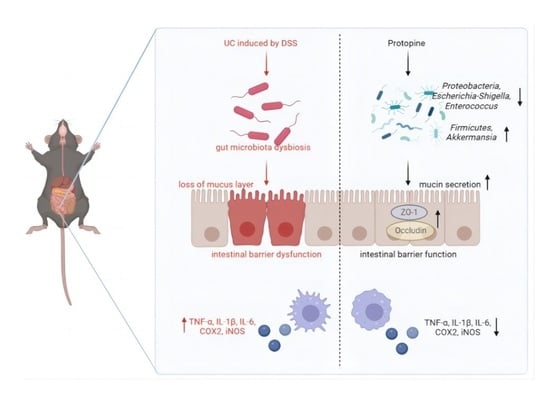
Protopine Alleviates Dextran Sodium Sulfate-Induced Ulcerative Colitis by Improving Intestinal Barrier Function and Regulating Intestinal Microbiota
Abstract
:1. Introduction
2. Results
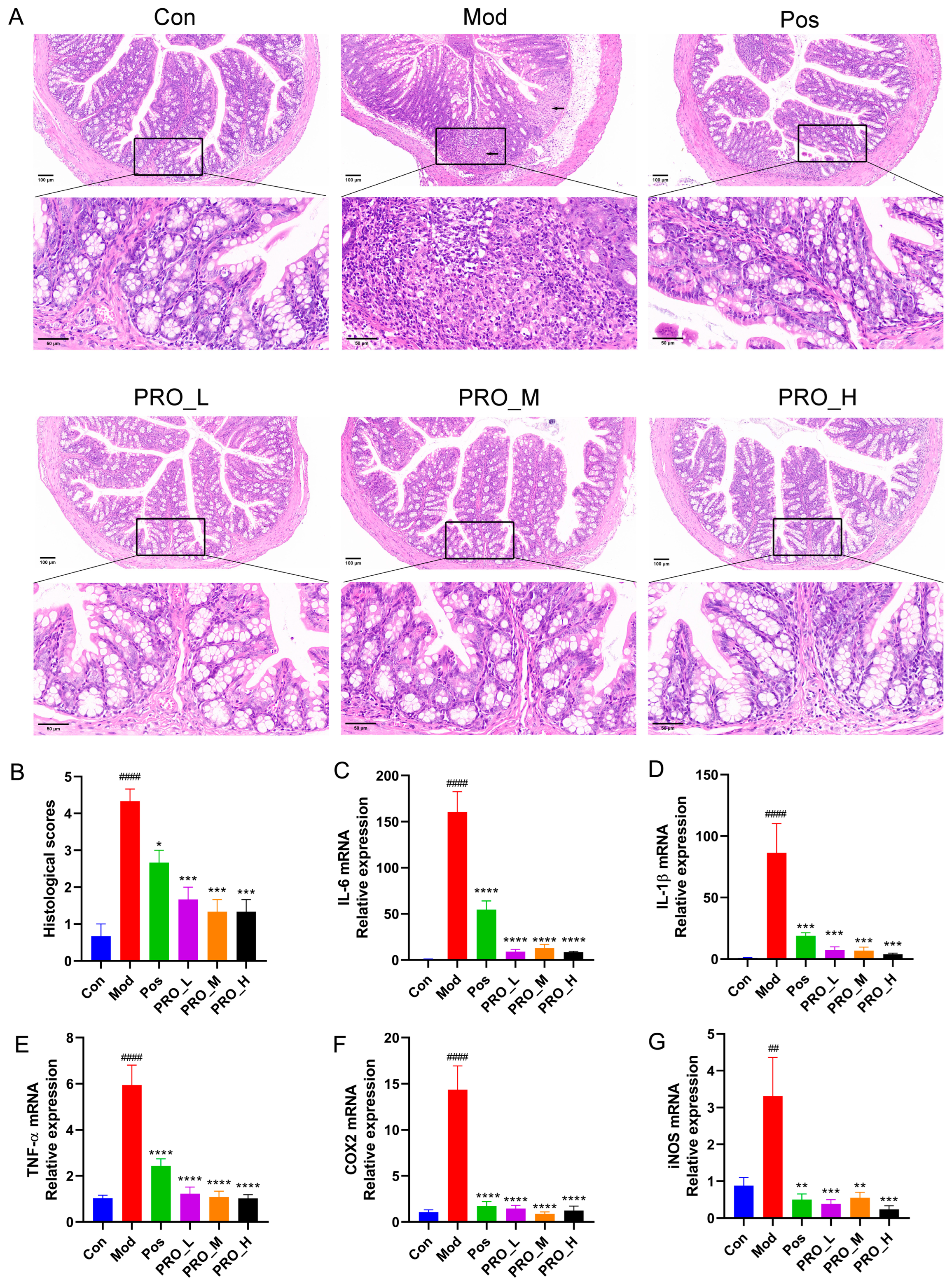
2.1. PRO Attenuated the Symptoms of DSS-Induced Colitis in Mice
2.2. PRO Inhibited the Expression of Inflammation-Related Genes in DSS-Induced Colitis
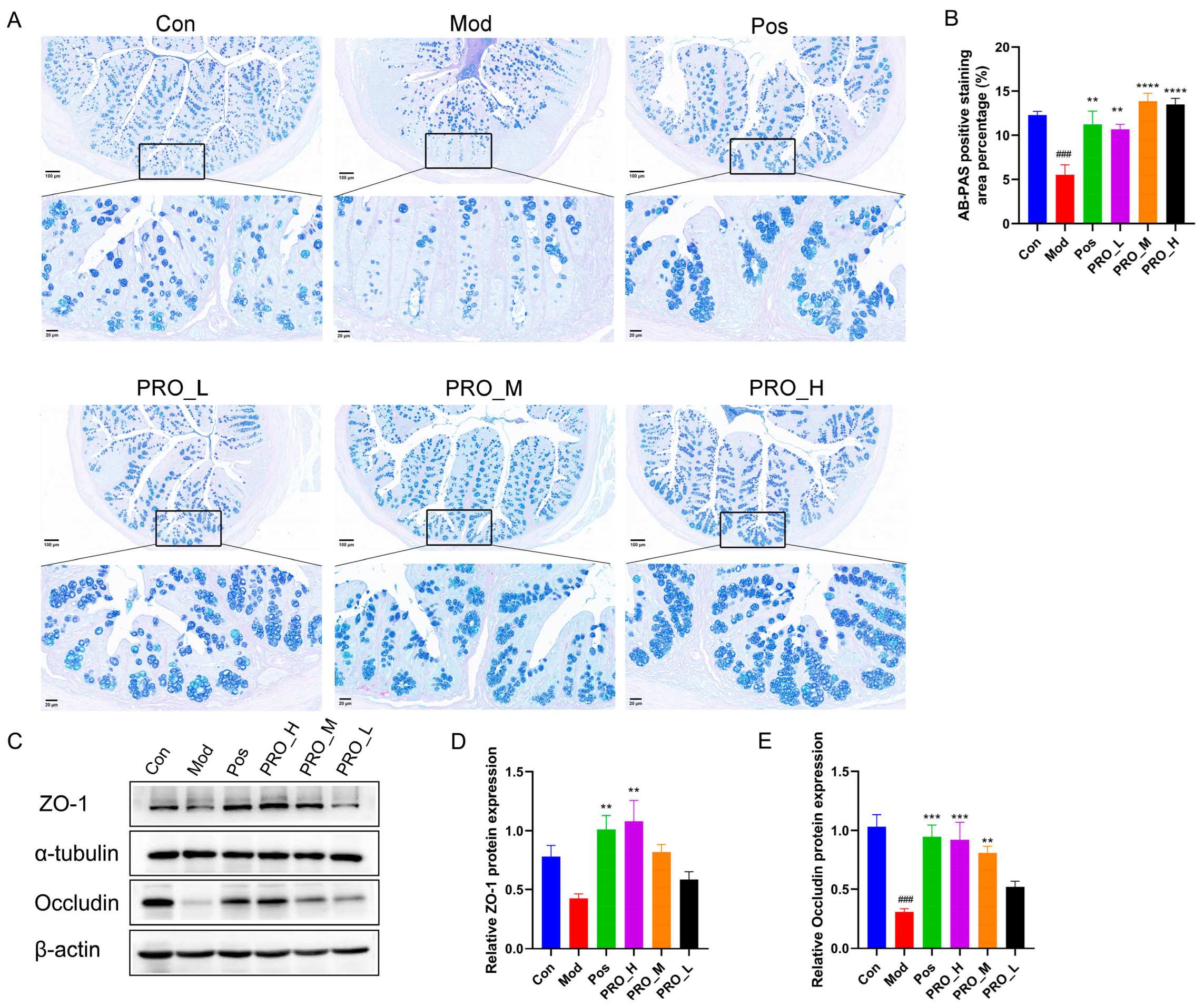
2.3. PRO Ameliorated Colonic Barrier Dysfunction in Mice with DSS-Induced Colitis
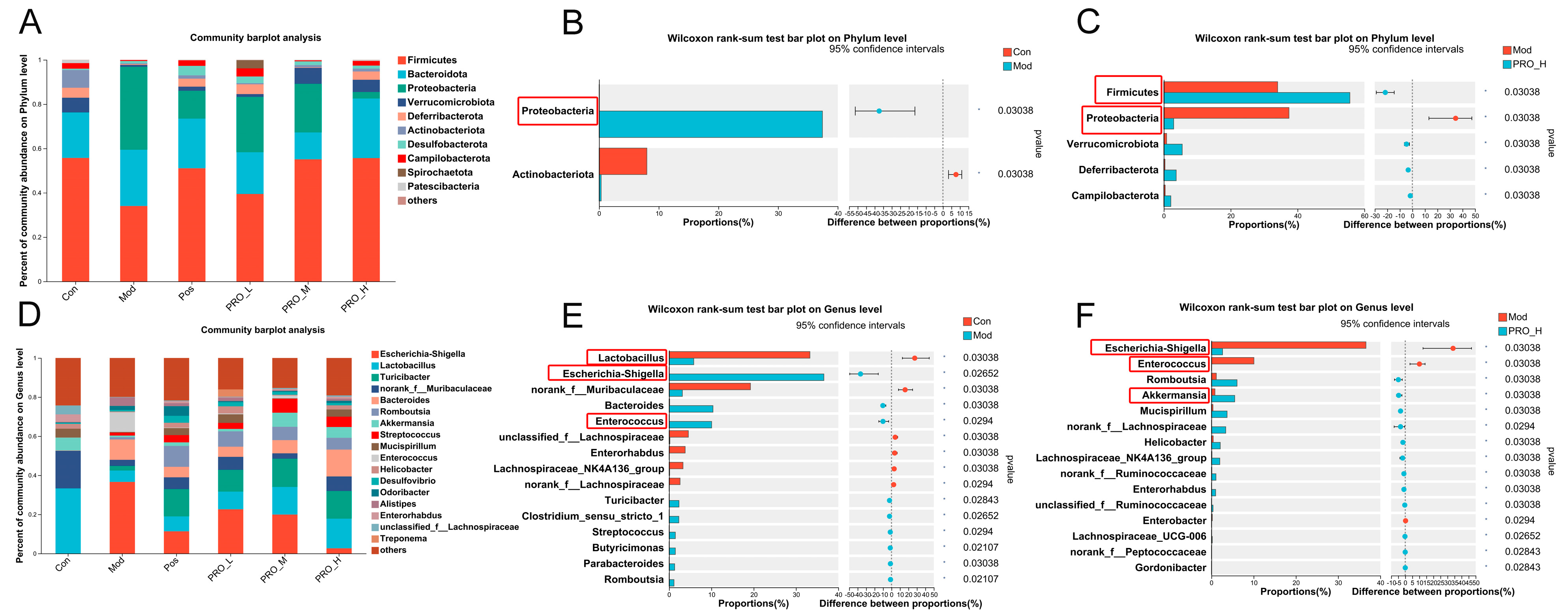
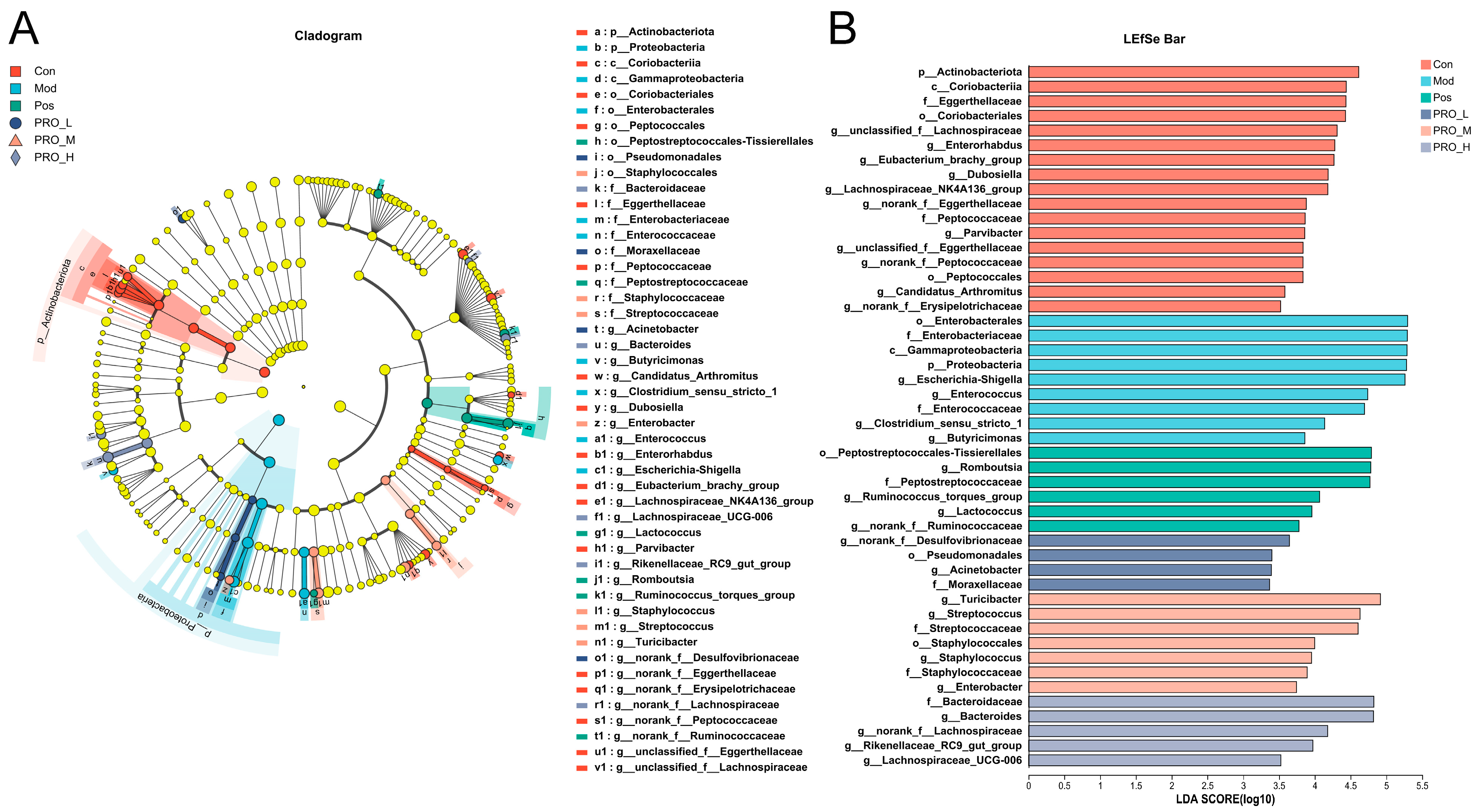
2.4. PRO Modulated Gut Microbiota Dysbiosis in DSS-Induced Colitis Mice
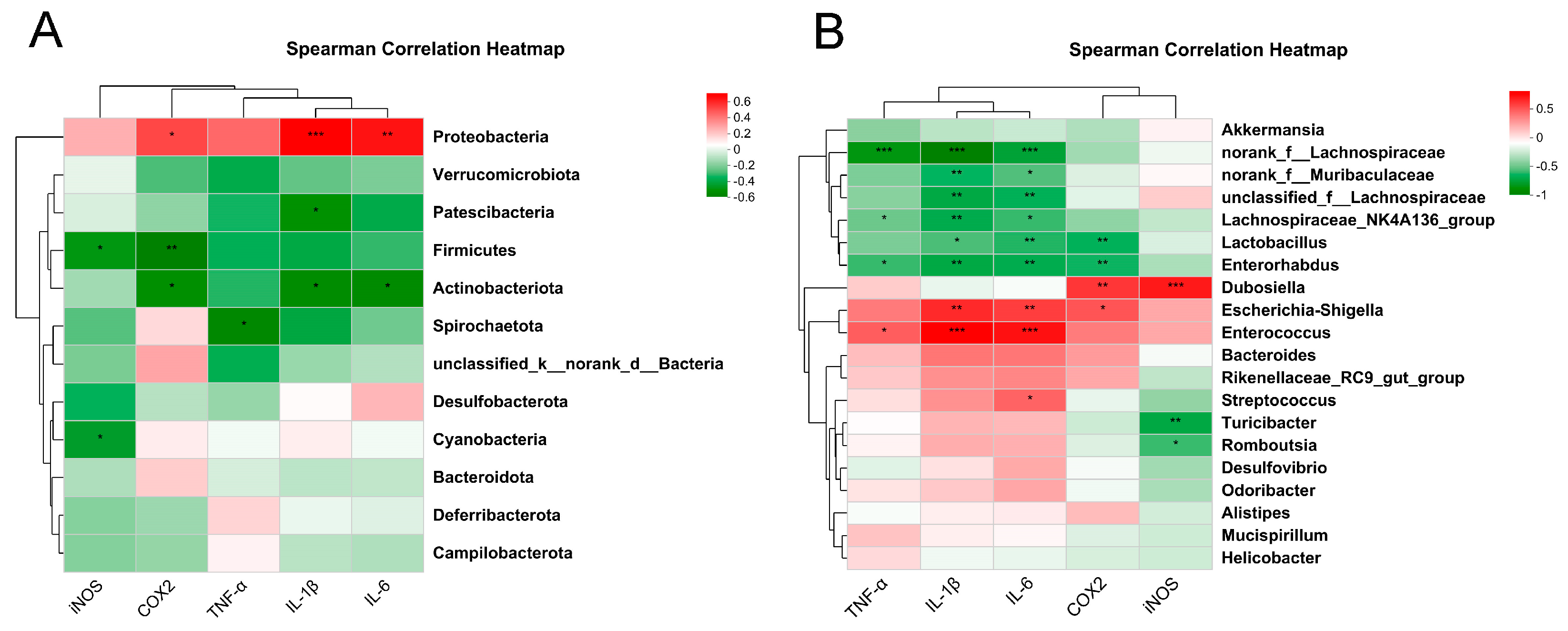
2.5. Correlation Analysis between Inflammation-Related Genes and Intestinal Microbiota
3. Discussion
4. Materials and Methods
4.1. Animals and Experimental Design
4.2. HE and AB-PAS Staining
4.3. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
4.4. Western Blot Analysis
4.5. Gut Microbiota Analysis
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Ungaro, R.; Mehandru, S.; Allen, P.B.; Peyrin-Biroulet, L.; Colombel, J.F. Ulcerative colitis. Lancet 2017, 389, 1756–1770. [Google Scholar] [CrossRef]
- Chelakkot, C.; Ghim, J.; Ryu, S.H. Mechanisms regulating intestinal barrier integrity and its pathological implications. Exp. Mol. Med. 2018, 50, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, L.; Ha, C. Epidemiology and Pathogenesis of Ulcerative Colitis. Gastroenterol. Clin. N. Am. 2020, 49, 643–654. [Google Scholar] [CrossRef]
- Lee, S.H.; Kwon, J.E.; Cho, M.L. Immunological pathogenesis of inflammatory bowel disease. Intest. Res. 2018, 16, 26–42. [Google Scholar] [CrossRef] [Green Version]
- Tatiya-Aphiradee, N.; Chatuphonprasert, W.; Jarukamjorn, K. Immune response and inflammatory pathway of ulcerative colitis. J. Basic Clin. Physiol. Pharmacol. 2018, 30, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Eichele, D.D.; Kharbanda, K.K. Dextran sodium sulfate colitis murine model: An indispensable tool for advancing our understanding of inflammatory bowel diseases pathogenesis. World J. Gastroenterol. 2017, 23, 6016–6029. [Google Scholar] [CrossRef] [PubMed]
- Pelaseyed, T.; Bergstrom, J.H.; Gustafsson, J.K.; Ermund, A.; Birchenough, G.M.; Schutte, A.; van der Post, S.; Svensson, F.; Rodriguez-Pineiro, A.M.; Nystrom, E.E.; et al. The mucus and mucins of the goblet cells and enterocytes provide the first defense line of the gastrointestinal tract and interact with the immune system. Immunol. Rev. 2014, 260, 8–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pei, L.Y.; Ke, Y.S.; Zhao, H.H.; Wang, L.; Jia, C.; Liu, W.Z.; Fu, Q.H.; Shi, M.N.; Cui, J.; Li, S.C. Role of colonic microbiota in the pathogenesis of ulcerative colitis. BMC Gastroenterol. 2019, 19, 10. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.; Huang, C.; Xu, J.; Xu, H.; Liu, L.; Zhao, H.; Wang, J.; Huang, W.; Peng, W.; Chen, Y.; et al. Gut Microbiota Is a Potential Biomarker in Inflammatory Bowel Disease. Front. Nutr. 2021, 8, 818902. [Google Scholar] [CrossRef]
- Chang, J.T. Pathophysiology of Inflammatory Bowel Diseases. N. Engl. J. Med. 2020, 383, 2652–2664. [Google Scholar] [CrossRef]
- Huttenhower, C.; Kostic, A.D.; Xavier, R.J. Inflammatory bowel disease as a model for translating the microbiome. Immunity 2014, 40, 843–854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danielewski, M.; Zielinska, S.; Matuszewska, A.; Slupski, W.; Wlodarczyk, M.; Jeskowiak, I.; Wiatrak, B.; Kowalski, K.; Jezierska-Domaradzka, A.; Ziolkowski, P.; et al. Sanguinarine-Chelerythrine Fraction of Coptis chinensis Exerts Anti-inflammatory Activity in Carrageenan Paw Oedema Test in Rats and Reveals Reduced Gastrotoxicity. Oxid. Med. Cell Longev. 2022, 2022, 1504929. [Google Scholar] [CrossRef] [PubMed]
- Li, C.M.; Yang, X.Y.; Zhong, Y.R.; Yu, J.P. Chemical composition, antioxidant and antimicrobial activity of the essential oil from the leaves of Macleaya cordata (Willd) R. Br. Nat. Prod. Res. 2016, 30, 438–442. [Google Scholar] [CrossRef] [PubMed]
- Ke, W.; Lin, X.; Yu, Z.; Sun, Q.; Zhang, Q. Molluscicidal activity and physiological toxicity of Macleaya cordata alkaloids components on snail Oncomelania hupensis. Pestic. Biochem. Physiol. 2017, 143, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Yin, Y.; Yang, M.; Chen, J.; Fu, C.; Huang, K. Effects of Combined Supplementation of Macleaya cordata Extract and Benzoic Acid on the Growth Performance, Immune Responses, Antioxidant Capacity, Intestinal Morphology, and Microbial Composition in Weaned Piglets. Front. Vet. Sci. 2021, 8, 708597. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhang, J.; Huang, X.; Liu, Y.; Zeng, J. Effects of Dietary Macleaya cordata Extract on Growth Performance, Biochemical Indices, and Intestinal Microbiota of Yellow-Feathered Broilers Subjected to Chronic Heat Stress. Animals 2022, 12, 2197. [Google Scholar] [CrossRef]
- Dong, Z.; Wang, Y.H.; Tang, Z.S.; Li, C.H.; Jiang, T.; Yang, Z.H.; Zeng, J.G. Exploring the Anti-inflammatory Effects of Protopine Total Alkaloids of Macleaya Cordata (Willd.) R. Br. Front. Vet. Sci. 2022, 9, 935201. [Google Scholar] [CrossRef]
- Xiao, X.; Liu, J.; Hu, J.; Zhu, X.; Yang, H.; Wang, C.; Zhang, Y. Protective effects of protopine on hydrogen peroxide-induced oxidative injury of PC12 cells via Ca(2+) antagonism and antioxidant mechanisms. Eur. J. Pharmacol. 2008, 591, 21–27. [Google Scholar] [CrossRef]
- Nie, C.; Wang, B.; Wang, B.; Lv, N.; Yu, R.; Zhang, E. Protopine triggers apoptosis via the intrinsic pathway and regulation of ROS/PI3K/Akt signalling pathway in liver carcinoma. Cancer Cell Int. 2021, 21, 396. [Google Scholar] [CrossRef]
- Zhang, B.; Zeng, M.; Li, M.; Kan, Y.; Li, B.; Xu, R.; Wu, Y.; Wang, S.; Zheng, X.; Feng, W. Protopine Protects Mice against LPS-Induced Acute Kidney Injury by Inhibiting Apoptosis and Inflammation via the TLR4 Signaling Pathway. Molecules 2019, 25, 15. [Google Scholar] [CrossRef] [Green Version]
- Alam, M.B.; Ju, M.K.; Kwon, Y.G.; Lee, S.H. Protopine attenuates inflammation stimulated by carrageenan and LPS via the MAPK/NF-kappaB pathway. Food Chem. Toxicol. 2019, 131, 110583. [Google Scholar] [CrossRef]
- Bae, D.S.; Kim, Y.H.; Pan, C.H.; Nho, C.W.; Samdan, J.; Yansan, J.; Lee, J.K. Protopine reduces the inflammatory activity of lipopolysaccharide-stimulated murine macrophages. BMB Rep. 2012, 45, 108–113. [Google Scholar] [CrossRef] [Green Version]
- Son, Y.; An, Y.; Jung, J.; Shin, S.; Park, I.; Gwak, J.; Ju, B.G.; Chung, Y.H.; Na, M.; Oh, S. Protopine isolated from Nandina domestica induces apoptosis and autophagy in colon cancer cells by stabilizing p53. Phytother. Res. 2019, 33, 1689–1696. [Google Scholar] [CrossRef]
- Li, J.; Xu, Z.; OuYang, C.; Wu, X.; Xie, Y.; Xie, J. Protopine alleviates lipopolysaccharide-triggered intestinal epithelial cell injury through retarding the NLRP3 and NF-kappaB signaling pathways to reduce inflammation and oxidative stress. Allergol. Immunopathol. 2022, 50, 84–92. [Google Scholar]
- Dong, Y.; Fan, H.; Zhang, Z.; Jiang, F.; Li, M.; Zhou, H.; Guo, W.; Zhang, Z.; Kang, Z.; Gui, Y.; et al. Berberine ameliorates DSS-induced intestinal mucosal barrier dysfunction through microbiota-dependence and Wnt/beta-catenin pathway. Int. J. Biol. Sci. 2022, 18, 1381–1397. [Google Scholar] [CrossRef]
- Cao, J.; Chen, M.; Xu, R.; Guo, M. Therapeutic Mechanisms of Berberine to Improve the Intestinal Barrier Function via Modulating Gut Microbiota, TLR4/NF-kappa B/MTORC Pathway and Autophagy in Cats. Front. Microbiol. 2022, 13, 961885. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wu, X.; Wang, Q.; Xu, W.; Zhao, Q.; Xu, N.; Hu, X.; Ye, Z.; Yu, S.; Liu, J.; et al. Sanguinarine ameliorates DSS induced ulcerative colitis by inhibiting NLRP3 inflammasome activation and modulating intestinal microbiota in C57BL/6 mice. Phytomedicine 2022, 104, 154321. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ding, T.; Xiao, G.; Huang, J.; Luo, D.; Yue, M.; Su, Y.; Jiang, S.; Zeng, J.; Liu, Y. Anti-Inflammatory Effects of Allocryptopine via the Target on the CX3CL1-CX3CR1 axis/GNB5/AKT/NF-kappaB/Apoptosis in Dextran Sulfate-Induced Mice. Biomedicines 2023, 11, 464. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, M.; Pohin, M.; Powrie, F. Cytokine Networks in the Pathophysiology of Inflammatory Bowel Disease. Immunity 2019, 50, 992–1006. [Google Scholar] [CrossRef] [Green Version]
- Niu, S.; Jing, M.; Wen, J.; Wei, S.; Li, H.; Li, X.; Ma, X.; Zhao, Y. Jatrorrhizine Alleviates DSS-Induced Ulcerative Colitis by Regulating the Intestinal Barrier Function and Inhibiting TLR4/MyD88/NF-kappaB Signaling Pathway. Evid. Based Complement. Alternat. Med. 2022, 2022, 3498310. [Google Scholar] [CrossRef]
- Capaldo, C.T.; Powell, D.N.; Kalman, D. Layered defense: How mucus and tight junctions seal the intestinal barrier. J. Mol. Med. 2017, 95, 927–934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, G.; Jiang, H.; Feng, J.; Zheng, X.; Zhang, D.; Jiang, C.; Zhang, J. Aloe vera mitigates dextran sulfate sodium-induced rat ulcerative colitis by potentiating colon mucus barrier. J. Ethnopharmacol. 2021, 279, 114108. [Google Scholar] [CrossRef] [PubMed]
- Sanchez de Medina, F.; Romero-Calvo, I.; Mascaraque, C.; Martinez-Augustin, O. Intestinal inflammation and mucosal barrier function. Inflamm. Bowel Dis. 2014, 20, 2394–2404. [Google Scholar] [CrossRef]
- Qu, Y.; Li, X.; Xu, F.; Zhao, S.; Wu, X.; Wang, Y.; Xie, J. Kaempferol Alleviates Murine Experimental Colitis by Restoring Gut Microbiota and Inhibiting the LPS-TLR4-NF-kappaB Axis. Front. Immunol. 2021, 12, 679897. [Google Scholar] [CrossRef] [PubMed]
- Shin, N.R.; Whon, T.W.; Bae, J.W. Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef]
- Sokol, H.; Seksik, P.; Furet, J.P.; Firmesse, O.; Nion-Larmurier, I.; Beaugerie, L.; Cosnes, J.; Corthier, G.; Marteau, P.; Dore, J. Low counts of Faecalibacterium prausnitzii in colitis microbiota. Inflamm. Bowel Dis. 2009, 15, 1183–1189. [Google Scholar] [CrossRef]
- Wu, X.; Fu, S.; Jiang, M.; Wang, J.; Tang, H.; Fang, C.; Li, W.; Fu, C. Sanhuang Xiexin decoction ameliorates DSS-induced colitis in mice by regulating intestinal inflammation, intestinal barrier, and intestinal flora. J. Ethnopharmacol. 2022, 297, 115537. [Google Scholar] [CrossRef]
- Wang, Y.; Tao, H.; Huang, H.; Xiao, Y.; Wu, X.; Li, M.; Shen, J.; Xiao, Z.; Zhao, Y.; Du, F.; et al. The dietary supplement Rhodiola crenulata extract alleviates dextran sulfate sodium-induced colitis in mice through anti-inflammation, mediating gut barrier integrity and reshaping the gut microbiome. Food Funct. 2021, 12, 3142–3158. [Google Scholar] [CrossRef]
- Dempsey, E.; Corr, S.C. Lactobacillus spp. for Gastrointestinal Health: Current and Future Perspectives. Front. Immunol. 2022, 13, 840245. [Google Scholar] [CrossRef]
- Schneider, K.M.; Mohs, A.; Gui, W.; Galvez, E.J.C.; Candels, L.S.; Hoenicke, L.; Muthukumarasamy, U.; Holland, C.H.; Elfers, C.; Kilic, K.; et al. Imbalanced gut microbiota fuels hepatocellular carcinoma development by shaping the hepatic inflammatory microenvironment. Nat. Commun. 2022, 13, 3964. [Google Scholar] [CrossRef]
- Grander, C.; Adolph, T.E.; Wieser, V.; Lowe, P.; Wrzosek, L.; Gyongyosi, B.; Ward, D.V.; Grabherr, F.; Gerner, R.R.; Pfister, A.; et al. Recovery of ethanol-induced Akkermansia muciniphila depletion ameliorates alcoholic liver disease. Gut 2018, 67, 891–901. [Google Scholar] [CrossRef] [PubMed]
- Alex, P.; Zachos, N.C.; Nguyen, T.; Gonzales, L.; Chen, T.E.; Conklin, L.S.; Centola, M.; Li, X. Distinct cytokine patterns identified from multiplex profiles of murine DSS and TNBS-induced colitis. Inflamm. Bowel Dis. 2009, 15, 341–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erben, U.; Loddenkemper, C.; Doerfel, K.; Spieckermann, S.; Haller, D.; Heimesaat, M.M.; Zeitz, M.; Siegmund, B.; Kühl, A.A. A guide to histomorphological evaluation of intestinal inflammation in mouse models. Int. J. Clin. Exp. Pathol. 2014, 7, 4557–4576. [Google Scholar] [PubMed]


| Scores | Weight Loss | Fecal Consistency | Rectal Bleeding |
|---|---|---|---|
| 0 | none | normal | no bleeding |
| 1 | 1–5% | ||
| 2 | 5–10% | loose stools | slight bleeding |
| 3 | 10–15% | ||
| 4 | >15% | watery diarrhea | gross bleeding |
| Gene Name | Primer Sequence (5′–3′) |
|---|---|
| IL-6 | F:AGACTTCCATCCAGTTGCCT R:CAGGTCTGTTGGGAGTGGTA |
| IL-1β | F:ACTCATTGTGGCTGTGGAGA R:TTGTTCATCTCGGAGCCTGT |
| TNF-α | F:ATGTCTCAGCCTCTTCTCATTC R:GCTTGTCACTCGAATTTTGAGA |
| COX2 | F:ATTCCAAACCAGCAGACTCATA R:CTTGAGTTTGAAGTGGTAACCG |
| iNOS | F:ATCTTGGAGCGAGTTGTGGATTGTC R:TAGGTGAGGGCTTGGCTGAGTG |
| β-Actin | F:CTGTGCCCATCTACGAGGGCTAT R:TTTGATGTCACGCACGATTTCC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yue, M.; Huang, J.; Ma, X.; Huang, P.; Liu, Y.; Zeng, J. Protopine Alleviates Dextran Sodium Sulfate-Induced Ulcerative Colitis by Improving Intestinal Barrier Function and Regulating Intestinal Microbiota. Molecules 2023, 28, 5277. https://doi.org/10.3390/molecules28135277
Yue M, Huang J, Ma X, Huang P, Liu Y, Zeng J. Protopine Alleviates Dextran Sodium Sulfate-Induced Ulcerative Colitis by Improving Intestinal Barrier Function and Regulating Intestinal Microbiota. Molecules. 2023; 28(13):5277. https://doi.org/10.3390/molecules28135277
Chicago/Turabian StyleYue, Meishan, Jialu Huang, Xiaolan Ma, Peng Huang, Yisong Liu, and Jianguo Zeng. 2023. "Protopine Alleviates Dextran Sodium Sulfate-Induced Ulcerative Colitis by Improving Intestinal Barrier Function and Regulating Intestinal Microbiota" Molecules 28, no. 13: 5277. https://doi.org/10.3390/molecules28135277
APA StyleYue, M., Huang, J., Ma, X., Huang, P., Liu, Y., & Zeng, J. (2023). Protopine Alleviates Dextran Sodium Sulfate-Induced Ulcerative Colitis by Improving Intestinal Barrier Function and Regulating Intestinal Microbiota. Molecules, 28(13), 5277. https://doi.org/10.3390/molecules28135277