Antimicrobial and Apoptotic Efficacy of Plant-Mediated Silver Nanoparticles
Abstract
1. Introduction
2. Results
2.1. Antioxidant Capacity of Extracts Evaluated Using SNPAC and FRAP Tests
2.1.1. SNPAC
2.1.2. FRAP
2.1.3. DPPH
2.1.4. Total Phenolic and Flavonoid Content
2.2. Tanacetum Vulgare-Ag-NPs Characteristic
2.2.1. UV–vis Spectroscopy
2.2.2. Scanning Electron Microscopy (SEM) with Energy-Dispersive X-ray Spectroscopy (EDX)
2.2.3. Zeta Potential and Size of Ag-NPs
2.2.4. FT-IR Measurements
2.3. Kinetics of Ag+ Ion Release from Ag-NPs in Aqueous Suspensions
2.4. Antimicrobial Activity
2.5. Anticancer Activity
2.5.1. HeLa Cancer Cells
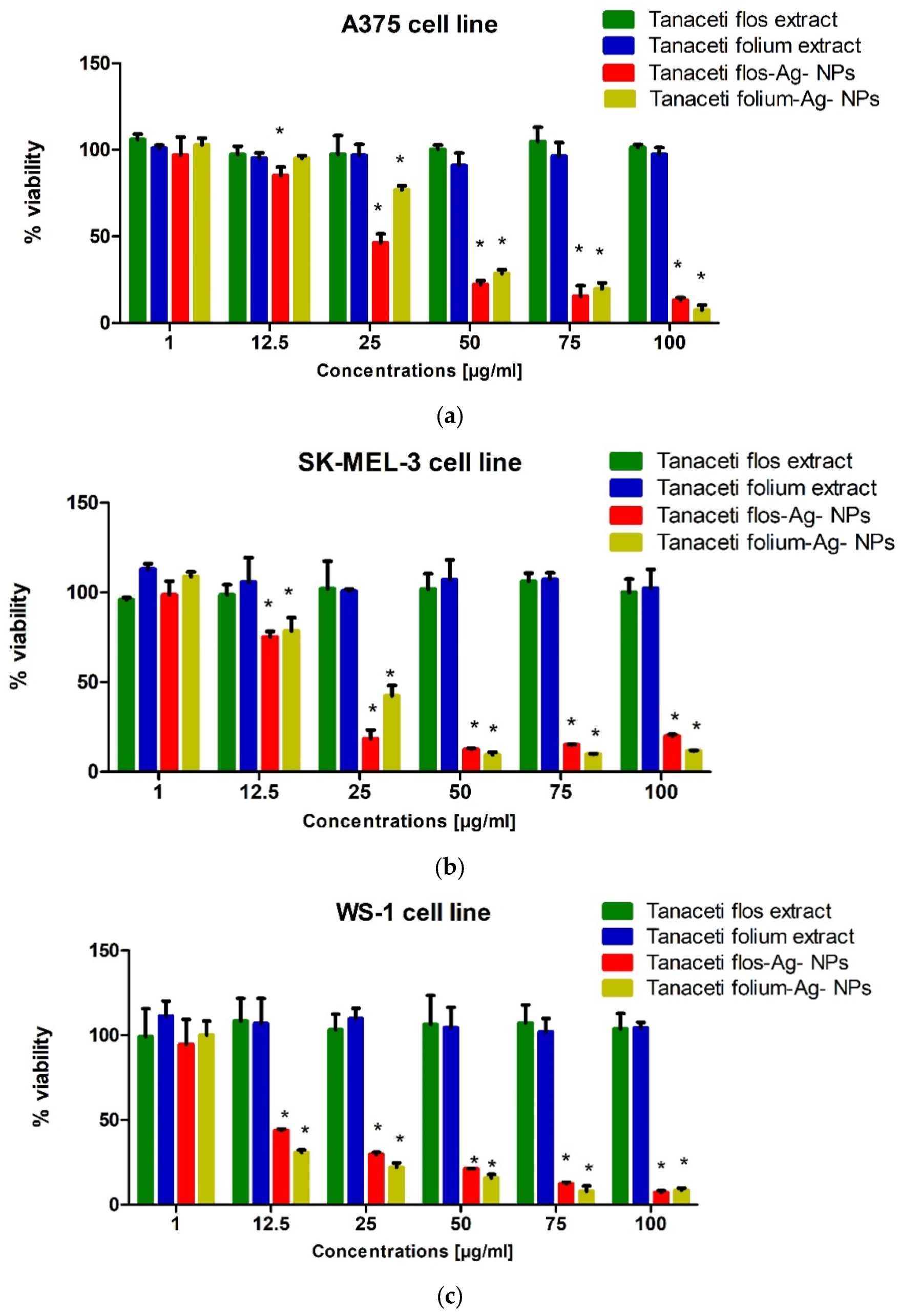
2.5.2. Melanoma Cells
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Collection of Plant Material and Sample Preparation
4.3. Estimation of the Total Phenolic (TPC) and the Total Flavonoid Content (TFC)
4.4. Antioxidant Activity Assays
4.4.1. The Silver NanoParticle Antioxidant Capacity (SNPAC)
4.4.2. Ferric Reducing Antioxidant Power (FRAP) Assay
4.4.3. The 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) Radical Scavenging Assay
4.5. Synthesis of Ag-NPs by Plant Extracts
4.6. Characterisation of Ag-NPs
4.6.1. SEM and EDS
4.6.2. FT-IR
4.6.3. DLS
4.6.4. Conductivity Measurements
4.7. Antimicrobial Activity Assay
4.8. Cytotoxicity Assay
4.8.1. Cells
4.8.2. Cytotoxicity Assay on HeLa Cells
4.8.3. Cytotoxicity Assay on Melanoma Cells
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Das, S.K.; Dickinson, C.; Lafir, F.; Brougham, D.F.; Marsili, E. Synthesis, characterization and catalytic activity of gold nanoparticles biosynthesized with Rhizopus oryzaeprotein extract. Green Chem. 2012, 14, 1322–1334. [Google Scholar] [CrossRef]
- Cheng, F.; Betts, J.W.; Kelly, S.M.; Schaller, J.; Heinze, T. Synthesis and antibacterial effects of aqueous colloidal solutions of silver nanoparticles using aminocellulose as a combined reducing and capping reagent. Green Chem. 2013, 15, 989–998. [Google Scholar] [CrossRef]
- Karczmarska, A.; Adamek, M.; El Houbbadi, S.; Kowalczyk, P.; Laskowska, M. Carbon-Supported Noble-Metal Nanoparticles for Catalytic Applications—A Review. Crystals 2022, 12, 584. [Google Scholar] [CrossRef]
- Habibullah, G.; Viktorova, J.; Ruml, T. Current Strategies for Noble Metal Nanoparticle Synthesis. Nanoscale Res. Lett. 2021, 16, 47. [Google Scholar] [CrossRef]
- Liu, L.; Corma, A. Metal Catalysts for Heterogeneous Catalysis: From Single Atoms to Nanoclusters and Nanoparticles. Chem. Rev. 2018, 118, 4981–5079. [Google Scholar] [CrossRef]
- Klębowski, B.; Depciuch, J.; Parlińska-Wojtan, M.; Baran, J. Applications of Noble Metal-Based Nanoparticles in Medicine. Int. J. Mol. Sci. 2018, 19, 4031. [Google Scholar] [CrossRef]
- Arvizo, R.R.; Bhattacharyya, S.; Kudgus, R.A.; Giri, K.; Bhattacharya, R.; Mukherjee, P. Intrinsic therapeutic applications of noble metal nanoparticles: Past, present and future. Chem. Soc. Rev. 2012, 41, 2943–2970. [Google Scholar] [CrossRef]
- Kong, F.-Y.; Zhang, J.-W.; Li, R.-F.; Wang, Z.-X.; Wang, W.-J.; Wang, W. Unique Roles of Gold Nanoparticles in Drug Delivery, Targeting and Imaging Applications. Molecules 2017, 22, 1445. [Google Scholar] [CrossRef]
- Song, J.; Cheng, L.; Liu, A.; Yin, J.; Kuang, M.; Duan, H. Plasmonic vesicles of amphiphilic gold nanocrystals: Self-assembly and external-stimuli-triggered destruction. J. Am. Chem. Soc. 2011, 133, 10760–10763. [Google Scholar] [CrossRef]
- Riley, M.K., II; Vermerris, W. Recent Advances in Nanomaterials for Gene Delivery—A Review. Nanomaterials 2017, 7, 94. [Google Scholar] [CrossRef]
- Joshi, P.; Chakraborti, S.; Ramirez-Vick, J.E.; Ansari, Z.A.; Shanker, V.; Chakrabarti, P.; Singh, S.P. The anticancer activity of chloroquine-gold nanoparticles against MCF-7 breast cancer cells. Colloids Surf. B Biointerfaces 2012, 95, 195–200. [Google Scholar] [CrossRef]
- Vijayakumar, S.; Ganesan, S. Biocompatibility and Toxicity of Nanobiomaterials. J. Nanomater. 2012, 2012, 734398. [Google Scholar] [CrossRef]
- Asharani, P.V.; Low, G.; Mun, K.; Hande, M.P.; Valiyaveettil, S. Cytotoxicity and Genotoxicity of Silver Nanoparticles in Human Cells. ACS Nano 2009, 3, 279–290. [Google Scholar] [CrossRef]
- Holzinger, M.; Le Goff, A.; Cosnier, S. Nanomaterials for biosensing applications: A review. Front. Chem. 2014, 2, 63. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, G.; Li, Q.; Jiang, H.; Liu, C.; Amatore, C.; Wang, X. In vivo self-bio-imaging of tumors through in situ biosynthesized fluorescent gold nanoclusters. Sci. Rep. 2013, 3, 1157. [Google Scholar] [CrossRef]
- Chien, C.C.; Chen, H.H.; Lai, S.F.; Wu, K.C.; Cai, X.; Hwu, Y.; Petibois, C.; Chu, Y.; Margaritondo, G. Gold nanoparticles as high-resolution X-ray imaging contrast agents for the analysis of tumor-related micro-vasculature. J. Nanobiotechnol. 2012, 10, 10. [Google Scholar] [CrossRef]
- Huang, X.; El-Sayed, I.H.; Qian, W.; El-Sayed, M.A. Cancer cells assemble and align gold nanorods conjugated to antibodies to produce highly enhanced, sharp, and polarized surface Raman spectra: A potential cancer diagnostic marker. Nano Lett. 2007, 7, 1591–1597. [Google Scholar] [CrossRef]
- El-Sayed, I.H.; Huang, X.; El-Sayed, M.A. Surface plasmon resonance scattering and absorption of anti-EGFR antibody conjugated gold nanoparticles in cancer diagnostics: Applications in oral cancer. Nano Lett. 2005, 5, 829–834. [Google Scholar] [CrossRef]
- Pan, Y.; Neuss, S.; Leifert, A.; Fischler, M.; Wen, F.; Simon, U.; Schmid, G.; Brandau, W.; Jahnen-Dechent, W. Size-dependent cytotoxicity of gold nanoparticles. Small 2007, 3, 1941–1949. [Google Scholar] [CrossRef]
- Shankar, S.S.; Rai, A.; Ankamwar, B.; Singh, A.; Ahmad, A.; Sastry, M. Biological synthesis of triangular gold nanoprisms. Nat. Mater. 2004, 3, 482–488. [Google Scholar] [CrossRef]
- Bhattacharya, R.; Mukherjee, P. Biological properties of “naked” metal nanoparticles. Adv. Drug Deliv. Rev. 2008, 60, 1289–1306. [Google Scholar] [CrossRef] [PubMed]
- Magner, L.N.; Oliver, J.K. A History of Medicine; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Burdușel, A.C.; Gherasim, O.; Grumezescu, A.M.; Mogoantă, L.; Ficai, A.; Andronescu, E. Biomedical Applications of Silver Nanoparticles: An Up-to-Date Overview. Nanomaterials 2018, 8, 681. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-R.; Sun, T.-L.; Zhou, S.-L.; Ma, Y.-K.; Shi, Q.-S.; Xie, X.-B.; Huang, X.-M. A comparative analysis of antibacterial activity, dynamics, and effects of silver ions and silver nanoparticles against four bacterial strains. Int. Biodeterior. Biodegrad. 2017, 123, 304–310. [Google Scholar] [CrossRef]
- Mokhena, T.C.; Luyt, A.S. Electrospun alginate nanofibres impregnated with silver nanoparticles: Preparation, morphology and antibacterial properties. Carbohydr. Polym. 2017, 165, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Alshareef, A.; Laird, K.; Cross, R.B.M. Shape-dependent antibacterial activity of silver nanoparticles on Escherichia coli and Enterococcus faecium bacterium. Appl. Surf. Sci. 2017, 424, 310–315. [Google Scholar] [CrossRef]
- Adur, A.J.; Nandini, N.; Shilpashree Mayachar, K.; Ramya, R.; Srinatha, N. Bio-synthesis and antimicrobial activity of silver nanoparticles using anaerobically digested parthenium slurry. J. Photochem. Photobiol. B Biol. 2018, 183, 30–34. [Google Scholar] [CrossRef]
- Zeng, J.; Xiong, X.; Hu, F.; Li, J.; Li, P. Dialdehyde Cellulose Solution as Reducing Agent: Preparation of Uniform Silver Nanoparticles and In Situ Synthesis of Antibacterial Composite Films with High Barrier Properties. Molecules 2023, 28, 2956. [Google Scholar] [CrossRef]
- Etemadzade, M.; Ghamarypour, A.; Zabihollahi, R.; Shabbak, G.; Shirazi, M.; Sahebjamee, H.; Vaziri, A.Z.; Assadi, A.; Ardestani, M.S.; Shandiz, S.A.S.; et al. Synthesis and evaluation of antiviral activities of novel sonochemical silver nanorods against hiv and hsv viruses. Asian Pac. J. Trop. Dis. 2016, 6, 854–858. [Google Scholar] [CrossRef]
- Tamilselvan, S.; Ashokkumar, T.; Govindaraju, K. Microscopy based studies on the interaction of bio-based silver nanoparticles with bombyx mori nuclear polyhedrosis virus. J. Virol. Methods 2017, 242, 58–66. [Google Scholar] [CrossRef]
- Kalaivani, R.; Maruthupandy, M.; Muneeswaran, T.; Hameedha Beevi, A.; Anand, M.; Ramakritinan, C.M.; Kumaraguru, A.K. Synthesis of chitosan mediated silver nanoparticles (Ag NPs) for potential antimicrobial applications. Front. Lab. Med. 2018, 2, 30–35. [Google Scholar] [CrossRef]
- Dojčilović, R.; Pajović, J.D.; Božanić, D.K.; Bogdanović, U.; Vodnik, V.V.; Dimitrijević-Branković, S.; Miljković, M.G.; Kaščaková, S.; Réfrégiers, M.; Djoković, V. Interaction of amino acid-functionalized silver nanoparticles and Candida albicans polymorphs: A deep-UV fluorescence imaging study. Colloids Surf. B Biointerfaces 2017, 155, 341–348. [Google Scholar] [CrossRef]
- Raszewska-Famielec, M.; Flieger, J. Nanoparticles for Topical Application in the Treatment of Skin Dysfunctions—An Overview of Dermo-Cosmetic and Dermatological Products. Int. J. Mol. Sci. 2022, 23, 15980. [Google Scholar] [CrossRef]
- Durán, N.; Durán, M.; de Jesus, M.B.; Seabra, A.B.; Fávaro, W.J.; Nakazato, G. Silver nanoparticles: A new view on mechanistic aspects on antimicrobial activity. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 789–799. [Google Scholar] [CrossRef]
- Koduru, J.R.; Kailasa, S.K.; Bhamore, J.R.; Kim, K.-H.; Dutta, T.; Vellingiri, K. Phytochemical-assisted synthetic approaches for silver nanoparticles antimicrobial applications: A review. Adv. Colloid Interface Sci. 2018, 256, 326–339. [Google Scholar] [CrossRef]
- Lee, J.-H.; Lim, J.-M.; Velmurugan, P.; Park, Y.-J.; Park, Y.-J.; Bang, K.-S.; Oh, B.-T. Photobiologic-mediated fabrication of silver nanoparticles with antibacterial activity. J. Photochem. Photobiol. B Biol. 2016, 162, 93–99. [Google Scholar] [CrossRef]
- Ghiuță, I.; Cristea, D.; Croitoru, C.; Kost, J.; Wenkert, R.; Vyrides, I.; Anayiotos, A.; Munteanu, D. Characterization and antimicrobial activity of silver nanoparticles, biosynthesized using bacillus species. Appl. Surf. Sci. 2018, 438, 66–73. [Google Scholar] [CrossRef]
- De Faria, A.F.; Martinez, D.S.T.; Meira, S.M.M.; de Moraes, A.C.M.; Brandelli, A.; Filho, A.G.S.; Alves, O.L. Anti-adhesion and antibacterial activity of silver nanoparticles supported on graphene oxide sheets. Colloids Surf. B Biointerfaces 2014, 113, 115–124. [Google Scholar] [CrossRef]
- Zhou, Y.; Hu, K.; Guo, Z.; Fang, K.; Wang, X.; Yang, F.; Gu, N. Plla microcapsules combined with silver nanoparticles and chlorhexidine acetate showing improved antibacterial effect. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 78, 349–353. [Google Scholar] [CrossRef]
- Amooaghaie, R.; Saeri, M.R.; Azizi, M. Synthesis, characterization and biocompatibility of silver nanoparticles synthesized from nigella sativa leaf extract in comparison with chemical silver nanoparticles. Ecotoxicol. Environ. Saf. 2015, 120, 400–408. [Google Scholar] [CrossRef]
- Bakadia, B.M.; Lamboni, L.; Ahmed, A.A.Q.; Zheng, R.; Ode Boni, B.O.; Shi, Z.; Song, S.; Souho, T.; Mukole, B.M.; Qi, F.; et al. Antibacterial silk sericin/poly (vinyl alcohol) hydrogel with antifungal property for potential infected large burn wound healing: Systemic evaluation. Smart Mater. Med. 2023, 4, 37–58. [Google Scholar] [CrossRef]
- Thapa, R.K.; Kim, J.H.; Jeong, J.H.; Shin, B.S.; Choi, H.G.; Yong, C.S.; Kim, J.O. Silver nanoparticle-embedded graphene oxide-methotrexate for targeted cancer treatment. Colloids Surf. B Biointerfaces 2017, 153, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Rajeshkumar, S.; Malarkodi, C.; Vanaja, M.; Annadurai, G. Anticancer and enhanced antimicrobial activity of biosynthesizd silver nanoparticles against clinical pathogens. J. Mol. Struct. 2016, 1116, 165–173. [Google Scholar] [CrossRef]
- Stensberg, M.C.; Wei, Q.; McLamore, E.S.; Porterfield, D.M.; Wei, A.; Sepúlveda, M.S. Toxicological studies on silver nanoparticles: Challenges and opportunities in assessment, monitoring and imaging. Nanomedicine 2011, 6, 879–898. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Munoz, R.; Borrego, B.; Juarez-Moreno, K.; Garcia-Garcia, M.; Mota Morales, J.D.; Bogdanchikova, N.; Huerta-Saquero, A. Toxicity of silver nanoparticles in biological systems: Does the complexity of biological systems matter? Toxicol. Lett. 2017, 276, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Salarian, A.A.; Bahari, Y.; Hami, Z.; Soltani-Rezaee-Rad, M. Cephalexin nanoparticles: Synthesis, cytotoxicity and their synergistic antibacterial study in combination with silver nanoparticles. Mater. Chem. Phys. 2017, 198, 125–130. [Google Scholar] [CrossRef]
- Mohamed El Mahdy, M.; Salah, T.; Sayed Aly, H.; Mohammed, F.; Shaalan, M. Evaluation of hepatotoxic and genotoxic potential of silver nanoparticles in albino rats. Exp. Toxicol. Pathol. 2015, 67, 21–29. [Google Scholar] [CrossRef]
- Pinzaru, I.; Coricovac, D.; Dehelean, C.; Moaca, E.A.; Mioc, M.; Baderca, F.; Sizemore, I.; Brittle, S.; Marti, D.; Calina, C.D.; et al. Stable peg-coated silver nanoparticles—A comprehensive toxicological profile. Food Chem. Toxicol. 2018, 111, 546–556. [Google Scholar] [CrossRef]
- Jacob, J.A.; Sivalingam, P.; Chen, B. Toxicological effects of silver nanoparticles. Environ. Toxicol. Pharmacol. 2015, 40, 729–732. [Google Scholar] [CrossRef]
- Sharma, N.C.; Sahi, S.V.; Nath, S.; Parsons, J.G.; Gardea-Torresdey, J.L.; Pal, T. Synthesis of plant-mediated gold nanoparticles and catalytic role of biomatrix-embedded nanomaterials. Environ. Sci. Technol. 2007, 41, 5137–5142. [Google Scholar] [CrossRef]
- Park, M.V.; Neigh, A.M.; Vermeulen, J.P.; de la Fonteyne, L.J.; Verharen, H.W.; Briedé, J.J.; van Loveren, H.; de Jong, W.H. The effect of particle size on the cytotoxicity, inflammation, developmental toxicity and genotoxicity of silver nanoparticles. Biomaterials 2011, 32, 9810–9817. [Google Scholar] [CrossRef]
- Kajani, A.A.; Bordbar, A.K.; Zarkesh Esfahani, S.H.; Khosropour, A.R.; Razmjou, A. Green synthesis of anisotropic silver nanoparticles with potent anticancer activity using Taxus baccata extract. RSC Adv. 2014, 4, 61394–61403. [Google Scholar] [CrossRef]
- Mittal, A.K.; Bhaumik, J.; Kumar, S.; Banerjee, U.C. Biosynthesis of silver nanoparticles: Elucidation of prospective mechanism and therapeutic potential. J. Colloid Interface Sci. 2014, 415, 39–47. [Google Scholar] [CrossRef]
- Iravani, S. Green synthesis of metal nanoparticles using plants. Green Chem. 2011, 13, 2638. [Google Scholar] [CrossRef]
- Mittal, A.K.; Chisti, Y.; Banerjee, U.C. Synthesis of metallic nanoparticles using plant extracts. Biotechnol. Adv. 2013, 31, 346–356. [Google Scholar] [CrossRef]
- Khan, J.A.; Pillai, B.; Das, T.K.; Singh, Y.; Maiti, S. Molecular effects of uptake of gold nanoparticles in HeLa cells. Chembiochem. 2007, 8, 1237–1240. [Google Scholar] [CrossRef]
- Fratoddi, I.; Venditti, I.; Cametti, C.; Russo, M.V. Gold nanoparticles and gold nanoparticle-conjugates for delivery of therapeutic molecules. Progress and challenges. J. Mater. Chem. B 2014, 2, 4204–4220. [Google Scholar] [CrossRef]
- Virkutyte, J.; Varma, R.S. Green synthesis of metal nanoparticles: Biodegradable polymers and enzymes in stabilization and surface functionalization. Chem. Sci. 2011, 2, 837. [Google Scholar] [CrossRef]
- Baram-Pinto, D.; Shukla, S.; Gedanken, A.; Sarid, R. Inhibition of HSV-1 attachment, entry, and cell-to-cell spread by functionalized multivalent gold nanoparticles. Small 2010, 6, 1044–1050. [Google Scholar] [CrossRef]
- Arino, A.; Arberas, I.; Renobales, G.; DomÌnguez, J.B. Influence of extraction method and storage conditions on the volatile oil of wormwood (Artemisia absinthium L.). Eur. Food Technol. 1999, 209, 126–129. [Google Scholar] [CrossRef]
- Setzer, W.N.; Vogler, B.; Schmidt, J.M.; Leahy, J.G.; Rives, R. Antimicrobial activity of Artemisia douglasiana leaf essential oil. Fitoterapia 2004, 75, 192–200. [Google Scholar] [CrossRef]
- Judzentiene, A.; Mockute, D. The inflorescence and leaf essential oils of Tanacetum vulgare L. var. vulgare growing wild in Lithuania. Biochem. Syst. Ecol. 2005, 33, 487–498. [Google Scholar] [CrossRef]
- Larocque, N.; Vincent, C.; Bélanger, A.; Bourassa, J.-P. Effects of Tansy Essential Oil from Tanacetum vulgare on Biology of Oblique-Banded Leafroller, Choristoneura rosaceana. J. Chem. Ecol. 1999, 25, 1319–1330. [Google Scholar] [CrossRef]
- Ivanescu, B.; Vlase, L.; Corciova, A.; Lazar, M.I. HPLC-DAD-MS study of polyphenols from Artemisia absinthium, A. annua, and A. vulgaris. Chem. Nat. Compd. 2010, 46, 468–470. [Google Scholar] [CrossRef]
- Fraisse, D.; Carnat, A.; Viala, D.; Pradel, P.; Besle, J.M.; Coulon, J.-B.; Felgines, C.; Lamaison, J.-L. Polyphenolic composition of a permanent pasture: Variations related to the period of harvesting. J. Sci. Food Agric. 2007, 87, 2427–2435. [Google Scholar] [CrossRef]
- Ferracane, R.; Graziani, G.; Gallo, M.; Fogliano, V.; Ritieni, A. Metabolic profile of the bioactive compounds of burdock (Arctium lappa) seeds, roots and leaves. J. Pharm. Biomed. Anal. 2010, 51, 399–404. [Google Scholar] [CrossRef]
- Kurkin, V.A.; Sharova, O.V. Flavonoids from Calendula officinalis flowers. Chem. Nat. Comp. 2007, 43, 216–217. [Google Scholar] [CrossRef]
- Kalvatchev, Z.; Walder, R.; Garzaro, D. Anti-HIV activity of extracts from Calendula officinalis flowers. Biomed. Pharmacother. 1997, 51, 176–180. [Google Scholar] [CrossRef]
- Zidorn, C.; Grass, S.; Ellmerer, E.P.; Ongania, K.H.; Stuppner, H. Stilbenoids from Tragopogon orientalis. Phytochemistry 2006, 67, 2182–2188. [Google Scholar] [CrossRef]
- Williams, C.A.; Harborne, J.B.; Geiger, H.; Hoult, J.R. The flavonoids of Tanacetum parthenium and T. vulgare and their anti-inflammatory properties. Phytochemistry 1999, 51, 417–423. [Google Scholar] [CrossRef]
- Xie, G.; Schepetkin, I.A.; Quinn, M.T. Immunomodulatory activity of acidic polysaccharides isolated from Tanacetum vulgare L. Int. Immunopharmacol. 2007, 7, 1639–1650. [Google Scholar] [CrossRef]
- Lachenmeier, D.W. Wormwood (Artemisia absinthium L.): A curious plant with both neurotoxic and neuroprotective properties? J. Ethnopharmacol. 2010, 131, 224–227. [Google Scholar] [CrossRef]
- Dembitsky, V.M. Bioactive peroxides as potential therapeutic agents. Eur. J. Med. Chem. 2008, 43, 223–241. [Google Scholar] [CrossRef]
- Szakiel, A.; Ruszkowski, D.; Janiszowska, W. Saponins in Calendula officinalis L.—Structure, Biosynthesis, Transport and Biological Activity. Phytochem. Rev. 2005, 4, 151–158. [Google Scholar] [CrossRef]
- Hamburger, M.; Adler, S.; Baumann, D.; Forg, A.; Weinreich, B. Preparative purification of the major anti-inflammatory triterpenoid esters from marigold (Calendula officinalis). Fitoterapia 2003, 74, 328–338. [Google Scholar] [CrossRef]
- Zitterl-Eglseer, K.; Sosa, S.; Jurenitsch, J.; Schubert-Zsilavecz, M.; Della Loggia, R.; Tubaro, A.; Bertoldi, M.; Franz, C. Anti-oedematous activities of the main triterpendiol esters of marigold (Calendula officinalis L.). J. Ethnopharmacol. 1997, 57, 139–144. [Google Scholar] [CrossRef]
- Wang, B.S.; Yen, G.C.; Chang, L.W.; Yen, W.J.; Duh, P.D. Protective effects of burdock (Arctium lappa Linne) on oxidation of low-density lipoprotein and oxidative stress in RAW 264.7 macrophages. Food Chem. 2006, 101, 729–738. [Google Scholar] [CrossRef]
- Sarker, S.D.; Laird, A.; Nahar, L.; Kumarasamy, Y.; Jaspars, M. Indole alkaloids from the seeds of Centaurea cyanus (Asteraceae). Phytochemistry 2001, 57, 1273–1276. [Google Scholar] [CrossRef]
- Wegiera, M.; Smolarz, H.D.; Jedruch, M.; Korczak, M.; Koproń, K. Cytotoxic effect of some medicinal plants from Asteraceae family on J-45.01 leukemic cell line–pilot study. Acta Pol. Pharm. 2012, 69, 263–268. [Google Scholar]
- Nibret, E.; Wink, M. Volatile components of four Ethiopian Artemisia species extracts and their in vitro antitrypanosomal and cytotoxic activities. Phytomedicine 2010, 17, 369–374. [Google Scholar] [CrossRef]
- Bora, K.S.; Sharma, A. Neuroprotective effect of Artemisia absinthium L. on focal ischemia and reperfusion-induced cerebral injury. J. Ethnopharmacol. 2010, 129, 403–409. [Google Scholar] [CrossRef]
- Duh, P.D. Antioxidant activity of burdock (Arctium lappa Linné): Its scavenging effect on free-radical and active oxygen. J. Amer. Oil Chem. Soc. 1998, 75, 455–461. [Google Scholar] [CrossRef]
- Ramos, A.; Edreira, A.; Vizoso, A.; Betancourt, J.; López, M.; Décalo, M. Genotoxicity of an extract of Calendula officinalis L. J. Ethnopharmacol. 1998, 61, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Guarrera, P.M. Food medicine and minor nourishment in the folk traditions of Central Italy (Marche, Abruzzo and Latium). Fitoterapia 2003, 74, 515–544. [Google Scholar] [CrossRef] [PubMed]
- Keskitalo, M.; Pehu, E.; Simon, J.E. Variation in volatile compounds from tansy (Tanacetum vulgare L.) related to genetic and morphological differences of genotypes. Biochem. Syst. Ecol. 2001, 29, 267–285. [Google Scholar] [CrossRef]
- Goudarzi, T.; Saharkhiz, M.J.; Rowshan, V. Ontogenetic variation of essential oil content and constituents in tansy (Tanacetum vulgare L.). J. App. Res. Med. Arom. Plants 2015, 2, 48–53. [Google Scholar] [CrossRef]
- Devrnja, N.; Anđelković, B.; Aranđelović, S.; Radulović, S.; Soković, M.; Krstić-Milošević, D.; Ristić, M.; Ćalić, D. Comparative studies on the antimicrobial and cytotoxic activities of Tanacetum vulgare L. essential oil and methanol extracts. South Afr. J. Bot. 2017, 111, 212–221. [Google Scholar] [CrossRef]
- Ekundayo, O. Essential oils IV. Composition of the volatile leaf (needle) oils of Pinus species. Z. Für Pflanzenphysiol. 1980, 99, 235–239. [Google Scholar] [CrossRef]
- Von Rudloff, E.; Underhill, E.W. Gas-liquid chromatography of terpenes—XII: Seasonal variation in the volatile oil from Tanacetum vulgare L. Phytochemistry 1965, 4, 11–17. [Google Scholar] [CrossRef]
- Bungau, S.; Baldea, I.; Copolovici, L. The determination of ascorbic acid from fruits using a Landolt type method. Rev. Chim. 2003, 54, 213–216. [Google Scholar]
- Oprea, O.B.; Apostol, L.; Bungau, S.; Cioca, G.; Samuel, A.D.; Badea, M.; Gaceu, L. Researches on the chemical composition and the rheological properties of wheat and grape epicarp flour mixes. Rev. Chim. 2018, 69, 70–75. [Google Scholar] [CrossRef]
- Mosteanu, D.; Epure, G.; Barsan, G.; Giurgiu, L. Research Regarding the Obtaining of Volatile Oils. Rev. Chim. 2009, 60, 290–292. [Google Scholar]
- Mot, C.A.; Lupitu, A.I.; Bungau, S.; Iovan, C.; Copolovici, D.M.; Purza, L.; Melinte, C.E.; Copolovici, L. Composition and Antioxidant Activity of Aqueous Extracts Obtained from Herb of Tansy (Tanacetum vulgare L.). Rev. Chim. 2018, 69, 1041–1044. [Google Scholar] [CrossRef]
- Lahlou, S.; Tahraoui, A.; Israili, Z.; Lyoussi, B. Diuretic activity of the aqueous extracts of Carum carvi and Tanacetum vulgare in normal rats. J. Ethnopharmacol. 2007, 110, 458–463. [Google Scholar] [CrossRef]
- Samuel, A.D.; Brejea, R.C.; Domuta, C.; Bungau, S.; Cenusa, N.D.M.; Tit, D.M. Enzymatic Indicators of Soil Quality. J. Environ. Prot. Ecology 2017, 18, 871–878. [Google Scholar]
- Samuel, A.D.; Tit, D.M.; Melinte, C.E.; Iovan, C.; Purza, L.; Gitea, M.; Bungau, S. Enzymological and Physicochemical Evaluation of the Effects of Soil Management Practices. Rev. Chim. 2017, 68, 2243–2247. [Google Scholar] [CrossRef]
- Taschina, M.; Copolovici, D.M.; Bungau, S.; Lupitu, A.I.; Copolovici, L.; Iovan, C. The influence of residual acetaminophen on Phaseolus vulgaris L. secondary metabolites. Farmacia 2017, 65, 709–713. [Google Scholar]
- Copolovici, L.; Timis, D.; Taschina, M.; Copolovici, D.; Cioca, G.; Bungau, S. Diclofenac influence on photosynthetic parameters and volatile organic compounds emission from Phaseolus vulgaris L. plants. Rev. Chim. 2017, 68, 2076–2078. [Google Scholar] [CrossRef]
- Copolovici, L.; Kännaste, A.; Remmel, T.; Niinemets, Ü. Volatile organic compound emissions from Alnus glutinosa under interacting drought and herbivory stresses. Environ. Exp. Bot. 2014, 100, 55–63. [Google Scholar] [CrossRef]
- Tit, D.M.; Bungau, S.; Nistor Cseppento, C.; Copolovici, D.M.; Buhas, C. Disposal of Unused Medicines Resulting from Home Treatment in Romania. J. Environ. Prot. Ecol. 2016, 17, 1425–1433. [Google Scholar]
- Opriş, O.; Copaciu, F.; Soran, M.L.; Ristoiu, D.; Niinemets, Ü.; Copolovici, L. Influence of nine antibiotics on key secondary metabolites and physiological characteristics in Triticum aestivum: Leaf volatiles as a promising new tool to assess toxicity. Ecotoxicol. Environ. Saf. 2013, 87, 70–79. [Google Scholar] [CrossRef]
- Balint, R.; Nechifor, G.; Ajmone-Marsan, F. Leaching potential of heavy metals from contaminated soils under anoxia. Environ. Sci. Process Impacts 2014, 16, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Balint, R.; Orbeci, C.; Nechifor, G.; Pleşca, M.; Ajmone-Marsan, F. Effect of Redox Conditions on the Crystallinity of Fe Oxides in Soil. Rev. Chim. 2013, 64, 1218–1223. [Google Scholar]
- Diaconu, I.; Gîrdea, R.; Cristea, C.; Nechifor, G.; Ruse, E.; Totu, E.E. Removal and recovery of some phenolic pollutants using liquid membranes. Rom. Biotechnol. Lett. 2010, 15, 5702–5708. [Google Scholar]
- Copolovici, L.; Niinemets, U. Flooding induced emissions of volatile signalling compounds in three tree species with differing waterlogging tolerance. Plant Cell Environ. 2010, 33, 1582. [Google Scholar] [CrossRef] [PubMed]
- Copaciu, F.; Opriş, O.; Coman, V.; Ristoiu, D.; Niinemets, Ü.; Copolovici, L. Diffuse Water Pollution by Anthraquinone and Azo Dyes in Environment Importantly Alters Foliage Volatiles, Carotenoids and Physiology in Wheat (Triticum aestivum). Water Air Soil Pollut. 2013, 224, 1478. [Google Scholar] [CrossRef]
- Orbeci, C.; Nechifor, G.; Stănescu, R. Removing toxic compounds from wastewater. Environ. Eng. Manag. J. 2014, 13, 2153–2158. [Google Scholar] [CrossRef]
- Ghimpusan, M.; Nechifor, G.; Din, I.S.; Nechifor, A.C.; Passeri, P. Application of Hollow Fiber Membrane Bioreactor Instead of Granular Activated Carbon Filtration for Treatment of Wastewater from Car Dismantler Activity. Mat. Plast. 2016, 53, 578–584. [Google Scholar]
- Kamar, F.H.; Mohammed, A.A.; Faisal, A.A.H.; Nechifor, A.C.; Nechifor, G. Biosorption of Lead, Copper and Cadmium Ions from Industrial Wastewater Using Fluidized Bed of Dry Cabbage Leaves. Rev. Chim. 2016, 67, 1039–1046. [Google Scholar]
- Kamar, F.H.; Nechifor, A.C.; Alwan, G.M.; Craciun, M.E.; Nechifor, G. Comparative Removal of Lead, Copper and Cadmium Ions from Wastewater in Single and Ternary Batch Biosorption Systems onto Dry Walnut Shells. Rev. Chim. 2015, 66, 1083–1087. [Google Scholar]
- Coté, H.; Boucher, M.-A.; Pichette, A.; Legault, J. Anti-Inflammatory, Antioxidant, Antibiotic, and Cytotoxic Activities of Tanacetum vulgare L. Essential Oil and Its Constituents. Medicines 2017, 4, 34. [Google Scholar] [CrossRef]
- Bączek, K.B.; Kosakowska, O.; Przybył, J.L.; Pióro-Jabrucka, E.; Costa, R.; Mondello, L.; Gniewosz, M.; Synowiec, A.; Węglarz, Z. Antibacterial and antioxidant activity of essential oils and extracts from costmary (Tanacetum balsamita L.) and tansy (Tanacetum vulgare L.). Ind. Crops Prod. 2017, 102, 154–163. [Google Scholar] [CrossRef]
- Lahlou, S.; Tangi, K.C.; Lyoussi, B.; Morel, N. Vascular effects of Tanacetum vulgare L. leaf extract: In vitro pharmacological study. J. Ethnopharmacol. 2008, 120, 98–102. [Google Scholar] [CrossRef]
- Schinella, G.R.; Giner, R.M.; Recio, M.D.C.; Buschiazzo, P.M.; Rios, J.L.; Manez, S. Anti-Inflammatory Effects of South American Tanacetum vulgare. J. Pharm. Pharmacol. 1998, 50, 1069–1074. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- Flieger, J.; Flieger, W.; Baj, J.; Maciejewski, R. Antioxidants: Classification, Natural Sources, Activity/Capacity Measurements, and Usefulness for the Synthesis of Nanoparticles. Materials 2021, 14, 4135. [Google Scholar] [CrossRef]
- Özyürek, M.; Güngör, N.; Baki, S.; Güclü, K.; Apak, R. Development of a Silver Nanoparticle-Based Method for the Antioxidant Capacity Measurement of Polyphenols. Anal. Chem. 2012, 84, 8052–8059. [Google Scholar] [CrossRef]
- Liberato, M.D.C.T.C.; De Morais, S.M.; Siqueira, S.M.C.; De Menezes, J.E.S.A.; Ramos, D.N.; Machado, L.K.A.; Magalhães, I.L. Phenolic Content and Antioxidant and Antiacetylcholinesterase Properties of Honeys from Different Floral Origins. J. Med. Food 2011, 14, 658–663. [Google Scholar] [CrossRef]
- Shameli, K.; Bin Ahmad, M.; Jaffar, E.A.; Mulla, A.; Ibrahim, N.A.; Shabanzadeh, P.; Rustaiyan, A.; Abdollahi, Y.; Bagheri, S.; Abdolmohammadi, S.; et al. Green Biosynthesis of Silver Nanoparticles Using Callicarpa maingayi Stem Bark Extraction. Molecules 2012, 17, 8506–8517. [Google Scholar] [CrossRef]
- Rodríguez-León, E.; Iñiguez-Palomares, R.; Navarro, R.E.; Herrera-Urbina, R.; Tánori, J.; Iñiguez-Palomares, C.; Maldonado, A. Synthesis of silver nanoparticles using reducing agents obtained from natural sources (Rumex hymenosepalus extracts). Nanoscale Res. Lett. 2013, 8, 318. [Google Scholar] [CrossRef]
- Rodríguez-León, E.; Rodríguez-Vázquez, B.E.; Martínez-Higuera, A.; Rodríguez-Beas, C.; Larios-Rodríguez, E.; Navarro, R.E.; López-Esparza, R.; Iñiguez-Palomares, R.A. Synthesis of Gold Nanoparticles Using Mimosa tenuiflora Extract, Assessments of Cytotoxicity, Cellular Uptake, and Catalysis. Nanoscale Res. Lett. 2019, 14, 334. [Google Scholar] [CrossRef]
- Siakavella, I.K.; Lamari, F.; Papoulis, D.; Orkoula, M.; Gkolfi, P.; Lykouras, M.; Avgoustakis, K.; Hatziantoniou, S. Effect of Plant Extracts on the Characteristics of Silver Nanoparticles for Topical Application. Pharmaceutics 2020, 12, 1244. [Google Scholar] [CrossRef] [PubMed]
- Guerrini, L.; Alvarez-Puebla, R.A.; Pazos-Perez, N. Surface Modifications of Nanoparticles for Stability in Biological Fluids. Materials 2018, 11, 1154. [Google Scholar] [CrossRef] [PubMed]
- Meléndrez, M.F.; Cárdenas, G.; Arbiol, J. Synthesis and characterization of gallium colloidal nanoparticles. J. Colloid Interface Sci. 2010, 346, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Raval, N.; Maheshwari, R.; Kalyane, D.; Youngren-Ortiz, S.R.; Chougule, M.B.; Tekade, R.K. Importance of Physicochemical Characterization of Nanoparticles in Pharmaceutical Product Development. Basic Fundam. Drug Deliv. 2019, 369–400. [Google Scholar] [CrossRef]
- Baldassarre, F.; Cacciola, M.; Ciccarella, G. A predictive model of iron oxide nanoparticles flocculation tuning Z-potential in aqueous environment for biological application. J. Nanopart. Res. 2015, 17, 377. [Google Scholar] [CrossRef]
- Saeb, A.T.; Alshammari, A.S.; Al-Brahim, H.; Al-Rubeaan, K.A. Production of silver nanoparticles with strong and stable antimicrobial activity against highly pathogenic and multidrug resistant bacteria. Sci. World J. 2014, 2014, 704708. [Google Scholar] [CrossRef]
- Perera, G.; Zipser, M.; Bonengel, S.; Salvenmoser, W.; Bernkop-Schnürch, A. Development of phosphorylated nanoparticles as zeta potential inverting systems. Eur. J. Pharm. Biopharm. 2015, 97 Pt A, 250–256. [Google Scholar] [CrossRef]
- Németh, Z.; Csóka, I.; Semnani Jazani, R.; Sipos, B.; Haspel, H.; Kozma, G.; Kónya, Z.; Dobó, D.G. Quality by Design-Driven Zeta Potential Optimisation Study of Liposomes with Charge Imparting Membrane Additives. Pharmaceutics 2022, 14, 1798. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef]
- Nurzyńska-Wierdak, R.; Sałata, A.; Kniaziewicz, M. Tansy (Tanacetum vulgare L.)—A Wild-Growing Aromatic Medicinal Plant with a Variable Essential Oil Composition. Agronomy 2022, 12, 277. [Google Scholar] [CrossRef]
- Alharbi, N.S.; Alsubhi, N.S.; Felimban, A.I. Green synthesis of silver nanoparticles using medicinal plants: Characterization and application. J. Radiat. Res. Appl. Sci. 2022, 15, 109–124. [Google Scholar] [CrossRef]
- Jasuja, N.D.; Gupta, D.K.; Reza, M.; Joshi, S.C. Green Synthesis of AgNPs Stabilized with biowaste and their antimicrobial activities. Braz. J. Microbiol. 2014, 45, 1325–1332. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Shen, Y.; Xie, A.; Yu, X.; Qiu, L.; Zhang, L.; Zhang, Q. Green synthesis of silver nanoparticles using Capsicum annuum L. extract. Green Chem. 2007, 9, 852–858. [Google Scholar] [CrossRef]
- Mallikarjuna, K.; Narasimha, G.; Dillip, G.R.; Praveen, B.; Shreedhar, B.; Lakshmi, C.S.; Reddy, B.V.S.; Raju, B.D.P. Green synthesis of silver nanoparticles using Ocimum leaf extract and their characterization. Dig. J. Nanomater. Biostruct. 2011, 6, 181–186. [Google Scholar]
- Akintelu, S.A.; Bo, Y.; Folorunso, A.S. A review on synthesis, optimization, mechanism, characterization, and antibacterial application of silver nanoparticles synthesized from plants. J. Chem. 2020, 2020, 3189043. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies. Tables and Charts; John Wiley&Sons, Ltd.: Chichester, UK, 2001. [Google Scholar]
- Bernabé-Antonio, A.; Martínez-Ceja, A.; Romero-Estrada, A.; Sánchez-Carranza, J.N.; Columba-Palomares, M.C.; Rodríguez-López, V.; Meza-Contreras, J.C.; Silva-Guzmán, J.A.; Gutiérrez-Hernández, J.M. Green Synthesis of Silver Nanoparticles Using Randia aculeata L. Cell Culture Extracts, Characterization, and Evaluation of Antibacterial and Antiproliferative Activity. Nanomaterials 2022, 12, 4184. [Google Scholar] [CrossRef]
- Kowalonek, J.; Stachowiak, N.; Bolczak, K.; Richert, A. Physicochemical and Antibacterial Properties of Alginate Films Containing Tansy (Tanacetum vulgare L.) Essential Oil. Polymers 2023, 15, 260. [Google Scholar] [CrossRef]
- Rónavári, A.; Kovács, D.; Igaz, N.; Vágvölgyi, C.; Boros, I.M.; Kónya, Z.; Pfeiffer, I.; Kiricsi, M. Biological activity of green-synthesized silver nanoparticles depends on the applied natural extracts: A comprehensive study. Int. J. Nanomed. 2017, 12, 871–883. [Google Scholar] [CrossRef]
- Goudarzi, M.; Mir, N.; Mousavi-Kamazani, M.; Bagheri, S.; Salavati-Niasari, M. Biosynthesis and characterization of silver nanoparticles prepared from two novel natural precursors by facile thermal decomposition methods. Sci. Rep. 2016, 6, 32539. [Google Scholar] [CrossRef]
- Qing, Y.; Cheng, L.; Li, R.; Liu, G.; Zhang, Y.; Tang, X.; Wang, J.; Liu, H.; Qin, Y. Potential antibacterial mechanism of silver nanoparticles and the optimization of orthopedic implants by advanced modification technologies. Int. J. Nanomed. 2018, 13, 3311–3327. [Google Scholar] [CrossRef]
- Auffan, M.; Rose, J.; Bottero, J.-Y.; Lowry, G.V.; Jolivet, J.-P.; Wiesner, M.R. Towards a Definition of Inorganic Nanoparticles from an Environmental, Health and Safety Perspective. Nat. Nanotechnol. 2009, 4, 634–641. [Google Scholar] [CrossRef]
- Liu, J.Y.; Sonshine, D.A.; Shervani, S.; Hurt, R.H. Controlled Release of Biologically Active Silver from Nanosilver Surfaces. ACS Nano 2010, 4, 6903–6913. [Google Scholar] [CrossRef]
- Navarro, E.; Piccapietra, F.; Wagner, B.; Marconi, F.; Kaegi, R.; Odzak, N.; Sigg, L.; Behra, R. Toxicity of Silver Nanoparticles to Chlamydomonas Reinhardtii. Environ. Sci. Technol. 2008, 42, 8959–8964. [Google Scholar] [CrossRef]
- Sotiriou, G.A.; Pratsinis, S.E. Antibacterial Activity of Nanosilver Ions and Particles. Environ. Sci. Technol. 2010, 44, 5649–5654. [Google Scholar] [CrossRef] [PubMed]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.B.; Ramirez, J.T.; Yacaman, M.J. The Bactericidal Effect of Silver Nanoparticles. Nanotechnology 2005, 16, 2346–2353. [Google Scholar] [CrossRef]
- Gunawan, C.; Teoh, W.Y.; Marquis, C.P.; Lifia, J.; Amal, R. Reversible Antimicrobial Photoswitching in Nanosilver. Small 2009, 5, 341–344. [Google Scholar] [CrossRef] [PubMed]
- Kittler, S.; Greulich, C.; Diendorf, J.; Koller, M.; Epple, M. Toxicity of Silver Nanoparticles Increases During Storage Because of Slow Dissolution under Release of Silver Ions. Chem. Mater. 2010, 22, 4548–4554. [Google Scholar] [CrossRef]
- Liu, J.Y.; Hurt, R.H. Ion Release Kinetics and Particle Persistence in Aqueous Nano-Silver Colloids. Environ. Sci. Technol. 2010, 44, 2169–2175. [Google Scholar] [CrossRef]
- Sotiriou, G.A.; Teleki, A.; Camenzind, A.; Krumeich, F.; Meyer, A.; Panke, S.; Pratsinis, S.E. Nanosilver on Nanostructured Silica: Antibacterial Activity and Ag Surface Area. Chem. Eng. J. 2011, 170, 547–554. [Google Scholar] [CrossRef]
- Sotiriou, G.A.; Hirt, A.M.; Lozach, P.Y.; Teleki, A.; Krumeich, F.; Pratsinis, S.E. Hybrid, Silica-Coated, Janus-Like PlasmonicMagnetic Nanoparticles. Chem. Mater. 2011, 23, 1985–1992. [Google Scholar] [CrossRef]
- Sotiriou, G.A.; Sannomiya, T.; Teleki, A.; Krumeich, F.; Vörös, J.; Pratsinis, S.E. Non-Toxic Dry-Coated Nanosilver for Plasmonic Biosensors. Adv. Funct. Mater. 2010, 20, 4250–4257. [Google Scholar] [CrossRef]
- Fabrega, J.; Fawcett, S.R.; Renshaw, J.C.; Lead, J.R. Silver Nanoparticle Impact on Bacterial Growth: Effect of pH, Concentration, and Organic Matter. Environ. Sci. Technol. 2009, 43, 7285–7290. [Google Scholar] [CrossRef] [PubMed]
- Levard, C.; Reinsch, B.C.; Michel, F.M.; Oumahi, C.; Lowry, G.V.; Brown, G.E. Sulfidation Processes of PVP-Coated Silver Nanoparticles in Aqueous Solution: Impact on Dissolution Rate. Environ. Sci. Technol. 2011, 45, 5260–5266. [Google Scholar] [CrossRef]
- Choi, O.; Cleuenger, T.E.; Deng, B.L.; Surampalli, R.Y.; Ross, L.; Hu, Z.Q. Role of Sulfide and Ligand Strength in Controlling Nanosilver Toxicity. Water Res. 2009, 43, 1879–1886. [Google Scholar] [CrossRef]
- Reinsch, B.C.; Levard, C.; Li, Z.; Ma, R.; Wise, A.; Gregory, K.B.; Brown, G.E.; Lowry, G.V. Sulfidation of Silver Nanoparticles Decreases Escherichia Coli Growth Inhibition. Environ. Sci. Technol. 2012, 46, 6992–7000. [Google Scholar] [CrossRef]
- Sotiriou, G.A.; Meyer, A.; Knijnenburg, J.T.; Panke, S.; Pratsinis, S.E. Quantifying the origin of released Ag+ ions from nanosilver. Langmuir 2012, 28, 15929–15936. [Google Scholar] [CrossRef]
- Rizwana, H.; Alwhibi, M.S.; Aldarsone, H.A.; Awad, M.A.; Soliman, D.A.; Bhat, R.S. Green synthesis, characterization, and antimicrobial activity of silver nanoparticle prepared using Trigonella foenum-graecum L. leaves grown in Saudi Arabia. Green Process. Synth. 2021, 10, 421–429. [Google Scholar] [CrossRef]
- Kovács, D.; Igaz, N.; Gopisetty, M.K.; Kiricsi, M. CancerTherapy by Silver Nanoparticles:Fiction or Reality? Int. J. Mol. Sci. 2022, 23, 839. [Google Scholar] [CrossRef] [PubMed]
- Almukaynizi, F.B.; Daghestani, M.H.; Awad, M.A.; Althomali, A.; Merghani, N.M.; Bukhari, W.I.; Algahtani, N.M.; Al-Zuhairy, S.S.; ALOthman, A.M.; Alsenani, E.A.; et al. Cytotoxicity of green-synthesized silver nanoparticles by Adansonia digitata fruit extract against HTC116 and SW480 human colon cancer cell lines. Green Process. Synth. 2022, 11, 411–422. [Google Scholar] [CrossRef]
- Al-Zahrani, S.A.; Bhat, R.S.; Al-Onazi, M.A.; Alwhibi, M.S.; Soliman, D.A.; Aljebrin, N.A.; Al-Suhaibani, L.S.; Al Daihan, S. Anticancer potential of biogenic silver nanoparticles using the stem extract of Commiphora gileadensis against human colon cancer cells. Green Process. Synth. 2022, 11, 435–444. [Google Scholar] [CrossRef]
- Althomali, A.; Daghestani, M.H.; Almukaynizi, B.; Al-Zahrani, F.; Ahmed, S.; Awad, M.A.; Merghani, N.M.; Bukhari, W.I.; Ibrahim, E.M.; Alzahrani, S.M.; et al. Anti-colon cancer activities of green-synthesized Moringa oleifera-AgNPs against human colon cancer cells. Green Process. Synth. 2022, 11, 545–554. [Google Scholar] [CrossRef]
- Gherasim, O.; Puiu, R.A.; Birca, A.C.; Burdusel, A.C.; Grumezescu, A.M. An updated review on silver nanoparticles in biomedicine. Nanomaterials 2020, 10, 2318. [Google Scholar] [CrossRef] [PubMed]
- Kong, I.C.; Ko, K.S.; Koh, D.C. Evaluation of the Effects of Particle Sizes of Silver Nanoparticles on Various Biological Systems. Int. J. Mol. Sci. 2020, 21, 8465. [Google Scholar] [CrossRef]
- Donlan, R.M.; Costerton, J.W. Biofilms: Survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193. [Google Scholar] [CrossRef]
- Mah, T.F.; O’Toole, G.A. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001, 9, 34–39. [Google Scholar] [CrossRef]
- Kalishwaralal, K.; Barath Mani Kanth, S.; Pandian, S.R.K.; Deepak, V.; Gurunathan, S. Silver nanoparticles impede the biofilm formation by Pseudomonas aeruginosa and Staphylococcus epidermidis. Colloids Sur. B Biointerfaces 2010, 79, 340–344. [Google Scholar] [CrossRef]
- Siddique, M.H.; Aslam, B.; Imran, M.; Ashraf, A.; Nadeem, H.; Hayat, S.; Khurshid, M.; Afzal, M.; Malik, I.R.; Shahzad, M.; et al. Effect of Silver Nanoparticles on Biofilm Formation and EPS Production of Multidrug-Resistant Klebsiella pneumoniae. Biomed. Res. Int. 2020, 2020, 6398165. [Google Scholar] [CrossRef] [PubMed]
- Hamida, R.S.; Ali, M.A.; Goda, D.A.; Khalil, M.I.; Al-Zaban, M.I. Novel Biogenic Silver Nanoparticle-Induced Reactive Oxygen Species Inhibit the Biofilm Formation and Virulence Activities of Methicillin-Resistant Staphylococcus aureus (MRSA) Strain. Front. Bioeng. Biotechnol. 2020, 8, 433. [Google Scholar] [CrossRef]
- Jafari, A.; Nagheli, A.; Alavi Foumani, A.; Soltani, B.; Goswami, R. The Role of Metallic Nanoparticles in Inhibition of Mycobacterium Tuberculosis and Enhances Phagosome Maturation into the Infected Macrophage. Oman Med. J. 2020, 35, e194. [Google Scholar] [CrossRef]
- Li, X.; Sun, L.; Zhang, P.; Wang, Y. Novel Approaches to Combat Medical Device-Associated BioFilms. Coatings 2021, 11, 294. [Google Scholar] [CrossRef]
- Jaison, J.P.; Balasubramanian, B.; Gangwar, J.; James, N.; Pappuswamy, M.; Anand, A.V.; Al-Dhabi, N.A.; Valan Arasu, M.; Liu, W.-C.; Sebastian, J.K. Green Synthesis of Bioinspired Nanoparticles Mediated from Plant Extracts of Asteraceae Family for Potential Biological Applications. Antibiotics 2023, 12, 543. [Google Scholar] [CrossRef] [PubMed]
- Dubey, S.P.; Lahtinen, M.; Sillanpää, M. Tansy Fruit Mediated Greener Synthesis of Silver and Gold Nanoparticles. Process Biochem. 2010, 45, 1065–1071. [Google Scholar] [CrossRef]
- Babich, O.; Larina, V.; Krol, O.; Ulrikh, E.; Sukhikh, S.; Gureev, M.A.; Prosekov, A.; Ivanova, S. In Vitro Study of Biological Activity of Tanacetum vulgare Extracts. Pharmaceutics 2023, 15, 616. [Google Scholar] [CrossRef] [PubMed]
- Devrnja, N.; Krstić-Milošević, D.; Janošević, D.; Tešević, V.; Vinterhalter, B.; Savić, J.; Ćalić, D. In vitro cultivation of tansy (Tanacetum vulgare L.): A tool for the production of potent pharmaceutical agents. Protoplasma 2021, 258, 587–599. [Google Scholar] [CrossRef] [PubMed]
- Seong, M.; Lee, D.G. Silver nanoparticles against Salmonella enterica serotype typhimurium: Role of inner membrane dysfunction. Curr. Microbiol. 2017, 74, 661–670. [Google Scholar] [CrossRef]
- Khalandi, B.; Asadi, N.; Milani, M.; Davaran, S.; Najaf Abadi, A.J.; Abasi, E.; Akbarzadeh, A. A review on potential role of silver nanoparticles and possible mechanisms of their actions on bacteria. Drug Res. 2017, 67, 70–76. [Google Scholar] [CrossRef]
- Van Der Wal, A.; Norde, W.; Zehnder, A.J.B.; Lyklema, J. Determination of the total charge in the cell walls of Gram-positive bacteria. Colloids Surf. B Biointerfaces 1997, 9, 81–100. [Google Scholar] [CrossRef]
- Mandal, D.; Kumar Dash, S.; Das, B.; Chattopadhyay, S.; Ghosh, T.; Das, D.; Roy, S. Bio-fabricated silver nanoparticles preferentially targets Gram positive depending on cell surface charge. Biomed. Pharmacother. 2016, 83, 548–558. [Google Scholar] [CrossRef]
- Jin, J.C.; Wu, X.J.; Xu, J.; Wang, B.B.; Jiang, F.L.; Liu, Y. Ultrasmall silver nanoclusters: Highly efficient antibacterial activity and their mechanisms. Biomater. Sci. 2017, 5, 247–257. [Google Scholar] [CrossRef]
- Lombardo, P.C.; Poli, A.L.; Castro, L.F.; Perussi, J.R.; Schmitt, C.C. Photochemical deposition of silver nanoparticles on clays and exploring their antibacterial activity. ACS Appl. Mater. Interfaces 2016, 8, 21640–21647. [Google Scholar] [CrossRef]
- Kim, T.; Braun, G.B.; She, Z.G.; Hussain, S.; Rouslahti, E.; Sailor, M.J. Composite porous silicon-silver nanoparticles as theranostic antibacterial agents. ACS Appl. Mater. Interfaces 2016, 8, 30449–30457. [Google Scholar] [CrossRef]
- Zawadzka, K.; Kądzioła, K.; Felczak, A.; Wrońska, N.; Piwoński, I.; Kisielewska, A.; Lisowska, K. Surface area or diameter—Which factor really determines the antibacterial activity of silver nanoparticles grown on TiO2 coatings? New J. Chem. 2014, 38, 3275–3281. [Google Scholar] [CrossRef]
- Long, Y.M.; Hu, L.G.; Yan, X.T.; Zhao, X.C.; Zhou, Q.F.; Cai, Y.; Jiang, G.B. Surface ligand controls silver ion release of nanosilver and its antibacterial activity against Escherichia coli. Int. J. Nanomed. 2017, 12, 3193–3206. [Google Scholar] [CrossRef] [PubMed]
- Hatchett, D.W.; White, H.S. Electrochemistry of sulfur adlayers on the low-index faces of silver. J. Phys. Chem. 1996, 100, 9854–9859. [Google Scholar] [CrossRef]
- Monteiro, D.R.; Gorup, L.F.; Takamiya, A.S.; de Camargo, E.R.; Filho, A.C.; Barbosa, D.B. Silver distribution and release from an antimicrobial denture base resin containing silver colloidal nanoparticles. J. Prosthodont. 2012, 21, 7–15. [Google Scholar] [CrossRef]
- Bruna, T.; Maldonado-Bravo, F.; Jara, P.; Caro, N. Silver Nanoparticles and Their Antibacterial Applications. Int. J. Mol. Sci. 2021, 22, 7202. [Google Scholar] [CrossRef]
- Sukhanova, A.; Bozrova, S.; Sokolov, P.; Berestovoy, M.; Karaulov, A.; Nabiev, I. Dependence of Nanoparticle Toxicity on Their Physical and Chemical Properties. Nanoscale Res. Lett. 2018, 13, 44. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, L.; Chen, Q.; Chen, C. Cytotoxic potential of silver nanoparticles. Yonsei Med. J. 2014, 55, 283–291. [Google Scholar] [CrossRef]
- Kittler, S.; Greulich, C.; Gebauer, J.S.; Diendorf, J.; Treuel, L.; Ruiz, L. The influence of proteins on the dispersability and cell-biological activity of silver nanoparticles. J. Mater. Chem. 2010, 20, 512–518. [Google Scholar] [CrossRef]
- Quettier-Deleu, C.; Gressier, B.; Vasseur, J.; Dine, T.; Brunet, C.; Luyckx, M.C.; Cazin, J.C.; Bailleul, F.; Trotin, F. Phenolic compounds and antioxidant activities of buckweat (Fagopyrum esculentum Moench) hulls and flour. J. Ethnopharmacol. 2000, 72, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Lawag, I.L.; Yoo, O.; Lim, L.Y.; Hammer, K.; Locher, C. Optimisation of Bee Pollen Extraction to Maximise Extractable Antioxidant Constituents. Antioxidants 2021, 10, 1113. [Google Scholar] [CrossRef]
- De Camargo, A.C.; Vieira, T.M.F.S.; Regitano-d’Arce, M.A.B.; de Alencar, S.M.; Calori-Domingues, M.A.; Spoto, M.H.F.; Canniatti-Brazaca, S.G. Gamma irradiation of in-shell and blanched peanuts protects against mycotoxic fungi and retains their nutraceutical components during long-term storage. Int. J. Mol. Sci. 2012, 13, 10935–10958. [Google Scholar] [CrossRef] [PubMed]
- Flieger, J.; Franus, W.; Panek, R.; Szymańska-Chargot, M.; Flieger, W.; Flieger, M.; Kołodziej, P. Green Synthesis of Silver Nanoparticles Using Natural Extracts with Proven Antioxidant Activity. Molecules 2021, 26, 4986. [Google Scholar] [CrossRef] [PubMed]
- Cañadas, A.; Gualle, A.; Vizuete, K.; Debut, A.; Rojas-Silva, P.; Ponce, S.; Orejuela-Escobar, L.M. Green Synthesis of Antibacterial Silver Nanocolloids with Agroindustrial Waste Extracts, Assisted by LED Light. Colloids Interfaces 2022, 6, 74. [Google Scholar] [CrossRef]
- Oyaizu, M. Studies on products of browning reaction. Jpn. J. Nutr. Diet. 1986, 44, 307315. [Google Scholar] [CrossRef]
- Hatano, T.; Kagawa, H.; Yasuhara, T.; Okuda, T. Two New Flavonoids and Other Constituents in Licorice Root Their Relative Astringency and Radical Scavenging Effects. Chem. Pharm. Bull. 1988, 36, 2090–2097. [Google Scholar] [CrossRef]
- Widelski, J.; Okińczyc, P.; Paluch, E.; Mroczek, T.; Szperlik, J.; Żuk, M.; Sroka, Z.; Sakipova, Z.; Chinou, I.; Skalicka-Woźniak, K.; et al. The Antimicrobial Properties of Poplar and Aspen-Poplar Propolises and Their Active Components against Selected Microorganisms, including Helicobacter pylori. Pathogens 2022, 11, 191. [Google Scholar] [CrossRef]
- Rajtar, B.; Skalicka-Woźniak, K.; Świątek, Ł.; Stec, A.; Boguszewska, A.; Polz-Dacewicz, M. Antiviral effect of compounds derived from Angelica archangelica L. on Herpes simplex virus-1 and Coxsackievirus B3 infections. Food Chem. Toxicol. 2017, 109 Pt 2, 1026–1031. [Google Scholar] [CrossRef]
- Treuel, L.; Nienhaus, G.U. Toward a molecular understanding of nanoparticle-protein interactions. Biophys. Rev. 2012, 4, 137–147. [Google Scholar] [CrossRef]
- Walczyk, D.; Bombelli, F.B.; Monopoli, M.P.; Lynch, I.; Dawson, K.A. What the cell “sees” in bionanoscience. J. Am. Chem. Soc. 2010, 132, 5761–5768. [Google Scholar] [CrossRef]
- Monopoli, M.P.; Walczyk, D.; Campbell, A.; Elia, G.; Lynch, I.; Bombelli, F.B.; Dawson, K.A. Physical-chemical aspects of protein corona: Relevance to in vitro and in vivo biological impacts of nanoparticles. J. Am. Chem. Soc. 2011, 133, 2525–2534. [Google Scholar] [CrossRef]
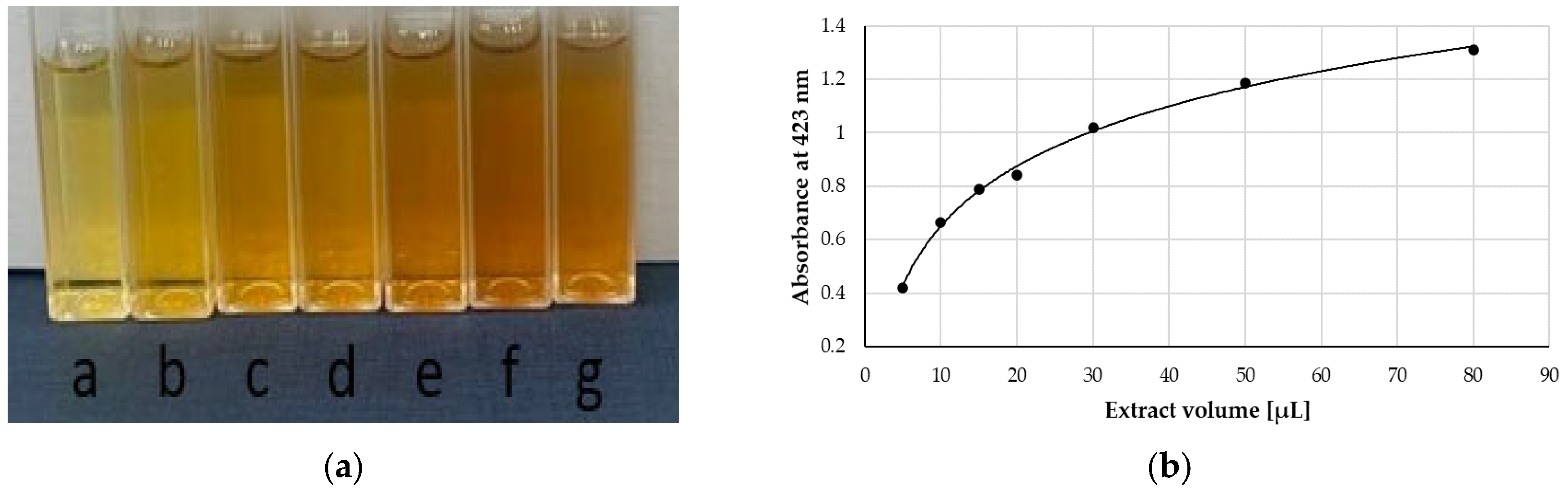
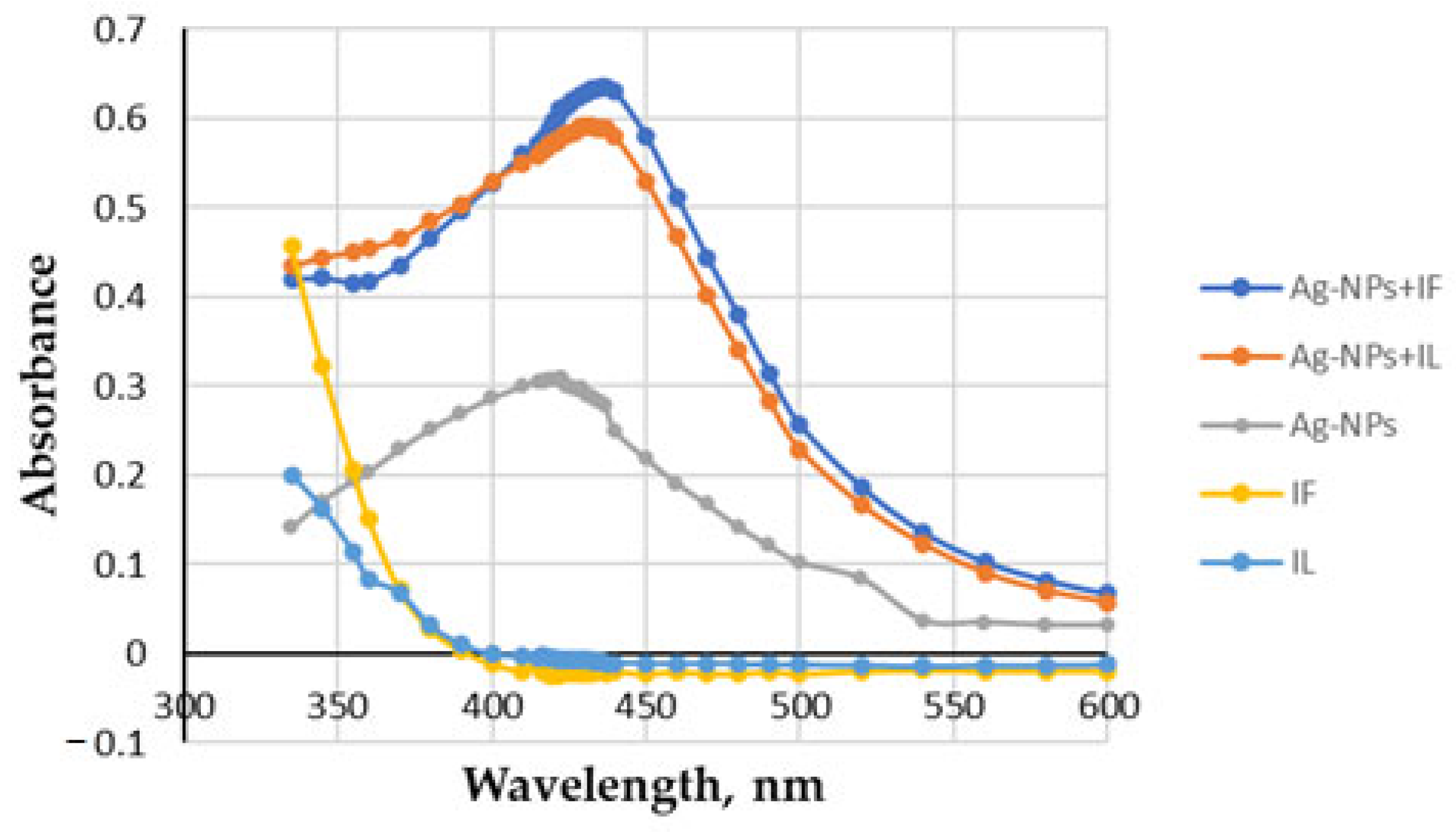
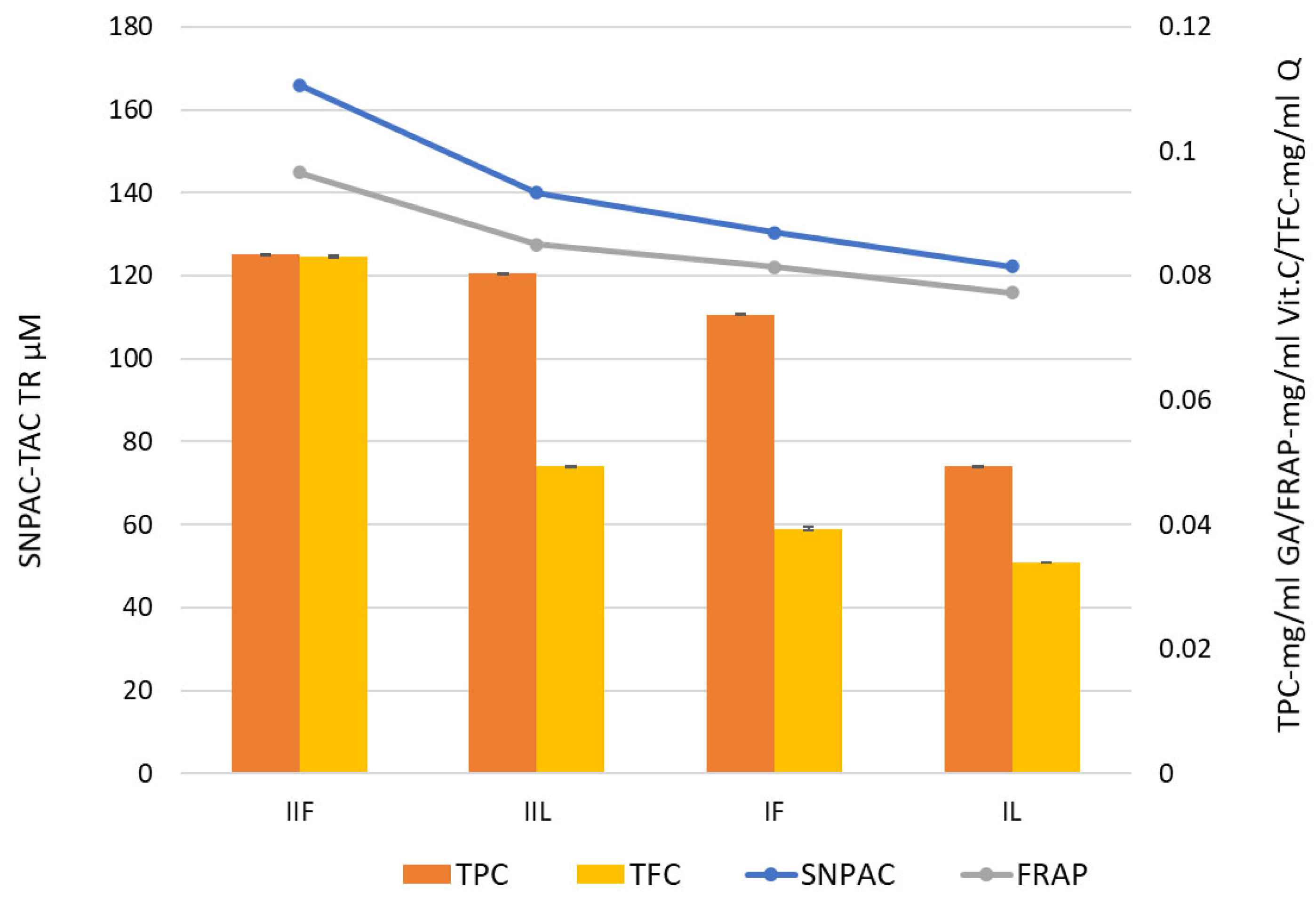


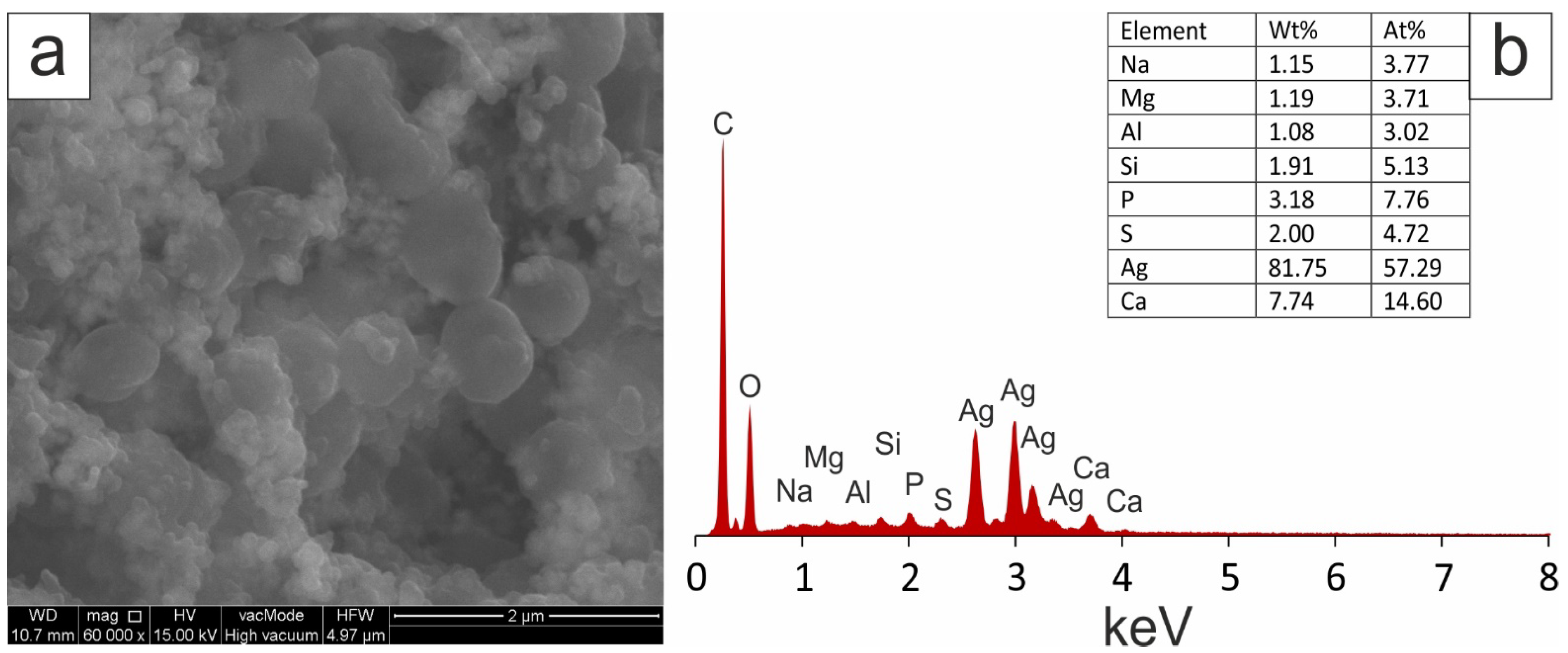

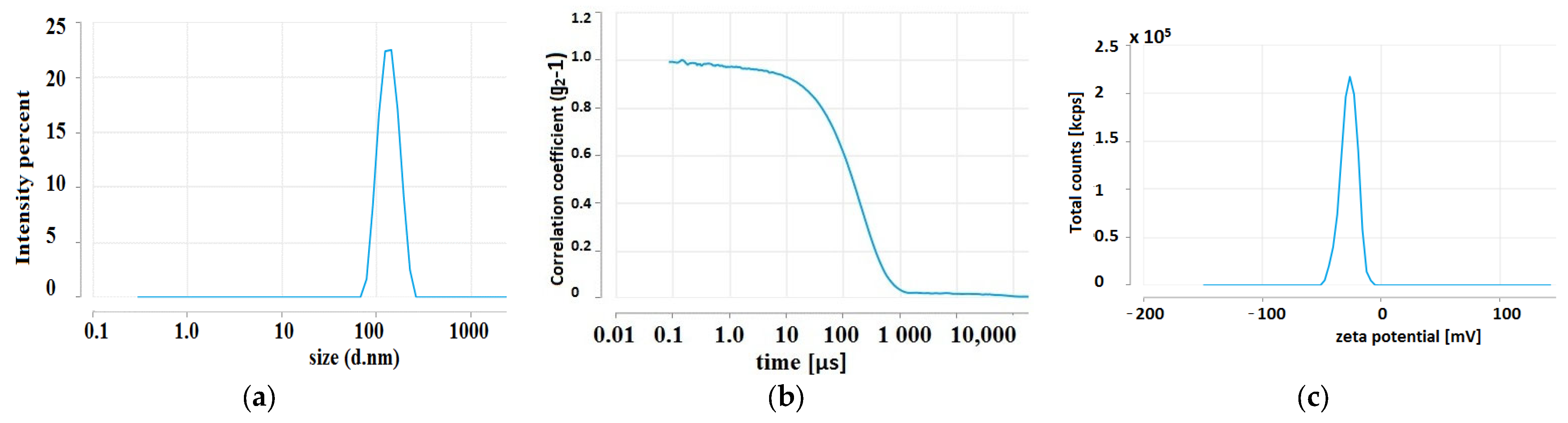
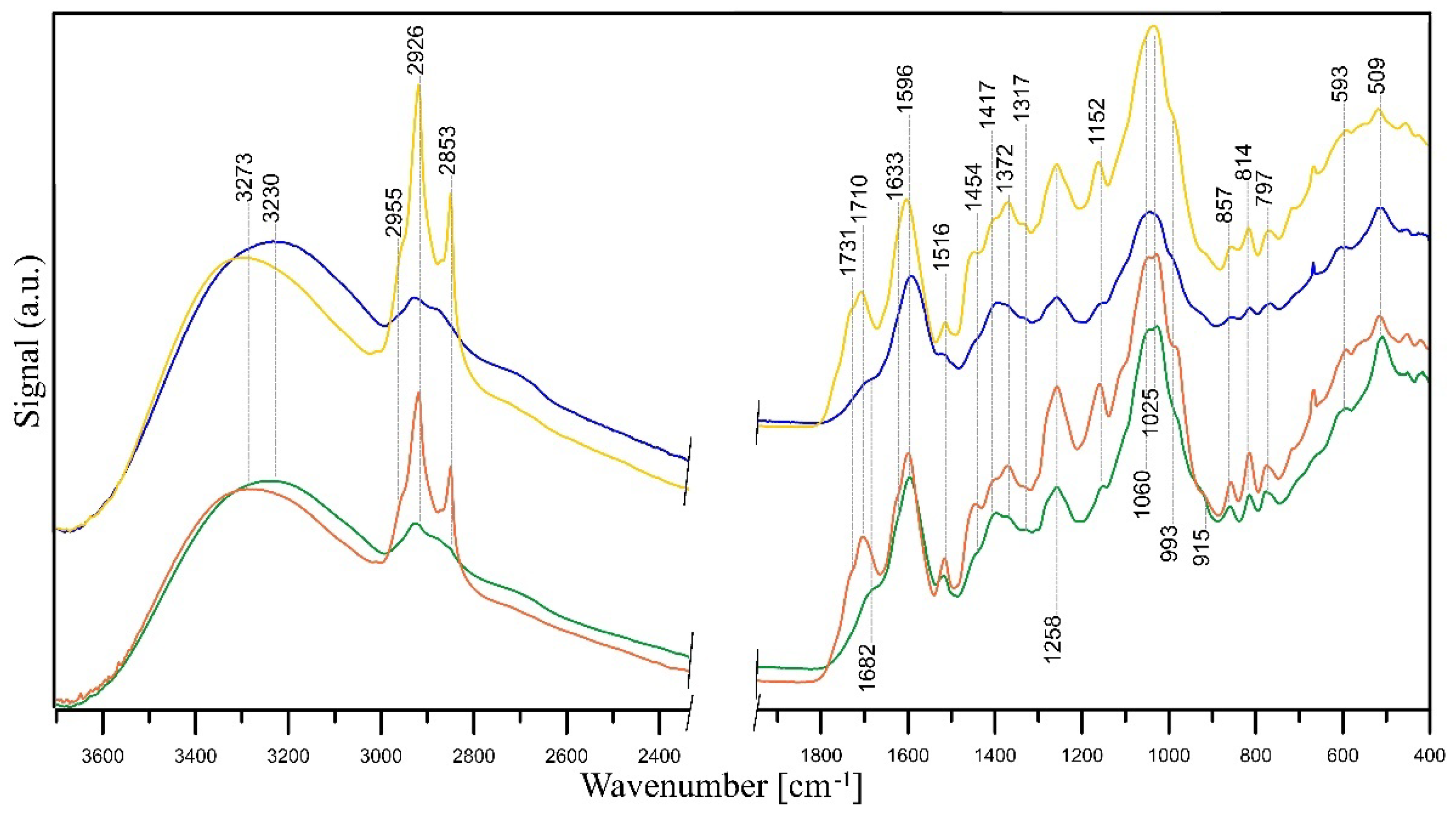


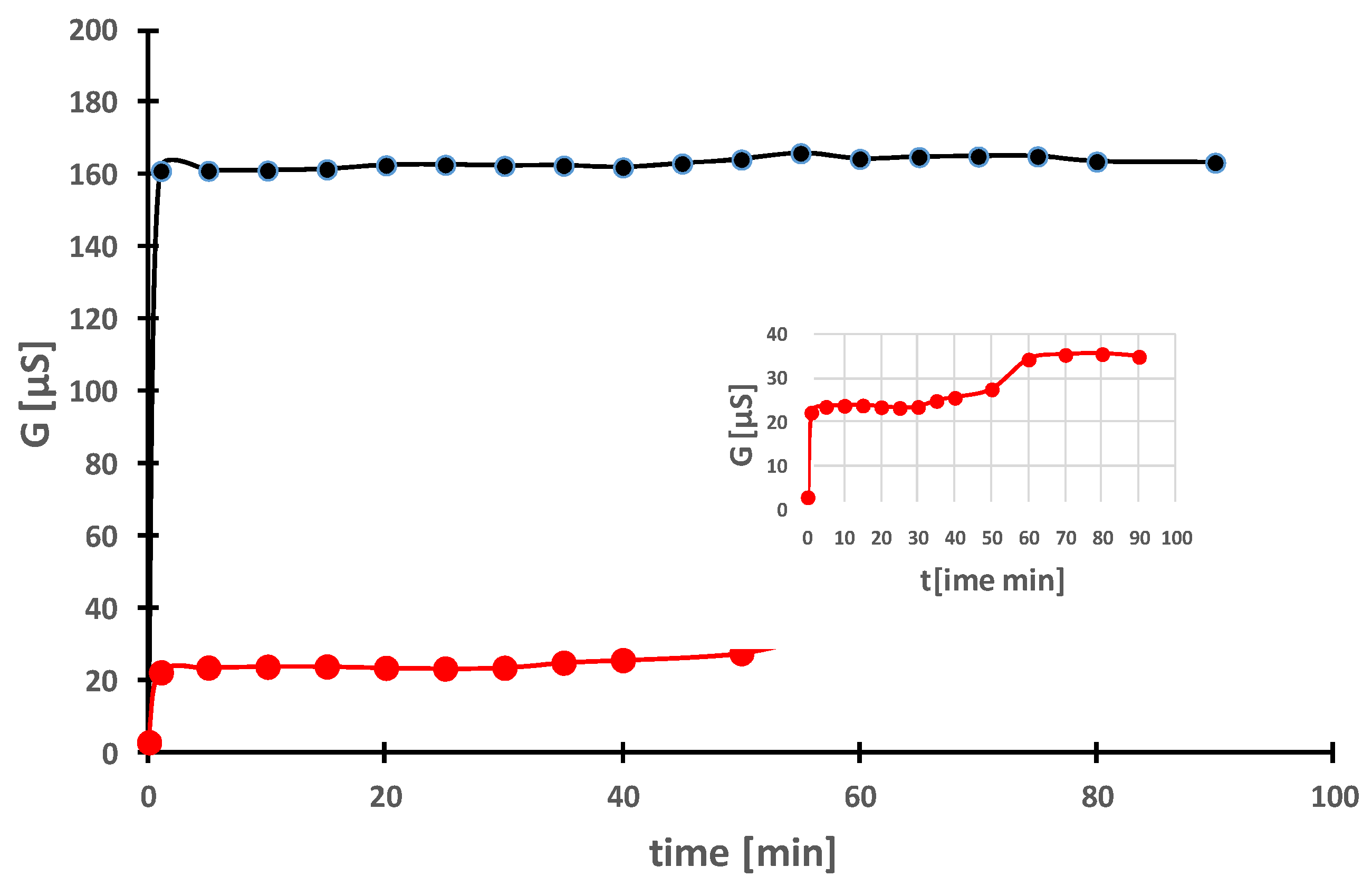
| Extract | FRAP | SNPAC | DPPH | |||
|---|---|---|---|---|---|---|
| µg vit.C mL−1 Extract | RSD% | TAC µM | RSD% | IC50 mg CGA mL−1 Extract | RSD% | |
| aqueous extract of flowers | 81.44 | 0.01 | 130.49 | 0.12 | 0.94 | 0 |
| aqueous extract of leaves | 77.25 | 0.01 | 122.31 | 0.13 | 0.94 | 1.08 |
| hydroalcoholic extract of flowers | 96.65 | 0.02 | 165.99 | 0.13 | 0.94 | 1.02 |
| hydroalcoholic extract of leaves | 85.03 | 0.03 | 140.12 | 0.14 | 0.94 | 1.25 |
| Extract | Total Phenolics | Total Flavonoids | ||
|---|---|---|---|---|
| Equivalents | µg GA mL−1 Extract | RSD% | µg Q mL−1 Extract | RSD% |
| aqueous extract of flowers | 73.76 | 0.03 | 39.31 | 0.03 |
| aqueous extract of leaves | 49.43 | 0.01 | 33.84 | 0.02 |
| hydroalcoholic extract of flowers | 83.46 | 0.03 | 83.02 | 0.01 |
| hydroalcoholic extract of leaves | 80.48 | 0.03 | 49.40 | 0.03 |
| Parameter | Mean | Std | RSD% | Min | Max |
|---|---|---|---|---|---|
| ζ-potential (mV) | −23.30 | 1.09 | −4.68 | −22.15 | −24.32 |
| conductivity (mS/cm) | 0.61 | - | - | 0.61 | 0.61 |
| wall ζ-potential (mV) | −31.26 | 0.89 | −2.87 | −30.46 | −32.23 |
| quality factor | 1.53 | 0.42 | 27.65 | 1.10 | 1.94 |
| Z-average (nm) | 88.73 | 0.81 | 0.92 | 88.02 | 89.87 |
| polydispersity index (PDI) | 0.24 | 0.01 | 4.51 | 0.23 | 0.25 |
| mean by intensity ordered by area (nm) | 114.85 | 2.85 | 2.48 | 111.60 | 118 |
| area by intensity ordered by area (%) | 100 | 0 | 0 | 100 | 100 |
| Parameter | Mean | Std | RSD% | Min | Max |
|---|---|---|---|---|---|
| ζ-potential (mV) | −27.38 | 0.94 | −3.44 | −26.72 | −28.46 |
| conductivity (mS/cm) | 0.94 | 0 | 0 | 0.94 | 0.94 |
| wall ζ-potential (mV) | −27.67 | 1.65 | −5.96 | −25.82 | −28.99 |
| quality factor | 2.39 | 0.67 | 27.92 | 1.95 | 3.17 |
| Z-average (nm) | 147.13 | 0.85 | 0.58 | 145.90 | 147.80 |
| polydispersity index (PDI) | 0.22 | 0.02 | 8.13 | 0.21 | 0.25 |
| mean by intensity ordered by area (nm) | 140.85 | 4.75 | 3.37 | 135 | 146.60 |
| area by intensity ordered by area (%) | 100 | 0 | 0 | 100 | 100 |
| Microorganism | Tanaceti flos Extract | Tanaceti folium Extract | Tanaceti flos Ag-NPs | Tanaceti folium Ag-NPs | ||||
|---|---|---|---|---|---|---|---|---|
| MIC | MIC | MIC | MIC | MIC | MBC | MIC | MBC | |
| Gram-positive bacteria | ||||||||
| S. aureus ATCC 25923 | 2000 | 2000 | 2000 | 2000 | 31.3 | 62.5 | 31.3 | 62.5 |
| S. aureus ATCC BAA-1707 a | 8000 | 8000 | 4000 | 4000 | 31.3 | 125 | 31.3 | 125 |
| S. epidermidis ATCC 12228 | 2000 | 4000 | 4000 | 4000 | 15.6 | 125 | 15.6 | 62.5 |
| M. luteus ATCC 10240 | 8000 | 8000 | 4000 | 8000 | 7.8 | 125 | 7.8 | 62.5 |
| B. cereus ATCC 10876 | 8000 | >8000 | 8000 | 8000 | 62.5 | 1000 | 62.5 | 250 |
| E. faecalis ATCC 29212 | >8000 | >8000 | >8000 | >8000 | 62.5 | 250 | 62.5 | 250 |
| Gram-negative bacteria | ||||||||
| S. typhimurium ATCC 14028 | >8000 | Nd | >8000 | Nd | 31.3 | 31.3 | 31.3 | 62.5 |
| E. coli ATCC 25922 | >8000 | Nd | >8000 | Nd | 31.3 | 62.5 | 31.3 | 62.5 |
| P. mirabilis ATCC 12453 | 8000 | Nd | >8000 | Nd | 62.5 | 62.5 | 62.5 | 62.5 |
| K. pneumoniae ATCC 13883 | >8000 | Nd | >8000 | Nd | 31.3 | 125 | 31.3 | 62.5 |
| P. aeruginosa ATCC 9027 | >8000 | Nd | >8000 | Nd | 15.6 | 31.3 | 15.6 | 31.3 |
| Yeasts | ||||||||
| C. glabrata ATCC 90030 | >8000 | Nd | >8000 | Nd | 15.6 | 1000 | 15.6 | 62.5 |
| C. albicans ATCC 102231 | >8000 | Nd | >8000 | Nd | 15.6 | 500 | 7.8 | 62.5 |
| C. parapsilosis ATCC 22019 | >8000 | Nd | >8000 | Nd | 15.6 | 1000 | 7.8 | 1000 |
| The Investigated Sample | Cell Line | SI | |
|---|---|---|---|
| Vero | HeLa | ||
| CC50 (µg mL−1) a | |||
| Tanaceti flos extract | 596.6 ± 6.0 | 681.0 ± 6.0 | 0.9 |
| Tanaceti folium extract | 861.3 ± 102.5 | 753.1 ± 46.3 | 1.1 |
| Tanaceti flos Ag-NPs | 22.1 ± 5.4 | 67.3 ± 0.2 | 0.3 |
| Tanaceti folium Ag-NPs | 14.1 ± 0.3 | 57.9 ± 3.3 | 0.2 |
| The Investigated Sample | SK-MEL-3 Cell Line | A375 Cell Line | WS1 Cell Line |
|---|---|---|---|
| Tanaceti flos extract | >100 | >100 | >100 |
| Tanaceti folium extract | >100 | >100 | >100 |
| Tanaceti flos Ag-NPs | 14.53 | 22.04 | 4.83 |
| Tanaceti folium Ag-NPs | 18.57 | 34.98 | 4.87 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radzikowska-Büchner, E.; Flieger, W.; Pasieczna-Patkowska, S.; Franus, W.; Panek, R.; Korona-Głowniak, I.; Suśniak, K.; Rajtar, B.; Świątek, Ł.; Żuk, N.; et al. Antimicrobial and Apoptotic Efficacy of Plant-Mediated Silver Nanoparticles. Molecules 2023, 28, 5519. https://doi.org/10.3390/molecules28145519
Radzikowska-Büchner E, Flieger W, Pasieczna-Patkowska S, Franus W, Panek R, Korona-Głowniak I, Suśniak K, Rajtar B, Świątek Ł, Żuk N, et al. Antimicrobial and Apoptotic Efficacy of Plant-Mediated Silver Nanoparticles. Molecules. 2023; 28(14):5519. https://doi.org/10.3390/molecules28145519
Chicago/Turabian StyleRadzikowska-Büchner, Elżbieta, Wojciech Flieger, Sylwia Pasieczna-Patkowska, Wojciech Franus, Rafał Panek, Izabela Korona-Głowniak, Katarzyna Suśniak, Barbara Rajtar, Łukasz Świątek, Natalia Żuk, and et al. 2023. "Antimicrobial and Apoptotic Efficacy of Plant-Mediated Silver Nanoparticles" Molecules 28, no. 14: 5519. https://doi.org/10.3390/molecules28145519
APA StyleRadzikowska-Büchner, E., Flieger, W., Pasieczna-Patkowska, S., Franus, W., Panek, R., Korona-Głowniak, I., Suśniak, K., Rajtar, B., Świątek, Ł., Żuk, N., Bogucka-Kocka, A., Makuch-Kocka, A., Maciejewski, R., & Flieger, J. (2023). Antimicrobial and Apoptotic Efficacy of Plant-Mediated Silver Nanoparticles. Molecules, 28(14), 5519. https://doi.org/10.3390/molecules28145519










