Enzymatic Characterization of a Novel HSL Family IV Esterase EstD04 from Pseudomonas sp. D01 in Mealworm Gut Microbiota
Abstract
:1. Introduction
2. Results
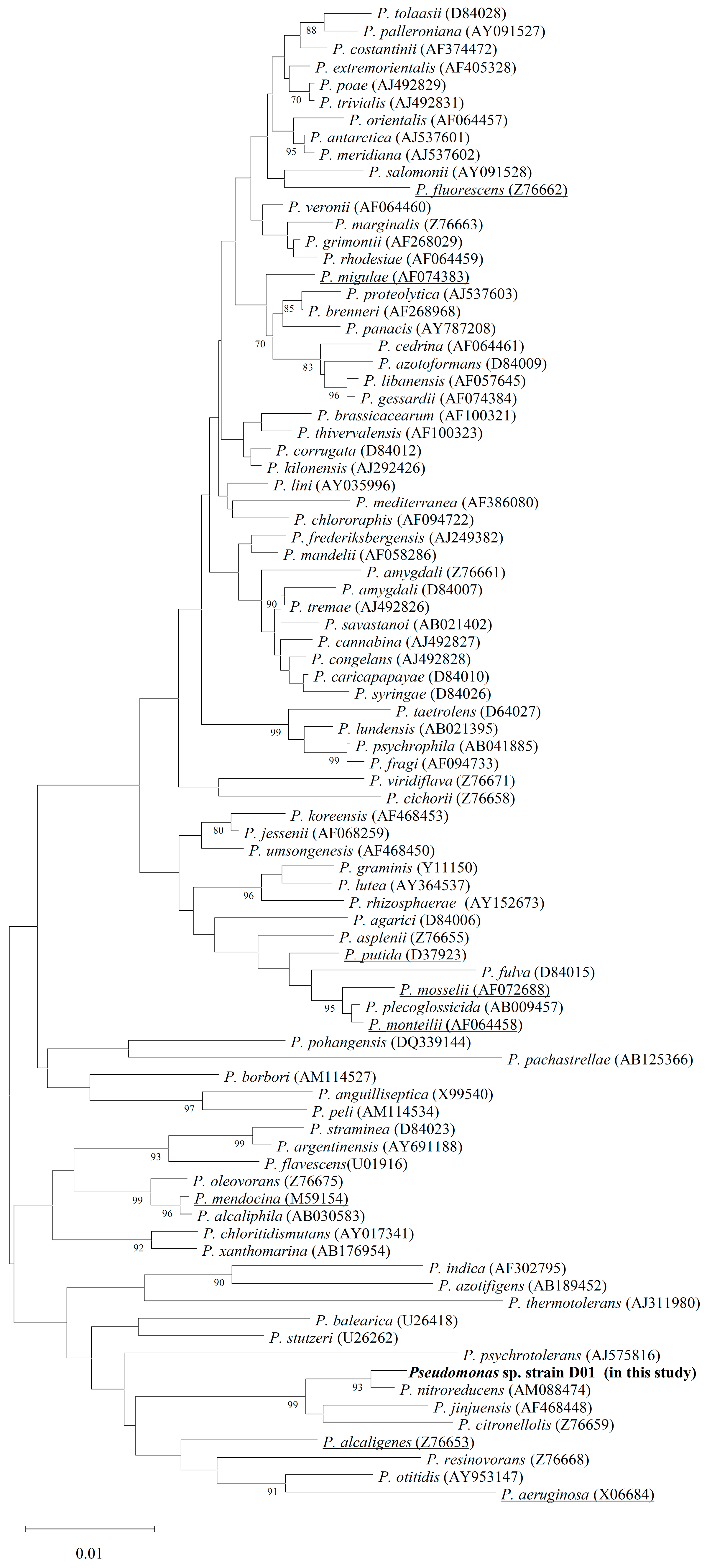
2.1. Screening and Identification of Bacterial Strain D01 on Tributyrin-Agar-Plate
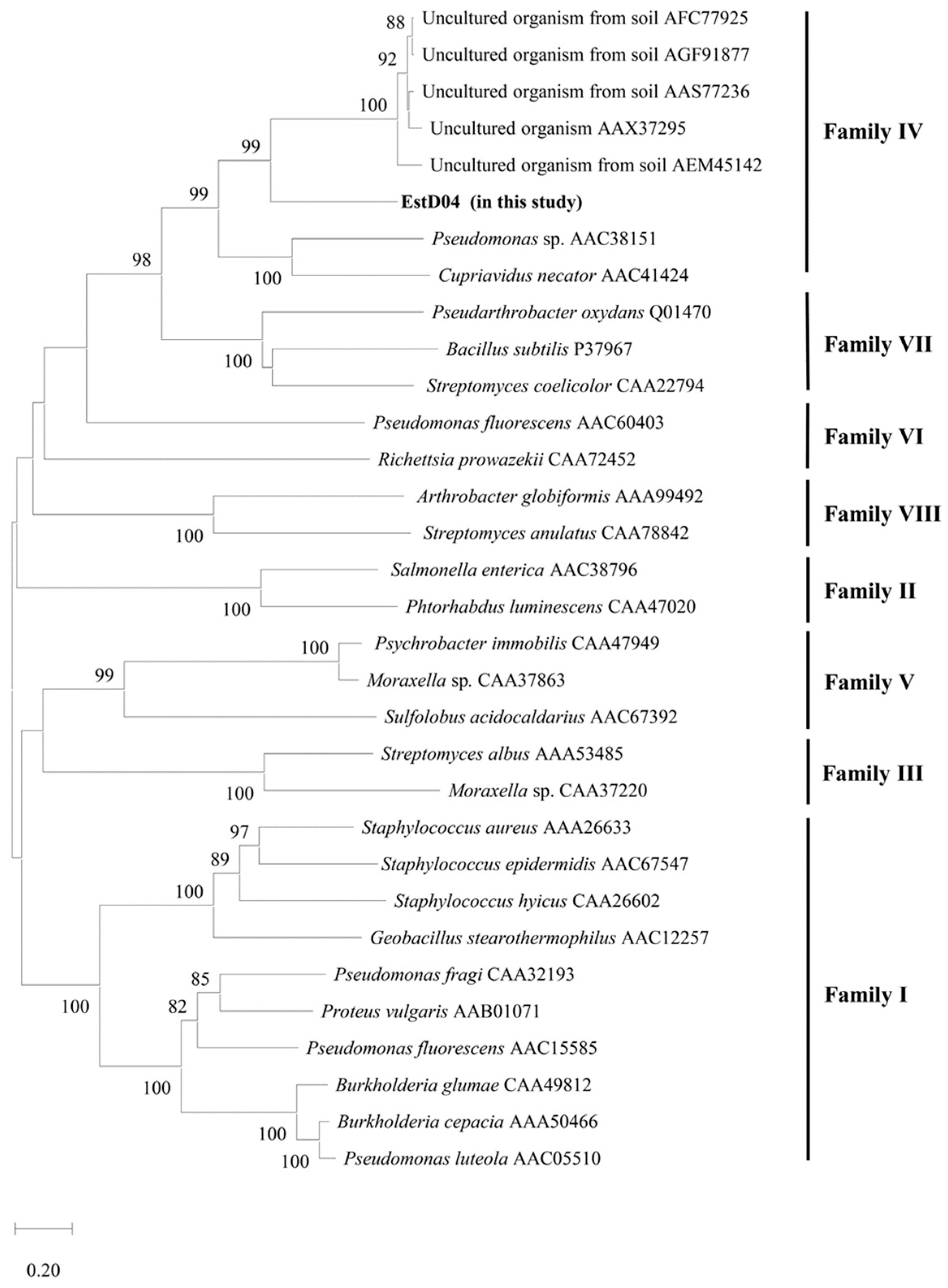
2.2. Identification of estD04+ Gene from D01 Strain and Classification of EstD04 as an Esterase of bHSL Family IV
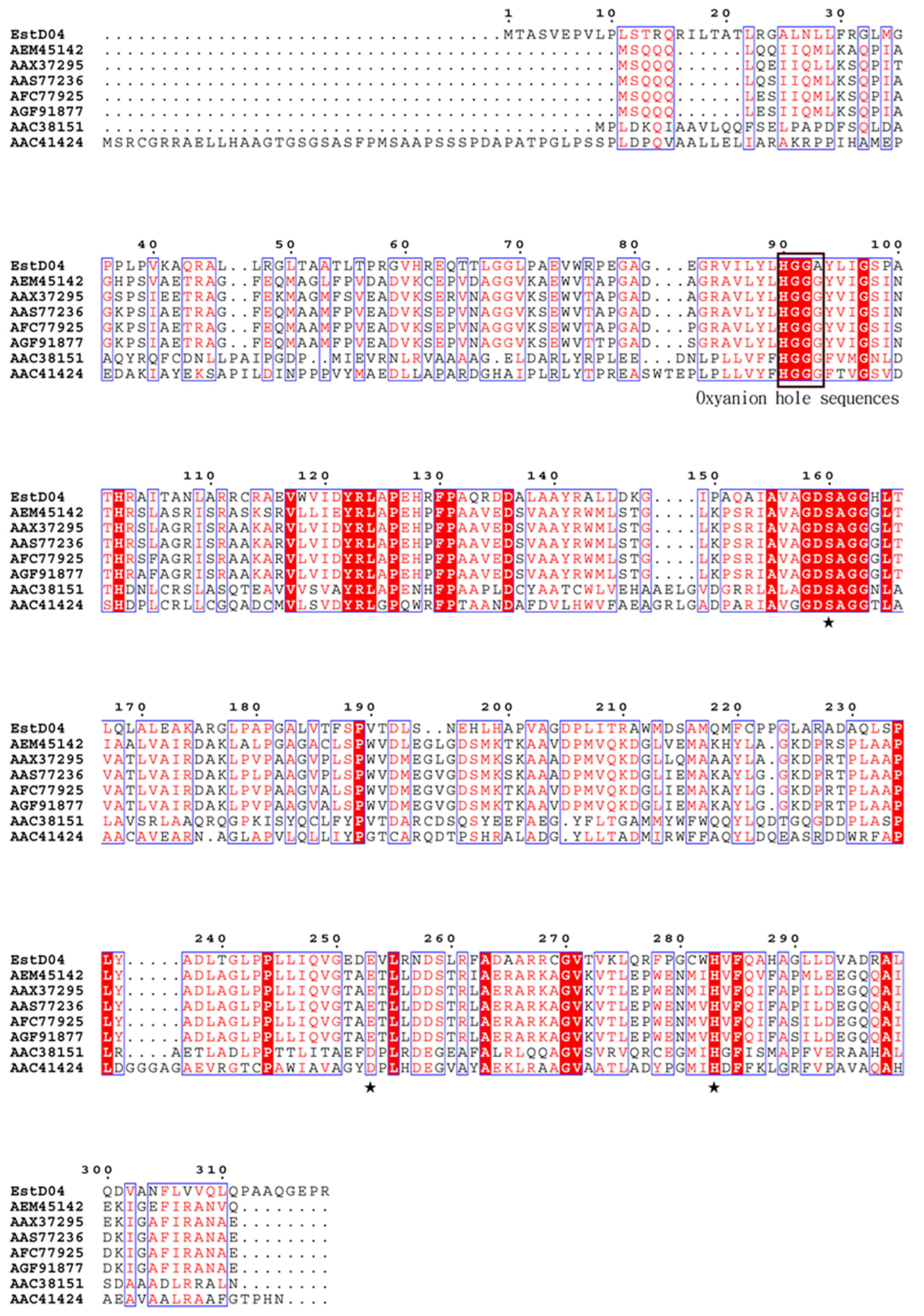
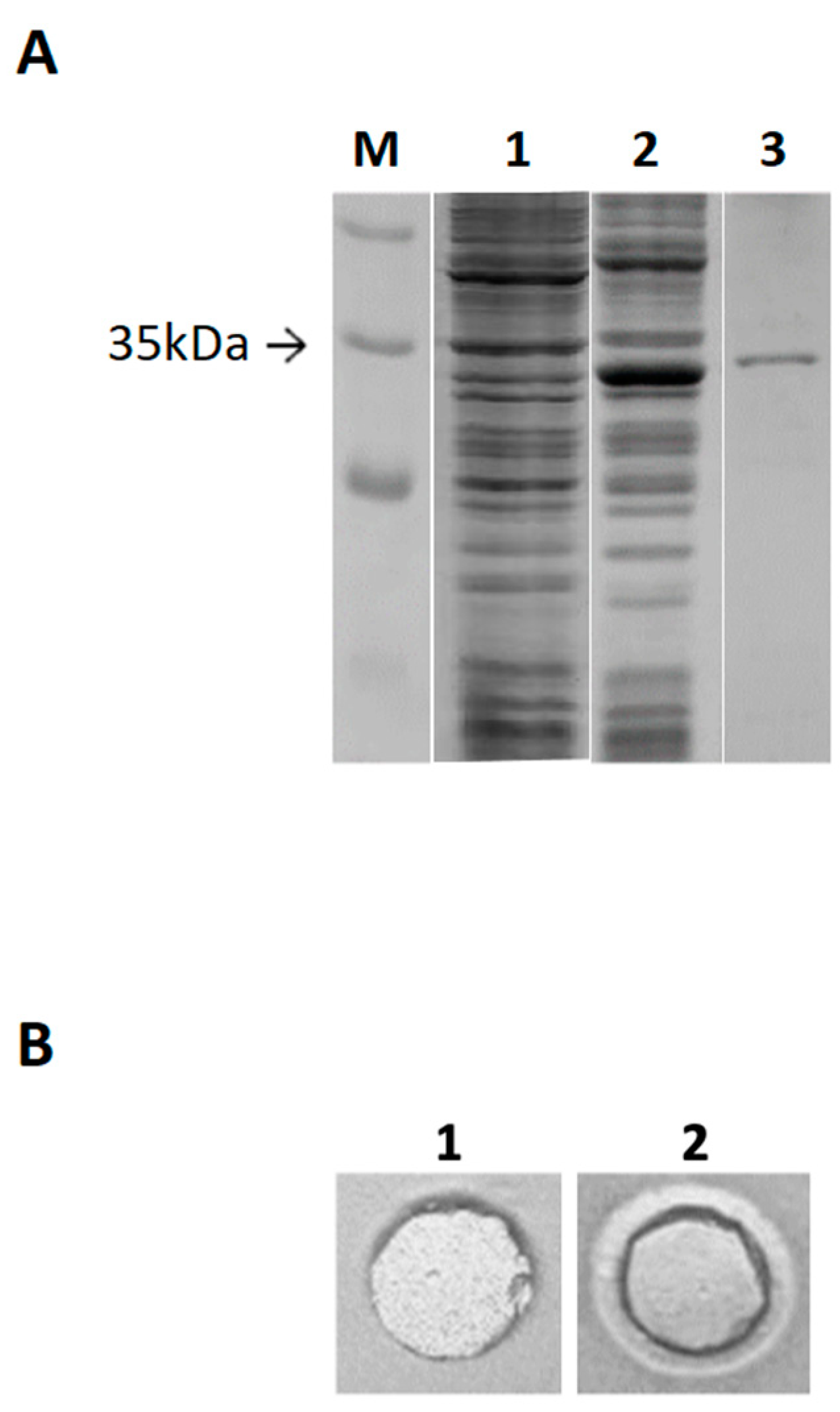
2.3. Cloning of estD04+ Gene and Purification of EstD04-His(6x) Protein
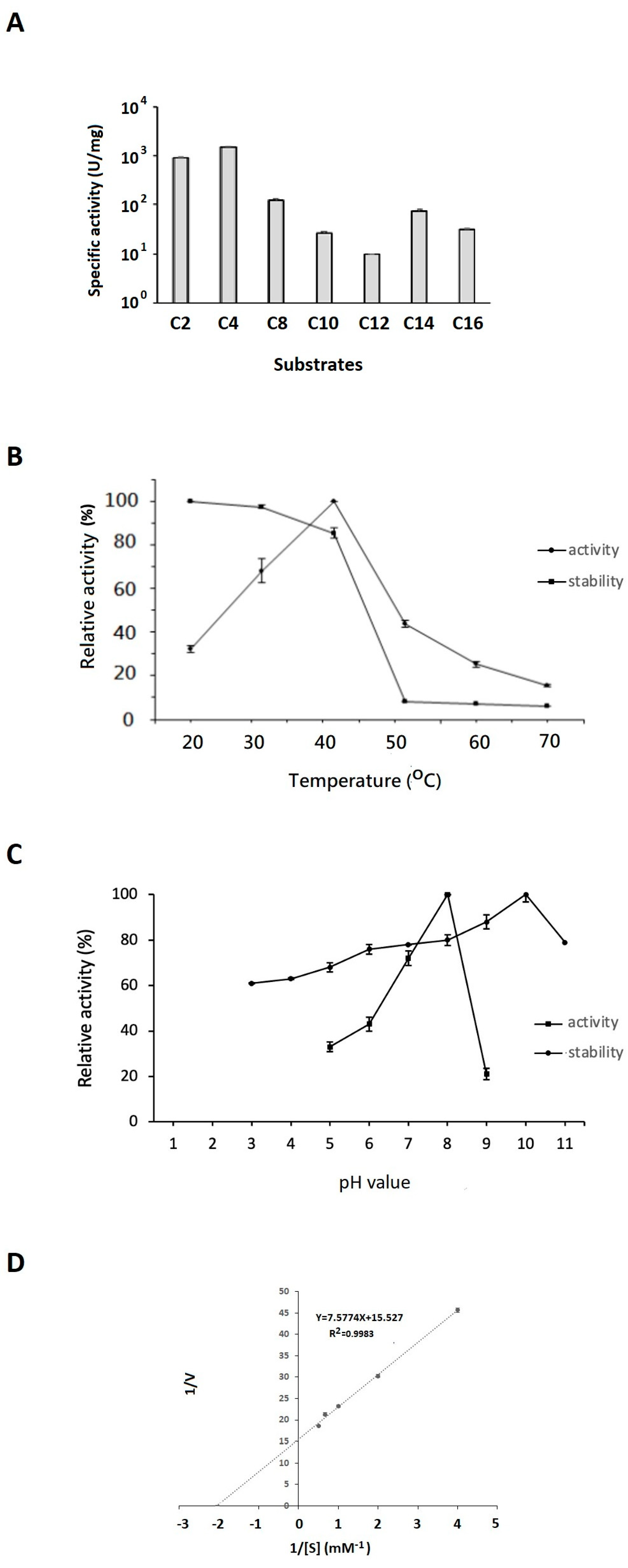
2.4. Effects of Substrate Chain Length, Temperature, and pH Value on EstD04 Activity and Stability as Well as the Enzyme’s Kinetic Analyses
2.5. Effects of Cations, Organic Solvents, and Detergents on EstD04 Activity
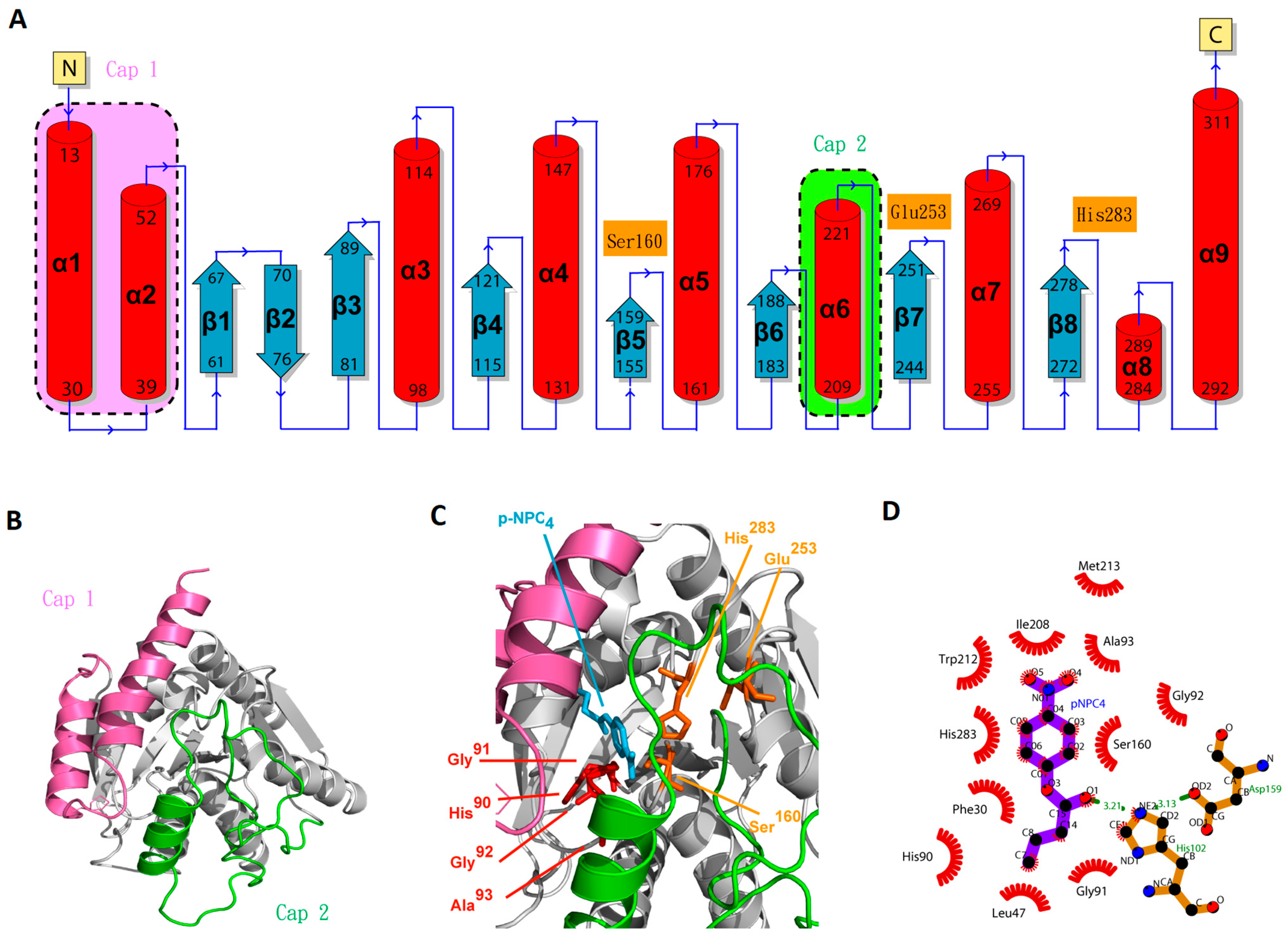
2.6. Molecular Modeling of EstD04 and Ligplot+ Analyses
3. Discussion
4. Materials and Methods
4.1. Reagents, Enzymes, Plasmids, Strains, and Culture Medium
4.2. Isolation and Identification of a Lipolytic Enzyme-Producing Microorganism
4.3. Extraction of Pseudomonas sp. D01 Chromosomal DNA
4.4. Prediction of estD04+ Gene and Phylogenetic Analyses of Its Encoding Protein
4.5. Cloning, Overexpression, and Purification of the Recombinant EstD04
4.6. Substrate Specificity Assays
4.7. Effects of Temperature and pH on EstD04 Enzyme Activity
4.8. Kinetic Analyses
4.9. Effects of Cations, Organic Solvents, or Surfactants on EstD04 Activity
4.10. Modeling and Protein-Substrate Interaction Analyses
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Ali, Y.B.; Verger, R.; Abousalham, A. Lipases or esterases: Does it really matter? Toward a new bio-physico-chemical classification. Methods Mol. Biol. 2012, 861, 31–51. [Google Scholar] [PubMed]
- Gupta, R.; Gupta, N.; Rathi, P. Bacterial lipases: An overview of production, purification and biochemical properties. Appl. Microbiol. Biotechnol. 2004, 64, 763–781. [Google Scholar] [CrossRef]
- Holmquist, M. Alpha/Beta-hydrolase fold enzymes: Structures, functions and mechanisms. Curr. Protein Pept. Sci. 2000, 1, 209–235. [Google Scholar] [CrossRef] [PubMed]
- Johan, U.U.M.; Rahman, R.; Kamarudin, N.H.A.; Ali, M.S.M. An integrated overview of bacterial carboxylesterase: Structure, function and biocatalytic applications. Colloids Surf. B Biointerfaces 2021, 205, 111882. [Google Scholar] [CrossRef] [PubMed]
- Sarmah, N.; Revathi, D.; Sheelu, G.; Yamuna Rani, K.; Sridhar, S.; Mehtab, V.; Sumana, C. Recent advances on sources and industrial applications of lipases. Biotechnol. Prog. 2018, 34, 5–28. [Google Scholar] [CrossRef]
- Kapoor, M.; Gupta, M.N. Lipase promiscuity and its biochemical applications. Process Biochem. 2012, 47, 555–569. [Google Scholar] [CrossRef]
- Hasan, F.; Shah, A.A.; Hameed, A. Methods for detection and characterization of lipases: A comprehensive review. Biotechnol. Adv. 2009, 27, 782–798. [Google Scholar] [CrossRef]
- Javed, S.; Azeem, F.; Hussain, S.; Rasul, I.; Siddique, M.H.; Riaz, M.; Afzal, M.; Kouser, A.; Nadeem, H. Bacterial lipases: A review on purification and characterization. Prog. Biophys. Mol. Biol. 2018, 132, 23–34. [Google Scholar] [CrossRef]
- Dumorne, K.; Cordova, D.C.; Astorga-Elo, M.; Renganathan, P. Extremozymes: A potential source for industrial applications. J. Microbiol. Biotechnol. 2017, 27, 649–659. [Google Scholar] [CrossRef]
- Chandra, P.; Enespa; Singh, R.; Arora, P.K. Microbial lipases and their industrial applications: A comprehensive review. Microb. Cell Fact. 2020, 19, 169. [Google Scholar] [CrossRef]
- Kim, T.D. Bacterial hormone-sensitive lipases (bHSLs): Emerging enzymes for biotechnological applications. J. Microbiol. Biotechnol. 2017, 27, 1907–1915. [Google Scholar] [CrossRef] [Green Version]
- Jia, M.L.; Zhong, X.L.; Lin, Z.W.; Dong, B.X.; Li, G. Expression and characterization of an esterase belonging to a new family via isolation from a metagenomic library of paper mill sludge. Int. J. Biol. Macromol. 2019, 126, 1192–1200. [Google Scholar] [CrossRef]
- Kryukova, M.V.; Petrovskaya, L.E.; Kryukova, E.A.; Lomakina, G.Y.; Yakimov, S.A.; Maksimov, E.G.; Boyko, K.M.; Popov, V.O.; Dolgikh, D.A.; Kirpichnikov, M.P. Thermal Inactivation of a cold-active esterase PMGL3 isolated from the Permafrost metagenomic library. Biomolecules 2019, 9, 880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tutuncu, H.E.; Balci, N.; Tuter, M.; Karaguler, N.G. Recombinant production and characterization of a novel esterase from a hypersaline lake, Acigol, by metagenomic approach. Extremophiles 2019, 23, 507–520. [Google Scholar] [CrossRef] [PubMed]
- Miguel-Ruano, V.; Rivera, I.; Rajkovic, J.; Knapik, K.; Torrado, A.; Otero, J.M.; Beneventi, E.; Becerra, M.; Sanchez-Costa, M.; Hidalgo, A.; et al. Biochemical and structural characterization of a novel thermophilic esterase EstD11 provide catalytic insights for the HSL family. Comput. Struct. Biotechnol. J. 2021, 19, 1214–1232. [Google Scholar] [CrossRef]
- Park, J.E.; Jeong, G.S.; Lee, H.W.; Kim, H. Molecular haracterization of ovel family IV and VIII esterases from a compost metagenomic library. Microorganisms 2021, 9, 1614. [Google Scholar] [CrossRef] [PubMed]
- Park, J.E.; Jeong, G.S.; Lee, H.W.; Kim, S.K.; Kim, J.; Kim, H. Characterization of a novel family IV esterase containing a predicted CzcO domain and a family V esterase with broad substrate specificity from an oil-polluted mud flat metagenomic library. Appl. Sci. 2021, 11, 5905. [Google Scholar] [CrossRef]
- Distaso, M.; Cea-Rama, I.; Coscolin, C.; Chernikova, T.N.; Tran, H.; Ferrer, M.; Sanz-Aparicio, J.; Golyshin, P.N. The mobility of the cap domain is essential for the substrate promiscuity of a family IV esterase from sorghum rhizosphere microbiome. Appl. Environ. Microbiol. 2023, 89, e0180722. [Google Scholar] [CrossRef]
- Sood, S.; Sharma, A.; Sharma, N.; Kanwa, S.S. Carboxylesterases: Sources, characterization and boader applications. Insights Enzym. Res. 2016, 1, 1–11. [Google Scholar] [CrossRef]
- Farrokh, P.; Yakhchali, B.; Karkhane, A.A. Cloning and characterization of newly isolated lipase from Enterobacter sp. Bn12. Braz. J. Microbiol. 2014, 45, 677–687. [Google Scholar] [CrossRef] [Green Version]
- Faouzi, L.; Fatimazahra, E.B.; Moulay, S.; Adel, S.; Wifak, B.; Soumya, E.; Iraqui, M.; Saad, K.I. Higher tolerance of a novel lipase from Aspergillus flavus to the presence of free fatty acids at lipid/water interface. Afr. J. Biochem. Res. 2015, 9, 9–17. [Google Scholar] [CrossRef] [Green Version]
- Jochens, H.; Hesseler, M.; Stiba, K.; Padhi, S.K.; Kazlauskas, R.J.; Bornscheuer, U.T. Protein engineering of alpha/beta-hydrolase fold enzymes. Chembiochem 2011, 12, 1508–1517. [Google Scholar] [CrossRef] [PubMed]
- Le, L.; Yoo, W.; Lee, C.; Wang, Y.; Jeon, S.; Kim, K.K.; Lee, J.H.; Kim, T.D. Molecular characterization of a novel cold-active hormone-sensitive lipase (HaHSL) from Halocynthiibacter arcticus. Biomolecules 2019, 9, 704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hitch, T.C.A.; Clavel, T. A proposed update for the classification and description of bacterial lipolytic enzymes. PeerJ 2019, 7, e7249. [Google Scholar] [CrossRef]
- Huang, J.; Huo, Y.Y.; Ji, R.; Kuang, S.; Ji, C.; Xu, X.W.; Li, J. Structural insights of a hormone sensitive lipase homologue Est22. Sci. Rep. 2016, 6, 28550. [Google Scholar] [CrossRef]
- Ren, L.; Lin, Z.; Liu, H.; Hu, H. Bacteria-mediated phthalic acid esters degradation and related molecular mechanisms. Appl. Microbiol. Biotechnol. 2018, 102, 1085–1096. [Google Scholar] [CrossRef]
- Hoppner, A.; Bollinger, A.; Kobus, S.; Thies, S.; Coscolin, C.; Ferrer, M.; Jaeger, K.E.; Smits, S.H.J. Crystal structures of a novel family IV esterase in free and substrate-bound form. FEBS J. 2021, 288, 3570–3584. [Google Scholar] [CrossRef]
- Lang, E.; Griese, B.; Sproer, C.; Schumann, P.; Steffen, M.; Verbarg, S. Characterization of ‘Pseudomonas azelaica’ DSM 9128, leading to emended descriptions of Pseudomonas citronellolis Seubert 1960 (Approved Lists 1980) and Pseudomonas nitroreducens Iizuka and Komagata 1964 (Approved Lists 1980), including Pseudomonas multiresinivorans as its later heterotypic synonym. Int. J. Syst. Evol. Microbiol. 2007, 57, 878–882. [Google Scholar]
- Wagner, J.; Short, K.; Catto-Smith, A.G.; Cameron, D.J.; Bishop, R.F.; Kirkwood, C.D. Identification and characterisation of Pseudomonas 16S ribosomal DNA from ileal biopsies of children with Crohn’s disease. PLoS ONE 2008, 3, e3578. [Google Scholar] [CrossRef] [Green Version]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Consortium, T.U. UniProt: The universal protein knowledgebase in 2023. Nucleic Acids Res. 2022, 51, D523–D531. [Google Scholar] [CrossRef] [PubMed]
- Ison, J.; Kalas, M.; Jonassen, I.; Bolser, D.; Uludag, M.; McWilliam, H.; Malone, J.; Lopez, R.; Pettifer, S.; Rice, P. EDAM: An ontology of bioinformatics operations, types of data and identifiers, topics and formats. Bioinformatics 2013, 29, 1325–1332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teufel, F.; Almagro Armenteros, J.J.; Johansen, A.R.; Gíslason, M.H.; Pihl, S.I.; Tsirigos, K.D.; Winther, O.; Brunak, S.; von Heijne, G.; Nielsen, H. SignalP 6.0 predicts all five types of signal peptides using protein language models. Nat. Biotechnol. 2022, 40, 1023–1025. [Google Scholar] [CrossRef]
- Huo, Y.Y.; Jian, S.L.; Cheng, H.; Rong, Z.; Cui, H.L.; Xu, X.W. Two novel deep-sea sediment metagenome-derived esterases: Residue 199 is the determinant of substrate specificity and preference. Microb. Cell Fact. 2018, 17, 16. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Zhou, M.; Xing, S.; Wu, T.; He, H.; Bielicki, J.K.; Chen, J. Identification and biochemical characterization of a novel hormone-sensitive lipase family esterase Est19 from the Antarctic bacterium Pseudomonas sp. E2-15. Biomolecules 2021, 11, 1552. [Google Scholar] [CrossRef] [PubMed]
- Nacke, H.; Will, C.; Herzog, S.; Nowka, B.; Engelhaupt, M.; Daniel, R. Identification of novel lipolytic genes and gene families by screening of metagenomic libraries derived from soil samples of the German biodiversity exploratories. FEMS Microbiol. Ecol. 2011, 78, 188–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.W.; Won, K.; Lim, H.K.; Kim, J.C.; Choi, G.J.; Cho, K.Y. Screening for novel lipolytic enzymes from uncultured soil microorganisms. Appl. Microbiol. Biotechnol. 2004, 65, 720–726. [Google Scholar] [CrossRef]
- Jin, P.; Pei, X.; Du, P.; Yin, X.; Xiong, X.; Wu, H.; Zhou, X.; Wang, Q. Overexpression and characterization of a new organic solvent-tolerant esterase derived from soil metagenomic DNA. Bioresour. Technol. 2012, 116, 234–240. [Google Scholar] [CrossRef]
- Zhang, A.; Zhao, R.; Jin, P.; Ma, L.; Xiong, X.; Xie, T.; Pei, X.; Yu, L.; Yin, X.; Wang, Q. Discovery of a novel esterase subfamily sharing an identified arm sequence (ArmEst) by gene-specific metagenomic PCR. Biotechnol. Lett. 2013, 35, 1937–1944. [Google Scholar] [CrossRef]
- Choo, D.W.; Kurihara, T.; Suzuki, T.; Soda, K.; Esaki, N. A cold-adapted lipase of an Alaskan psychrotroph, Pseudomonas sp. strain B11-1: Gene cloning and enzyme purification and characterization. Appl. Environ. Microbiol. 1998, 64, 486–491. [Google Scholar] [CrossRef] [Green Version]
- Valentin, H.E.; Zwingmann, G.; Schonebaum, A.; Steinbuchel, A. Metabolic pathway for biosynthesis of poly(3-hydroxybutyrate-co-4-hydroxybutyrate) from 4-hydroxybutyrate by Alcaligenes eutrophus. Eur. J. Biochem. 1995, 227, 43–60. [Google Scholar] [CrossRef]
- Rahman, R.N.; Baharum, S.N.; Basri, M.; Salleh, A.B. High-yield purification of an organic solvent-tolerant lipase from Pseudomonas sp. strain S5. Anal. Biochem. 2005, 341, 267–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, J.; Liu, Y.; Qin, Y.; Pan, L.; Li, Y.; Liang, G.; Wang, Q. Isolation and characterization of a novel bacterium Burkholderia gladioli Bsp-1 producing alkaline lipase. J. Microbiol. Biotechnol. 2019, 29, 1043–1052. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Mukhia, S.; Kumar, N.; Acharya, V.; Kumar, S.; Kumar, R. A broad temperature active lipase purified from a psychrotrophic bacterium of sikkim himalaya with potential application in detergent formulation. Front. Bioeng. Biotechnol. 2020, 8, 642. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Nie, K.; Xu, H.; Xiong, X.; Krastev, R.; Wang, F.; Tan, T.; Liu, L. Insight into the mechanism behind the activation phenomenon of lipase from Thermus thermophilus HB8 in polar organic solvents. J. Mol. Catal. B Enzym. 2016, 133, 5400–5409. [Google Scholar] [CrossRef]
- Vaezzadeh, M.; Sabbaghian, M.; Yaghmaei, P.; Ebrahim-Habibi, A. Effect of organic solvents on porcine pancreatic lipase thermal aggregation. Protein Pept. Lett. 2017, 24, 955–961. [Google Scholar] [CrossRef]
- Ogino, H.; Nakagawa, S.; Shinya, K.; Muto, T.; Fujimura, N.; Yasuda, M.; Ishikawa, H. Purification and characterization of organic solvent-stable lipase from organic solvent-tolerant Pseudomonas aeruginosa LST-03. J. Biosci. Bioeng. 2000, 89, 451–457. [Google Scholar] [CrossRef]
- Chen, S.-X.; Qian, L.-L.; Shi, B.-Z. Purification and properties of enantioselective lipase from a newly isolated Bacillus cereus C71. Process Biochem. 2007, 42, 988–994. [Google Scholar]
- Rehdorf, J.; Behrens, G.A.; Nguyen, G.S.; Kourist, R.; Bornscheuer, U.T. Pseudomonas putida esterase contains a GGG(A)X-motif confering activity for the kinetic resolution of tertiary alcohols. Appl. Microbiol. Biotechnol. 2012, 93, 1119–1126. [Google Scholar] [CrossRef]
- Martinez-Martinez, M.; Coscolin, C.; Santiago, G.; Chow, J.; Stogios, P.J.; Bargiela, R.; Gertler, C.; Navarro-Fernandez, J.; Bollinger, A.; Thies, S.; et al. Determinants and prediction of esterase substrate promiscuity patterns. ACS Chem. Biol. 2018, 13, 225–234. [Google Scholar] [CrossRef] [Green Version]
- Edwards, U.; Rogall, T.; Blocker, H.; Emde, M.; Bottger, E.C. Isolation and direct complete nucleotide determination of entire genes. Characterization of a gene coding for 16S ribosomal RNA. Nucleic Acids Res. 1989, 17, 7843–7853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stackebrandt, E.; Liesack, W.; Goebel, B.M. Bacterial diversity in a soil sample from a subtropical Australian environment as determined by 16S rDNA analysis. FASEB J. 1993, 7, 232–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Lineweaver, H.; Burk, D. The determination of enzyme dissociation constants. J. Am. Chem. Soc. 1934, 56, 658. [Google Scholar] [CrossRef]
- Laskowski, R.A.; Swindells, M.B. LigPlot+: Multiple ligand-protein interaction diagrams for drug discovery. J. Chem. Inf. Model. 2011, 51, 2778–2786. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuan, J.-E.; Tsai, C.-H.; Chou, C.-C.; Wu, C.; Wu, W.-F. Enzymatic Characterization of a Novel HSL Family IV Esterase EstD04 from Pseudomonas sp. D01 in Mealworm Gut Microbiota. Molecules 2023, 28, 5410. https://doi.org/10.3390/molecules28145410
Kuan J-E, Tsai C-H, Chou C-C, Wu C, Wu W-F. Enzymatic Characterization of a Novel HSL Family IV Esterase EstD04 from Pseudomonas sp. D01 in Mealworm Gut Microbiota. Molecules. 2023; 28(14):5410. https://doi.org/10.3390/molecules28145410
Chicago/Turabian StyleKuan, Jung-En, Chih-Hsuan Tsai, Chun-Chi Chou, Cindy Wu, and Whei-Fen Wu. 2023. "Enzymatic Characterization of a Novel HSL Family IV Esterase EstD04 from Pseudomonas sp. D01 in Mealworm Gut Microbiota" Molecules 28, no. 14: 5410. https://doi.org/10.3390/molecules28145410




