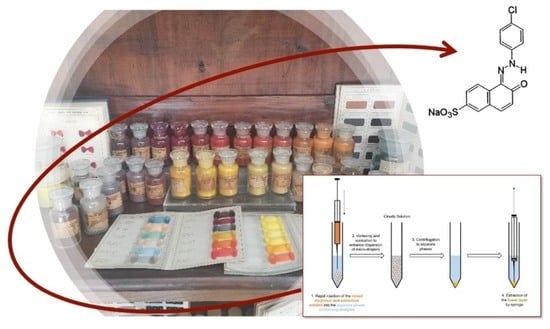
Inside the History of Italian Coloring Industries: An Investigation of ACNA Dyes through a Novel Analytical Protocol for Synthetic Dye Extraction and Characterization
Abstract
1. Introduction
2. Results and Discussion
2.1. Development of IP-dLLME Protocol
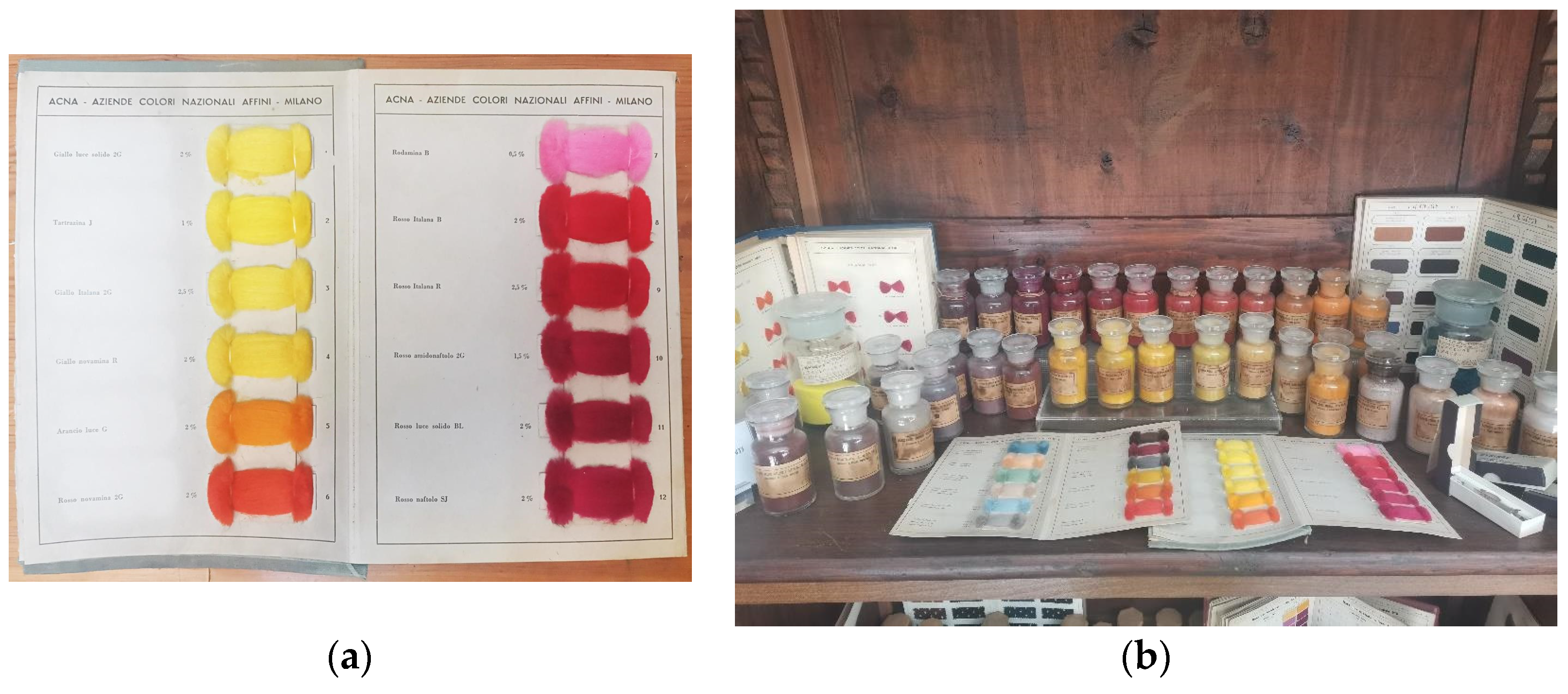
2.2. Application to the Case Study: ACNA Industries Samples
2.3. HRMS Analyses—Analytical Challenges
- Assignments of “Arancio Luce G”
- Assignment of “Giallo Eliaminia RL”
- Assignment of “Giallo Italana 2G”
- Assignment of “Giallo Luce Solido 2G”
- Assignment of “Giallo Novamina 2G”
- Assignment of “Rosso Amidonaftolo 2G”
- Assignment of “Tartrazina J”
- Assignment of “Rosso Italana B”
- Assignment of “Rosso Luce Solido BL” and “Rosso Italana R”
- Assignment of “Rosso Naftolo SJ”
- Assignment of “Rosso Novamina 2G”
- Assignment of “SEII Azoico Acido Pag”
3. Materials and Methods
3.1. Solvents and Reagents
3.2. Development of Clean Up for Synthetic Dyes: dLLME
3.2.1. Evaluation of Disperser for Synthetic Dyes
3.2.2. Trial of the Complete Analytical Protocol for Synthetic Dyes
3.2.3. Analysis of Historical Textile and Powder Samples
3.3. HPLC-MS Analyses
3.3.1. Targeted HPLC-MS Analyses
- i.
- Phase A: 0.1% formic acid in acetonitrile; Phase B: 0.1% formic acid in water;
- ii.
- Phase A: 5 mM ammonium acetate in acetonitrile; Phase B: 5 mM ammonium acetate in water;
- iii.
- Phase A: methanol; Phase B: 5 mM ammonium acetate in water.
3.3.2. Untargeted HPLC-MS Analyses
3.4. Sampling from ACNA Collection
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holme, I. Sir William Henry Perkin: A review of his life, work and legacy. Color. Technol. 2006, 122, 235–251. [Google Scholar] [CrossRef]
- Mcclure, K.R. Development of New Extraction Methods for Analysis of Natural and Synthetic Organic Colourants from Historical and Artistic Matrices. Master’s Thesis, Sapienza University of Rome, Rome, Italy, 2020. [Google Scholar]
- Liu, J.; Zhou, Y.; Zhao, F.; Peng, Z.; Wang, S. Identification of early synthetic dyes in historical Chinese textiles of the late nineteenth century by high-performance liquid chromatography coupled with diode array detection and mass spectrometry. Color. Technol. 2016, 132, 177–185. [Google Scholar] [CrossRef]
- Chequer, F.D.; de Oliveira, G.A.; Ferraz, E.A.; Cardoso, J.C.; Zanoni, M.B.; de Oliveira, D.P. Textile dyes: Dyeing process and environmental impact. Eco-Friendly Text. Dye. Finish. 2013, 6, 151–176. [Google Scholar] [CrossRef]
- Shahid, M.; Wertz, J.; Degano, I.; Aceto, M.; Khan, M.I.; Quye, A. Analytical methods for determination of anthraquinone dyes in historical textiles: A review. Anal. Chim. Acta 2019, 1083, 58–87. [Google Scholar] [CrossRef] [PubMed]
- Khatri, A.; Peerzada, M.H.; Mohsin, M.; White, M. A review on developments in dyeing cotton fabrics with reactive dyes for reducing effluent pollution. J. Clean. Prod. 2015, 87, 50–57. [Google Scholar] [CrossRef]
- Colombo, S. American Institute of Chemical Engineers. Global Outlook: Innovation and Italy’s Chemicals Industries. 2014. Available online: https://www.aiche.org/sites/default/files/cep/20140454_2.pdf (accessed on 1 November 2020).
- Souto, C.S. Analysis of Early Synthetic Dyes with HPLC-DAD-MS: An Important Database for Analysis of Colorants Used in Cultural Heritage. Ph.D. Thesis, Faculdade de Ciências e Tecnologia Department of Conservation and Restoration, Universidade Nova de Lisboa, Lisbon, Portugal, 2010. [Google Scholar]
- Ciccola, A.; McClure, K.R.; Serafini, I.; Vincenti, F.; Montesano, C.; Gentili, A.; Curini, R.; Favero, G.; Postorino, P. The XXth Century and its new colours: Investigating the molecular structures of historical synthetic dyes using Raman spectroscopy, submitted to Journal of Raman Spectroscopy. Rome, Italy, 2023; Available online: https://www.mdpi.com/journal/molecules/instructions#references (accessed on 1 November 2020).
- Cooksey, C.J. Quirks of dye nomenclature. 2. Congo red. Biotech. Histochem. 2014, 89, 384–387. [Google Scholar] [CrossRef]
- Fremout, W.; Saverwyns, S. Identification of synthetic organic pigments: The role of a comprehensive digital Raman spectral library. J. Raman Spectrosc. 2012, 43, 1536–1544. [Google Scholar] [CrossRef]
- Smith, G.D.; Esson, J.M.; Chen, V.J.; Hanson, R.M. Forensic dye analysis in cultural heritage: Unraveling the authenticity of the earliest Persian knotted-pile silk carpet. Forensic. Sci. Int. Synergy 2021, 3, 100130. [Google Scholar] [CrossRef]
- Leona, M.; Decuzzi, P.; Kubic, T.A.; Gates, G.; Lombardi, J.R. Nondestructive identification of natural and synthetic organic colorants in works of art by surface enhanced Raman scattering. Anal. Chem. 2011, 83, 3990–3993. [Google Scholar] [CrossRef] [PubMed]
- Sessa, C.; Weiss, R.; Niessner, R.; Ivleva, N.P.; Stege, H. Towards a Surface Enhanced Raman Scattering (SERS) spectra database for synthetic organic colourants in cultural heritage. The effect of using different metal substrates on the spectra. Microchem. J. 2018, 138, 209–225. [Google Scholar] [CrossRef]
- Pirok, B.W.; Moro, G.; Meekel, N.; Berbers, S.V.; Schoenmakers, P.J.; Van Bommel, M.R. Mapping degradation pathways of natural and synthetic dyes with LC-MS: Influence of solvent on degradation mechanisms. J. Cult. Herit. 2019, 38, 29–36. [Google Scholar] [CrossRef]
- Weisz, A.; Lazo-Portugal, R.; Perez-Gonzalez, M.; Ridge, C.D. Identification, synthesis, and quantification of a novel carbazole derivative as an impurity in the anthraquinone dye Ext. D&C Violet No. 2 (Acid Violet 43). Dyes Pigment. 2022, 200, 110161. [Google Scholar]
- Sandstrom, E.; Wyld, H.; Mackay, C.L.; Troalen, L.G. An optimised small-scale sample preparation workflow for historical dye analysis using UHPLC- PDA applied to Scottish and English Renaissance embroidery. Anal. Methods 2021, 13, 4220–4227. [Google Scholar] [CrossRef]
- Serafini, I.; Lombardi, L.; Fasolato, C.; Sergi, M.; Di Ottavio, F.; Sciubba, F.; Montesano, C.; Guiso, M.; Costanza, R.; Nucci, L.; et al. A new multi analytical approach for the identification of synthetic and natural dyes mixtures. The case of orcein-mauveine mixture in a historical dress of a Sicilian noblewoman of nineteenth century. Nat. Prod. Res. 2019, 33, 1040–1051. [Google Scholar] [CrossRef]
- Chen, V.J.; Smith, G.D.; Holden, A.; Paydar, N.; Kiefer, K. Chemical analysis of dyes on an Uzbek ceremonial coat: Objective evidence for artifact dating and the chemistry of early synthetic dyes. Dyes Pigment. 2016, 131, 320–332. [Google Scholar] [CrossRef]
- Lombardi, L.; Serafini, I.; Guiso, M.; Sciubba, F.; Bianco, A. A new approach to the mild extraction of madder dyes from lake and textile. Microchem. J. 2016, 126, 373–380. [Google Scholar] [CrossRef]
- Serafini, I.; Lombardi, L.; Vannutelli, G.; Montesano, C.; Sciubba, F.; Guiso, M.; Curini, R.; Bianco, A. How the extraction method could be crucial in the characterization of natural dyes from dyed yarns and lake pigments: The case of American and Armenian cochineal dyes, extracted through the new ammonia-EDTA method. Microchem. J. 2017, 134, 237–245. [Google Scholar] [CrossRef]
- Christie, R. Colour Chemistry, 2nd ed.; Royal Society of Chemistry: London, UK, 2014. [Google Scholar]
- Piccinini, P.; Senaldi, C.; Buriova, E. European Survey on the Presence of Banned Azodyes in Textiles; JRC Scientific and Technical Reports; JRC European Commission: Brussels, Belgium, 2008; Available online: https://publications.jrc.ec.europa.eu/repository/handle/JRC44198 (accessed on 1 July 2023).
- Faraji, M.; Nasiri Sahneh, B.; Javanshir, R. An ion-pair dispersive liquid-liquid microextraction for simultaneous determination of synthetic dyes in ice cream samples by HPLC. Anal. Bioanal. Chem. Res. 2017, 4, 213–225. [Google Scholar]
- Unsal, Y.E.; Tuzen, M.; Soylak, M. Spectrophotometric determination of Sudan Blue II in environmental samples after dispersive liquid-liquid microextraction. Quim. Nova 2014, 37, 1128–1131. [Google Scholar] [CrossRef]
- Long, C.; Mai, Z.; Yang, X.; Zhu, B.; Xu, X.; Huang, X.; Zou, X. A new liquid-liquid extraction method for determination of 6 azo-dyes in chilli products by high-performance liquid chromatography. Food Chem. 2011, 126, 1324–1329. [Google Scholar] [CrossRef]
- Germinario, G.; Ciccola, A.; Serafini, I.; Ruggiero, L.; Sbroscia, M.; Vincenti, F.; Fasolato, C.; Curini, R.; Ioele, M.; Postorino, P.; et al. Gel substrates and ammonia-EDTA extraction solution: A new non- destructive combined approach for the identification of anthraquinone dyes from wool textiles. Microchem. J. 2020, 155, 104780. [Google Scholar] [CrossRef]
- Hunger, K.; Mischke, P.; Rieper, W.; Raue, R.; Kunde, K.; Engel, A. Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2000; Volume 4. [Google Scholar]
- Serafini, I.; Bosi, A.; Vincenti, F.; Peruzzi, P.; McClure, K.R.; Ciccola, A.; Hamza, N.M.; Moricca, C.; Sadori, L.; Montesano, C.; et al. Overcoming the limit of in situ gel supported liquid microextraction: Development of the new InGeL-LC-MS analyses, a smart methodology for the identification of natural dyes from Tutankhamun tomb relics. Anal. Chim. Acta, 2023; to be resubmitted. [Google Scholar]
- Rezaee, M.; Assadi, Y.; Hosseini, M.R.M.; Aghaee, E.; Ahmadi, F.; Berijani, S. Determination of organic compounds in water using dispersive liquid–liquid microextraction. J. Chromatog A 2006, 1116, 1–9. [Google Scholar] [CrossRef]
- Yan, H.; Wang, H. Recent development and applications of dispersive liquid–liquid microextraction. J. Chromatogr. A 2013, 1295, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Vincenti, F.; Montesano, C.; Cellucci, L.; Gregori, A.; Fanti, F.; Compagnone, D.; Curini, R.; Sergi, M. Combination of pressurized liquid extraction with dispersive liquid liquid micro extraction for the determination of sixty drugs of abuse in hair. J. Chromatogr. A 2019, 1605, 360348. [Google Scholar] [CrossRef]
- Quigley, A.; Cummins, W.; Connolly, D. Dispersive liquid-liquid microextraction in the analysis of milk and dairy products: A review. J. Chem-NY 2016, 2016, 4040165. [Google Scholar] [CrossRef]
- El-Sheikh, A.H.; Al-Degs, Y.S. Spectrophotometric determination of food dyes in soft drinks by second order multivariate calibration of the absorbance spectra-pH data matrices. Dyes Pigments 2013, 97, 330–339. [Google Scholar] [CrossRef]
- Brändström, A. Principles of phase-transfer catalysis by quaternary ammonium salts. In Advances in Physical Organic Chemistry; Academic Press: New York, NY, USA, 1977; Volume 15, pp. 267–330. [Google Scholar]
- Colour Index, 2nd ed., The Society of Dyers and Colorists. Available online: https://www.worldcat.org/title/colour-index/oclc/1171036 (accessed on 2 November 2022).
- World Dye Variety. Acid Orange 31. Available online: http://www.worlddyevariety.com/acid-dyes/acid-orange-31.html (accessed on 2 November 2020).
- World Dye Variety. Acid Yellow 11. Available online: http://www.worlddyevariety.com/acid-dyes/acid-yellow-11.html (accessed on 2 November 2020).
- PubChem. Compound Summary Acid Yellow 25. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Acid-Yellow-25 (accessed on 2 November 2020).
- Martin, F.; Oberson, J.-M.; Meschiari, M.; Munari, C. Determination of 18 water-soluble artificial dyes by LC–MS in selected matrices. Food Chem. 2016, 197, 1249–1255. [Google Scholar] [CrossRef] [PubMed]
- PubChem. Compound Summary Red 2G. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Acid-red-1 (accessed on 2 November 2020).
- PubChem. Compound Summary Acid Yellow 17. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/22842 (accessed on 2 November 2020).
- Gao, H.-G.; Gong, W.-J.; Zhao, Y.-G. Rapid Method for quantification of seven Synthetic Pigments in Colored Chinese Steamed Buns using UFLC-MS/MS without SPE. Anal. Sci. 2015, 31, 205–210. [Google Scholar] [CrossRef]
- Giustiniani, P.; Natta, G.; Mazzanti, G.; Crespi, G. Composizioni Poliofiniche Adatte Alla Produzione di Fibre Tessili Crystalline Aventi una Migliorata Affinita per Coloranti. 1959. Available online: http://www.giulionatta.it/pdf/brevetti/img157.pdf (accessed on 2 November 2020).
- World Dye Variety. Acid Orange 19. Available online: http://www.worlddyevariety.com/acid-dyes/acid-orange-19.html (accessed on 2 November 2020).
- Liu, X.; Yang, J.L.; Li, J.H.; Li, X.L.; Li, J.; Lu, X.Y.; Shen, J.Z.; Wang, Y.W.; Zhang, Z.H. Analysis of water-soluble azo dyes in soft drinks by high resolution UPLC–MS. Food Addit. Contam—Chem. Anal. Control Expo. Risk Assess. 2011, 28, 1315–1323. [Google Scholar] [CrossRef]
- PubChem. Compound Summary Acid Orange 7. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Acid-orange-7 (accessed on 2 November 2020).
- Scripo Museum. Aziende Chimiche Nazionali Associate. Available online: https://scripomuseum.com/aziende-chimiche-nazionali-associate/ (accessed on 2 November 2020).


| Sample | Possible Match or Possible Chemical Features | Chemical Structure |
|---|---|---|
| Arancio Luce G | Acid Orange 31 | 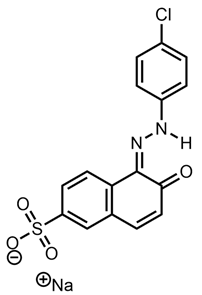 |
| Giallo Eliaminia RL | Further studies ongoing | |
| Giallo Italana 2G | Structural similarities with Acid Orange 31 | |
| Giallo Luce Solido 2G—powder | Acid Yellow 11 | 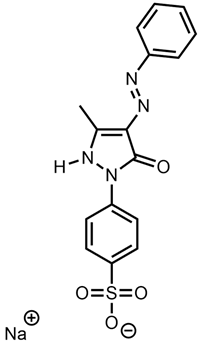 |
| Giallo Luce Solido 2G—fiber | Further studies ongoing | |
| Giallo Novamina 2G | Acid Yellow 25 |  |
| Rosso Amidonaftolo 2G | Red 2G | 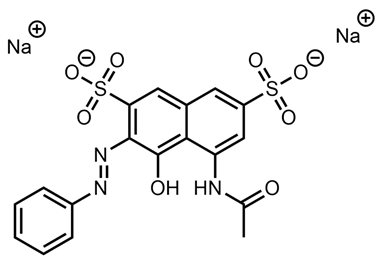 |
| Tartrazine J | Further studies ongoing | |
| Rosso Italana B | Further studies ongoing | |
| Rosso Luce Solido BL; Rosso Italana R | Further studies ongoing | |
| Rosso Naftolo SJ | A naphthalene group (as suggested by the name) as well as at least one sulfonate group and one azo group | |
| Rosso Novamina 2G | Acid Orange 19 | 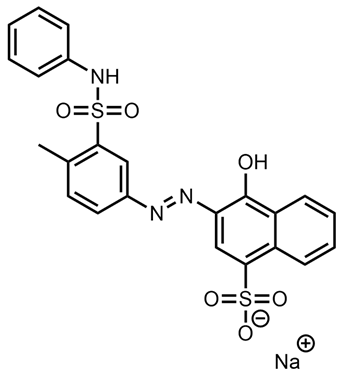 |
| SEII Azoico Acido Pag | Acid Orange 7 | 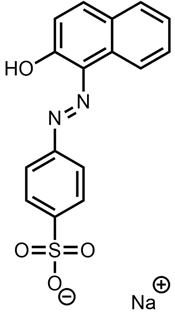 |
| Time (min) | Phase A (MeOH) | Phase B (5 mM Ammonium Acetate in H2O) |
|---|---|---|
| 0.0 | 0% | 100% |
| 3.0 | 100% | 0% |
| 4.5 | 100% | 0% |
| 5.0 | 0% | 100% |
| 7.0 | 0% | 100% |
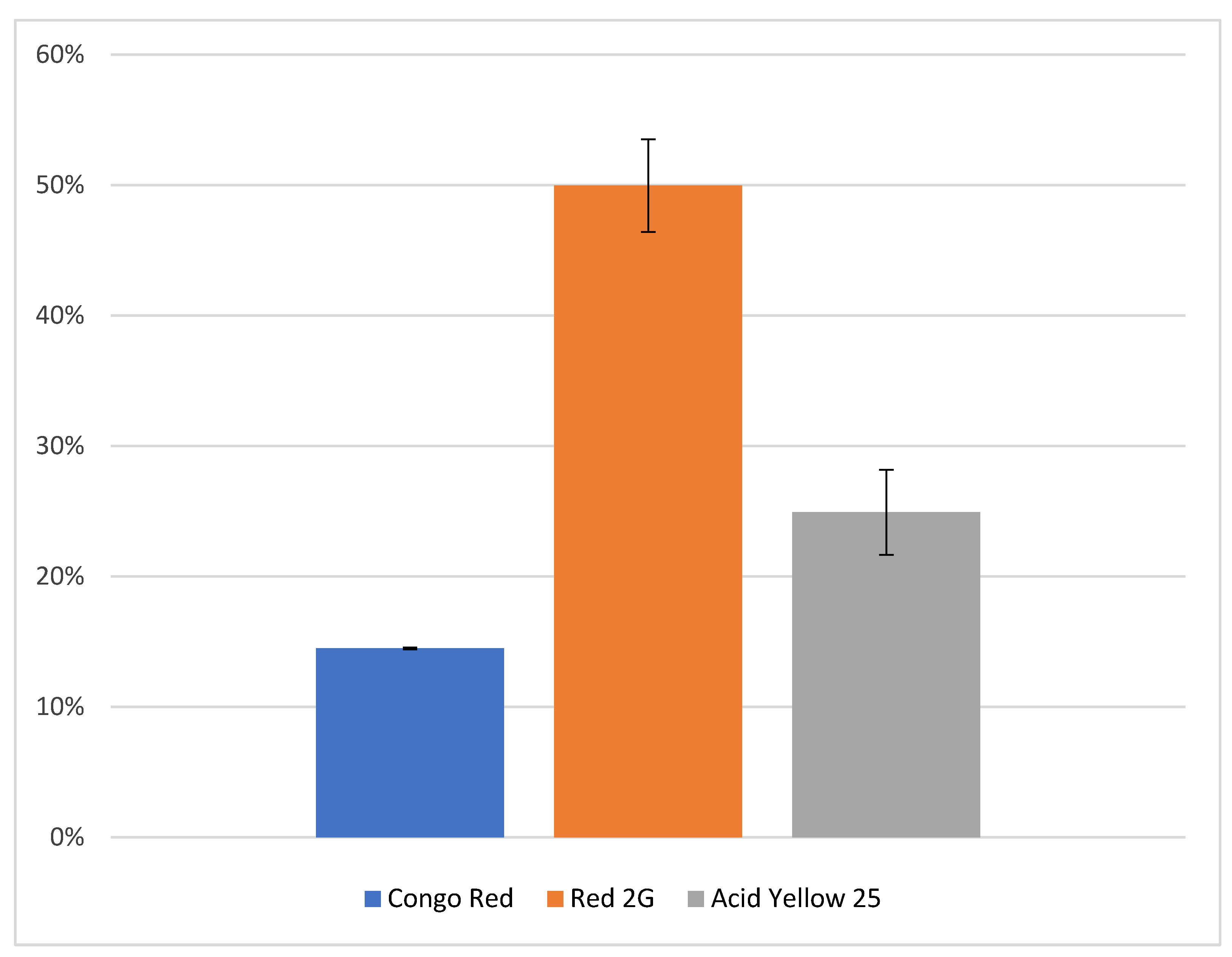
| Dye | Exact Mass | Parent Ion (m/z) | DP (V) | FP (V) | EP (V) | CEP (V) |
|---|---|---|---|---|---|---|
| Acid Yellow 25 | 549.5537 | 526.2 | −101 | −140 | −9 | −29.66 |
| Congo Red | 696.6622 | 325.3 | −38 | −365 | −9 | −24.79 |
| Red 2G | 509.4200 | 231.5 | −15 | −326 | −7 | −22.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serafini, I.; McClure, K.R.; Ciccola, A.; Vincenti, F.; Bosi, A.; Peruzzi, G.; Montesano, C.; Sergi, M.; Favero, G.; Curini, R. Inside the History of Italian Coloring Industries: An Investigation of ACNA Dyes through a Novel Analytical Protocol for Synthetic Dye Extraction and Characterization. Molecules 2023, 28, 5331. https://doi.org/10.3390/molecules28145331
Serafini I, McClure KR, Ciccola A, Vincenti F, Bosi A, Peruzzi G, Montesano C, Sergi M, Favero G, Curini R. Inside the History of Italian Coloring Industries: An Investigation of ACNA Dyes through a Novel Analytical Protocol for Synthetic Dye Extraction and Characterization. Molecules. 2023; 28(14):5331. https://doi.org/10.3390/molecules28145331
Chicago/Turabian StyleSerafini, Ilaria, Kathryn Raeburn McClure, Alessandro Ciccola, Flaminia Vincenti, Adele Bosi, Greta Peruzzi, Camilla Montesano, Manuel Sergi, Gabriele Favero, and Roberta Curini. 2023. "Inside the History of Italian Coloring Industries: An Investigation of ACNA Dyes through a Novel Analytical Protocol for Synthetic Dye Extraction and Characterization" Molecules 28, no. 14: 5331. https://doi.org/10.3390/molecules28145331
APA StyleSerafini, I., McClure, K. R., Ciccola, A., Vincenti, F., Bosi, A., Peruzzi, G., Montesano, C., Sergi, M., Favero, G., & Curini, R. (2023). Inside the History of Italian Coloring Industries: An Investigation of ACNA Dyes through a Novel Analytical Protocol for Synthetic Dye Extraction and Characterization. Molecules, 28(14), 5331. https://doi.org/10.3390/molecules28145331