Bioinorganic Chemistry of Micronutrients Related to Alzheimer’s and Parkinson’s Diseases
Abstract
1. Introduction
2. Vitamins in Parkinson’s and Alzheimer’s Diseases
2.1. Parkinson’s Disease
| Vitamin | Type of Study | Intervention | Results | Ref. |
|---|---|---|---|---|
| B1 | Case-control | Plasma levels | ↓ B1 | [35] |
| B6, B9, B12 | Rotterdam | Dietary intake | ↑ B9, B12 ↔ PD risk; ↑ B6 ↓ PD risk | [36] |
| B2, B6, B9, B12 | Case-control | Dietary intake | ↑ B2, B9, B12 ↔ PD risk; ↓ B6 ↑ PD risk | [37] |
| B7 | In vivo | Drosophila | ↓ B7 | [38] |
| Rodents | ↓ Biotin carboxylase | |||
| In vitro | Neurons | ↓ B7 ↑ Mitochondrial stress | ||
| B12 | Clinical | Serum levels | ↑ B12 ↓ PD risk | [40] |
| B5 | Ex vivo | Human PD brains | ↓ B5 | [41] |
| B1 | Clinical | Intramuscular administration | ↑ B1 ↑ Motor and non-motor functions | [42] |
| B2 | Clinical | Oral administration | ↑ B2 ↑ Motor functions | [43] |
| B3 | In vivo | Drosophila | ↑ B3 ↑ Motor functions | [44] |
| In vitro | SK-N-MC neurons | ↑ B3 ↓ Cytotoxicity | ||
| B12 | In vivo | C. elegans | ↑ B12 ↓ ROS ↑ Motor functions | [45] |
| Rodents | ↑ B12 ↑ Motor functions; | |||
| In vitro | SH-SY5Y neurons | ↑ B12 ↓ Ox. stress ↓ Apoptosis | ||
| B3 | In vitro | RAW264.7 cells | ↑ B3 ↓ Neuroinflammation | [56] |
| D | Cross-sectional | Serum levels | ↓ D | [64] |
| D3 | In vivo | Rodents | ↑ D3 ↑ Motor and non-motor functions ↓ Ox. stress | [65] |
| K2 | Case-control | Serum levels | ↓ K2 | [72] |
| K2 | In vivo | Drosophila | ↑ K2 ↑ Survival rate | [73] |
| In vitro | Mitochondria | ↑ K2 ↑ ATP | ||
| K | In vitro | αSyn | ↑ K ↓ Fibrillization | [76] |
| A | In vivo | Rodents | ↑ A ↑Neuroprotection | [78] |
| In vitro | E14–15 neurons | ↑ A ↑ Viability | ||
| A | In vivo | Rodents | ↑ A ↔ Neuroprotection | [79] |
| C, E | In vivo | Drosophila | ↑ C, E ↓ Ox. stress | [82] |
| E | In vivo | Rodents | ↑ E ↑ Neuroprotection ↑ Motor functions | [83] |
| A, C, E | Clinical | Plasma levels | ↔ A, C, E | [84] |
| C | Clinical | Lymphocyte levels | ↓ C | [85] |
| A, C, E | Prospective cohort | Dietary intake | ↑ A, C, E ↔ PD risk | [86] |
2.2. Alzheimer’s Disease
| Vitamin | Type of Study | Intervention | Results | Ref. |
|---|---|---|---|---|
| B6, B9, B12 | Clinical | Serum levels | ↔ B6 ↓ B9, B12 | [88] |
| B9, B12 | Case-control | Plasma levels | ↓ B9, B12 | [89] |
| B6, B9, B12 | RCT * | Supplementation | ↑ B6, B9, B12 ↔ Cognitive func. | [37,92] |
| B9, B12 | Cross-sectional | Serum levels | ↑ B9, B12 ↓ AD risk | [94] |
| B1, B2, B9, B12, C, A | Cross-sectional | Plasma levels | ↓ B2, C, A ↔ B1, B9, B12 | [95] |
| B6, B9, B12 | In vivo | Rodents | ↑ B6, B9, B12 ↑ Cognitive func. | [97] |
| B12 | In vitro | Tau | ↑ B12 ↓ Fibrillization | [99] |
| B2 | In vivo | Rodents | ↑ B2 ↑ Cognitive func. ↓ Ox. stress | [101] |
| B3 | Prosp. cohort | Dietary intake | ↑ B3 ↑ Cognitive func. | [107] |
| B3 | Clinical | Supplementation | ↑ B3 ↔ Cognitive func. | [108] |
| B3 | In vivo | Rodents | ↑ B3 ↑ Cognitive func. ↑ Microtubule stability | [109] |
| B3 | In vivo | Rodents | ↑ B3 ↓ Ox. stress | [111] |
| B7 | In vivo | Drosophila | ↓ B7 | [38] |
| Rodents | ↓ Biotin carboxylase | |||
| Ex vivo | Human AD brains | ↓ Biotin carboxylase | ||
| In vitro | Neurons | ↓ B7 ↑ Mitochondrial stress | ||
| B7 | In vivo | Rodents | ↑ B7 ↓ Neuroinflammation | [112] |
| B5 | Ex vivo | Human AD brains | ↓ B5 | [113] |
| C, E | Clinical | Supplementation | ↑ C, E ↓ AD risk | [115] |
| E | Clinical | CSF, Plasma levels | ↓ E | [117,118] |
| C, E | Prosp. cohort | Dietary intake | ↑ C, E ↔ AD risk | [119] |
| C | In vivo | Rodents | ↑ C ↑ Cognitive func. ↓ Ox. stress ↓ Aβ oligomerization | [120] |
| C, E | Clinical | Supplementation | ↑ C, E ↔ AD | [122] |
| E | Clinical | Supplementation | ↑ E ↓ Functional decline | [125] |
| E | In vitro | E18 neurons | ↑ E ↓ Ox. stress | [127] |
| A, E | Case-control | Serum levels | ↓ A, E | [130] |
| A | In vitro | Several cell lines | ↑ A ↓ γ-secretase | [132] |
| A | In vivo | Rodents | ↑ A ↓ AChE | [133] |
| In silico | Molecular docking | A—A ChE | [134] | |
| D | Rotterdam | Serum levels | ↓ D ↑ AD risk | [135] |
| D3 | Clinical | Supplementation | ↑ D3 ↑ Neuroprotection | [136] |
| D | Clinical | Dietary intake | ↑ D ↓ AD risk | [137] |
| D3 | In vivo | Rodents | ↑ D3 ↓ Ox.stress ↑ Cholinergic | [138] |
| K2 | In vivo | Drosophila | ↑ K2 ↑ Neuroprotection | [142] |
| K2 | In vitro | PC12 | ↑ K2 ↓ Aβ42 cytotoxicity | [143] |
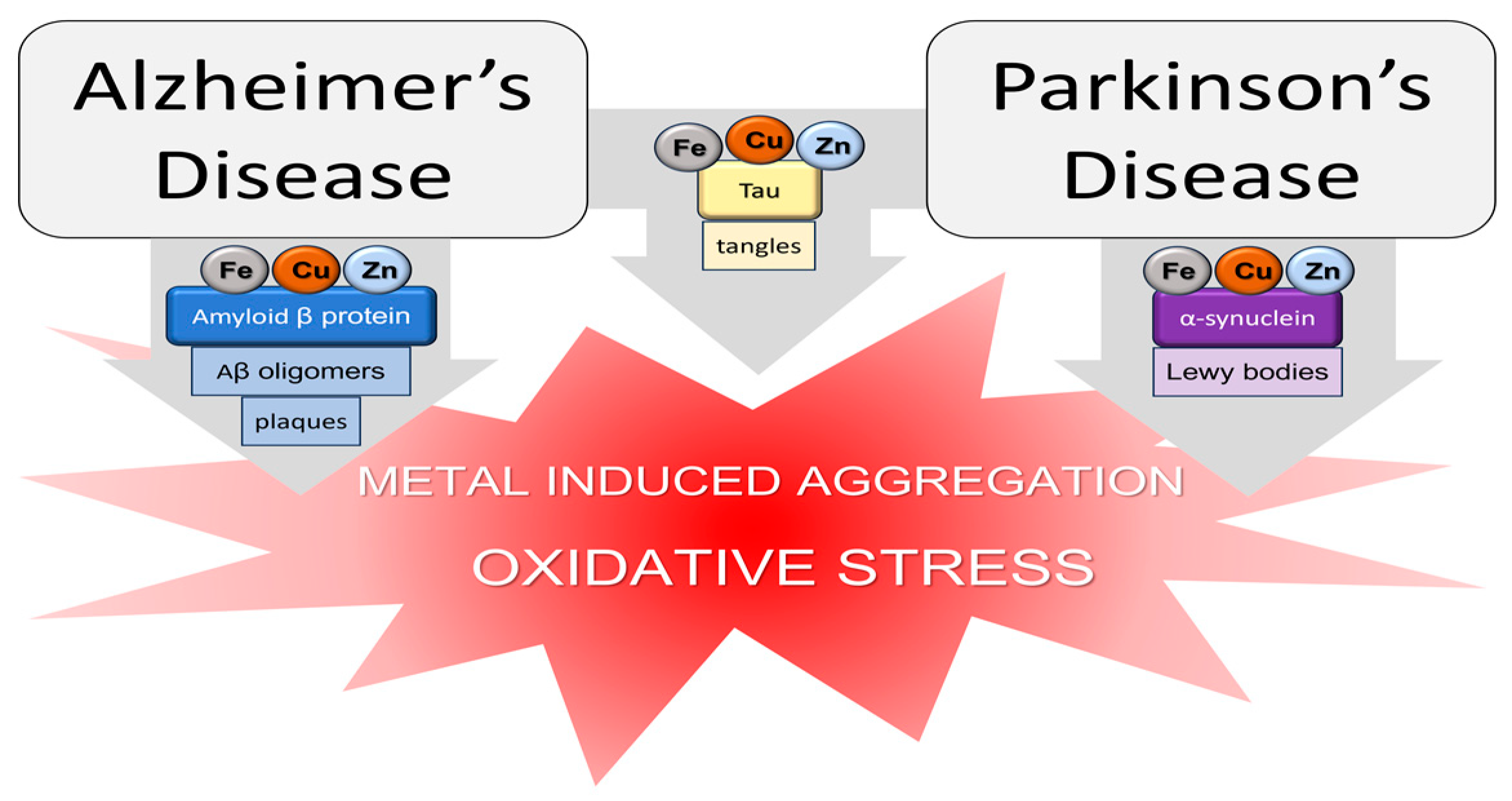
3. Metals in Parkinson’s and Alzheimer’s Diseases
3.1. Zinc, Copper, and Iron
3.2. Manganese, Nickel, and Aluminum
4. Interaction between Vitamins and Metal Ions
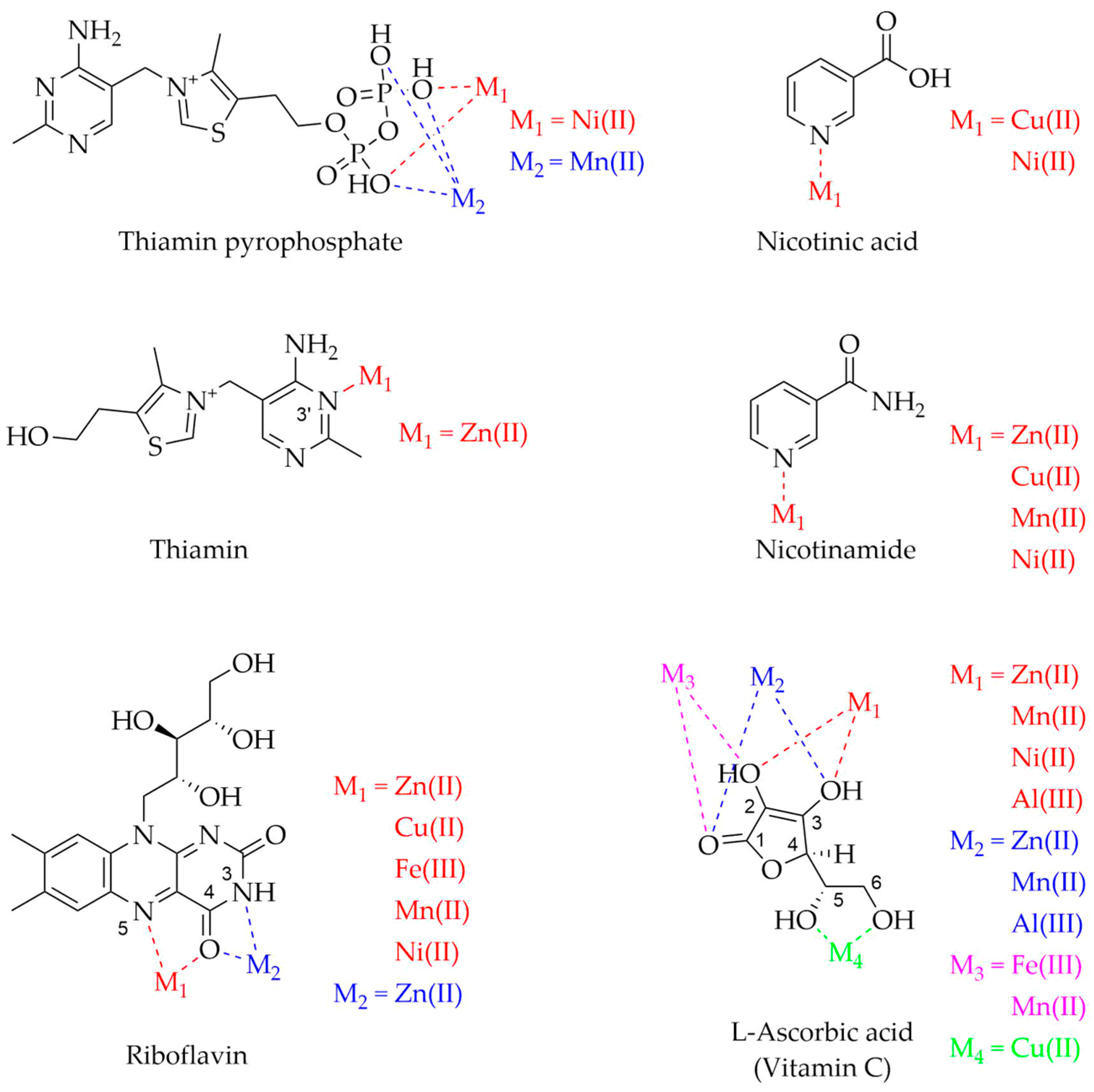

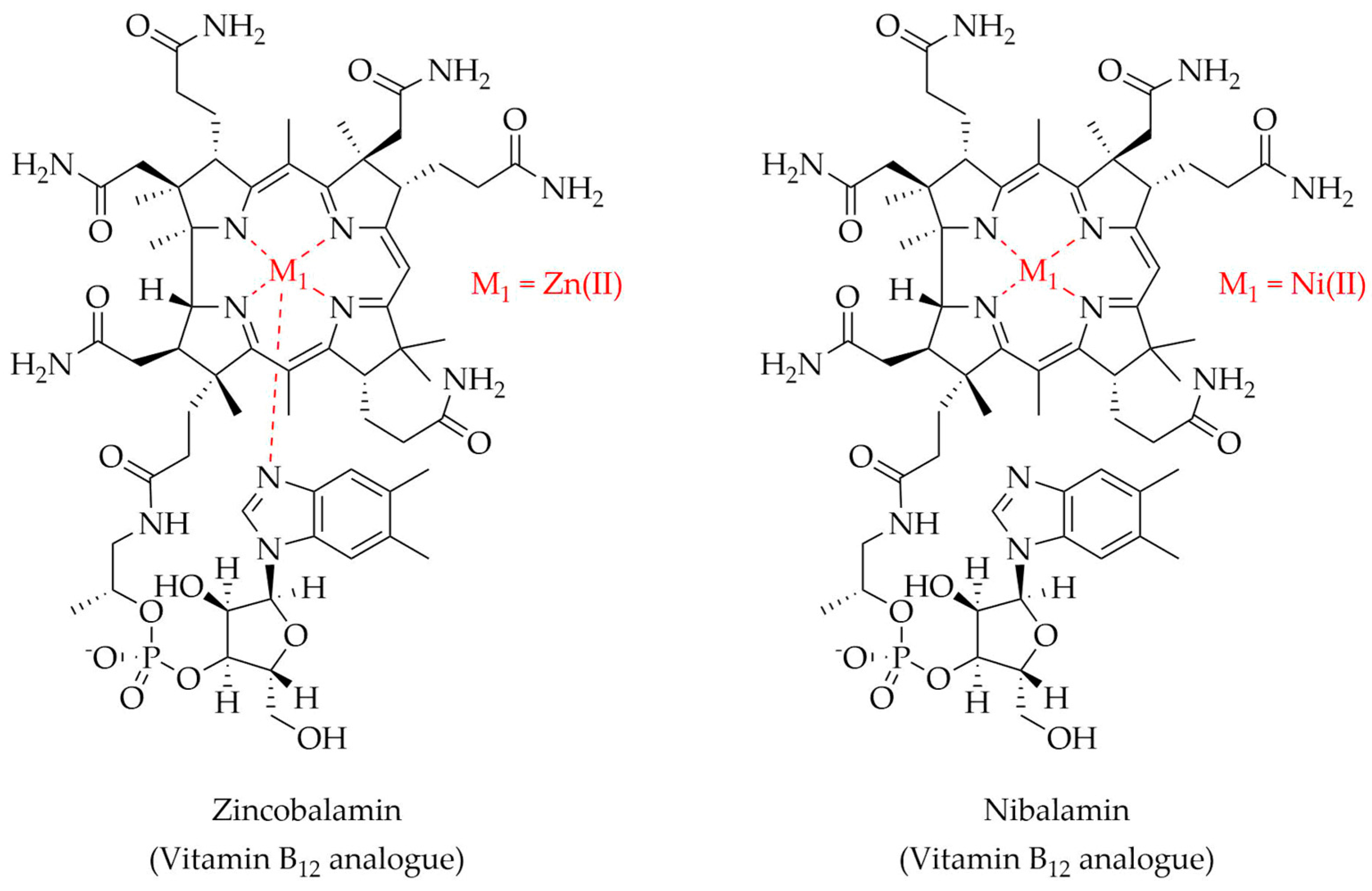
| Vitamin | Metal Ions | Experimental Techniques | pH | References |
|---|---|---|---|---|
| Thiamin | Zn(II) | NMR, IR | - | [245] |
| Mn(II) | NMR, EPR | 6.6 | [244] | |
| Ni(II) | NMR | 6.9 | [242,243] | |
| Riboflavin | Zn(II) | PT | - | [246] |
| FT-IR, LC-MS, AS | - | [247] | ||
| Cu(II) | PT | - | [246] | |
| Fe(III) | PT | - | [246] | |
| Mn(II) | PT | - | [246] | |
| Ni(II) | PT | - | [246] | |
| Niacin | Zn(II) | UV-Vis, FT-IR, TGA, CV, MSM, MPD | - | [250] |
| Cu(II) | PT, SP, UV-Vis | 5.0 | [248] | |
| UV-Vis, FT-IR, TGA, CV, MSM, MPD | - | [250] | ||
| Mn(II) | UV-Vis, FT-IR, TGA, CV, MSM, MPD | - | [250] | |
| PT, TPD | 4.0 | [251] | ||
| Ni(II) | PT, SP, UV-Vis | 5.0 | [248] | |
| UV-Vis, FT-IR, TGA, CV, MSM, MPD | - | [250] | ||
| Vitamin B6 | Zn(II) | X-ray | - | [259] |
| X-ray, DTA, FT-IR | - | [260] | ||
| PT, SP | 2.4–7.4 a | [261] | ||
| Cu(II) | PT, SP | 3.8–8.8 a | [261] | |
| Mn(II) | NMR | 6.2, 7.0 | [257] | |
| Ni(II) | UV-Vis, MCV, TPD | - | [258] | |
| PT, SP | 3.8–8.4 a | [261] | ||
| Biotin | Zn(II) | PT, NMR | 3.5–8.5 b | [262] |
| NMR, UV-Vis | 2.0 | [263] | ||
| Cu(II) | PT, NMR | 3.5–8.5 b | [262] | |
| NMR, UV-Vis | 2.0 | [263] | ||
| Mn(II) | PT, NMR | 3.5–8.5 b | [262] | |
| NMR, UV-Vis | 2.0 | [263] | ||
| Folate | Cu(II) | PT, conductometry | >4.0 | [264] |
| EA, AA, polarimetry, FT-IR, DAEB | 7.6–7.8 | [265] | ||
| Fe(III) | PT, conductometry | >4.0 | [264] | |
| EA, AA, polarimetry, FT-IR, DAEB | 7.6–7.8 | [265] | ||
| Mn(II) | PT, conductometry | >4.0 | [264] | |
| Ni(II) | PT, conductometry | >4.0 | [264] | |
| Al(III) | PT, conductometry | >4.0 | [264] | |
| Vitamin B12 | Zn(II) | NMR, UV-Vis, CD, F, MS, HPLC-DAD, X-ray, DFTC, SC | 6.0 | [267] |
| Ni(II) | NMR, UV-Vis, CD, F, MS, HPLC-DAD, X-ray, DFTC, SC | 6.0 | [268] | |
| Vitamin C | Zn(II) | NMR, FT-IR | 6.0–7.0 | [252] |
| Cu(II) | NMR, MS, IR, TGA, EA, SDCu, MSM | - | [256] | |
| Fe(III) | EA, MMD, UV-Vis, IR, AS | 8.0 | [255] | |
| Mn(II) | NMR, FT-IR | 6.0–7.0 | [252] | |
| EA, MMD, UV-Vis, IR, AS | 8.0 | [255] | ||
| Ni(II) | PT, NMR, DFTC | >4.0 | [253] | |
| Al(III) | NMR, FT-IR | 6.0–7.0 | [254] | |
| PT, NMR, DFTC | >4.0 | [253] | ||
| Vitamin D | Zn(II) | PT, SP | >7.0 | [269] |
| Cu(II) | PT, SP | >2.0 | [266] | |
| Fe(II) | PT, SP | >7.0 | [269] | |
| Fe(III) | PT, SP | >2.0 | [269] | |
| Mn(II) | PT, SP | >8.5 | [269] | |
| Ni(II) | PT, SP | >8.0 | [266] |
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Z.; Pierson, R.; Heymsfield, S. The Five-Level Model: A New Approach to Organizing Body-Composition Research. Am. J. Clin. Nutr. 1992, 56, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Andrews, N.C. Disorders of Iron Metabolism. N. Engl. J. Med. 1999, 341, 1986–1995. [Google Scholar] [CrossRef] [PubMed]
- Moustakas, M. The Role of Metal Ions in Biology, Biochemistry and Medicine. Materials 2021, 14, 549. [Google Scholar] [CrossRef] [PubMed]
- Farina, M.; Avila, D.S.; da Rocha, J.B.T.; Aschner, M. Metals, Oxidative Stress and Neurodegeneration: A Focus on Iron, Manganese and Mercury. Neurochem. Int. 2013, 62, 575–594. [Google Scholar] [CrossRef]
- Slobodian, M.R.; Petahtegoose, J.D.; Wallis, A.L.; Levesque, D.C.; Merritt, T.J.S. The Effects of Essential and Non-Essential Metal Toxicity in the Drosophila Melanogaster Insect Model: A Review. Toxics 2021, 9, 269. [Google Scholar] [CrossRef]
- Lachowicz, J.I.; Lecca, L.I.; Meloni, F.; Campagna, M. Metals and Metal-Nanoparticles in Human Pathologies: From Exposure to Therapy. Molecules 2021, 26, 6639. [Google Scholar] [CrossRef]
- Chen, P.; Miah, M.R.; Aschner, M. Metals and Neurodegeneration. F1000Research 2016, 5, 366. [Google Scholar] [CrossRef]
- Cicero, C.E.; Mostile, G.; Vasta, R.; Rapisarda, V.; Signorelli, S.S.; Ferrante, M.; Zappia, M.; Nicoletti, A. Metals and Neurodegenerative Diseases. A Systematic Review. Environ. Res. 2017, 159, 82–94. [Google Scholar] [CrossRef]
- Shah, H.; Dehghani, F.; Ramezan, M.; Gannaban, R.B.; Haque, Z.F.; Rahimi, F.; Abbasi, S.; Shin, A.C. Revisiting the Role of Vitamins and Minerals in Alzheimer’s Disease. Antioxidants 2023, 12, 415. [Google Scholar] [CrossRef]
- Yamauchi, O.; Odani, A.; Takani, M. Metal–Amino Acid Chemistry. Weak Interactions and Related Functions of Side Chain Groups. J. Chem. Soc. Dalton Trans. 2002, 34, 3411–3421. [Google Scholar] [CrossRef]
- Liu, X.; Wu, M.; Li, C.; Yu, P.; Feng, S.; Li, Y.; Zhang, Q. Interaction Structure and Affinity of Zwitterionic Amino Acids with Important Metal Cations (Cd2+, Cu2+, Fe3+, Hg2+, Mn2+, Ni2+ and Zn2+) in Aqueous Solution: A Theoretical Study. Molecules 2022, 27, 2407. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Chen, S.; Qiao, F.; Zhang, X. Interaction of Peptide Backbones and Transition Metal Ions: 1. an IM-MS and DFT Study of the Binding Pattern, Structure and Fragmentation of Pd(II)/Ni(II)-Polyalanine Complexes. Int. J. Mass. Spectrom. 2019, 438, 87–96. [Google Scholar] [CrossRef]
- Di Natale, C.; De Benedictis, I.; De Benedictis, A.; Marasco, D. Metal–Peptide Complexes as Promising Antibiotics to Fight Emerging Drug Resistance: New Perspectives in Tuberculosis. Antibiotics 2020, 9, 337. [Google Scholar] [CrossRef] [PubMed]
- Witkowska, D.; Rowińska-Żyrek, M. Biophysical Approaches for the Study of Metal-Protein Interactions. J. Inorg. Biochem. 2019, 199, 110783. [Google Scholar] [CrossRef]
- Guo, C.; Cheng, M.; Gross, M.L. Protein-Metal-Ion Interactions Studied by Mass Spectrometry-Based Footprinting with Isotope-Encoded Benzhydrazide. Anal. Chem. 2019, 91, 1416–1423. [Google Scholar] [CrossRef]
- Wu, G. Amino Acids: Metabolism, Functions, and Nutrition. Amino Acids 2009, 37, 1–17. [Google Scholar] [CrossRef]
- Morris, R.; Black, K.A.; Stollar, E.J. Uncovering Protein Function: From Classification to Complexes. Essays Biochem. 2022, 66, 255–285. [Google Scholar] [CrossRef]
- Potocki, S.; Rowinska-Zyrek, M.; Witkowska, D.; Pyrkosz, M.; Szebesczyk, A.; Krzywoszynska, K.; Kozlowski, H. Metal Transport and Homeostasis within the Human Body: Toxicity Associated with Transport Abnormalities. Curr. Med. Chem. 2012, 19, 2738–2759. [Google Scholar] [CrossRef]
- Combs, G.F.; McClung, J.P. Chapter 1—What Is a Vitamin? In The Vitamins, 5th ed.; Combs, G.F., McClung, J.P., Eds.; Academic Press: Amsterdam, NL, USA; Boston, MA, USA, 2017; pp. 3–6. ISBN 978-0-12-802965-7. [Google Scholar]
- Fukuwatari, T.; Shibata, K. Nutritional Aspect of Tryptophan Metabolism. Int. J. Tryptophan Res. 2013, 6, 3–8. [Google Scholar] [CrossRef]
- Wacker, M.; Holick, M.F. Sunlight and Vitamin D: A Global Perspective for Health. Dermatoendocrinol 2013, 5, 51–108. [Google Scholar] [CrossRef]
- Combs, G.F.; McClung, J.P. Chapter 3—General Properties of Vitamins. In The Vitamins, 5th ed.; Combs, G.F., McClung, J.P., Eds.; Academic Press: Amsterdam, NL, USA; Boston, MA, USA, 2017; pp. 33–58. ISBN 978-0-12-802965-7. [Google Scholar]
- Rai, S.N.; Singh, P.; Steinbusch, H.W.M.; Vamanu, E.; Ashraf, G.; Singh, M.P. The Role of Vitamins in Neurodegenerative Disease: An Update. Biomedicines 2021, 9, 1284. [Google Scholar] [CrossRef] [PubMed]
- Przedborski, S.; Vila, M.; Jackson-Lewis, V. Series Introduction: Neurodegeneration: What Is It and Where Are We? J. Clin. Investig. 2003, 111, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.M.; Cookson, M.R.; Van Den Bosch, L.; Zetterberg, H.; Holtzman, D.M.; Dewachter, I. Hallmarks of Neurodegenerative Diseases. Cell 2023, 186, 693–714. [Google Scholar] [CrossRef] [PubMed]
- Forrest, S.L.; Kovacs, G.G. Current Concepts of Mixed Pathologies in Neurodegenerative Diseases. Can. J. Neurol. Sci. 2023, 50, 329–345. [Google Scholar] [CrossRef] [PubMed]
- Lamptey, R.N.L.; Chaulagain, B.; Trivedi, R.; Gothwal, A.; Layek, B.; Singh, J. A Review of the Common Neurodegenerative Disorders: Current Therapeutic Approaches and the Potential Role of Nanotherapeutics. Int. J. Mol. Sci. 2022, 23, 1851. [Google Scholar] [CrossRef] [PubMed]
- Wyss-Coray, T. Ageing, Neurodegeneration and Brain Rejuvenation. Nature 2016, 539, 180–186. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef]
- Hou, Y.; Dan, X.; Babbar, M.; Wei, Y.; Hasselbalch, S.G.; Croteau, D.L.; Bohr, V.A. Ageing as a Risk Factor for Neurodegenerative Disease. Nat. Rev. Neurol. 2019, 15, 565–581. [Google Scholar] [CrossRef]
- Sandeep; Sahu, M.R.; Rani, L.; Kharat, A.S.; Mondal, A.C. Could Vitamins Have a Positive Impact on the Treatment of Parkinson’s Disease? Brain Sci. 2023, 13, 272. [Google Scholar] [CrossRef]
- Kumar, R.R.; Singh, L.; Thakur, A.; Singh, S.; Kumar, B. Role of Vitamins in Neurodegenerative Diseases: A Review. CNS Neurol. Disord. Drug Targets 2022, 21, 766–773. [Google Scholar] [CrossRef]
- Modica, J.S.; Déry, C.; Canissario, R.; Logigian, E.; Bonno, D.; Stanton, M.; Dupré, N.; McDermott, M.P.; Bouchard, M.; Lang, A.E.; et al. A Systematic Review of the Potential Consequences of Abnormal Serum Levels of Vitamin B6 in People Living with Parkinson’s Disease. J. Neurol. Sci. 2023, 450, 120690. [Google Scholar] [CrossRef] [PubMed]
- Håglin, L.; Johansson, I.; Forsgren, L.; Bäckman, L. Intake of Vitamin B before Onset of Parkinson’s Disease and Atypical Parkinsonism and Olfactory Function at the Time of Diagnosis. Eur. J. Clin. Nutr. 2017, 71, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Håglin, L.; Domellöf, M.; Bäckman, L.; Forsgren, L. Low Plasma Thiamine and Phosphate in Male Patients with Parkinson’s Disease Is Associated with Mild Cognitive Impairment. Clin. Nutr. ESPEN 2020, 37, 93–99. [Google Scholar] [CrossRef] [PubMed]
- De Lau, L.M.L.; Koudstaal, P.J.; Witteman, J.C.M.; Hofman, A.; Breteler, M.M.B. Dietary Folate, Vitamin B12, and Vitamin B6 and the Risk of Parkinson Disease. Neurology 2006, 67, 315–318. [Google Scholar] [CrossRef]
- Murakami, K.; Miyake, Y.; Sasaki, S.; Tanaka, K.; Fukushima, W.; Kiyohara, C.; Tsuboi, Y.; Yamada, T.; Oeda, T.; Miki, T.; et al. Dietary Intake of Folate, Vitamin B6, Vitamin B12 and Riboflavin and Risk of Parkinson’s Disease: A Case–Control Study in Japan. Br. J. Nutr. 2010, 104, 757–764. [Google Scholar] [CrossRef]
- Lohr, K.M.; Frost, B.; Scherzer, C.; Feany, M.B. Biotin Rescues Mitochondrial Dysfunction and Neurotoxicity in a Tauopathy Model. Proc. Natl. Acad. Sci. USA 2020, 117, 33608–33618. [Google Scholar] [CrossRef]
- Shen, L. Associations between B Vitamins and Parkinson’s Disease. Nutrients 2015, 7, 7197–7208. [Google Scholar] [CrossRef]
- McCarter, S.J.; Stang, C.; Turcano, P.; Mielke, M.M.; Ali, F.; Bower, J.H.; Savica, R. Higher Vitamin B12 Level at Parkinson’s Disease Diagnosis Is Associated with Lower Risk of Future Dementia. Park. Relat. Disord. 2020, 73, 19–22. [Google Scholar] [CrossRef]
- Scholefield, M.; Church, S.J.; Xu, J.; Patassini, S.; Hooper, N.M.; Unwin, R.D.; Cooper, G.J.S. Substantively Lowered Levels of Pantothenic Acid (Vitamin B5) in Several Regions of the Human Brain in Parkinson’s Disease Dementia. Metabolites 2021, 11, 569. [Google Scholar] [CrossRef]
- Costantini, A.; Fancellu, R. An Open-Label Pilot Study with High-Dose Thiamine in Parkinson’s Disease. Neural Regen. Res. 2016, 11, 406. [Google Scholar] [CrossRef]
- Coimbra, C.G.; Junqueira, V.B.C. High Doses of Riboflavin and the Elimination of Dietary Red Meat Promote the Recovery of Some Motor Functions in Parkinson’s Disease Patients. Braz. J. Med. Biol. Res. 2003, 36, 1409–1417. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Li, X.; Gao, H.; Feng, Z.; Li, X.; Zhao, L.; Jia, X.; Zhang, H.; Liu, J. High Doses of Nicotinamide Prevent Oxidative Mitochondrial Dysfunction in a Cellular Model and Improve Motor Deficit in a Drosophila Model of Parkinson’s Disease. J. Neurosci. Res. 2008, 86, 2083–2090. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhao, Z.; Yang, N.; Xin, C.; Li, Z.; Xu, J.; Ma, B.; Lim, K.-L.; Li, L.; Wu, Q.; et al. Vitamin B12 Ameliorates the Pathological Phenotypes of Multiple Parkinson’s Disease Models by Alleviating Oxidative Stress. Antioxidants 2023, 12, 153. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Matsumine, H.; Matuda, S.; Mizuno, Y. Association between the Gene Encoding the E2 Subunit of the Alpha-Ketoglutarate Dehydrogenase Complex and Parkinson’s Disease. Ann. Neurol. 1998, 43, 120–123. [Google Scholar] [CrossRef]
- Naseri, N.N.; Xu, H.; Bonica, J.; Vonsattel, J.P.G.; Cortes, E.P.; Park, L.C.; Arjomand, J.; Gibson, G.E. Abnormalities in the Tricarboxylic Acid Cycle in Huntington Disease and in a Huntington Disease Mouse Model. J. Neuropathol. Exp. Neurol. 2015, 74, 527–537. [Google Scholar] [CrossRef]
- Tretter, L.; Adam-Vizi, V. Alpha-Ketoglutarate Dehydrogenase: A Target and Generator of Oxidative Stress. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2005, 360, 2335–2345. [Google Scholar] [CrossRef]
- Hansen, G.E.; Gibson, G.E. The α-Ketoglutarate Dehydrogenase Complex as a Hub of Plasticity in Neurodegeneration and Regeneration. Int. J. Mol. Sci. 2022, 23, 12403. [Google Scholar] [CrossRef]
- Mooney, S.; Leuendorf, J.-E.; Hendrickson, C.; Hellmann, H. Vitamin B6: A Long Known Compound of Surprising Complexity. Molecules 2009, 14, 329–351. [Google Scholar] [CrossRef]
- Sato, K. Why Is Vitamin B6 Effective in Alleviating the Symptoms of Autism? Med. Hypotheses 2018, 115, 103–106. [Google Scholar] [CrossRef]
- Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Elewa, Y.H.A.; Zahran, M.H.; Alexiou, A.; Papadakis, M.; Batiha, G.E.-S. Parkinson’s Disease Risk and Hyperhomocysteinemia: The Possible Link. Cell. Mol. Neurobiol. 2023, 43, 2743–2759. [Google Scholar] [CrossRef]
- Price, B.R.; Wilcock, D.M.; Weekman, E.M. Hyperhomocysteinemia as a Risk Factor for Vascular Contributions to Cognitive Impairment and Dementia. Front. Aging Neurosci. 2018, 10, 350. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Zhang, L.; Li, H.; Chen, G.; Qi, G.; Ma, X.; Jin, Y. Role of Homocysteine in the Development and Progression of Parkinson’s Disease. Ann. Clin. Transl. Neurol. 2020, 7, 2332–2338. [Google Scholar] [CrossRef]
- Luzzi, S.; Cherubini, V.; Falsetti, L.; Viticchi, G.; Silvestrini, M.; Toraldo, A. Homocysteine, Cognitive Functions, and Degenerative Dementias: State of the Art. Biomedicines 2022, 10, 2741. [Google Scholar] [CrossRef]
- Giri, B.; Belanger, K.; Seamon, M.; Bradley, E.; Purohit, S.; Chong, R.; Morgan, J.C.; Baban, B.; Wakade, C. Niacin Ameliorates Neuro-Inflammation in Parkinson’s Disease via GPR109A. Int. J. Mol. Sci. 2019, 20, 4559. [Google Scholar] [CrossRef]
- Wakade, C.; Chong, R.; Bradley, E.; Thomas, B.; Morgan, J. Upregulation of GPR109A in Parkinson’s Disease. PLoS ONE 2014, 9, e109818. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, Y.; Meng, X. Vitamin D and Neurodegenerative Diseases. Heliyon 2023, 9, e12877. [Google Scholar] [CrossRef] [PubMed]
- Palermo, S.; Stanziano, M.; Nigri, A.; Civilotti, C.; Celeghin, A. Parkinson’s Disease, SARS-CoV-2, and Frailty: Is There a Vicious Cycle Related to Hypovitaminosis D? Brain Sci. 2023, 13, 528. [Google Scholar] [CrossRef]
- Lasoń, W.; Jantas, D.; Leśkiewicz, M.; Regulska, M.; Basta-Kaim, A. The Vitamin D Receptor as a Potential Target for the Treatment of Age-Related Neurodegenerative Diseases Such as Alzheimer’s and Parkinson’s Diseases: A Narrative Review. Cells 2023, 12, 660. [Google Scholar] [CrossRef]
- Behl, T.; Arora, A.; Singla, R.K.; Sehgal, A.; Makeen, H.A.; Albratty, M.; Meraya, A.M.; Najmi, A.; Bungau, S.G. Understanding the Role of “Sunshine Vitamin D” in Parkinson’s Disease: A Review. Front. Pharmacol. 2022, 13, 993033. [Google Scholar] [CrossRef]
- Barichella, M.; Garrì, F.; Caronni, S.; Bolliri, C.; Zocchi, L.; Macchione, M.C.; Ferri, V.; Calandrella, D.; Pezzoli, G. Vitamin D Status and Parkinson’s Disease. Brain Sci. 2022, 12, 790. [Google Scholar] [CrossRef]
- Lv, L.; Tan, X.; Peng, X.; Bai, R.; Xiao, Q.; Zou, T.; Tan, J.; Zhang, H.; Wang, C. The Relationships of Vitamin D, Vitamin D Receptor Gene Polymorphisms, and Vitamin D Supplementation with Parkinson’s Disease. Transl. Neurodegener. 2020, 9, 34. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-J.; Zhang, J.-R.; Mao, C.-J.; Li, K.; Wang, F.; Chen, J.; Liu, C.-F. Relationship between 25-Hydroxyvitamin D, Bone Density, and Parkinson’s Disease Symptoms. Acta Neurol. Scand. 2019, 140, 274–280. [Google Scholar] [CrossRef]
- Bayo-Olugbami, A.; Nafiu, A.B.; Amin, A.; Ogundele, O.M.; Lee, C.C.; Owoyele, B.V. Vitamin D Attenuated 6-OHDA-Induced Behavioural Deficits, Dopamine Dysmetabolism, Oxidative Stress, and Neuro-Inflammation in Mice. Nutr. Neurosci. 2022, 25, 823–834. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Albornoz, M.C.; García-Guáqueta, D.P.; Velez-van-Meerbeke, A.; Talero-Gutiérrez, C. Maternal Nutrition and Neurodevelopment: A Scoping Review. Nutrients 2021, 13, 3530. [Google Scholar] [CrossRef] [PubMed]
- Anjum, I.; Jaffery, S.S.; Fayyaz, M.; Samoo, Z.; Anjum, S. The Role of Vitamin D in Brain Health: A Mini Literature Review. Cureus 2018, 10, e2960. [Google Scholar] [CrossRef]
- Gezen-Ak, D.; Dursun, E.; Yilmazer, S. The Effect of Vitamin D Treatment On Nerve Growth Factor (NGF) Release From Hippocampal Neurons. Noro Psikiyatr. Ars. 2014, 51, 157–162. [Google Scholar] [CrossRef]
- Pertile, R.A.N.; Brigden, R.; Raman, V.; Cui, X.; Du, Z.; Eyles, D. Vitamin D: A Potent Regulator of Dopaminergic Neuron Differentiation and Function. J. Neurochem. 2023. online ahead of print. [Google Scholar] [CrossRef]
- Emekli-Alturfan, E.; Alturfan, A.A. The Emerging Relationship between Vitamin K and Neurodegenerative Diseases: A Review of Current Evidence. Mol. Biol. Rep. 2023, 50, 815–828. [Google Scholar] [CrossRef]
- Tamadon-Nejad, S.; Ouliass, B.; Rochford, J.; Ferland, G. Vitamin K Deficiency Induced by Warfarin Is Associated With Cognitive and Behavioral Perturbations, and Alterations in Brain Sphingolipids in Rats. Front. Aging Neurosci. 2018, 10, 213. [Google Scholar] [CrossRef]
- Yu, Y.-X.; Yu, X.-D.; Cheng, Q.; Tang, L.; Shen, M.-Q. The Association of Serum Vitamin K2 Levels with Parkinson’s Disease: From Basic Case-Control Study to Big Data Mining Analysis. Aging 2020, 12, 16410–16419. [Google Scholar] [CrossRef]
- Vos, M.; Esposito, G.; Edirisinghe, J.N.; Vilain, S.; Haddad, D.M.; Slabbaert, J.R.; Van Meensel, S.; Schaap, O.; De Strooper, B.; Meganathan, R.; et al. Vitamin K2 Is a Mitochondrial Electron Carrier That Rescues Pink1 Deficiency. Science 2012, 336, 1306–1310. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Zheng, Z.; Wang, H.; Wang, L.; Zhao, G.; Wang, P. Vitamin K2 Modulates Mitochondrial Dysfunction Induced by 6-Hydroxydopamine in SH-SY5Y Cells via Mitochondrial Quality-Control Loop. Nutrients 2022, 14, 1504. [Google Scholar] [CrossRef] [PubMed]
- Ferland, G. Vitamin K, an Emerging Nutrient in Brain Function. BioFactors 2012, 38, 151–157. [Google Scholar] [CrossRef] [PubMed]
- da Silva, F.L.; Coelho Cerqueira, E.; de Freitas, M.S.; Gonçalves, D.L.; Costa, L.T.; Follmer, C. Vitamins K Interact with N-Terminus α-Synuclein and Modulate the Protein Fibrillization in Vitro. Exploring the Interaction between Quinones and α-Synuclein. Neurochem. Int. 2013, 62, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Yamada, M. Vitamin A Potently Destabilizes Preformed α-Synuclein Fibrils in Vitro: Implications for Lewy Body Diseases. Neurobiol. Dis. 2007, 25, 446–454. [Google Scholar] [CrossRef]
- Yin, L.-H.; Shen, H.; Diaz-Ruiz, O.; Bäckman, C.M.; Bae, E.; Yu, S.-J.; Wang, Y. Early Post-Treatment with 9-Cis Retinoic Acid Reduces Neurodegeneration of Dopaminergic Neurons in a Rat Model of Parkinson’s Disease. BMC Neurosci. 2012, 13, 120. [Google Scholar] [CrossRef]
- Kunzler, A.; Ribeiro, C.T.; Gasparotto, J.; Petiz, L.L.; da Rosa Silva, H.T.; da Silva, J.D.; Bortolin, R.; de Souza, P.O.; Barreto, F.; Espitia-Perez, P.; et al. The Effects of Retinol Oral Supplementation in 6-Hydroxydopamine Dopaminergic Denervation Model in Wistar Rats. Neurochem. Int. 2019, 125, 25–34. [Google Scholar] [CrossRef]
- Rusu, M.E.; Fizesan, I.; Pop, A.; Mocan, A.; Gheldiu, A.-M.; Babota, M.; Vodnar, D.C.; Jurj, A.; Berindan-Neagoe, I.; Vlase, L.; et al. Walnut (Juglans Regia L.) Septum: Assessment of Bioactive Molecules and In Vitro Biological Effects. Molecules 2020, 25, 2187. [Google Scholar] [CrossRef]
- Lloret, A.; Esteve, D.; Monllor, P.; Cervera-Ferri, A.; Lloret, A. The Effectiveness of Vitamin E Treatment in Alzheimer’s Disease. Int. J. Mol. Sci. 2019, 20, 879. [Google Scholar] [CrossRef]
- Casani, S.; Gómez-Pastor, R.; Matallana, E.; Paricio, N. Antioxidant Compound Supplementation Prevents Oxidative Damage in a Drosophila Model of Parkinson’s Disease. Free Radic. Biol. Med. 2013, 61, 151–160. [Google Scholar] [CrossRef]
- Sharma, N.; Nehru, B. Beneficial Effect of Vitamin E in Rotenone Induced Model of PD: Behavioural, Neurochemical and Biochemical Study. Exp. Neurobiol. 2013, 22, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Paraskevas, G.P.; Kapaki, E.; Petropoulou, O.; Anagnostouli, M.; Vagenas, V.; Papageorgiou, C. Plasma Levels of Antioxidant Vitamins C and E Are Decreased in Vascular Parkinsonism. J. Neurol. Sci. 2003, 215, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Ide, K.; Yamada, H.; Umegaki, K.; Mizuno, K.; Kawakami, N.; Hagiwara, Y.; Matsumoto, M.; Yoshida, H.; Kim, K.; Shiosaki, E.; et al. Lymphocyte Vitamin C Levels as Potential Biomarker for Progression of Parkinson’s Disease. Nutrition 2015, 31, 406–408. [Google Scholar] [CrossRef]
- Ying, A.F.; Khan, S.; Wu, Y.; Jin, A.; Wong, A.S.Y.; Tan, E.-K.; Yuan, J.-M.; Koh, W.-P.; Tan, L.C.S. Dietary Antioxidants and Risk of Parkinson’s Disease in the Singapore Chinese Health Study. Mov. Disord. 2020, 35, 1765–1773. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.C.; Kwak, S.G.; Kwak, S. Effect of Dietary Vitamins C and E on the Risk of Parkinson’s Disease: A Meta-Analysis. Clin. Nutr. 2021, 40, 3922–3930. [Google Scholar] [CrossRef]
- Malaguarnera, M.; Ferri, R.; Bella, R.; Alagona, G.; Carnemolla, A.; Pennisi, G. Homocysteine, Vitamin B12 and Folate in Vascular Dementia and in Alzheimer Disease. Clin. Chem. Lab. Med. CCLM 2004, 42, 1032–1035. [Google Scholar] [CrossRef]
- Chen, H.; Liu, S.; Ji, L.; Wu, T.; Ma, F.; Ji, Y.; Zhou, Y.; Zheng, M.; Zhang, M.; Huang, G. Associations between Alzheimer’s Disease and Blood Homocysteine, Vitamin B12, and Folate: A Case-Control Study. Curr. Alzheimer Res. 2015, 12, 88–94. [Google Scholar] [CrossRef]
- Froese, D.S.; Fowler, B.; Baumgartner, M.R. Vitamin B12, Folate, and the Methionine Remethylation Cycle-Biochemistry, Pathways, and Regulation. J. Inherit. Metab. Dis. 2019, 42, 673–685. [Google Scholar] [CrossRef]
- Durga, J.; van Boxtel, M.P.J.; Schouten, E.G.; Kok, F.J.; Jolles, J.; Katan, M.B.; Verhoef, P. Effect of 3-Year Folic Acid Supplementation on Cognitive Function in Older Adults in the FACIT Trial: A Randomised, Double Blind, Controlled Trial. Lancet Lond. Engl. 2007, 369, 208–216. [Google Scholar] [CrossRef]
- Sun, Y.; Lu, C.-J.; Chien, K.-L.; Chen, S.-T.; Chen, R.-C. Efficacy of Multivitamin Supplementation Containing Vitamins B6 and B12 and Folic Acid as Adjunctive Treatment with a Cholinesterase Inhibitor in Alzheimer’s Disease: A 26-Week, Randomized, Double-Blind, Placebo-Controlled Study in Taiwanese Patients. Clin. Ther. 2007, 29, 2204–2214. [Google Scholar] [CrossRef]
- Aisen, P.S.; Schneider, L.S.; Sano, M.; Diaz-Arrastia, R.; van Dyck, C.H.; Weiner, M.F.; Bottiglieri, T.; Jin, S.; Stokes, K.T.; Thomas, R.G.; et al. High Dose B Vitamin Supplementation and Cognitive Decline in Alzheimer’s Disease: A Randomized Controlled Trial. JAMA J. Am. Med. Assoc. 2008, 300, 1774–1783. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Li, Y.; Zhang, W.; Zhao, Y.; Niu, X.; Guo, J. The Relationship between Cognitive Impairment and Homocysteine in a B12 and Folate Deficient Population in China: A Cross-Sectional Study. Med. Baltim. 2019, 98, e17970. [Google Scholar] [CrossRef] [PubMed]
- Lanyau-Domínguez, Y.; Macías-Matos, C.; Llibre-Rodríguez, J.d.J.; Pita-Rodríguez, G.M.; Suárez-Medina, R.; Quintero-Alejo, M.E.; Noriega-Fernández, L.; Guerra-Hernández, M.; Calvo-Rodríguez, M.; Sánchez-Gil, Y.; et al. Levels of Vitamins and Homocysteine in Older Adults with Alzheimer Disease or Mild Cognitive Impairment in Cuba. MEDICC Rev. 2020, 22, 40–47. [Google Scholar] [PubMed]
- Ma, F.; Wu, T.; Zhao, J.; Ji, L.; Song, A.; Zhang, M.; Huang, G. Plasma Homocysteine and Serum Folate and Vitamin B12 Levels in Mild Cognitive Impairment and Alzheimer’s Disease: A Case-Control Study. Nutrients 2017, 9, 725. [Google Scholar] [CrossRef]
- Yu, L.; Chen, Y.; Wang, W.; Xiao, Z.; Hong, Y. Multi-Vitamin B Supplementation Reverses Hypoxia-Induced Tau Hyperphosphorylation and Improves Memory Function in Adult Mice. J. Alzheimers Dis. 2016, 54, 297–306. [Google Scholar] [CrossRef]
- Lauer, A.A.; Grimm, H.S.; Apel, B.; Golobrodska, N.; Kruse, L.; Ratanski, E.; Schulten, N.; Schwarze, L.; Slawik, T.; Sperlich, S.; et al. Mechanistic Link between Vitamin B12 and Alzheimer’s Disease. Biomolecules 2022, 12, 129. [Google Scholar] [CrossRef]
- Rafiee, S.; Asadollahi, K.; Riazi, G.; Ahmadian, S.; Saboury, A.A. Vitamin B12 Inhibits Tau Fibrillization via Binding to Cysteine Residues of Tau. ACS Chem. Neurosci. 2017, 8, 2676–2682. [Google Scholar] [CrossRef]
- Moore, K.; Hughes, C.F.; Ward, M.; Hoey, L.; McNulty, H. Diet, Nutrition and the Ageing Brain: Current Evidence and New Directions. Proc. Nutr. Soc. 2018, 77, 152–163. [Google Scholar] [CrossRef]
- Zhao, R.; Wang, H.; Qiao, C.; Zhao, K. Vitamin B2 Blocks Development of Alzheimer’s Disease in APP/PS1 Transgenic Mice via Anti-Oxidative Mechanism. Trop. J. Pharm. Res. 2018, 17, 1049. [Google Scholar] [CrossRef]
- Seshadri, S.; Beiser, A.; Selhub, J.; Jacques, P.F.; Rosenberg, I.H.; D’Agostino, R.B.; Wilson, P.W.F.; Wolf, P.A. Plasma Homocysteine as a Risk Factor for Dementia and Alzheimer’s Disease. N. Engl. J. Med. 2002, 346, 476–483. [Google Scholar] [CrossRef]
- Guenther, B.D.; Sheppard, C.A.; Tran, P.; Rozen, R.; Matthews, R.G.; Ludwig, M.L. The Structure and Properties of Methylenetetrahydrofolate Reductase from Escherichia Coli Suggest How Folate Ameliorates Human Hyperhomocysteinemia. Nat. Struct. Biol. 1999, 6, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.E.; Sheu, K.F.; Blass, J.P.; Baker, A.; Carlson, K.C.; Harding, B.; Perrino, P. Reduced Activities of Thiamine-Dependent Enzymes in the Brains and Peripheral Tissues of Patients with Alzheimer’s Disease. Arch. Neurol. 1988, 45, 836–840. [Google Scholar] [CrossRef] [PubMed]
- Gold, M.; Hauser, R.A.; Chen, M.F. Plasma Thiamine Deficiency Associated with Alzheimer’s Disease but Not Parkinson’s Disease. Metab. Brain Dis. 1998, 13, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.E.; Hirsch, J.A.; Fonzetti, P.; Jordon, B.D.; Cirio, R.T.; Elder, J. Vitamin B1 (Thiamine) and Dementia. Ann. N. Y. Acad. Sci. 2016, 1367, 21–30. [Google Scholar] [CrossRef]
- Morris, M.; Evans, D.; Bienias, J.; Scherr, P.; Tangney, C.; Hebert, L.; Bennett, D.; Wilson, R.; Aggarwal, N. Dietary Niacin and the Risk of Incident Alzheimer’s Disease and of Cognitive Decline. J. Neurol. Neurosurg. Psychiatry 2004, 75, 1093–1099. [Google Scholar] [CrossRef]
- Rainer, M.; Kraxberger, E.; Haushofer, M.; Mucke, H.A.M.; Jellinger, K.A. No Evidence for Cognitive Improvement from Oral Nicotinamide Adenine Dinucleotide (NADH) in Dementia. J. Neural Transm. 2000, 107, 1475–1481. [Google Scholar] [CrossRef]
- Green, K.N.; Steffan, J.S.; Martinez-Coria, H.; Sun, X.; Schreiber, S.S.; Thompson, L.M.; LaFerla, F.M. Nicotinamide Restores Cognition in Alzheimer’s Disease Transgenic Mice via a Mechanism Involving Sirtuin Inhibition and Selective Reduction of Thr231-Phosphotau. J. Neurosci. 2008, 28, 11500–11510. [Google Scholar] [CrossRef]
- Ewers, M.; Buerger, K.; Teipel, S.J.; Scheltens, P.; Schröder, J.; Zinkowski, R.P.; Bouwman, F.H.; Schönknecht, P.; Schoonenboom, N.S.M.; Andreasen, N.; et al. Multicenter Assessment of CSF-Phosphorylated Tau for the Prediction of Conversion of MCI. Neurology 2007, 69, 2205–2212. [Google Scholar] [CrossRef]
- Turunc Bayrakdar, E.; Uyanikgil, Y.; Kanit, L.; Koylu, E.; Yalcin, A. Nicotinamide Treatment Reduces the Levels of Oxidative Stress, Apoptosis, and PARP-1 Activity in Aβ(1–42)-Induced Rat Model of Alzheimer’s Disease. Free Radic. Res. 2014, 48, 146–158. [Google Scholar] [CrossRef]
- Attia, H.; Albuhayri, S.; Alaraidh, S.; Alotaibi, A.; Yacoub, H.; Mohamad, R.; Al-Amin, M. Biotin, Coenzyme Q10, and Their Combination Ameliorate Aluminium Chloride-Induced Alzheimer’s Disease via Attenuating Neuroinflammation and Improving Brain Insulin Signaling. J. Biochem. Mol. Toxicol. 2020, 34, e22519. [Google Scholar] [CrossRef]
- Xu, J.; Patassini, S.; Begley, P.; Church, S.; Waldvogel, H.J.; Faull, R.L.M.; Unwin, R.D.; Cooper, G.J.S. Cerebral Deficiency of Vitamin B5 (d-Pantothenic Acid; Pantothenate) as a Potentially-Reversible Cause of Neurodegeneration and Dementia in Sporadic Alzheimer’s Disease. Biochem. Biophys. Res. Commun. 2020, 527, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Sang, C.; Philbert, S.A.; Hartland, D.; Unwin, R.D.; Dowsey, A.W.; Xu, J.; Cooper, G.J.S. Coenzyme A-Dependent Tricarboxylic Acid Cycle Enzymes Are Decreased in Alzheimer’s Disease Consistent With Cerebral Pantothenate Deficiency. Front. Aging Neurosci. 2022, 14, 893159. [Google Scholar] [CrossRef] [PubMed]
- Basambombo, L.L.; Carmichael, P.-H.; Côté, S.; Laurin, D. Use of Vitamin E and C Supplements for the Prevention of Cognitive Decline. Ann. Pharmacother. 2017, 51, 118–124. [Google Scholar] [CrossRef] [PubMed]
- de Wilde, M.C.; Vellas, B.; Girault, E.; Yavuz, A.C.; Sijben, J.W. Lower Brain and Blood Nutrient Status in Alzheimer’s Disease: Results from Meta-Analyses. Alzheimers Dement. Transl. Res. Clin. Interv. 2017, 3, 416–431. [Google Scholar] [CrossRef]
- Jiménez-Jiménez, F.J.; De Bustos, F.; Molina, J.A.; Benito-León, J.; Tallón-Barranco, A.; Gasalla, T.; Ortí-Pareja, M.; Guillamón, F.; Rubio, J.C.; Arenas, J.; et al. Cerebrospinal Fluid Levels of Alpha-Tocopherol (Vitamin E) in Alzheimer’s Disease. J. Neural Transm. 1997, 104, 703–710. [Google Scholar] [CrossRef]
- Casati, M.; Boccardi, V.; Ferri, E.; Bertagnoli, L.; Bastiani, P.; Ciccone, S.; Mansi, M.; Scamosci, M.; Rossi, P.D.; Mecocci, P.; et al. Vitamin E and Alzheimer’s Disease: The Mediating Role of Cellular Aging. Aging Clin. Exp. Res. 2020, 32, 459–464. [Google Scholar] [CrossRef]
- Gray, S.L.; Anderson, M.L.; Crane, P.K.; Breitner, J.C.S.; McCormick, W.; Bowen, J.D.; Teri, L.; Larson, E. Antioxidant Vitamin Supplement Use and Risk of Dementia or Alzheimer’s Disease in Older Adults. J. Am. Geriatr. Soc. 2008, 56, 291–295. [Google Scholar] [CrossRef]
- Murakami, K.; Murata, N.; Ozawa, Y.; Kinoshita, N.; Irie, K.; Shirasawa, T.; Shimizu, T. Vitamin C Restores Behavioral Deficits and Amyloid-β Oligomerization without Affecting Plaque Formation in a Mouse Model of Alzheimer’s Disease. J. Alzheimers Dis. 2011, 26, 7–18. [Google Scholar] [CrossRef]
- Sil, S.; Ghosh, T.; Gupta, P.; Ghosh, R.; Kabir, S.N.; Roy, A. Dual Role of Vitamin C on the Neuroinflammation Mediated Neurodegeneration and Memory Impairments in Colchicine Induced Rat Model of Alzheimer Disease. J. Mol. Neurosci. 2016, 60, 421–435. [Google Scholar] [CrossRef]
- Arlt, S.; Müller-Thomsen, T.; Beisiegel, U.; Kontush, A. Effect of One-Year Vitamin C-and E-Supplementation on Cerebrospinal Fluid Oxidation Parameters and Clinical Course in Alzheimer’s Disease. Neurochem. Res. 2012, 37, 2706–2714. [Google Scholar] [CrossRef]
- Ide, K.; Yamada, H.; Kawasaki, Y.; Yamanaka, M.; Kawakami, N.; Katsuyama, Y.; Yoshida, H.; Kim, K.; Shiosaki, E.; Sonoda, A.; et al. Peripheral Vitamin C Levels in Alzheimer’s Disease: A Cross-Sectional Study. J. Nutr. Sci. Vitaminol. 2016, 62, 432–436. [Google Scholar] [CrossRef]
- Shen, J.; Griffiths, P.T.; Campbell, S.J.; Utinger, B.; Kalberer, M.; Paulson, S.E. Ascorbate Oxidation by Iron, Copper and Reactive Oxygen Species: Review, Model Development, and Derivation of Key Rate Constants. Sci. Rep. 2021, 11, 7417. [Google Scholar] [CrossRef]
- Dysken, M.W.; Sano, M.; Asthana, S.; Vertrees, J.E.; Pallaki, M.; Llorente, M.; Love, S.; Schellenberg, G.D.; McCarten, J.R.; Malphurs, J.; et al. Effect of Vitamin E and Memantine on Functional Decline in Alzheimer Disease. JAMA J. Am. Med. Assoc. 2014, 311, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Mehrabadi, S.; Sadr, S.S. Administration of Vitamin D3 and E Supplements Reduces Neuronal Loss and Oxidative Stress in a Model of Rats with Alzheimer’s Disease. Neurol. Res. 2020, 42, 862–868. [Google Scholar] [CrossRef] [PubMed]
- Yatin, S.M.; Varadarajan, S.; Butterfield, D.A. Vitamin E Prevents Alzheimer’s Amyloid Beta-Peptide (1-42)-Induced Neuronal Protein Oxidation and Reactive Oxygen Species Production. J. Alzheimers Dis. JAD 2000, 2, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zhao, Y.; Jin, S.; Hu, Y.; Wang, T.; Tian, R.; Han, Z.; Xu, D.; Jiang, Q. Circulating Vitamin E Levels and Alzheimer’s Disease: A Mendelian Randomization Study. Neurobiol. Aging 2018, 72, 189.e1–189.e9. [Google Scholar] [CrossRef]
- Lloret, A.; Badía, M.-C.; Mora, N.J.; Pallardó, F.V.; Alonso, M.-D.; Viña, J. Vitamin E Paradox in Alzheimer’s Disease: It Does Not Prevent Loss of Cognition and May Even Be Detrimental. J. Alzheimers Dis. 2009, 17, 143–149. [Google Scholar] [CrossRef]
- Mullan, K.; Williams, M.A.; Cardwell, C.R.; McGuinness, B.; Passmore, P.; Silvestri, G.; Woodside, J.V.; McKay, G.J. Serum Concentrations of Vitamin E and Carotenoids Are Altered in Alzheimer’s Disease: A Case-Control Study. Alzheimers Dement. Transl. Res. Clin. Interv. 2017, 3, 432–439. [Google Scholar] [CrossRef]
- Lopes da Silva, S.; Vellas, B.; Elemans, S.; Luchsinger, J.; Kamphuis, P.; Yaffe, K.; Sijben, J.; Groenendijk, M.; Stijnen, T. Plasma Nutrient Status of Patients with Alzheimer’s Disease: Systematic Review and Meta-Analysis. Alzheimers Dement. 2014, 10, 485–502. [Google Scholar] [CrossRef]
- Kapoor, A.; Wang, B.-J.; Hsu, W.-M.; Chang, M.-Y.; Liang, S.-M.; Liao, Y.-F. Retinoic Acid-Elicited RARα/RXRα Signaling Attenuates Aβ Production by Directly Inhibiting γ-Secretase-Mediated Cleavage of Amyloid Precursor Protein. ACS Chem. Neurosci. 2013, 4, 1093–1100. [Google Scholar] [CrossRef]
- Hira, S.; Saleem, U.; Anwar, F.; Sohail, M.F.; Raza, Z.; Ahmad, B. β-Carotene: A Natural Compound Improves Cognitive Impairment and Oxidative Stress in a Mouse Model of Streptozotocin-Induced Alzheimer’s Disease. Biomolecules 2019, 9, 441. [Google Scholar] [CrossRef]
- Geng, T.; Lu, Q.; Wan, Z.; Guo, J.; Liu, L.; Pan, A.; Liu, G. Association of Serum 25-Hydroxyvitamin D Concentrations with Risk of Dementia among Individuals with Type 2 Diabetes: A Cohort Study in the UK Biobank. PLOS Med. 2022, 19, e1003906. [Google Scholar] [CrossRef] [PubMed]
- Licher, S.; De Bruijn, R.F.A.G.; Wolters, F.J.; Zillikens, M.C.; Ikram, M.A.; Ikram, M.K. Vitamin D and the Risk of Dementia: The Rotterdam Study. J. Alzheimers Dis. 2017, 60, 989–997. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Hu, J.; Huo, X.; Miao, R.; Zhang, Y.; Ma, F. Effects of Vitamin D Supplementation on Cognitive Function and Blood Aβ-Related Biomarkers in Older Adults with Alzheimer’s Disease: A Randomised, Double-Blind, Placebo-Controlled Trial. J. Neurol. Neurosurg. Psychiatry 2019, 90, 1347–1352. [Google Scholar] [CrossRef] [PubMed]
- Annweiler, C.; Rolland, Y.; Schott, A.M.; Blain, H.; Vellas, B.; Herrmann, F.R.; Beauchet, O. Higher Vitamin D Dietary Intake Is Associated With Lower Risk of Alzheimer’s Disease: A 7-Year Follow-Up. J. Gerontol. Ser. A 2012, 67, 1205–1211. [Google Scholar] [CrossRef]
- Yamini, P.; Ray, R.S.; Chopra, K. Vitamin D3 Attenuates Cognitive Deficits and Neuroinflammatory Responses in ICV-STZ Induced Sporadic Alzheimer’s Disease. Inflammopharmacology 2018, 26, 39–55. [Google Scholar] [CrossRef]
- Stein, M.S.; Scherer, S.C.; Ladd, K.S.; Harrison, L.C. A Randomized Controlled Trial of High-Dose Vitamin D2 Followed by Intranasal Insulin in Alzheimer’s Disease. J. Alzheimers Dis. 2011, 26, 477–484. [Google Scholar] [CrossRef]
- Presse, N.; Shatenstein, B.; Kergoat, M.-J.; Ferland, G. Low Vitamin K Intakes in Community-Dwelling Elders at an Early Stage of Alzheimer’s Disease. J. Am. Diet. Assoc. 2008, 108, 2095–2099. [Google Scholar] [CrossRef]
- Booth, S.L.; Shea, M.K.; Barger, K.; Leurgans, S.E.; James, B.D.; Holland, T.M.; Agarwal, P.; Fu, X.; Wang, J.; Matuszek, G.; et al. Association of Vitamin K with Cognitive Decline and Neuropathology in Community-Dwelling Older Persons. Alzheimers Dement. Transl. Res. Clin. Interv. 2022, 8, e12255. [Google Scholar] [CrossRef]
- Lin, X.; Wen, X.; Wei, Z.; Guo, K.; Shi, F.; Huang, T.; Wang, W.; Zheng, J. Vitamin K2 Protects against Aβ42-Induced Neurotoxicity by Activating Autophagy and Improving Mitochondrial Function in Drosophila. Neuroreport 2021, 32, 431. [Google Scholar] [CrossRef]
- Hadipour, E.; Tayarani-Najaran, Z.; Fereidoni, M. Vitamin K2 Protects PC12 Cells against Aβ(1-42) and H2O2-Induced Apoptosis via P38 MAP Kinase Pathway. Nutr. Neurosci. 2018, 23, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Gaggelli, E.; Kozlowski, H.; Valensin, D.; Valensin, G. Copper Homeostasis and Neurodegenerative Disorders (Alzheimer’s, Prion, and Parkinson’s Diseases and Amyotrophic Lateral Sclerosis). Chem. Rev. 2006, 106, 1995–2044. [Google Scholar] [CrossRef]
- Kozlowski, H.; Janicka-Klos, A.; Brasun, J.; Gaggelli, E.; Valensin, D.; Valensin, G. Copper, Iron, and Zinc Ions Homeostasis and Their Role in Neurodegenerative Disorders (Metal Uptake, Transport, Distribution and Regulation). Coord. Chem. Rev. 2009, 253, 2665–2685. [Google Scholar] [CrossRef]
- Kozlowski, H.; Luczkowski, M.; Remelli, M.; Valensin, D. Copper, Zinc and Iron in Neurodegenerative Diseases (Alzheimer’s, Parkinson’s and Prion Diseases). Coord. Chem. Rev. 2012, 256, 2129–2141. [Google Scholar] [CrossRef]
- Liu, Y.; Nguyen, M.; Robert, A.; Meunier, B. Metal Ions in Alzheimer’s Disease: A Key Role or Not? Acc. Chem. Res. 2019, 52, 2026–2035. [Google Scholar] [CrossRef]
- Wang, L.; Yin, Y.-L.; Liu, X.-Z.; Shen, P.; Zheng, Y.-G.; Lan, X.-R.; Lu, C.-B.; Wang, J.-Z. Current Understanding of Metal Ions in the Pathogenesis of Alzheimer’s Disease. Transl. Neurodegener. 2020, 9, 10. [Google Scholar] [CrossRef]
- Foley, P.B.; Hare, D.J.; Double, K.L. A Brief History of Brain Iron Accumulation in Parkinson Disease and Related Disorders. J. Neural Transm. Vienna Austria 1996 2022, 129, 505–520. [Google Scholar] [CrossRef]
- Balachandran, R.C.; Mukhopadhyay, S.; McBride, D.; Veevers, J.; Harrison, F.E.; Aschner, M.; Haynes, E.N.; Bowman, A.B. Brain Manganese and the Balance between Essential Roles and Neurotoxicity. J. Biol. Chem. 2020, 295, 6312–6329. [Google Scholar] [CrossRef]
- Cheignon, C.; Tomas, M.; Bonnefont-Rousselot, D.; Faller, P.; Hureau, C.; Collin, F. Oxidative Stress and the Amyloid Beta Peptide in Alzheimer’s Disease. Redox Biol. 2018, 14, 450–464. [Google Scholar] [CrossRef]
- Meneghini, R. Iron Homeostasis, Oxidative Stress, and DNA Damage. Free Radic. Biol. Med. 1997, 23, 783–792. [Google Scholar] [CrossRef]
- Barnham, K.J.; Bush, A.I. Metals in Alzheimer’s and Parkinson’s Diseases. Curr. Opin. Chem. Biol. 2008, 12, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Swartz, H.M.; Sarna, T.; Zecca, L. Modulation by Neuromelanin of the Availability and Reactivity of Metal Ions. Ann. Neurol. 1992, 32, S69–S75. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hong, L.; Kempf, V.R.; Wakamatsu, K.; Ito, S.; Simon, J.D. Ion-Exchange and Adsorption of Fe(III) by Sepia Melanin. Pigment. Cell Res. 2004, 17, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Zecca, L.; Pietra, R.; Goj, C.; Mecacci, C.; Radice, D.; Sabbioni, E. Iron and Other Metals in Neuromelanin, Substantia Nigra, and Putamen of Human Brain. J. Neurochem. 1994, 62, 1097–1101. [Google Scholar] [CrossRef] [PubMed]
- Horning, K.J.; Caito, S.W.; Tipps, K.G.; Bowman, A.B.; Aschner, M. Manganese Is Essential for Neuronal Health. Annu. Rev. Nutr. 2015, 35, 71–108. [Google Scholar] [CrossRef]
- Myhre, O.; Utkilen, H.; Duale, N.; Brunborg, G.; Hofer, T. Metal Dyshomeostasis and Inflammation in Alzheimer’s and Parkinson’s Diseases: Possible Impact of Environmental Exposures. Oxid. Med. Cell. Longev. 2013, 2013, e726954. [Google Scholar] [CrossRef]
- Li, B.; Xia, M.; Zorec, R.; Parpura, V.; Verkhratsky, A. Astrocytes in Heavy Metal Neurotoxicity and Neurodegeneration. Brain Res. 2021, 1752, 147234. [Google Scholar] [CrossRef]
- Genchi, G.; Carocci, A.; Lauria, G.; Sinicropi, M.S.; Catalano, A. Nickel: Human Health and Environmental Toxicology. Int. J. Environ. Res. Public. Health 2020, 17, 679. [Google Scholar] [CrossRef]
- Babić Leko, M.; Langer Horvat, L.; Španić Popovački, E.; Zubčić, K.; Hof, P.R.; Šimić, G. Metals in Alzheimer’s Disease. Biomedicines 2023, 11, 1161. [Google Scholar] [CrossRef]
- Brewer, G.J.; Kanzer, S.H.; Zimmerman, E.A.; Molho, E.S.; Celmins, D.F.; Heckman, S.M.; Dick, R. Subclinical Zinc Deficiency in Alzheimer’s Disease and Parkinson’s Disease. Am. J. Alzheimers Dis. Dementiasr 2010, 25, 572–575. [Google Scholar] [CrossRef]
- Ahmed, S.S.S.J.; Santosh, W. Metallomic Profiling and Linkage Map Analysis of Early Parkinson’s Disease: A New Insight to Aluminum Marker for the Possible Diagnosis. PLoS ONE 2010, 5, e11252. [Google Scholar] [CrossRef] [PubMed]
- Giacoppo, S.; Galuppo, M.; Calabrò, R.S.; D’Aleo, G.; Marra, A.; Sessa, E.; Bua, D.G.; Potortì, A.G.; Dugo, G.; Bramanti, P.; et al. Heavy Metals and Neurodegenerative Diseases: An Observational Study. Biol. Trace Elem. Res. 2014, 161, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.-W.; Lin, J.; Wang, X.-B.; Cheng, X.; Wang, J.-Y.; Hu, B.-L.; Zhang, Y.; Zhang, X.; Zhu, J.-H. Assessing Plasma Levels of Selenium, Copper, Iron and Zinc in Patients of Parkinson’s Disease. PLoS ONE 2013, 8, e83060. [Google Scholar] [CrossRef] [PubMed]
- Yadav, J.; Verma, A.K.; Ahmad, M.K.; Garg, R.K.; Shiuli; Mahdi, A.A.; Srivastava, S. Metals Toxicity and Its Correlation with the Gene Expression in Alzheimer’s Disease. Mol. Biol. Rep. 2021, 48, 3245–3252. [Google Scholar] [CrossRef]
- Paglia, G.; Miedico, O.; Cristofano, A.; Vitale, M.; Angiolillo, A.; Chiaravalle, A.E.; Corso, G.; Di Costanzo, A. Distinctive Pattern of Serum Elements During the Progression of Alzheimer’s Disease. Sci. Rep. 2016, 6, 22769. [Google Scholar] [CrossRef]
- Wang, Z.-X.; Tan, L.; Wang, H.-F.; Ma, J.; Liu, J.; Tan, M.-S.; Sun, J.-H.; Zhu, X.-C.; Jiang, T.; Yu, J.-T. Serum Iron, Zinc, and Copper Levels in Patients with Alzheimer’s Disease: A Replication Study and Meta-Analyses. J. Alzheimers Dis. 2015, 47, 565–581. [Google Scholar] [CrossRef]
- Koç, E.R.; Ilhan, A.; Zübeyde Aytürk, A.; Acar, B.; Gürler, M.; Altuntaş, A.; Karapirli, M.; Bodur, A.S. A Comparison of Hair and Serum Trace Elements in Patients with Alzheimer Disease and Healthy Participants. Turk. J. Med. Sci. 2015, 45, 1034–1039. [Google Scholar] [CrossRef]
- Rembach, A.; Doecke, J.D.; Roberts, B.R.; Watt, A.D.; Faux, N.G.; Volitakis, I.; Pertile, K.K.; Rumble, R.L.; Trounson, B.O.; Fowler, C.J.; et al. Longitudinal Analysis of Serum Copper and Ceruloplasmin in Alzheimer’s Disease. J. Alzheimers Dis. 2013, 34, 171–182. [Google Scholar] [CrossRef]
- Alsadany, M.A.; Shehata, H.H.; Mohamad, M.I.; Mahfouz, R.G. Histone Deacetylases Enzyme, Copper, and IL-8 Levels in Patients With Alzheimer’s Disease. Am. J. Alzheimers Dis. Other Demen. 2013, 28, 54–61. [Google Scholar] [CrossRef]
- Mariani, S.; Ventriglia, M.; Simonelli, I.; Donno, S.; Bucossi, S.; Vernieri, F.; Melgari, J.-M.; Pasqualetti, P.; Rossini, P.M.; Squitti, R. Fe and Cu Do Not Differ in Parkinson’s Disease: A Replication Study plus Meta-Analysis. Neurobiol. Aging 2013, 34, 632–633. [Google Scholar] [CrossRef]
- Crespo, Â.C.; Silva, B.; Marques, L.; Marcelino, E.; Maruta, C.; Costa, S.; Timóteo, Â.; Vilares, A.; Couto, F.S.; Faustino, P.; et al. Genetic and Biochemical Markers in Patients with Alzheimer’s Disease Support a Concerted Systemic Iron Homeostasis Dysregulation. Neurobiol. Aging 2014, 35, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, T.; Tan, X.; Luo, Y.; Kanda, H. Relationship between Blood Levels of Heavy Metals and Parkinson’s Disease in China. Neuroepidemiology 2010, 34, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Dexter, D.T.; Carayon, A.; Javoy-Agid, F.; Agid, Y.; Wells, F.R.; Daniel, S.E.; Lees, A.J.; Jenner, P.; Marsden, C.D. Alterations in the Levels of Iron, Ferritin and Other Trace Metals in Parkinson’s Disease and Other Neurodegenerative Diseases Affecting the Basal Ganglia. Brain 1991, 114, 1953–1975. [Google Scholar] [CrossRef] [PubMed]
- Babić Leko, M.; Jurasović, J.; Nikolac Perković, M.; Španić, E.; Sekovanić, A.; Orct, T.; Lukinović Škudar, V.; Bačić Baronica, K.; Kiđemet-Piskač, S.; Vogrinc, Ž.; et al. The Association of Essential Metals with APOE Genotype in Alzheimer’s Disease. J. Alzheimers Dis. 2021, 82, 661–672. [Google Scholar] [CrossRef]
- Hozumi, I.; Hasegawa, T.; Honda, A.; Ozawa, K.; Hayashi, Y.; Hashimoto, K.; Yamada, M.; Koumura, A.; Sakurai, T.; Kimura, A.; et al. Patterns of Levels of Biological Metals in CSF Differ among Neurodegenerative Diseases. J. Neurol. Sci. 2011, 303, 95–99. [Google Scholar] [CrossRef]
- Squitti, R.; Ventriglia, M.; Simonelli, I.; Bonvicini, C.; Costa, A.; Perini, G.; Binetti, G.; Benussi, L.; Ghidoni, R.; Koch, G.; et al. Copper Imbalance in Alzheimer’s Disease: Meta-Analysis of Serum, Plasma, and Brain Specimens, and Replication Study Evaluating ATP7B Gene Variants. Biomolecules 2021, 11, 960. [Google Scholar] [CrossRef]
- Miller, L.M.; Wang, Q.; Telivala, T.P.; Smith, R.J.; Lanzirotti, A.; Miklossy, J. Synchrotron-Based Infrared and X-Ray Imaging Shows Focalized Accumulation of Cu and Zn Co-Localized with β-Amyloid Deposits in Alzheimer’s Disease. J. Struct. Biol. 2006, 155, 30–37. [Google Scholar] [CrossRef]
- Dong, J.; Atwood, C.S.; Anderson, V.E.; Siedlak, S.L.; Smith, M.A.; Perry, G.; Carey, P.R. Metal Binding and Oxidation of Amyloid-Beta within Isolated Senile Plaque Cores: Raman Microscopic Evidence. Biochemistry 2003, 42, 2768–2773. [Google Scholar] [CrossRef]
- Lovell, M.A.; Robertson, J.D.; Teesdale, W.J.; Campbell, J.L.; Markesbery, W.R. Copper, Iron and Zinc in Alzheimer’s Disease Senile Plaques. J. Neurol. Sci. 1998, 158, 47–52. [Google Scholar] [CrossRef]
- Smith, M.A.; Harris, P.L.R.; Sayre, L.M.; Perry, G. Iron Accumulation in Alzheimer Disease Is a Source of Redox-Generated Free Radicals. Proc. Natl. Acad. Sci. USA 1997, 94, 9866–9868. [Google Scholar] [CrossRef]
- Liu, B.; Moloney, A.; Meehan, S.; Morris, K.; Thomas, S.E.; Serpell, L.C.; Hider, R.; Marciniak, S.J.; Lomas, D.A.; Crowther, D.C. Iron Promotes the Toxicity of Amyloid β Peptide by Impeding Its Ordered Aggregation. J. Biol. Chem. 2011, 286, 4248–4256. [Google Scholar] [CrossRef]
- Sóvágó, I.; Várnagy, K.; Kállay, C.; Grenács, Á. Interactions of Copper(II) and Zinc(II) Ions with the Peptide Fragments of Proteins Related to Neurodegenerative Disorders: Similarities and Differences. Curr. Med. Chem. 2023, 30, 4050–4071. [Google Scholar] [CrossRef] [PubMed]
- Nath, A.K.; Dey, S.G. Simultaneous Binding of Heme and Cu with Amyloid β Peptides: Active Site and Reactivities. Dalton Trans. 2022, 51, 4986–4999. [Google Scholar] [CrossRef]
- Stefaniak, E.; Bal, W. CuII Binding Properties of N-Truncated Aβ Peptides: In Search of Biological Function. Inorg. Chem. 2019, 58, 13561–13577. [Google Scholar] [CrossRef]
- Arena, G.; Rizzarelli, E. Zn2+ Interaction with Amyloid-Β: Affinity and Speciation. Mol. Basel Switz. 2019, 24, 2796. [Google Scholar] [CrossRef] [PubMed]
- Atrián-Blasco, E.; Conte-Daban, A.; Hureau, C. Mutual Interference of Cu and Zn Ions in Alzheimer’s Disease: Perspectives at the Molecular Level. Dalton Trans. 2017, 46, 12750–12759. [Google Scholar] [CrossRef]
- Wärmländer, S.K.T.S.; Österlund, N.; Wallin, C.; Wu, J.; Luo, J.; Tiiman, A.; Jarvet, J.; Gräslund, A. Metal Binding to the Amyloid-β Peptides in the Presence of Biomembranes: Potential Mechanisms of Cell Toxicity. J. Biol. Inorg. Chem. JBIC Publ. Soc. Biol. Inorg. Chem. 2019, 24, 1189–1196. [Google Scholar] [CrossRef] [PubMed]
- De Gregorio, G.; Biasotto, F.; Hecel, A.; Luczkowski, M.; Kozlowski, H.; Valensin, D. Structural Analysis of Copper(I) Interaction with Amyloid β Peptide. J. Inorg. Biochem. 2019, 195, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Trapani, G.; Satriano, C.; La Mendola, D. Peptides and Their Metal Complexes in Neurodegenerative Diseases: From Structural Studies to Nanomedicine Prospects. Curr. Med. Chem. 2018, 25, 715–747. [Google Scholar] [CrossRef]
- Ahmadi, S.; Zhu, S.; Sharma, R.; Wilson, D.J.; Kraatz, H.-B. Interaction of Metal Ions with Tau Protein. The Case for a Metal-Mediated Tau Aggregation. J. Inorg. Biochem. 2019, 194, 44–51. [Google Scholar] [CrossRef]
- Binolfi, A.; Quintanar, L.; Bertoncini, C.W.; Griesinger, C.; Fernández, C.O. Bioinorganic Chemistry of Copper Coordination to Alpha-Synuclein: Relevance to Parkinson’s Disease. Coord. Chem. Rev. 2012, 256, 2188–2201. [Google Scholar] [CrossRef]
- Valensin, D.; Dell’Acqua, S.; Kozlowski, H.; Casella, L. Coordination and Redox Properties of Copper Interaction with α-Synuclein. J. Inorg. Biochem. 2016, 163, 292–300. [Google Scholar] [CrossRef] [PubMed]
- González, N.; Arcos-López, T.; König, A.; Quintanar, L.; Menacho Márquez, M.; Outeiro, T.F.; Fernández, C.O. Effects of Alpha-Synuclein Post-Translational Modifications on Metal Binding. J. Neurochem. 2019, 150, 507–521. [Google Scholar] [CrossRef]
- Atrián-Blasco, E.; Gonzalez, P.; Santoro, A.; Alies, B.; Faller, P.; Hureau, C. Cu and Zn Coordination to Amyloid Peptides: From Fascinating Chemistry to Debated Pathological Relevance. Coord. Chem. Rev. 2018, 375, 38–55. [Google Scholar] [CrossRef] [PubMed]
- Leal, S.S.; Botelho, H.M.; Gomes, C.M. Metal Ions as Modulators of Protein Conformation and Misfolding in Neurodegeneration. Coord. Chem. Rev. 2012, 256, 2253–2270. [Google Scholar] [CrossRef]
- Gamez, P.; Caballero, A.B. Copper in Alzheimer’s Disease: Implications in Amyloid Aggregation and Neurotoxicity. AIP Adv. 2015, 5, 092503. [Google Scholar] [CrossRef]
- Faller, P.; Hureau, C.; La Penna, G. Metal Ions and Intrinsically Disordered Proteins and Peptides: From Cu/Zn Amyloid-β to General Principles. Acc. Chem. Res. 2014, 47, 2252–2259. [Google Scholar] [CrossRef]
- DeToma, A.S.; Salamekh, S.; Ramamoorthy, A.; Lim, M.H. Misfolded Proteins in Alzheimer’s Disease and Type II Diabetes. Chem. Soc. Rev. 2012, 41, 608–621. [Google Scholar] [CrossRef]
- Ke, P.C.; Sani, M.-A.; Ding, F.; Kakinen, A.; Javed, I.; Separovic, F.; Davis, T.P.; Mezzenga, R. Implications of Peptide Assemblies in Amyloid Diseases. Chem. Soc. Rev. 2017, 46, 6492–6531. [Google Scholar] [CrossRef]
- Viles, J.H. Metal Ions and Amyloid Fiber Formation in Neurodegenerative Diseases. Copper, Zinc and Iron in Alzheimer’s, Parkinson’s and Prion Diseases. Coord. Chem. Rev. 2012, 256, 2271–2284. [Google Scholar] [CrossRef]
- Miller, Y.; Ma, B.; Nussinov, R. Zinc Ions Promote Alzheimer Abeta Aggregation via Population Shift of Polymorphic States. Proc. Natl. Acad. Sci. USA 2010, 107, 9490–9495. [Google Scholar] [CrossRef]
- Sharma, A.K.; Pavlova, S.T.; Kim, J.; Kim, J.; Mirica, L.M. The Effect of Cu2+ and Zn2+ on the Aβ42 Peptide Aggregation and Cellular Toxicity. Metallomics 2013, 5, 1529–1536. [Google Scholar] [CrossRef] [PubMed]
- Bush, A.I.; Pettingell, W.H.; Multhaup, G.; d Paradis, M.; Vonsattel, J.P.; Gusella, J.F.; Beyreuther, K.; Masters, C.L.; Tanzi, R.E. Rapid Induction of Alzheimer A Beta Amyloid Formation by Zinc. Science 1994, 265, 1464–1467. [Google Scholar] [CrossRef] [PubMed]
- Carboni, E.; Lingor, P. Insights on the Interaction of Alpha-Synuclein and Metals in the Pathophysiology of Parkinson’s Disease. Metallomics 2015, 7, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Drew, S.C. The N Terminus of α-Synuclein Forms CuII-Bridged Oligomers. Chem. Eur. J. 2015, 21, 7111–7118. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-J.; Jiang, H.; Song, N.; Xie, J.-X. Dose- and Time-Dependent α-Synuclein Aggregation Induced by Ferric Iron in SK-N-SH Cells. Neurosci. Bull. 2010, 26, 205–210. [Google Scholar] [CrossRef]
- Rasia, R.M.; Bertoncini, C.W.; Marsh, D.; Hoyer, W.; Cherny, D.; Zweckstetter, M.; Griesinger, C.; Jovin, T.M.; Fernández, C.O. Structural Characterization of Copper(II) Binding to α-Synuclein: Insights into the Bioinorganic Chemistry of Parkinson’s Disease. Proc. Natl. Acad. Sci. USA 2005, 102, 4294–4299. [Google Scholar] [CrossRef]
- Li, X.; Du, X.; Ni, J. Zn2+ Aggravates Tau Aggregation and Neurotoxicity. Int. J. Mol. Sci. 2019, 20, 487. [Google Scholar] [CrossRef]
- Zubčić, K.; Hof, P.R.; Šimić, G.; Jazvinšćak Jembrek, M. The Role of Copper in Tau-Related Pathology in Alzheimer’s Disease. Front. Mol. Neurosci. 2020, 13, 572308. [Google Scholar] [CrossRef]
- Ahmadi, S.; Wu, B.; Song, R.; Zhu, S.; Simpson, A.; Wilson, D.J.; Kraatz, H.-B. Exploring the Interactions of Iron and Zinc with the Microtubule Binding Repeats R1 and R4. J. Inorg. Biochem. 2020, 205, 110987. [Google Scholar] [CrossRef]
- Soragni, A.; Zambelli, B.; Mukrasch, M.D.; Biernat, J.; Jeganathan, S.; Griesinger, C.; Ciurli, S.; Mandelkow, E.; Zweckstetter, M. Structural Characterization of Binding of Cu(II) to Tau Protein. Biochemistry 2008, 47, 10841–10851. [Google Scholar] [CrossRef]
- Balogh, B.D.; Szakács, B.; Di Natale, G.; Tabbì, G.; Pappalardo, G.; Sóvágó, I.; Várnagy, K. Copper (II) Binding Properties of an Octapeptide Fragment from the R3 Region of Tau Protein: A Combined Potentiometric, Spectroscopic and Mass Spectrometric Study. J. Inorg. Biochem. 2021, 217, 111358. [Google Scholar] [CrossRef]
- Bacchella, C.; Gentili, S.; Bellotti, D.; Quartieri, E.; Draghi, S.; Baratto, M.C.; Remelli, M.; Valensin, D.; Monzani, E.; Nicolis, S.; et al. Binding and Reactivity of Copper to R1 and R3 Fragments of Tau Protein. Inorg. Chem. 2020, 59, 274–286. [Google Scholar] [CrossRef]
- Jing, J.; Tu, G.; Yu, H.; Huang, R.; Ming, X.; Zhan, H.; Zhan, F.; Xue, W. Copper (Cu2+) Ion-Induced Misfolding of Tau Protein R3 Peptide Revealed by Enhanced Molecular Dynamics Simulation. Phys. Chem. Chem. Phys. 2021, 23, 11717–11726. [Google Scholar] [CrossRef]
- Rivers-Auty, J.; Tapia, V.S.; White, C.S.; Daniels, M.J.D.; Drinkall, S.; Kennedy, P.T.; Spence, H.G.; Yu, S.; Green, J.P.; Hoyle, C.; et al. Zinc Status Alters Alzheimer’s Disease Progression through NLRP3-Dependent Inflammation. J. Neurosci. 2021, 41, 3025–3038. [Google Scholar] [CrossRef] [PubMed]
- Singh, I.; Sagare, A.P.; Coma, M.; Perlmutter, D.; Gelein, R.; Bell, R.D.; Deane, R.J.; Zhong, E.; Parisi, M.; Ciszewski, J.; et al. Low Levels of Copper Disrupt Brain Amyloid-β Homeostasis by Altering Its Production and Clearance. Proc. Natl. Acad. Sci. USA 2013, 110, 14771–14776. [Google Scholar] [CrossRef] [PubMed]
- Sparks, D.L.; Schreurs, B.G. Trace Amounts of Copper in Water Induce β-Amyloid Plaques and Learning Deficits in a Rabbit Model of Alzheimer’s Disease. Proc. Natl. Acad. Sci. USA 2003, 100, 11065–11069. [Google Scholar] [CrossRef] [PubMed]
- Prasanthi, J.R.P.; Schrag, M.; Dasari, B.; Marwarha, G.; Dickson, A.; Kirsch, W.M.; Ghribi, O. Deferiprone Reduces Amyloid-β and Tau Phosphorylation Levels but Not Reactive Oxygen Species Generation in Hippocampus of Rabbits Fed a Cholesterol-Enriched Diet. J. Alzheimers Dis. 2012, 30, 167–182. [Google Scholar] [CrossRef]
- Chen, P.; Chakraborty, S.; Mukhopadhyay, S.; Lee, E.; Paoliello, M.M.B.; Bowman, A.B.; Aschner, M. Manganese Homeostasis in the Nervous System. J. Neurochem. 2015, 134, 601–610. [Google Scholar] [CrossRef]
- Shen, X.; Liu, J.; Fujita, Y.; Liu, S.; Maeda, T.; Kikuchi, K.; Obara, T.; Takebe, A.; Sayama, R.; Takahashi, T.; et al. Iron Treatment Inhibits Aβ42 Deposition in Vivo and Reduces Aβ42/Aβ40 Ratio. Biochem. Biophys. Res. Commun. 2019, 512, 653–658. [Google Scholar] [CrossRef]
- Alimonti, A.; Ristori, G.; Giubilei, F.; Stazi, M.A.; Pino, A.; Visconti, A.; Brescianini, S.; Monti, M.S.; Forte, G.; Stanzione, P.; et al. Serum Chemical Elements and Oxidative Status in Alzheimer’s Disease, Parkinson Disease and Multiple Sclerosis. NeuroToxicology 2007, 28, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Guilarte, T.R. Manganese Neurotoxicity: New Perspectives from Behavioral, Neuroimaging, and Neuropathological Studies in Humans and Non-Human Primates. Front. Aging Neurosci. 2013, 5, 23. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, E.C.; Brandel, J.-P.; Galle, P.; Javoy-Agid, F.; Agid, Y. Iron and Aluminum Increase in the Substantia Nigra of Patients with Parkinson’s Disease: An X-Ray Microanalysis. J. Neurochem. 1991, 56, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Altschuler, E. Aluminum-Containing Antacids as a Cause of Idiopathic Parkinson’s Disease. Med. Hypotheses 1999, 53, 22–23. [Google Scholar] [CrossRef]
- Zatta, P.; Zambenedetti, P.; Milanese, M. Activation of Monoamine Oxidase Type-B by Aluminum in Rat Brain Homogenate. NeuroReport 1999, 10, 3645–3648. [Google Scholar] [CrossRef]
- Baum, L.; Chan, I.H.S.; Cheung, S.K.-K.; Goggins, W.B.; Mok, V.; Lam, L.; Leung, V.; Hui, E.; Ng, C.; Woo, J.; et al. Serum Zinc Is Decreased in Alzheimer’s Disease and Serum Arsenic Correlates Positively with Cognitive Ability. BioMetals 2010, 23, 173–179. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Zhao, Y.; Hill, J.M.; Culicchia, F.; Kruck, T.P.A.; Percy, M.E.; Pogue, A.I.; Walton, J.R.; Lukiw, W.J. Selective Accumulation of Aluminum in Cerebral Arteries in Alzheimer’s Disease (AD). J. Inorg. Biochem. 2013, 126, 35–37. [Google Scholar] [CrossRef]
- González-Domínguez, R.; García-Barrera, T.; Gómez-Ariza, J.L. Characterization of Metal Profiles in Serum during the Progression of Alzheimer’s Disease. Metallomics 2014, 6, 292–300. [Google Scholar] [CrossRef]
- Smorgon, C.; Mari, E.; Atti, A.R.; Dalla Nora, E.; Zamboni, P.F.; Calzoni, F.; Passaro, A.; Fellin, R. Trace Elements and Cognitive Impairment: An Elderly Cohort Study. Arch. Gerontol. Geriatr. 2004, 38, 393–402. [Google Scholar] [CrossRef]
- Du, K.; Liu, M.; Pan, Y.; Zhong, X.; Wei, M. Association of Serum Manganese Levels with Alzheimer’s Disease and Mild Cognitive Impairment: A Systematic Review and Meta-Analysis. Nutrients 2017, 9, 231. [Google Scholar] [CrossRef]
- Koseoglu, E.; Koseoglu, R.; Kendirci, M.; Saraymen, R.; Saraymen, B. Trace Metal Concentrations in Hair and Nails from Alzheimer’s Disease Patients: Relations with Clinical Severity. J. Trace Elem. Med. Biol. 2017, 39, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Szabo, S.T.; Harry, G.J.; Hayden, K.M.; Szabo, D.T.; Birnbaum, L. Comparison of Metal Levels between Postmortem Brain and Ventricular Fluid in Alzheimer’s Disease and Nondemented Elderly Controls. Toxicol. Sci. 2016, 150, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Gorantla, N.V.; Das, R.; Balaraman, E.; Chinnathambi, S. Transition Metal Nickel Prevents Tau Aggregation in Alzheimer’s Disease. Int. J. Biol. Macromol. 2020, 156, 1359–1365. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Yang, H.; Tian, X.; Wang, H.; Zhou, T.; Zhang, S.; Yu, J.; Zhang, T.; Fan, D.; Guo, X.; et al. High Manganese, A Risk for Alzheimer’s Disease: High Manganese Induces Amyloid-β Related Cognitive Impairment. J. Alzheimers Dis. 2014, 42, 865–878. [Google Scholar] [CrossRef] [PubMed]
- Walton, J.R.; Wang, M.-X. APP Expression, Distribution and Accumulation Are Altered by Aluminum in a Rodent Model for Alzheimer’s Disease. J. Inorg. Biochem. 2009, 103, 1548–1554. [Google Scholar] [CrossRef]
- Bouras, C.; Giannakopoulos, P.; Good, P.F.; Hsu, A.; Hof, P.R.; Perl, D.P. A Laser Microprobe Mass Analysis of Brain Aluminum and Iron in Dementia Pugilistica: Comparison with Alzheimer’s Disease. Eur. Neurol. 2007, 38, 53–58. [Google Scholar] [CrossRef]
- Rondeau, V.; Jacqmin-Gadda, H.; Commenges, D.; Helmer, C.; Dartigues, J.-F. Aluminum and Silica in Drinking Water and the Risk of Alzheimer’s Disease or Cognitive Decline: Findings from 15-Year Follow-up of the PAQUID Cohort. Am. J. Epidemiol. 2009, 169, 489–496. [Google Scholar] [CrossRef]
- Zhang, Q.L.; Jia, L.; Jiao, X.; Guo, W.L.; Ji, J.W.; Yang, H.L.; Niu, Q. APP/PS1 Transgenic Mice Treated with Aluminum: An Update of Alzheimer’s Disease Model. Int. J. Immunopathol. Pharmacol. 2012, 25, 49–58. [Google Scholar] [CrossRef]
- Kola, A.; Dudek, D.; Valensin, D. Metal Complexation Mechanisms of Polyphenols Associated to Alzheimer’s Disease. Curr. Med. Chem. 2021, 28, 7278–7294. [Google Scholar] [CrossRef]
- Gallo, A.A.; Sable, H.Z. Conformation of Complexes of Thiamin Pyrophosphate with Divalent Cations as Studied by Nuclear Magnetic Resonance Spectroscopy. J. Biol. Chem. 1975, 250, 4986–4991. [Google Scholar] [CrossRef]
- Gallo, A.A.; Hansen, I.L.; Sable, H.Z.; Swift, T.J. Coenzyme Interactions—Vii. Proton Magnetic Resonance Studies of Complexes of Thiamine Pyrophosphate with Divalent Cations. J. Biol. Chem. 1972, 247, 5913–5920. [Google Scholar] [CrossRef]
- Grande, H.J.; Veeger, C.; Houghton, R.L. A Nuclear-Magnetic-Resonance Study of the Manganese · Thiamine-Pyrophosphate Complex in Solution. Eur. J. Biochem. 1973, 37, 563–569. [Google Scholar] [CrossRef]
- Gary, J.; Adeyemo, A. Interaction of Vitamin B1 (Thiamine Hydrochloride) with Zn(II), Cd(II) and Hg(II) in Deuterated Dimethyl Sulfoxide. Inorganica Chim. Acta 1981, 55, 93–98. [Google Scholar] [CrossRef]
- Albert, A. Quantitative Studies of the Avidity of Naturally Occurring Substances for Trace Metals. 3. Pteridines, Riboflavin and Purines. Biochem. J. 1953, 54, 646–654. [Google Scholar] [CrossRef] [PubMed]
- Aruna Kumari, S.; Kishore Babu, B.; Muralikrishna, N.; Mohana Rao, K.; Sinduri, V.L.; Praveen, K.; Padma Rao, C.V.; Swarna Latha, B.; Ramarao, K.; Veeraiah, V.; et al. Riboflavin Metal Complexes. Pharma Chem. 2015, 7, 307–315. [Google Scholar]
- Hernowo, E.; Angkawijaya, A.E.; Fazary, A.E.; Ismadji, S.; Ju, Y.-H. Complex Stability and Molecular Structure Studies of Divalent Metal Ion with l-Norleucine and Vitamin B3. J. Chem. Eng. Data 2011, 56, 4549–4555. [Google Scholar] [CrossRef]
- Irving, H.; Williams, R.J.P. The Stability of Transition-Metal Complexes. J. Chem. Soc. Resumed 1953, 3192–3210. [Google Scholar] [CrossRef]
- Hasan, M.M.; Hossain, M.E.; Halim, M.E.; Ehsan, M.Q. Preparation and Characterisation of Some Transition Metal Complexes of Niacinamide (Vitamin B3). Pak. J. Sci. Ind. Res. Ser. Phys. Sci. 2015, 58, 59–65. [Google Scholar] [CrossRef]
- Sismanoglu, T. Thermodynamics of Stability Constant of Binary Complex of Nicotinamide with Mn++. Chin. Chem. Lett. 2003, 14, 1207–1210. [Google Scholar]
- Tajmir-Riahi, H.A. Coordination Chemistry of Vitamin C. Part II. Interaction of L-Ascorbic Acid with Zn(II), Cd(II), Hg(II), and Mn(II) Ions in the Solid State and in Aqueous Solution. J. Inorg. Biochem. 1991, 42, 47–55. [Google Scholar] [CrossRef]
- Cesario, D.; Furia, E.; Mazzone, G.; Beneduci, A.; De Luca, G.; Sicilia, E. Complexation of Al3+ and Ni2+ by l-Ascorbic Acid: An Experimental and Theoretical Investigation. J. Phys. Chem. A 2017, 121, 9773–9781. [Google Scholar] [CrossRef] [PubMed]
- Tajmir-Riahi, H.A. Coordination Chemistry of Vitamin C. Part III. Interaction of L-Ascorbic Acid with Al(III), La(III), and Pb(II) Ions. Evidence for Metal Chelate Formation in the Solid and Aqueous Solution. J. Inorg. Biochem. 1991, 44, 39–45. [Google Scholar] [CrossRef]
- Obaleye, J.A.; Orjiekwe, C.L. Synthesis and Characterization of Some Metal Complexes of Vitamin C. Part 21 —Ascorbate Complexes of Mn(II), Fe(III) and Co(II). Synth. React. Inorg. Met.-Org. Chem. 1992, 22, 1015–1029. [Google Scholar] [CrossRef]
- Ünaleroğlu, C.; Mert, Y.; Zümreoğlu-Karan, B. Synthesis and Characterization of Copper Ascorbate. Synth. React. Inorg. Met.-Org. Chem. 2001, 31, 1531–1543. [Google Scholar] [CrossRef]
- Viswanathan, T.S.; Swift, T.J. A Nuclear Magnetic Resonance Study of Pyridoxal Phosphate—Metal Ion Interactions. II. Binding of Manganese(II). Can. J. Chem. 1979, 57, 1050–1055. [Google Scholar] [CrossRef]
- Jasim, M.A.; Al-Ani, H.N. Evaluating Spectra, Thermodynamic and Kinetic Parameters of the Complexation Reaction of Organic Compound or Chelation Therapy Drug with Some Heavy Metal Pollutants. Eurasian Chem. Commun. 2022, 4, 41–51. [Google Scholar] [CrossRef]
- Thompson, D.M.; Balenovich, W.; Hornich, L.H.M.; Richardson, M.F. Reactions of Metal Ions with Vitamins. IV. The Crystal Structure of a Zinc Complex of Pyridoxamine (Vitamin B6). Inorganica Chim. Acta 1980, 46, 199–203. [Google Scholar] [CrossRef]
- Furmanova, N.G.; Berdalieva, Z.I.; Chernaya, T.S.; Resnyanskiĭ, V.F.; Shiitieva, N.K.; Sulaĭmankulov, K.S. Synthesis and Crystal Structures of Coordination Compounds of Pyridoxine with Zinc and Cadmium Sulfates. Crystallogr. Rep. 2009, 54, 228–235. [Google Scholar] [CrossRef]
- Zhu, Y.C.; Zhang, M.Q.; Wu, J.G.; Deng, R.W. The Study of Equilibrium and Formation Constants of Some Transition Metal Complexes with Vitamin B6 in Solution by Potentiometry. Chem. Pap. 2001, 55, 229–232. [Google Scholar]
- Sigel, H.; McCormick, D.B.; Griesser, R.; Prijs, B.; Wright, L.D. Metal Ion Complexes with Biotin and Biotin Derivatives. Participation of Sulfur in the Orientation of Divalent Cations. Biochemistry 1969, 8, 2687–2695. [Google Scholar] [CrossRef]
- Sigel, H.; Scheller, K.H. Metal Ion Complexes of D-Biotin in Solution. Stability of the Stereoselective Thioether Coordination. J. Inorg. Biochem. 1982, 16, 297–310. [Google Scholar] [CrossRef]
- Yousef, W. Potentiometric and Conductometric Studies on Complexes of Folic Acid with Some Metal Ions. Int. J. Electrochem. Sci. 2017, 12, 1146–1156. [Google Scholar] [CrossRef] [PubMed]
- Hamed, E.; Attia, M.S.; Bassiouny, K. Synthesis, Spectroscopic and Thermal Characterization of Copper(II) and Iron(III) Complexes of Folic Acid and Their Absorption Efficiency in the Blood. Bioinorg. Chem. Appl. 2009, 2009, e979680. [Google Scholar] [CrossRef] [PubMed]
- Mercê, A.L.R.; Szpoganicz, B.; Dutra, R.C.; Khan, M.A.; Thanh, X.D.; Bouet, G. Potentiometric Study of Vitamin D3 Complexes with Cobalt (II), Nickel (II) and Copper (II) in Water–Ethanol Medium. J. Inorg. Biochem. 1998, 71, 87–91. [Google Scholar] [CrossRef]
- Kieninger, C.; Baker, J.A.; Podewitz, M.; Wurst, K.; Jockusch, S.; Lawrence, A.D.; Deery, E.; Gruber, K.; Liedl, K.R.; Warren, M.J.; et al. Zinc Substitution of Cobalt in Vitamin B12: Zincobyric Acid and Zincobalamin as Luminescent Structural B12-Mimics. Angew. Chem. Int. Ed. 2019, 58, 14568–14572. [Google Scholar] [CrossRef]
- Kieninger, C.; Wurst, K.; Podewitz, M.; Stanley, M.; Deery, E.; Lawrence, A.D.; Liedl, K.R.; Warren, M.J.; Kräutler, B. Replacement of the Cobalt Center of Vitamin B12 by Nickel: Nibalamin and Nibyric Acid Prepared from Metal-Free B12 Ligands Hydrogenobalamin and Hydrogenobyric Acid. Angew. Chem. 2020, 132, 20304–20311. [Google Scholar] [CrossRef]
- Mercê, A.L.R.; Szpoganicz, B.; Khan, M.A.; Do Thanh, X.; Bouet, G. Potentiometric Study of Vitamin D3 Complexes with Manganese(II), Iron(II), Iron(III) and Zinc(II) in Water-Ethanol Medium. J. Inorg. Biochem. 1999, 73, 167–172. [Google Scholar] [CrossRef]
- Das, N.; Raymick, J.; Sarkar, S. Role of Metals in Alzheimer’s Disease. Metab. Brain Dis. 2021, 36, 1627–1639. [Google Scholar] [CrossRef]
- Kennedy, D. B Vitamins and the Brain: Mechanisms, Dose and Efficacy—A Review. Nutrients 2016, 8, 68. [Google Scholar] [CrossRef]
- Scheiber, I.F.; Mercer, J.F.B.; Dringen, R. Metabolism and Functions of Copper in Brain. Prog. Neurobiol. 2014, 116, 33–57. [Google Scholar] [CrossRef]
- Oteiza, P.I. Zinc and the Modulation of Redox Homeostasis. Free Radic. Biol. Med. 2012, 53, 1748–1759. [Google Scholar] [CrossRef] [PubMed]
- Tassone, G.; Kola, A.; Valensin, D.; Pozzi, C. Dynamic Interplay between Copper Toxicity and Mitochondrial Dysfunction in Alzheimer’s Disease. Life 2021, 11, 386. [Google Scholar] [CrossRef] [PubMed]
- Spector, R. Vitamin Transport Diseases of Brain: Focus on Folates, Thiamine and Riboflavin. Brain Disord. Ther. 2014, 3, 2. [Google Scholar] [CrossRef]
- Spector, R.; Johanson, C.E. Vitamin Transport and Homeostasis in Mammalian Brain: Focus on Vitamins B and E. J. Neurochem. 2007, 103, 425–438. [Google Scholar] [CrossRef]
- Graham, S.F.; Nasaruddin, M.B.; Carey, M.; Holscher, C.; McGuinness, B.; Kehoe, P.G.; Love, S.; Passmore, P.; Elliott, C.T.; Meharg, A.A.; et al. Age-Associated Changes of Brain Copper, Iron, and Zinc in Alzheimer’s Disease and Dementia with Lewy Bodies. J. Alzheimers Dis. 2014, 42, 1407–1413. [Google Scholar] [CrossRef]
- Uchida, Y.; Ito, K.; Ohtsuki, S.; Kubo, Y.; Suzuki, T.; Terasaki, T. Major Involvement of Na+-Dependent Multivitamin Transporter (SLC5A6/SMVT) in Uptake of Biotin and Pantothenic Acid by Human Brain Capillary Endothelial Cells. J. Neurochem. 2015, 134, 97–112. [Google Scholar] [CrossRef]
- Ghasemzadeh, S.; Riazi, G.H. Inhibition of Tau Amyloid Fibril Formation by Folic Acid: In-Vitro and Theoretical Studies. Int. J. Biol. Macromol. 2020, 154, 1505–1516. [Google Scholar] [CrossRef]
- Espín, S.; Sánchez-Virosta, P. A Review of Metal-Induced Effects on Vitamins A, E and D3 in Birds. Ecotoxicology 2021, 30, 1–16. [Google Scholar] [CrossRef]
- Sampaio, I.; Quatroni, F.D.; Pincela Lins, P.M.; Nascimento, A.S.; Zucolotto, V. Modulation of Beta-Amyloid Aggregation Using Ascorbic Acid. Biochimie 2022, 200, 36–43. [Google Scholar] [CrossRef]
- Cannell, J.J.; Grant, W.B. What Is the Role of Vitamin D in Autism? Dermatoendocrinol. 2013, 5, 199–204. [Google Scholar] [CrossRef]
- Máčová, L.; Bičíková, M.; Ostatníková, D.; Hill, M.; Stárka, L. Vitamin D, Neurosteroids and Autism. Physiol. Res. 2017, 66, S333–S340. [Google Scholar] [CrossRef]
- Temova Rakuša, Ž.; Pišlar, M.; Kristl, A.; Roškar, R. Comprehensive Stability Study of Vitamin D3 in Aqueous Solutions and Liquid Commercial Products. Pharmaceutics 2021, 13, 617. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kola, A.; Nencioni, F.; Valensin, D. Bioinorganic Chemistry of Micronutrients Related to Alzheimer’s and Parkinson’s Diseases. Molecules 2023, 28, 5467. https://doi.org/10.3390/molecules28145467
Kola A, Nencioni F, Valensin D. Bioinorganic Chemistry of Micronutrients Related to Alzheimer’s and Parkinson’s Diseases. Molecules. 2023; 28(14):5467. https://doi.org/10.3390/molecules28145467
Chicago/Turabian StyleKola, Arian, Federico Nencioni, and Daniela Valensin. 2023. "Bioinorganic Chemistry of Micronutrients Related to Alzheimer’s and Parkinson’s Diseases" Molecules 28, no. 14: 5467. https://doi.org/10.3390/molecules28145467
APA StyleKola, A., Nencioni, F., & Valensin, D. (2023). Bioinorganic Chemistry of Micronutrients Related to Alzheimer’s and Parkinson’s Diseases. Molecules, 28(14), 5467. https://doi.org/10.3390/molecules28145467








