Synthesis and Characterization of Amphiphilic Diblock Polyphosphoesters Containing Lactic Acid Units for Potential Drug Delivery Applications
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization of Poly(ethylene glycol)lactate (PEG-LA)
2.2. Synthesis of Poly[poly(ethylene glycol) H-phosphonate-b-poly(ethylene glycol)lactate H-phosphonate]
2.3. One-Pot Synthesis of Poly[alkylpoly(ethylene glycol) phosphate-b-alkylpoly(ethylene glycol)lactate phosphate]s
One-Pot Synthesis of Poly[hexadecylpoly(ethylene glycol) phosphate)-b-hexadecylpoly(ethylene glycol)lactate phosphate]
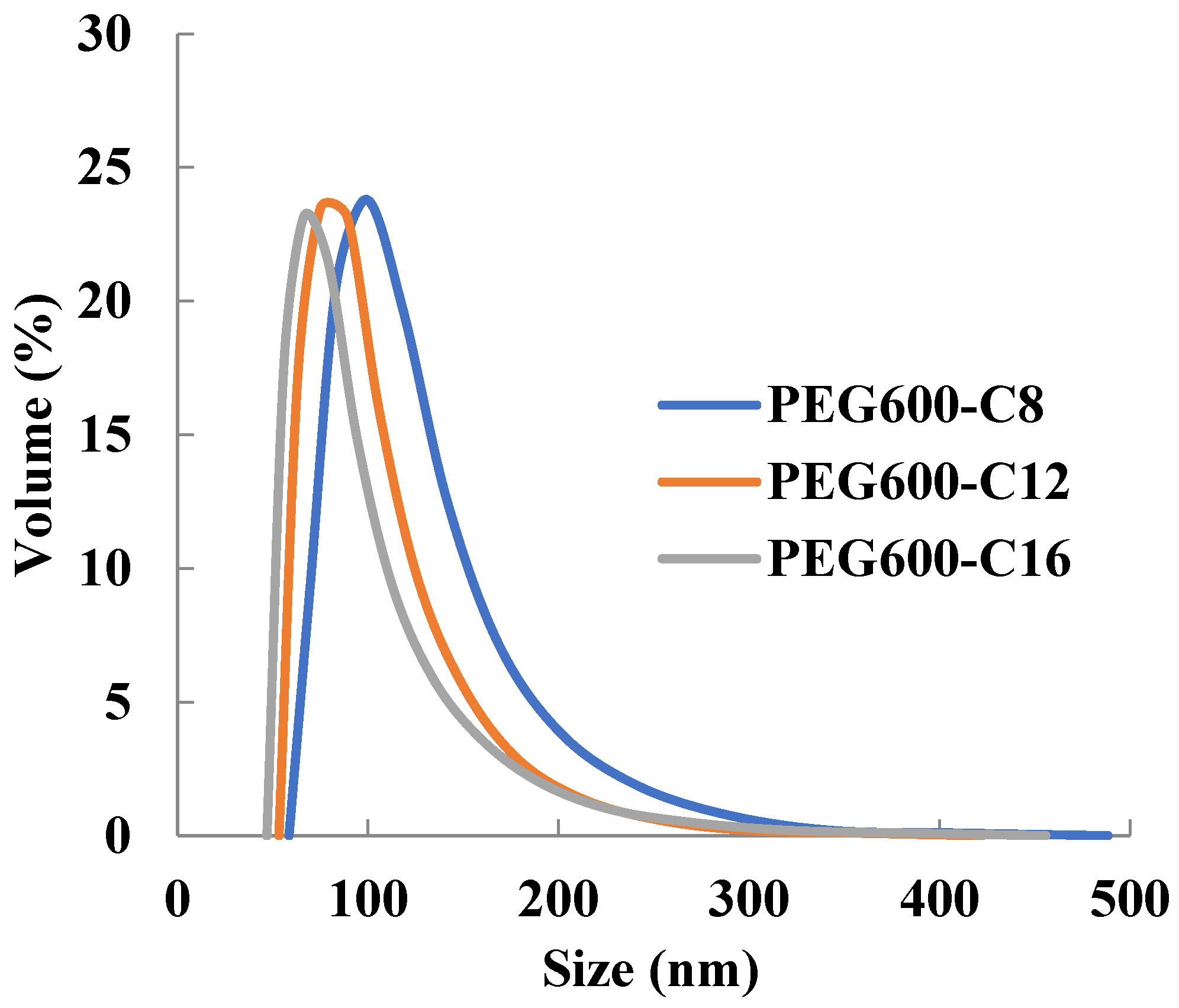
2.4. Self-Assembly of Poly[alkylpoly(ethylene glycol) phosphate-b-alkylpoly(ethylene glycol)lactate phosphate]s
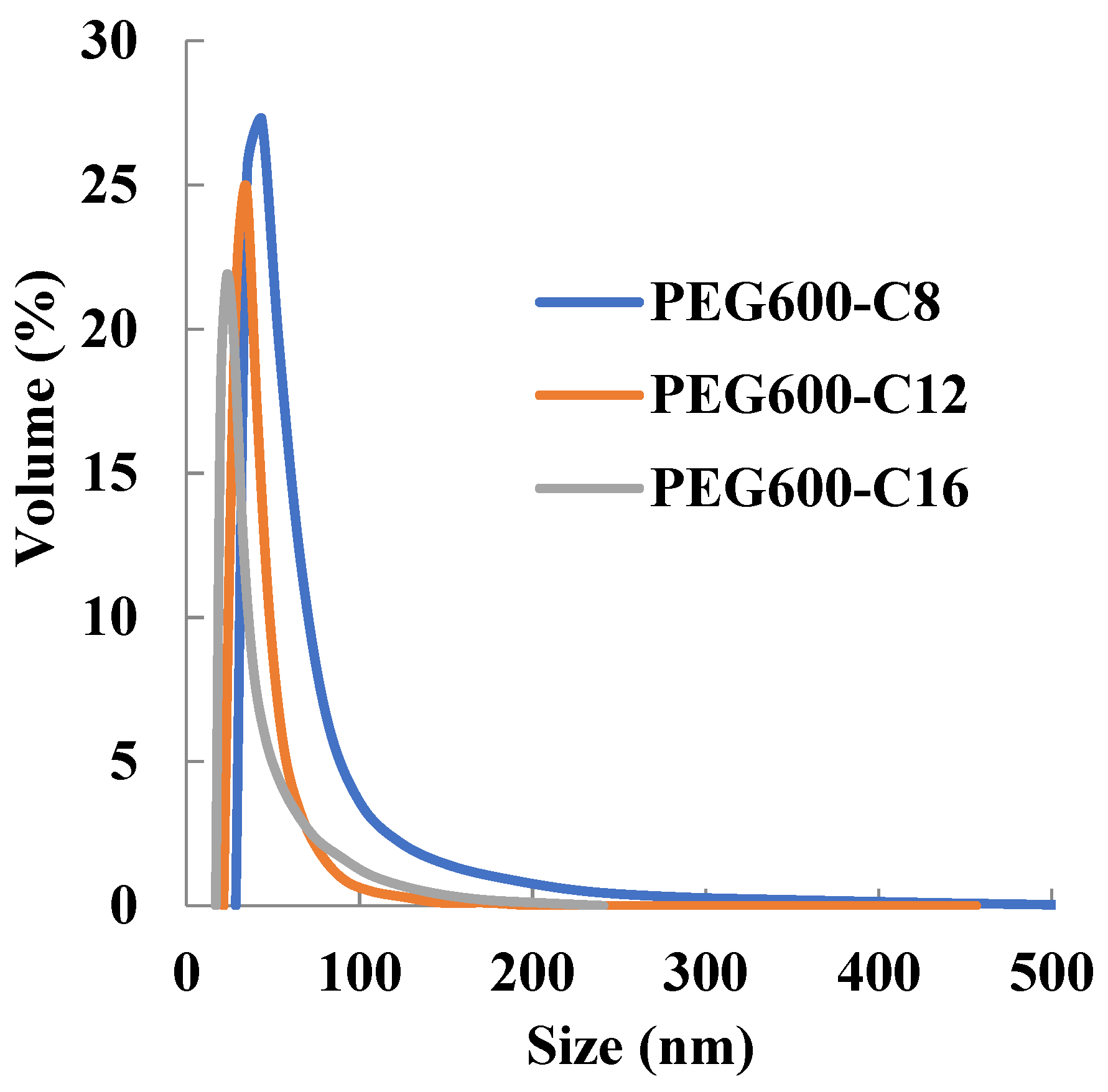
2.5. Particle Size Distribution and Loading Rate and Encapsulation Efficiency of Sudan III Encapsulating Micelles
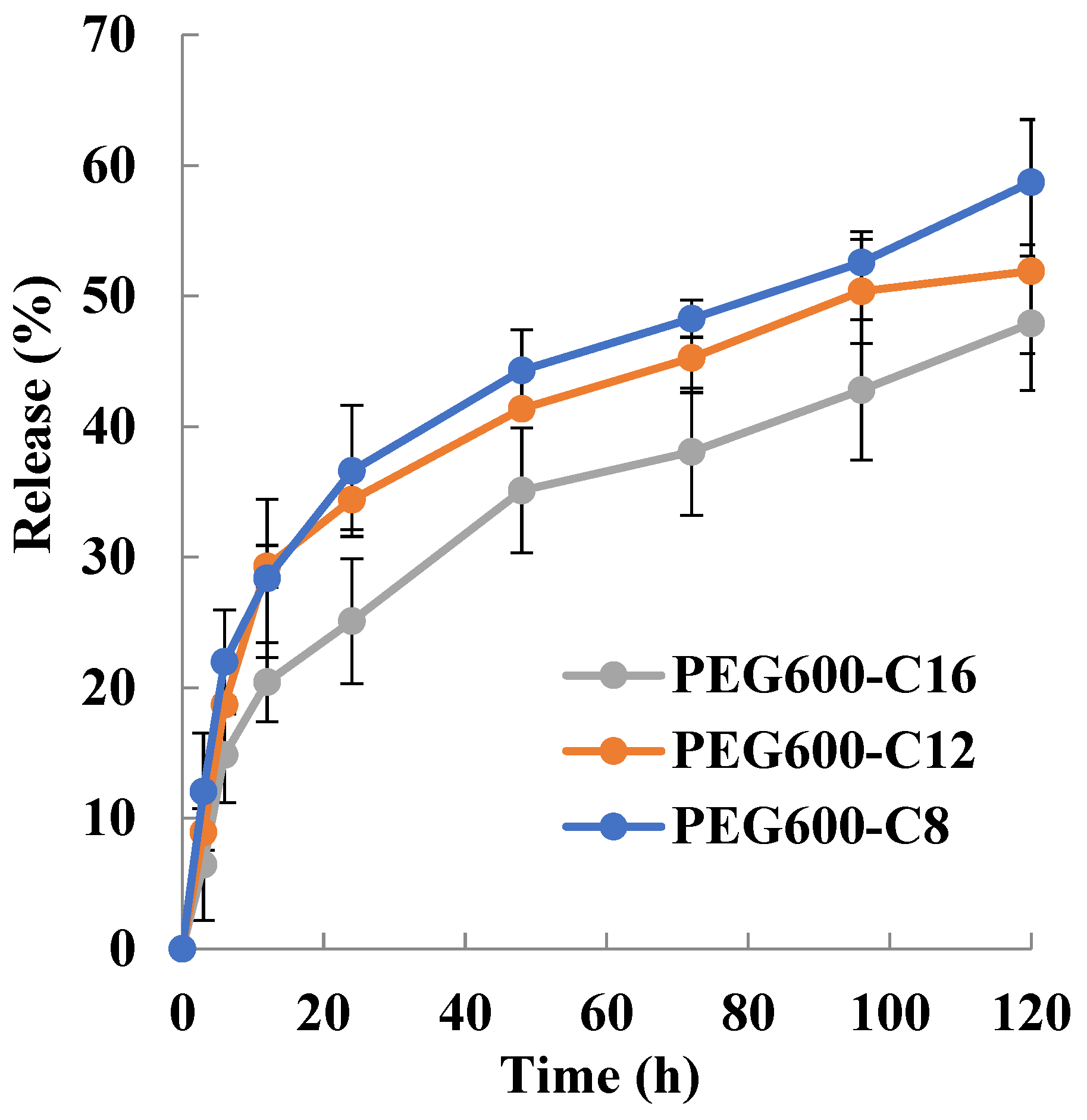
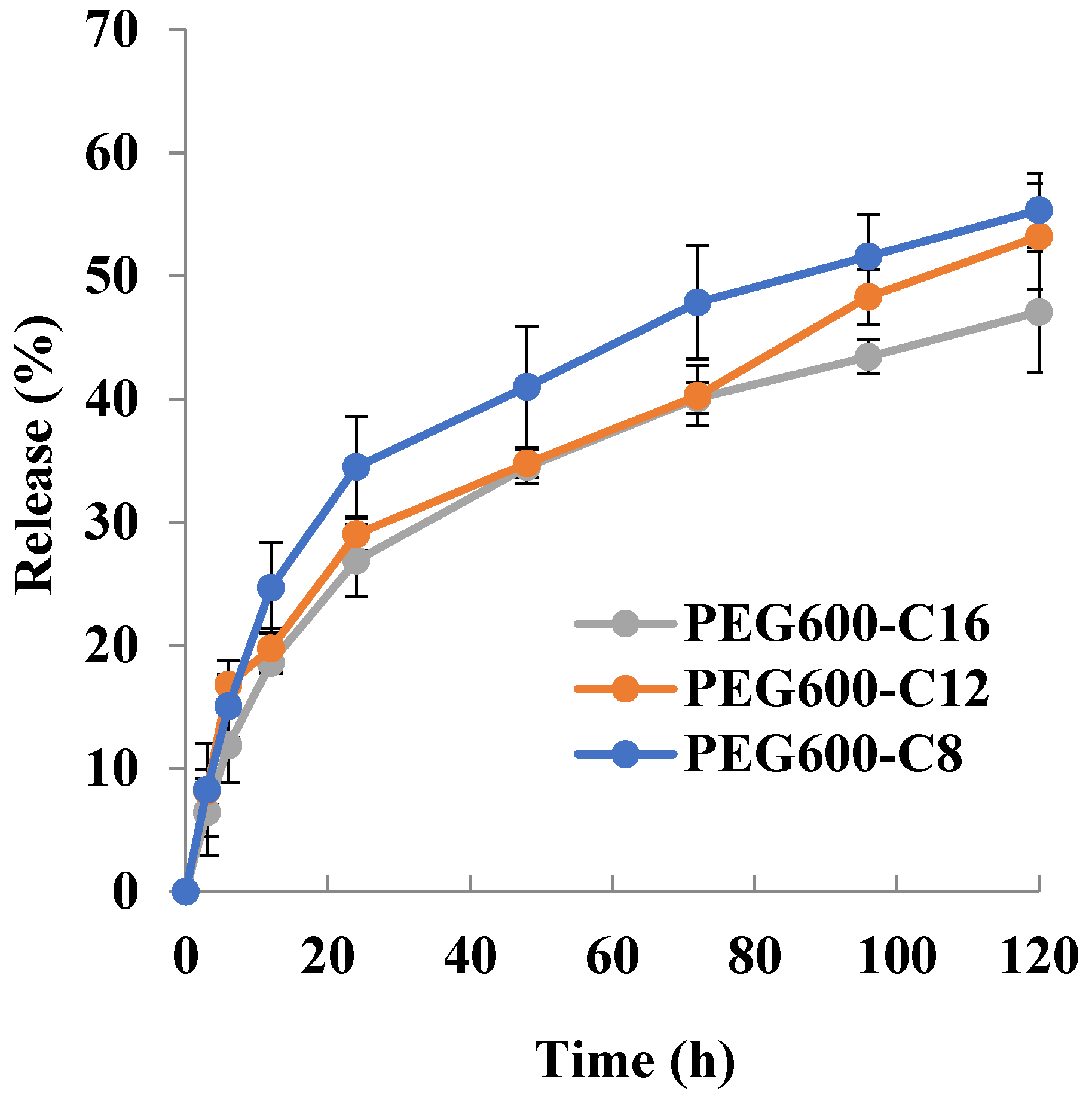
2.6. Particle Size Distribution and Drug Loading and Encapsulation Efficiency for Doxorubicin
3. Experimental Part
3.1. Materials
3.2. Characterization Methods and Instruments
3.3. Synthesis of Poly(ethylene glycol)lactate
3.4. Synthesis of Poly[poly(ethylene glycol) H-phosphonate-b-poly(ethylene glycol)lactate H-phosphonate]
3.5. Synthesis of Poly[alkylpoly(ethylene glycol) phosphate-b-alkylpoly(ethylene glycol)-lactate phosphate]s
3.5.1. Synthesis of Poly[hexadecylpoly(ethylene glycol) phosphate-b-[hexadecylpoly(ethylene glycol)lactate phosphate]
3.5.2. Synthesis of Poly[octylpoly(ethylene glycol) phosphate-b-octylpoly(ethylene glycol)lactate phosphate]
3.5.3. Synthesis of Poly[dodecylpoly(ethylene glycol) phosphate)-b-dodecylpoly(ethylene glycol)lactate phosphate]
3.6. Measurement of Polymeric Micelle Size
3.7. Solubilizing Test
3.7.1. Preparation of Sudan III Encapsulating Micelles
- A0: Absorbance of Sudan III encapsulating micelle on day 0
- An: Absorbance of Sudan III encapsulating micelle on day n.
3.7.2. Preparation of Doxorubicin-Encapsulating Micelles
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Troev, K.D. Polyphosphoesters: Chemistry and Application, 1st ed.; Elsevier: Amsterdam, The Netherlands; New York, NY, USA; Oxford, UK; Tokyo, Japan, 2012; p. 84. [Google Scholar]
- Tzevi, R.; Todorova, G.; Kossev, K.; Troev, K.; Georgiev, E.; Roundhill, D.M. Immobilization of bioactive substances on poly(a1kylenephosphate)s. Immobilization of 2-phenylethylamine. Macromol. Chem. 1993, 194, 3261–3269. [Google Scholar] [CrossRef]
- Troev, K.; Tsacheva, I.; Koseva, N.; Georgieva, R.; Gitsov, I. Immobilization of Aminothiols on Poly(oxyethylene H-phosphonate)s and Poly(oxyethylene phosphate)s—An Approach to Polymeric Protective Agents for Radiotherapy of Cancer. J. Polym. Sci. Part A Polym. Chem. 2007, 45, 1349–1363. [Google Scholar] [CrossRef]
- Myrex, R.D.; Farmer, B.; Gray, G.M.; Wright, Y.J.; Dees, J.; Bharara, P.C.; Byrd, H.; Branham, K.E. 31P and 1H NMR studies of the transesterification polymerization of polyphosphonate oligomers. Eur. Polym. J. 2003, 39, 1105–1115. [Google Scholar] [CrossRef]
- Oussadi, K.; Montemblault, V.; Fontaine, L. Synthesis of poly(oxyethylene phosphate)-g-poly(ethylene oxide) via the “grafting onto” approach by “click” chemistry. J. Polym. Sci. Part A Polymer Chem. 2011, 49, 5124–5128. [Google Scholar] [CrossRef]
- Oussadi, K.; Montemblault, V.; Belbachir, M.; Fontaine, L. Ring-opening bulk polymerization of five-and six-membered cyclic phosphonates using maghnite, a nontoxic proton exchanged montmorillonite clay. J. Appl. Polym. Sci. 2011, 122, 891–897. [Google Scholar] [CrossRef]
- Koseva, N.; Bogomilova, A.; Atkova, K.; Troev, K. New functional polyphosphoesters: Design and characterization. React. Funct. Polym. 2008, 68, 954–966. [Google Scholar] [CrossRef]
- Branham, K.E.; Mays, J.W.; Gray, G.M.; Bharara, P.C.; HByrd, H.; Bittenger, R.; Farmer, B. Polycondensations of dimethyl phosphonate with diols: SEC and 31P and 13C NMR spectroscopic studies. Polymer 2000, 41, 3371–3379. [Google Scholar] [CrossRef]
- Pretula, J.; Penczek, S. Poly(ethylene glycol) ionomers with phosphate diester linkages. Makromol. Chem. Rapid Commun. 1988, 9, 731–737. [Google Scholar] [CrossRef]
- Pretula, J.; Penczek, S. High-molecular-weight poly (alkylene phosphonate)s by condensation of dialkylphosphonates with diols. Makromol. Chem. 1990, 191, 671–680. [Google Scholar] [CrossRef]
- Penczek, S.; Pretula, J. High-molecular-weight poly (alkylene phosphates) and preparation of amphiphilic polymers thereof. Macromolecules 1993, 26, 2228–2233. [Google Scholar] [CrossRef]
- Gorjanova, B.; Gitsov, I.; Troev, K. Hydrolytic stability of poly (oxyethylene-H-phosphonate)s. In Proceedings of the 37th Middle Atlantic Regional Meeting of the American Chemical Society, New Brunswick, NJ, USA, 22–25 May 2005. [Google Scholar]
- Gitsov, I.; Johnson, F.E. Synthesis and hydrolytic stability of poly (oxyethylene-H-phosphonate)s. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 4130–4139. [Google Scholar] [CrossRef]
- Pretula, J.; Kaluzynski, K.; Szymanski, R.; Penczek, S. Preparation of poly (alkylene H-phosphonate) s and their derivatives by polycondensation of diphenyl H-phosphonate with diols and subsequent transformations. Macromolecules 1997, 30, 8172–8176. [Google Scholar] [CrossRef]
- Georgieva, R.; Tsevi, R.; Kossev, K.; Kusheva, R.; Balgjiska, M.; Petrova, R.; Tenchova, V.; Gitsov, I.; Troev, K. Immobilization of Aminothiols on Poly(oxyalkylene phosphates). Formation of Poly(oxyethylene phosphates)/Cysteamine Complexes and Their Radioprotective Efficiency. J. Med. Chem. 2002, 45, 5797–5801. [Google Scholar] [CrossRef]
- Pencheva, I.; Bogomilova, A.; Koseva, N.; Obreshkova, D.; Troev, K. HPLC study on the stability of bendamustine hydrochloride immobilized onto polyphosphoesters. J. Pharm. Biomed. Anal. 2008, 48, 1143–1150. [Google Scholar] [CrossRef] [PubMed]
- Kraicheva, I.; Bogomilova, A.; Tsacheva, I.; Momekov, G.; Momekova, D.; Troev, K. Synthesis, NMR characterization and in vitro cytotoxicity evaluation of new poly (oxyethylene aminophosphonate)s. Eur. J. Med. Chem. 2010, 45, 6039–6044. [Google Scholar] [CrossRef] [PubMed]
- Troev, K.; Mitova, V.; Ivanov, I. On the design of polymeric 5′-O-ester prodrugs of 3′-azido-2′, 3′-dideoxythymidine (AZT). Tetrahedron Lett. 2010, 51, 6123–6125. [Google Scholar] [CrossRef]
- Mitova, V.; Hristova, T.; Cherkezova, R.; Koseva, N.; Yusa, S.; Troev, K. Polyphosphoesters Based—Placlitaxel Complexes. Synthesis and Charcterization. J. Appl. Polym. Sci. 2015, 132, 42772. [Google Scholar] [CrossRef]
- Hirota, K.; Hristova, T.; Mitova, V.; Koda, T.; Fushimi, M.; Kuniya, M.; Makino, K.; Terada, H.; Cherkezova, R.; Yusa, S.-I.; et al. Polyphosphoesters Based—Placlitaxel Complexes. Biol. Eval. Anticancer. Res. 2016, 36, 1613–1620. [Google Scholar]
- Todorova, Z.; Koseva, N.; Ugrinova, I.K.; Troev, K. Synthesis of Poly(oxyethylene phosphoramidate)s and Glycopolymers via. Staudinger Reaction: Multivalent Binding Studies with Concanavalin A. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 1730. [Google Scholar] [CrossRef]
- Wang, J.; Mao, H.Q.; Leong, K.W. A novel biodegradable gene carrier based on polyphosphoester. J. Am. Chem. Soc. 2001, 123, 9480–9481. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, P.C.; Mao, H.Q.; Leong, K.W. Enhanced gene expression in mouse muscle by sustained release of plasmid DNA using PPE-EA as a carrier. Gene Ther. 2002, 9, 1254–1261. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, P.C.; Lu, H.F.; Ma, N.; Wang, S.; Mao, H.Q.; Leong, K.W. New polyphosphoramidate with a spermidine side chain as a gene carrier. J. Control. Release 2002, 83, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Jiang, X.; Pan, D.; Mao, H.Q. Charge density and molecular weight of polyphosphoramidate gene carrier are key parameters influencing its DNA compaction ability and transfection efficiency. Biomacromolecules 2010, 11, 3432–3439. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gao, S.J.; Zhang, P.C.; Wang, S.; Mao, H.Q.; Leong, K.W. Polyphosphoramidate gene carriers: Effect of charge group on gene transfer efficiency. Gene Ther. 2004, 11, 1001–1010. [Google Scholar] [CrossRef]
- Huang, S.W.; Wang, J.; Zhang, P.C.; Mao, H.Q.; Zhuo, R.X.; Leong, K.W. Water-soluble and nonionic polyphosphoester: Synthesis, degradation, biocompatibility and enhancement of gene expression in mouse muscle. Biomacromolecules 2004, 5, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Dai, H.; Ke, C.Y.; Mo, X.; Torbenson, M.S.; Li, Z.; Mao, H.Q. PEG-b-PPA/DNA micelles improve transgene expression in rat liver through intrabiliary infusion. J. Control. Release 2007, 122, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Yamakita, Y.; Tekauchi, I.; Makino, K.; Terada, H.; Kikuchi, A.; Troev, K. Thermoresponsive Polyphosphoester via Polycondensation Reactions: Synthesis, Characterization, and Self-Assembly. Molecules 2022, 27, 6006. [Google Scholar] [CrossRef]
- Ikada, Y.; Tsuji, H. Biodegradable polyesters for medical and ecological applications. Macromol. Rapid Commun. 2000, 21, 117–132. [Google Scholar] [CrossRef]
- Vert, M. Aliphatic polyesters: Great degradable polymers that cannot do everything. Biomacromolecules 2005, 6, 538–546. [Google Scholar] [CrossRef]
- Parrish, B.; Emrick, T. Aliphatic Polyesters with Pendant Cyclopentene Groups: Controlled Synthesis and Conversion to Polyester-graft-PEG Copolymers. Macromolecules 2004, 37, 5863–5865. [Google Scholar] [CrossRef]
- Langlois, V.; Vallee-Rehel, K.; Peron, J.J.; Borgne, A.; Walls, M.; Guerin, P. Synthesis and hydrolytic degradation of graft copolymers containing poly (lactic acid) side chains: In vitro release studies of bioactive molecules. Polym. Degrad. Stab. 2002, 76, 411–417. [Google Scholar] [CrossRef]
- Tsuji, H.; Miyase, T.; Tezuka, Y.; Saha, S.K. Physical Properties, Crystallization, and Spherulite Growth of Linear and 3-Arm Poly(l-lactide)s. Biomacromolecules 2005, 6, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Tuominen, J.; Kylma, J.; Seppala, J. Chain extending of lactic acid oligomers. 2. Increase of molecular weight with 1, 6-hexamethylene diisocyanate and 2, 2′-bis (2-oxazoline). Polymer 2002, 43, 3–10. [Google Scholar] [CrossRef]
- Andreopoulos, A.G.; Hatzi, G.E.; Doxastakis, M. Synthesis and properties. J. Mater. Sci. Mater. Med. 1999, 10, 29–33. [Google Scholar] [CrossRef]
- Stridsberg, K.; Albertsson, A.C. Controlled ring-opening polymerization of L-lactide and 1,5-dioxepan-2-one forming a triblock copolymer. J. Polym. Sci. Part A Polym. Chem. 2000, 38, 1774–1784. [Google Scholar] [CrossRef]
- Okada, M. Chemical syntheses of biodegradable polymers. Prog. Polym. Sci. 2002, 27, 87–133. [Google Scholar] [CrossRef]
- Hile, D.D.; Pishko, M.V. Emulsion copolymerization of D,L-lactide and glycolide in supercritical carbon dioxide. J. Polym. Sci. Part A Polym. Chem. 2001, 39, 562–570. [Google Scholar] [CrossRef]
- Tuominen, J.; Seppala, J.V. Synthesis and characterization of lactic acid based poly (ester−amide). Macromolecules 2000, 33, 3530–3535. [Google Scholar] [CrossRef]
- Bayan, M.F.; Jaradat, A.; Alyami, M.H.; Naser, A.Y. Smart pellets for controlled delivery of 5-fluorouracil. Molecules 2023, 28, 306. [Google Scholar] [CrossRef]
- Bayan, F.B.; Marji, S.M.; Salem, M.S.; Begum, M.Y.; Chidambaram, K.; Chandrasekaran, B. Development of polymeric-based formulation as potential smart colonic drug delivery system. Polymers 2022, 14, 3697. [Google Scholar] [CrossRef]
- Troev, K.; Naruoka, A.; Terada, H.; Kikuchi, A.; Makino, K. New Efficient Method of Oxidation of Poly(alkylene H-phosphonate)s: A Promising Route to Novel co-Polyphosphoesters. Macromolecules 2012, 45, 5698–5703. [Google Scholar] [CrossRef]
- Chaubal, M.V.; Wang, B.; Su, G.; Zhao, Z. Compositional analysis of biodegradable polyphosphoester copolymers using nmr spectroscopic methods. J. Appl. Polym. Sci. 2003, 90, 4021–4031. [Google Scholar] [CrossRef]
- Modro, A.M.; Modro, T.A. The phosphoryl and the carbonyl group as hydrogen bond acceptors. Phosphorus Sulfur Silicon 2002, 177, 2067. [Google Scholar] [CrossRef]
- La, S.B.; Okano, T.; Kataoka, K. Preparation and characterization of the micelle-forming polymeric drug indomethacin-incorporated poly(ethylene oxide)-poly(beta-benzyl L-aspartate) block copolymer micelles. J. Pharm. Sci. 1996, 85, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Kwon, G.; Naito, M.; Yokoyama, M.; Okano, T.; Sakurai, Y.; Kataoka, K. Block copolymer micelles for drug delivery: Loading and release of doxorubicin. J. Control. Release 1997, 48, 195–201. [Google Scholar] [CrossRef]
- Kim, S.Y.; Shin, I.G.; Lee, Y.M.; Cho, C.S.; Sung, Y.K. Methoxy poly(ethylene glycol) and e -caprolactone amphiphilic block copolymeric micelle containing indomethacin. II. Micelle formation and drug release behaviors. J. Control. Release 1998, 51, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.; Maysinger, D. Adi Eisenberg Nano-engineering block copolymer aggregates for drug delivery. Colloids Surf. B Biointerfaces 1999, 16, 3–27. [Google Scholar] [CrossRef]
- De Jaeghere, F.; Allemann, E.; Leroux, J.C.; Stevels, W.; Feijen, J.; Doelker, E.; Gurny, R. Formulation and lyoprotection of poly (lactic acid-co-ethyleneoxide) nanoparticles: Influence on physical stability and in vitro cell uptake. Pharm. Res. 1999, 16, 859–866. [Google Scholar] [CrossRef]
- Hussein, Y.H.A.; Youssry, M. Polymeric Micelles of Biodegradable Diblock Copolymers: Enhanced Encapsulation of Hydrophobic Drugs. Materials 2018, 11, 688–704. [Google Scholar] [CrossRef]
- Jeong, Y.-I.; Cheon, J.-B.; Kim, S.-H.; Nah, J.-W.; Lee, Y.-M.; Sung, Y.-K.; Akaike, T.; Cho, C.-S. Clonazepam release from core-shell type nanoparticles in vitro. J. Control. Release 1998, 51, 169–178. [Google Scholar] [CrossRef]

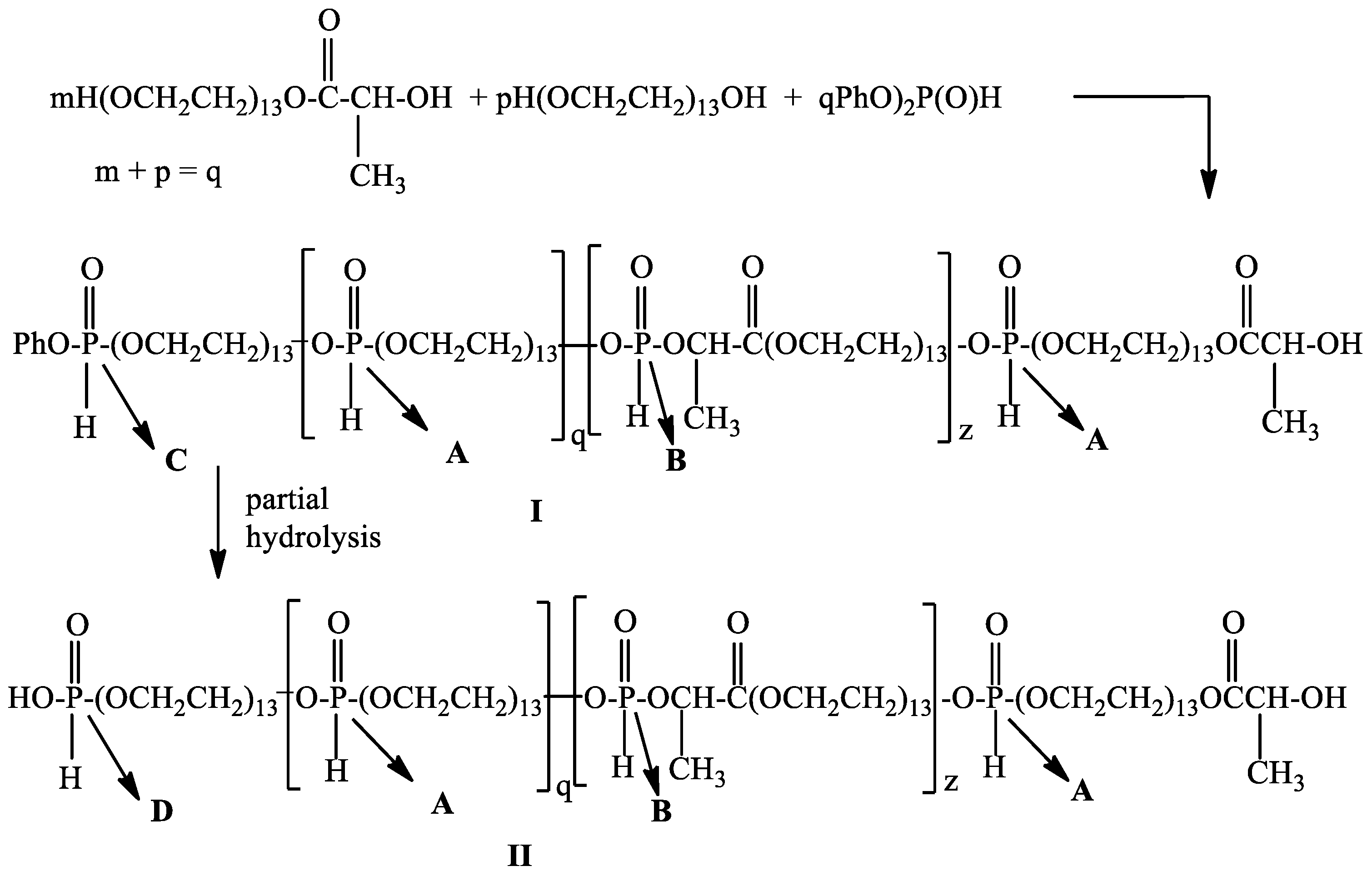
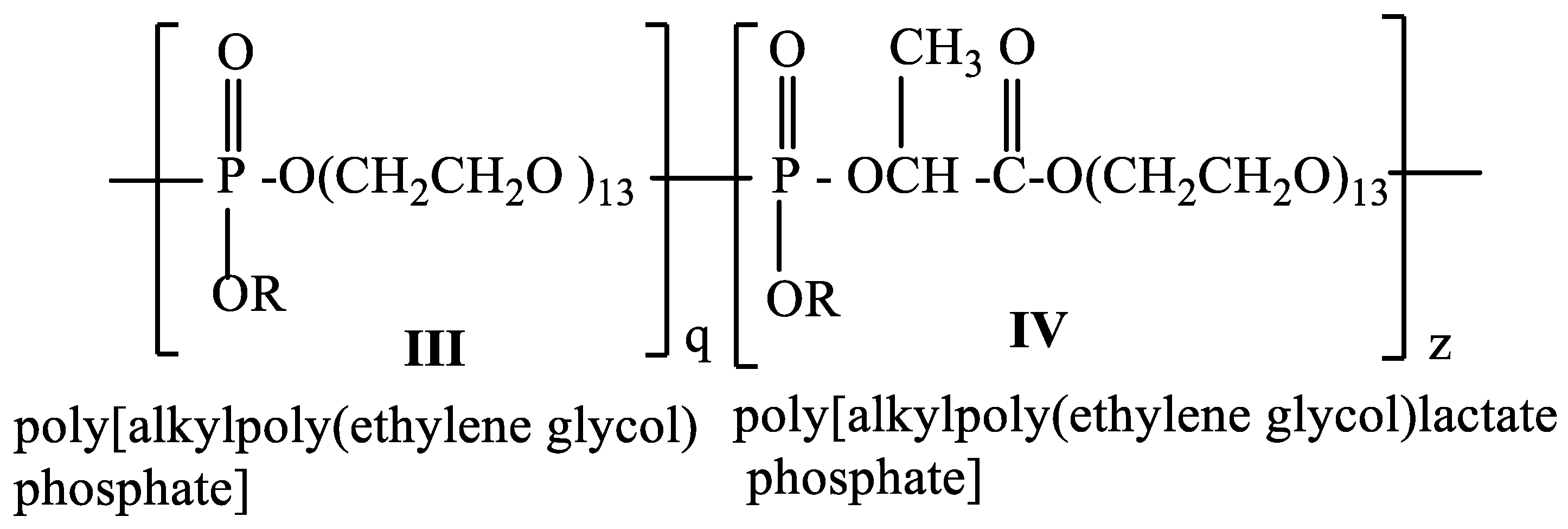


| Polymer | Molecular Weight (×103) | Molecular Weight/ Molecular Number |
|---|---|---|
| PEG600-OC8H17 | 16.8 | 1.56 |
| PEG600-OC12H25 | 16.9 | 1.65 |
| PEG600-OC16H33 | 17.2 | 1.57 |
| Polymer | Particle Size (nm) |
|---|---|
| PEG600-OC8H17 | 58.0 ± 28.4 |
| PEG600-OC12H25 | 40.2 ± 14.4 |
| PEG600-OC16H33 | 36.8 ± 15.7 |
| Polymer + Sudan III | Particle Size (nm) |
|---|---|
| PEG600-OC8H17 | 107.5 ± 41.9 |
| PEG600-OC12H25 | 86.8 ± 34.7 |
| PEG600-OC16H33 | 71.9 ± 36.5 |
| Polymer | Drug Loading (%) | Encapsulation Efficiency (%) |
|---|---|---|
| PEG600-OC8H17 | 0.48 ± 0.13 | 6.04 ± 1.39 |
| PEG600-OC12H25 | 0.66 ± 0.13 | 7.61 ± 1.48 |
| PEG600-OC16H33 | 0.75 ± 0.15 | 8.16 ± 1.58 |
| Polymer + Doxorubicin | Particle Size (nm) |
|---|---|
| PEG600-OC8H17 | 116.1 ± 41.9 |
| PEG600-OC12H25 | 94.4 ± 57.5 |
| PEG600-OC16H33 | 88.7 ± 37.0 |
| Polymer | Drug Loading (%) | Encapsulation Efficiency (%) |
|---|---|---|
| PEG600-OC8H17 | 1.46 ± 0.30 | 37.76 ± 7.66 |
| PEG600-OC12H25 | 1.61 ± 0.24 | 43.38 ± 6.56 |
| PEG600-OC16H33 | 1.63 ± 0.46 | 45.80 ± 12.82 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakuma, T.; Makino, K.; Terada, H.; Takeuchi, I.; Mitova, V.; Troev, K. Synthesis and Characterization of Amphiphilic Diblock Polyphosphoesters Containing Lactic Acid Units for Potential Drug Delivery Applications. Molecules 2023, 28, 5243. https://doi.org/10.3390/molecules28135243
Sakuma T, Makino K, Terada H, Takeuchi I, Mitova V, Troev K. Synthesis and Characterization of Amphiphilic Diblock Polyphosphoesters Containing Lactic Acid Units for Potential Drug Delivery Applications. Molecules. 2023; 28(13):5243. https://doi.org/10.3390/molecules28135243
Chicago/Turabian StyleSakuma, Tatsuya, Kimiko Makino, Hiroshi Terada, Issei Takeuchi, Violeta Mitova, and Kolio Troev. 2023. "Synthesis and Characterization of Amphiphilic Diblock Polyphosphoesters Containing Lactic Acid Units for Potential Drug Delivery Applications" Molecules 28, no. 13: 5243. https://doi.org/10.3390/molecules28135243
APA StyleSakuma, T., Makino, K., Terada, H., Takeuchi, I., Mitova, V., & Troev, K. (2023). Synthesis and Characterization of Amphiphilic Diblock Polyphosphoesters Containing Lactic Acid Units for Potential Drug Delivery Applications. Molecules, 28(13), 5243. https://doi.org/10.3390/molecules28135243







