Anti-Inflammatory and Antiatopic Effects of Rorippa cantoniensis (Lour.) Ohwi in RAW 264.7 and HaCaT Cells
Abstract
:1. Introduction
2. Results
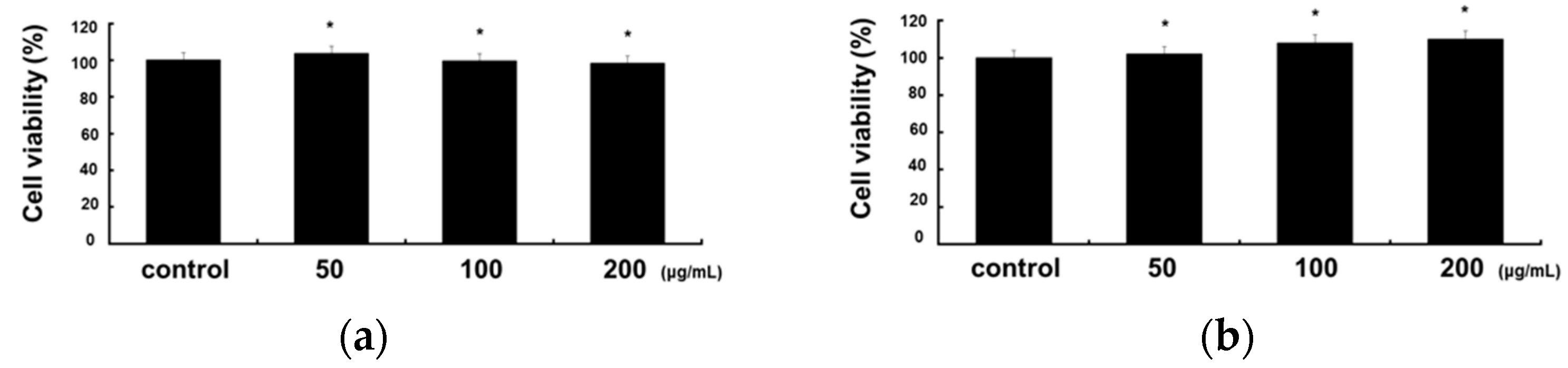
2.1. Cytotoxicity of RCE in RAW 264.7 and HaCaT Cells
2.2. Inhibitory Effects of RCE on LPS-Induced Inflammatory Mediator and Proinflammatory Cytokines in RAW 264.7 Cells
2.3. Inhibitory Effects of RCE on TNF-α/IFN-γ-Induced Proinflammatory Cytokines and Chemokines in HaCaT Cells
2.4. Inhibitory Effects of RCE on the Expression of NF-κB and STAT1 Signaling Pathway in TNF-α/IFN-γ-Induced HaCaT Cells
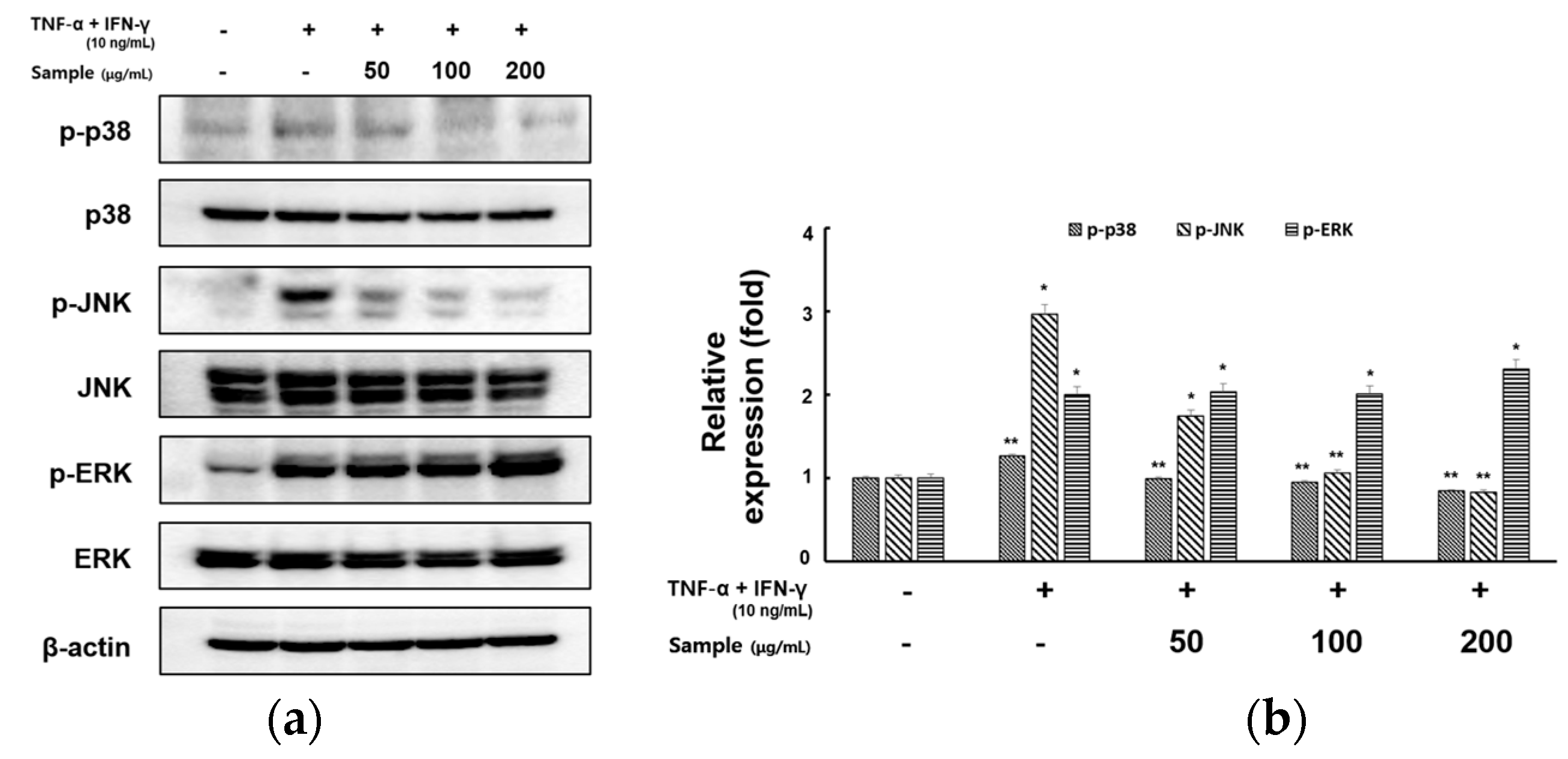
2.5. Inhibitory Effects of RCE on the Expression of MAPK Signaling Pathway in TNF-α/IFN-γ-Induced HaCaT Cells
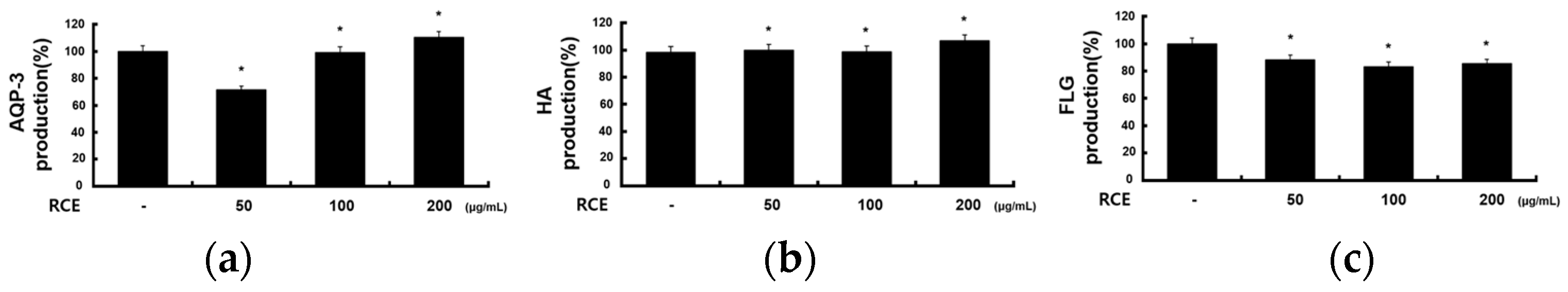
2.6. Effects of RCE on Other Molecules in HaCaT Cells
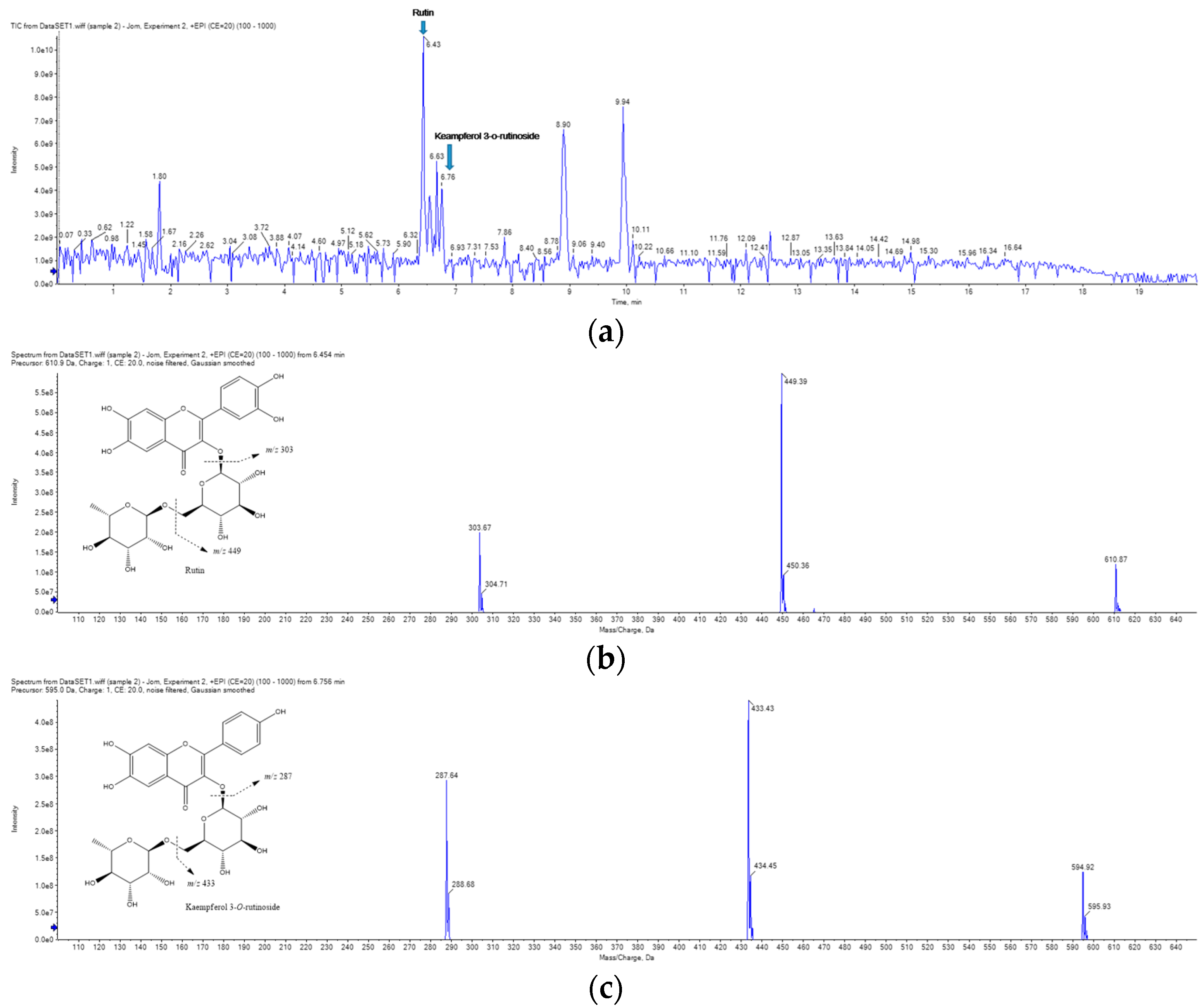
2.7. UPLC-MS/MS Analysis of RCE
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Plant Material
3.3. Cell Culture
3.4. Cell Viability Assay
3.5. Nitric Oxide (NO) Determination
3.6. Enzyme-Linked Immunosorbent Assay (ELISA)
3.7. Western Blot Analysis
3.8. UPLC-MS/MS Analysis
3.9. Data Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Kader, H.A.; Azeem, M.; Jwayed, S.A.; Al-Shehhi, A.; Tabassum, A.; Ayoub, M.A.; Hetta, H.F.; Waheed, Y.; Iratni, R.; Al-Dhaheri, A.; et al. Current insights into immunology and novel therapeutics of atopic dermatitis. Cells 2021, 10, 1392. [Google Scholar] [CrossRef] [PubMed]
- Weidinger, S.; Beck, L.A.; Bieber, T.; Kabashima, K.; Irvine, A.D. Atopic Dermatitis. Nat. Rev. Dis. Primers 2018, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Dainichi, T.; Kitoh, A.; Otsuka, A.; Nakajima, S.; Nomura, T.; Kaplan, D.H.; Kabashima, K. The Epithelial Immune Microenvironment (EIME) in Atopic Dermatitis and Psoriasis. Nat. Immunol. 2018, 19, 1286–1298. [Google Scholar] [CrossRef] [PubMed]
- Chieosilapatham, P.; Kiatsurayanon, C.; Umehara, Y.; Trujillo-Paez, J.V.; Peng, G.; Yue, H.; Nguyen, L.T.H.; Niyonsaba, F. Keratinocytes: Innate Immune Cells in Atopic Dermatitis. Clin. Exp. Immunol. 2021, 204, 296–309. [Google Scholar] [CrossRef] [PubMed]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J. Cell. Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef]
- Kandhaya-Pillai, R.; Yang, X.; Tchkonia, T.; Martin, G.M.; Kirkland, J.L.; Oshima, J. TNF-α/IFN-γ Synergy Amplifies Senescence-Associated Inflammation and SARS-CoV-2 Receptor Expression via Hyper-activated JAK/STAT1. Aging Cell 2022, 21, e13646. [Google Scholar] [CrossRef]
- Xiong, X.; Huang, C.; Wang, F.; Dong, J.; Zhang, D.; Jiang, J.; Feng, Y.; Wu, B.; Xie, T.; Cheng, L. Qingxue Jiedu Formulation Ameliorated DNFB-Induced Atopic Dermatitis by Inhibiting STAT3/MAPK/NF-κB Signaling Pathways. J. Ethnopharmacol. 2021, 270, 113773. [Google Scholar] [CrossRef]
- Piipponen, M.; Li, D.; Landén, N.X. The immune functions of keratinocytes in skin wound healing. Int. J. Mol. Sci. 2020, 21, 8790. [Google Scholar] [CrossRef]
- Renert-Yuval, Y.; Thyssen, J.P.; Bissonnette, R.; Bieber, T.; Kabashima, K.; Hijnen, D.J.; Guttman-Yassky, E. Biomarkers in atopic dermatitis—A review on behalf of the International Eczema Council. J. Allergy Clin. Immunol. 2021, 147, 1174–1190.e1. [Google Scholar] [CrossRef]
- Catherine, J.; Roufosse, F. What does elevated TARC/CCL17 expression tell us about eosinophilic disorders? Semin. Immunopathol. 2021, 43, 439–458. [Google Scholar] [CrossRef]
- Li, W.; Liu, Q.; Shi, J.; Xu, X.; Xu, J. The role of TNF-α in the fate regulation and functional reprogramming of mesenchymal stem cells in an inflammatory microenvironment. Front. Immunol. 2023, 14, 1074863. [Google Scholar] [CrossRef]
- Wang, R.; Moon, S.K.; Kim, W.J.; Dhandapani, S.; Kim, H.; Kim, Y.J. Biologically Synthesized Rosa rugosa-Based Gold Nanoparticles Suppress Skin Inflammatory Responses via MAPK and NF-κB Signaling Pathway in TNF-α/IFN-γ-Induced HaCaT Keratinocytes. ACS Omega 2022, 7, 35951–35960. [Google Scholar] [CrossRef]
- Choi, J.H.; Lee, G.H.; Jin, S.W.; Kim, J.Y.; Hwang, Y.P.; Han, E.H.; Kim, Y.H.; Jeong, H.G. Impressic Acid Ameliorates Atopic Dermatitis-Like Skin Lesions by Inhibiting ERK1/2-Mediated Phosphorylation of NF-κB and stat1. Int. J. Mol. Sci. 2021, 22, 2334. [Google Scholar] [CrossRef]
- Segarra, S.; Naiken, T.; Garnier, J.; Hamon, V.; Coussay, N.; Bernard, F.X. Enhanced In Vitro Expression of Filaggrin and Antimicrobial Peptides Following Application of Glycosaminoglycans and a Sphingomyelin-Rich Lipid Extract. Vet. Sci. 2022, 9, 323. [Google Scholar] [CrossRef]
- Tanei, R. Atopic Dermatitis in Older Adults: A Review of Treatment Options. Drugs Aging 2020, 37, 149–160. [Google Scholar] [CrossRef] [Green Version]
- Fleming, P.; Yang, Y.B.; Lynde, C.; O’Neill, B.; Lee, K.O. Diagnosis and Management of Atopic Dermatitis for Primary Care Providers. J. Am. Board Fam. Med. 2020, 33, 626–635. [Google Scholar] [CrossRef]
- Kim, M.J.; Hwang, B.S.; Oh, Y.T. Anti-inflammatory and Anti-atopic Effects of Rorippa cantoniensis Extracts of poster. In Proceedings of the International Meeting of the Microbiological Society of Korea, Jeju-si, Republic of Korea, 30 October–1 November 2022. [Google Scholar]
- Mosser, D.M.; Hamidzadeh, K.; Goncalves, R. Macrophages and the Maintenance of Homeostasis. Cell. Mol. Immunol. 2021, 18, 579–587. [Google Scholar] [CrossRef]
- Vu, Y.H.; Furue, M.; Tsuji, G. The Role of Interleukin-24 in Atopic Dermatitis. Explor. Immunol. 2021, 1, 4–15. [Google Scholar] [CrossRef]
- Klonowska, J.; Gleń, J.; Nowicki, R.J.; Trzeciak, M. New Cytokines in the Pathogenesis of Atopic Dermatitis—New Therapeutic Targets. Int. J. Mol. Sci. 2018, 19, 3086. [Google Scholar] [CrossRef] [Green Version]
- Albensi, B.C. What Is Nuclear Factor Kappa B (NF-κB) Doing in and to the Mitochondrion? Front. Cell Dev. Biol. 2019, 7, 154. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.H.; Do, H.J.; Lee, E.; Yim, N.H.; Cho, W.K.; Park, K.I.; Ma, J.Y. Jageum-Jung Improves 2,4-Dinitrochlorobenzene-Induced Atopic Dermatitis-Like Skin Lesions in Mice and Suppresses Pro-inflammatory Chemokine Production by Inhibiting TNF-α/IFN-γ-Induced STAT-1 and NFκB Signaling in HaCaT Cells. J. Ethnopharmacol. 2018, 221, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Chiu, K.M.; Hung, Y.L.; Wang, S.J.; Tsai, Y.J.; Wu, N.L.; Liang, C.W.; Chang, D.C.; Hung, C.F. Anti-allergic and Anti-Inflammatory Effects of Neferine on rbl-2h3 Cells. Int. J. Mol. Sci. 2021, 22, 10994. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.; Hu, Z.; Jia, C.; Yang, M.; Li, D.; Xu, A.; Jiang, J.; Chen, Z.; Li, Y.; Li, S.; et al. Deciphering the mechanisms of Yinlan Tiaozhi capsule in treating hyperlipidemia by combining network pharmacology, molecular docking and experimental verifcation. Sci. Rep. 2023, 13, 6424. [Google Scholar] [CrossRef] [PubMed]
- Hulshof, L.; Landvan’t, B.; Sprikkelman, A.B.; Garssen, J. Role of Microbial Modulation in Management of Atopic Dermatitis in Children. Nutrients 2017, 9, 854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakatsuji, T.; Chen, T.H.; Two, A.M.; Chun, K.A.; Narala, S.; Geha, R.S.; Hata, T.R.; Gallo, R.L. Staphylococcus aureus Exploits Epidermal Barrier Defects in Atopic Dermatitis to Trigger Cytokine Expression. J. Investig. Dermatol. 2016, 136, 2192–2200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woods, C.A. Overview of Atopic Dermatitis. Am. J. Manag. Care 2017, 23, 115–123. [Google Scholar]
- El-Zayat, S.R.; Sibaii, H.; Mannaa, F.A. Toll-Like Receptors Activation, Signaling, and Targeting: An Overview. Bull. Natl. Res. Cent. 2019, 43, 187. [Google Scholar] [CrossRef] [Green Version]
- Asahina, R.; Maeda, S. A Review of the Roles of Keratinocyte-Derived Cytokines and Chemokines in the Pathogenesis of Atopic Dermatitis in Humans and Dogs. Vet. Dermatol. 2017, 28, 15–25. [Google Scholar]
- Lee, H.; Lee, D.H.; Oh, J.H.; Chung, J.H. Skullcapflavone ii Suppresses tnf-α/ifn-γ-induced Tarc, mdc, and Ctss Production in Hacat Cells. Int. J. Mol. Sci. 2021, 22, 6428. [Google Scholar] [CrossRef]
- Townsley, H.; Crane, J.; Siebers, R. Effect of Haemolysis on the Determination of CCL17/Thymus and Activation-Regulated Chemokine (TARC) and CCL22/Macrophage-Derived Chemokine (MDC). Clin. Chem. Lab. Med. 2018, 56, 92–93. [Google Scholar] [CrossRef]
- Yeo, H.; Lee, Y.H.; Koh, D.; Lim, Y.; Shin, S.Y. Chrysin inhibits nf-κb-dependent ccl5 transcription by targeting iκb kinase in the atopic dermatitis-like inflammatory microenvironment. Int. J. Mol. Sci. 2020, 21, 7348. [Google Scholar] [CrossRef]
- Brown, R.; Nath, S.; Lora, A.; Samaha, G.; Elgamal, Z.; Kaiser, R.; Taggart, C.; Weldon, S.; Geraghty, P. Cathepsin S: Investigating an old player in lung disease pathogenesis, comorbidities, and potential therapeutics. Respir. Res. 2020, 21, 111. [Google Scholar] [CrossRef]
- Kumar, S.; Jeong, Y.; Ashraf, M.U.; Bae, Y.S. Dendritic Cell-Mediated th2 Immunity and Immune Disorders. Int. J. Mol. Sci. 2019, 20, 2159. [Google Scholar] [CrossRef] [Green Version]
- Park, J.W.; Kwon, O.K.; Yuniato, P.; Marwoto, B.; Lee, J.; Oh, S.R.; Kim, J.H.; Ahn, K.S. Amelioration of an LPS-Induced Inflammatory Response Using a Methanolic Extract of Lagerstroemia Ovalifolia to Suppress the Activation of NF-κB in RAW264.7 Macrophages. Int. J. Mol. Med. 2016, 38, 482–490. [Google Scholar] [CrossRef] [Green Version]
- Safdar, M.; Ozaslan, M.; Junejo, Y.; Channa, I.S. Cytotoxic and Anticancer Activity of a Novel Synthesized Tet-AuNPs Simultaneously Activates p53 and Inhibits NF-kB Signaling in SKBR3 Cell Line. Toxicol. Environ. Health Sci. 2022, 14, 69–76. [Google Scholar] [CrossRef]
- Gómez-Chávez, F.; Correa, D.; Navarrete-Meneses, P.; Cancino-Diaz, J.C.; Cancino-Diaz, M.E.; Rodríguez-Martínez, S. NF-κB and Its Regulators During Pregnancy. Front. Immunol. 2021, 12, 679106. [Google Scholar] [CrossRef]
- Yu, C.H.; Suh, B.; Shin, I.; Kim, E.H.; Kim, D.; Shin, Y.J.; Chang, S.Y.; Baek, S.H.; Kim, H.; Bae, O.N. Inhibitory Effects of a Novel Chrysin-Derivative, CPD 6, on Acute and Chronic Skin Inflammation. Int. J. Mol. Sci. 2019, 20, 2607. [Google Scholar] [CrossRef] [Green Version]
- Gil, T.Y.; Kang, Y.M.; Eom, Y.J.; Hong, C.H.; An, H.J. Anti-atopic Dermatitis Effect of Seaweed Fulvescens Extract via Inhibiting the STAT1 Pathway. Mediat. Inflamm. 2019, 2019, 3760934. [Google Scholar] [CrossRef] [Green Version]
- Ye, Q.; Luo, F.; Yan, T. Transcription Factor KLF4 Regulated STAT1 to Promote M1 Polarization of Macrophages in Rheumatoid Arthritis. Aging 2022, 14, 5669. [Google Scholar] [CrossRef]
- He, X.; Wang, C.; Wang, H.; Li, L.; Wang, C. The Function of MAPK Cascades in Response to Various Stresses in Horticultural Plants. Front. Plant Sci. 2020, 11, 952. [Google Scholar] [CrossRef]
- Ha, Y.; Lee, W.H.; Jeong, J.; Park, M.; Ko, J.Y.; Kwon, O.W.; Lee, J.; Kim, Y.J. Pyropia yezoensis Extract Suppresses ifn-gamma and tnf-Alpha-Induced Proinflammatory Chemokine Production in Hacat Cells via the Down-Regulation of NF-κB. Nutrients 2020, 12, 1238. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.J.; Pan, W.W.; Liu, S.B.; Shen, Z.F.; Xu, Y.; Hu, L.L. ERK/MAPK Signalling Pathway and Tumorigenesis. Exp. Ther. Med. 2020, 19, 1997–2007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furue, M. Regulation of Filaggrin, Loricrin, and Involucrin by IL-4, IL-13, IL-17A, IL-22, AHR, and NRF2: Pathogenic Implications in Atopic Dermatitis. Int. J. Mol. Sci. 2020, 21, 5382. [Google Scholar] [CrossRef]
- Kim, B.E.; Leung, D.Y.M. Significance of Skin Barrier Dysfunction in Atopic Dermatitis. Allergy Asthma Immunol. Res. 2018, 10, 207–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- How, K.N.; Yap, W.H.; Lim, C.L.H.; Goh, B.H.; Lai, Z.W. Hyaluronic Acid-Mediated Drug Delivery System Targeting for Inflammatory Skin Diseases: A Mini Review. Front. Pharmacol. 2020, 11, 1105. [Google Scholar] [CrossRef]
- Park, C.H.; Min, S.Y.; Yu, H.W.; Kim, K.; Kim, S.; Lee, H.J.; Kim, J.H.; Park, Y.J. Effects of Apigenin on rbl-2h3, raw264.7, and Hacat Cells: Anti-allergic, Anti-Inflammatory, and Skin-Protective Activities. Int. J. Mol. Sci. 2020, 21, 4620. [Google Scholar] [CrossRef]
- Bollag, W.B.; Aitkens, L.; White, J.; Hyndman, K.A. Aquaporin-3 in the Epidermis: More than Skin Deep. Am. J. Physiol. Cell Physiol. 2020, 318, C1144–C1153. [Google Scholar] [CrossRef]
- Li, B.; Ji, Y.; Yi, C.; Wang, X.; Liu, C.; Wang, C.; Lu, X.; Xu, X.; Wang, X. Rutin Inhibits Ox-LDL-Mediated Macrophage Inflammation and Foam Cell Formation by Inducing Autophagy and Modulating PI3K/ATK Signaling. Molecules 2022, 27, 4201. [Google Scholar] [CrossRef]
- Tian, C.; Liu, X.; Chang, Y.; Wang, R.; Yang, M.; Liu, M. Rutin prevents inflammation induced by lipopolysaccharide in RAW 264.7 cells via conquering the TLR4-MyD88-TRAF6-NF-κB signalling pathway. J. Pharm. Pharmacol. 2021, 73, 110–117. [Google Scholar] [CrossRef]
- Hwang, D.; Kang, M.J.; Kang, C.W.; Kim, G.D. Kaempferol-3-O-β-rutinoside suppresses the inflammatory responses in lipopolysaccharide-stimulated RAW264.7 cells via the NF-κB and MAPK pathways. Int. J. Mol. Med. 2019, 44, 2321–2328. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.-J.; Hwang, B.S.; Hwang, Y.; Jeong, Y.T.; Jeong, D.W.; Oh, Y.T. Anti-Inflammatory and Antiatopic Effects of Rorippa cantoniensis (Lour.) Ohwi in RAW 264.7 and HaCaT Cells. Molecules 2023, 28, 5463. https://doi.org/10.3390/molecules28145463
Kim M-J, Hwang BS, Hwang Y, Jeong YT, Jeong DW, Oh YT. Anti-Inflammatory and Antiatopic Effects of Rorippa cantoniensis (Lour.) Ohwi in RAW 264.7 and HaCaT Cells. Molecules. 2023; 28(14):5463. https://doi.org/10.3390/molecules28145463
Chicago/Turabian StyleKim, Min-Jin, Buyng Su Hwang, Yong Hwang, Yong Tae Jeong, Dae Won Jeong, and Young Taek Oh. 2023. "Anti-Inflammatory and Antiatopic Effects of Rorippa cantoniensis (Lour.) Ohwi in RAW 264.7 and HaCaT Cells" Molecules 28, no. 14: 5463. https://doi.org/10.3390/molecules28145463





