Polylysine-Containing Hydrogel Formulation of Fuzapladib, Inhibitor of Leukocyte-Function Associated Antigen-1 (LFA-1) Activation, for Sustained Release
Abstract
:1. Introduction
2. Results and Discussion
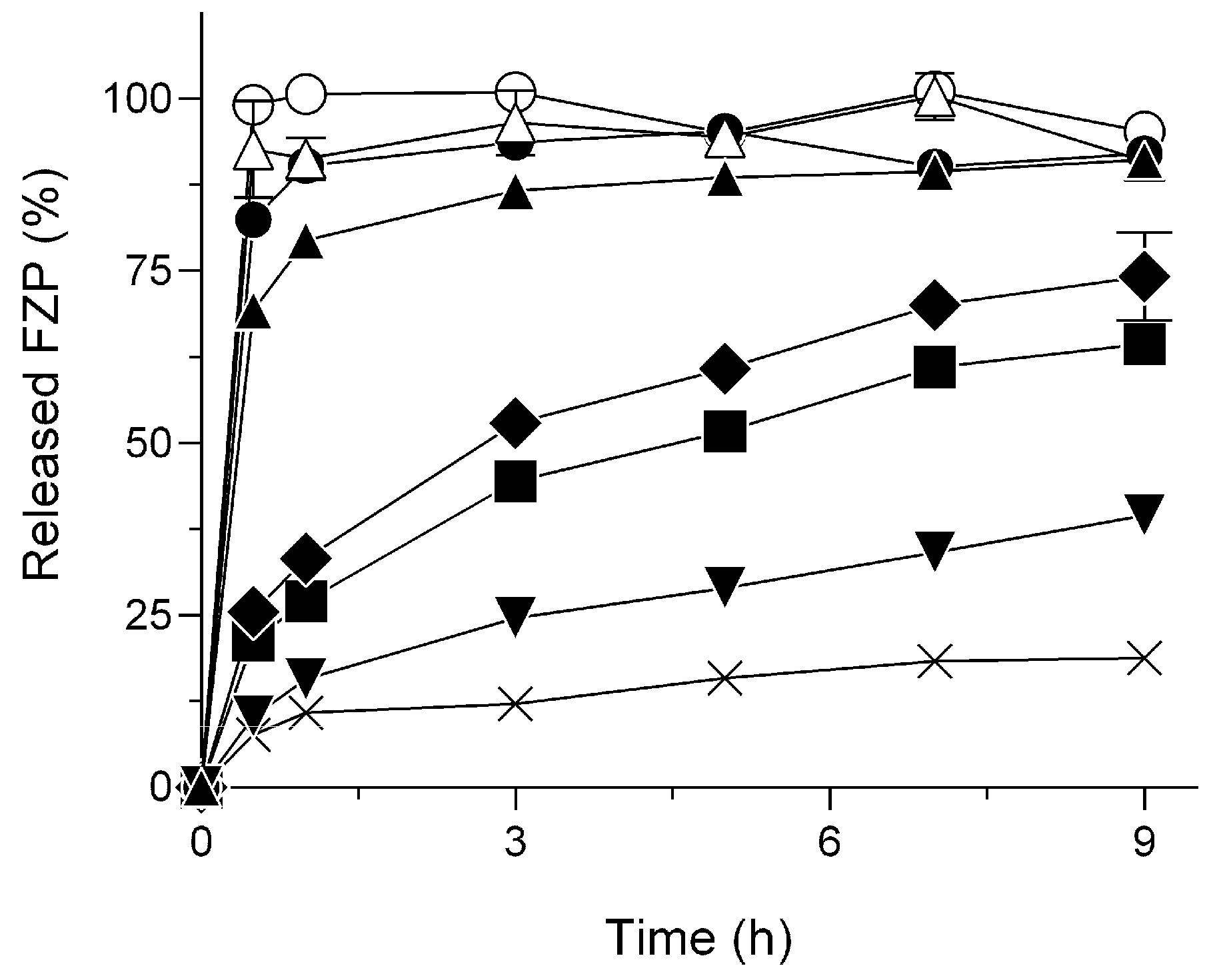
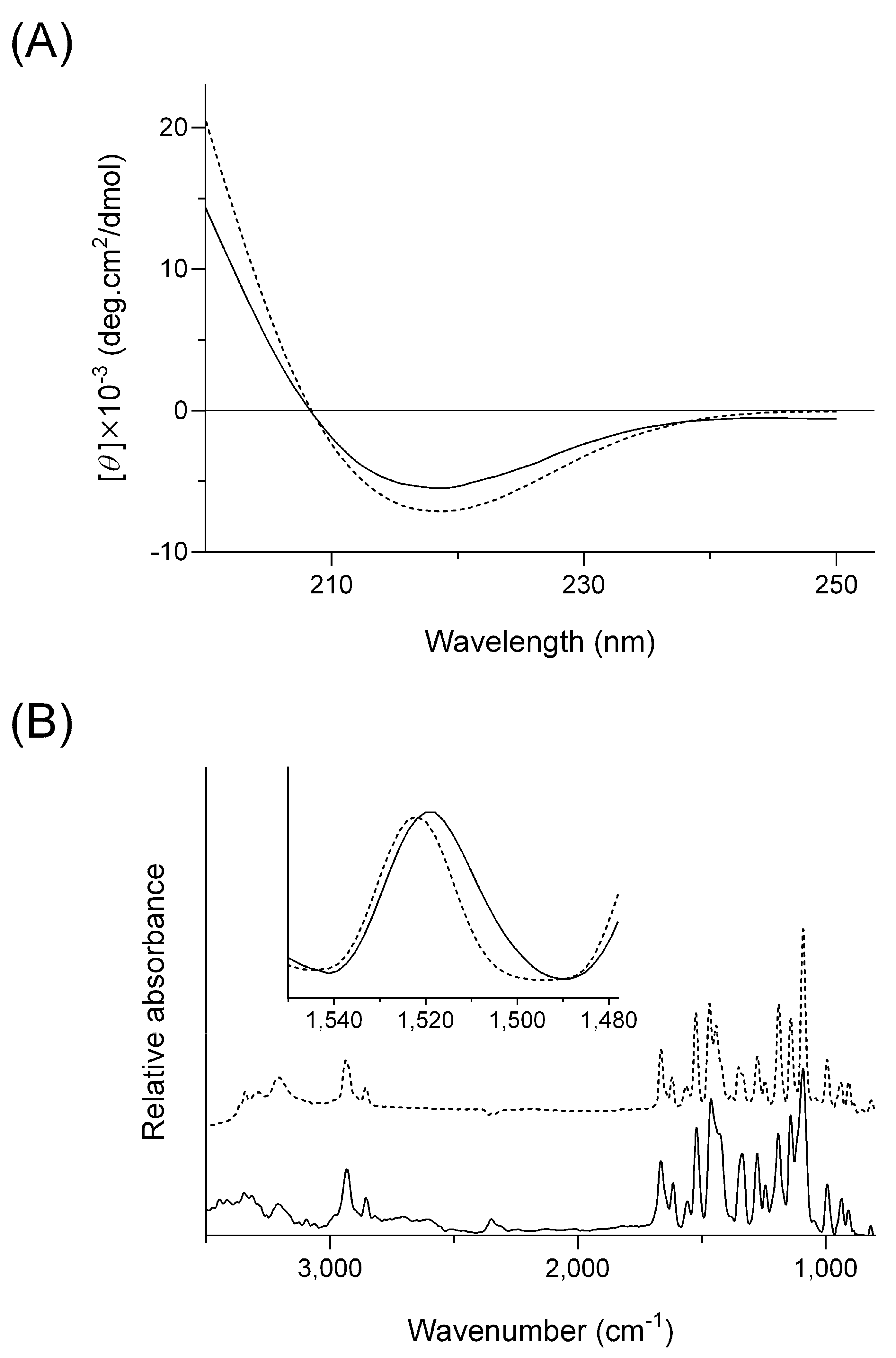
2.1. Delayed Dissolution Behavior of HG/FZP with pLys (HG/FZP-pLys)
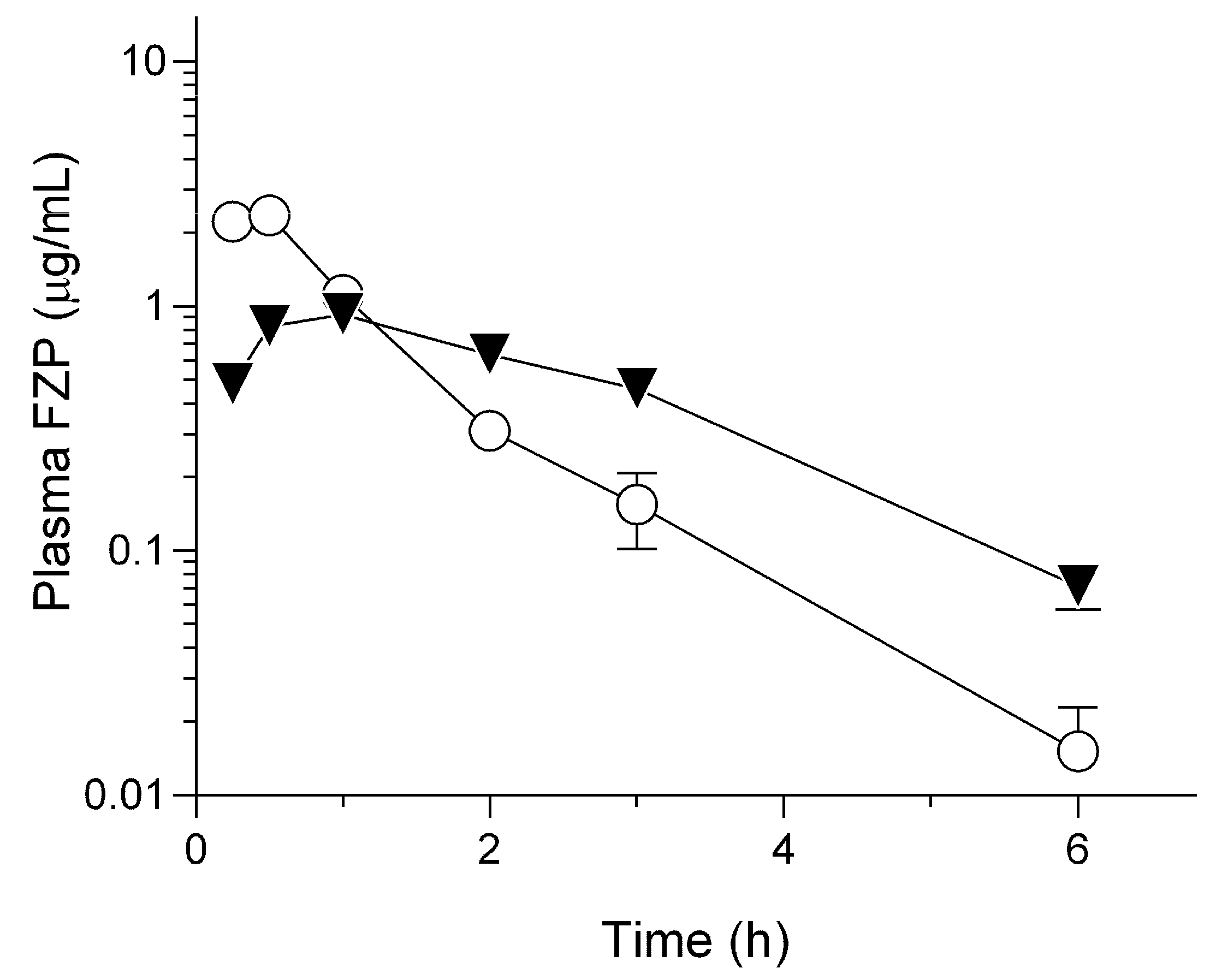
2.2. Pharmacokinetic Behavior of HG/FZP-pLys100 in Rats
3. Materials and Methods
3.1. Materials
3.2. Preparation
3.3. Release Test
3.4. Viscosity Measurement
3.5. Circular Dichroism Spectroscopy
3.6. Fourier Transformation Infrared Spectroscopy
3.7. Animals
3.8. Pharmacokinetic Study on Subcutaneous FZP Samples
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Cridge, H.; Lim, S.Y.; Algül, H.; Steiner, J.M. New Insights into the Etiology, Risk Factors, and Pathogenesis of Pancreatitis in Dogs: Potential Impacts on Clinical Practice. J. Vet. Intern. Med. 2022, 36, 847–864. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Freedom of Information Summary Conditional Approval Application Application Number 141-567. Available online: https://animaldrugsatfda.fda.gov/adafda/app/search/public/document/downloadFoi/13134 (accessed on 11 April 2023).
- Shikama, H.; Yotsuya, S.; Satake, S.; Sugi, H.; Kato, M. Effect of IS-741 on Cell Adhesion between Human Umbilical Vein Endothelial Cells and HK-60 Cells. Biol. Pharm. Bull. 1999, 22, 127–131. [Google Scholar] [CrossRef] [Green Version]
- Mansfield, C. Acute Pancreatitis in Dogs: Advances in Understanding, Diagnostics, and Treatment. Top. Companion Anim. Med. 2012, 27, 123–132. [Google Scholar] [CrossRef]
- Ohno, K. Update on Diagnosis and Therapy of Canine Acute and Chronic Pancreatitis 1. Pathogenesis and Clinicopathologic Diagnosis. J. Anim. Clin. Med. 2015, 24, 151–152. [Google Scholar]
- Berman, C.; Lobetti, R.; Lindquist, E. A Comparison of Ultrasonographic and Clinical Findings in 293 Dogs with Acute Pancreatitis: Different Clinical Presentation with Left Limb, Right Limb, or Diffuse Involvement of the Pancreas. J. S. Afr. Vet. Assoc. 2020, 91, 2022. [Google Scholar] [CrossRef] [Green Version]
- De Cock, H.E.V.; Forman, M.A.; Farver, T.B.; Marks, S.L. Prevalence and Histopathologic Characteristics of Pancreatitis in Cats. Vet. Pathol. 2007, 44, 39–49. [Google Scholar] [CrossRef]
- Forman, M.A.; Marks, S.L.; De Cock, H.E.V.; Hergesell, E.J.; Wisner, E.R.; Baker, T.W.; Kass, P.H.; Steiner, J.M.; Williams, D.A. Evaluation of Serum Feline Pancreatic Lipase Immunoreactivity and Helical Computed Tomography versus Conventional Testing for the Diagnosis of Feline Pancreatitis. J. Vet. Intern. Med. 2004, 18, 807–815. [Google Scholar] [CrossRef]
- Forman, M.A.; Steiner, J.M.; Armstrong, P.J.; Camus, M.S.; Gaschen, L.; Hill, S.L.; Mansfield, C.S.; Steiger, K. ACVIM Consensus Statement on Pancreatitis in Cats. J. Vet. Intern. Med. 2021, 35, 703–723. [Google Scholar] [CrossRef]
- Onoue, S.; Higuchi, K.; Tsukada, R.; Yamada, K.; Sasaki, K.; Yoshida, C.; Sato, H. Species Differences in the Biopharmaceutical Properties of Fuzapladib Sodium Monohydrate in Rats, Cats, and Dogs. Pharm. Pharmacol. Int. J. 2022, 10, 225–228. [Google Scholar] [CrossRef]
- Rivankar, S. An Overview of Doxorubicin Formulations in Cancer Therapy. J. Cancer Res. Ther. 2014, 10, 853–858. [Google Scholar] [CrossRef]
- Takada, N.; Kawabe, H. Drug Discovery in Japan: Investigating the Sources of Innovation; Nagaoka, S., Ed.; Springer: Singapore, 2019; ISBN 9789811389061. [Google Scholar]
- Karabin, N.B.; Allen, S.; Kwon, H.K.; Bobbala, S.; Firlar, E.; Shokuhfar, T.; Shull, K.R.; Scott, E.A. Sustained Micellar Delivery via Inducible Transitions in Nanostructure Morphology. Nat. Commun. 2018, 9, 624. [Google Scholar] [CrossRef] [Green Version]
- Peppas, N.A.; Bures, P.; Leobandung, W.; Ichikawa, H. Hydrogels in Pharmaceutical Formulations. Eur. J. Pharm. Biopharm. 2000, 50, 27–46. [Google Scholar] [CrossRef]
- Thambi, T.; Li, Y.; Lee, D.S. Injectable Hydrogels for Sustained Release of Therapeutic Agents. J. Control. Release 2017, 267, 57–66. [Google Scholar] [CrossRef]
- Vintiloiu, A.; Leroux, J.C. Organogels and Their Use in Drug Delivery—A Review. J. Control. Release 2008, 125, 179–192. [Google Scholar] [CrossRef]
- Hiraki, J.; Ichikawa, T.; Ninomiya, S.I.; Seki, H.; Uohama, K.; Seki, H.; Kimura, S.; Yanagimoto, Y.; Barnett, J.W. Use of ADME Studies to Confirm the Safety of ε-Polylysine as a Preservative in Food. Regul. Toxicol. Pharmacol. 2003, 37, 328–340. [Google Scholar] [CrossRef]
- Shukla, S.C.; Singh, A.; Pandey, A.K.; Mishra, A. Review on Production and Medical Applications of ε-Polylysine. Biochem. Eng. J. 2012, 65, 70–81. [Google Scholar] [CrossRef]
- Townend, R.; Kumosinski, T.F.; Timasheff, S.N.; Fasman, G.D.; Davidson, B. The Circular Dichroism of the β Structure of Poly-l-Lysine. Biochem. Biophys. Res. Commun. 1966, 23, 163–169. [Google Scholar] [CrossRef]
- Miles, A.J.; Janes, R.W.; Wallace, B.A. Tools and Methods for Circular Dichroism Spectroscopy of Proteins: A Tutorial Review. Chem. Soc. Rev. 2021, 50, 8400–8413. [Google Scholar] [CrossRef]
- Suzuki, H.; Yakushiji, K.; Matsunaga, S.; Yamauchi, Y.; Seto, Y.; Sato, H.; Onoue, S. Amorphous Solid Dispersion of Meloxicam Enhanced Oral Absorption in Rats With Impaired Gastric Motility. J. Pharm. Sci. 2018, 107, 446–452. [Google Scholar] [CrossRef] [Green Version]
- Kimura, H.; Yotsuya, S.; Yuki, S.; Sugi, H.; Shigehara, I.; Haga, T. Synthesis and Antipancretitis Activities of Novel N-(2-Sulfonylamino-5-Trifluoromethyl-3-Pyridyl)Carboxamide Derivatives as Phospholipase A2 Inhibitors. Chem. Pharm. Bull. 1995, 43, 1696–1700. [Google Scholar] [CrossRef] [Green Version]
- Louis-Jeune, C.; Andrade-Navarro, M.A.; Perez-Iratxeta, C. Prediction of Protein Secondary Structure from Circular Dichroism Using Theoretically Derived Spectra. Proteins. Proteins 2012, 80, 374–381. [Google Scholar] [CrossRef] [PubMed]
| Composition | ||||
|---|---|---|---|---|
| PEO (mg) | FZP (mg) | pLys (mg) | Water (mL) | |
| HG/FZP | 10 | 1 | 0 | 1 |
| HG/FZP-pLys5 | 10 | 1 | 5 | 1 |
| HG/FZP-pLys10 | 10 | 1 | 10 | 1 |
| HG/FZP-pLys20 | 10 | 1 | 20 | 1 |
| HG/FZP-pLys50 | 10 | 1 | 50 | 1 |
| HG/FZP-pLys100 | 10 | 1 | 100 | 1 |
| HG/FZP-pLys150 | 10 | 1 | 150 | 1 |
| Cmax (mg/mL) | tmax (h) | t1/2 (h) | MRT (h) | AUC0–inf (mg·h/mL) | BA (%) | |
|---|---|---|---|---|---|---|
| FZP | 2.3 ± 0.4 | 0.4 ± 0.1 | 0.7 ± 0.2 | 1.1 ± 0.2 | 2.9 ± 0.2 | 90.2 |
| HG/FZP-pLys100 | 0.9 ± 0.1 | 0.9 ± 0.2 | 1.7 ± 0.4 | 2.1 ± 0.1 | 2.9 ± 0.4 | 88.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Higuchi, K.; Yamada, K.; Kihara, T.; Makino, K.; Sasaki, K.; Shindo, T.; Shikama, H.; Sato, H.; Onoue, S. Polylysine-Containing Hydrogel Formulation of Fuzapladib, Inhibitor of Leukocyte-Function Associated Antigen-1 (LFA-1) Activation, for Sustained Release. Molecules 2023, 28, 5325. https://doi.org/10.3390/molecules28145325
Higuchi K, Yamada K, Kihara T, Makino K, Sasaki K, Shindo T, Shikama H, Sato H, Onoue S. Polylysine-Containing Hydrogel Formulation of Fuzapladib, Inhibitor of Leukocyte-Function Associated Antigen-1 (LFA-1) Activation, for Sustained Release. Molecules. 2023; 28(14):5325. https://doi.org/10.3390/molecules28145325
Chicago/Turabian StyleHiguchi, Koji, Kohei Yamada, Tsubasa Kihara, Keisuke Makino, Kenta Sasaki, Takeshi Shindo, Hiroshi Shikama, Hideyuki Sato, and Satomi Onoue. 2023. "Polylysine-Containing Hydrogel Formulation of Fuzapladib, Inhibitor of Leukocyte-Function Associated Antigen-1 (LFA-1) Activation, for Sustained Release" Molecules 28, no. 14: 5325. https://doi.org/10.3390/molecules28145325






