An Estimation of the Antiviral Activity and Toxicity of Biologically Active Substances Obtained from the Raw Materials of Artemisia cina Berg. In Vitro and In Vivo
Abstract
:1. Introduction
2. Results and Discussion
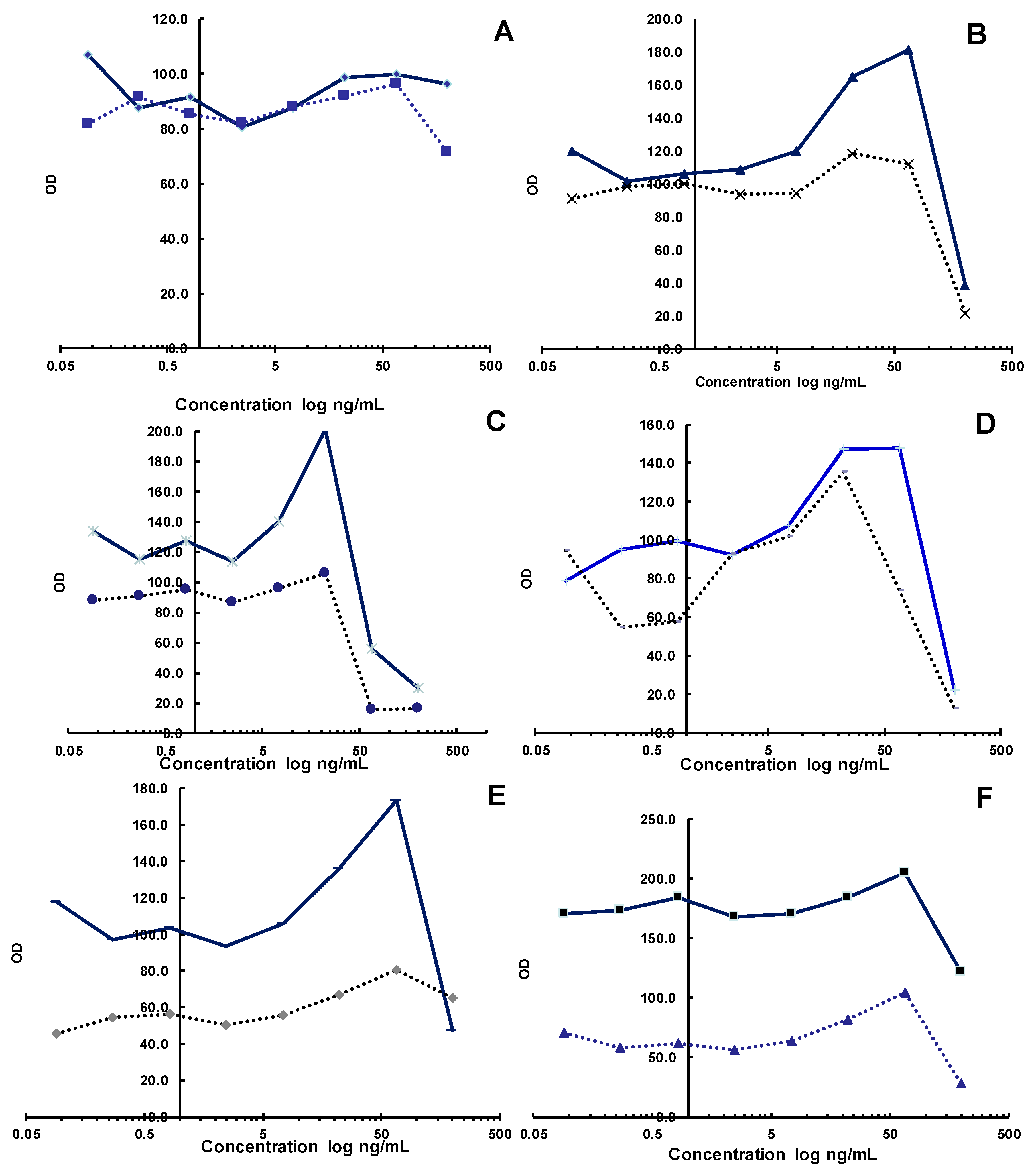
2.1. Evaluation of Biological Activity against SARS-CoV-2
2.2. Evaluation of Toxicity In Vivo
3. Materials and Methods
3.1. Collection and Preparation of Plant Raw Materials
3.2. Obtaining Extracts from Plant Raw Materials
3.2.1. Lyophilization of Thick Extracts
3.2.2. Selection of the Extractant
3.2.3. Selection of the Optimal Ratio of Extractants: Raw Material
- (a)
- No. 2 extract was prepared as follows: 50 g of raw material of Artemisia cina Berg. Was extracted with 250 mL of petroleum ether; then, the petroleum ether was distilled off and extracted with 250 mL of hexane into the obtained meal;
- (b)
- No. 3 extract, an aqueous extract of wormwood following fractional extraction, was prepared as follows: 50 g of Artemisia cina Berg. was sequentially extracted with petroleum ether, hexane, and ethyl alcohol. Subsequently, the remaining substances were extracted from the obtained meal with the help of 250 mL of hot water, and then the water was distilled from the obtained tincture (the extract was concentrated). Product yield: 4.91 g. The scheme of the extract is presented in Figure S1;
- (c)
- No. 4 extract was prepared as follows: 10 g of Artemisia cina Berg. was infused in 200 mL of hot water for 2 h, and then water was distilled from the resulting tincture (the extract was concentrated). Product yield: 7.31 g. The scheme of the extract is presented in Figure S2;
- (d)
- No. 5 extract was prepared as follows: 50 g of raw material of Artemisia cina Berg. was extracted with 250 mL of petroleum ether; then, the petroleum ether was distilled off and extracted with 250 mL of 96% alcohol into the obtained meal;
- (e)
- No. 6 extract (using 40% ethanol) was prepared as follows: 50 g of Artemisia cina Berg. was infused in 250 mL of 40% alcohol solution, and then the water and alcohol were distilled from the resulting tincture (i.e., the extract was concentrated). Product yield: 5.12 g. The scheme of the extract is exhibited in Figure S3;
- (f)
- No. 7 extract (Artemisia cina Berg., 1 tsp): A total of 5 g of wormwood was infused in 200 mL of hot water, and then the water was distilled from the resulting tincture (i.e., the extract was concentrated). Subsequently, 5 mL of hexane was added to the obtained concentrated extract to remove hexane-soluble toxic impurities. Product yield: 1.78 g. The scheme of the extract is illustrated in Figure S4.
3.2.4. Gas Chromatography with a Mass Spectrometer
- -
- Temperature: 80 °C for 1 min;
- -
- Heating at a rate of 10 °C per minute to 250 °C, and held for 5 min at this temperature;
- -
- Total analysis time: 23 min.
- -
- Quadrupole temperature: 150 °C;
- -
- Source temperature: 230 °C;
- -
- Interface temperature: 280 °C;
- -
- The voltage of the ion multiplier: 1388 V;
- -
- The polarity was positive.
3.2.5. Separation of Extracts into Separate Fractions and Individual Components
3.2.6. Method of Evaluation of Antiviral Activity
3.3. MTT Assay
3.4. Evaluation of Toxicity of Plant Extracts In Vivo
- (1)
- Group 1 (6 female mice): laboratory female mice in this study group were injected once, intragastrically, with the substance No. 7 Artemisia cina Berg. in the maximum technically achievable dose of 2 g/kg of body weight of mice;
- (2)
- Group 2 (6 female mice): laboratory female mice in this study group were injected once, intragastrically, with the substance No. 3 Artemisia cina Berg. in the maximum technically achievable dose of 2 g/kg of body weight of mice;
- (3)
- Group 3 (6 female mice): laboratory female mice in this study group were injected once, intragastrically, with the substance No. 4 Artemisia cina Berg. in the maximum technically achievable dose of 2 g/kg of body weight of mice;
- (4)
- Group 4 (6 female mice): laboratory female mice in this study group were injected intragastrically with the appropriate solvent (drinking water) in the same volume (0.5 mL) and according to the same scheme (once) as the test substance (manipulation control).
- (5)
- Group 5 (6 male mice): laboratory male mice in this study group were injected once, intragastrically, with the substance No. 7 Artemisia cina Berg. in the maximum technically achievable dose of 2 g/kg of body weight of mice;
- (6)
- Group 6 (6 male mice): laboratory male mice in this study group were injected once, intragastrically, with the substance No. 3 Artemisia cina Berg. in the maximum technically achievable dose of 2 g/kg of body weight of mice;
- (7)
- Group 7 (6 male mice): laboratory male mice in this study group were injected once, intragastrically, with the substance No. 4 Artemisia cina Berg. in the maximum technically achievable dose of 2 g/kg of body weight of mice;
- (8)
- Group 8 (6 female mice): laboratory female mice in this study group were injected intragastrically with the appropriate solvent (drinking water) in the same volume (0.5 mL) and according to the same scheme (once) as the test substance (handling control).
3.5. Ethical Aspects
3.6. Statistical Analysis of Data
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Hussain, A. A phylogenetic perspective of antiviral species of the genus Artemisia (Asteraceae-Anthemideae): A proposal of anti SARS-CoV-2 (COVID-19) candidate taxa. J. Herb. Med. 2022, 36, 100601. [Google Scholar] [CrossRef] [PubMed]
- Dogan, K.; Erol, E.; Orhan, M.D.; Degirmenci, Z.; Kan, T.; Gungor, A.; Yasa, B.; Avsar, T.; Cetin, Y.; Durdagi, S.; et al. Instant determination of the artemisinin from various Artemisia annua L. extracts by LC-ESI-MS/MS and their in-silico modelling and in vitro antiviral activity studies against SARS-CoV-2. Phytochem. Anal. 2022, 33, 303–319. [Google Scholar] [CrossRef] [PubMed]
- Nair, M.; Huang, Y.; Fidock, D.; Towler, M.; Weathers, P. Artemisia annua L. hot-water extracts show potent activity in vitro against COVID-19 variants including delta. J. Ethnopharmacol. 2022, 284, 114797. [Google Scholar] [CrossRef] [PubMed]
- Nair, M.; Huang, Y.; Fidock, D.; Polyak, S.; Wagoner, J.; Towler, M.; Weathers, P. Artemisia annua L. extracts inhibit the in vitro replication of SARS-CoV-2 and two of its variants. J. Ethnopharmacol. 2021, 274, 114016. [Google Scholar] [CrossRef] [PubMed]
- Babacan, Ü.; Cengiz, M.F.; Bouali, M.; Tongur, T.; Mutlu, S.S.; Gülmez, E. Determination, solvent extraction, and purification of artemisinin from Artemisia annua L. J. Appl. Res. Med. Aromat. Plants 2022, 28, 100363. [Google Scholar] [CrossRef]
- Mao, Z.Q.; Minakawa, N.; Moi, M.L. Novel Antiviral Efficacy of Hedyotis diffusa and Artemisia capillaris Extracts against Dengue Virus, Japanese Encephalitis Virus, and Zika Virus Infection and Immunoregulatory Cytokine Signatures. Plants 2022, 11, 2589. [Google Scholar] [CrossRef]
- Osunsanmi, F.O.; Yotwana, L.; Mosa, R.A.; Liu, A.-L.; Gao, L.; Du, G.-H.; Opoku, A.R. In vitro antiviral, antioxidant and in vivo antipyretic activity of three South Africa medicinal plants crude extracts. Bol. Latinoam. Caribe Plantas Med. Aromát. 2022, 21, 620–630. [Google Scholar] [CrossRef]
- Liu, P.; Zhong, L.; Xiao, J.; Hu, Y.; Liu, T.; Ren, Z.; Wang, Y.; Zheng, K. Ethanol extract from Artemisia argyi leaves inhibits HSV-1 infection by destroying the viral envelope. Virol. J. 2023, 20, 8. [Google Scholar] [CrossRef]
- Hegazy, A.; Mostafa, I.; Elshaier, Y.A.M.M.; Mahmoud, S.H.; Shama, N.M.A.; Shehata, M.; Yahya, G.; Nasr, N.F.; El-Halawany, A.M.; Ali, M.A.; et al. Robust Antiviral Activity of Santonica Flower Extract (Artemisia cina) against Avian and Human Influenza A Viruses: In Vitro and Chemoinformatic Studies. ACS Omega 2022, 7, 41212–41223. [Google Scholar] [CrossRef]
- Obul, M.; Wang, X.; Zhao, J.; Li, G.; Aisa, H.A.; Huang, G. Structural modification on rupestonic acid leads to highly potent inhibitors against influenza virus. Mol. Divers. 2019, 23, 1–9. [Google Scholar] [CrossRef]
- Ticona, L.A.; Bermejo, P.; Guerra, J.; Abad, M.; Beltrán, M.; Lázaro, R.M.; Alcamí, J.; Bedoya, L. Ethanolic extract of Artemisia campestris subsp. glutinosa (Besser) Batt. inhibits HIV–1 replication in vitro through the activity of terpenes and flavonoids on viral entry and NF–κB pathway. J. Ethnopharmacol. 2020, 263, 113163. [Google Scholar] [CrossRef]
- Zhang, P.; Jiang, J.; Zhang, K.; Liu, W.; Tu, P.; Li, J.; Song, Y.; Zheng, J.; Tang, L. Shotgun chemome characterization of Artemisia rupestris L. Using direct infusion-MS/MSALL. J. Chromatogr. B 2021, 1176, 122735. [Google Scholar] [CrossRef] [PubMed]
- Heleno, S.A.; Carocho, M.; Reis, F.S.; Pires, T.C.S.P.; Pintado, M.; Ferreira, I.C.F.R.; Barros, L. Plant Extracts and SARS-CoV-2: Research and Applications. Life 2023, 13, 386. [Google Scholar] [CrossRef] [PubMed]
- Staff, T.P.O. Correction: Quantification of santonin in eight species of Artemisia from Kazakhstan by means of HPLC-UV: Method development and validation. PLoS ONE 2017, 12, e0175088. [Google Scholar] [CrossRef] [Green Version]
- Tian, S.-H.; Zhang, C.; Zeng, K.-W.; Zhao, M.-B.; Jiang, Y.; Tu, P.-F. Sesquiterpenoids from Artemisia vestita. Phytochemistry 2018, 147, 194–202. [Google Scholar] [CrossRef]
- Greger, H.; Zdero, C.; Bohlmann, F. Eudesman-12,8β-olides and other terpenes from Artemisia species. Phytochemistry 1986, 25, 891–897. [Google Scholar] [CrossRef]
- Marco, J.; Sanz-Cervera, J.F.; Pareja, J.M.; Sancenon, F.; Valles-Xirau, J. Sesquiterpene lactones from North African Artemisia species. Phytochemistry 1994, 37, 477–485. [Google Scholar] [CrossRef]
- Zhurinov, M.; Miftakhova, A.; Shustov, A.; Keyer, V.; Solodova, E. Inhibitory activity of Artemisia annua L. extracts against SARS-CoV-2 coronavirus. Eurasian J. Appl. Biotechnol. 2022, 3, 25–31. [Google Scholar] [CrossRef]
- On Approval of the Rules of Good Practice for Cultivation, Collection, Processing and Storage of Raw Materials of Plant Origin Decision of the Council of the Eurasian Economic Commission Dated 26 January 2018 No. 15. Available online: https://adilet.zan.kz/rus/docs/H18EV000015 (accessed on 11 May 2021).
- Barnard, D.L.; Day, C.W.; Bailey, K.; Heiner, M.; Montgomery, R.; Lauridsen, L.; Winslow, S.; Hoopes, J.; Li, J.K.-K.; Lee, J.; et al. Enhancement of the infectivity of SARS-CoV in BALB/c mice by IMP dehydrogenase inhibitors, including ribavirin. Antivir. Res. 2006, 71, 53–63. [Google Scholar] [CrossRef]
- Available online: https://www.criver.com/products-services/discovery-services/pharmacology-studies/infectious-disease-models-assays/influenza-models?region=3701 (accessed on 30 May 2023).
- Miftakhova, A.; Syzdykova, L.; Keer, V.; Shustov, A.; Zhurinov, M. The plant Artemisia annua (“SWEET wormwood”) kazakhstan’s sourse of bioactive compounds potentially cure the SARS-CoV-2 infection. Eurasian J. Appl. Biotechnol. 2022, 3, 32–40. [Google Scholar] [CrossRef]
- Moazzam, M.; Shehzad, A.; Sultanova, D.; Mukasheva, F.; Trifonov, A.; Berillo, D.; Akilbekova, D. Macroporous 3D printed structures for regenerative medicine applications. Bioprinting 2022, 28, e00254. [Google Scholar] [CrossRef]
- Seidakhmetova, R.B.; Amanzhan, A.; Shults, E.E.; Goldaeva, K.V.; Adekenov, S.M.; Berillo, D.A. Analgesic and Antidepressant Activity of 8-Substituted Harmine Derivatives. Chem. Heterocycl. Compd. 2022, 58, 324–332. [Google Scholar] [CrossRef]
| Row of the 96-Well Plate | Extract Concentration, mg/mL |
|---|---|
| A | 0.004 |
| B | 0.01 |
| C | 0.04 |
| D | 0.12 |
| E | 0.37 |
| F | 1.11 |
| G | 3.33 |
| H | 10.0 |
| No. | Main Compounds | Relative Amount, % | Rt, Minutes |
|---|---|---|---|
| 1 | α-santonin | 66.3 | 18.23 |
| 2 | 6-nitro-2-phenyl-4-quinolinol | 8.0 | 18.33 |
| 3 | 3,5,5-trimethylcyclohexylisophosphofloride | 6.7 | 9.98 |
| 4 | Lumisantonin | 3.7 | 16.36 |
| 5 | 2,3-Dihydro-4H-pyran-4-one | 3.2 | 5.27 |
| No. | Main Compounds | Relative Amount, % | Rt, Minutes |
|---|---|---|---|
| 1 | α-santonin | 39.39 | 18.22 |
| 2 | Butanoic acid | 10.05 | 12.24 |
| 3 | Anhydro-β-retinol | 3.28 | 21.02 |
| No. | Main Compounds | Relative Amount, % | Rt, Minutes |
|---|---|---|---|
| 1 | α-santonin | 66.35 | 18.22 |
| 2 | 2,3-Dihydro-4H-pyran-4-one | 4.90 | 5.42 |
| 3 | 4-Nonanone, 7-ethyl- | 5.90 | 9.98 |
| 4 | Lumisantonin | 2.58 | 16.36 |
| Study Groups, Sex, Number | Initial Body Weight of Mice, g | Body Weight of Mice 1 Week after the Administration of the Test Substances, g | Body Weight of Mice 2 Weeks after the Administration of the Test Substances, g |
|---|---|---|---|
1st group, No. 7  , n = 6 , n = 6 | 29.3 ± 2.1 | 31.0 ± 2.2 | 32.7 ± 2.0 |
| p = 0.6970 | p = 0.7548 | p = 0.6317 | |
2nd group, No. 3,  , n = 6 , n = 6 | 29.5 ± 1.1 | 31.9 ± 0.9 | 33.4 ± 0.9 |
| p = 0.8836 | p = 0.9518 | p = 0.7700 | |
3rd group, No. 4,  , n = 6 , n = 6 | 29.4 ± 1.8 | 32.4 ± 2.0 | 34.6 ± 1.8 |
| p = 0.8763 | p = 0.9196 | p = 0.8907 | |
4th group, control,  , n = 6 , n = 6 | 29.9 ± 2.5 | 32.1 ± 2.5 | 34.2 ± 2.3 |
| 29.9 ± 2.5 | 33.4 ± 1.4 | 35.2 ± 1.2 | 36.3 ± 0.9 |
| p = 0.4729 | p = 0.7145 | p = 0.3984 | |
6th group, No. 3,  , n = 6 , n = 6 | 33.7 ± 2.1 | 35.8 ± 1.9 | 37.3 ± 1.4 |
| p = 0.5115 | p = 0.5978 | p = 0.4195 | |
7th group, No. 4,  , n = 6 , n = 6 | 32.4 ± 2.7 | 36.4 ± 1.2 | 37.3 ± 1.2 |
| p = 0.8926 | p = 0.2559 | p = 0.4010 | |
8th group, control,  , n = 6 , n = 6 | 32.0 ± 1.3 | 34.6 ± 1.0 | 35.2 ± 2.0 |
 is a symbol indicating males;
is a symbol indicating males;  is a symbol indicating females; p is the significance level, and a p < 0.05 indicated statistically significant differences compared to the corresponding values in the control group of animals.
is a symbol indicating females; p is the significance level, and a p < 0.05 indicated statistically significant differences compared to the corresponding values in the control group of animals.| Investigated Parameter | Group of Animal, n = 6,  | |||
|---|---|---|---|---|
| 1st Group, No. 7 | 2nd Group, No. 3 | 3rd Group, No. 4 | 4th Group, Control | |
| Intensity and nature of motor activity | The mice are active. The coordination of movements is not disturbed | |||
| The presence and nature of seizures | Absent | |||
| Condition of hair and skin | No changes detected (wool is white, clean, smooth) | |||
| Condition and color of mucous membranes | No changes found or observed | |||
| Reaction to sound, pain stimuli | Adequately react | |||
| Animal death | 0 | |||
| Urination (color of urine) | No changes found | |||
| Defecation | No changes found | |||
 is a symbol indicating females.
is a symbol indicating females.| Investigated Parameter | Group of Animal, n = 6,  | |||
|---|---|---|---|---|
| 5th Group, No. 7 | 6th Group, No. 3 | 7th Group, No. 4 | 8th Group, Control | |
| Intensity and nature of motor activity | The mice are active. The coordination of movements is not disturbed | |||
| The presence and nature of seizures | Absent | |||
| Condition of hair and skin | No changes detected (wool is white, clean, smooth) | |||
| Condition and color of mucous membranes | No changes found or observed | |||
| Reaction to sound, pain stimuli | Adequately react | |||
| Animal death | 0 | |||
| Urination (color of urine) | No changes found | |||
| Defecation | No changes found | |||
 is a symbol indicating males.
is a symbol indicating males.| Investigated Group | Researched Parameters | ||||
|---|---|---|---|---|---|
| Erythrocytes, Units/µL | Ketones, mmol/L | Protein, g/L | Glucose, mmol/L | pH | |
1st group, No. 3,  , n = 6 , n = 6 | 1/6—10 unit/μL 5/6—50 unit/μL | 6/6—0.5 mmol/L | 4/6—0.1 g/L 2/6—0.3 g/L | 6/6—negative | 6.0 ± 0.0 (6/6–6.0) |
2nd group, No. 3,  , n = 6 , n = 6 | 6/6—50 unit/μL | 6/6—0.5 mmol/L | 4/6—0.1 g/L 2/6—0.3 g/L | 6/6—negative | 6.0 ± 0.0 (6/6–6.0) |
3rd group, No. 4,  , n = 6 , n = 6 | 4/6—25 unit/μL 2/6—50 unit/μL | 6/6—negative | 2/6—0.1 g/L 4/6—0.3 g/L | 6/6—negative | 6.0 ± 0.0 (6/6–6.0) |
4th group, control,  , n = 6 , n = 6 | 2/6—negative 4/6—10 unit/μL | 6/6—negative | 3/6—0.1 g/L 3/6—0.3 g/L | 6/6—negative | 6.0 ± 0.0 (6/6–6.0) |
5th group, No. 7,  , n = 6 , n = 6 | 1/6—10 unit/μL 1/6—25 unit/μL 4/6—50 unit/μL | 6/6—0.5 mmol/L | 4/6—0.1 g/L 2/6—0.3 g/L | 6/6—negative | 6.0 ± 0.0 (6/6–6.0) |
6th group, No. 3,  , n = 6 , n = 6 | 3/6—25 unit/μL 3/6—50 unit/μL | 2/6—negative 4/6—0.5 mmol/L | 3/6—0.1 g/L 3/6—0.3 g/L | 6/6—negative | 6.0 ± 0.0 (6/6–6.0) |
7th group, No. 4,  , n = 6 , n = 6 | 4/6—25 unit/μL 2/6—50 unit/μL | 6/6—negative | 2/6—0.1 g/L 4/6—0.3 g/L | 6/6—negative | 6.0 ± 0.0 (6/6–6.0) |
8th group, control,  , n = 6 , n = 6 | 3/6—negative 3/6—10 unit/μL | 6/6—negative | 3/6—0.1 g/L 3/6—0.3 g/L | 6/6—negative | 6.0 ± 0.0 (6/6–6.0) |
 is a symbol indicating males;
is a symbol indicating males;  is a symbol indicating females; p is significance level, and a p < 0.05 indicates statistically significant differences compared to the corresponding values in the control group of animals; n is the number of animals in the group.
is a symbol indicating females; p is significance level, and a p < 0.05 indicates statistically significant differences compared to the corresponding values in the control group of animals; n is the number of animals in the group.| Investigated Group | Essential Physiological Parameters | ||||
|---|---|---|---|---|---|
| Erythrocytes, Units/µL | Ketones, mmol/L | Protein, g/L | Glucose, mmol/L | pH | |
1st group, No. 7,  , n = 6 , n = 6 | 2/6—10 unit/μL 2/6—25 unit/μL 2/6—50 unit/μL | 6/6—0.5 mmol/L | 4/6—0.1 g/L 2/6—0.3 g/L | 6/6—negative | 6.0 ± 0.0 (6/6–6.0) |
2nd group, No. 3,  , n = 6 , n = 6 | 4/6—10 unit/μL 1/6—25 unit/μL 1/6—50 unit/μL | 6/6—0.5 mmol/L | 4/6—0.1 g/L 2/6—0.3 g/L | 6/6—negative | 6.0 ± 0.0 (6/6–6.0) |
3rd group, No. 4,  , n = 6 , n = 6 | 3/6—10 unit/μL 3/6—25 unit/μL | 6/6—negative | 1/6—0.1 g/L 5/6—0.3 g/L | 6/6—negative | 6.0 ± 0.0 (6/6–6.0) |
4th group,  , n = 6 , n = 6 | 4/6—negative 2/6—10 unit/μL | 6/6—negative | 2/6—0.1 g/L 4/6—0.3 g/L | 6/6—negative | 6.0 ± 0.0 (6/6–6.0) |
5th group, No. 7,  , n = 6 , n = 6 | 5/6—10 unit/μL 1/6—25 unit/μL | 6/6—0.5 mmol/L | 4/6—0.1 g/L 2/6—0.3 g/L | 6/6—negative | 6.0 ± 0.0 (6/6–6.0) |
6th group, No. 3,  , n = 6 , n = 6 | 5/6—10 unit/μL 1/6—50 unit/μL | 2/6—negative 4/6—0.5 mmol/L | 4/6—0.1 g/L 2/6—0.3 g/L | 6/6—negative | 6.0 ± 0.0 (6/6–6.0) |
7th group, No. 4,  , n = 6 , n = 6 | 3/6—10 unit/μL 3/6—25 unit/μL | 6/6—negative | 2/6—0.1 g/L 4/6—0.3 g/L | 6/6—negative | 6.0 ± 0.0 (6/6–6.0) |
8th group, control,  , n = 6 , n = 6 | 4/6—negative 2/6—10 unit/μL | 6/6—negative | 2/6—0.1 g/L 4/6—0.3 g/L | 6/6—negative | 6.0 ± 0.0 (6/6–6.0) |
 is a symbol indicating males;
is a symbol indicating males;  is a symbol indicating females; p is significance level, and a p < 0.05 indicates statistically significant differences compared to the corresponding values in the control group of animals; n is the number of animals in the group.
is a symbol indicating females; p is significance level, and a p < 0.05 indicates statistically significant differences compared to the corresponding values in the control group of animals; n is the number of animals in the group.| Investigated Group | Weight of Internal Animals Organs, g | ||||||
|---|---|---|---|---|---|---|---|
| Brain | Heart | Lungs | Liver | Spleen | Kidneys | Gonads | |
1st group, No. 7,  , n = 6 , n = 6 | 0.44 ± 0.0207 p = 0.3090 | 0.163 ± 0.0188 p = 0.7740 | 0.245 ± 0.0190 p = 0.1595 | 1.68 ± 0.0791 p = 0.2223 | 0.198 ± 0.0130 p = 0.8804 | 0.19 ± 0.0146 p = 0.2551 | 0.0176 ± 0.0008 p = 0.9560 |
2nd group, No. 3,  , n = 6 , n = 6 | 0.45 ± 0.0088 p = 0.2180 | 0.15 ± 0.0104 p = 0.2781 | 0.265 ± 0.0361 p = 0.7717 | 1.815 ± 0.0944 p = 0.6229 | 0.186 ± 0.0105 p = 0.5616 | 0.198 ± 0.0071 p = 0.2907 | 0.0187 ± 0.0012 p = 0.5011 |
3rd group, No. 4,  , n = 6 , n = 6 | 0.45 ± 0.0176 p = 0.5598 | 0.17 ± 0.0148 p = 0.8722 | 0.27 ± 0.0203 p = 0.8412 | 2.11 ± 0.0818 p = 0.2958 | 0.20 ± 0.0122 p = 0.9774 | 0.21 ± 0.0063 p = 0.6120 | 0.018 ± 0.0007 p = 0.8967 |
4th group, control,  , n = 6 , n = 6 | 0.47 ± 0.0121 | 0.17 ± 0.0098 | 0.276 ± 0.0071 | 1.91 ± 0.1607 | 0.202 ± 0.0259 | 0.21 ± 0.0099 | 0.018 ± 0.0011 |
5th group, No. 7,  , n = 6 , n = 6 | 0.39 ± 0.0167 p = 0.9226 | 0.18 ± 0.0131 p = 0.7860 | 0.24 ± 0.0132 p = 0.4870 | 1.88 ± 0.0861 p = 0.6666 | 0.2069 ± 0.0132 p = 0.2501 | 0.22 ± 0.0050 p = 0.1597 | 0.102 ± 0.0026 p = 0.4869 |
6th group, No. 3,  , n = 6 , n = 6 | 0.39 ± 0.0162 p = 0.8859 | 0.17 ± 0.0105 p = 0.5547 | 0.27 ± 0.0330 p = 0.7836 | 1.88 ± 0.1745 p = 0.7810 | 0.21 ± 0.0150 p = 0.3370 | 0.22 ± 0.0048 p = 0.2061 | 0.102 ± 0.0018 p = 0.4140 |
7th group, No. 4,  , n = 6 , n = 6 | 0.397 ± 0.0073 p = 0.7855 | 0.17 ± 0.0076 p = 0.5711 | 0.25 ± 0.0109 p = 0.6138 | 1.99 ± 0.0865 p = 0.7212 | 0.208 ± 0.0129 p = 0.2638 | 0.22 ± 0.0025 p = 0.3128 | 0.11 ± 0.0023 p = 0.2694 |
8th group, control,  , n = 6 , n = 6 | 0.39 ± 0.0174 | 0.18 ± 0.0053 | 0.26 ± 0.0189 | 1.94 ± 0.1176 | 0.23 ± 0.0113 | 0.23 ± 0.0107 | 0.105 ± 0.0031 |
 is a symbol indicating males;
is a symbol indicating males;  is a symbol indicating females; p is significance level, and a p < 0.05 indicates statistically significant differences compared to the corresponding values in the control group of animals; n is the number of animals in the group.
is a symbol indicating females; p is significance level, and a p < 0.05 indicates statistically significant differences compared to the corresponding values in the control group of animals; n is the number of animals in the group.Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhurinov, M.; Berillo, D.; Bazhykova, K.B.; Rakhimov, K.D.; Bekezhanova, T. An Estimation of the Antiviral Activity and Toxicity of Biologically Active Substances Obtained from the Raw Materials of Artemisia cina Berg. In Vitro and In Vivo. Molecules 2023, 28, 5413. https://doi.org/10.3390/molecules28145413
Zhurinov M, Berillo D, Bazhykova KB, Rakhimov KD, Bekezhanova T. An Estimation of the Antiviral Activity and Toxicity of Biologically Active Substances Obtained from the Raw Materials of Artemisia cina Berg. In Vitro and In Vivo. Molecules. 2023; 28(14):5413. https://doi.org/10.3390/molecules28145413
Chicago/Turabian StyleZhurinov, Murat, Dmitriy Berillo, Kulzada Begalinovna Bazhykova, Kayrolla Dyusenbaevich Rakhimov, and Tolkyn Bekezhanova. 2023. "An Estimation of the Antiviral Activity and Toxicity of Biologically Active Substances Obtained from the Raw Materials of Artemisia cina Berg. In Vitro and In Vivo" Molecules 28, no. 14: 5413. https://doi.org/10.3390/molecules28145413
APA StyleZhurinov, M., Berillo, D., Bazhykova, K. B., Rakhimov, K. D., & Bekezhanova, T. (2023). An Estimation of the Antiviral Activity and Toxicity of Biologically Active Substances Obtained from the Raw Materials of Artemisia cina Berg. In Vitro and In Vivo. Molecules, 28(14), 5413. https://doi.org/10.3390/molecules28145413








