Single versus Double Coffee-Ring Effect Patterns in Thin-Layer Chromatography Coupled with Surface-Enhanced Raman Spectroscopic Analysis of Anti-Diabetic Drugs Adulterated in Herbal Products
Abstract
1. Introduction
2. Results
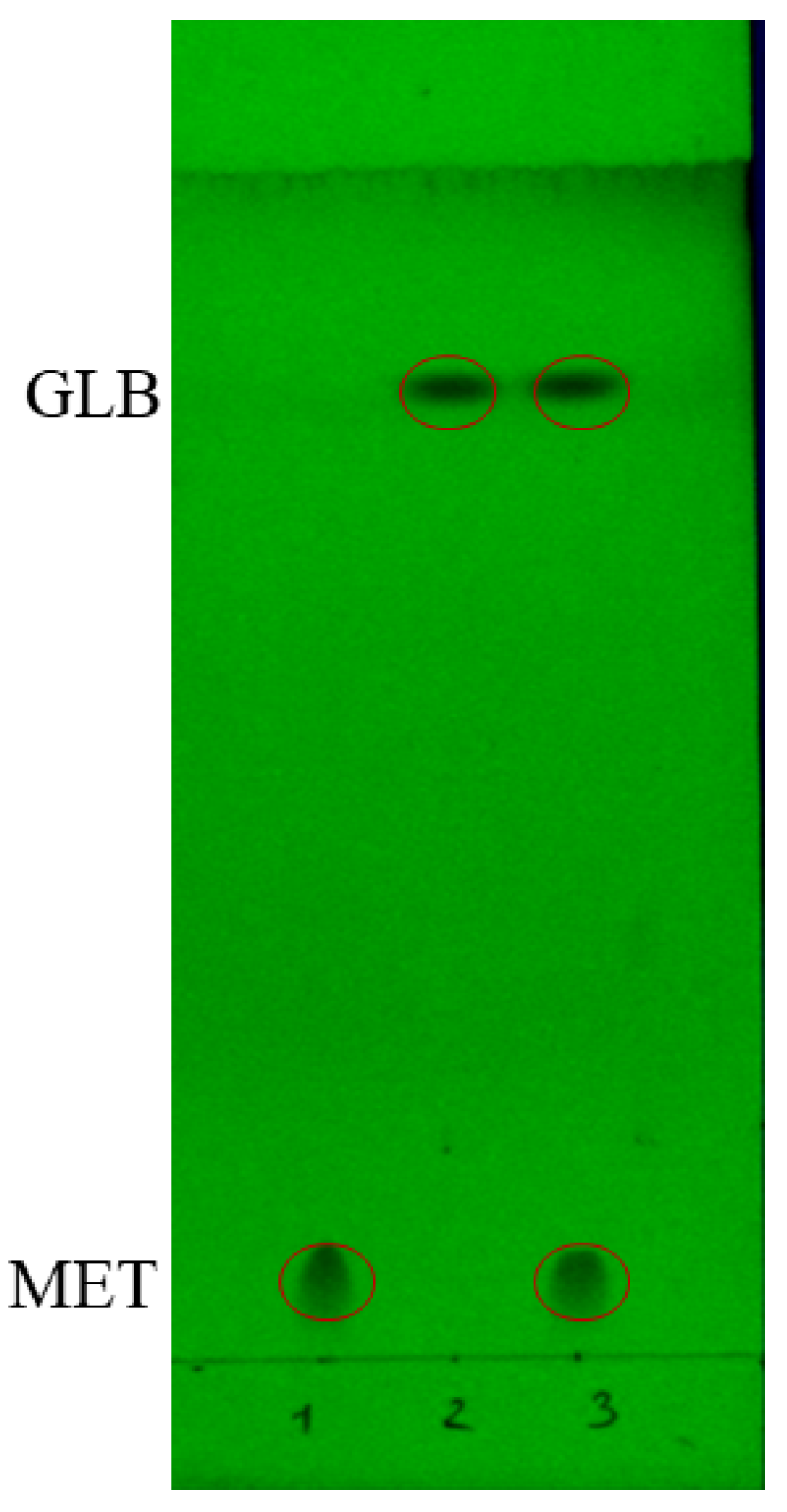
2.1. Preliminary TLC-SERS Method Development
2.1.1. Thin Layer Chromatographic Design
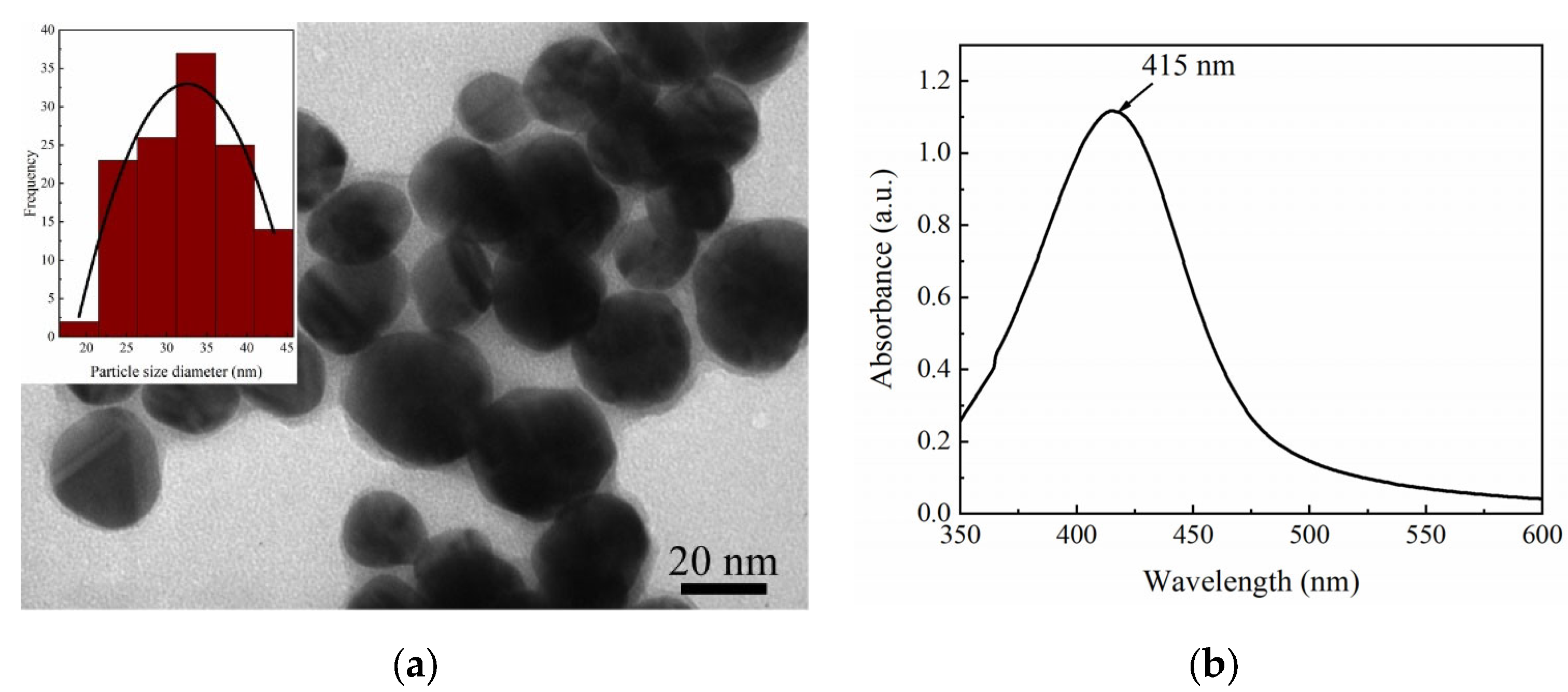
2.1.2. Silver Colloid Solution Preparation
2.1.3. Selection of Typical Raman Shifts for SERS Analysis
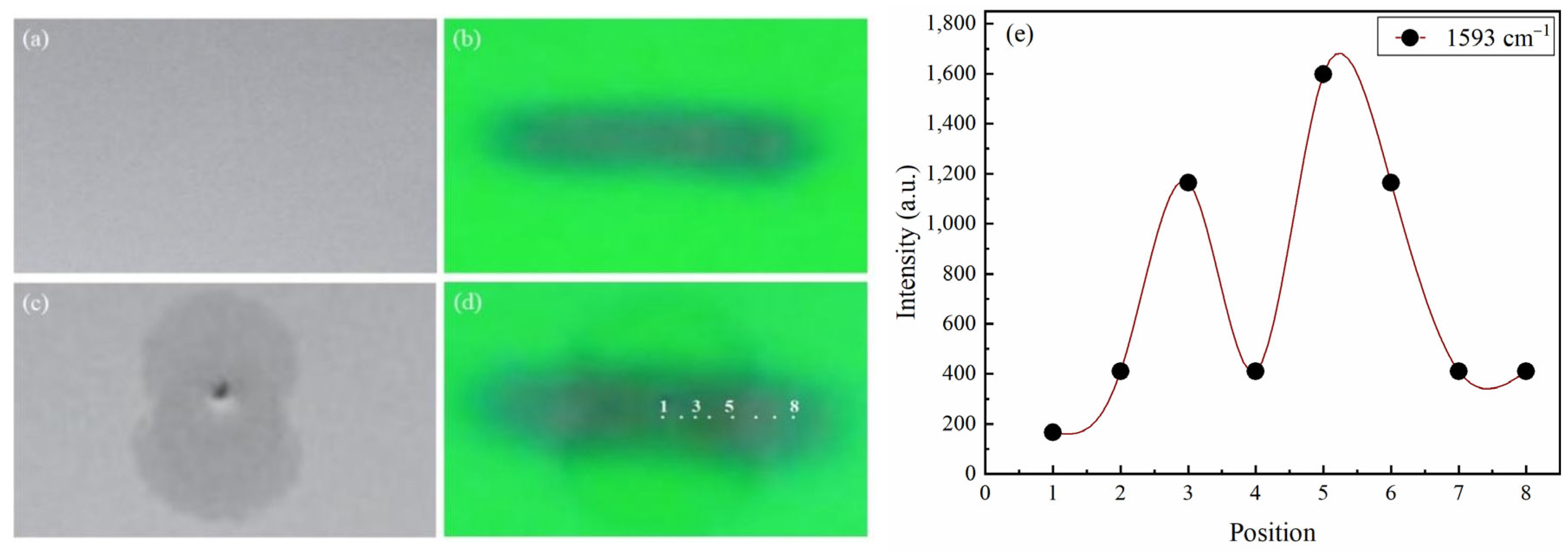
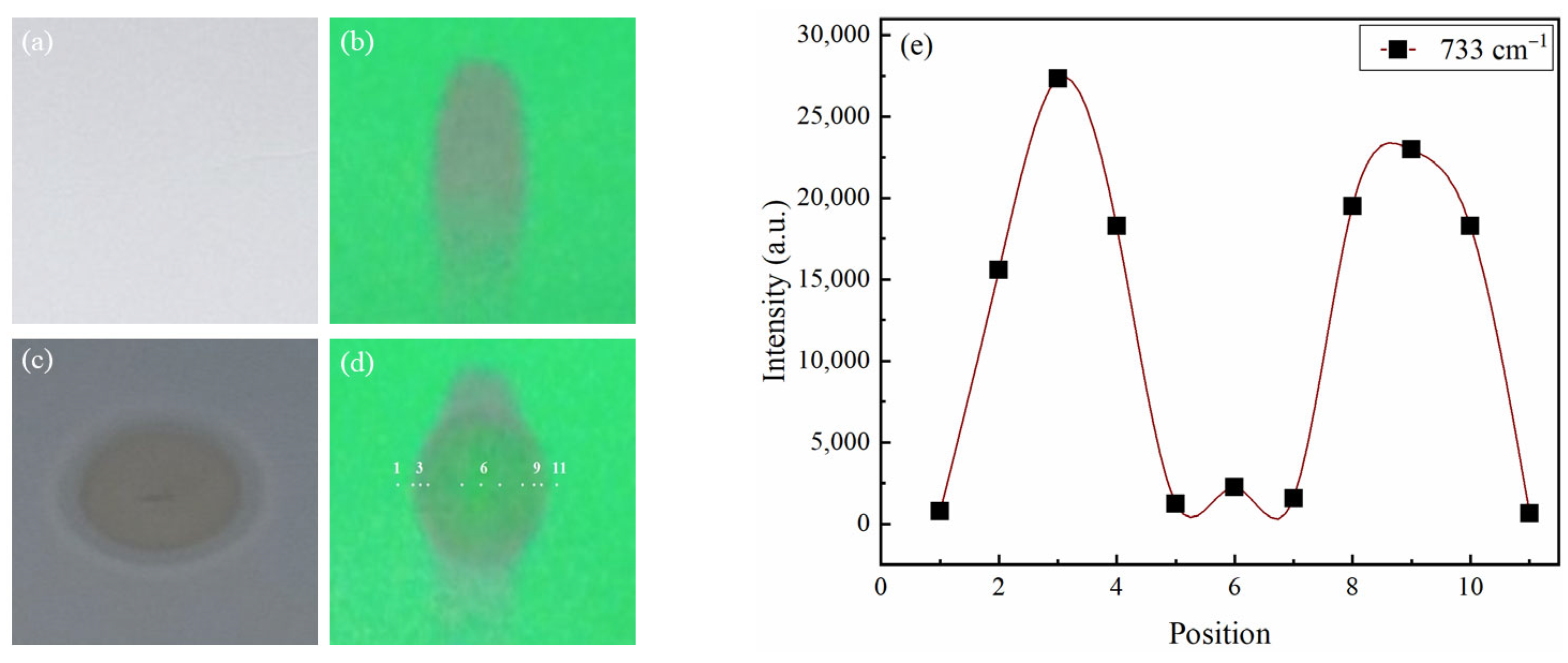
2.2. Determination of CRE Pattern
2.2.1. CRE Pattern of Glibenclamide
2.2.2. CRE Pattern of Metformin
2.3. CRE-Based TLC-SERS Method Optimization
2.3.1. CRE-Based Optimization for Glibenclamide
2.3.2. CRE-Based Optimization for Metformin
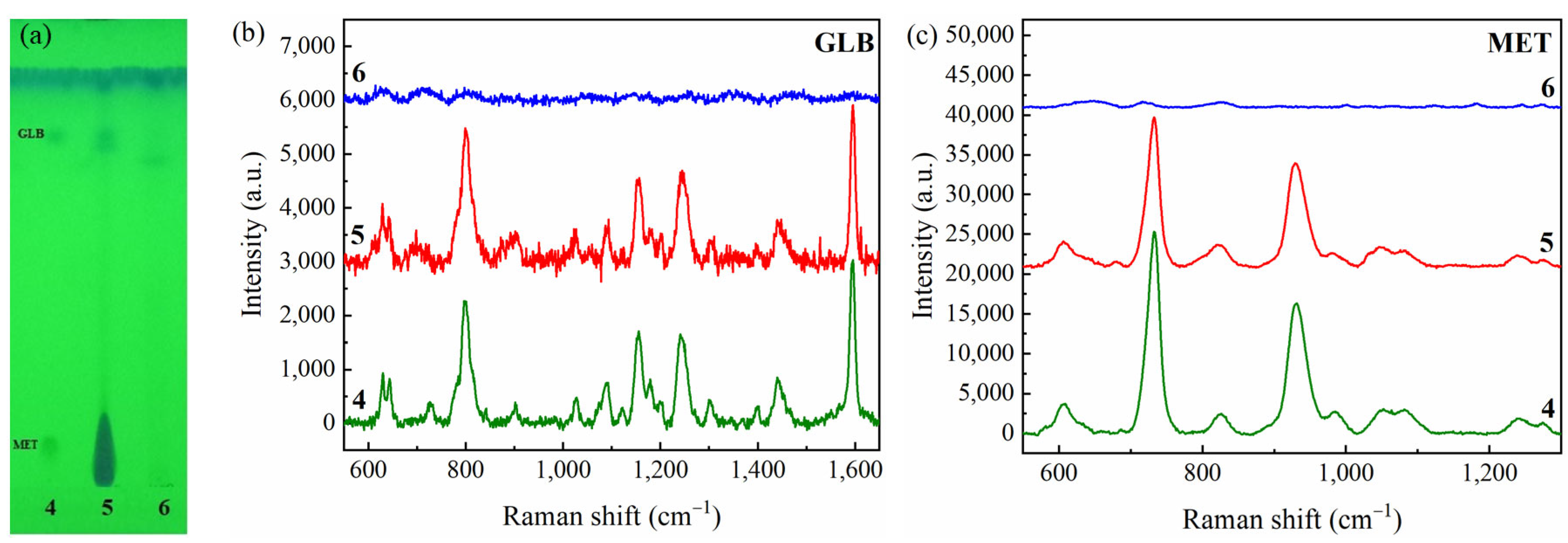
2.4. Validation of the Detection Method by TLC-SERS
2.4.1. Selectivity
2.4.2. Limit of Detection
2.5. Analysis of GLB and Adulterated MET in Herbal Products
2.5.1. CRE-Based TLC-SERS Analysis of Real Samples
2.5.2. Parallel HPLC Analysis of Real Samples
3. Materials and Methods
3.1. Chemical Reagents and Materials
3.2. Apparatus and Equipment
3.3. Sample Preparation
3.4. Silver Colloid Preparation
3.5. Analytical Conditions
3.5.1. For TLC-SERS Method
3.5.2. For the HPLC-DAD Method
4. Discussion
4.1. Remarks from TLC-SERS Method Development
4.2. Remarks from CRE-Based TLC-SERS Method Optimization
4.3. Remarks from TLC-SERS Method Application in Real Samples
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pratiwi, R.; Dipadharma, R.H.F.; Prayugo, I.J.; Layandro, O.A. Recent analytical method for detection of chemical adulterants in herbal medicine. Molecules 2021, 26, 6606. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhu, Q.; Chwee, T.; Wu, L.; Chai, Y.; Lu, F.; Yuan, Y. Detection of structurally similar adulterants in botanical dietary supplements by thin-layer chromatography and surface enhanced Raman spectroscopy combined with two-dimensional correlation spectroscopy. Anal. Chim. Acta 2015, 883, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Hu, Y.; Ha, T.; Ma, Y.; Yu, X. Fabrication of encapsulated and sticky surface-enhanced Raman spectroscopy substrate coupling with thin layer chromatography for determination of pesticide. Vib. Spectrosc. 2023, 125, 103499. [Google Scholar] [CrossRef]
- Hu, X.; Fang, G.; Han, A.; Fu, Y.; Tong, R.; Wang, S. Rapid detection of six phosphodiesterase type 5 enzyme inhibitors in healthcare products using thin-layer chromatography and surface enhanced Raman spectroscopy combined with BP neural network. J. Sep. Sci. 2017, 40, 2506–2514. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Fang, D.; Wu, Y.; Wu, Y.; Yao, W. Controllable Preparation of Gold Nanocrystals with Different Porous Structures for SERS Sensing. Molecules 2023, 28, 2316. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Xie, Y.; Mrozek, M.F.; Ortiz, C.; Davisson, V.J.; Ben-Amotz, D. Raman Detection of Proteomic Analytes. Anal. Chem. 2003, 75, 5703–5709. [Google Scholar] [CrossRef]
- Zhu, Q.; Li, X.; Li, D.; Lu, F.; Zhao, Y.; Yuan, Y. A Rapid Therapeutic Drug Monitoring Strategy of Carbamazepine in Serum by Using Coffee-Ring Effect Assisted Surface-Enhanced Raman Spectroscopy. Molecules 2023, 28, 128. [Google Scholar] [CrossRef]
- Tang, S.; Li, Y.; Huang, H.; Li, P.; Guo, Z.; Luo, Q.; Wang, Z.; Chu, P.K.; Li, J.; Yu, X.F. Efficient Enrichment and Self-Assembly of Hybrid Nanoparticles into Removable and Magnetic SERS Substrates for Sensitive Detection of Environmental Pollutants. ACS Appl. Mater. Interfaces 2017, 9, 7472–7480. [Google Scholar] [CrossRef]
- Yunker, P.J.; Still, T.; Lohr, M.A.; Yodh, A.G. Suppression of the coffee-ring effect by shape-dependent capillary interactions. Nature 2011, 476, 308–311. [Google Scholar] [CrossRef]
- Zhou, W.; Hu, A.; Bai, S.; Ma, Y.; Su, Q. Surface-enhanced Raman spectra of medicines with large-scale self-assembled silver nanoparticle films based on the modified coffee ring effect. Nanoscale Res. Lett. 2014, 9, 87. [Google Scholar] [CrossRef]
- Wang, J.; Feng, S.; Lin, J.; Zeng, Y.; Li, L.; Huang, Z.; Li, B.; Zeng, H.; Chen, R. Serum albumin and globulin analysis for hepatocellular carcinoma detection avoiding false-negative results from alpha-fetoprotein test negative subjects. Appl. Phys. Lett. 2013, 103, 204106. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, L.; Li, X.; Ong, L.; Soe, Y.G.; Sinsua, N.; Gras, S.L.; Tabor, R.F.; Wang, X.; Shen, W. Trace analysis and chemical identification on cellulose nanofibers-textured SERS substrates using the "coffee ring" effect. ACS Sens. 2017, 2, 1060–1067. [Google Scholar] [CrossRef]
- Zhu, Q.; Yu, X.; Wu, Z.; Lu, F.; Yuan, Y. Antipsychotic drug poisoning monitoring of clozapine in urine by using coffee ring effect based surface-enhanced Raman spectroscopy. Anal. Chim. Acta 2018, 1014, 64–70. [Google Scholar] [CrossRef]
- Minh, D.T.C.; Thi, L.A.; Huyen, N.T.T.; Van Vu, L.; Anh, N.T.K.; Ha, P.T.T. Detection of sildenafil adulterated in herbal products using thin layer chromatography combined with surface enhanced Raman spectroscopy: “Double coffee-ring effect” based enhancement. J. Pharm. Biomed. Anal. 2019, 174, 340–347. [Google Scholar] [CrossRef]
- Ramirez-Perez, J.C.; Reis, T.A.; Olivera, C.L.P.; Rizzutto, M.A. Impact of silver nanoparticles size on SERS for detection and identification of filamentous fungi. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 272, 120980. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Y.-J.; Meng, J.-H.; Chen, S.; Panneerselvam, R.; Li, C.-Y.; Jamali, S.B.; Li, X.; Yang, Z.-L.; Li, J.-F.; et al. A facile method for the synthesis of large-size Ag nanoparticles as efficient SERS substrates. J. Raman Spectrosc. 2016, 47, 662–667. [Google Scholar] [CrossRef]
- Sidhu, M.; Sharma, T. Antihyperglycemic activity of petroleum ether leaf extract of Ficus krishnae L. on alloxan-induced diabetic rats. Indian J. Pharm. Sci. 2014, 76, 323–331. [Google Scholar]
- Meng, W.; Hu, F.; Jiang, X.; Lu, L. Preparation of silver colloids with improved uniformity and stable surface-enhanced Raman scattering. Nanoscale Res. Lett. 2015, 10, 34. [Google Scholar] [CrossRef]
- Bayramov, F.B.; Toporov, V.V.; Chakchir, O.B.; Anisimov, V.N.; Rud, V.Y.; Glinushkin, A.P.; Bairamov, B.H. Raman spectroscopic characterization of anti-diabetic drug metformin hydrochloride. J. Phys. Conf. Ser. 2019, 1400, 033010. [Google Scholar] [CrossRef]
- Zhu, Q.; Cao, Y.; Cao, Y.; Chai, Y.; Lu, F. Rapid on-site TLC-SERS detection of four antidiabetes drugs used as adulterants in botanical dietary supplements. Anal. Bioanal. Chem. 2014, 406, 1877–1884. [Google Scholar] [CrossRef]
- Ren, X.; Li, X. Flower-like Ag coated with molecularly imprinted polymers as a surface-enhanced Raman scattering substrate for the sensitive and selective detection of glibenclamide. Anal. Methods 2020, 12, 2858–2864. [Google Scholar] [CrossRef] [PubMed]
- Castro, R.C.; Ribeiro, D.S.M.; Santos, J.L.M.; Nunes, C.; Reis, S.; Páscoa, N.M.J. Chemometric-assisted surface-enhanced Raman spectroscopy for metformin determination using gold nanoparticles as substrate. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2023, 287 (Pt 2), 122118. [Google Scholar] [CrossRef] [PubMed]
- Borah, B.; Devi, T.G. Molecular property analysis of the interacting state of L-Threonine and Metformin: An experimental and computational approach. J. Mol. Struct. 2020, 1221, 128819. [Google Scholar] [CrossRef]
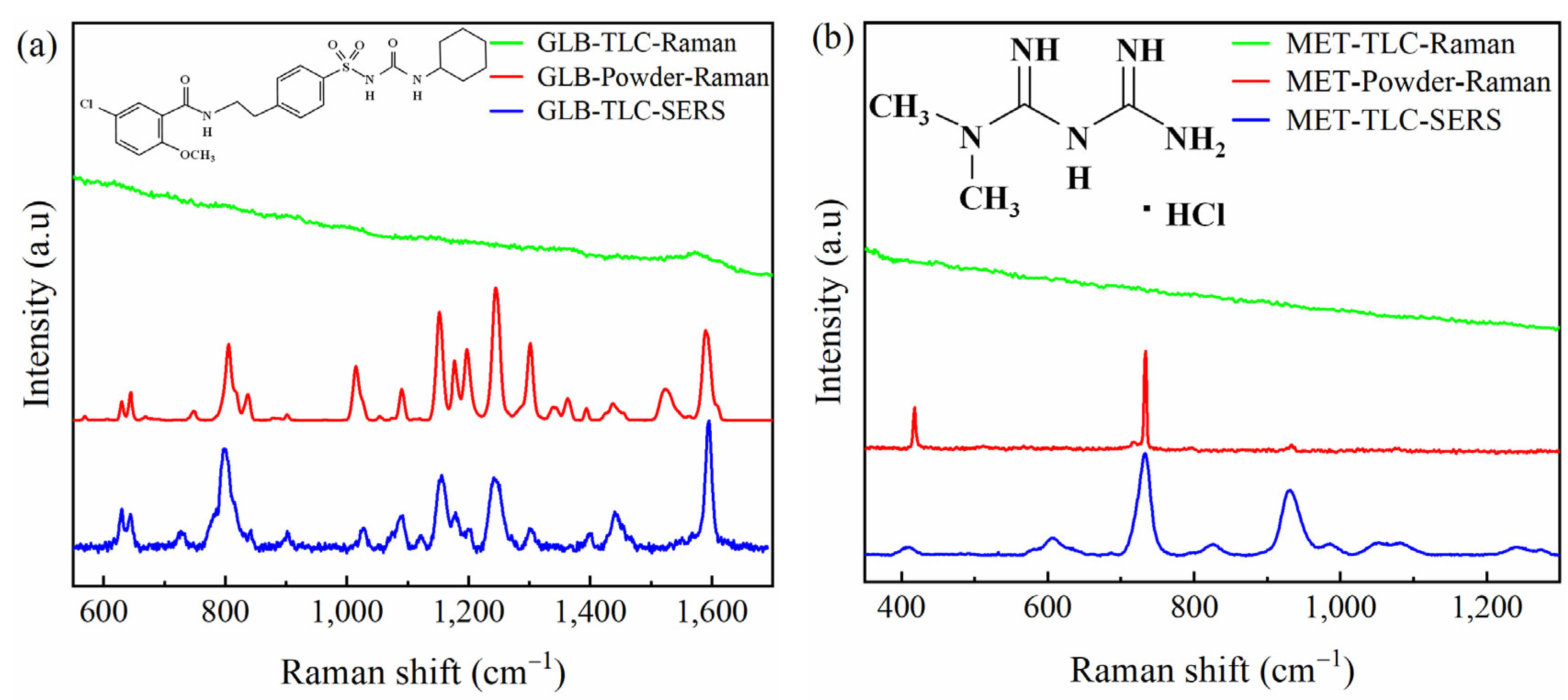


| Samples | Powder Raman Shift (cm−1) | Solution SERS Shift (cm−1) | Ref. | Description |
|---|---|---|---|---|
| GLB | 644 | 643 | 653 a; 653 b | C-Cl stretching |
| 745 | 726 | 763 a; 787 b | C-H bending | |
| 805 | 802 | 816 a; 810 b | C-H bending | |
| 839 | 839 | 842 a; 843 b | C-H bending | |
| 903 | 903 | 855 a; 878 b | C-H bending | |
| 1014 | 1008 | 1027 a; 1030 b | C-H bending | |
| 1054 | 1025 | 1053 a; 1088 b | C-O stretching | |
| 1090 | 1087 | 1110 a | C-H bending | |
| 1151 | 1155 | 1163 a;1158 b | Sulfonyl stretching | |
| 1244 | 1241 | 1261 a; 1203 b | C-H bending | |
| 1362 | 1368 | 1344 a; 1336 b | C-H bending | |
| 1394 | 1398 | 1399 a; | Sulfonyl stretching | |
| 1435 | 1441 | 1433 a; 1442 b | C=C stretching | |
| 1523 | 1545 | 1530 b | C=C stretching | |
| 1589 | 1593 | 1592 a; 1582 b | C=O stretching | |
| MET | 418 | 409 | 561 a, 490 c; 428 d | C-N-C deformation |
| 606 | 622 a, 623 d | C-N-C deformation | ||
| 733 | 733 | 739 a, 750 c | N-H wagging | |
| 826 | 849 a, | N-H wagging | ||
| 931 | 932 | 950 c; 931 d | N-H wagging | |
| 987 | 1003 a | N-H wagging | ||
| 1047 | 1033 a; 1057 d | C-N stretching | ||
| 1081 | 1045 d | C-N stretching | ||
| 1240 | 1209 a; 1248 d | C-N stretching | ||
| 1275 | 1300 c; 1266 d | C-N stretching |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minh, D.T.C.; Tram, L.T.B.; Phong, N.H.; Huong, H.T.L.; Vu, L.V.; Thi, L.A.; Anh, N.T.K.; Ha, P.T.T. Single versus Double Coffee-Ring Effect Patterns in Thin-Layer Chromatography Coupled with Surface-Enhanced Raman Spectroscopic Analysis of Anti-Diabetic Drugs Adulterated in Herbal Products. Molecules 2023, 28, 5492. https://doi.org/10.3390/molecules28145492
Minh DTC, Tram LTB, Phong NH, Huong HTL, Vu LV, Thi LA, Anh NTK, Ha PTT. Single versus Double Coffee-Ring Effect Patterns in Thin-Layer Chromatography Coupled with Surface-Enhanced Raman Spectroscopic Analysis of Anti-Diabetic Drugs Adulterated in Herbal Products. Molecules. 2023; 28(14):5492. https://doi.org/10.3390/molecules28145492
Chicago/Turabian StyleMinh, Dao Thi Cam, Le Thi Bao Tram, Nguyen Hai Phong, Hoang Thi Lan Huong, Le Van Vu, Le Anh Thi, Nguyen Thi Kieu Anh, and Pham Thi Thanh Ha. 2023. "Single versus Double Coffee-Ring Effect Patterns in Thin-Layer Chromatography Coupled with Surface-Enhanced Raman Spectroscopic Analysis of Anti-Diabetic Drugs Adulterated in Herbal Products" Molecules 28, no. 14: 5492. https://doi.org/10.3390/molecules28145492
APA StyleMinh, D. T. C., Tram, L. T. B., Phong, N. H., Huong, H. T. L., Vu, L. V., Thi, L. A., Anh, N. T. K., & Ha, P. T. T. (2023). Single versus Double Coffee-Ring Effect Patterns in Thin-Layer Chromatography Coupled with Surface-Enhanced Raman Spectroscopic Analysis of Anti-Diabetic Drugs Adulterated in Herbal Products. Molecules, 28(14), 5492. https://doi.org/10.3390/molecules28145492







