Solvent-Free Enzymatic Synthesis of Dietary Triacylglycerols from Cottonseed Oil in a Fluidized Bed Reactor
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of Cottonseed Oil
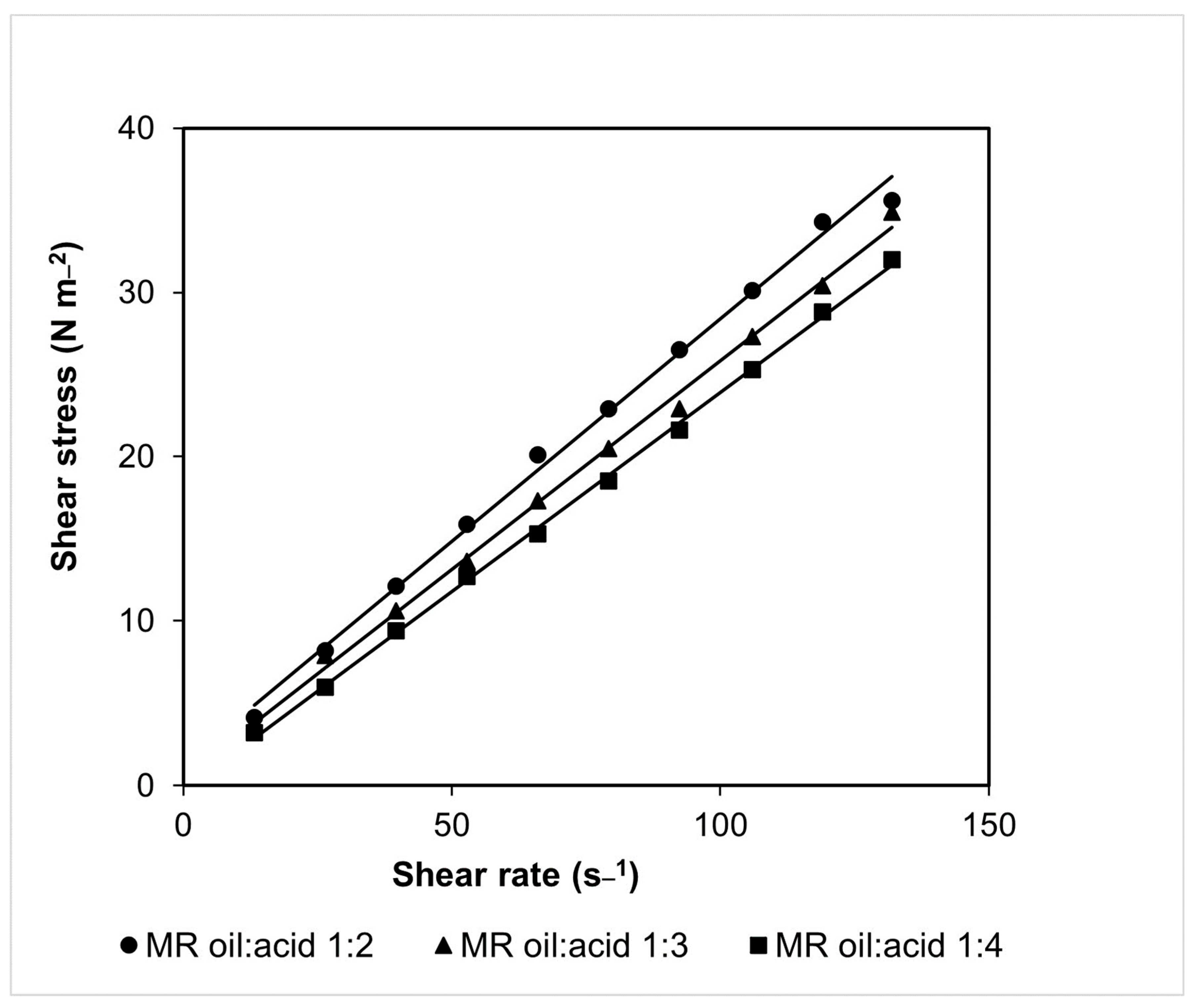
2.2. Density and Viscosity of Substrate Mixtures at Different Molar Ratios
2.3. Hydrodynamic Characterization of the FBR System
2.3.1. Minimum Fluidization Velocity
2.3.2. Hydrodynamic Characterization of FBR
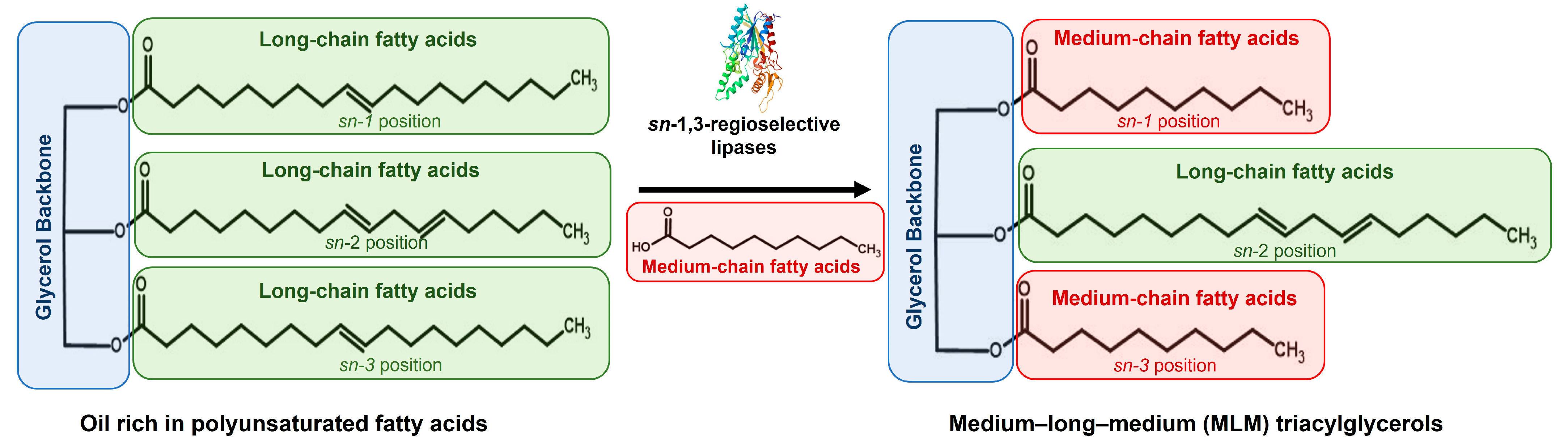
2.4. Acidolysis of Cottonseed Oil in FBR
2.4.1. Preliminary Studies of Residence Time
2.4.2. Evaluation of the Influence of Residence Time and Oil/Acid Molar Ratio on the Synthesis of Low-Calorie MLM Triacylglycerols
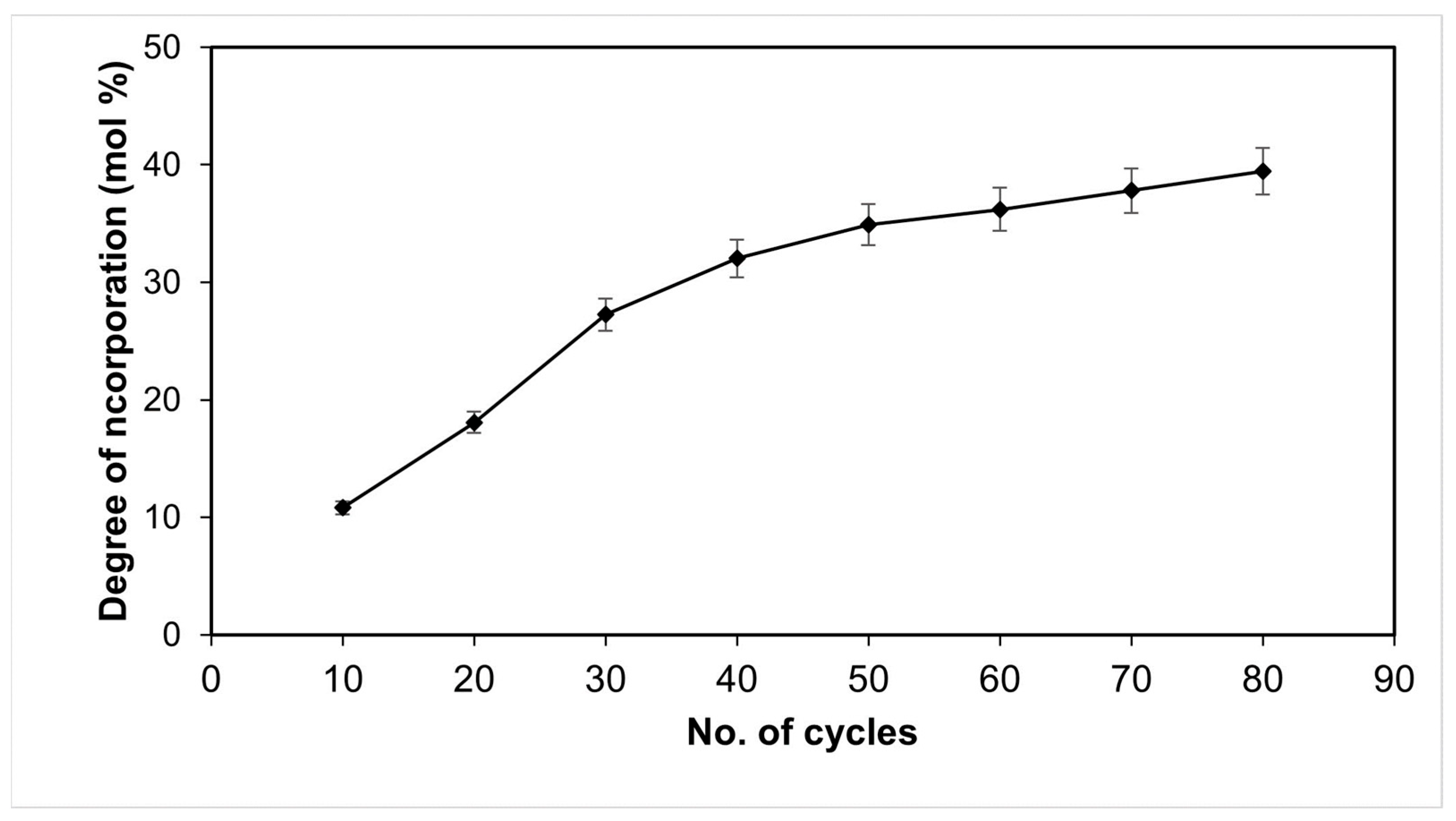
2.5. Operational Stability
2.6. sn-2 Position Analysis of Modified Triacylglycerols
3. Materials and Methods
3.1. Materials
3.2. Analytical Methods
3.2.1. Determination of Esterification Activity
3.2.2. Determination of Hydrolytic Activity
3.2.3. Characterization of Raw Materials
3.2.4. Determination of Fatty Acid Profile
3.2.5. Determination of the Degree of Incorporation (DI)
3.2.6. Analysis of sn-2 Fatty Acids
3.2.7. Statistical Analysis
3.3. Instrumental Methods
3.3.1. Physical Characterization of Reaction Mixtures at Different Molar Ratios
3.3.2. Hydrodynamic Characterization of the FBR
3.3.3. Minimum Fluidization Velocity as a Function of Biocatalyst Mass
3.3.4. Synthesis of MLM Triacylglycerols
3.3.5. Effect of Cycle Number and Substrate Molar Ratio on MLM Triacylglycerols Synthesis
3.3.6. Analysis of Operational Stability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Soni, S. Trends in Lipase Engineering for Enhanced Biocatalysis. Biotechnol. Appl. Biochem. 2022, 69, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Remonatto, D.; Miotti, R.H.; Monti, R.; Bassan, J.C.; de Paula, A.V. Applications of Immobilized Lipases in Enzymatic Reactors: A Review. Process Biochem. 2022, 114, 1–20. [Google Scholar] [CrossRef]
- Arana-Peña, S.; Carballares, D.; Berenguer-Murcia, Á.; Alcántara, A.R.; Rodrigues, R.C.; Fernandez-Lafuente, R. One Pot Use of Combilipases for Full Modification of Oils and Fats: Multifunctional and Heterogeneous Substrates. Catalysts 2020, 10, 605. [Google Scholar] [CrossRef]
- Bornscheuer, U.T. The Fourth Wave of Biocatalysis Is Approaching. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2018, 376, 20170063. [Google Scholar] [CrossRef]
- Mehta, A.; Guleria, S.; Sharma, R.; Gupta, R. The Lipases and Their Applications with Emphasis on Food Industry. In Microbial Biotechnology in Food and Health; Academic Press: Cambridge, MA, USA, 2021; pp. 143–164. ISBN 9780128198131. [Google Scholar]
- Akram, F.; Mir, A.S.; Haq, I.U.; Roohi, A. An Appraisal on Prominent Industrial and Biotechnological Applications of Bacterial Lipases. Mol. Biotechnol. 2022, 65, 521–543. [Google Scholar] [CrossRef]
- Remonatto, D.; Ribeiro Ferrari, B.; Bassan, J.C.; Mussagy, C.U.; de Carvalho Santos-Ebinuma, V.; Veloso de Paula, A. Utilization of Clay Materials as Support for Aspergillus Japonicus Lipase: An Eco-Friendly Approach. Catalysts 2021, 11, 1173. [Google Scholar] [CrossRef]
- Guo, Y.; Cai, Z.; Xie, Y.; Ma, A.; Zhang, H.; Rao, P.; Wang, Q. Synthesis, Physicochemical Properties, and Health Aspects of Structured Lipids: A Review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 759–800. [Google Scholar] [CrossRef]
- Bassan, N.; Rodrigues, R.H.; Monti, R.; Tecelão, C.; Ferreira-Dias, S.; Paula, A.V. Enzymatic Modification of Grapeseed (Vitis vinifera L.) Oil Aiming to Obtain Dietary Triacylglycerols in a Batch Reactor. LWT 2019, 99, 600–606. [Google Scholar] [CrossRef]
- Jadhav, H.B.; Annapure, U. Designer Lipids -Synthesis and Application—A Review. Trends Food Sci. Technol. 2021, 116, 884–902. [Google Scholar] [CrossRef]
- Shiki, P.S.; Pereira, G.N.; de Meneses, A.C.; de Oliveira, D.; Lerin, L.A. Novozym ® 435 and Lipozyme ® RM IM as Biocatalysts for Benzyl Benzoate Synthesis. Biointerface Res. Appl. Chem. 2022, 12, 8271–8284. [Google Scholar] [CrossRef]
- Basso, A.; Serban, S. Industrial Applications of Immobilized Enzymes—A Review. Mol. Catal. 2019, 479, 110607. [Google Scholar] [CrossRef]
- Monteiro, R.R.C.; Arana-Peña, S.; da Rocha, T.N.; Miranda, L.P.; Berenguer-Murcia, Á.; Tardioli, P.W.; dos Santos, J.C.S.; Fernandez-Lafuente, R. Liquid Lipase Preparations Designed for Industrial Production of Biodiesel. Is It Really an Optimal Solution? Renew. Energy 2021, 164, 1566–1587. [Google Scholar] [CrossRef]
- Nunes, P.A.; Pires-Cabral, P.; Ferreira-Dias, S. Production of Olive Oil Enriched with Medium Chain Fatty Acids Catalysed by Commercial Immobilised Lipases. Food Chem. 2011, 127, 993–998. [Google Scholar] [CrossRef]
- Simões, T.; Ferreira, J.; Lemos, M.F.L.; Augusto, A.; Félix, R.; Silva, S.F.J.; Ferreira-Dias, S.; Tecelão, C. Argan Oil as a Rich Source of Linoleic Fatty Acid for Dietetic Structured Lipids Production. Life 2021, 11, 1114. [Google Scholar] [CrossRef]
- De Cozentino, I.S.C.; de Rodrigues, M.F.; Mazziero, V.T.; Cerri, M.O.; Cavallini, D.C.U.; de Paula, A.V. Enzymatic Synthesis of Structured Lipids from Grape Seed (Vitis vinifera L.) Oil in Associated Packed Bed Reactors. Biotechnol. Appl. Biochem. 2022, 69, 101–109. [Google Scholar] [CrossRef]
- Abed, S.M.; Wei, W.; Ali, A.H.; Korma, S.A.; Mousa, A.H.; Hassan, H.M.; Jin, Q.; Wang, X. Synthesis of Structured Lipids Enriched with Medium-Chain Fatty Acids via Solvent-Free Acidolysis of Microbial Oil Catalyzed by Rhizomucor Miehei Lipase. LWT 2018, 93, 306–315. [Google Scholar] [CrossRef]
- Giovannini, P.P.; Catani, M.; Massi, A.; Sacchetti, G.; Tacchini, M.; de Oliveira, D.; Lerin, L.A. Continuous Production of Eugenol Esters Using Enzymatic Packed-Bed Microreactors and an Evaluation of the Products as Antifungal Agents. Flavour Fragr. J. 2019, 34, 201–210. [Google Scholar] [CrossRef]
- Akoh, C.C. Food Lipids Chemistry, Nutrition, and Biotechnology, 4th ed.; Akoh, C.C., Ed.; CRC Press: Boca Raton, FL, USA, 2017; ISBN 9781498744874. [Google Scholar]
- Morales-Medina, R.; Munio, M.; Guadix, A.; Guadix, E.M. Development of an Up-Grading Process to Produce MLM Structured Lipids from Sardine Discards. Food Chem. 2017, 228, 634–642. [Google Scholar] [CrossRef]
- Li, Y.; Li, C.; Feng, F.; Wei, W.; Zhang, H. Synthesis of Medium and Long-Chain Triacylglycerols by Enzymatic Acidolysis of Algal Oil and Lauric Acid. LWT 2021, 136, 110309. [Google Scholar] [CrossRef]
- Sharif, I.; Farooq, J.; Chohan, S.M.; Saleem, S.; Kainth, R.A.; Mahmood, A.; Sarwar, G. Strategies to Enhance Cottonseed Oil Contents and Reshape Fatty Acid Profile Employing Different Breeding and Genetic Engineering Approaches. J. Integr. Agric. 2019, 18, 2205–2218. [Google Scholar] [CrossRef]
- Riaz, T.; Iqbal, M.W.; Mahmood, S.; Yasmin, I.; Leghari, A.A.; Rehman, A.; Mushtaq, A.; Ali, K.; Azam, M.; Bilal, M. Cottonseed Oil: A Review of Extraction Techniques, Physicochemical, Functional, and Nutritional Properties. Crit. Rev. Food Sci. Nutr. 2021, 63, 1219–1237. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.N.; Mahesar, S.A.; Abro, K.A.; Sherazi, S.T.H.; Nizamani, S.M.; Laghari, Z.H.; Panhwar, T.; Shaikh, T.H.; Mugheri, G.A. FTIR Characterization and Physicochemical Evaluation of Cottonseed Oil. Pak. J. Anal. Environ. Chem. 2017, 18, 46–53. [Google Scholar] [CrossRef]
- Kouser, S.; Mahmood, K.; Anwar, F. Variations in physicochemical attributes of seed oil among different varieties of cotton (Gossypium hirsutum L.). Pak. J. Bot. 2015, 47, 723–729. [Google Scholar]
- Damnjanović, J.J.; Žuža, M.G.; Savanović, J.K.; Bezbradica, D.I.; Mijin, D.Ž.; Bošković-Vragolović, N.; Knežević-Jugović, Z.D. Covalently Immobilized Lipase Catalyzing High-Yielding Optimized Geranyl Butyrate Synthesis in a Batch and Fluidized Bed Reactor. J. Mol. Catal. B Enzym. 2012, 75, 50–59. [Google Scholar] [CrossRef]
- Utama, Q.D.; Sitanggang, A.B.; Adawiyah, D.R.; Hariyadi, P. Lipase-Catalyzed Interesterification for the Synthesis of Medium-Long-Medium (MLM) Structured Lipids—A Review. Food Technol. Biotechnol. 2019, 57, 305–318. [Google Scholar] [CrossRef]
- Remonatto, D.; Lerin, L.A. Biocatalysis and Bioactive Molecules: Future and Development. Int. J. Mol. Sci. 2023, 24, 5571. [Google Scholar] [CrossRef]
- Tofani, G.; Petri, A.; Piccolo, O. Preparation of Enantiomerically Pure N-Heterocyclic Amino Alcohols by Enzymatic Kinetic Resolution. Tetrahedron Asymmetry 2015, 26, 638–643. [Google Scholar] [CrossRef]
- Remonatto, D.; Oliveira, J.V.; Guisan, J.M.; Oliveira, D.; Ninow, J.; Fernandez-Lorente, G. Immobilization of Eversa Lipases on Hydrophobic Supports for Ethanolysis of Sunflower Oil Solvent-Free. Appl. Biochem. Biotechnol. 2022, 194, 2151–2167. [Google Scholar] [CrossRef]
- Remonatto, D.; de Oliveira, J.V.; Manuel Guisan, J.; de Oliveira, D.; Ninow, J.; Fernandez-Lorente, G. Production of FAME and FAEE via Alcoholysis of Sunflower Oil by Eversa Lipases Immobilized on Hydrophobic Supports. Appl. Biochem. Biotechnol. 2018, 185, 705–716. [Google Scholar] [CrossRef]
- Pacheco, B.J.S.; Domingues, O.; Reina, M.P.; de Neto, A.B.; Andrade, G.S.S.; de Paula, A.V. Improved Synthesis of Dietary Triglycerides by Using Lipase Supported on Clay Carriers. Biotechnol. J. 2022, 17, 2100491. [Google Scholar] [CrossRef]
- Paula, A.V.; Nunes, G.F.M.; de Castro, H.F.; Santos, J.C. Synthesis of Structured Lipids by Enzymatic Interesterification of Milkfat and Soybean Oil in a Basket-Type Stirred Tank Reactor. Ind. Eng. Chem. Res. 2015, 54, 1731–1737. [Google Scholar] [CrossRef]
- Korma, S.A.; Zou, X.; Ali, A.H.; Abed, S.M.; Jin, Q.; Wang, X. Preparation of Structured Lipids Enriched with Medium- and Long-Chain Triacylglycerols by Enzymatic Interesterification for Infant Formula. Food Bioprod. Process. 2018, 107, 121–130. [Google Scholar] [CrossRef]
- Hamam, F.; Budge, S.M. Structured and Specialty Lipids in Continuous Packed Column Reactors: Comparison of Production Using One and Two Enzyme Beds. J. Am. Oil Chem. Soc. 2010, 87, 385–394. [Google Scholar] [CrossRef]
- Paula, A.V.; Nunes, G.F.M.; Santos, J.C.; de Castro, H.F. Interesterification of Milkfat with Soybean Oil Catalysed by Rhizopus Oryzae Lipase Immobilised on SiO2-PVA on Packed Bed Reactor. Int. J. Food Sci. Technol. 2011, 46, 2124–2130. [Google Scholar] [CrossRef]
- de Paula, A.V.; Nunes, G.F.M.; de Castro, H.F.; dos Santos, J.C. Performance of Packed Bed Reactor on the Enzymatic Interesterification of Milk Fat with Soybean Oil to Yield Structure Lipids. Int. Dairy J. 2018, 86, 1–8. [Google Scholar] [CrossRef]
- Willis, W.M.; Marangoni Alejandro, G. Enzymatic Interesterification. In Food Lipids; Akoh, C.C., Min, D.B., Eds.; CRC Press: Boca Raton, FL, USA, 2020; pp. 821–854. [Google Scholar]
- Osório, N.M.; Dubreucq, E.; da Fonseca, M.M.R.; Ferreira-Dias, S. Operational Stability of Immobilised Lipase/Acyltransferase during Interesterification of Fat Blends. Eur. J. Lipid Sci. Technol. 2009, 111, 358–367. [Google Scholar] [CrossRef]
- Hidayat, C.; Fitria, K.; Supriyanto; Hastuti, P. Enzymatic Synthesis of Bio-Surfactant Fructose Oleic Ester Using Immobilized Lipase on Modified Hydrophobic Matrix in Fluidized Bed Reactor. Agric. Agric. Sci. Procedia 2016, 9, 353–362. [Google Scholar] [CrossRef]
- Balcão, V.M.; Paiva, A.L.; Malcata, F.X. Bioreactors with Immobilized Lipases: State of the Art. Enzyme Microb. Technol. 1996, 18, 392–416. [Google Scholar] [CrossRef]
- Wassef, E.A.; Shalaby, S.H.; Saleh, N.E. Cottonseed Oil as a Complementary Lipid Source in Diets for Gilthead Seabream Sparus Aurata Juveniles. Aquac. Res. 2015, 46, 2469–2480. [Google Scholar] [CrossRef]
- Wu, P.; Xu, X.; Li, J.; Zhang, J.; Chang, S.; Yang, X.; Guo, X. Seed-Specific Overexpression of Cotton GhDGAT1 Gene Leads to Increased Oil Accumulation in Cottonseed. Crop J. 2021, 9, 487–490. [Google Scholar] [CrossRef]
- FAO Section2. Codex Standards for Fats and Oils from Vegetable Sources. Available online: https://www.fao.org/3/y2774e/y2774e04.htm (accessed on 17 March 2022).
- Zia, M.A.; Shah, S.H.; Shoukat, S.; Hussain, Z.; Khan, S.U.; Shafqat, N. Physicochemical Features, Functional Characteristics, and Health Benefits of Cottonseed Oil: A Review. Braz. J. Biol. 2022, 82, e243511. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Hong, B.; Jiang, S.; Luo, X.; Lin, H.; Zhou, Y.; Wu, J.; Yue, X.; Shi, H.; Wu, R. Recent Advances on Essential Fatty Acid Biosynthesis and Production: Clarifying the Roles of Δ12/Δ15 Fatty Acid Desaturase. Biochem. Eng. J. 2022, 178, 108306. [Google Scholar] [CrossRef]
- Baker, E.J.; Miles, E.A.; Burdge, G.C.; Yaqoob, P.; Calder, P.C. Metabolism and Functional Effects of Plant-Derived Omega-3 Fatty Acids in Humans. Prog. Lipid Res. 2016, 64, 30–56. [Google Scholar] [CrossRef] [PubMed]
- Ramsden, C.E.; Zamora, D.; Faurot, K.R.; Macintosh, B.; Horowitz, M.; Keyes, G.S.; Yuan, Z.X.; Miller, V.; Lynch, C.; Honvoh, G.; et al. Dietary Alteration of N-3 and n-6 Fatty Acids for Headache Reduction in Adults with Migraine: Randomized Controlled Trial. BMJ 2021, 374, n1448. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Chen, H.; Stanton, C.; Ross, R.P.; Zhang, H.; Chen, Y.Q.; Chen, W. Review of the Roles of Conjugated Linoleic Acid in Health and Disease. J. Funct. Foods 2015, 15, 314–325. [Google Scholar] [CrossRef]
- Viladomiu, M.; Hontecillas, R.; Bassaganya-Riera, J. Modulation of Inflammation and Immunity by Dietary Conjugated Linoleic Acid. Eur. J. Pharmacol. 2016, 785, 87–95. [Google Scholar] [CrossRef]
- Kammili, A.; Yadav, P. Enhancing Oleic Acid and Oil Content in Low Oil and Oleic Type Indian Safflower (Carthamus tinctorius L.). Ind. Crops Prod. 2022, 175, 114254. [Google Scholar] [CrossRef]
- Igarashi, M.; Iwasa, K.; Yoshikawa, K. Feeding Regulation by Oleoylethanolamide Synthesized from Dietary Oleic Acid. Prostaglandins Leukot Essent Fat. Acids 2021, 165, 102228. [Google Scholar] [CrossRef]
- Martínez-Galán, J.P.; Ontibón-Echeverri, C.M.; Campos Costa, M.; Batista-Duharte, A.; Guerso Batista, V.; Mesa, V.; Monti, R.; Veloso de Paula, A.; Martins Baviera, A. Enzymatic Synthesis of Capric Acid-Rich Structured Lipids and Their Effects on Mice with High-Fat Diet-Induced Obesity. Food Res. Int. 2021, 148, 110602. [Google Scholar] [CrossRef]
- Cao, Y.; Qi, S.; Zhang, Y.; Wang, X.; Yang, B.; Wang, Y. Synthesis of Structured Lipids by Lipase-Catalyzed Interesterification of Triacetin with Camellia Oil Methyl Esters and Preliminary Evaluation of Their Plasma Lipid-Lowering Effect in Mice. Molecules 2013, 18, 3733–3744. [Google Scholar] [CrossRef]
- Brasil Instrução Normativa—In N° 87, de 15 de Março de 2021—Instrução Normativa—In N° 87, DE 15 DE MARÇO DE 2021—Dou—Imprensa Nacional. Available online: https://www.in.gov.br/en/web/dou/-/instrucao-normativa-in-n-87-de-15-de-marco-de-2021-309008143 (accessed on 16 March 2022).
- Kunii, D.; Levenspiel, O. Fluidization Engineering, 2nd ed.; Butterworth-Heinemann: Oxford, UK, 1991; ISBN 9780080506647. [Google Scholar]
- Fogler, H.S. Elementos de Engenharia Das Reações Químicas, 4th ed.; LCT: Shanghai, China, 2009; Volume 1, ISBN 978-8521617167. [Google Scholar]
- De Óleos, M.; Gorduras, E.; Biotransformação, P.; De Castro, H.F.; Mendes, A.A.; Dos Santos, J.C.; De Aguiar, C.L. Modificação de Óleos e Gorduras Por Biotransformação. Quim Nova 2004, 27, 146–156. [Google Scholar] [CrossRef]
- Gòdia, F.; Solà, C. Fluidized-Bed Bioreactors. Biotechnol. Prog. 1995, 11, 479–497. [Google Scholar] [CrossRef]
- Choi, J.H.; Kim, B.H.; Hong, S.I.; Kim, Y.; Kim, I.H. Synthesis of Structured Lipids Containing Pinolenic Acid at the Sn-2 Position via Lipase-Catalyzed Acidolysis. JAOCS J. Am. Oil Chem. Soc. 2012, 89, 1449–1454. [Google Scholar] [CrossRef]
- Remonatto, D.; Fantatto, R.R.; Pietro, R.C.L.R.; Monti, R.; Oliveira, J.V.; de Paula, A.V.; Bassan, J.C. Enzymatic Synthesis of Geranyl Acetate in Batch and Fed-Batch Reactors and Evaluation of Its Larvicidal Activity against Rhipicephalus (Boophilus). Microplus. Process Biochem. 2022, 120, 287–300. [Google Scholar] [CrossRef]
- De Meneses, A.C.; Balen, M.; de Andrade Jasper, E.; Korte, I.; de Araújo, P.H.H.; Sayer, C.; de Oliveira, D. Enzymatic Synthesis of Benzyl Benzoate Using Different Acyl Donors: Comparison of Solvent-Free Reaction Techniques. Process Biochem. 2020, 92, 261–268. [Google Scholar] [CrossRef]
- Kuo, S.J.; Parkin, K.L. Substrate Preferences for Lipase-Mediated Acyl-Exchange Reactions with Butteroil Are Concentration-Dependent. J. Am. Oil Chem. Soc. 1993, 70, 393–399. [Google Scholar] [CrossRef]
- Sousa, R.R.; Silva, A.S.A.; Fernandez-Lafuente, R.; Ferreira-Leitão, V.S. Solvent-Free Esterifications Mediated by Immobilized Lipases: A Review from Thermodynamic and Kinetic Perspectives. Catal. Sci. Technol. 2021, 11, 5696–5711. [Google Scholar] [CrossRef]
- Wang, L.; Chen, G.; Tang, J.; Ming, M.; Jia, C.; Feng, B. Continuous Biosynthesis of Geranyl Butyrate in a Circulating Fluidized Bed Reactor. Food Biosci. 2019, 27, 60–65. [Google Scholar] [CrossRef]
- Hajar, M.; Vahabzadeh, F. Biolubricant Production from Castor Oil in a Magnetically Stabilized Fluidized Bed Reactor Using Lipase Immobilized on Fe3O4 Nanoparticles. Ind. Crops Prod. 2016, 94, 544–556. [Google Scholar] [CrossRef]
- Venturi, V.; Presini, F.; Trapella, C.; Bortolini, O.; Giovannini, P.P.; Lerin, L.A. Microwave-Assisted Enzymatic Synthesis of Geraniol Esters in Solvent-Free Systems: Optimization of the Reaction Parameters, Purification and Characterization of the Products, and Biocatalyst Reuse. Mol. Divers. 2023. Online first. [Google Scholar] [CrossRef]
- Paula, A.V.; Nunes, G.F.M.; Osório, N.M.; Santos, J.C.; de Castro, H.F.; Ferreira-Dias, S. Continuous Enzymatic Interesterification of Milkfat with Soybean Oil Produces a Highly Spreadable Product Rich in Polyunsaturated Fatty Acids. Eur. J. Lipid Sci. Technol. 2015, 117, 608–619. [Google Scholar] [CrossRef]
- Poppe, J.K.; Fernandez-Lafuente, R.; Rodrigues, R.C.; Ayub, M.A.Z. Enzymatic Reactors for Biodiesel Synthesis: Present Status and Future Prospects. Biotechnol. Adv. 2015, 33, 511–525. [Google Scholar] [CrossRef] [PubMed]
- Ricca, E.; de Paola, M.G.; Calabrò, V.; Curcio, S.; Iorio, G. Olive Husk Oil Transesterification in a Fluidized Bed Reactor with Immobilized Lipases. Asia Pac. J. Chem. Eng. 2009, 4, 365–368. [Google Scholar] [CrossRef]
- de Sousa, I.G.; Mota, G.F.; Cavalcante, A.L.G.; Rocha, T.G.; da Silva Sousa, P.; Holanda Alexandre, J.Y.N.; da Silva Souza, J.E.; Neto, F.S.; Cavalcante, F.T.T.; Lopes, A.A.S.; et al. Renewable Processes of Synthesis of Biolubricants Catalyzed by Lipases. J. Environ. Chem. Eng. 2023, 11, 109006. [Google Scholar] [CrossRef]
- Ferreira-Dias, S.; Osório, N.M.; Tecelão, C. Lipase-Catalyzed Synthesis of Structured Lipids at Laboratory Scale. Methods Mol. Biol. 2018, 1835, 315–336. [Google Scholar] [CrossRef]
- Tecelão, C.; Silva, J.; Dubreucq, E.; Ribeiro, M.H.; Ferreira-Dias, S. Production of Human Milk Fat Substitutes Enriched in Omega-3 Polyunsaturated Fatty Acids Using Immobilized Commercial Lipases and Candida Parapsilosis Lipase/Acyltransferase. J. Mol. Catal. B Enzym. 2010, 65, 122–127. [Google Scholar] [CrossRef]
- Yang, T.; Fruekilde, M.B.; Xu, X. Suppression of Acyl Migration in Enzymatic Production of Structured Lipids through Temperature Programming. Food Chem. 2005, 92, 101–107. [Google Scholar] [CrossRef]
- Peng, B.; Chen, F.; Liu, X.; Hu, J.N.; Zheng, L.F.; Li, J.; Deng, Z.Y. Trace Water Activity Could Improve the Formation of 1,3-Oleic-2-Medium Chain-Rich Triacylglycerols by Promoting Acyl Migration in the Lipase RM IM Catalyzed Interesterification. Food Chem. 2020, 313, 126130. [Google Scholar] [CrossRef]
- Pinto, M.C.C.; Freire, D.M.G.; Pinto, J.C. Influence of the Morphology of Core-Shell Supports on the Immobilization of Lipase B from Candida Antarctica. Molecules 2014, 19, 12509–12530. [Google Scholar] [CrossRef]
- AOCS. Official Methods and Recommended Practices of the AOCS, 5th ed.; AOCS: Urbana, IL, USA, 2004. [Google Scholar]
- Wang, Y.; Xia, L.; Xu, X.; Xie, L.; Duan, Z. Lipase-Catalyzed Acidolysis of Canola Oil with Caprylic Acid to Produce Medium-, Long- and Medium-Chain-Type Structured Lipids. Food Bioprod. Process. 2012, 90, 707–712. [Google Scholar] [CrossRef]
- ISO 12966-2:2017; Animal and Vegetable Fats and Oils—Gas Chromatography of Fatty Acid Methyl Esters—Part 2: Preparation of Methyl Esters of Fatty Acids. International Organization for Standardization (ISO). Available online: https://www.iso.org/standard/72142.html (accessed on 10 July 2023).
- Casas-Godoy, L.; Marty, A.; Sandoval, G.; Ferreira-Dias, S. Optimization of Medium Chain Length Fatty Acid Incorporation into Olive Oil Catalyzed by Immobilized Lip2 from Yarrowia Lipolytica. Biochem. Eng. J. 2013, 77, 20–27. [Google Scholar] [CrossRef]
- Silva, W.C.E.; Teixeira, L.F.; Carvalho, A.K.F.; Mendes, A.A.; de Castro, H.F. Influence of Feedstock Source on the Biocatalyst Stability and Reactor Performance in Continuous Biodiesel Production. J. Ind. Eng. Chem. 2014, 20, 881–886. [Google Scholar] [CrossRef]


| Fatty Acid | Concentration (%, w/w) |
|---|---|
| Palmitic acid (C16:0) | 11.27 |
| Stearic acid (C18:0) | 4.83 |
| Oleic acid (C18:1n9) | 26.94 |
| Linoleic acid (C18:2n6) | 50.24 |
| Linolenic acid (C18:3n3) | 4.28 |
| Gamma-linolenic acid (C18:3n6) | 0.22 |
| Unidentified fatty acids | 2.22 |
| Cottonseed Oil/Capric Acid Molar Ratio | Density (g mL−1) |
|---|---|
| 1:2 | 0.9215 a ± 0.0416 |
| 1:3 | 0.9108 a ± 0.0252 |
| 1:4 | 0.9079 a ± 0.0920 |
| Run | Oil/Acid Molar Ratio 1 | Cycle Number 1 | Reaction Time (h) | Experimental Degree of Incorporation 2 (mol%) | Predicted Degree of Incorporation 3 (mol%) | Relative Error 4 (%) |
|---|---|---|---|---|---|---|
| 1 | −1 (1:2) | −1 (20) | 4.34 | 19 | 18.65491 | 1.842105 |
| 2 | −1 (1:2) | 1 (70) | 15.17 | 25.74 | 27.98653 | 8.74126 |
| 3 | 1 (1:4) | −1 (20) | 4.3 | 35.7 | 31.22069 | 12.57703 |
| 4 | 1 (1:4) | 1 (70) | 15.17 | 40.88 | 40.55231 | 0.807241 |
| 5 | 0 (1:3) | −1.41 (10) | 2.17 | 22.84 | 23.02482 | 0.76751 |
| 6 | 0 (1:3) | 1.41 (80) | 17.34 | 40.78 | 36.1824 | 11.26851 |
| 7 | −1.41 (1:1.59) | 0 (45) | 9.75 | 20.83 | 20.74474 | 0.407105 |
| 8 | 1.41 (1:4.41) | 0 (45) | 9.75 | 33.79 | 38.46248 | 13.8053 |
| 9 | 0 (1:3) | 0 (45) | 9.75 | 29.67 | 29.60361 | 0.235929 |
| 10 | 0 (1:3) | 0 (45) | 9.75 | 30.61 | 29.60361 | 3.299575 |
| 11 | 0 (1:3) | 0 (45) | 9.75 | 28.54 | 29.60361 | 3.71409 |
| Sample | Fatty Acid | Concentration (%, w/w) |
|---|---|---|
| Cottonseed oil | Palmitic acid (C16:0) | 2.87 |
| Stearic acid (C18:0) | 0.79 | |
| Oleic acid (C18:1n9) | 32.24 | |
| Linoleic acid (C18:2n6) | 60.78 | |
| Linolenic acid (C18:3n3) | 3.32 | |
| Modified triglyceride oil | Capric acid (C10:0) | 1.80 |
| Palmitic acid (C16:0) | 1.06 | |
| Stearic acid (C18:0) | 0.48 | |
| Oleic acid (C18:1n9) | 26.28 | |
| Linoleic acid (C18:2n6) | 65.68 | |
| Linolenic acid (C18:3n3) | 4.7 |
| Independent Variable | Level | ||||
|---|---|---|---|---|---|
| −1.41 | −1 | 0 | +1 | +1.41 | |
| Cottonseed oil/capric acid molar ratio | 1.59 | 2 | 3 | 4 | 4.41 |
| Cycle number | 10 | 20 | 45 | 70 | 80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Remonatto, D.; Santaella, N.; Lerin, L.A.; Bassan, J.C.; Cerri, M.O.; de Paula, A.V. Solvent-Free Enzymatic Synthesis of Dietary Triacylglycerols from Cottonseed Oil in a Fluidized Bed Reactor. Molecules 2023, 28, 5384. https://doi.org/10.3390/molecules28145384
Remonatto D, Santaella N, Lerin LA, Bassan JC, Cerri MO, de Paula AV. Solvent-Free Enzymatic Synthesis of Dietary Triacylglycerols from Cottonseed Oil in a Fluidized Bed Reactor. Molecules. 2023; 28(14):5384. https://doi.org/10.3390/molecules28145384
Chicago/Turabian StyleRemonatto, Daniela, Núbia Santaella, Lindomar Alberto Lerin, Juliana Cristina Bassan, Marcel Otávio Cerri, and Ariela Veloso de Paula. 2023. "Solvent-Free Enzymatic Synthesis of Dietary Triacylglycerols from Cottonseed Oil in a Fluidized Bed Reactor" Molecules 28, no. 14: 5384. https://doi.org/10.3390/molecules28145384
APA StyleRemonatto, D., Santaella, N., Lerin, L. A., Bassan, J. C., Cerri, M. O., & de Paula, A. V. (2023). Solvent-Free Enzymatic Synthesis of Dietary Triacylglycerols from Cottonseed Oil in a Fluidized Bed Reactor. Molecules, 28(14), 5384. https://doi.org/10.3390/molecules28145384







