Phytochemical, Morphological and Genetic Characterisation of Anacyclus pyrethrum var. depressus (Ball.) Maire and Anacyclus pyrethrum var. pyrethrum (L.) Link
Abstract
:1. Introduction
2. Results and Discussion
2.1. Morphological Characterisation
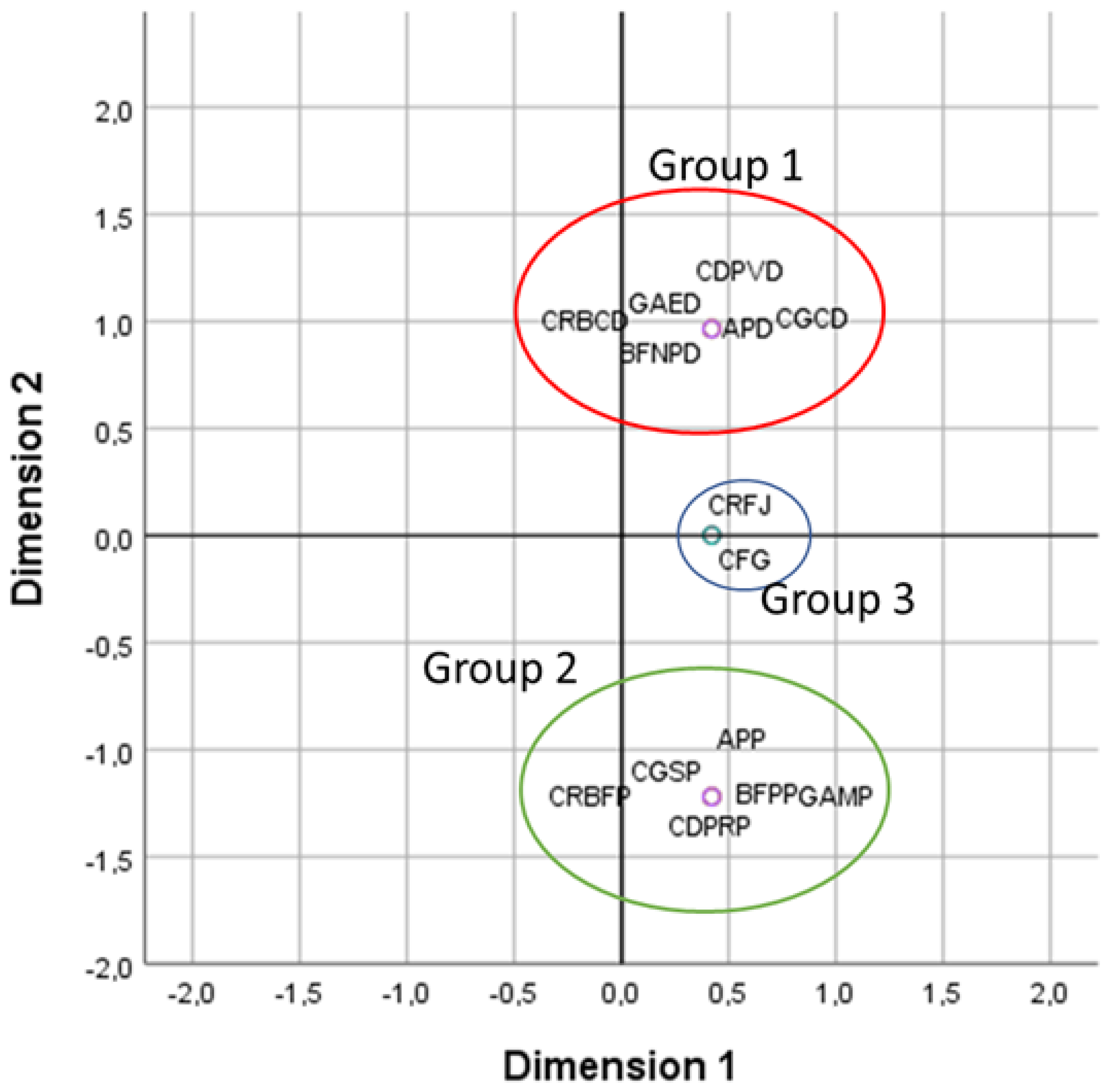
2.1.1. Descriptive Analysis of Qualitative Characteristics
2.1.2. Descriptive Analysis of Quantitative Morphological Traits Studied
- Diversity of quantitative morphological characteristics
- Descriptive statistics for quantitative characteristics
- Correlation between quantitative morphological characteristics
2.2. Phytochemical Characterisation
2.2.1. Phytochemical Screening
2.2.2. Physicochemical Characterisation by UHPLC
2.3. Genetic Characterisation
3. Materials and Methods
3.1. Plant Material
3.2. Morphological Characterisation
3.3. Phytochemical Characterisation
3.3.1. Preparation of Extracts
3.3.2. Phytochemical Screening
- -
- Frankly positive reaction: +++;
- -
- Positive reaction: ++;
- -
- Moderately positive reaction: +;
- -
- Negative reaction: −.
3.3.3. Physicochemical Characterisation by UHPLC
3.4. Molecular Characterisation
3.4.1. DNA Extraction
3.4.2. DNA Amplification and Sequencing
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Dutuit, P.; Gorenflot, R. Unité du Monde Vivant et Développement Durable; Collection—Titre Associé Other Edition; Educagri éditions: Dijon, France, 2016; ISBN 979-10-275-0077-2. [Google Scholar]
- Chebbi, H.; Pascual-Villalobos, M.J.; Cenis, J.L.; Correal, E. Caractérisation morphologique et moléculaire des espèces ligneuses du genre Medicago. Fourrages 1995, 142, 191–206. [Google Scholar]
- El Hansali, M.; Zinelabidine, L.H.; Haddioui, A. Variabilité des caractères morphologiques des populations naturelles de Medicago truncatula Gaertn. au Maroc. Acta Bot. Gall. 2007, 154, 643–649. [Google Scholar] [CrossRef]
- Gorenflot, R. Niveaux et diversité des variations intra-individuelles. Bull. de la Société Bot. de France. Actual. Bot. 1985, 132, 7–17. [Google Scholar] [CrossRef] [Green Version]
- Lesins, K.A.; Lesins, I. Genus Medicago (Leguminosae); Springer: Dordrecht, The Netherlands, 1979; ISBN 978-94-009-9636-6. [Google Scholar]
- Polhill, J.B. Paul: Theology Born of Mission. Rev. Expo. 1981, 78, 233–247. [Google Scholar] [CrossRef]
- Al Naser, O. Effet Des Conditions Environnementales sur les Caratéristiques Morpho-Physiologiques et la Teneur en Métabolites Secondaires Chez Inula Montana: Une Plante de la Médecine Traditionnelle Provençale; Université d’Avignon: Avignon, France, 2018. [Google Scholar]
- Gross, C.L. Floral Structure, Breeding System and Fruit-Set in the Threatened Sub-Shrub Tetratheca Juncea Smith (Tremandraceae). Ann. Bot. 2003, 92, 771–777. [Google Scholar] [CrossRef] [Green Version]
- Tandon, R. Reproductive Biology of Butea Monosperma (Fabaceae). Ann. Bot. 2003, 92, 715–723. [Google Scholar] [CrossRef] [Green Version]
- Aprotosoaie, A.C.; Spac, A.; Miron, A.; Floria, V.; Dorneanu, V. The chemical profile of essential oils obtained from fennel fruits (Foeniculum vulgare Mill.). Farmacia 2010, 58, 46–53. [Google Scholar]
- Aprotosoaie, A.C.; Răileanu, E.; Trifan, A.; Cioanca, O. The Polyphenolic Content of Common Lamiaceae Species Available as Herbal Tea Products in Romanian Pharmacies. Rev. Med. Chir. Soc. Med. Nat. Iasi. 2013, 117, 233–237. [Google Scholar]
- Bruneton, J. Pharmacognosie, Phytochimie Ŕ Plantes Médicinales, 4ème ed.; Techniques et Documentations; lavoisier: Paris, France, 1999. [Google Scholar]
- Chowdhury, M.S.H.; Koike, M.; Muhammed, N.; Halim, M.A.; Saha, N.; Kobayashi, H. Use of Plants in Healthcare: A Traditional Ethno-Medicinal Practice in Rural Areas of Southeastern Bangladesh. Int. J. Biodivers. Sci. Manag. 2009, 5, 41–51. [Google Scholar] [CrossRef] [Green Version]
- Deschepper, R. Variabilité de la Composition Des Huiles Essentielles et Intérêt de la Notion de Chémotype en Aromathérapie; Faculté de pharmacie de Marseille: Marseille, France, 2017. [Google Scholar]
- Barro Kondombo, C.P. Diversités Agro-Morphologique et Génétique de Variétés Locales de Sorgho (Sorghum Bicolor [L.] Moench) du Burkina Faso; Eléments pour la Valorisation des Ressources Génétiques Locales; Université de Ouagadougou: Burkina Faso, Africa, 2010. [Google Scholar]
- Frankham, R.; Briscoe, D.A.; Ballou, J.D. Introduction to Conservation Genetics; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2002; ISBN 978-0-521-63014-6. [Google Scholar]
- Freeland, J. Molecular Ecology; John Wiley & Sons Ltd.: Chichester, UK, 2005; ISBN 13. [Google Scholar]
- Hamrick, J.L.; Godt, M.J.W. Allozyme Diversity in Cultivated Crops. Crop Sci. 1997, 37, 26–30. [Google Scholar] [CrossRef]
- Bautista Salas, A.M. Caractérisation Agro-Morphologique et Moléculaire D’Une Collection de Landraces Péruviennes de Pigeonpea (Cajanus Cajan L. Millsp.) Pour L’Analyse de SA Diversité; Département de Biologie Unité de Recherche en Biologie Cellulaire et Moléculaire Végétale: Namur, Belgium, 2009. [Google Scholar]
- Falińska, K. Seed Bank Dynamics in Abandoned Meadows during a 20-year Period in the Białowieża National Park. J. Ecol. 1999, 87, 461–475. [Google Scholar] [CrossRef]
- Judd, W.S.; Campbell, C.S.; Kellogg, E.A.; Stevens, P.; Bouharmont, J.; Evrard, C.-M. Botanique Systématique. Une Perspective Phylogénétique; Plant Systematics: A Phylogenetic Approach; De Boeck Université: Bruxelles, Belgium; Paris, France, 2002; ISBN 2-7445-0123-9. [Google Scholar]
- Kazan, K.; Manners, J.M.; Cameron, D.F. Genetic Variation in Agronomically Important Species of Stylosanthes Determined Using Random Amplified Polymorphic DNA Markers. Theoret. Appl. Genet. 1993, 85, 882–888. [Google Scholar] [CrossRef]
- Angeles-Shim, R.B.; Asano, K.; Takashi, T.; Kitano, H.; Ashikari, M. Mapping of the Glabrous Gene in Rice Using CSSLs Derived from the Cross Oryza Sativa Subsp. Japonica Cv. Koshihikari times O. Glaberrima. In Proceedings of the 6th International Rice Genetics Symposium, Manila, Philippines, 16–19 November 2009; pp. 16–19. [Google Scholar]
- Gnacadja, C.; Berthouly-Salazar, C.; Nourou Sall, S.; Zekraoui, L.; Sabot, F.; Pegalepo, E.; Manneh, B.; Vieira-Dalode, G.; Moreira, J.; Alaoui El Belghiti, M.; et al. Caractérisation phénotypique et génétique du riz africain (oryza glaberrima steud) phenotypic and genetic characterization of african rice (oryza glaberrima steud). IJAR 2018, 6, 1389–1398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirotsu, N.; Murakami, N.; Kashiwagi, T.; Ujiie, K.; Ishimaru, K. MPertohotdoolocgoy l: A Simple Gel-Free Method for SNP Genotyping Using Allele-Specific Primers in Rice and Other Plant Species. Plant Methods 2010, 6, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Humphries, C.J. A Revision of the Genus Anacyclus L. (Compositae: Anthemidaea). Bull. Br. Mus. Nat. Hist. (Bot.) 1979, 7, 83–142. [Google Scholar]
- Ouarghidi, A.; Powell, B.; Martin, G.J.; Abbad, A. Traditional Sustainable Harvesting Knowledge and Distribution of a Vulnerable Wild Medicinal Root (Anacyclus pyrethrum var. pyrethrum) in Ait M’hamed Valley, Morocco. Econ. Bot. 2017, 71, 83–95. [Google Scholar] [CrossRef]
- Ouarghidi, A.; Abbad, A. Étude ethnobotanique, ethno-taxonomique et ethnoécologique de Anacyclus pyrethrum var. pyrethrum (L.) Link. (Asteraceae) dans la vallée d’Ait Mhamed (Région d’Azilal, Maroc). Rev. D’ethnoécologie 2019, 16. [Google Scholar] [CrossRef] [Green Version]
- Jawhari, F.; Imtara, H.; El Moussaoui, A.; Khalis, H.; Es-Safi, I.; Al Kamaly, O.; Saleh, A.; Parvez, M.K.; Guemmouh, R.; Bari, A. Reproductive Biology of the Two Varieties of Anacyclus pyrethrum L.—Anacyclus pyrethrum var. pyrethrum (L.) Link and Anacyclus pyrethrum var. depressus (Ball.) Maire—An Endemic Endangered Species. Plants 2022, 11, 2299. [Google Scholar] [CrossRef]
- Macheteau, S.; Desvaux, C. Miraculeuses Plantes D’Hildegarde de Bingen: Usages et Remèdes; “Rustica” Éditions: Paris, France, 2017; ISBN 978-2-8153-1048-2. [Google Scholar]
- Abbas Zaidi, S.M.; Pathan, S.A.; Singh, S.; Jamil, S.; Ahmad, F.J.; Khar, R.K. Anticonvulsant, Anxiolytic and Neurotoxicity Profile of Aqarqarha (Anacyclus pyrethrum) DC (Compositae) Root Ethanolic Extract. Pharmacol. Pharm. 2013, 4, 535–541. [Google Scholar] [CrossRef] [Green Version]
- Boonen, J.; Sharma, V.; Dixit, V.; Burvenich, C.; De Spiegeleer, B. LC-MS N-Alkylamide Profiling of an Ethanolic Anacyclus pyrethrum Root Extract. Planta Med. 2012, 78, 1787–1795. [Google Scholar] [CrossRef]
- Boonen, J.; Sharma, V.; Dixit, V.; De Spiegeleer, B. New N-Alkylamides from Anacyclus pyrethrum. Planta Med. 2011, 77, s-0031-1282578. [Google Scholar] [CrossRef]
- Shahraki, S.; Rad, J.S.; Rostami, F.M.; Shahraki, M.R.; Arab, M.R. Effects of aqueous root extracts of Anacyclus pyrethrum on gonadotropins and testosterone serum in adult male rats. Am. J. Phytomed. Clin. Ther. 2014, 6, 767–772. [Google Scholar]
- Sharma, V. Evaluation of the Anabolic, Aphrodisiac and Reproductive Activity of Anacyclus pyrethrum DC in Male Rats. Sci. Pharm. 2009, 77, 97–110. [Google Scholar] [CrossRef] [Green Version]
- Sujith, K.; Darwin, C.R.; Suba, V. Antioxidant Activity of Ethanolic Root Extract of Anacyclus pyrethrum. Int. Res. J. Pharm. 2011, 2, 2109. [Google Scholar]
- Sharma, V.; Thakur, M.; Chauhan, N.S.; Dixit, V.K. Immunomodulatory Activity of Petroleum Ether Extract of Anacyclus pyrethrum. Pharm. Biol. 2010, 48, 1247–1254. [Google Scholar] [CrossRef]
- Sharma, V.; Boonen, J.; Chauhan, N.S.; Thakur, M.; De Spiegeleer, B.; Dixit, V.K. Spilanthes Acmella Ethanolic Flower Extract: LC–MS Alkylamide Profiling and Its Effects on Sexual Behavior in Male Rats. Phytomedicine 2011, 18, 1161–1169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, V.; Boonen, J.; Spiegeleer, B.D.; Dixit, V.K. Androgenic and Spermatogenic Activity of Alkylamide-Rich Ethanol Solution Extract of Anacyclus pyrethrum DC: Androgenic and Spermatogenic Activity of Anacyclus pyrethrum. Phytother. Res. 2013, 27, 99–106. [Google Scholar] [CrossRef]
- Pahuja, M.; Mehla, J.; Reeta, K.H.; Tripathi, M.; Gupta, Y.K. Effect of Anacyclus pyrethrum on Pentylenetetrazole-Induced Kindling, Spatial Memory, Oxidative Stress and Rho-Kinase II Expression in Mice. Neurochem Res 2013, 38, 547–556. [Google Scholar] [CrossRef]
- Bendjeddou, D.; Lalaoui, K.; Satta, D. Immunostimulating Activity of the Hot Water-Soluble Polysaccharide Extracts of Anacyclus pyrethrum, Alpinia Galanga and Citrullus Colocynthis. J. Ethnopharmacol. 2003, 88, 155–160. [Google Scholar] [CrossRef]
- Gautam, O.P.; Verma, S.; Jain, S.K. Anticonvulsant and Myorelaxation Activity of Anacyclus pyrethrum Dc. (Akarkara) Root Extract. Pharmacologyonline 2011, 5, 121–125. [Google Scholar]
- Doudach, L.; Meddah, B.; Alnamer, R.; Chibani, F.; Cherrah, Y. In Vitro Antibacterial Activity of the Methanolic and Aqueous Extracts of Anacyclus pyrethrum Used in Moroccan Traditional Medicine. Int. J. Pharm. Pharm. Sci. 2012, 4, 4. [Google Scholar]
- Elazzouzi, H.; Khennouchi, S.; Bentayeb, A.; Elhilali, F.; Zair, T. Effets biocides des alcaloïdes extraits des racines d’Anacyclus pyrethrum L. (Astéracées) sur Callosobruchus maculatus (Fab.) (Coléoptera: Bruchidae). Int. J. Innov. Appl. Stud. 2015, 13, 19. [Google Scholar]
- Kushwaha, M.; Vijay, S. Plant Anacyclus pyrethrum -A Review. Res. J. Pharmacogn. Phytochem. 2012, 4, 164–170. [Google Scholar]
- Daoudi, A.; Mohamed, B.; Jamal, I.; Laila, N. Antibacterial Activity of Aqueous Extracts of Anacyclus pyrethrum (L.) Link and Corrigiola Telephiifolia Pourr. From the Middle Atlas Region-Morocco. ESJ 2017, 13, 116. [Google Scholar] [CrossRef] [Green Version]
- Jalayer Naderi, N.; Niakan, M.; Khodadadi, E. Determination of Antibacterial Activity of Anacyclus pyrethrum Extract against Some of the Oral Bacteria: An In Vitro Study. J. Dent. Shiraz. Univ. Med. Scien. 2012, 13, 5. [Google Scholar]
- Selles, C.; Djabou, N.; Beddou, F.; Muselli, A.; Tabti, B.; Costa, J.; Hammouti, B. Antimicrobial Activity and Evolution of the Composition of Essential Oil from Algerian Anacyclus pyrethrum L. through the Vegetative Cycle. Nat. Prod. Res. 2013, 27, 2231–2234. [Google Scholar] [CrossRef]
- Jawhari, F.Z.; Moussaoui, A.E.L.; Bourhia, M.; Imtara, H.; Saghrouchni, H.; Ammor, K.; Ouassou, H.; Elamine, Y.; Ullah, R.; Ezzeldin, E.; et al. Anacyclus pyrethrum var. pyrethrum (L.) and Anacyclus pyrethrum var. depressus (Ball) Maire: Correlation between Total Phenolic and Flavonoid Contents with Antioxidant and Antimicrobial Activities of Chemically Characterized Extracts. Plants 2021, 10, 149. [Google Scholar] [CrossRef]
- Hamimed, S. Caractérisation chimique des principes à effet antidermatophyte des racines d’Anacyclus pyrethrum L. Master’s Thesis, Université Constantine 1, Constantine, Algeria, 2009. [Google Scholar]
- Patel, V.K.; Patel, R.V.; Venkatakrishna-Bhatt, H.; Gopalakrishna, G.; Devasankariah, G. A Clinical Appraisal of Anacyclus pyrethrum Root Extract in Dental Patients. Phytother. Res. 1992, 6, 158–159. [Google Scholar] [CrossRef]
- Sijelmassi, A. Les Plantes Médicinales du Maroc De. Available online: https://sites.google.com/site/tiomenmafe/les-plantes-medicinales-du-maroc-badu (accessed on 23 August 2019).
- Van Hecken, L.; Practoner, G. Literature Review on Anacyclus pyrethrum and Profile of Company Jura in Germany Who Supplies the pyrethrum Root Powder Belgium. 2004, p. 28. Available online: https://docplayer.net/amp/47803539-Literature-revieuw-on-anacyclus-pyrethrum-and-profile-of-company-jura-in-germany-who-supplies-the-pyrethrum-root-powder.html (accessed on 7 July 2023).
- Annalakshmi, R.; Uma, R. A Treasure of Medicinal Herb—Anacyclus pyrethrum A Review. Indian J. Drugs Dis. 2012, 3, 9. [Google Scholar]
- Manouze, H.; Bouchatta, O.; Bennis, M.; Sokar, Z.; Ba-M’hamed, S. Anticonvulsive and Neuroprotective Effects of Aqueous and Methanolic Extracts of Anacyclus pyrethrum Root in Kainic Acid-Induced-Status Epilepticus in Mice. Epilepsy Res. 2019, 158, 106225. [Google Scholar] [CrossRef]
- Sujith, K.; Suba, V.; Darwin, C.R. Neuropharmacological Profile of Ethanolic Extract of Anacyclus pyrethrum in Albino Wistar Rats. Int. J. Pharm. Sci. Res. 2011, 2, 2109. [Google Scholar]
- Muralikrishnan, K.; Asokan, S.; Geetha Priya, P.; Ahmed, K.Z.; Ayyappadasan, G. Comparative Evaluation of the Local Anesthetic Activity of Root Extract of Anacyclus pyrethrum and Its Interaction at the Site of Injection in Guinea Pigs. Anesth. Essays Res. 2017, 11, 444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jawhari, F.Z.; El Moussaoui, A.; Bourhia, M.; Imtara, H.; Mechchate, H.; Es-Safi, I.; Ullah, R.; Ezzeldin, E.; Mostafa, G.A.; Grafov, A.; et al. Anacyclus pyrethrum (L.): Chemical Composition, Analgesic, Anti-Inflammatory, and Wound Healing Properties. Molecules 2020, 25, 5469. [Google Scholar] [CrossRef] [PubMed]
- Manouze, H.; Bouchatta, O.; Gadhi, A.C.; Bennis, M.; Sokar, Z.; Ba-M’hamed, S. Anti-Inflammatory, Antinociceptive, and Antioxidant Activities of Methanol and Aqueous Extracts of Anacyclus pyrethrum Roots. Front. Pharmacol. 2017, 8, 598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rimbau, V.; Cerdan, C.; Vila, R.; Iglesias, J. Antiinflammatory Activity of Some Extracts from Plants Used in the Traditional Medicine of North-African Countries (II). Phytother. Res. 1999, 13, 128–132. [Google Scholar] [CrossRef]
- Benali, O.; Selles, C.; Salghi, R. Inhibition of Acid Corrosion of Mild Steel by Anacyclus pyrethrum L. Extracts. Res. Chem. Intermed. 2014, 40, 259–268. [Google Scholar] [CrossRef]
- Azzi, R.; Djaziri, R.; Lahfa, F.; Sekkal, F.Z.; Benmehdi, H.; Belkacem, N. Ethnopharmacological Survey of Medicinal Plants Used in the Traditional Treatment of Diabetes Mellitus in the North Western and South Western Algeria. J. Med. Plants Res. 2012, 10, 2041–2050. [Google Scholar] [CrossRef]
- Selles, C. Valorisation D’Une Plante Médicinale à Activité Antidiabétique de la Région de Tlemcen: Anacyclus pyrethrum L. Application de L’Extrait Aqueux à L’Inhibition de Corrosion D’Un Acier Doux Dans h2so4 0.5M; Universite Abou Bekr Belkaid, Tlemcen Faculte Des Sciences Departement De Chimie: Tlemcen, Algérie, 2012. [Google Scholar]
- Tyagi, S.; Mansoori, M.H.; Singh, N.K.; Shivhare, M.K.; Bhardwaj, P.; Singh, R.K. Antidiabetic Effect of Anacyclus pyrethrum DC in Alloxan Induced Diabetic Rats. Eur. J. Biol. Sci. 2011, 3, 117–120. [Google Scholar]
- Usmani, A.; Khushtar, M.; Arif, M.; Siddiqui, M.; Sing, S.; Mujahid, M. Pharmacognostic and Phytopharmacology Study of Anacyclus pyrethrum: An Insight. J. App. Pharm. Sci. 2016, 6, 144–150. [Google Scholar] [CrossRef] [Green Version]
- Mohammadi, A.; Mansoori, B.; Baradaran, P.C.; Baradaran, S.C.; Baradaran, B. Anacyclus pyrethrum Extract Exerts Anticancer Activities on the Human Colorectal Cancer Cell Line (HCT) by Targeting Apoptosis, Metastasis and Cell Cycle Arrest. J. Gastrointest. Canc. 2017, 48, 333–340. [Google Scholar] [CrossRef]
- Gupta, P.K. Toxicological Testing. In Fundamentals of Toxicology; Elsevier: Amsterdam, The Netherlands, 2016; pp. 131–150. ISBN 978-0-12-805426-0. [Google Scholar]
- Sujith, K.; Darwin, C.R.; Sathish, S.V. Memory-Enhancing Activity of Anacyclus pyrethrum in Albino Wistar Rats. Asian Pac. J. Trop. Dis. 2012, 2, 307–311. [Google Scholar] [CrossRef]
- Crombie, L. Amides of Vegetable Origin. Part IV. the Nature of Pellitorine and Anacyclin. J. Chem. Soc. 1955, 999–1006. [Google Scholar] [CrossRef]
- Crombie, L. Isolation and Structure of an N-Isobutyldienediynamide from Pellitory (Anacyclus pyrethrum DC.). Nature 1954, 174, 832. [Google Scholar] [CrossRef]
- Canli, K.; Yetgin, A.; Akata, I.; Altuner, E.M. Antimicrobial Activity and Chemical Composition Screening of Anacyclus pyrethrum Root. IJPER 2017, 51, s244–s248. [Google Scholar] [CrossRef]
- Chaabane, D.A. Flore et Végétations Méditerranéennes. 2010, p. 74. Available online: https://www.mcours.net/cours/pdf/hasclic1/hasclic141.pdf (accessed on 7 July 2023).
- Chen, Q.-B.; Gao, J.; Zou, G.-A.; Xin, X.-L.; Aisa, H.A. Piperidine Alkaloids with Diverse Skeletons from Anacyclus pyrethrum. J. Nat. Prod. 2018, 81, 1474–1482. [Google Scholar] [CrossRef] [PubMed]
- Elazzouzi, H.; Soro, A.; Elhilali, F.; Bentayeb, A.; Belghiti, M.A.E. Phytochemical Study of Anacyclus pyrethrum (L.) of Middle Atlas (Morocco), and in Vitro Study of Antibacterial Activity of pyrethrum. Adv. Nat. Appl. Sci. 2014, 8, 131–141. [Google Scholar]
- Elazzouzi, H.; Fadili, K.; Cherrat, A.; Amalich, S.; Zekri, N.; Zerkani, H.; Tagnaout, I.; Hano, C.; Lorenzo, J.M.; Zair, T. Phytochemistry, Biological and Pharmacological Activities of the Anacyclus pyrethrum (L.) Lag: A Systematic Review. Plants 2022, 11, 2578. [Google Scholar] [CrossRef]
- Gorji, A.; Khaleghi Ghadiri, M. History of Epilepsy in Medieval Iranian Medicine. Neurosci. Biobehav. Rev. 2001, 25, 455–461. [Google Scholar] [CrossRef]
- Sukumaran, K.; Kuttan, R. Inhibition of Tobacco-Induced Mutagenesis by Eugenol and Plant Extracts. Mutat. Res./Genet. Toxicol. 1995, 343, 25–30. [Google Scholar] [CrossRef]
- Kaur, S.; Jindal, S.; Dhailwal, M.; Chawla, N.; Meena, O. Genetic Diversity Analysis in Elite Lines of Tomato (Solanum Lycopersicum L.) for Growth, Yield and Quality Parameters. Genetika 2017, 49, 329–344. [Google Scholar] [CrossRef]
- Kenneth, T.O. Agro-Morphological and Nutritional Characterization of Tomato Landraces (Lycopersicon Species) in Africa; University of Nairobi: Kenya, Africa, 2016. [Google Scholar]
- Sondo, K. Caractérisation Agro-Morphologique Des Morphotypes de Tomate Issus D’Accession Collectées AU Burkina Faso; Université Polytechnique de Bobo-Dioulasso: Bobodioulasso, Burkina Faso, 2017. [Google Scholar]
- Bellakhdar, J. Contribution À L’étude de la Pharmacopée Traditionnelle Au Maroc: La Situation Actuelle, les Produits’ les Sources du Savoir. Ph.D. Thesis, Université Paul Verlaine, Lorraine, France, 1997. [Google Scholar]
- Cherrat, A.; Amalich, S.; Regragui, M.; Bouzoubae, A.; Elamrani, M.; Mahjoubi, M.; Bourakhouadar, M.; Zair, T. Polyphenols Content and Evaluation of Antioxidant Activity of Anacyclus pyrethrum (L.) Lag. From Timahdite a Moroccan Middle Atlas Region. IJAR 2017, 5, 569–577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hmamouchi, M. Les Plantes Médicinales et Aromatiques Marocaines: Utilisation, Biologie, éCologie, Chimie, Pharmacologie, Toxicologie, Lexiques. Available online: http://www.idpc.ma/view/documentation/bibliopci:35?titleinitial=h&num=3 (accessed on 23 August 2019).
- Jawhari, F.Z.; El Moussaoui, A.; Imtara, H.; Mechchate, H.; Es-Safi, I.; Bouhrim, M.; Kharchoufa, L.; Miry, A.; Bousta, D.; Bari, A. Evaluation of the Acute Toxicity of the Extracts of Anacyclus pyrethrum var. pyrethrum (L.) and Anacyclus pyrethrum var. depressus Maire in Swiss Mice. Vet. World 2021, 14, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Adesina, S.K.; Reisch, J. Arnottianamide and Other Constituents of Zanthoxylum Gillettii Root. J. Nat. Prod. 1988, 51, 601–602. [Google Scholar] [CrossRef]
- Insecticides of Plant Origin; Arnason, J.T.; Philogène, B.J.R.; Morand, P. (Eds.) ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1989; Volume 387, ISBN 978-0-8412-1569-6. [Google Scholar]
- Althaus, J.B.; Malyszek, C.; Kaiser, M.; Brun, R.; Schmidt, T.J. Alkamides from Anacyclus pyrethrum L. and Their in Vitro Antiprotozoal Activity. Molecules 2017, 22, 796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Auhman, A. Contribution à L’éTude Chimique et Pharmacologique D’Anacyclus pyrethrum DC; Faculté des Sciences Semalia: Marrakech, Morocco, 1995. [Google Scholar]
- Ee, G.C.L.; Lim, C.M.; Rahmani, M.; Shaari, K.; Bong, C.F.J. Pellitorine, a Potential Anti-Cancer Lead Compound against HL60 and MCT-7 Cell Lines and Microbial Transformation of Piperine from Piper Nigrum. Molecules 2010, 15, 2398–2404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ku, S.-K.; Lee, I.-C.; Kim, J.A.; Bae, J.-S. Antithrombotic Activities of Pellitorine In Vitro and In Vivo. Fitoterapia 2013, 91, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Ku, S.-K.; Min, B.-W.; Lee, S.; Jee, J.-G.; Kim, J.A.; Bae, J.-S. Vascular Barrier Protective Effects of Pellitorine in LPS-Induced Inflammation In Vitro and In Vivo. Fitoterapia 2014, 92, 177–187. [Google Scholar] [CrossRef]
- El Mokhtari, K.; EL Kouali, M.; Talbi, M.; Hajji, L.; El Brouzi, A. Chemical Composition and Insecticidal Activity of Anacyclus pyrethrum Essential Oil from the Bensliman Area against Culex Pipiens. Mediterr. J. Chem. 2020, 10, 13–21. [Google Scholar] [CrossRef] [Green Version]
- Chaaib Kouri, F. Investigation Phytochimique D’Une Brosse à Dents Africaine Zanthoxylum Zanthoxyloides (Lam.) Zepernick et Timler (Syn. Fagara Zanthoxyloides L.) (Rutaceae); Université de Lausanne: Genève, Switzerland, 2004. [Google Scholar]
- Ho, H.-H.; Chang, C.-S.; Ho, W.-C.; Liao, S.-Y.; Wu, C.-H.; Wang, C.-J. Anti-Metastasis Effects of Gallic Acid on Gastric Cancer Cells Involves Inhibition of NF-ΚB Activity and Downregulation of PI3K/AKT/Small GTPase Signals. Food Chem. Toxicol. 2010, 48, 2508–2516. [Google Scholar] [CrossRef]
- Kang, M.-S.; Jang, H.-S.; Oh, J.-S.; Yang, K.-H.; Choi, N.-K.; Lim, H.-S.; Kim, S.-M. Effects of Methyl Gallate and Gallic Acid on the Production of Inflammatory Mediators Interleukin-6 and Interleukin-8 by Oral Epithelial Cells Stimulated with Fusobacterium Nucleatum. J. Microbiol. 2009, 47, 760–767. [Google Scholar] [CrossRef]
- Lo, C.; Lai, T.-Y.; Yang, J.-S.; Yang, J.-H.; Ma, Y.-S.; Weng, S.-W.; Lin, H.-Y.; Chen, H.-Y.; Lin, J.-G.; Chung, J.-G. Gallic Acid Inhibits the Migration and Invasion of A375.S2 Human Melanoma Cells through the Inhibition of Matrix Metalloproteinase-2 and Ras. Melanoma Res. 2011, 21, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Lee, H.-J.; Jiang, C.; Zhang, J.; Wang, L.; Zhao, Y.; Xiang, Q.; Lee, E.-O.; Kim, S.-H.; Lu, J. Penta-1,2,3,4,6-O-Galloyl- -D-Glucose Induces P53 and Inhibits STAT3 in Prostate Cancer Cells in Vitro and Suppresses Prostate Xenograft Tumor Growth in Vivo. Mol. Cancer Ther. 2008, 7, 2681–2691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Na, H.-J.; Lee, G.; Oh, H.-Y.; Jeon, K.-S.; Kwon, H.-J.; Ha, K.-S.; Lee, H.; Kwon, Y.-G.; Kim, Y.-M. 4-O-Methylgallic Acid Suppresses Inflammation-Associated Gene Expression by Inhibition of Redox-Based NF-ΚB Activation. Int. Immunopharmacol. 2006, 6, 1597–1608. [Google Scholar] [CrossRef] [PubMed]
- Yoon, C.-H.; Chung, S.-J.; Lee, S.-W.; Park, Y.-B.; Lee, S.-K.; Park, M.-C. L’acide gallique, acide polyphénolique naturel, induit l’apoptose et inhibe l’expression des gènes pro-inflammatoires dans les synoviocytes fibroblastiques de polyarthrite rhumatoïde. Rev. du Rhum. 2013, 80, 271–278. [Google Scholar] [CrossRef]
- Genaro-Mattos, T.C.; Maurício, Â.Q.; Rettori, D.; Alonso, A.; Hermes-Lima, M. Antioxidant Activity of Caffeic Acid against Iron-Induced Free Radical Generation—A Chemical Approach. PLoS ONE 2015, 10, e0129963. [Google Scholar] [CrossRef] [Green Version]
- Huang, Q.; Lin, Y.; Yan, Y. Caffeic Acid Production Enhancement by Engineering a Phenylalanine Over-Producing Escherichia Coli Strain: Caffeic Acid Production. Biotechnol. Bioeng. 2013, 110, 3188–3196. [Google Scholar] [CrossRef]
- Lee, K.W.; Kang, N.J.; Kim, J.H.; Lee, K.M.; Lee, D.E.; Hur, H.J.; Lee, H.J. Caffeic Acid Phenethyl Ester Inhibits Invasion and Expression of Matrix Metalloproteinase in SK-Hep1 Human Hepatocellular Carcinoma Cells by Targeting Nuclear Factor Kappa B. Genes. Nutr. 2008, 2, 319–322. [Google Scholar] [CrossRef] [Green Version]
- McGlynn, K.A.; Petrick, J.L.; London, W.T. Global Epidemiology of Hepatocellular Carcinoma. Clin. Liver Dis. 2015, 19, 223–238. [Google Scholar] [CrossRef] [Green Version]
- Tosovic, J. Spectroscopic Features of Caffeic Acid: Theoretical Study. Kragujev. J. Sci 2017, 39, 99–108. [Google Scholar] [CrossRef] [Green Version]
- Verma, R.P.; Hansch, C. An Approach towards the Quantitative Structure-Activity Relationships of Caffeic Acid and Its Derivatives. ChemBioChem 2004, 5, 1188–1195. [Google Scholar] [CrossRef]
- Yang, S.-Y.; Hong, C.-O.; Lee, G.P.; Kim, C.-T.; Lee, K.-W. The Hepatoprotection of Caffeic Acid and Rosmarinic Acid, Major Compounds of Perilla Frutescens, against t-BHP-Induced Oxidative Liver Damage. Food Chem. Toxicol. 2013, 55, 92–99. [Google Scholar] [CrossRef]
- Kikuzaki, H.; Hisamoto, M.; Hirose, K.; Akiyama, K.; Taniguchi, H. Antioxidant Properties of Ferulic Acid and Its Related Compounds. J. Agric. Food Chem. 2002, 50, 2161–2168. [Google Scholar] [CrossRef] [PubMed]
- Ou, S.; Kwok, K.-C. Ferulic Acid: Pharmaceutical Functions, Preparation and Applications in Foods. J. Sci. Food Agric. 2004, 84, 1261–1269. [Google Scholar] [CrossRef]
- Chiang, L.C.; Chiang, W.; Chang, M.Y.; Ng, L.T.; Lin, C.C. Antiviral Activity of Plantago Major Extracts and Related Compounds in Vitro. Antivir. Res. 2002, 55, 53–62. [Google Scholar] [CrossRef]
- dos Santos, M.D.; Almeida, M.C.; Lopes, N.P.; de Souza, G.E.P. Evaluation of the Anti-Inflammatory, Analgesic and Antipyretic Activities of the Natural Polyphenol Chlorogenic Acid. Biol. Pharm. Bull. 2006, 29, 2236–2240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, M.T.H.; Ather, A.; Thompson, K.D.; Gambari, R. Extracts and Molecules from Medicinal Plants against Herpes Simplex Viruses. Antivir. Res. 2005, 67, 107–119. [Google Scholar] [CrossRef]
- McDougall, B.; King, P.J.; Wu, B.W.; Hostomsky, Z.; Reinecke, M.G.; Robinson, W.E. Dicaffeoylquinic and Dicaffeoyltartaric Acids Are Selective Inhibitors of Human Immunodeficiency Virus Type 1 Integrase. Antimicrob. Agents Chemother. 1998, 42, 140–146. [Google Scholar] [CrossRef] [Green Version]
- Tamura, H.; Akioka, T.; Ueno, K.; Chujyo, T.; Okazaki, K.; King, P.J.; Robinson, W.E. Anti-Human Immunodeficiency Virus Activity of 3,4,5-Tricaffeoylquinic Acid in Cultured Cells of Lettuce Leaves. Mol. Nutr. Food Res. 2006, 50, 396–400. [Google Scholar] [CrossRef]
- Wang, G.-F.; Shi, L.-P.; Ren, Y.-D.; Liu, Q.-F.; Liu, H.-F.; Zhang, R.-J.; Li, Z.; Zhu, F.-H.; He, P.-L.; Tang, W.; et al. Anti-Hepatitis B Virus Activity of Chlorogenic Acid, Quinic Acid and Caffeic Acid in Vivo and in Vitro. Antivir. Res. 2009, 83, 186–190. [Google Scholar] [CrossRef]
- Isemura, M. Catechin in Human Health and Disease. Molecules 2019, 24, 528. [Google Scholar] [CrossRef] [Green Version]
- Boots, A.W.; Haenen, G.R.M.M.; Bast, A. Health Effects of Quercetin: From Antioxidant to Nutraceutical. Eur. J. Pharmacol. 2008, 585, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Dajas, F. Life or Death: Neuroprotective and Anticancer Effects of Quercetin. J. Ethnopharmacol. 2012, 143, 383–396. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, G. Quercetin: A Flavonol with Multifaceted Therapeutic Applications? Fitoterapia 2015, 106, 256–271. [Google Scholar] [CrossRef] [PubMed]
- Lesjak, M.; Beara, I.; Simin, N.; Pintać, D.; Majkić, T.; Bekvalac, K.; Orčić, D.; Mimica-Dukić, N. Antioxidant and Anti-Inflammatory Activities of Quercetin and Its Derivatives. J. Funct. Foods 2018, 40, 68–75. [Google Scholar] [CrossRef]
- Russo, M.; Spagnuolo, C.; Tedesco, I.; Bilotto, S.; Russo, G.L. The Flavonoid Quercetin in Disease Prevention and Therapy: Facts and Fancies. Biochem. Pharmacol. 2012, 83, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Cooray, H.C.; Janvilisri, T.; van Veen, H.W.; Hladky, S.B.; Barrand, M.A. Interaction of the Breast Cancer Resistance Protein with Plant Polyphenols. Biochem. Biophys. Res. Commun. 2004, 317, 269–275. [Google Scholar] [CrossRef]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid Antioxidants: Chemistry, Metabolism and Structure-Activity Relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Roohbakhsh, A.; Parhiz, H.; Soltani, F.; Rezaee, R.; Iranshahi, M. Molecular Mechanisms behind the Biological Effects of Hesperidin and Hesperetin for the Prevention of Cancer and Cardiovascular Diseases. Life Sci. 2015, 124, 64–74. [Google Scholar] [CrossRef]
- Medini, M.; Hamza, S.; Rebai, A.; Baum, M. Analysis of Genetic Diversity in Tunisian Durum Wheat Cultivars and Related Wild Species by SSR and AFLP Markers. Genet. Resour. Crop Evol. 2005, 52, 21–31. [Google Scholar] [CrossRef]
- Álvarez, I.; Agudo, A.B.; Herrero, A.; Torices, R. The Mendelian Inheritance of Gynomonoecy: Insights from Anacyclus Hybridizing Species. Am. J. Bot. 2020, 107, 116–125. [Google Scholar] [CrossRef] [Green Version]
- Humphries, C.J. Cytogenetic and Cladistic Studies in Anacyclus (Compositae: Anthemideae). Nord. J. Bot. 1981, 1, 83–96. [Google Scholar] [CrossRef]
- Oberprieler, C. On the Taxonomic Status and the Phylogenetic Relationships of Some Unispecific Mediterranean Genera of Compositae-Anthemideae I. Brocchia, Endopappus and Heliocauta. Willdenowia 2004, 34, 39. [Google Scholar] [CrossRef]
- Vitales, D.; Feliner, G.N.; Vallès, J.; Garnatje, T.; Firat, M.; Álvarez, I. A New Circumscription of the Mediterranean Genus Anacyclus (Anthemideae, Asteraceae) Based on Plastid and Nuclear DNA Markers. Phytotaxa 2018, 349, 1. [Google Scholar] [CrossRef]
- Fennane, M.; Ibn Tattou, M.; El Oualidi, J. Flore Pratique du Maroc—Volume 3; Institut Scientifique: Rabat, Morocco, 2014; Volume 3. [Google Scholar]
- Quézel, P.; Santa, S.; Emberger, L.; Schotter, O. Nouvelle Flore de L’Algérie et Des Régions Désertiques Méridionales; Éditions du Centre National de la Recherche Scientifique: Paris, France, 1963. [Google Scholar]
- Diallo, A. Étude de la Phytochimie et Des Activités Biologiques de Syzygium Guineense Willd. (Myrtaceae); Université de Bamako: Bamako, Republic of Mali, 2005. [Google Scholar]
- Dohou, N.; Yamni, K.; Tahrouch, S. Screening phytochimique d’une endémique iberomarocaine, Thymelaea lythroides. Bull.-Société de Pharm. de Bordx. 2003, 142, 61–78. [Google Scholar]
- Fong, H.H.S.; Tin, W.A.M.; Farnsworth, N. Phytochemical Screening Review; University of Illinois: Chicago, IL, USA, 1977; p. 126. [Google Scholar]
- Niare A ÉTude de la Phytochimie et Des Activités Pharmacologiques de Syzygium Guineense Willd. (Myrtaceae). Ph.D. Thesis, Université de Bamako, Bamako, Republic of Mali, 2005.
- Senhadji, O.; Faid, M.; Elyachioui, M. Étude de l’activité antifongique de divers extraits de cannelle. J. de Mycol. Méd. 2005, 15, 220–229. [Google Scholar] [CrossRef]
- Judith, M.D. Etude Phytochimique et Pharmacologique de Cassia Nigricans Vahl (Caesalpiniaceae) Utilisé Dans Le Traitement Des Dermatoses AU Tchad; Université de Bamako: Bamako, Republic of Mali, 2005. [Google Scholar]
- Boukhira, S. Développement de Conservateurs Naturels Pour la Cosmétique: Applications du Challenge Test et éValuation de Leurs Activités Biologiques; Université Sidi Mohammed Ben Abde llah Facultédes Sciences Dhar El Mahraz-Fès: Fes, Morocco, 2017. [Google Scholar]
- Zekri, N. ÉTude Phytochimique et Activités Biologiques Des Huiles Essentielles et Des Extraits Des M. Pulegium (L.), M. Suaveolens (Ehrh.) et M. Spicata (L.) du Moyen-Atlas Marocain; Chimie de L’environnement, Université Mohammed v Faculté des Sciences Rabat: Rabat, Morocco, 2017. [Google Scholar]
- Ammor, K. Réduction Massique Des Calculs Rénaux Par L’Utilisation Des Végétaux; Université Sidi Mohammed Ben Abdel lah Faculté des Sciences Dhar El Mahraz-Fès: Fes, Morocco, 2020. [Google Scholar]
- Cota-Sánchez, J.H.; Remarchuk, K.; Ubayasena, K. Ready-to-Use DNA Extracted with a CTAB Method Adapted for Herbarium Specimens and Mucilaginous Plant Tissue. Plant Mol. Biol. Rep. 2006, 24, 161–167. [Google Scholar] [CrossRef]
- Parvathy, V.A.; Swetha, V.P.; Sheeja, T.E.; Sasikumar, B. A Two Locus Barcode for Discriminating Piper Nigrum from Its Related Adulterant Species. Indian J. Biotechnol. 2018, 17, 346–350. [Google Scholar]


| Qualitative Characteristics | A.P var. pyrethrum | A.P var. despressus |
|---|---|---|
| Roots | ||
| Colour | Dark brown (CRBFP) | Light brown (CRBCD) |
| Leaves | ||
| Colour | Glaucous (CFGP) | Glaucous (CFGD) |
| Base appearance | Evergreen (BFPP) | Not evergreen (BFNPD) |
| Capitula | ||
| Flower ray colour | Yellow (CRFJP) | Yellow (CRFJD) |
| Petal back colour | Red (CDPRP) | Violet (CDPVD) |
| Seeds | ||
| Colour | Dark (CGSP) | Clear (CGCD) |
| Wing | Thin (GAMP) | Thick (GAED) |
| Variables | Minimum Value | Maximum Value | Mean/Standard Deviation |
|---|---|---|---|
| Roots | |||
| Length (cm) (LOR) | 5 | 9 | 6.637 ± 1.110 |
| Width (cm) (LAR) | 0.9 | 1.3 | 1.065 ± 0.142 |
| Leaves | |||
| Number of branches/individual (FNR) | 41 | 102 | 52.38 ± 20.188 |
| Capitula | |||
| Number/individual (NC) | 50 | 320 | 89.32 ± 29.80 |
| Length (cm) (LOC) | 0.7 | 1.2 | 0.958 ± 0.139 |
| Width (cm) (LAC) | 0.8 | 1.2 | 0.97 ± 0.138 |
| Ligulate flowers | |||
| Number/capitula (NFL) | 12 | 15 | 13.15 ± 0.978 |
| Length (mm) (LOFL) | 7.8 | 13 | 9 ± 0.105 |
| Width (mm) (LAFL) | 2 | 3 | 2.4 ± 0.038 |
| Tubular flowers | |||
| Number/capitula (NFT) | 34 | 130 | 78.05 ± 25.920 |
| Length (mm) (LOFT) | 3 | 5.6 | 4.21 ± 0.090 |
| Width (mm) (LAFT) | 1 | 1.2 | 1.02 ± 0.006 |
| Seeds | |||
| Number/capitula (NG) | 40 | 143 | 81.73 ± 22.45 |
| Length (mm) (LOG) | 2.8 | 3.5 | 3.267 ± 0.404 |
| Width (mm) (LAG) | 2.2 | 2.6 | 2.433 ± 0.208 |
| Weight of 100 seeds (g) (PG) | 0.04 | 0.06 | 0.05 ± 0.005 |
| Variables | Minimum Value | Maximum Value | Mean/Standard Deviation |
|---|---|---|---|
| Roots | |||
| Length (cm) (LOR) | 10 | 18 | 13.979 ± 2.188 |
| Width (cm) (LAR) | 0.9 | 1.8 | 1.424 ± 0.282 |
| Leaves | |||
| Number of branches/individual (FNR) | 16 | 63 | 34.15 ± 10.80 |
| Capitula | |||
| Number/individual (NC) | 29 | 69 | 46.33 ± 10.094 |
| Length (cm) (LOC) | 1.3 | 2.3 | 1.79 ± 0.247 |
| Width (cm) (LAC) | 1.3 | 2.2 | 1.714 ± 0.224 |
| Ligulate flowers | |||
| Number/capitula (NFL) | 9 | 13 | 10.92 ± 1.284 |
| Length (mm) (LOFL) | 14 | 17 | 15.44 ± 1.031 |
| Width (mm) (LAFL) | 2.1 | 4.2 | 3.192 ± 0.79 |
| Tubular flowers | |||
| Number/capitula (NFT) | 36 | 188 | 117.36 ± 27.509 |
| Length (mm) (LOFT) | 5.9 | 8 | 7.16 ± 0.56 |
| Width (mm) (LAFT) | 1.2 | 2.6 | 1.913 ± 0.30 |
| Seeds | |||
| Number/capitula (NG) | 81 | 175 | 116.98 ± 21.75 |
| Length (mm) (LOG) | 3.9 | 4.2 | 4.033 ± 0.153 |
| Width (mm) (LAG) | 3.6 | 4 | 3.767 ± 0.208 |
| Weight of 100 seeds (g) (PG) | 0.11 | 0.14 | 0.13 ± 0.01 |
| Variables | Variation Coefficient between Individuals of A.P var. depressus | Variation Coefficient between Individuals of A.P var. pyrethrum | Variation Coefficient between the Two Varieties | Significance p = 0.001 |
|---|---|---|---|---|
| Roots | ||||
| Length (cm) (LOR) | 16.72% | 15.65% | 39.45% | *** |
| Width (cm) (LAR) | 13.37% | 19.81% | 23.02% | ns |
| Leaves | ||||
| Number of branches/individual (FNR) | 24.86% | 31.62% | 34.71% | *** |
| Capitula | ||||
| Number/individual (NC) | 33.36% | 21.78% | 45.47% | ** |
| Length (cm) (LOC) | 14.55% | 13.83% | 33.67% | ns |
| Width (cm) (LAC) | 14.31% | 13.11% | 31.06% | ns |
| Ligulate flowers | ||||
| Number/capitula (NFL) | 7.43% | 11.76% | 13.25% | ns |
| Length (mm) (LOFL) | 16.05% | 6.67% | 48.83% | *** |
| Width (mm) (LAFL) | 11.72% | 24.96% | 26.35% | * |
| Tubular flowers | ||||
| Number/capitula (NFT) | 33.21% | 23.44% | 33.92% | ns |
| Length (mm) (LOFT) | 21.50% | 7.93% | 29.11% | * |
| Width (mm) (LAFT) | 5.94% | 16.08% | 33.83% | ns |
| Seeds | ||||
| Number/capitula (NG) | 25.59% | 18 % | 29.50% | *** |
| Length (mm) (LOG) | 12.36% | 3.79% | 14.35% | ns |
| Width (mm) (LAG) | 8.54% | 5.52% | 23.40% | ns |
| Weight of 100 seeds (g) (PG) | 10% | 7.69% | 43.85% | ** |
| Compounds/Extracts | A.P var. pyrethrum | A.P var. depressus | ||||||
|---|---|---|---|---|---|---|---|---|
| Capitula (CPP) | Seeds (GPP) | Roots (RPP) | Leaves (FPP) | Capitula (CPD) | Seeds (GPD) | Roots (RPD) | Leaves (FPD) | |
| Tannins | − | − | + | − | + | − | + | + |
| Catechic tannins | − | − | + | − | − | − | + | + |
| Gallic tannins | − | − | − | − | + | − | − | − |
| Flavonoids | + | ++ | + | ++ | ++ | ++ | +++ | ++ |
| Sterols | + | + | − | + | + | + | + | + |
| Alkaloids | ||||||||
| Dragondorf test | + | ++ | ++ | + | + | + | ++ | + |
| Mayer’s test | ++ | + | ++ | ++ | ++ | + | + | ++ |
| Saponosides | + | + | − | − | +++ | + | + | − |
| Cardiac glycosides | + | − | − | + | + | − | − | + |
| Oses and holosides | + | − | − | + | + | − | − | + |
| Mucilages | − | − | − | − | − | − | − | − |
| Free quinones | + | +++ | − | ++ | + | − | − | +++ |
| Sterols and terpenes | + | +++ | +++ | − | ++ | +++ | +++ | − |
| Steroidal heterosides | ++ | + | − | ++ | ++ | + | − | ++ |
| Triterpenes heterosides | + | + | + | ++ | + | + | + | ++ |
| No | RT | m/z | Structural Formula | Compounds | % Area | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A.P var. pyrethrum | A.P var. depressus | |||||||||||
| Roots (RPP) | Seeds (GPP) | Leaves (FPP) | Capitula (CPP) | Roots (RPD) | Seeds (GPD) | Leaves (FPD) | Capitula (CPD) | |||||
| 1 | 0.60 | 180 | C9H8O4 | Caffeic acid | − | − | − | 0.55 | − | − | − | − |
| 2 | 4.60 | 154 | C8H10O3 | Hydroxytyrosol | 1.11 | 5.56 | 2.62 | 3.02 | 1.37 | 3.73 | 1.89 | 3.87 |
| 3 | 5.95 | 174 | C6H14N4O2 | L-arginine | 15.76 | 16.12 | 2.29 | 3.20 | 15.02 | 1.68 | 1.49 | 2.82 |
| 4 | 6.64 | 170 | C7H6O5 | Gallic acid | 6.52 | 6.09 | 8.95 | 11.00 | − | 4.54 | 7.16 | 11.91 |
| 5 | 7.26 | 223 | C14H25NO | Pellitorine | 0.18 | 1.07 | 0.85 | 1.25 | 0.15 | 0.35 | 0.57 | 0.46 |
| 6 | 12.77 | 290 | C15H14O6 | Catechin | − | − | − | − | 7.23 | − | − | − |
| 7 | 16.98 | 168 | C8H8O4 | Vanillic acid | 3.07 | − | 1.10 | 4.60 | 0.45 | − | 1.11 | 2.28 |
| 8 | 17.61 | 354 | C16H18O9 | Chlorogenic acid | − | − | − | − | 1.05 | − | − | − |
| 9 | 21.69 | 146 | C9H6O2 | Coumarin | 2.40 | 12.56 | 19.29 | 12.98 | 3.37 | 11.41 | 20.53 | 13.73 |
| 10 | 21.79 | 148 | C9H8O2 | Cinnamic acid | 1.07 | − | − | − | 2.42 | − | − | − |
| 11 | 21.86 | 164 | C9H8O3 | P-coumaric acid | − | − | − | − | 0.88 | − | − | − |
| 12 | 22.03 | 194 | C10H10O4 | Transferulic acid | − | − | − | − | − | 1.00 | − | − |
| 13 | 22.15 | 194 | C10H10O4 | Ferulic acid | − | − | − | − | − | − | 1.52 | − |
| 14 | 23.21 | 540 | C25H32O13 | Oleuropein | − | 0.41 | 3.07 | 0.84 | − | 0.32 | 1.65 | 0.44 |
| 15 | 24.63 | 580 | C27H32O14 | Naringin | − | 0.58 | 0.46 | 0.44 | − | 0.31 | 0.26 | − |
| 16 | 24.86 | 302 | C15H10O7 | Quercetin | − | − | − | − | − | − | 0.18 | − |
| 17 | 25.96 | 154 | C10H18O | Geraniol | − | − | 3.67 | 1.49 | − | − | − | − |
| 18 | 28.59 | 302 | C16H14O6 | Hesperetin | − | − | − | − | 6.79 | 2.83 | 8.36 | |
| 19 | 38.72 | 237 | C15H27NO | Deca-2E,4E-dienoic acid N-Me IBA | − | 0.39 | − | − | − | − | − | − |
| 20 | 42.03 | 271 | C18H25NO | Anacyclin | 0.67 | − | − | − | 0.44 | − | − | − |
| 21 | 54.25 | 318 | C15H10O8 | ((2E,4E)-N-(2-methylpropyl)tetradeca-2,4-diene-8,10-diynamide) | − | − | − | − | − | − | 0.27 | − |
| Primer Name | Sequence | Number of Nucleotides |
|---|---|---|
| rbcL a-f | 5′ ATG TCA CCA CAA ACA GAG ACT AAA GC3′ | 26 |
| rbcL a-r | 5′ GTA AAA TCA AGT CCA CCG CG 3′ | 20 |
| rpoC1-2 | 5′ GGC AAA GAG GGA AGA TTT CG3′ | 20 |
| rpoC1-4 | 5′ CCA TAA GCA TAT CTT GAG TTG G 3′ | 22 |
| Reaction Condition | Locus | |
|---|---|---|
| rbcL | rpoC1 | |
| Initial denaturation | 95 °C—4 min | 94 °C—1 min |
| Denaturation | 94 °C—30 s | 94 °C—30 s |
| Annealing | 55 °C—1 min | 50 °C—40 s |
| Extension | 72 °C—1 min | 72 °C—40 s |
| Final extension | 72 °C—10 min | 72 °C—5 min |
| Number of cycles | 35 | 40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jawhari, F.Z.; Imtara, H.; Radouane, N.; El Moussaoui, A.; Es-safi, I.; Amaghnouje, A.; N. AlZain, M.; Noman, O.; Parvez, M.K.; Bousta, D.; et al. Phytochemical, Morphological and Genetic Characterisation of Anacyclus pyrethrum var. depressus (Ball.) Maire and Anacyclus pyrethrum var. pyrethrum (L.) Link. Molecules 2023, 28, 5378. https://doi.org/10.3390/molecules28145378
Jawhari FZ, Imtara H, Radouane N, El Moussaoui A, Es-safi I, Amaghnouje A, N. AlZain M, Noman O, Parvez MK, Bousta D, et al. Phytochemical, Morphological and Genetic Characterisation of Anacyclus pyrethrum var. depressus (Ball.) Maire and Anacyclus pyrethrum var. pyrethrum (L.) Link. Molecules. 2023; 28(14):5378. https://doi.org/10.3390/molecules28145378
Chicago/Turabian StyleJawhari, Fatima Zahra, Hamada Imtara, Nabil Radouane, Abdelfattah El Moussaoui, Imane Es-safi, Amal Amaghnouje, Mashail N. AlZain, Omer Noman, Mohammad Khalid Parvez, Dalila Bousta, and et al. 2023. "Phytochemical, Morphological and Genetic Characterisation of Anacyclus pyrethrum var. depressus (Ball.) Maire and Anacyclus pyrethrum var. pyrethrum (L.) Link" Molecules 28, no. 14: 5378. https://doi.org/10.3390/molecules28145378









