos, Ameni Ben Alaya, Dorra Gharbi, Islem Abid, Monerah Rashed Alothman, Ferid Limam, and et al. 2023. "Datura stramonium Flowers as a Potential Natural Resource of Bioactive Molecules: Identification of Anti-Inflammatory Agents and Molecular Docking Analysis" Molecules 28, no. 13: 5195. https://doi.org/10.3390/molecules28135195







