Preparation of Multicolor Fluorescent Carbon Dots Based on Catechol and o-Phthalaldehyde
Abstract
:1. Introduction
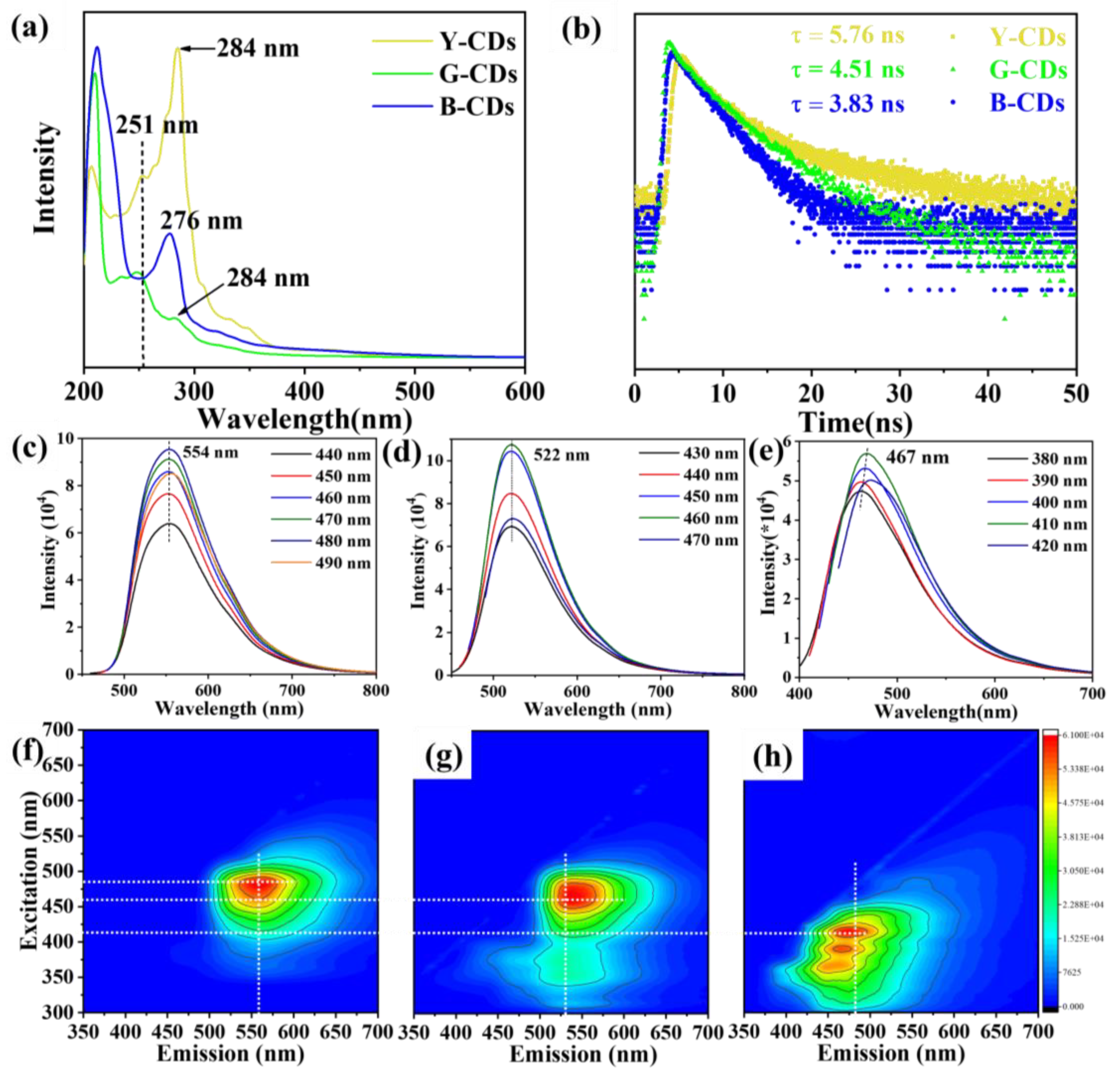
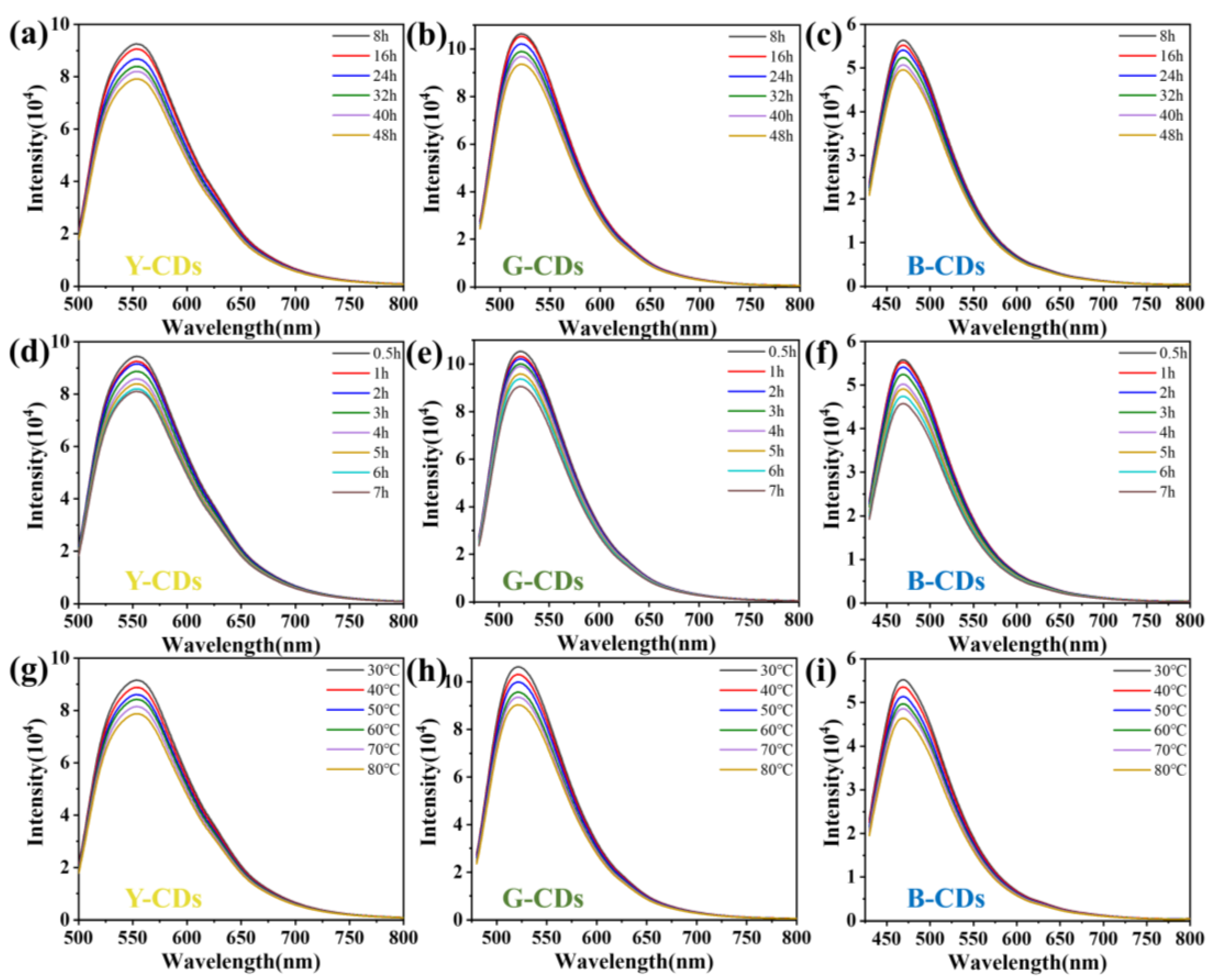
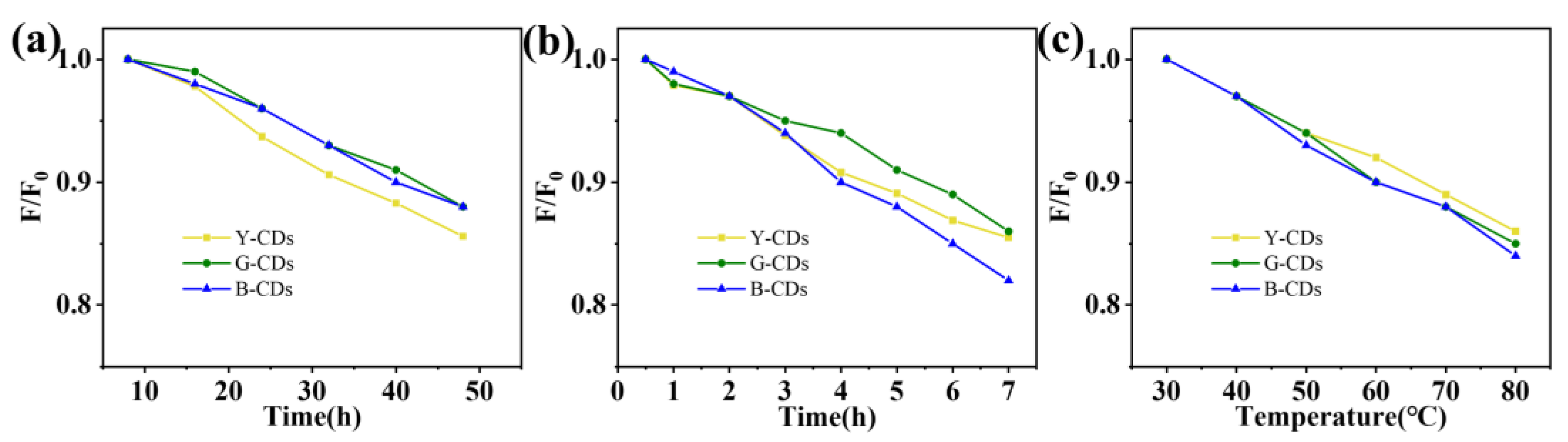
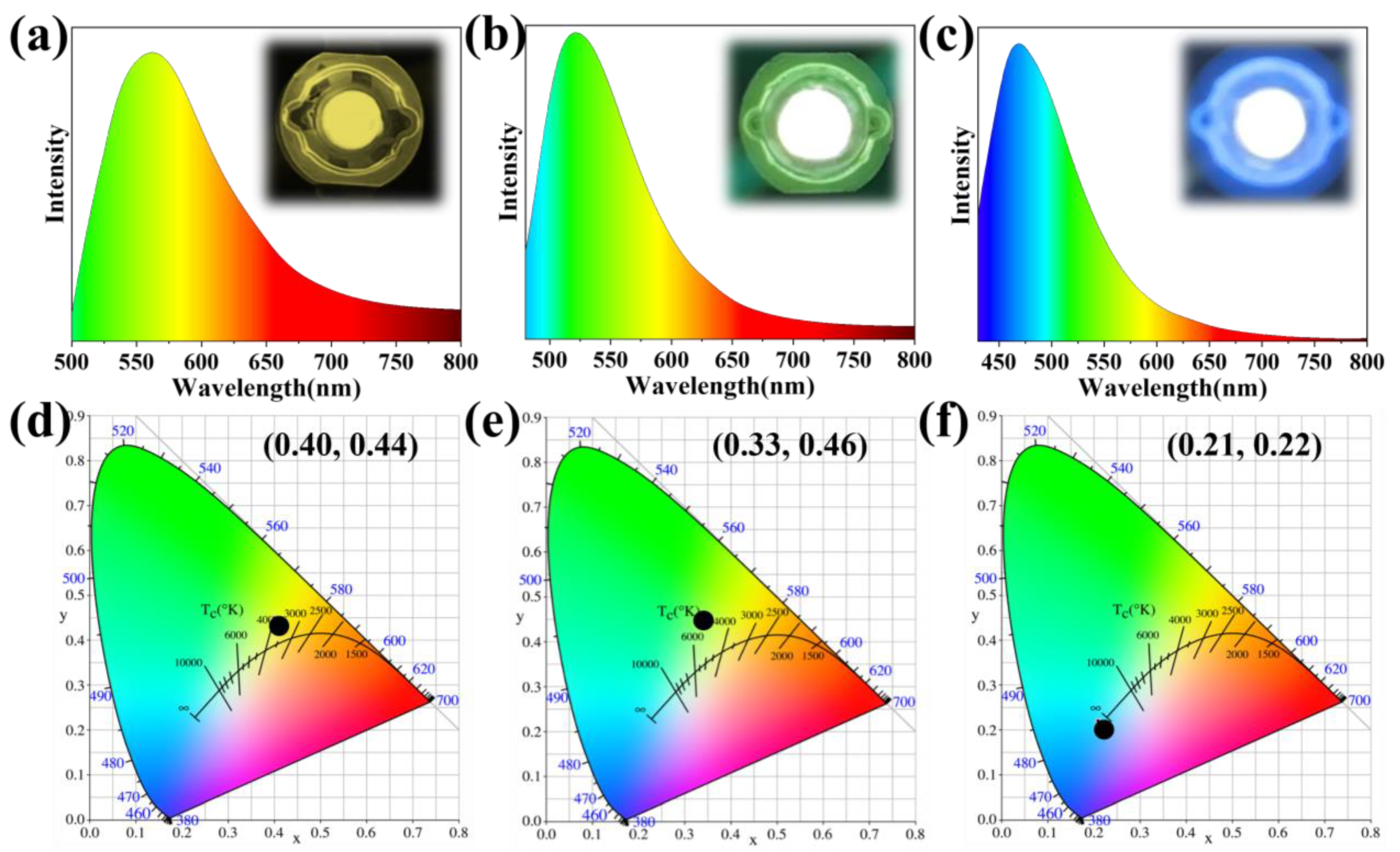
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Instruments and Methodology
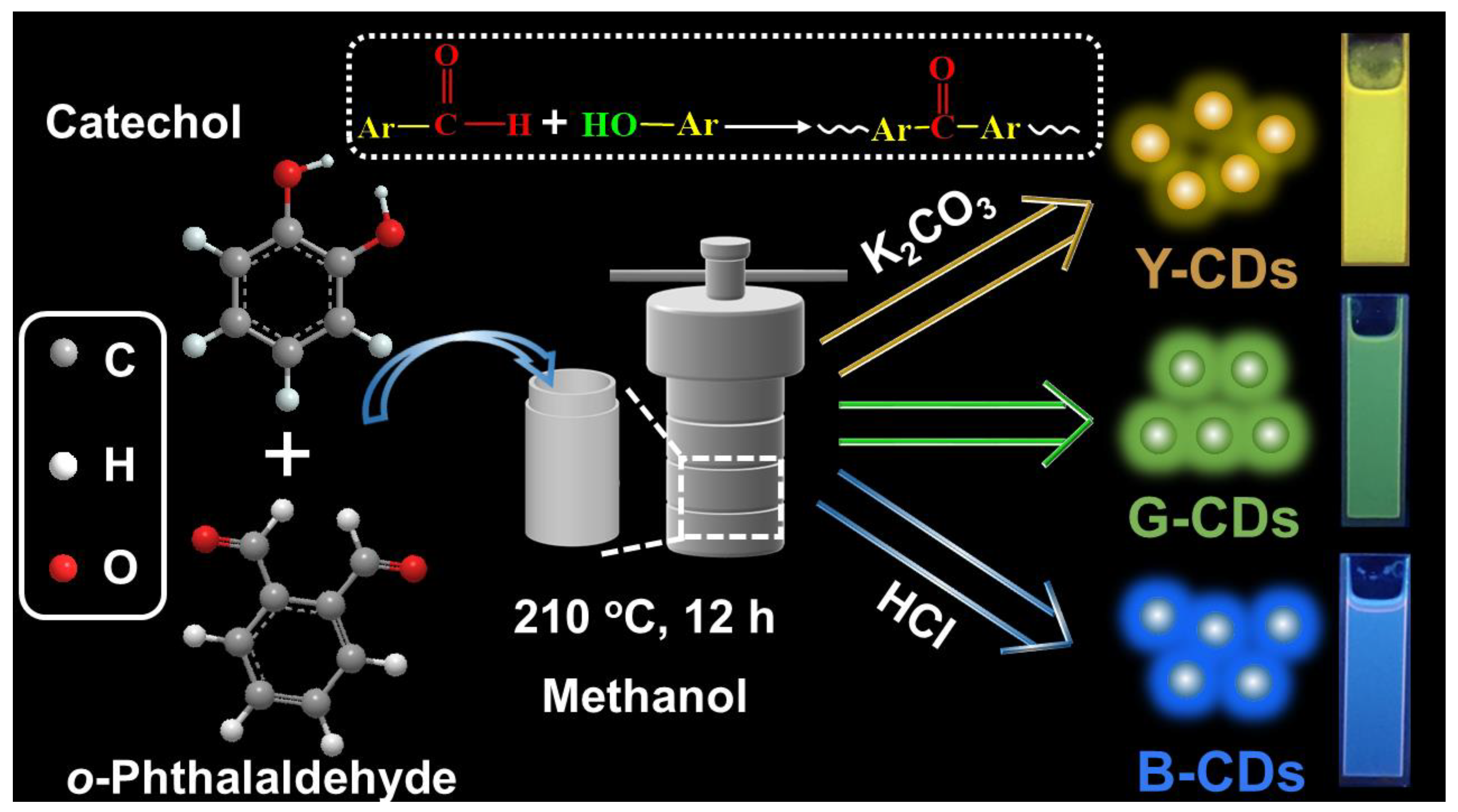
3.3. Synthesis of Multicolour CDs
3.4. Preparation of CDs-LEDs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Xu, X.; Ray, R.; Gu, Y.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Li, C.; Dong, L.; Fu, D.; Chen, Z. Imaging biofilm-encased microorganisms using carbon dots derived from L. plantarum. Nanoscale 2017, 9, 9056–9064. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Xu, P.; Zhang, X.; Long, W. The synthetic strategies, photoluminescence mechanisms and promising applications of carbon dots: Current state and future perspective. Carbon 2022, 186, 91–127. [Google Scholar] [CrossRef]
- Chandra, A.; Singh, N. Cell microenvironment pH sensing in 3D microgels using fluorescent carbon dots. ACS Biomater. Sci. Eng. 2017, 3, 3620–3627. [Google Scholar] [CrossRef]
- Gao, N.; Yang, W.; Nie, H.; Gong, Y.; Jing, J.; Gao, L.; Zhang, X. Turn-on theranostic fluorescent nanoprobe by electrostatic self-assembly of carbon dots with doxorubicin for targeted cancer cell imaging, in vivo hyaluronidase analysis, and targeted drug delivery. Biosens. Bioelectron. 2017, 96, 300–307. [Google Scholar] [CrossRef]
- Zou, S.; Hou, C.; Fa, H.; Zhang, L.; Ma, Y.; Dong, L.; Li, D.; Huo, D.; Yang, M. An efficient fluorescent probe for fluazinam using N, S co-doped carbon dots from L-cysteine. Sens. Actuators B Chem. 2017, 239, 1033–1041. [Google Scholar] [CrossRef]
- Chen, J.; Wei, J.-S.; Zhang, P.; Niu, X.-Q.; Zhao, W.; Zhu, Z.-Y.; Ding, H.; Xiong, H.-M. Red-emissive carbon dots for fingerprints detection by spray method: Coffee ring effect and unquenched fluorescence in drying process. ACS Appl. Mater. Interfaces 2017, 9, 18429–18433. [Google Scholar] [CrossRef]
- Lu, S.; Xiao, G.; Sui, L.; Feng, T.; Yong, X.; Zhu, S.; Li, B.; Liu, Z.; Zou, B.; Jin, M.; et al. Piezochromic carbon dots with two-photon fluorescence. Angew. Chem. 2017, 129, 6283–6287. [Google Scholar] [CrossRef]
- Li, D.; Liang, C.; Ushakova, E.; Sun, M.; Huang, X.; Zhang, X.; Jing, P.; Yoo, S.J.; Kim, J.; Liu, E.; et al. Thermally activated upconversion near-infrared photoluminescence from carbon dots synthesized via microwave assisted exfoliation. Small 2019, 15, 1905050. [Google Scholar] [CrossRef]
- Mewada, A.; Pandey, S.; Thakur, M.; Jadhav, D.; Sharon, M. Swarming carbon dots for folic acid mediated delivery of doxorubicin and biological imaging. J. Mater. Chem. B 2014, 2, 698–705. [Google Scholar] [CrossRef]
- Ding, H.; Du, F.; Liu, P.; Chen, Z.; Shen, J. DNA-carbon dots function as fluorescent vehicles for drug delivery. ACS Appl. Mater. Interfaces 2015, 7, 6889–6897. [Google Scholar] [CrossRef] [PubMed]
- Miao, X.; Yan, X.; Qu, D.; Li, D.; Tao, F.F.; Sun, Z. Red emissive sulfur, nitrogen codoped carbon dots and their application in ion detection and theraonostics. ACS Appl. Mater. Interfaces 2017, 9, 18549–18556. [Google Scholar] [CrossRef]
- Li, Y.; Liu, C.; Chen, M.; An, Y.; Zheng, Y.; Tian, H.; Shi, R.; He, X.; Lin, X. Solvent-Free Preparation of Tannic Acid Carbon Dots for Selective Detection of Ni2+ in the Environment. Int. J. Mol. Sci. 2022, 23, 6681. [Google Scholar] [CrossRef]
- Guo, X.; Wang, C.-F.; Yu, Z.-Y.; Chen, L.; Chen, S. Facile access to versatile fluorescent carbon dots toward light-emitting diodes. Chem. Commun. 2012, 48, 2692–2694. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.-H.; Tang, W.-Q.; Deng, Z.-Y.; Liu, S.-S.; Wang, C.-F.; Chen, S. Facile access to white fluorescent carbon dots toward light-emitting devices. Ind. Eng. Chem. Res. 2014, 53, 6417–6425. [Google Scholar] [CrossRef]
- Sun, Y.-P.; Zhou, B.; Lin, Y.; Wang, W.; Fernando, K.A.; Pathak, P.; Meziani, M.J.; Harruff, B.A.; Wang, X.; Wang, H.; et al. Quantum-sized carbon dots for bright and colorful photoluminescence. J. Am. Chem. Soc. 2006, 128, 7756–7757. [Google Scholar] [CrossRef]
- Ge, G.; Li, L.; Wang, D.; Chen, M.; Zeng, Z.; Xiong, W.; Wu, X.; Guo, C. Carbon dots: Synthesis, properties and biomedical applications. J. Mater. Chem. B 2021, 9, 6553–6575. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, X.; Li, Y.; Wang, Z.; Yang, F.; Yang, X. Microwave synthesis of fluorescent carbon nanoparticles with electrochemiluminescence properties. Chem. Commun. 2009, 34, 5118–5120. [Google Scholar] [CrossRef]
- Tang, L.; Ji, R.; Cao, X.; Lin, J.; Jiang, H.; Li, X.; Teng, K.S.; Luk, C.M.; Zeng, S.; Hao, J.; et al. Deep ultraviolet photoluminescence of water-soluble self-passivated graphene quantum dots. ACS Nano 2012, 6, 5102–5110. [Google Scholar] [CrossRef]
- Lu, W.; Qin, X.; Liu, S.; Chang, G.; Zhang, Y.; Luo, Y.; Asiri, A.M.; Al-Youbi, A.O.; Sun, X. Economical, green synthesis of fluorescent carbon nanoparticles and their use as probes for sensitive and selective detection of mercury (II) ions. Anal. Chem. 2012, 84, 5351–5357. [Google Scholar] [CrossRef]
- Tyagi, A.; Tripathi, K.M.; Singh, N.; Choudhary, S.; Gupta, R.K. Green synthesis of carbon quantum dots from lemon peel waste: Applications in sensing and photocatalysis. RSC Adv. 2016, 6, 72423–72432. [Google Scholar] [CrossRef]
- Zhou, J.; Cheiftz, J.; Li, R.; Wang, F.; Zhou, X.; Sham, T.-K.; Sun, X.; Ding, Z. Tailoring multi-wall carbon nanotubes for smaller nanostructures. Carbon 2009, 47, 829–838. [Google Scholar] [CrossRef]
- Li, H.; Ming, H.; Liu, Y.; Yu, H.; He, X.; Huang, H.; Pan, K.; Kang, Z.; Lee, S.-T. Fluorescent carbon nanoparticles: Electrochemical synthesis and their pH sensitive photoluminescence properties. New J. Chem. 2011, 35, 2666–2670. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, D.Y.; Lee, J.; Lee, S.B.; Han, H.; Kim, Y.Y.; Mun, S.C.; Im, S.H.; Kim, T.H.; Park, O.O. Synthesis of single-crystalline hexagonal graphene quantum dots from solution chemistry. Nano Lett. 2019, 19, 5437–5442. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Sun, C.; Chen, X.; Zhang, Y.; Colvin, V.L.; Rice, Q.; Seo, J.; Feng, S.; Wang, S.; Yu, W.W. Excitation wavelength independent visible color emission of carbon dots. Nanoscale 2017, 9, 1909–1915. [Google Scholar] [CrossRef]
- Ge, J.; Jia, Q.; Liu, W.; Guo, L.; Liu, Q.; Lan, M.; Zhang, H.; Meng, X.; Wang, P. Red-emissive carbon dots for fluorescent, photoacoustic, and thermal theranostics in living mice. Adv. Mater. 2015, 27, 4169–4177. [Google Scholar] [CrossRef]
- Zuo, G.; Xie, A.; Li, J.; Su, T.; Pan, X.; Dong, W. Large emission red-shift of carbon dots by fluorine doping and their applications for red cell imaging and sensitive intracellular Ag+ detection. J. Phys. Chem. C 2017, 121, 26558–26565. [Google Scholar] [CrossRef]
- Jiang, K.; Sun, S.; Zhang, L.; Lu, Y.; Wu, A.; Cai, C.; Lin, H. Red, green, and blue luminescence by carbon dots: Full-color emission tuning and multicolor cellular imaging. Angew. Chem. Int. Ed. 2015, 54, 5360–5363. [Google Scholar] [CrossRef]
- Lin, S.; Cheng, Y.; Lin, C.; Fang, J.; Xiang, W.; Liang, X. Carbon nanodots with intense emission from green to red and their multifunctional applications. J. Alloys Compd. 2018, 742, 212–219. [Google Scholar] [CrossRef]
- Yuan, F.; Wang, Z.; Li, X.; Li, Y.; Tan, Z.; Fan, L.; Yang, S. Bright multicolor bandgap fluorescent carbon quantum dots for electroluminescent light-Emitting diodes. Adv. Mater. 2017, 29, 1604436. [Google Scholar] [CrossRef]
- Li, Y.; Liu, C.; Chen, M.; Zheng, Y.; Tian, H.; Shi, R.; He, X.; Lin, X. Preparing Colour-Tunable Tannic Acid-Based Carbon Dots by Changing the pH Value of the Reaction System. Nanomaterials 2022, 12, 3062. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, C.; Sun, H.; Chen, M.; Hou, D.; Zheng, Y.; Xie, H.; Zhou, B.; Lin, X. Formation and Band Gap Tuning Mechanism of Multicolor Emissive Carbon Dots from m-Hydroxybenzaldehyde. Adv. Sci. 2023, 10, e2300543. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lian, H.; Wei, Y.; He, X.; Chen, Y.; Wang, B.; Zeng, Q.; Lin, J. Concentration-induced multi-colored emissions in carbon dots: Origination from triple fluorescent centers. Nanoscale 2018, 10, 6734–6743. [Google Scholar] [CrossRef] [PubMed]
- Gharat, P.M.; Chethodil, J.M.; Srivastava, A.P.; Praseetha, P.K.; Pal, H.; Choudhury, S.D. An insight into the molecular and surface state photoluminescence of carbon dots revealed through solvent-induced modulations in their excitation wavelength dependent emission properties. Photochem. Photobiol. Sci. 2019, 18, 110–119. [Google Scholar] [CrossRef]
- Yuan, F.; Ding, L.; Li, Y.; Li, X.; Fan, L.; Zhou, S.; Fang, D.; Yang, S. Multicolor fluorescent graphene quantum dots colorimetrically responsive to all-pH and a wide temperature range. Nanoscale 2015, 7, 11727–11733. [Google Scholar] [CrossRef]
- Karami, S.; Shamsipur, M.; Taherpour, A.A. In situ chromophore doping: A new mechanism for the long-wavelength emission of carbon dots. J. Phys. Chem. C 2020, 124, 10638–10646. [Google Scholar] [CrossRef]
- Jiao, Y.; Liu, Y.; Meng, Y.; Gao, Y.; Lu, W.; Liu, Y.; Gong, Y.; Shuang, S.; Dong, C. Novel processing for color-tunable luminescence carbon dots and their advantages in biological systems. ACS Sustain. Chem. Eng. 2020, 8, 8585–8592. [Google Scholar] [CrossRef]
- Meng, W.; Wang, B.; Ai, L.; Song, H.; Lu, S. Engineering white light-emitting diodes with high color rendering index from biomass carbonized polymer dots. J. Colloid Interface Sci 2021, 598, 274–282. [Google Scholar] [CrossRef]
- Wei, X.; Mei, S.; Yang, D.; Zhang, G.; Xie, F.; Zhang, W.; Guo, R. Surface states induced photoluminescence enhancement of nitrogen-doped carbon dots via post-treatments. Nanoscale Res. Lett. 2019, 14, 172. [Google Scholar] [CrossRef]
- Huo, F.; Liu, Y.; Zhu, M.; Gao, E.; Zhao, B.; Yang, X. Ultrabright full color carbon dots by fine-tuning crystal morphology controllable synthesis for multicolor bioimaging and sensing. ACS Appl. Mater. Interfaces 2019, 11, 27259–27268. [Google Scholar] [CrossRef]

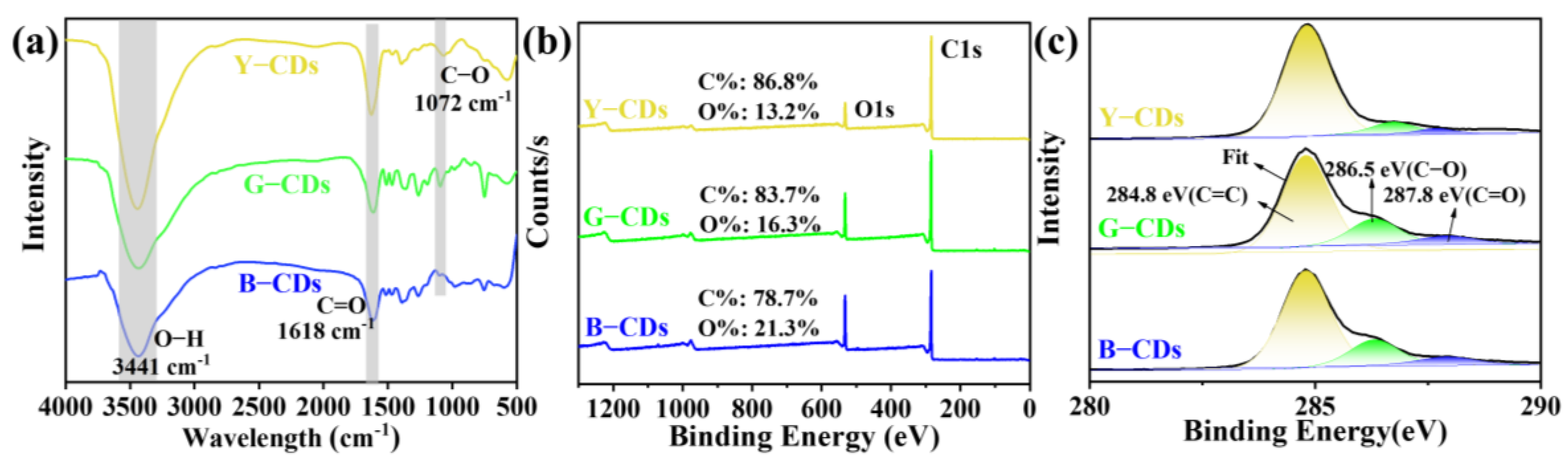
| Y-CDs (%) | G-CDs (%) | B-CDs (%) | ||
|---|---|---|---|---|
| XPS survey | C1s | 86.8 | 83.7 | 78.7 |
| O1s | 13.2 | 16.3 | 21.3 | |
| C 1s | C=C/C–C | 81.7 | 77.2 | 76.2 |
| C–O | 14.7 | 20.1 | 20.3 | |
| C=O | 3.6 | 2.7 | 3.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, M.; Yang, F.; Hou, D.; Zheng, Y.; Liu, C.; Lin, X.; Li, Y.; Sun, H. Preparation of Multicolor Fluorescent Carbon Dots Based on Catechol and o-Phthalaldehyde. Molecules 2023, 28, 5303. https://doi.org/10.3390/molecules28145303
Chen M, Yang F, Hou D, Zheng Y, Liu C, Lin X, Li Y, Sun H. Preparation of Multicolor Fluorescent Carbon Dots Based on Catechol and o-Phthalaldehyde. Molecules. 2023; 28(14):5303. https://doi.org/10.3390/molecules28145303
Chicago/Turabian StyleChen, Ming, Fulin Yang, Defa Hou, Yunwu Zheng, Can Liu, Xu Lin, Yan Li, and Hao Sun. 2023. "Preparation of Multicolor Fluorescent Carbon Dots Based on Catechol and o-Phthalaldehyde" Molecules 28, no. 14: 5303. https://doi.org/10.3390/molecules28145303
APA StyleChen, M., Yang, F., Hou, D., Zheng, Y., Liu, C., Lin, X., Li, Y., & Sun, H. (2023). Preparation of Multicolor Fluorescent Carbon Dots Based on Catechol and o-Phthalaldehyde. Molecules, 28(14), 5303. https://doi.org/10.3390/molecules28145303







