A New HPLC-UV Method Using Hydrolyzation with Sodium Hydroxide for Quantitation of Trans-p-Hydroxycinnamic Acid and Total Trans-p-Hydroxycinnamic Acid Esters in the Leaves of Ligustrum robustum
Abstract
1. Introduction
2. Results and Discussion
2.1. Method Development
2.1.1. Optimization of the Chromatographic Conditions
2.1.2. Optimization of the Hydrolyzed Conditions of Trans-p-Hydroxycinnamic Acid Esters
2.1.3. Calculation of the Total Concentration of Trans-p-hydroxycinnamic Esters in the Extracting Solution of L. robustum
2.2. Method Validation
2.2.1. Specificity
2.2.2. Linearity and Calibration Curve
2.2.3. Limit of Detection and Limit of Quantification
2.2.4. Precision
2.2.5. Accuracy
2.2.6. System Suitability Parameters
2.2.7. Robustness
2.3. Quantification of Trans-p-Hydroxycinnamic Acid and Total Trans-p-Hydroxycinnamic Acid Esters in Various Extracts of L. robustum
2.4. Similarity of HPLC Chromatograms
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Plant Material
3.3. Hydrolyzation of Extracting Solution and Preparation of Solutions
3.4. HPLC Determination of Trans-p-Hydroxycinnamic Acid and Total Trans-p-Hydroxycinnamic Acid Esters
3.5. HPLC Validation
3.5.1. Specificity
3.5.2. Linearity
3.5.3. LOD and LOQ
3.5.4. Precision
3.5.5. Accuracy
3.5.6. System Suitability
3.5.7. Robustness
3.6. Similarity of HPLC Chromatograms
3.7. Statistical Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Lau, K.M.; He, Z.D.; Dong, H.; Fung, K.P.; But, P.P.-H. Anti-oxidative, anti-inflammatory and hepato-protective effects of Ligustrum robustum. J. Ethnopharmacol. 2002, 83, 63–71. [Google Scholar] [CrossRef]
- He, Z.D.; Lau, K.M.; But, P.P.-H.; Jiang, R.W.; Dong, H.; Ma, S.C.; Fung, K.P.; Ye, W.C.; Sun, H.D. Antioxidative glycosides from the leaves of Ligustrum robustum. J. Nat. Prod. 2003, 66, 851–854. [Google Scholar] [CrossRef]
- Zhu, F.; Cai, Y.Z.; Sun, M.; Ke, J.X.; Lu, D.Y.; Corke, H. Comparison of major phenolic constituents and in vitro antioxidant activity of diverse kudingcha genotypes from Ilex kudingcha, Ilex cornuta, and Ligustrum robustum. J. Agric. Food Chem. 2009, 57, 6082–6089. [Google Scholar] [CrossRef]
- Yang, R.M.; Liu, F.; He, Z.D.; Ji, M.; Chu, X.X.; Kang, Z.Y.; Cai, D.Y.; Gao, N.N. Anti-obesity effect of total phenylpropanoid glycosides from Ligustrum robustum Blume in fatty diet-fed mice via up-regulating leptin. J. Ethnopharmacol. 2015, 169, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Peng, Y.; Xu, L.J.; Wu-Lan, T.N.; Shi, R.B.; Xiao, P.G. Chemical constituents from Ligustrum robustum Bl. Biochem. Syst. Ecol. 2010, 38, 398–401. [Google Scholar] [CrossRef]
- Li, L.; Peng, Y.; Liu, Y.; Xu, L.J.; Guo, N.; Shi, R.B.; Xiao, P.G. Two new phenethanol glycosides from Ligustrum robustum. Chin. Chem. Lett. 2011, 22, 326–329. [Google Scholar] [CrossRef]
- Tian, J.; Zhang, H.J.; Sun, H.D.; Pan, L.T.; Yao, P.; Chen, D.Y. Monoterpenoid glycosides from Ligustrum robustum. Phytochemistry 1998, 48, 1013–1018. [Google Scholar] [CrossRef]
- Tian, J.; Sun, H.D. New monoterpenoid glycosides from Ligustrum robustum. Chin. J. Appl. Environ. Biol. 1999, 5, 501–506. [Google Scholar]
- Yu, Z.L.; Zeng, W.C. Antioxidant, antibrowning, and cytoprotective activities of Ligustrum robustum (Roxb.) Blume extract. J. Food Sci. 2013, 78, 1354–1362. [Google Scholar] [CrossRef]
- Yu, Z.L.; Gao, H.X.; Zhang, Z.; He, Z.; He, Q.; Jia, L.R.; Zeng, W.C. Inhibitory effects of Ligustrum robustum (Roxb.) Blume extract on α-amylase and α-glucosidase. J. Funct. Foods 2015, 19, 204–213. [Google Scholar] [CrossRef]
- Ito, H.; Otsuki, A.; Mori, H.; Li, P.; Kinoshita, M.; Kawakami, Y.; Tsuji, H.; Fang, D.Z.; Takahashi, Y. Two new monoterpene glycosides from Qing Shan Lu Shui tea with inhibitory effects on leukocyte-type 12-lipoxygenase activity. Molecules 2013, 18, 4257–4266. [Google Scholar] [CrossRef]
- Kawakami, Y.; Otsuki, A.; Mori, Y.; Kanzaki, K.; Suzuki-Yamamoto, T.; Fang, D.Z.; Ito, H.; Takahashi, Y. Involvement of the hydroperoxy group in the irreversible inhibition of leukocyte-type 12-lipoxygenase by monoterpene glycosides contained in the Qing Shan Lu Shui tea. Molecules 2019, 24, 304. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Zhang, Z.-W.; Li, C.-C.; Yang, R.-M.; Pan, R.-L. Chemical constituents of alcoholic extract from Ligustrum robustum (Roxb.) Blume. Mod. Chin. Med. 2018, 20, 540–544. [Google Scholar]
- Lu, S.-H.; Zuo, H.-J.; Shi, J.-X.; Li, C.-R.; Li, Y.-H.; Wang, X.; Li, L.-R.; Huang, J. Two new glycosides from the leaves of Ligustrum robustum and their antioxidant activities and inhibitory effects on α-glucosidase and α-amylase. S. Afr. J. Bot. 2019, 125, 521–526. [Google Scholar] [CrossRef]
- Lu, S.-H.; Huang, J.; Zuo, H.-J.; Zhou, Z.-B.; Yang, C.-Y.; Huang, Z.-L. Monoterpenoid glycosides from the leaves of Ligustrum robustum and their bioactivities. Molecules 2022, 27, 3709. [Google Scholar] [CrossRef]
- Lu, S.-H.; Zuo, H.-J.; Huang, J.; Chen, R.; Pan, J.-P.; Li, X.-X. Phenylethanoid and phenylmethanoid glycosides from the leaves of Ligustrum robustum and their bioactivities. Molecules 2022, 27, 7390. [Google Scholar] [CrossRef]
- Lu, S.-H.; Zuo, H.-J.; Huang, J.; Li, W.-N.; Huang, J.-L.; Li, X.-X. Chemical constituents from the leaves of Ligustrum robustum and their bioactivities. Molecules 2023, 28, 362. [Google Scholar] [CrossRef]
- You, Q.-D. Drug metabolism. In Medicinal Chemistry, 8th ed.; People’s Medical Publishing House: Beijing, China, 2016; pp. 46–62. ISBN 978-7-117-22151-1. [Google Scholar]
- Zhang, Y.-T.; Sun, Y.-M.; Zhang, J.-H.; Huo, Y.; Li, X.; Li, W.-B.; Zhao, A.-P.; Wang, R. Interaction between gut microbiota and drugs. Chin. Pharm. Bull. 2020, 36, 1650–1655. [Google Scholar]
- Kang, X.R.; Wang, Q.H.; Ao, S.; Moxiyele; Bao, W.L.; Zhao, C.L. HPLC analysis of 16 compounds from Artemisia ordosica. Chin. Herb. Med. 2023, 15, 337–342. [Google Scholar] [CrossRef]
- Chelly, S.; Chelly, M.; Salah, H.B.; Athmouni, K.; Bitto, A.; Sellami, H.; Kallel, C.; Bouaziz-Ketata, H. HPLC-DAD analysis, antioxidant and protective effects of Tunisian Rhanterium suaveolens against acetamiprid induced oxidative stress on mice erythrocytes. Chem. Biodivers. 2019, 16, e1900428. [Google Scholar] [CrossRef]
- Al-Huqail, A.A.; Behiry, S.I.; Salem, M.Z.M.; Ali, H.M.; Siddiqui, M.H.; Salem, A.Z.M. Antifungal, antibacterial, and antioxidant activities of Acacia Saligna (Labill.) H. L. Wendl. flower extract: HPLC analysis of phenolic and flavonoid compounds. Molecules 2019, 24, 700. [Google Scholar] [CrossRef]
- Pencheva, D.; Teneva, D.; Denev, P. Validation of HPLC method for analysis of gamma-amino butyric and glutamic acids in plant foods and medicinal plants. Molecules 2023, 28, 84. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Shin, H.Y.; Park, S.Y.; Kim, H.; Chung, D.K. Development and validation of a method for determining the quercetin-3-O-glucuronide and ellagic acid content of common evening primrose (Oenothera biennis) by HPLC-UVD. Molecules 2021, 26, 267. [Google Scholar] [CrossRef]
- Attimarad, M.; Venugopala, K.N.; Sreeharsha, N.; Chohan, M.S.; Shafi, S.; Nair, A.B.; Pottathil, S. A rapid HPLC method for the concurrent determination of several antihypertensive drugs from binary and ternary formulations. Separations 2021, 8, 86. [Google Scholar] [CrossRef]
- Duncan, L.F.; Wang, G.Q.; Ilyichova, O.V.; Scanlon, M.J.; Heras, B.; Abbott, B.M. The fragment-based development of a benzofuran hit as a new class of Escherichia coli DsbA inhibitors. Molecules 2019, 24, 3756. [Google Scholar] [CrossRef] [PubMed]
- Naseef, H.; Moqadi, R.; Qurt, M. Development and validation of an HPLC method for determination of antidiabetic drug alogliptin benzoate in bulk and tablets. J. Anal. Methods Chem. 2018, 2018, 1902510. [Google Scholar] [CrossRef]
- Wang, S.-H.; Cao, X.-L.; Song, S.-S.; Long, L.-M. Development of certified reference materials for Xinyang Maojian tea grading based on HPLC fingerprints of flavor components. Food Sci. 2019, 40, 293–301. [Google Scholar] [CrossRef]


| Parameter | Condition | Content of Potential Trans-p-hydroxycinnamic Acid (mg·g−1) a |
|---|---|---|
| Catalyst b | hydrochloride | 62.0 ± 0.5 a |
| sodium hydroxide | 66.0 ± 0.5 b | |
| Incubation temperature (°C) c | 30 | 46.5 ± 0.4 a |
| 40 | 50.1 ± 0.5 b | |
| 50 | 56.1 ± 0.4 c | |
| 60 | 59.0 ± 0.5 d | |
| 70 | 62.5 ± 0.4 e | |
| 80 | 66.2 ± 0.5 f | |
| 90 | 66.1 ± 0.5 f | |
| Period of incubation (h) d | 1 | 62.8 ± 0.5 a |
| 2 | 66.0 ± 0.5 c | |
| 4 | 65.4 ± 0.4 c | |
| 6 | 64.5 ± 0.5 b |
| No. | Regression Equation | r2 | Linear Range (μg·mL−1) | Residual STD (σ) | Calibration Curve Slope (S) | LOD (μg·mL−1) | LOQ (μg·mL−1) |
|---|---|---|---|---|---|---|---|
| 1 | Y = 69.34X + 349.8 | 1.000 | 11.0–352.0 | 51.25 | 69.34 | 2.44 | 7.39 |
| 2 | Y = 69.58X + 327.1 | 1.000 | 11.0–352.0 | 53.44 | 69.58 | 2.53 | 7.68 |
| 3 | Y = 69.46X + 347.1 | 1.000 | 11.0–352.0 | 21.83 | 69.46 | 1.04 | 3.14 |
| Integration (n = 3) | Y = 69.46X + 341.3 | 1.000 | 11.0–352.0 | 42.17 | 69.42 | 2.00 | 6.07 |
| Concentration (μg·mL−1) | Intra-Day Variability (n = 3) | Inter-Day Variability (n = 3) | ||
|---|---|---|---|---|
| Mean ± SD | RSD (%) | Mean ± SD | RSD (%) | |
| 66.0 | 65.4 ± 1.0 | 1.6 | 65.2 ± 1.1 | 1.7 |
| 132.0 | 130.9 ± 1.2 | 1.0 | 130.4 ± 1.3 | 1.0 |
| 264.0 | 262.7 ± 1.3 | 0.5 | 261.8 ± 1.3 | 0.5 |
| Level | Amount Spiked (mg) | Amount Recovered (mg) a | Recovery (%) a |
|---|---|---|---|
| 50% | 1.3 | 1.36 ± 0.02 | 104.6 ± 1.6 |
| 100% | 2.6 | 2.68 ± 0.02 | 103.1 ± 0.8 |
| 150% | 3.9 | 3.98 ± 0.03 | 102.1 ± 0.8 |
| Average (n = 9) | 103.3 ± 1.1 |
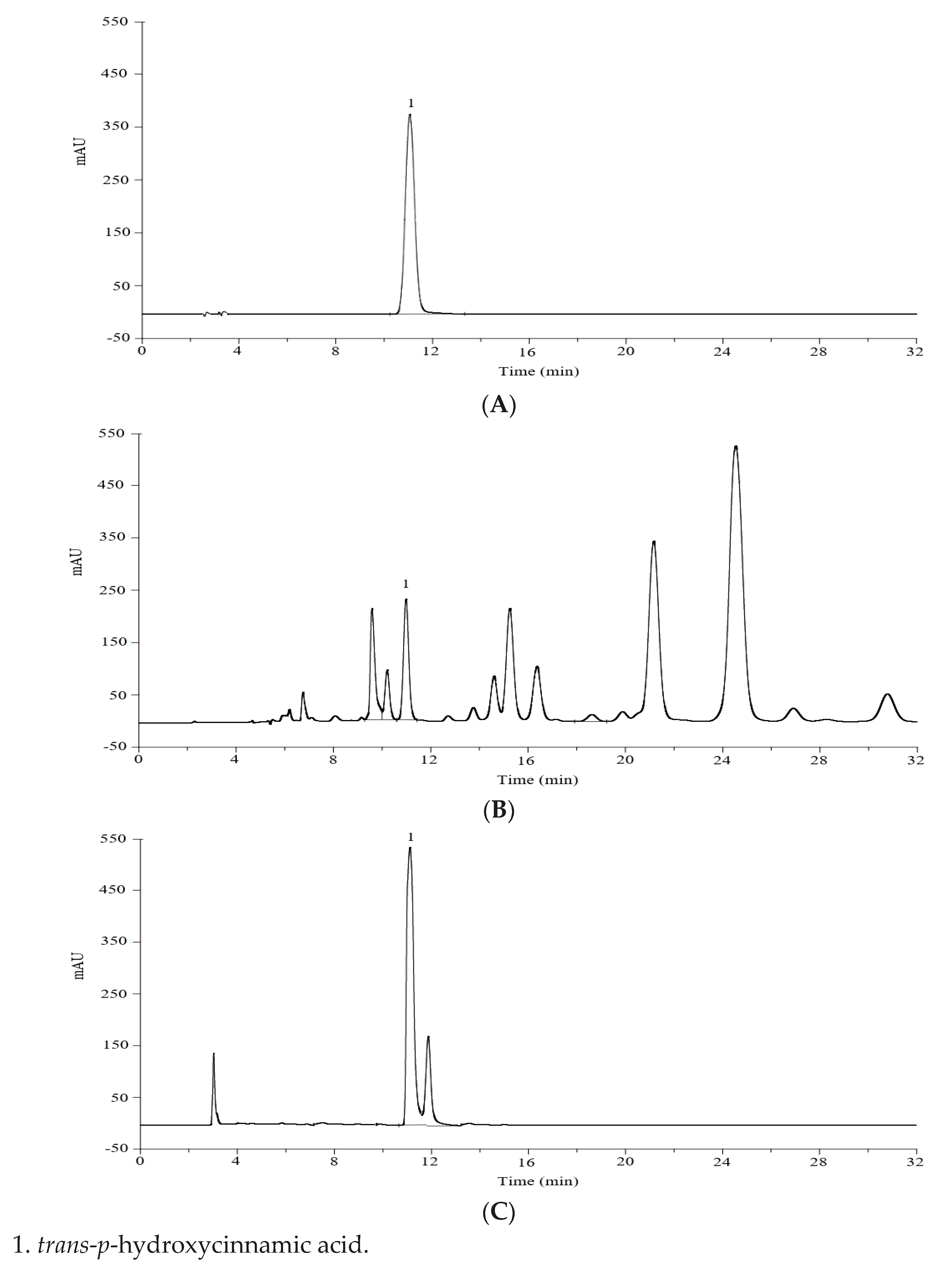
| Parameter | Figure 2B | Figure 2C | Reference Value |
|---|---|---|---|
| Retention time (min) a | 10.86 ± 0.03 | 10.88 ± 0.02 | - |
| Resolution a | 2.45 ± 0.10 | 1.58 ± 0.09 | R > 1.5 |
| Tailing factor a | 1.08 ± 0.02 | 1.29 ± 0.02 | ≈1 |
| Theoretical plates a | 27,534 ± 439 | 14,640 ± 257 | N ≥ 2000 |
| Injection precision (Area)-% | 0.9 | 0.3 | %RSD ≤ 2 |
| Parameter | Condition | Peak Area |
|---|---|---|
| Column temperature (°C) | 25 | 3407.45 |
| 30 | 3428.19 | |
| 35 | 3457.14 | |
| Mean | 3430.93 | |
| %RSD | 0.73 | |
| Flow rate (mL·min−1) | 0.8 | 3445.89 |
| 1.0 | 3424.12 | |
| 1.2 | 3408.46 | |
| Mean | 3426.16 | |
| %RSD | 0.55 | |
| Mobile phase composition | 38% methanol | 3414.56 |
| 40% methanol | 3418.79 | |
| 42% methanol | 3467.14 | |
| Mean | 3433.50 | |
| %RSD | 0.85 | |
| Wavelength (nm) | 308 | 3388.45 |
| 310 | 3435.36 | |
| 312 | 3370.89 | |
| Mean | 3398.23 | |
| %RSD | 0.98 |
| Sample | Free Trans-p-hydroxycinnamic Acid (mg·g−1) a | Potential Trans-p-hydroxycinnamic Acid (mg·g−1) a | Total Trans-p-hydroxycinnamic Acid Esters (mg·g−1) a | Similarity of Figure 2B with Figure 2C b |
|---|---|---|---|---|
| 30% EtOH | 2.26 ± 0.02 a | 37.1 ± 0.7 a | 125.8 ± 2.6 a | 0.119 |
| 40% EtOH | 3.34 ± 0.03 b | 54.8 ± 0.9 b | 185.8 ± 3.3 b | 0.101 |
| 50% EtOH | 3.77 ± 0.02 c | 59.9 ± 1.0 c | 202.6 ± 3.6 c | 0.100 |
| 60% EtOH | 3.99 ± 0.03 e | 62.2 ± 0.8 d | 210.1 ± 2.9 c | 0.100 |
| 70% EtOH | 3.96 ± 0.03 e | 62.3 ± 1.0 d | 210.6 ± 3.6 c | 0.099 |
| 80% EtOH | 3.91 ± 0.02 d | 61.5 ± 0.8 d | 207.9 ± 2.9 c | 0.100 |
| Average | 0.103 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, S.-H.; Liang, X.-N.; Nong, X.-J.; Chen, R.; Li, X.-X. A New HPLC-UV Method Using Hydrolyzation with Sodium Hydroxide for Quantitation of Trans-p-Hydroxycinnamic Acid and Total Trans-p-Hydroxycinnamic Acid Esters in the Leaves of Ligustrum robustum. Molecules 2023, 28, 5309. https://doi.org/10.3390/molecules28145309
Lu S-H, Liang X-N, Nong X-J, Chen R, Li X-X. A New HPLC-UV Method Using Hydrolyzation with Sodium Hydroxide for Quantitation of Trans-p-Hydroxycinnamic Acid and Total Trans-p-Hydroxycinnamic Acid Esters in the Leaves of Ligustrum robustum. Molecules. 2023; 28(14):5309. https://doi.org/10.3390/molecules28145309
Chicago/Turabian StyleLu, Shi-Hui, Xiao-Na Liang, Xiao-Jin Nong, Ran Chen, and Xiu-Xia Li. 2023. "A New HPLC-UV Method Using Hydrolyzation with Sodium Hydroxide for Quantitation of Trans-p-Hydroxycinnamic Acid and Total Trans-p-Hydroxycinnamic Acid Esters in the Leaves of Ligustrum robustum" Molecules 28, no. 14: 5309. https://doi.org/10.3390/molecules28145309
APA StyleLu, S.-H., Liang, X.-N., Nong, X.-J., Chen, R., & Li, X.-X. (2023). A New HPLC-UV Method Using Hydrolyzation with Sodium Hydroxide for Quantitation of Trans-p-Hydroxycinnamic Acid and Total Trans-p-Hydroxycinnamic Acid Esters in the Leaves of Ligustrum robustum. Molecules, 28(14), 5309. https://doi.org/10.3390/molecules28145309







