Investigation of Bioactive Complexes of Chitosan and Green Coffee Bean or Artichoke Extracts
Abstract
1. Introduction
2. Results
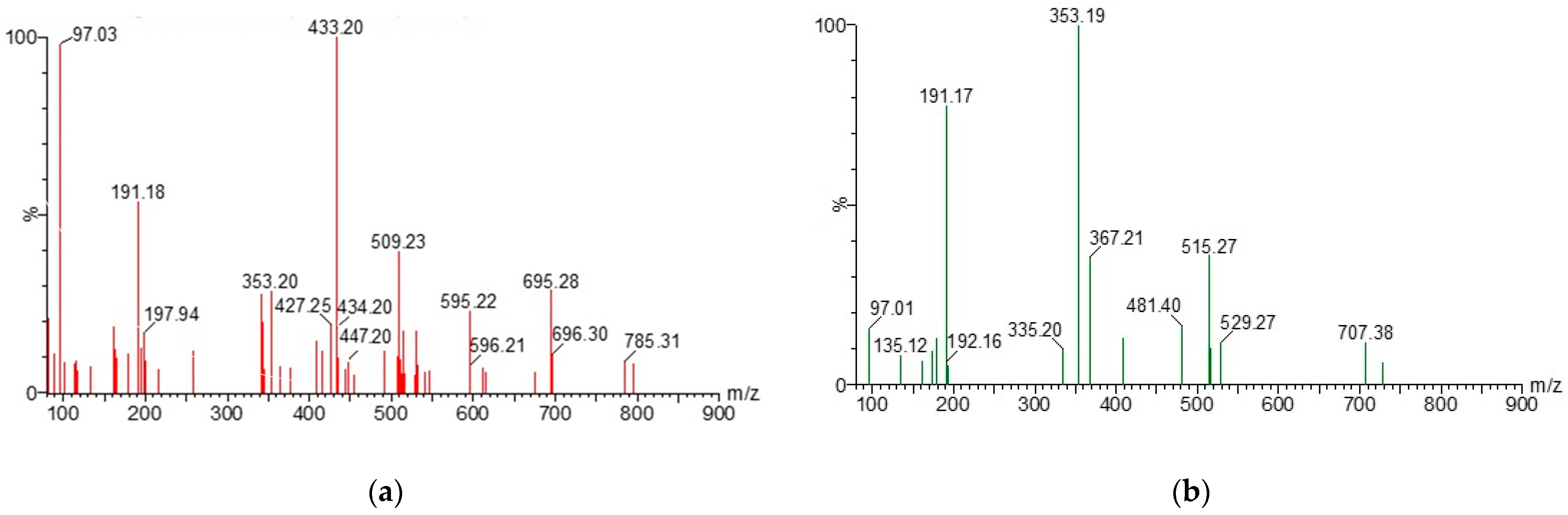
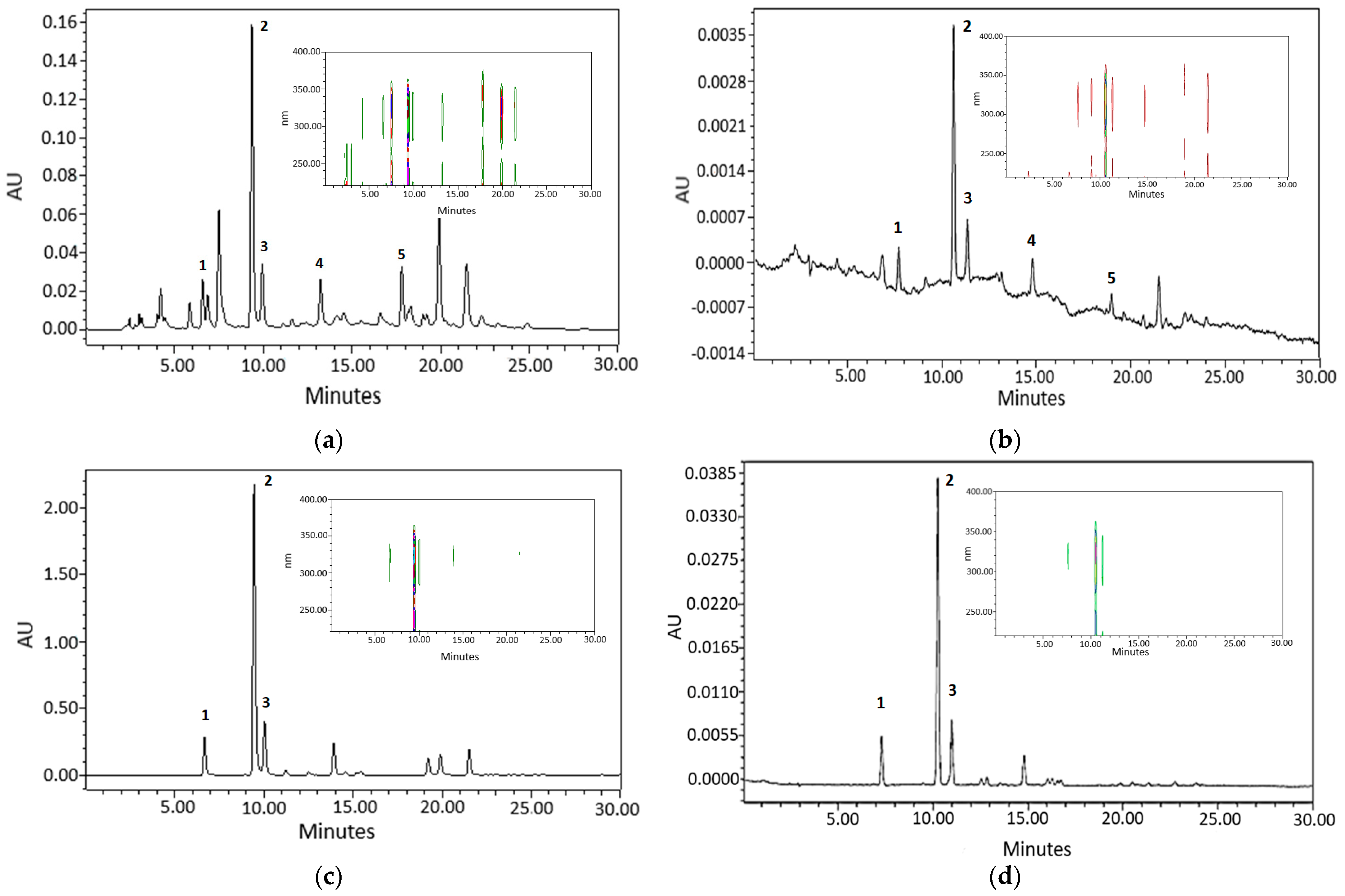
2.1. Chromatographic Analysis of AE and GCBE
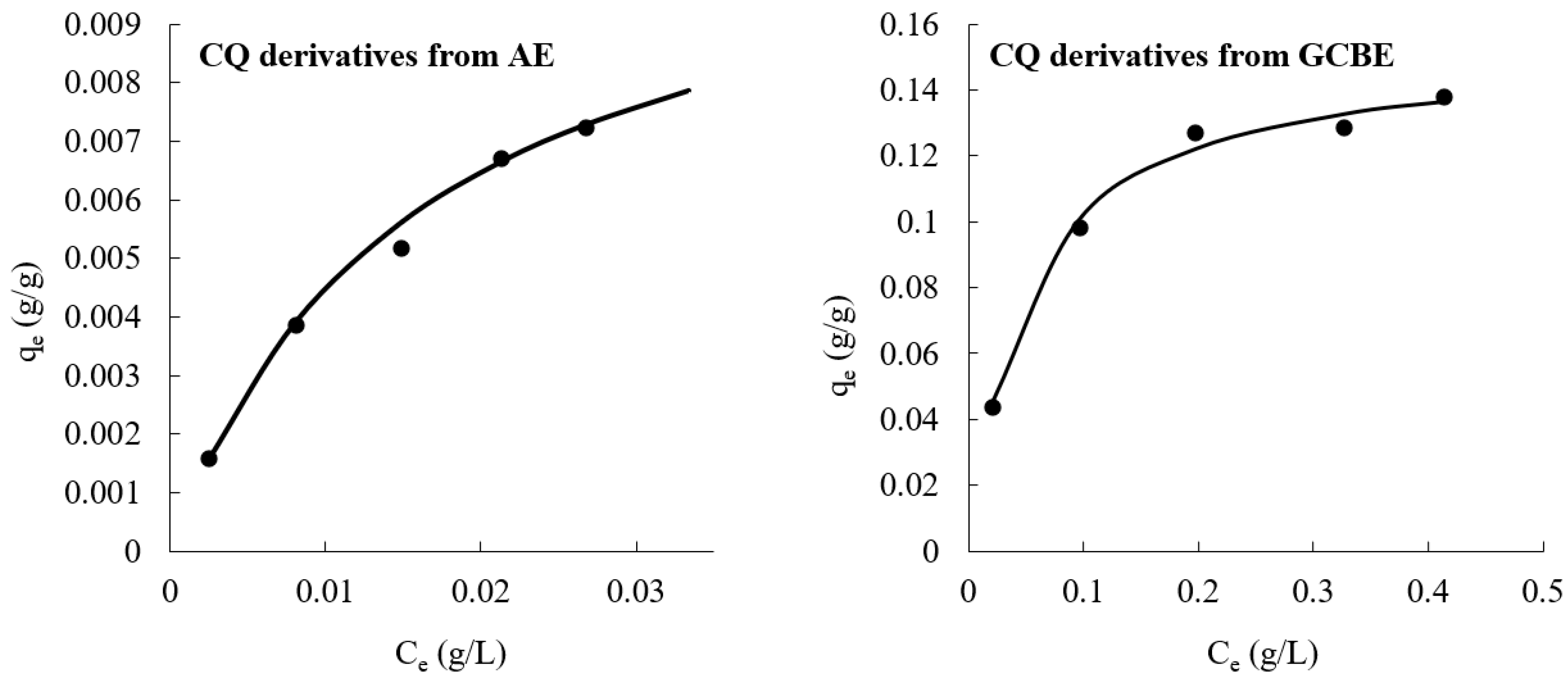
2.2. Equilibrium Adsorption of AE and GCBE onto ChS
2.3. Release of AE and GCBE from Complexes
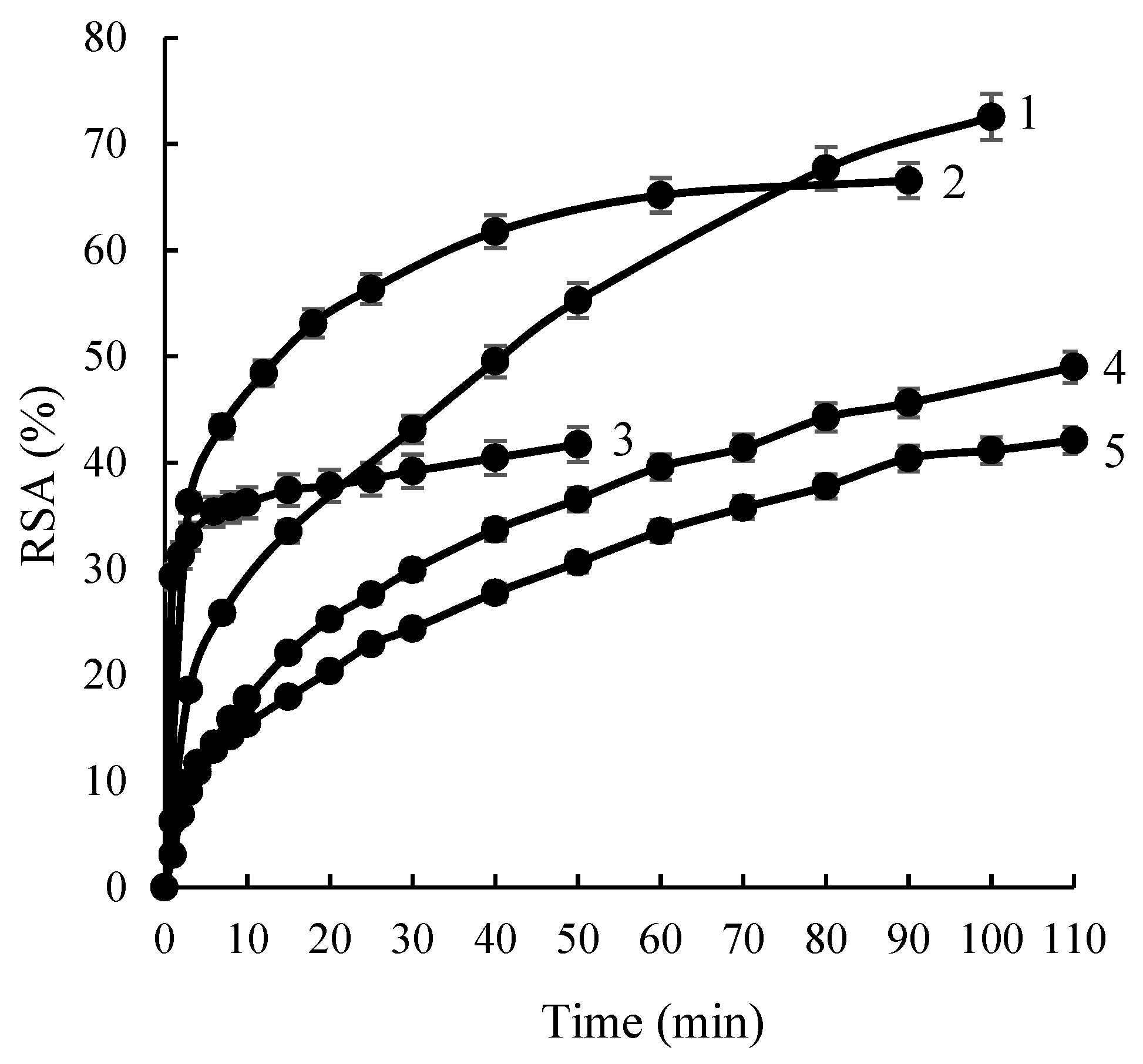
2.4. Bioactive Properties of Complexes
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Equilibrium Adsorption Studies
4.3. Chromatographic Analysis
4.4. Preparation of Complexes
4.5. Release Study
4.6. Antioxidant Activity Study
4.7. Antifungal Activity Study
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Farah, A.; Monteiro, M.; Donangelo, C.M.; Lafay, S. Chlorogenic acids from green coffee extract are highly bioavailable in humans. J. Nutr. 2008, 138, 2309–2315. [Google Scholar] [CrossRef]
- Upadhyay, R.; Rao, L.J.M. An outlook on chlorogenic acids-occurrence chemistry, technology, and biological activities. Crit. Rev. Food Sci. Nutr. 2013, 53, 968–984. [Google Scholar] [CrossRef]
- Carneiro, A.A.; De Barros, Y.Y.; De Freitas, M.M.; Simeoni, L.A.; Magalhães, P.O.; Silveira, D.; Fonseca-Bazzo, Y.M. Identification and quantification of caffeoylquinic acid derivatives in Cynara scolymus L. tablets and capsules. Afr. J. Pharm. Pharmacol. 2017, 11, 94–102. [Google Scholar]
- Nakatani, N.; Kayano, S.I.; Kikuzaki, H.; Sumino, K.; Katagiri, K.; Mitani, T. Identification, quantitative determination, and antioxidative activities of chlorogenic acid isomers in prune (Prunus domestica L.). J. Agric. Food Chem. 2000, 48, 5512–5516. [Google Scholar] [CrossRef]
- Lee, T.K.; Lee, J.Y.; Cho, Y.J.; Kim, J.E.; Kim, S.Y.; Han, J.; Park, Y.; Yang, H.; Lee, K.W. Optimization of the extraction process of high levels of chlorogenic acid and ginsenosides from short-term hydroponic-cultured ginseng and evaluation of the extract for the prevention of atopic dermatitis. J. Ginseng. Res. 2022, 46, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Rosliuk, D.; Rutkaite, R.; Ivanauskas, L.; Jakstas, V. Interaction between cross-linked cationic starch microgranules and chlorogenic acid isomers in artichoke and green coffee bean aqueous extracts. J. Chromatogr. B 2020, 1160, 122385. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.Y.; Hu, R.Z.; Zhang, X.Q.; Wan, Y.; Yao, X.H.; Chen, T.; Fu, Y.J.; Liu, Z.Y.; Hua, S. Preparation of modified cellulose microspheres and application of fast enrichment of chlorogenic acid from mulberry leaf extract. J. Mater. Res. Technol. 2021, 15, 911–923. [Google Scholar] [CrossRef]
- Liu, B.; Dong, B.; Yuan, X.; Kuang, Q.; Zhao, Q.; Yang, M.; Liu, J.; Zhao, B. Enrichment and separation of chlorogenic acid from the extract of Eupatorium adenophorum Spreng by macroporous resin. J. Chromatogr. B 2016, 1008, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Marinova, E.M.; Toneva, A.; Yanishlieva, N. Comparison of the antioxidative properties of caffeic and chlorogenic acids. Food Chem. 2009, 114, 1498–1502. [Google Scholar] [CrossRef]
- Santos, M.D.; Almeida, M.C.; Lopes, N.P.; Souza, G.E.P. Evaluation of the antiinflamatory, analgesic and antipyretic activity of the natural polyphenol chlorogenic acid. Biol. Pharm. Bull. 2006, 29, 2236–2240. [Google Scholar] [CrossRef]
- Ranheim, T.; Halvorsen, B. Coffee consumption and human health: Beneficial or detrimental? Mechanisms for effects of coffee consumption on different risk factors for cardiovascular disease and type 2 diabetes mellitus. Mol. Nutr. Food Res. 2005, 49, 274–284. [Google Scholar] [CrossRef]
- Almeida, A.A.; Farah, A.; Silva, D.A.M.; Nunam, E.A.; Glória, M.B.A. Antibacterial activity of coffee extracts and selected coffee chemical compounds against enterobacteria. J. Agric. Food Chem. 2006, 54, 8738–8743. [Google Scholar] [CrossRef]
- Ding, Y.; Cao, Z.; Cao, L.; Ding, G.; Wang, Z.; Xiao, W. Antiviral activity of chlorogenic acids against influenza A (H1N1/H3N2) virus and its inhibition of neuraminidase. Sci. Rep. 2017, 7, 45723. [Google Scholar] [CrossRef] [PubMed]
- Saqib, M.; Iqbal, S.; Mahmood, A.; Akram, R. Theoretical investigation for exploring the antioxidant potential of chlorogenic acid: A density functional theory study. Int. J. Food Prop. 2016, 19, 745–751. [Google Scholar] [CrossRef]
- Umeno, A.; Horie, M.; Murotomi, K.; Nakajima, Y.; Yoshida, Y. Antioxidative and antidiabetic effects of natural polyphenols and isoflavones. Molecules 2016, 21, 708. [Google Scholar] [CrossRef] [PubMed]
- Choquenet, B.; Couteau, C.; Paparis, E.; Coiffard, L.J. Flavonoids and polyphenols, molecular families with sunscreen potential: Determining effectiveness with an in vitro method. Nat. Prod. Commun. 2009, 4, 227–230. [Google Scholar] [CrossRef]
- Azuma, K.; Ippoushi, K.; Nakayama, M.; Ito, H.; Higashio, H.; Terao, J. Absorption of chlorogenic acid and caffeic acid in rats after oral administration. J. Agric. Food Chem. 2000, 48, 5496–5500. [Google Scholar] [CrossRef]
- Shi, G.; Rao, L.; Yu, H.; Xiang, H.; Shi, G.; Pen, G.; Long, S. Yeast-cell-based microencapsulation of chlorogenic acid as a water-soluble antioxidant. J. Food Eng. 2007, 80, 1060–1067. [Google Scholar] [CrossRef]
- Jaiswal, R.; Matei, M.F.; Subedi, P.; Kuhnert, N. Does roasted coffee contain chlorogenic acid lactones or/and cinnamoylshikimate esters? Food Res. Int. 2014, 61, 214–227. [Google Scholar] [CrossRef]
- Cai, Y.; Zhang, Z.; Jiang, S.; Yu, M.; Huang, C.; Qiu, R.; Zou, Y.; Zhang, Q.; Ou, S.; Zhou, H.; et al. Chlorogenic acid increased acrylamide formation through promotion of HMF formation and 3-aminopropionamide deamination. J. Hazard Mater. 2014, 268, 1–5. [Google Scholar] [CrossRef]
- Fang, Z.; Bhandari, B. Encapsulation of polyphenols—A review. Trends Food Sci. Technol. 2010, 21, 510–523. [Google Scholar] [CrossRef]
- Le-Bail, P.; Lorentz, C.; Pencreac’h, G.; Soultani-Vigneron, S.; Pontoire, B.; Lopez Giraldo, L.J.; Villeneuve, P.; Hendrickx, J.; Tran, V. Trapping by amylose of the aliphatic chain grafted onto chlorogenic acid: Importance of the graft position. Carbohydr. Polym. 2015, 117, 910–916. [Google Scholar] [CrossRef]
- Nallamuthu, I.; Devi, I.A.; Khanum, F. Chlorogenic acid loaded chitosan nanoparticles with sustained release property, retained antioxidant activity and enhanced bioavailability. Asian J. Pharm. Sci. 2015, 10, 203–211. [Google Scholar] [CrossRef]
- Lorentz, C.; Pencreac’h, G.; Soultani-Vigneron, S.; Rondeau-Mouro, C.; de Carvalho, M.; Pontoire, B.; Ergan, F.; Le Bail, P. Coupling lipophilization and amylose complexation to encapsulate chlorogenic acid. Carbohydr. Polym. 2012, 90, 152–158. [Google Scholar] [CrossRef]
- Simanaviciute, D.; Klimaviciute, R.; Rutkaite, R. Equilibrium adsorption of caffeic, chlorogenic and rosmarinic acids on cationic cross-linked starch with quaternary ammonium groups. Int. J. Biol. Macromol. 2017, 95, 788–795. [Google Scholar] [CrossRef] [PubMed]
- Simanaviciute, D.; Liudvinaviciute, D.; Klimaviciute, R.; Rutkaite, R. Cross-linked cationic starch derivatives for immobilization of chlorogenic acid. Eur. Polym. J. 2017, 93, 833–842. [Google Scholar] [CrossRef]
- Kou, S.; Peters, L.M.; Mucalo, M.R. Chitosan: A review of sources and preparation methods. Int. J. Biol. Macromol. 2021, 169, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.K.; Mishra, A.K.; Arotiba, O.A.; Mamba, B.B. Chitosan-based nanomaterials: A state-of-the-art review. Int. J. Biol. Macromol. 2013, 59, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Wan Ngah, W.S.; Teong, L.; Hanafiah, M.A.K.M. Adsorption of dyes and heavy metal ions by chitosan composites: A review. Carbohydr. Polym. 2011, 83, 1446–1456. [Google Scholar] [CrossRef]
- Zhang, L.; Zeng, Y.; Cheng, Z. Removal of heavy metal ions using chitosan and modified chitosan: A review. J. Mol. Liq. 2016, 214, 175–191. [Google Scholar] [CrossRef]
- Crini, G.; Badot, P.M. Application of chitosan, a natural aminopolysaccharide, for dye removal from aqueous solutions by adsorption processes using batch studies: A review of recent literature. Prog. Polym. Sci. 2008, 33, 399–447. [Google Scholar] [CrossRef]
- Vakili, M.; Rafatullah, M.; Salamatinia, B.; Abdullah, A.Z.; Ibrahim, M.H.; Tan, K.B.; Amouzgar, P. Application of chitosan and its derivatives as adsorbents for dye removal from water and wastewater: A review. Carbohydr. Polym. 2014, 113, 115–130. [Google Scholar] [CrossRef] [PubMed]
- Kyzas, G.Z.; Fu, J.; Lazaridis, N.K.; Bikiaris, D.N.; Matis, K.A. New approaches on the removal of pharmaceuticals from wastewaters with adsorbent materials. J. Mol. Liq. 2015, 209, 87–93. [Google Scholar] [CrossRef]
- Oladoja, N.A.; Adelagun, R.O.A.; Ahmad, A.L.; Unuabonah, E.I.; Bello, H.A. Preparation of magnetic, macro-reticulated cross-linked chitosan for tetracycline removal from aquatic systems. Colloids Surf. B Biointerfaces 2014, 117, 51–59. [Google Scholar] [CrossRef]
- McClements, D.J. Encapsulation, protection, and release of hydrophilic active components: Potential and limitations of colloidal delivery systems. Adv. Colloid Interface Sci. 2015, 219, 27–53. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, H.; Xu, C.; Gu, L. A review: Using nanoparticles to enhance absorption and bioavailability of phenolic phytochemicals. Food Hydrocoll. 2015, 43, 153–164. [Google Scholar] [CrossRef]
- Cirillo, G.; Curcio, M.; Vittorio, O.; Iemma, F.; Restuccia, D.; Spizzirri, U.G.; Puoci, F.; Picci, N. Polyphenol conjugates and human health: A perspective review. Crit. Rev. Food Sci. Nutr. 2016, 56, 326–337. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Pu, J.H.; Liu, S.; Kan, J.; Jin, C. Synthesis, characterization, bioactivity and potential application of phenolic acid grafted chitosan: A review. Carbohydr. Polym. 2017, 174, 999–1017. [Google Scholar] [CrossRef]
- Kavi Rajan, R.; Hussein, M.Z.; Fakurazi, S.; Yusoff, K.; Masarudin, M.J. Increased ROS Scavenging and Antioxidant Efficiency of Chlorogenic Acid Compound Delivered via a Chitosan Nanoparticulate System for Efficient In Vitro Visualization and Accumulation in Human Renal Adenocarcinoma Cells. Int. J. Mol. Sci. 2019, 20, 4667. [Google Scholar] [CrossRef]
- Wei, Z.; Gao, Y. Evaluation of structural and functional properties of chitosan/chlorogenic acid complexes. Int. J. Biol. Macromol. 2016, 86, 376–382. [Google Scholar] [CrossRef]
- Li, F.; Yan, Y.; Gu, C.; Sun, C.; Han, J.; Huangfu, Z.; Song, F. Preparation and Characterization of Phenolic Acid-Chitosan Derivatives as an Edible Coating for Enhanced Preservation of Saimaiti Apricots. Foods 2022, 11, 3548. [Google Scholar] [CrossRef] [PubMed]
- McDougall, B.; King, P.J.; Wu, B.W.; Hostomsky, Z.; Reinecke, M.G.; Robinson, W.E. Dicaffeoylquinic and dicaffeoyltartaric acids were selective inhibitors of human immunodeficiency virus type 1 integrase. Antimicrob. Agents Chemother. 1998, 42, 140–146. [Google Scholar] [CrossRef]
- Alonso-Salces, R.M.; Guillou, C.; Berrueta, L.A. Liquid chromatography coupled with ultraviolet absorbance detection, electrospray ionization, collision induced dissociation and tandem mass spectrometry on a triple quadrupole for the on-line characterization of polyphenols and methylxanthines in green coffee beans. Rapid Commun. Mass. Spectrom. 2009, 23, 363–383. [Google Scholar] [PubMed]
- Biel, W.; Witkowicz, R.; Podsiadlo, C. Proximate composition, minerals and antioxidant activity of artichoke leaf extracts. Biol. Trace Elem. Res. 2020, 194, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Wang, Q. Recent development of chitosan-based polyelectrolyte complexes with natural polysaccharides for drug delivery. Int. J. Biol. Macromol. 2014, 64, 353–367. [Google Scholar] [CrossRef]
- Wong, Y.C.; Szeto, Y.S.; Cheung, W.H.; McKay, G. Adsorption of acid dyes on chitosan—Equilibrium isotherm analysis. Process Biochem 2004, 39, 693–702. [Google Scholar] [CrossRef]
- Langmuir, I. Adsorption of gases on plane surfaces of glass, mica and platinu. J. Am. Chem. Soc. 1918, 40, 212–223. [Google Scholar] [CrossRef]
- Toan, N.V.; Hahn, T.T. Application of chitosan solutions for rice production in Vietnam. Afr. J. Biotechnol. 2013, 12, 382–384. [Google Scholar]
- Matthews, S.; Mila, I.; Scalbert, A.; Donnely, M.X. Extractable and non-extractable poanthocyanidins in barks. Phytochemistry 1997, 45, 693–702. [Google Scholar] [CrossRef]
- Woo, J.Y.; Je, J.Y. Antioxidant and tyrosinase inhibitory activities of a novel chitosan–phloroglucinol conjugate. Int. J. Food Sci. 2013, 48, 1172–1178. [Google Scholar] [CrossRef]
- Schöneberg, T.; Kibler, K.; Sulyok, M.; Musa, T.; Bucheli, T.D.; Mascher, F.; Bertossa, M.; Voegele, R.T.; Vogelgsang, S. Can Plant Phenolic Compounds Reduce Fusarium Growth and Mycotoxin Production in Cereals? Food Addit. Contam. Part A 2018, 35, 2455–2470. [Google Scholar] [CrossRef] [PubMed]
- Miller, N.J.; Rice-Evans, C.; Davies, M.J.; Gopinathan, V.; Milner, A. A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clin. Sci. 1993, 84, 407–412. [Google Scholar] [CrossRef] [PubMed]

| T (°C) | QL (g/g) | KL (L/g) | R2 |
|---|---|---|---|
| Adsorption of AE | |||
| 30 | 0.61 | 1.24 | 0.999 |
| 40 | 0.46 | 1.45 | 0.994 |
| 60 | 0.31 | 1.40 | 0.999 |
| Adsorption of GCBE | |||
| 30 | 2.45 | 38.20 | 0.991 |
| 40 | 2.49 | 29.52 | 0.998 |
| 60 | 2.51 | 22.89 | 0.999 |
| Natural Extract | QL (g/g) | KL (L/g) | R2 |
|---|---|---|---|
| AE | 0.01 | 62.94 | 0.999 |
| GCBE | 0.15 | 20.07 | 0.996 |
| Release Medium | Concentration 1 of Phenolics, (mg/L)/(%) | |
|---|---|---|
| AE | GCBE | |
| 0.1 mol/L HCl (pH = 1) | Dissolved | Dissolved |
| Acetate buffer (pH = 4.1) | Dissolved | 283/92.8 |
| Phosphate buffer (pH = 6.8) | 104/50.0 | 129/42.3 |
| Distilled water (pH = 5) | 13.3/6.4 | 13/4.3 |
| Sample | Concentration of Phenolics in PDA (g/L) | Concentration of ChS in PDA (g/L) | Inhibition of Growth at Day 4 (%) | |
|---|---|---|---|---|
| B. cinerea mycelium | F. graminearum spores | |||
| ChS | - | 5 | 54 ± 1 | 21 ± 1 |
| AE/ChS | 0.52 | 5 | 50 ± 1 | 16 ± 1 |
| GCBE/ChS | 0.89 | 5 | 37 ± 1 | 6 ± 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosliuk, D.; Jakstas, V.; Ivanauskas, L.; Liudvinaviciute, D.; Coma, V.; Rutkaite, R. Investigation of Bioactive Complexes of Chitosan and Green Coffee Bean or Artichoke Extracts. Molecules 2023, 28, 5356. https://doi.org/10.3390/molecules28145356
Rosliuk D, Jakstas V, Ivanauskas L, Liudvinaviciute D, Coma V, Rutkaite R. Investigation of Bioactive Complexes of Chitosan and Green Coffee Bean or Artichoke Extracts. Molecules. 2023; 28(14):5356. https://doi.org/10.3390/molecules28145356
Chicago/Turabian StyleRosliuk, Deimante, Valdas Jakstas, Liudas Ivanauskas, Dovile Liudvinaviciute, Veronique Coma, and Ramune Rutkaite. 2023. "Investigation of Bioactive Complexes of Chitosan and Green Coffee Bean or Artichoke Extracts" Molecules 28, no. 14: 5356. https://doi.org/10.3390/molecules28145356
APA StyleRosliuk, D., Jakstas, V., Ivanauskas, L., Liudvinaviciute, D., Coma, V., & Rutkaite, R. (2023). Investigation of Bioactive Complexes of Chitosan and Green Coffee Bean or Artichoke Extracts. Molecules, 28(14), 5356. https://doi.org/10.3390/molecules28145356







