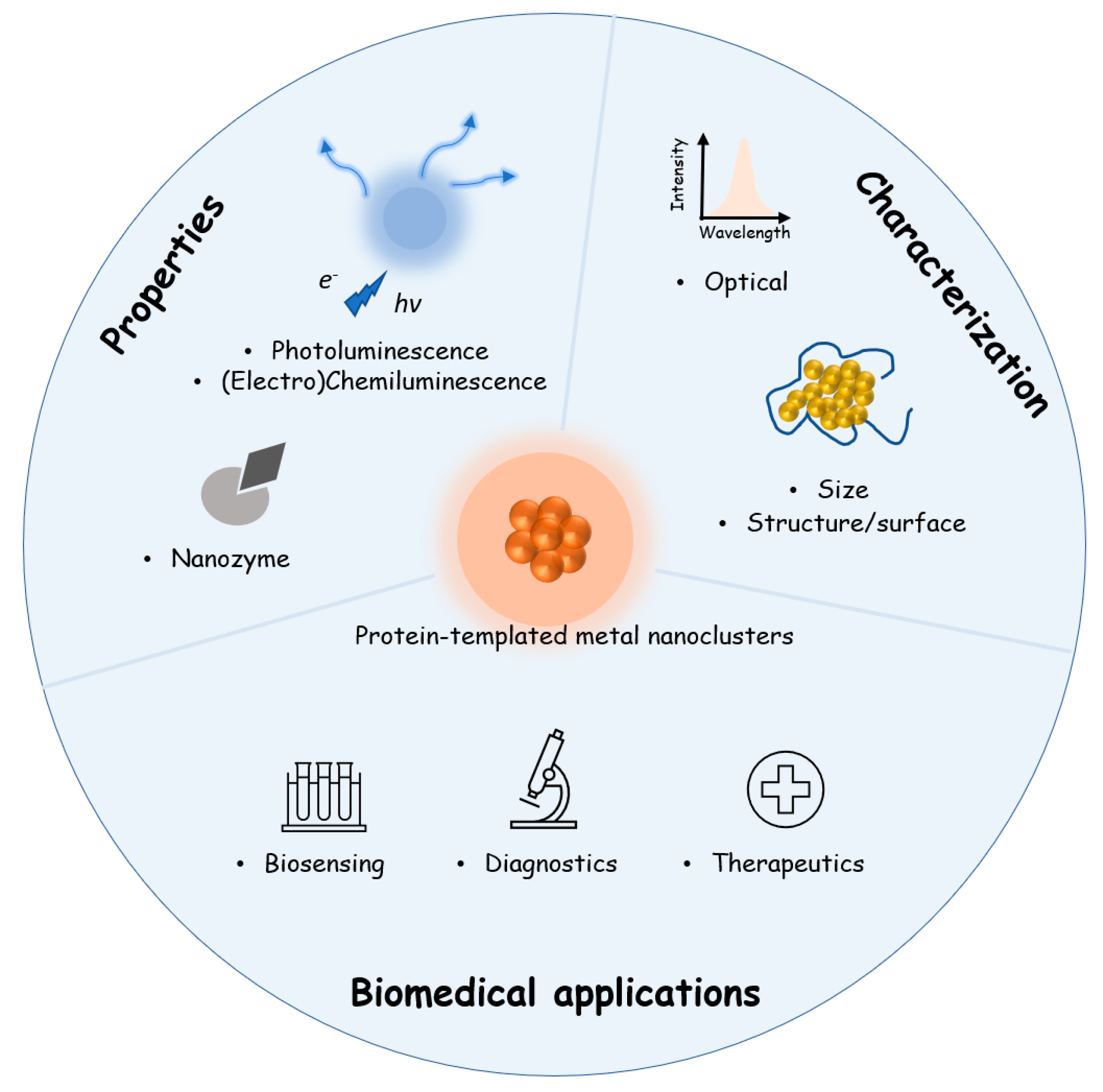
Protein-Templated Metal Nanoclusters: Molecular-like Hybrids for Biosensing, Diagnostics and Pharmaceutics
Abstract
:1. Introduction
2. Synthesis Approaches of Protein-Templated Metal Nanoclusters
3. Physicochemical Properties of Protein-Templated Metal Nanoclusters
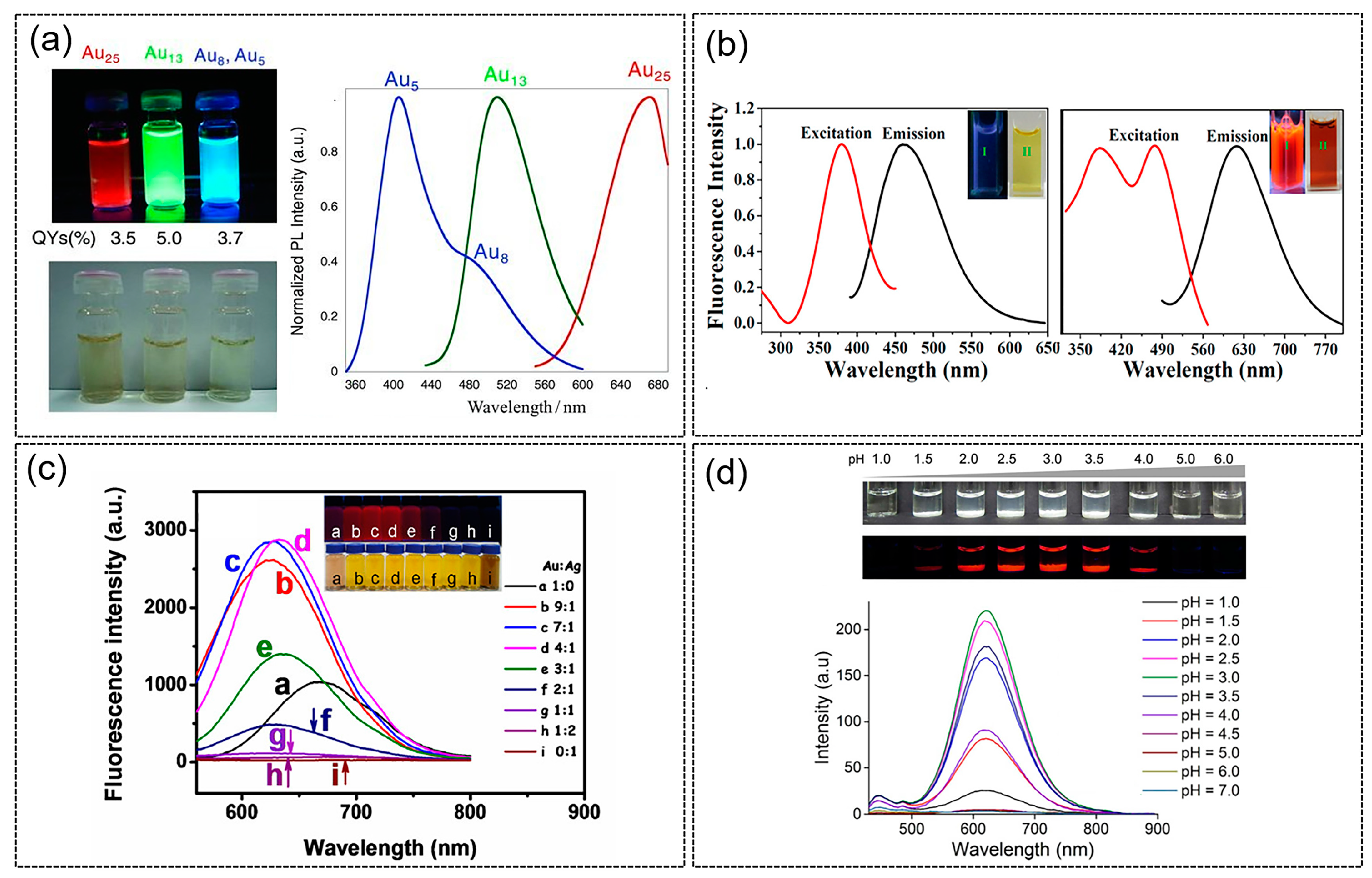
3.1. Photoluminescence Properties of Protein-Templated Metal Nanoclusters
3.2. Chemiluminescence/Electrochemiluminescence of Protein-Templated Metal Nanoclusters

3.3. Nanozyme Properties of Protein-Templated Metal Nanoclusters
4. Characterization Methods for Protein-Templated Metal Nanoclusters
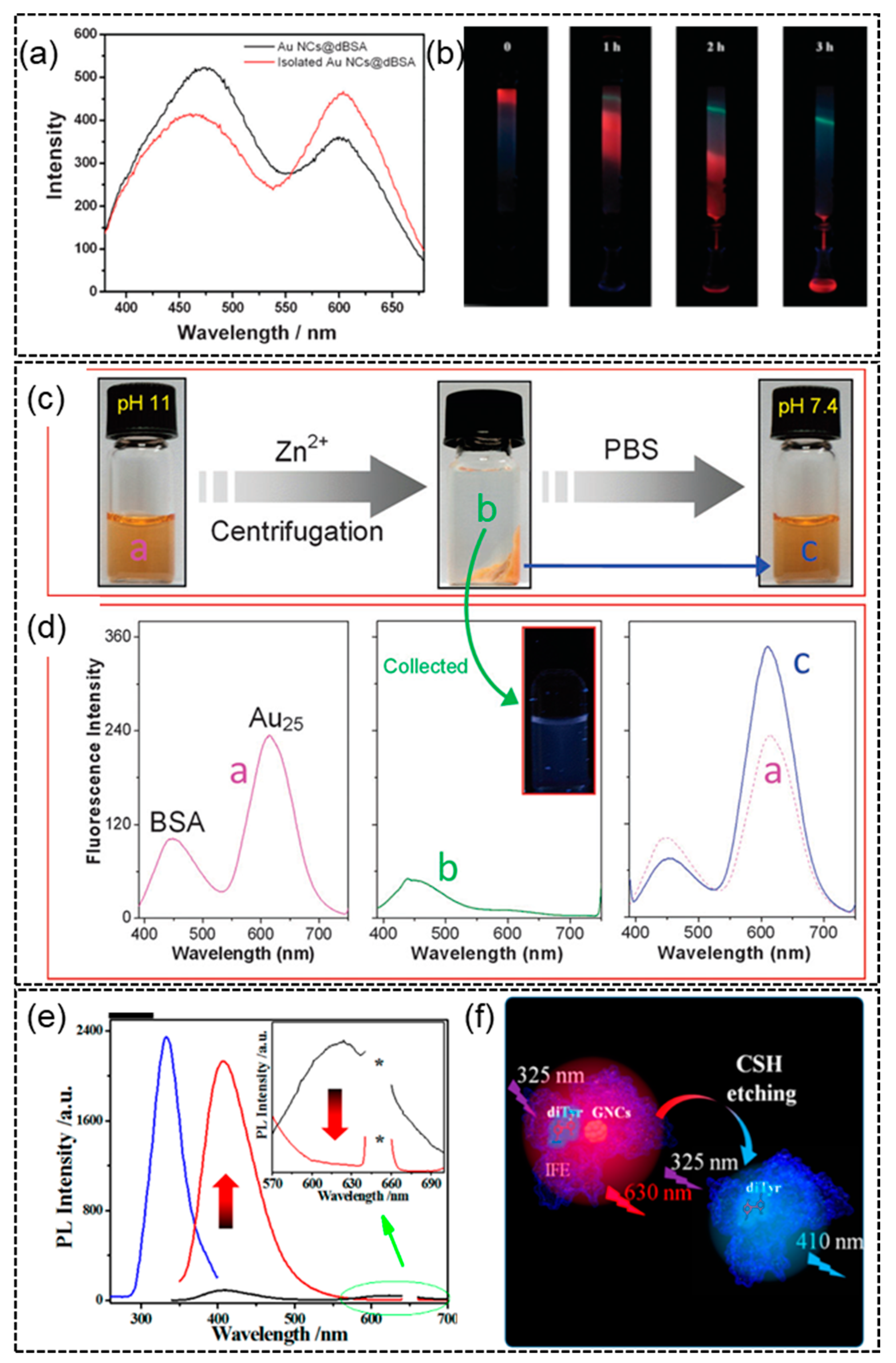
4.1. Case Study 1—The Origin of the Blue Emission in Protein-Templated AuNCs
4.2. Case Study 2—Protein Structure Change and Unfolding/Binding during Formation of AuNCs
5. Biomedical Applications of Protein-Templated Metal Nanoparticles
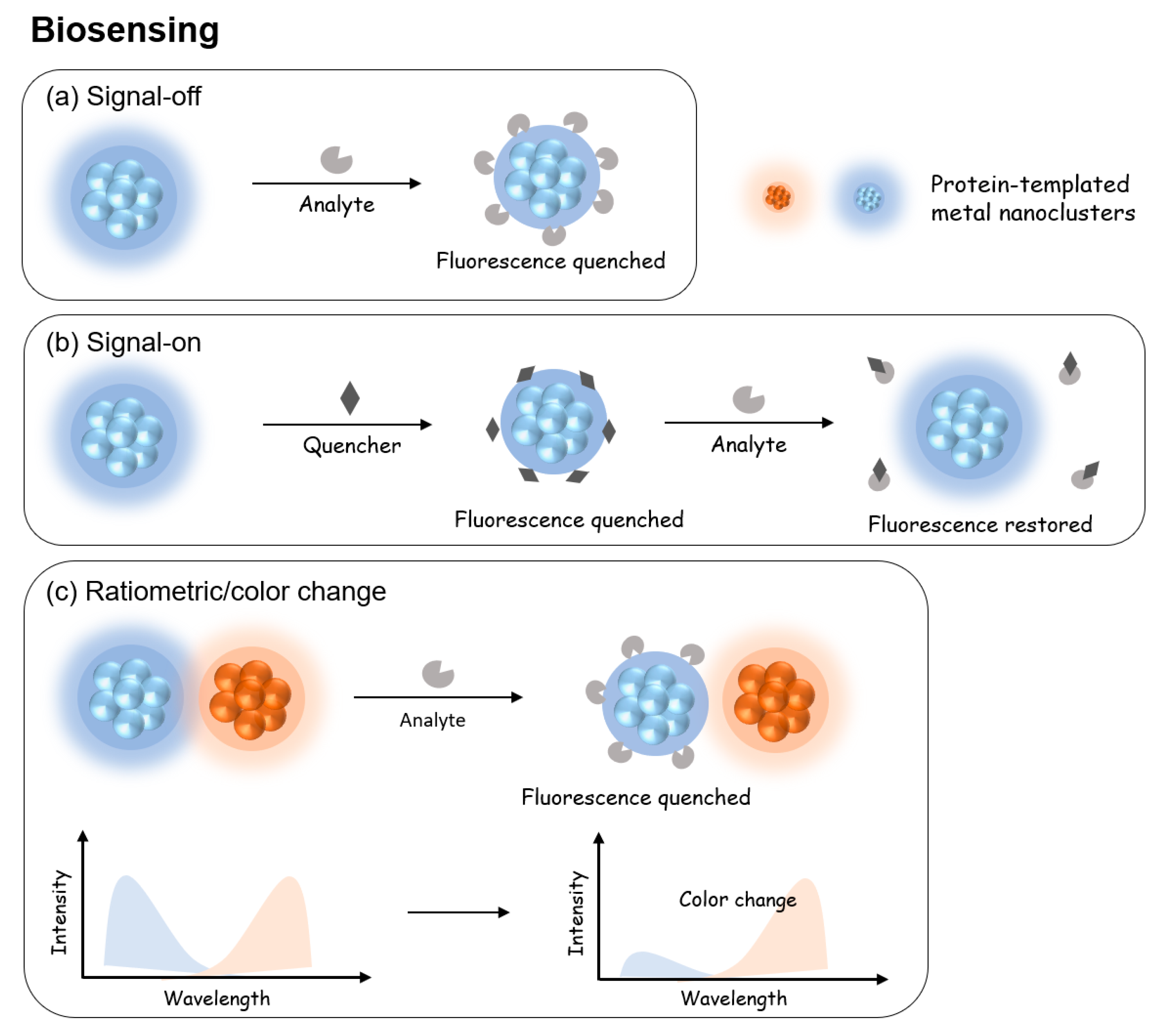
5.1. Protein-Templated Metal Nanoparticles for Biosensing
5.1.1. Signal-Off
5.1.2. Signal-On
5.1.3. Ratiometric
| Sensing Principle | Protein | Metal | Optical Property ex/em, QY | Size (nm) | Analyte | Sample | Linear Range | LOD | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Signal off | Aprotinin | Gold | 550/640 nm, 5.3% | 2.84 | Trypsin/mercury/ copper | Nil | 0–150 μg/mL | 10.18 μg/mL | [155] |
| Signal off | Amylase | Gold | 383/660 nm, 7.9% | 1.75 | Deltamethrin/ Glutathione | Water/urine and plasma | 0.01–5 μM/0.05–5 μM | 6/10 nM | [156] |
| Signal off | BSA | Gold | 450/650 nm, 8% | 4–6 | L-dopamine | Cerebrospinal fluid (CSF) | 0–10 nM | 0.622 nM | [137] |
| Signal off | BSA | Gold | 365/600 nm | ~2 | Uric acid | Blood | 0.7–80 μM | 120 nM | [157] |
| Signal off | BSA | bimetallic gold-silver | 270/630 nm | 1.9 | PPase activity | Bioassay for enzyme activity | 0.1–30 mU/mL | 0.03 mU/mL | [76] |
| Signal off | BSA | bimetallic gold-silver | 370/620 nm | 4.5 | Hg2+ Cu2+ | Blood samples | 1.0–2000 nM 2.0–2500 nM | 0.30 nM 0.30 nM | [134] |
| Signal off | BSA | Copper | 320/405 nm | 3 | Fe3+ | Wastewater and human blood serum | 0.2–2.4 μM | 10 nM | [158] |
| Signal off | HSA | Copper | 325/405 nm | 3 ± 0.3 | bilirubin | Human urine and blood serum | Two linear range: 1.25–7.50 μM; 5.00–28.75 μM | 35.0 nM 145 nM | [159] |
| Signal off | Lysozyme | Gold | 370/650 nm, 5.2% | 4 | CN− | Nil | 5–120 μM | 0.19 μM | [160] |
| Signal off | Ovalbumin | Gold | 470/630 nm | 3.8 | Cu2+ | Serum | 5.0–100.0 μM | 640.0 nM | [140] |
| Signal off | Pepsin | Gold | 416/655 nm, 7.4% | 2 | Spermine | Plasma & urine | 0.0075–10 μM | 1.75 nM | [161] |
| Signal on | BSA | Gold | 370/610 nm, 6% | 1.95 | Alkaline phosphatase | Human serum plasma | 1.0–200.0 U/L | 0.05 U/L | [144] |
| Signal on | BSA | Gold | 480/640 nm | 4 | Cysteine/ Homocysteine | Serum | 0.0057–5 μM/8–25 μM | 9 nM/12 nM | [138] |
| Signal on | BSA | Copper | 325/406 nm | 2.5 | Dopamine | Urine samples | 0.5 to 50 μM | 0.28μM | [139] |
| Signal on | Chicken egg ovalbumin | Gold | 370/640 nm, 6.6% | 2.6 | ATP/PPI | Serum | 42–324 μΜ/9–70 μM | 19 μM/5 μM | [42] |
| Signal on | Human serum albumin | Copper | 325/405 nm | - | Human serum albumin | Serum and urine | ~0.03–0.50 g L−1 | 1.8 ± 0.1 mg L−1 | [53] |
| Signal on | Papain | Gold | 490/639 nm | 5.7 | D-penicillamine | Rat serum | 30.0 μM–2.0 mM | 5.0 μM | [143] |
| Signal on | Papaya juice (papain, chymopapain) | Gold | 360/440 nm | 6.9 | L-lysine | Urine | 10.0–1000.0 μM | 6.0 μM | [162] |
| Signal on | Transferrin | Gold | 382/663 nm | 2.2 | 5-HT | Human serum | 0.2–50 μM | 0.049 μM | [142] |
| Ratiometric | BSA | Gold | 365/615 nm | ~2 | H2O2 | Blood | 0.05–10 μM | 7.7 nM | [148,149] |
| Ratiometric | BSA | Bimetallic gold/silver | 275/690 nm | 5 | Uric acid | Blood | 5.0–50 μM | 5.1 μM | [148] |
5.2. Diagnostics
5.2.1. In Vitro Imaging
5.2.2. In Vivo Imaging
5.3. Drug/Gene Delivery
5.4. Phototherapy
6. Conclusions and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Chakraborty, I.; Pradeep, T. Atomically Precise Clusters of Noble Metals: Emerging Link between Atoms and Nanoparticles. Chem. Rev. 2017, 117, 8208–8271. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Zeng, C.; Zhou, M.; Chen, Y. Atomically Precise Colloidal Metal Nanoclusters and Nanoparticles: Fundamentals and Opportunities. Chem. Rev. 2016, 116, 10346–10413. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Jin, R. Gold Nanoclusters: Size-Controlled Synthesis and Crystal Structures; Springer: Berlin/Heidelberg, Germany, 2014; pp. 1–29. [Google Scholar]
- Maity, P.; Xie, S.; Yamauchi, M.; Tsukuda, T. Stabilized gold clusters: From isolation toward controlled synthesis. Nanoscale 2012, 4, 4027–4037. [Google Scholar] [CrossRef]
- Lu, Y.; Chen, W. Sub-nanometre sized metal clusters: From synthetic challenges to the unique property discoveries. Chem. Soc. Rev. 2012, 41, 3594–3623. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Mu, Y.; Cao, W.; Zhuang, Q.; Wang, Y. Water-Soluble Photoluminescent Adenosine-Functionalized Gold Nanoclusters as Highly Sensitive and Selective Receptors for Riboflavin Detection in Rat Brain. Anal. Chem. 2023, 95, 1671–1679. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Yuan, X.; Yu, Y.; Zhang, Q.; Leong, D.T.; Lee, J.Y.; Xie, J. From Aggregation-Induced Emission of Au(I)–Thiolate Complexes to Ultrabright Au(0)@Au(I)–Thiolate Core—Shell Nanoclusters. J. Am. Chem. Soc. 2012, 134, 16662–16670. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, H.-W.; Zang, Y.; Wang, J.-J.; Xu, L.; Xu, S.-P.; Kong, L.-P. Poly(vinyl alcohol)-Coated Au Nanoclusters with High Stability and Quantum Yields of Fluorescence for Application in pH Sensing. ACS Appl. Nano Mater. 2023, 6, 332–341. [Google Scholar] [CrossRef]
- Ishii, W.; Okayasu, Y.; Kobayashi, Y.; Tanaka, R.; Katao, S.; Nishikawa, Y.; Kawai, T.; Nakashima, T. Excited State Engineering in Ag29 Nanocluster through Peripheral Modification with Silver(I) Complexes for Bright Near-Infrared Photoluminescence. J. Am. Chem. Soc. 2023, 145, 11236–11244. [Google Scholar] [CrossRef]
- McCoy, R.S.; Choi, S.; Collins, G.; Ackerson, B.J.; Ackerson, C.J. Superatom Paramagnetism Enables Gold Nanocluster Heating in Applied Radiofrequency Fields. ACS Nano 2013, 7, 2610–2616. [Google Scholar] [CrossRef]
- Pelayo, J.J.; Whetten, R.L.; Garzón, I.L. Geometric Quantification of Chirality in Ligand—Protected Metal Clusters. J. Phys. Chem. C 2015, 119, 28666–28678. [Google Scholar] [CrossRef]
- Shi, L.; Zhu, L.; Guo, J.; Zhang, L.; Shi, Y.; Zhang, Y.; Hou, K.; Zheng, Y.; Zhu, Y.; Lv, J.; et al. Self-Assembly of Chiral Gold Clusters into Crystalline Nanocubes of Exceptional Optical Activity. Angew. Chem. Int. Ed. 2017, 56, 15397–15401. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Dong, X.-Y.; Luo, P.; Li, S.; Wang, Z.-Y.; Zang, S.-Q.; Mak, T.C.W. Ultrastable atomically precise chiral silver clusters with more than 95% quantum efficiency. Sci. Adv. 2020, 6, eaay0107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kazem-Rostami, M.; Orte, A.; Ortuño, A.M.; David, A.H.G.; Roy, I.; Miguel, D.; Garci, A.; Cruz, C.M.; Stern, C.L.; Cuerva, J.M.; et al. Helically Chiral Hybrid Cyclodextrin Metal—Organic Framework Exhibiting Circularly Polarized Luminescence. J. Am. Chem. Soc. 2022, 144, 9380–9389. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Tang, C.; Wang, Y.; Wang, C.; Zhang, Y.; Qi, W.; Su, R.; He, Z. Circularly Polarized Luminescent Chiral Photonic Films Based on the Coassembly of Cellulose Nanocrystals and Gold Nanoclusters. Langmuir 2022, 38, 4147–4155. [Google Scholar] [CrossRef] [PubMed]
- Jia, T.; Guan, Z.-J.; Zhang, C.; Zhu, X.-Z.; Chen, Y.-X.; Zhang, Q.; Yang, Y.; Sun, D. Eight-Electron Superatomic Cu31 Nanocluster with Chiral Kernel and NIR-II Emission. J. Am. Chem. Soc. 2023, 145, 10355–10363. [Google Scholar] [CrossRef]
- Monti, M.; Matus, M.F.; Malola, S.; Fortunelli, A.; Aschi, M.; Stener, M.; Häkkinen, H. What Contributes to the Measured Chiral Optical Response of the Glutathione-Protected Au25 Nanocluster? ACS Nano 2023, 17, 11481–11491. [Google Scholar] [CrossRef]
- Kawawaki, T.; Negishi, Y. Gold Nanoclusters as Electrocatalysts for Energy Conversion. Nanomaterials 2020, 10, 238. [Google Scholar] [CrossRef] [Green Version]
- Kim, B.; Seong, H.; Song, J.T.; Kwak, K.; Song, H.; Tan, Y.C.; Park, G.; Lee, D.; Oh, J. Over a 15.9% Solar-to-CO Conversion from Dilute CO2 Streams Catalyzed by Gold Nanoclusters Exhibiting a High CO2 Binding Affinity. ACS Energy Lett. 2020, 5, 749–757. [Google Scholar] [CrossRef]
- Wang, H.; Liu, X.; Yang, W.; Mao, G.; Meng, Z.; Wu, Z.; Jiang, H.-L. Surface-Clean Au25 Nanoclusters in Modulated Microenvironment Enabled by Metal—Organic Frameworks for Enhanced Catalysis. J. Am. Chem. Soc. 2022, 144, 22008–22017. [Google Scholar] [CrossRef]
- Wang, S.; Tang, L.; Cai, B.; Yin, Z.; Li, Y.; Xiong, L.; Kang, X.; Xuan, J.; Pei, Y.; Zhu, M. Ligand Modification of Au25 Nanoclusters for Near-Infrared Photocatalytic Oxidative Functionalization. J. Am. Chem. Soc. 2022, 144, 3787–3792. [Google Scholar] [CrossRef]
- Li, Y.; Kim, H.K.; McGillicuddy, R.D.; Zheng, S.-L.; Anderton, K.J.; Stec, G.J.; Lee, J.; Cui, D.; Mason, J.A. A Double Open-Shelled Au43 Nanocluster with Increased Catalytic Activity and Stability. J. Am. Chem. Soc. 2023, 145, 9304–9312. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Gharib, M.; Zeng, Y.; Roy, S.; Nandi, C.K.; Chakraborty, I. Advances in bovine serum albumin-protected gold nanoclusters: From understanding the formation mechanisms to biological applications. Mater. Today Chem. 2023, 29, 101460. [Google Scholar] [CrossRef]
- Qiao, Z.; Zhang, J.; Hai, X.; Yan, Y.; Song, W.; Bi, S. Recent advances in templated synthesis of metal nanoclusters and their applications in biosensing, bioimaging and theranostics. Biosens. Bioelectron. 2021, 176, 112898. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Chen, H.; Chen, B.; Zhao, G. Highly fluorescent gold nanoclusters stabilized by food proteins: From preparation to application in detection of food contaminants and bioactive nutrients. Crit. Rev. Food Sci. Nutr. 2018, 58, 689–699. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhu, W.; Wan, A.; Liu, L. The mechanism and application of the protein-stabilized gold nanocluster sensing system. Analyst 2017, 142, 567–581. [Google Scholar] [CrossRef]
- Carter, D.C.; Ho, J.X. Structure of Serum Albumin. In Advances in Protein Chemistry; Anfinsen, C.B., Ed.; Academic Press: Cambridge, MA, USA, 1994; pp. 153–203. [Google Scholar]
- Dickerson, M.B.; Sandhage, K.H.; Naik, R.R. Protein- and Peptide-Directed Syntheses of Inorganic Materials. Chem. Rev. 2008, 108, 4935–4978. [Google Scholar] [CrossRef]
- Xie, J.; Zheng, Y.; Ying, J.Y. Protein-Directed Synthesis of Highly Fluorescent Gold Nanoclusters. J. Am. Chem. Soc. 2009, 131, 888–889. [Google Scholar] [CrossRef]
- Shang, L.; Dong, S.J.; Nienhaus, G.U. Ultra-small fluorescent metal nanoclusters: Synthesis and biological applications. Nano Today 2011, 6, 401–418. [Google Scholar] [CrossRef]
- Jin, R.; Zhu, Y.; Qian, H. Quantum-Sized Gold Nanoclusters: Bridging the Gap between Organometallics and Nanocrystals. Chem. A Eur. J. 2011, 17, 6584–6593. [Google Scholar] [CrossRef]
- Chen, L.-Y.; Wang, C.-W.; Yuan, Z.; Chang, H.-T. Fluorescent Gold Nanoclusters: Recent Advances in Sensing and Imaging. Anal. Chem. 2015, 87, 216–229. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, E. Metal nanoclusters: New fluorescent probes for sensors and bioimaging. Nano Today 2014, 9, 132–157. [Google Scholar] [CrossRef]
- Li, Z.; Sun, Q.; Zhu, Y.; Tan, B.; Xu, Z.P.; Dou, S.X. Ultra-small fluorescent inorganic nanoparticles for bioimaging. J. Mater. Chem. B 2014, 2, 2793–2818. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Jin, R. Atomically Precise Gold Nanoclusters as New Model Catalysts. Acc. Chem. Res. 2013, 46, 1749–1758. [Google Scholar] [CrossRef] [PubMed]
- Goswami, N.; Luo, Z.; Yuan, X.; Leong, D.T.; Xie, J. Engineering gold-based radiosensitizers for cancer radiotherapy. Mater. Horiz. 2017, 4, 817–831. [Google Scholar] [CrossRef]
- Zare, I.; Chevrier, D.M.; Cifuentes-Rius, A.; Moradi, N.; Xianyu, Y.; Ghosh, S.; Trapiella-Alfonso, L.; Tian, Y.; Shourangiz-Haghighi, A.; Mukherjee, S.; et al. Protein-protected metal nanoclusters as diagnostic and therapeutic platforms for biomedical applications. Mater. Today 2023, 66, 159–193. [Google Scholar] [CrossRef]
- Yue, Y.; Liu, T.Y.; Li, H.W.; Liu, Z.Y.; Wu, Y.Q. Microwave-assisted synthesis of BSA-protected small gold nanoclusters and their fluorescence—Enhanced sensing of silver(I) ions. Nanoscale 2012, 4, 2251–2254. [Google Scholar] [CrossRef]
- Yan, L.; Cai, Y.; Zheng, B.; Yuan, H.; Guo, Y.; Xiao, D.; Choi, M.M.F. Microwave-assisted synthesis of BSA-stabilized and HSA-protected gold nanoclusters with red emission. J. Mater. Chem. 2012, 22, 1000–1005. [Google Scholar] [CrossRef]
- Tian, J.; Yan, L.; Sang, A.; Yuan, H.; Zheng, B.; Xiao, D. Microwave-Assisted Synthesis of Red-Light Emitting Au Nanoclusters with the Use of Egg White. J. Chem. Educ. 2014, 91, 1715–1719. [Google Scholar] [CrossRef]
- Hsu, N.-Y.; Lin, Y.-W. Microwave-assisted synthesis of bovine serum albumin–gold nanoclusters and their fluorescence-quenched sensing of Hg2+ ions. New J. Chem. 2016, 40, 1155–1161. [Google Scholar] [CrossRef]
- Selvaprakash, K.; Chen, Y.-C. Using protein-encapsulated gold nanoclusters as photoluminescent sensing probes for biomolecules. Biosens. Bioelectron. 2014, 61, 88–94. [Google Scholar] [CrossRef]
- Yu, Y.; Luo, Z.; Teo, C.S.; Tan, Y.N.; Xie, J. Tailoring the protein conformation to synthesize different-sized gold nanoclusters. Chem. Commun. 2013, 49, 9740–9742. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, C.; Xu, L.; Cheng, H.; Lin, Q.; Zhang, C. Protein-directed synthesis of pH-responsive red fluorescent copper nanoclusters and their applications in cellular imaging and catalysis. Nanoscale 2014, 6, 1775–1781. [Google Scholar] [CrossRef]
- Yu, Y.; Geng, J.; Ong, E.Y.X.; Chellappan, V.; Tan, Y.N. Bovine Serum Albulmin Protein-Templated Silver Nanocluster (BSA-Ag13): An Effective Singlet Oxygen Generator for Photodynamic Cancer Therapy. Adv. Healthc. Mater. 2016, 5, 2528–2535. [Google Scholar] [CrossRef]
- Aires, A.; Llarena, I.; Moller, M.; Castro-Smirnov, J.; Cabanillas-Gonzalez, J.; Cortajarena, A.L. A Simple Approach to Design Proteins for the Sustainable Synthesis of Metal Nanoclusters. Angew. Chem. Int. Ed. 2019, 58, 6214–6219. [Google Scholar] [CrossRef]
- Chen, C.-T.; Chen, W.-J.; Liu, C.-Z.; Chang, L.-Y.; Chen, Y.-C. Glutathione-bound gold nanoclusters for selective-binding and detection of glutathione S-transferase-fusion proteins from cell lysates. Chem. Commun. 2009, 48, 7515–7517. [Google Scholar] [CrossRef] [PubMed]
- Matus, M.F.; Häkkinen, H. Understanding ligand-protected noble metal nanoclusters at work. Nat. Rev. Mater. 2023, 8, 372–389. [Google Scholar] [CrossRef]
- Xu, Y.; Sherwood, J.; Qin, Y.; Crowley, D.; Bonizzoni, M.; Bao, Y. The role of protein characteristics in the formation and fluorescence of Au nanoclusters. Nanoscale 2014, 6, 1515–1524. [Google Scholar] [CrossRef]
- Guo, Y.; Amunyela, H.T.N.N.; Cheng, Y.; Xie, Y.; Yu, H.; Yao, W.; Li, H.-W.; Qian, H. Natural protein-templated fluorescent gold nanoclusters: Syntheses and applications. Food Chem. 2021, 335, 127657. [Google Scholar] [CrossRef]
- Liu, X.; Astruc, D. Atomically precise copper nanoclusters and their applications. Coord. Chem. Rev. 2018, 359, 112–126. [Google Scholar] [CrossRef]
- Cao, H.; Chen, Z.; Zheng, H.; Huang, Y. Copper nanoclusters as a highly sensitive and selective fluorescence sensor for ferric ions in serum and living cells by imaging. Biosens. Bioelectron. 2014, 62, 189–195. [Google Scholar] [CrossRef]
- Lettieri, M.; Palladino, P.; Scarano, S.; Minunni, M. Protein-templated copper nanoclusters for fluorimetric determination of human serum albumin. Microchim. Acta 2021, 188, 116. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Wang, Z.; Yang, L.; Tian, S.; Hou, C.; Lu, Y. Lysozyme-stabilized gold fluorescent cluster: Synthesis and application as Hg2+ sensor. Analyst 2010, 135, 1406–1410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, T.-H.; Tseng, W.-L. (Lysozyme Type VI)-Stabilized Au8 Clusters: Synthesis Mechanism and Application for Sensing of Glutathione in a Single Drop of Blood. Small 2012, 8, 1912–1919. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, H.; Hamaguchi, K.; Osaka, I.; Arakawa, R. ph-Dependent Synthesis of Pepsin-Mediated Gold Nanoclusters with Blue Green and Red Fluorescent Emission. Adv. Funct. Mater. 2011, 21, 3508–3515. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Y.; Wang, C.; Li, W.; Zhou, H.; Jiao, H.; Lin, Q.; Yu, C. Papain-directed synthesis of luminescent gold nanoclusters and the sensitive detection of Cu2+. J. Colloid Interface Sci. 2013, 396, 63–68. [Google Scholar] [CrossRef]
- Liu, J.-M.; Chen, J.-T.; Yan, X.-P. Near Infrared Fluorescent Trypsin Stabilized Gold Nanoclusters as Surface Plasmon Enhanced Energy Transfer Biosensor and in Vivo Cancer Imaging Bioprobe. Anal. Chem. 2013, 85, 3238–3245. [Google Scholar] [CrossRef]
- Lv, C.; Yin, S.; Zhang, X.; Hu, J.; Zhang, T.; Zhao, G. 16-Mer ferritin-like protein templated gold nanoclusters for bioimaging detection of methylmercury in the brain of living mice. Anal. Chim. Acta 2020, 1127, 149–155. [Google Scholar] [CrossRef]
- Nain, A.; Tseng, Y.-T.; Wei, S.-C.; Periasamy, A.P.; Huang, C.-C.; Tseng, F.-G.; Chang, H.-T. Capping 1,3-propanedithiol to boost the antibacterial activity of protein-templated copper nanoclusters. J. Hazard. Mater. 2020, 389, 121821. [Google Scholar] [CrossRef]
- Rajamanikandan, R.; Ilanchelian, M. Protein-protected red emittive copper nanoclusters as a fluorometric probe for highly sensitive biosensing of creatinine. Anal. Methods 2018, 10, 3666–3674. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, J.; Yang, G.; Ma, S.; Zhang, M.; Yang, J. Cysteine-rich protein-templated silver nanoclusters as a fluorometric probe for mercury(ii) detection. Anal. Methods 2019, 11, 733–738. [Google Scholar] [CrossRef]
- Thawari, A.G.; Kumar, P.; Srivastava, R.; Rao, C.P. Lysozyme coated copper nanoclusters for green fluorescence and their utility in cell imaging. Mater. Adv. 2020, 1, 1439–1447. [Google Scholar] [CrossRef]
- Sarkar, P.; Saha, M.; Nandi, N.; Sahu, D.K.; Sahu, K. Red-Emitting Silver Nanoclusters for Dual-Mode Detection of Cu2+ and Vitamin B12 in Living Cells. ACS Appl. Nano Mater. 2022, 5, 7670–7678. [Google Scholar] [CrossRef]
- Sabarinathan, D.; Sharma, A.S.; Agyekum, A.A.; Murugavelu, M.; Sabapathy, P.C.; Ali, S.; Hassan, H.; Li, H.; Chen, Q. Thunnus albacares protein-mediated synthesis of water-soluble Copper nanoclusters as sensitive fluorescent probe for Ferric ion detection. J. Mol. Struct. 2022, 1254, 132333. [Google Scholar] [CrossRef]
- Wu, Z.; Jin, R. On the Ligand’s Role in the Fluorescence of Gold Nanoclusters. Nano Lett. 2010, 10, 2568–2573. [Google Scholar] [CrossRef]
- Zheng, J.; Zhang, C.; Dickson, R.M. Highly Fluorescent, Water-Soluble, Size-Tunable Gold Quantum Dots. Phys. Rev. Lett. 2004, 93, 077402. [Google Scholar] [CrossRef]
- Mu, J.; Yang, J.-L.; Zhang, D.-W.; Jia, Q. Progress in Preparation of Metal Nanoclusters and Their Application in Detection of Environmental Pollutants. Chin. J. Anal. Chem. 2021, 49, 319–329. [Google Scholar] [CrossRef]
- Zhang, X.-H.; Zhou, T.-Y.; Chen, X. Applications of Metal Nanoclusters in Environmental Monitorin. Chin. J. Anal. Chem. 2015, 43, 1296–1305. [Google Scholar] [CrossRef]
- Ghosh, S.; Anand, U.; Mukherjee, S. Luminescent silver nanoclusters acting as a label-free photoswitch in metal ion sensing. Anal. Chem. 2014, 86, 3188–3194. [Google Scholar] [CrossRef]
- Luo, Z.; Zheng, K.; Xie, J. Engineering ultrasmall water-soluble gold and silver nanoclusters for biomedical applications. Chem. Commun. 2014, 50, 5143–5155. [Google Scholar] [CrossRef]
- Tan, X.; Jin, R. Ultrasmall metal nanoclusters for bio-related applications. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2013, 5, 569–581. [Google Scholar] [CrossRef]
- Chakraborty, I.; Udayabhaskararao, T.; Pradeep, T. Luminescent sub-nanometer clusters for metal ion sensing: A new direction in nanosensors. J. Hazard. Mater. 2012, 211–212, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yang, F.; Zhang, C.; He, X.; Jin, R. Metal Nanoclusters as Biomaterials for Bioapplications: Atomic Precision as the Next Goal. ACS Mater. Lett. 2022, 4, 1279–1296. [Google Scholar] [CrossRef]
- Anand, U.; Ghosh, S.; Mukherjee, S. Toggling Between Blue- and Red-Emitting Fluorescent Silver Nanoclusters. J. Phys. Chem. Lett. 2012, 3, 3605–3609. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Lin, Y.; Xu, M.; Gao, Z.; Yang, H.; Tang, D. Facile Synthesis of Enhanced Fluorescent Gold–Silver Bimetallic Nanocluster and Its Application for Highly Sensitive Detection of Inorganic Pyrophosphatase Activity. Anal. Chem. 2016, 88, 8886–8892. [Google Scholar] [CrossRef] [PubMed]
- Goswami, N.; Lin, F.; Liu, Y.; Leong, D.T.; Xie, J. Highly Lumin. Thiolated Gold Nanoclusters Impregnated Nanogel. Chem. Mater. 2016, 28, 4009–4016. [Google Scholar] [CrossRef]
- Su, X.; Liu, J. pH-Guided Self-Assembly of Copper Nanoclusters with Aggregation-Induced Emission. ACS Appl. Mater. Interfaces 2017, 9, 3902–3910. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xiong, Y.; Kershaw, S.V.; Chen, B.; Yang, X.; Goswami, N.; Lai, W.-F.; Xie, J.; Rogach, A.L. In Situ Fabrication of Flexible, Thermally Stable, Large-Area, Strongly Luminescent Copper Nanocluster/Polymer Composite Films. Chem. Mater. 2017, 29, 10206–10211. [Google Scholar] [CrossRef]
- Mostafa, I.M.; Abdussalam, A.; Zholudov, Y.T.; Snizhko, D.V.; Zhang, W.; Hosseini, M.; Guan, Y.; Xu, G. Recent Applications and Future Perspectives of Chemiluminescent and Bioluminescent Imaging Technologies. Chem. Biomed. Imaging 2023. [Google Scholar] [CrossRef]
- Yang, M.; Huang, J.; Fan, J.; Du, J.; Pu, K.; Peng, X. Chemiluminescence for bioimaging and therapeutics: Recent advances and challenges. Chem. Soc. Rev. 2020, 49, 6800–6815. [Google Scholar] [CrossRef]
- Zhang, C.; Su, Y.; Liang, Y.; Lai, W.; Jiang, J.; Wu, H.; Mao, X.; Zheng, L.; Zhang, R. Chemiluminescence and Its Biomedical Applications. In Nanophotonics in Biomedical Engineering; Zhao, X., Lu, M., Eds.; Springer: Singapore, 2021; pp. 143–195. [Google Scholar]
- Wang, Z.; Huang, J.; Huang, J.; Yu, B.; Pu, K.; Xu, F.-J. Chemiluminescence: From mechanism to applications in biological imaging and therapy. Aggregate 2021, 2, e14020212021. [Google Scholar] [CrossRef]
- Sheng, Y.; Yang, H.; Wang, Y.; Han, L.; Zhao, Y.; Fan, A. Silver nanoclusters-catalyzed luminol chemiluminescence for hydrogen peroxide and uric acid detection. Talanta 2017, 166, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Wang, Y.; Zhou, D.; Kuang, M.; Fang, D.; Yang, W.; Wei, S.; Ma, L. A novel chemiluminescence sensor for sensitive detection of cholesterol based on the peroxidase-like activity of copper nanoclusters. Sci. Rep. 2016, 6, 39157. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.; Xu, S.; Chen, F. Enhanced chemiluminescence of the luminol-hydrogen peroxide system by BSA-stabilized Au nanoclusters as a peroxidase mimic and its application. Anal. Methods 2014, 6, 3117–3123. [Google Scholar] [CrossRef]
- Yang, D.; He, Y.; Sui, Y.; Chen, F. Determination of catechol in water based on gold nanoclusters-catalyzed chemiluminescence. J. Lumin. 2017, 187, 186–192. [Google Scholar] [CrossRef]
- Li, Y.; Peng, W.; You, X. Determination of dopamine by exploiting the catalytic effect of hemoglobin–stabilized gold nanoclusters on the luminol–NaIO4 chemiluminescence system. Microchim. Acta 2017, 184, 3539–3545. [Google Scholar] [CrossRef]
- Yang, D.; He, Y.; Sui, Y.; Chen, F. Gold nanoclusters-catalyzed rhodamine 6G–K3Fe(CN)6 chemiluminescence and its application. Anal. Methods 2016, 8, 7272–7278. [Google Scholar] [CrossRef]
- You, X.; Li, Y.; Li, B.; Ma, J. Gold nanoclusters-based chemiluminescence resonance energy transfer method for sensitive and label-free detection of trypsin. Talanta 2016, 147, 63–68. [Google Scholar] [CrossRef]
- You, X.; Li, Y. Direct chemiluminescence of fluorescent gold nanoclusters with classic oxidants for hydrogen peroxide sensing. Arab. J. Chem. 2019, 12, 69–74. [Google Scholar] [CrossRef] [Green Version]
- Vahid, B.; Hassanzadeh, J.; Khodakarami, B. CdSe quantum dots-sensitized chemiluminescence system and quenching effect of gold nanoclusters for cyanide detection. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 212, 322–329. [Google Scholar] [CrossRef]
- Luo, Q.-X.; Li, Y.; Liang, R.-P.; Cao, S.-P.; Jin, H.-J.; Qiu, J.-D. Gold nanoclusters enhanced electrochemiluminescence of g-C3N4 for protein kinase activity analysis and inhibition. J. Electroanal. Chem. 2020, 856, 113706. [Google Scholar] [CrossRef]
- Valenti, G.; Rampazzo, E.; Kesarkar, S.; Genovese, D.; Fiorani, A.; Zanut, A.; Palomba, F.; Marcaccio, M.; Paolucci, F.; Prodi, L. Electrogenerated chemiluminescence from metal complexes-based nanoparticles for highly sensitive sensors applications. Coord. Chem. Rev. 2018, 367, 65–81. [Google Scholar] [CrossRef]
- Song, Q.; Shi, Y.; He, D.; Xu, S.; Ouyang, J. Sequence-Dependent dsDNA-Templated Formation of Fluorescent Copper Nanoparticles. Chem. A Eur. J. 2015, 21, 2417–2422. [Google Scholar] [CrossRef] [PubMed]
- Babamiri, B.; Salimi, A.; Hallaj, R.; Hasanzadeh, M. Nickel nanoclusters as a novel emitter for molecularly imprinted electrochemiluminescence based sensor toward nanomolar detection of creatinine. Biosens. Bioelectron. 2018, 107, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Yang, L.; Xue, J.; Ren, X.; Zhang, N.; Fan, D.; Wei, Q.; Ma, H. Highly-branched Cu2O as well-ordered co-reaction accelerator for amplifying electrochemiluminescence response of gold nanoclusters and procalcitonin analysis based on protein bioactivity maintenance. Biosens. Bioelectron. 2019, 144, 111676. [Google Scholar] [CrossRef]
- Peng, H.; Jian, M.; Deng, H.; Wang, W.; Huang, Z.; Huang, K.; Liu, A.; Chen, W. Valence States Effect on Electrogenerated Chemiluminescence of Gold Nanocluster. ACS Appl. Mater. Interfaces 2017, 9, 14929–14934. [Google Scholar] [CrossRef]
- Kim, J.M.; Jeong, S.; Song, J.K.; Kim, J. Near-infrared electrochemiluminescence from orange fluorescent Au nanoclusters in water. Chem. Commun. 2018, 54, 2838–2841. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, S.; Luo, X.; Chai, Y.; Yuan, R. Ternary Electrochemiluminescence Nanostructure of Au Nanoclusters as a Highly Efficient Signal Label for Ultrasensitive Detection of Cancer Biomarkers. Anal. Chem. 2018, 90, 10024–10030. [Google Scholar] [CrossRef]
- Wang, G.-L.; Jin, L.-Y.; Dong, Y.-M.; Wu, X.-M.; Li, Z.-J. Intrinsic enzyme mimicking activity of gold nanoclusters upon visible light triggering and its application for colorimetric trypsin detection. Biosens. Bioelectron. 2015, 64, 523–529. [Google Scholar] [CrossRef]
- Liu, Q.; Wan, K.; Shang, Y.; Wang, Z.-G.; Zhang, Y.; Dai, L.; Wang, C.; Wang, H.; Shi, X.; Liu, D.; et al. Cofactor-free oxidase-mimetic nanomaterials from self-assembled histidine-rich peptides. Nat. Mater. 2021, 20, 395–402. [Google Scholar] [CrossRef]
- He, S.-B.; Deng, H.-H.; Liu, A.-L.; Li, G.-W.; Lin, X.-H.; Chen, W.; Xia, X.-H. Synthesis and Peroxidase-Like Activity of Salt-Resistant Platinum Nanoparticles by Using Bovine Serum Albumin as the Scaffold. ChemCatChem 2014, 6, 1543–1548. [Google Scholar] [CrossRef]
- He, S.-B.; Lin, M.-T.; Yang, L.; Noreldeen, H.A.A.; Peng, H.-P.; Deng, H.-H.; Chen, W. Protein-Assisted Osmium Nanoclusters with Intrinsic Peroxidase-like Activity and Extrinsic Antifouling Behavior. ACS Appl. Mater. Interfaces 2021, 13, 44541–44548. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Liu, Z.; Zhang, J.-R.; Zhou, Y.; Wang, L.; Zhu, J.-J. General Synergistic Hybrid Catalyst Synthesis Method Using a Natural Enzyme Scaffold-Confined Metal Nanocluster. ACS Appl. Mater. Interfaces 2022, 15, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Kelly, K.L.; Coronado, E.; Zhao, L.L.; Schatz, G.C. The Optical Properties of Metal Nanoparticles: The Influence of Size, Shape, and Dielectric Environment. J. Phys. Chem. B 2003, 107, 668–677. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, Q.; Xie, J.; Lee, J.Y. Engineering the architectural diversity of heterogeneous metallic nanocrystals. Nat. Commun. 2013, 4, 1454. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Meng, X.; Das, A.; Li, T.; Song, Y.; Cao, T.; Zhu, X.; Zhu, M.; Jin, R. A 200-fold Quantum Yield Boost in the Photoluminescence of Silver-Doped AgxAu25−x Nanoclusters: The 13th Silver Atom Matters. Angew. Chem. Int. Ed. 2014, 53, 2376–2380. [Google Scholar] [CrossRef]
- Andolina, C.M.; Dewar, A.C.; Smith, A.M.; Marbella, L.E.; Hartmann, M.J.; Millstone, J.E. Photoluminescent Gold–Copper Nanoparticle Alloys with Composition-Tunable Near-Infrared Emission. J. Am. Chem. Soc. 2013, 135, 5266–5269. [Google Scholar] [CrossRef]
- Chakraborty, S.; Mukherjee, S. Effects of protecting groups on luminescent metal nanoclusters: Spectroscopic signatures and applications. Chem. Commun. 2022, 58, 29–47. [Google Scholar] [CrossRef]
- Yuan, X.; Goswami, N.; Chen, W.; Yao, Q.; Xie, J. Insights into the effect of surface ligands on the optical properties of thiolated Au25 nanoclusters. Chem. Commun. 2016, 52, 5234–5237. [Google Scholar] [CrossRef]
- Aiken, J.D.; Finke, R.G. A review of modern transition-metal nanoclusters: Their synthesis, characterization, and applications in catalysis. J. Mol. Catal. A Chem. 1999, 145, 1–44. [Google Scholar] [CrossRef]
- Lim, J.; Yeap, S.P.; Che, H.X.; Low, S.C. Characterization of magnetic nanoparticle by dynamic light scattering. Nanoscale Res. Lett. 2013, 8, 381. [Google Scholar] [CrossRef] [Green Version]
- Maguire, C.M.; Rosslein, M.; Wick, P.; Prina-Mello, A. Characterisation of particles in solution—A perspective on light scattering and comparative technologies. Sci. Technol. Adv. Mater. 2018, 19, 732–745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hackley, V.A.; Clogston, J.D. Measuring the Hydrodynamic Size of Nanoparticles in Aqueous Media Using Batch-Mode Dynamic Light Scattering. In Characterization of Nanoparticles Intended for Drug Delivery; McNeil, S.E., Ed.; Humana Press: Totowa, NJ, USA, 2011; pp. 35–52. [Google Scholar]
- Chen, T.; Yao, Q.; Nasaruddin, R.R.; Xie, J. Electrospray Ionization Mass Spectrometry: A Powerful Platform for Noble-Metal Nanocluster Analysis. Angew. Chem. Int. Ed. 2019, 58, 11967–11977. [Google Scholar] [CrossRef] [PubMed]
- Shekhar, S.; Mahato, P.; Yadav, R.; Verma, S.D.; Mukherjee, S. White Light Generation through l-Ascorbic Acid-Templated Thermoresponsive Copper Nanoclusters. ACS Sustain. Chem. Eng. 2022, 10, 1379–1389. [Google Scholar] [CrossRef]
- Lin, Y.; Charchar, P.; Christofferson, A.J.; Thomas, M.R.; Todorova, N.; Mazo, M.M.; Chen, Q.; Doutch, J.; Richardson, R.; Yarovsky, I.; et al. Surface Dynamics and Ligand–Core Interactions of Quantum Sized Photoluminescent Gold Nanoclusters. J. Am. Chem. Soc. 2018, 140, 18217–18226. [Google Scholar] [CrossRef] [Green Version]
- Ungor, D.; Barbasz, A.; Czyżowska, A.; Csapó, E.; Oćwieja, M. Cytotoxicity studies of protein-stabilized fluorescent gold nanoclusters on human lymphocytes. Colloids Surf. B Biointerfaces 2021, 200, 111593. [Google Scholar] [CrossRef]
- Le Guével, X.; Hötzer, B.; Jung, G.; Hollemeyer, K.; Trouillet, V.; Schneider, M. Formation of Fluorescent Metal (Au, Ag) Nanoclusters Capped in Bovine Serum Albumin Followed by Fluorescence and Spectroscopy. J. Phys. Chem. C 2011, 115, 10955–10963. [Google Scholar] [CrossRef]
- Wen, X.; Yu, P.; Toh, Y.-R.; Tang, J. Structure-Correlated Dual Fluorescent Bands in BSA-Protected Au25 Nanoclusters. J. Phys. Chem. C 2012, 116, 11830–11836. [Google Scholar] [CrossRef]
- Micsonai, A.; Wien, F.; Kernya, L.; Lee, Y.-H.; Goto, Y.; Réfrégiers, M.; Kardos, J. Accurate secondary structure prediction and fold recognition for circular dichroism spectroscopy. Proc. Natl. Acad. Sci. USA 2015, 112, E3095–E3103. [Google Scholar] [CrossRef]
- Kompany-Zareh, M.; Akbarian, S.; Najafpour, M.M. Unsupervised recognition of components from the interaction of BSA with Fe cluster in different conditions utilizing 2D fluorescence spectroscopy. Sci. Rep. 2022, 12, 16875. [Google Scholar] [CrossRef]
- Wang, Q.; Dou, X.; Chen, X.; Zhao, Z.; Wang, S.; Wang, Y.; Sui, K.; Tan, Y.; Gong, Y.; Zhang, Y.; et al. Reevaluating Protein Photoluminescence: Remarkable Visible Luminescence upon Concentration and Insight into the Emission Mechanism. Angew. Chem. Int. Ed. 2019, 58, 12667–12673. [Google Scholar] [CrossRef]
- Li, H.-W.; Ai, K.; Wu, Y. Fluorescence visual gel-separation of dansylated BSA-protected gold-nanoclusters. Chem. Commun. 2011, 47, 9852–9854. [Google Scholar] [CrossRef] [PubMed]
- Guan, G.; Zhang, S.-Y.; Cai, Y.; Liu, S.; Bharathi, M.S.; Low, M.; Yu, Y.; Xie, J.; Zheng, Y.; Zhang, Y.-W.; et al. Convenient purification of gold clusters by co-precipitation for improved sensing of hydrogen peroxide, mercury ions and pesticides. Chem. Commun. 2014, 50, 5703–5705. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Shu, T.; Wang, J.; Zhang, Z.; Zhang, X. Hidden Dityrosine Residues in Protein-Protected Gold Nanoclusters. J. Phys. Chem. C 2015, 119, 12065–12070. [Google Scholar] [CrossRef]
- Chaudhari, K.; Xavier, P.L.; Pradeep, T. Understanding the Evolution of Luminescent Gold Quantum Clusters in Protein Templates. ACS Nano 2011, 5, 8816–8827. [Google Scholar] [CrossRef]
- Yu, Y.; New, S.Y.; Xie, J.; Su, X.; Tan, Y.N. Protein-based fluorescent metal nanoclusters for small molecular drug screening. Chem. Commun. 2014, 50, 13805–13808. [Google Scholar] [CrossRef]
- Yu, Y.; Mok, B.Y.L.; Loh, X.J.; Tan, Y.N. Rational Design of Biomolecular Templates for Synthesizing Multifunctional Noble Metal Nanoclusters toward Personalized Theranostic Applications. Adv. Healthc. Mater. 2016, 5, 1844–1859. [Google Scholar] [CrossRef]
- Xiong, H.; Wang, W.; Liang, J.; Wen, W.; Zhang, X.; Wang, S. A convenient purification method for metal nanoclusters based on pH-induced aggregation and cyclic regeneration and its applications in fluorescent pH sensors. Sens. Actuators B Chem. 2017, 239, 988–992. [Google Scholar] [CrossRef]
- Wu, Y.-T.; Shanmugam, C.; Tseng, W.-B.; Hiseh, M.-M.; Tseng, W.-L. A gold nanocluster-based fluorescent probe for simultaneous pH and temperature sensing and its application to cellular imaging and logic gates. Nanoscale 2016, 8, 11210–11216. [Google Scholar] [CrossRef]
- Ding, C.; Tian, Y. Gold nanocluster-based fluorescence biosensor for targeted imaging in cancer cells and ratiometric determination of intracellular pH. Biosens. Bioelectron. 2015, 65, 183–190. [Google Scholar] [CrossRef]
- Zhang, N.; Si, Y.; Sun, Z.; Chen, L.; Li, R.; Qiao, Y.; Wang, H. Rapid, Selective, and Ultrasensitive Fluorimetric Analysis of Mercury and Copper Levels in Blood Using Bimetallic Gold–Silver Nanoclusters with “Silver Effect”-Enhanced Red Fluorescence. Anal. Chem. 2014, 86, 11714–11721. [Google Scholar] [CrossRef]
- Nath, P.; Chatterjee, M.; Chanda, N. Dithiothreitol-Facilitated Synthesis of Bovine Serum Albumin—Gold Nanoclusters for Pb(II) Ion Detection on Paper Substrates and in Live Cells. ACS Appl. Nano Mater. 2018, 1, 5108–5118. [Google Scholar] [CrossRef]
- Chakraborty, S.; Nandy, A.; Ghosh, S.; Das, N.K.; Parveen, S.; Datta, S.; Mukherjee, S. Protein-templated gold nanoclusters as specific bio-imaging probes for the detection of Hg(ii) ions in in vivo and in vitro systems: Discriminating between MDA-MB-231 and MCF10A cells. Analyst 2021, 146, 1455–1463. [Google Scholar] [CrossRef] [PubMed]
- Govindaraju, S.; Ankireddy, S.R.; Viswanath, B.; Kim, J.; Yun, K. Fluorescent Gold Nanoclusters for Selective Detection of Dopamine in Cerebrospinal fluid. Sci. Rep. 2017, 7, 40298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nebu, J.; Anjali Devi, J.S.; Aparna, R.S.; Aswathy, B.; Lekha, G.M.; Sony, G. Potassium triiodide-quenched gold nanocluster as a fluorescent turn-on probe for sensing cysteine/homocysteine in human serum. Anal. Bioanal. Chem. 2019, 411, 997–1007. [Google Scholar] [CrossRef]
- Miao, Z.; Hou, W.; Liu, M.; Zhang, Y.; Yao, S. BSA capped bi-functional fluorescent Cu nanoclusters as pH sensor and selective detection of dopamine. New J. Chem. 2018, 42, 1446–1456. [Google Scholar] [CrossRef]
- Chen, Y.; Qiao, J.; Liu, Q.; Qi, L. Ovalbumin-stabilized gold nanoclusters with ascorbic acid as reducing agent for detection of serum copper. Chin. Chem. Lett. 2018, 29, 366–370. [Google Scholar] [CrossRef]
- Liu, Y.; Ding, D.; Zhen, Y.; Guo, R. Amino acid-mediated ‘turn-off/turn-on’ nanozyme activity of gold nanoclusters for sensitive and selective detection of copper ions and histidine. Biosens. Bioelectron. 2017, 92, 140–146. [Google Scholar] [CrossRef]
- Sha, Q.; Sun, B.; Yi, C.; Guan, R.; Fei, J.; Hu, Z.; Liu, B.; Liu, X. A fluorescence turn-on biosensor based on transferrin encapsulated gold nanoclusters for 5-hydroxytryptamine detection. Sens. Actuators B Chem. 2019, 294, 177–184. [Google Scholar] [CrossRef]
- Chen, Y.; Qiao, J.; Liu, Q.; Zhang, M.; Qi, L. Fluorescence turn-on assay for detection of serum D-penicillamine based on papain@AuNCs-Cu2+ complex. Anal. Chim. Acta 2018, 1026, 133–139. [Google Scholar] [CrossRef]
- Halawa, M.I.; Gao, W.; Saqib, M.; Kitte, S.A.; Wu, F.; Xu, G. Sensitive detection of alkaline phosphatase by switching on gold nanoclusters fluorescence quenched by pyridoxal phosphate. Biosens. Bioelectron. 2017, 95, 8–14. [Google Scholar] [CrossRef]
- Shu, T.; Wang, J.; Su, L.; Zhang, X. Chemical Etching of Bovine Serum Albumin-Protected Au25 Nanoclusters for Label-Free and Separation-Free Ratiometric Fluorescent Detection of Tris(2-carboxyethyl)phosphine. Anal. Chem. 2016, 88, 11193–11198. [Google Scholar] [CrossRef] [PubMed]
- Chandirasekar, S.; You, J.-G.; Xue, J.-H.; Tseng, W.-L. Synthesis of gold nanocluster-loaded lysozyme nanoparticles for label-free ratiometric fluorescent pH sensing: Applications to enzyme–substrate systems and cellular imaging. J. Mater. Chem. B 2019, 7, 3876–3883. [Google Scholar] [CrossRef]
- Duan, B.; Wang, M.; Li, Y.; Jiang, S.; Liu, Y.; Huang, Z. Dual-emitting zein-protected gold nanoclusters for ratiometric fluorescence detection of Hg2+/Ag+ ions in both aqueous solution and self-assembled protein film. New J. Chem. 2019, 43, 14678–14683. [Google Scholar] [CrossRef]
- Wang, X.-y.; Zhu, G.-b.; Cao, W.-d.; Liu, Z.-j.; Pan, C.-g.; Hu, W.-j.; Zhao, W.-y.; Sun, J.-f. A novel ratiometric fluorescent probe for the detection of uric acid in human blood based on H2O2-mediated fluorescence quenching of gold/silver nanoclusters. Talanta 2019, 191, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Luo, M.; Di, J.; Tu, Y.; Yan, J. Gold nanocluster-based ratiometric fluorescent probes for hydrogen peroxide and enzymatic sensing of uric acid. Mikrochim. Acta 2018, 185, 305. [Google Scholar] [CrossRef]
- Xiong, Q.; Liu, J.; Xu, Y. Effects of Uric Acid on Diabetes Mellitus and Its Chronic Complications. J. Endocrinol. 2019, 2019, 9691345. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.-X.; Liu, H.-J.; Chen, Y. Ultrabright gold-silver bimetallic nanoclusters: Synthesis and their potential application in cysteine sensing. Colloids Surf. A Physicochem. Eng. Asp. 2018, 555, 572–579. [Google Scholar] [CrossRef]
- Goswami, N.; Yao, Q.; Luo, Z.; Li, J.; Chen, T.; Xie, J. Luminescent Metal Nanoclusters with Aggregation-Induced Emission. J. Phys. Chem. Lett. 2016, 7, 962–975. [Google Scholar] [CrossRef]
- You, J.-G.; Tseng, W.-L. Peptide-induced aggregation of glutathione-capped gold nanoclusters: A new strategy for designing aggregation-induced enhanced emission probes. Anal. Chim. Acta 2019, 1078, 101–111. [Google Scholar] [CrossRef]
- Xie, M.; Wang, Y.; Liu, L.; Wang, X.; Jiang, H. Luminescent gold-peptide spheric aggregates: Selective and effective cellular targeting. J. Colloid Interface Sci. 2022, 614, 502–510. [Google Scholar] [CrossRef]
- Gao, P.; Wu, S.; Chang, X.; Liu, F.; Zhang, T.; Wang, B.; Zhang, K.-Q. Aprotinin Encapsulated Gold Nanoclusters: A Fluorescent Bioprobe with Dynamic Nuclear Targeting and Selective Detection of Trypsin and Heavy Metal. Bioconjugate Chem. 2018, 29, 4140–4148. [Google Scholar] [CrossRef] [PubMed]
- Bhamore, J.R.; Jha, S.; Singhal, R.K.; Murthy, Z.V.P.; Kailasa, S.K. Amylase protected gold nanoclusters as chemo- and bio- sensor for nanomolar detection of deltamethrin and glutathione. Sens. Actuators B Chem. 2019, 281, 812–820. [Google Scholar] [CrossRef]
- Xu, P.; Li, R.; Tu, Y.; Yan, J. A gold nanocluster-based sensor for sensitive uric acid detection. Talanta 2015, 144, 704–709. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Majhi, S.; Sharma, K.; Ali, M.; Sharma, S.; Choudhary, D.; Tripathi, C.S.P.; Guin, D. BSA stabilized copper nanoclusters as a highly sensitive and selective probe for fluorescence sensing of Fe3+ ions. Chem. Phys. Lett. 2022, 787, 139226. [Google Scholar] [CrossRef]
- Rajamanikandan, R.; Ilanchelian, M. Red emitting human serum albumin templated copper nanoclusters as effective candidates for highly specific biosensing of bilirubin. Mater. Sci. Eng. C 2019, 98, 1064–1072. [Google Scholar] [CrossRef]
- Lu, D.; Liu, L.; Li, F.; Shuang, S.; Li, Y.; Choi, M.M.F.; Dong, C. Lysozyme-stabilized gold nanoclusters as a novel fluorescence probe for cyanide recognition. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 121, 77–80. [Google Scholar] [CrossRef]
- Bhamore, J.R.; Murthy, Z.V.P.; Kailasa, S.K. Fluorescence turn-off detection of spermine in biofluids using pepsin mediated synthesis of gold nanoclusters as a probe. J. Mol. Liq. 2019, 280, 18–24. [Google Scholar] [CrossRef]
- Yu, T.; Xu, C.; Qiao, J.; Zhang, R.; Qi, L. Green synthesis of gold nanoclusters using papaya juice for detection of l-lysine. Chin. Chem. Lett. 2019, 30, 660–663. [Google Scholar] [CrossRef]
- Pan, Y.; Li, Q.; Zhou, Q.; Zhang, W.; Yue, P.; Xu, C.; Qin, X.; Yu, H.; Zhu, M. Cancer cell specific fluorescent methionine protected gold nanoclusters for in-vitro cell imaging studies. Talanta 2018, 188, 259–265. [Google Scholar] [CrossRef]
- Brzezicka, K.; Vogel, U.; Serna, S.; Johannssen, T.; Lepenies, B.; Reichardt, N.-C. Influence of Core β-1,2-Xylosylation on Glycoprotein Recognition by Murine C-type Lectin Receptors and Its Impact on Dendritic Cell Targeting. ACS Chem. Biol. 2016, 11, 2347–2356. [Google Scholar] [CrossRef] [Green Version]
- Brzezicka, K.A.; Serna, S.; Reichardt, N.C. Fluorescent Neoglycoprotein Gold Nanoclusters: Synthesis and Applications in Plant Lectin Sensing and Cell Imaging. Nanoscale Res. Lett. 2018, 13, 360. [Google Scholar] [CrossRef] [PubMed]
- Chattoraj, S.; Bhattacharyya, K. Fluorescent Gold Nanocluster Inside a Live Breast Cell: Etching and Higher Uptake in Cancer Cell. J. Phys. Chem. C 2014, 118, 22339–22346. [Google Scholar] [CrossRef]
- Zhang, X.-D.; Luo, Z.; Chen, J.; Song, S.; Yuan, X.; Shen, X.; Wang, H.; Sun, Y.; Gao, K.; Zhang, L.; et al. Ultrasmall Glutathione-Protected Gold Nanoclusters as Next Generation Radiotherapy Sensitizers with High Tumor Uptake and High Renal Clearance. Sci. Rep. 2015, 5, 8669. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; DeGraff, W.; Friedman, N.; Mitchell, J.B. Selective Modulation of Glutathione Levels in Human Normal versus Tumor Cells and Subsequent Differential Response to Chemotherapy Drugs1. Cancer Res. 1986, 46, 2845–2848. [Google Scholar]
- Zhao, J.-Y.; Cui, R.; Zhang, Z.-L.; Zhang, M.; Xie, Z.-X.; Pang, D.-W. Cytotoxicity of nucleus-targeting fluorescent gold nanoclusters. Nanoscale 2014, 6, 13126–13134. [Google Scholar] [CrossRef]
- Tian, L.; Zhao, W.; Li, L.; Tong, Y.; Peng, G.; Li, Y. Multi-talented applications for cell imaging, tumor cells recognition, patterning, staining and temperature sensing by using egg white-encapsulated gold nanoclusters. Sens. Actuators B Chem. 2017, 240, 114–124. [Google Scholar] [CrossRef]
- Li, Z.; Peng, H.; Liu, J.; Tian, Y.; Yang, W.; Yao, J.; Shao, Z.; Chen, X. Plant Protein-Directed Synthesis of Luminescent Gold Nanocluster Hybrids for Tumor Imaging. ACS Appl. Mater. Interfaces 2018, 10, 83–90. [Google Scholar] [CrossRef]
- Zheng, B.; Wu, Q.; Jiang, Y.; Hou, M.; Zhang, P.; Liu, M.; Zhang, L.; Li, B.; Zhang, C. One-pot synthesis of 68Ga-doped ultrasmall gold nanoclusters for PET/CT imaging of tumors. Mater. Sci. Eng. C 2021, 128, 112291. [Google Scholar] [CrossRef]
- Yang, J.; Wang, T.; Zhao, L.; Rajasekhar, V.K.; Joshi, S.; Andreou, C.; Pal, S.; Hsu, H.-t.; Zhang, H.; Cohen, I.J.; et al. Gold/alpha-lactalbumin nanoprobes for the imaging and treatment of breast cancer. Nat. Biomed. Eng. 2020, 4, 686–703. [Google Scholar] [CrossRef]
- Ma, H.; Wang, J.; Zhang, X.-D. Near-infrared II emissive metal clusters: From atom physics to biomedicine. Coord. Chem. Rev. 2021, 448, 214184. [Google Scholar] [CrossRef]
- Zhu, S.; Tian, R.; Antaris, A.L.; Chen, X.; Dai, H. Near-Infrared-II Molecular Dyes for Cancer Imaging and Surgery. Adv. Mater. 2019, 31, 1900321. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Wei, Z.; Song, C.; Tang, C.; Han, W.; Dong, X. Optical nano-agents in the second near-infrared window for biomedical applications. Chem. Soc. Rev. 2019, 48, 22–37. [Google Scholar] [CrossRef]
- Wang, W.; Kong, Y.; Jiang, J.; Xie, Q.; Huang, Y.; Li, G.; Wu, D.; Zheng, H.; Gao, M.; Xu, S.; et al. Engineering the Protein Corona Structure on Gold Nanoclusters Enables Red-Shifted Emissions in the Second Near-infrared Window for Gastrointestinal Imaging. Angew. Chem. Int. Ed. 2020, 59, 22431–22435. [Google Scholar] [CrossRef] [PubMed]
- Porret, E.; Le Guével, X.; Coll, J.-L. Gold nanoclusters for biomedical applications: Toward in vivo studies. J. Mater. Chem. B 2020, 8, 2216–2232. [Google Scholar] [CrossRef]
- Li, H.; Li, H.; Wan, A. Luminescent gold nanoclusters for in vivo tumor imaging. Analyst 2020, 145, 348–363. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Wu, Z.; Yao, Q.; Xie, J. Luminescent metal nanoclusters: Biosensing strategies and bioimaging applications. Aggregate 2021, 2, 114–132. [Google Scholar] [CrossRef]
- Chen, H.; Li, S.; Li, B.; Ren, X.; Li, S.; Mahounga, D.M.; Cui, S.; Gu, Y.; Achilefu, S. Folate-modified gold nanoclusters as near-infrared fluorescent probes for tumor imaging and therapy. Nanoscale 2012, 4, 6050–6064. [Google Scholar] [CrossRef]
- Chen, D.; Li, B.; Cai, S.; Wang, P.; Peng, S.; Sheng, Y.; He, Y.; Gu, Y.; Chen, H. Dual targeting luminescent gold nanoclusters for tumor imaging and deep tissue therapy. Biomaterials 2016, 100, 1–16. [Google Scholar] [CrossRef]
- Khandelia, R.; Bhandari, S.; Pan, U.N.; Ghosh, S.S.; Chattopadhyay, A. Gold Nanocluster Embedded Albumin Nanoparticles for Two-Photon Imaging of Cancer Cells Accompanying Drug Delivery. Small 2015, 11, 4075–4081. [Google Scholar] [CrossRef]
- Sarparast, M.; Noori, A.; Ilkhani, H.; Bathaie, S.Z.; El-Kady, M.F.; Wang, L.J.; Pham, H.; Marsh, K.L.; Kaner, R.B.; Mousavi, M.F. Cadmium nanoclusters in a protein matrix: Synthesis, characterization, and application in targeted drug delivery and cellular imaging. Nano Res. 2016, 9, 3229–3246. [Google Scholar] [CrossRef]
- Chen, H.; Li, B.; Ren, X.; Li, S.; Ma, Y.; Cui, S.; Gu, Y. Multifunctional near-infrared-emitting nano-conjugates based on gold clusters for tumor imaging and therapy. Biomaterials 2012, 33, 8461–8476. [Google Scholar] [CrossRef] [PubMed]
- Naldini, L. Gene therapy returns to centre stage. Nature 2015, 526, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Dutta, D.; Chattopadhyay, A.; Ghosh, S.S. Cationic BSA Templated Au–Ag Bimetallic Nanoclusters as a Theranostic Gene Delivery Vector for HeLa Cancer Cells. ACS Biomater. Sci. Eng. 2016, 2, 2090–2098. [Google Scholar] [CrossRef] [PubMed]
- Dutta, D.; Sailapu, S.K.; Simon, A.T.; Ghosh, S.S.; Chattopadhyay, A. Gold-Nanocluster-Embedded Mucin Nanoparticles for Photodynamic Therapy and Bioimaging. Langmuir 2019, 35, 10475–10483. [Google Scholar] [CrossRef]
- Van Straten, D.; Mashayekhi, V.; De Bruijn, H.S.; Oliveira, S.; Robinson, D.J. Oncologic Photodynamic Therapy: Basic Principles, Current Clinical Status and Future Directions. Cancers 2017, 9, 19. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Lee, W.D.; Tan, Y.N. Protein-protected gold/silver alloy nanoclusters in metal-enhanced singlet oxygen generation and their correlation with photoluminescence. Mater. Sci. Eng. C 2020, 109, 110525. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, X.; Wang, X.; Guan, X.; Zhang, W.; Ma, J. Recent advances in selective photothermal therapy of tumor. J. Nanobiotechnol. 2021, 19, 335. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, T.; Ke, H.; Zhu, A.; Wang, Y.; Wang, J.; Shen, J.; Liu, G.; Chen, C.; Zhao, Y.; et al. Smart Albumin-Biomineralized Nanocomposites for Multimodal Imaging and Photothermal Tumor Ablation. Adv. Mater. 2015, 27, 3874–3882. [Google Scholar] [CrossRef]
- Pan, U.N.; Khandelia, R.; Sanpui, P.; Das, S.; Paul, A.; Chattopadhyay, A. Protein-Based Multifunctional Nanocarriers for Imaging, Photothermal Therapy, and Anticancer Drug Delivery. ACS Appl. Mater. Interfaces 2017, 9, 19495–19501. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, Y.; Zhang, M.; Li, C.; Yang, R.; Li, J.; Qian, C.; Sun, M. Size Switchable Nanoclusters Fueled by Extracellular ATP for Promoting Deep Penetration and MRI-Guided Tumor Photothermal Therapy. Adv. Funct. Mater. 2019, 29, 1904144. [Google Scholar] [CrossRef]
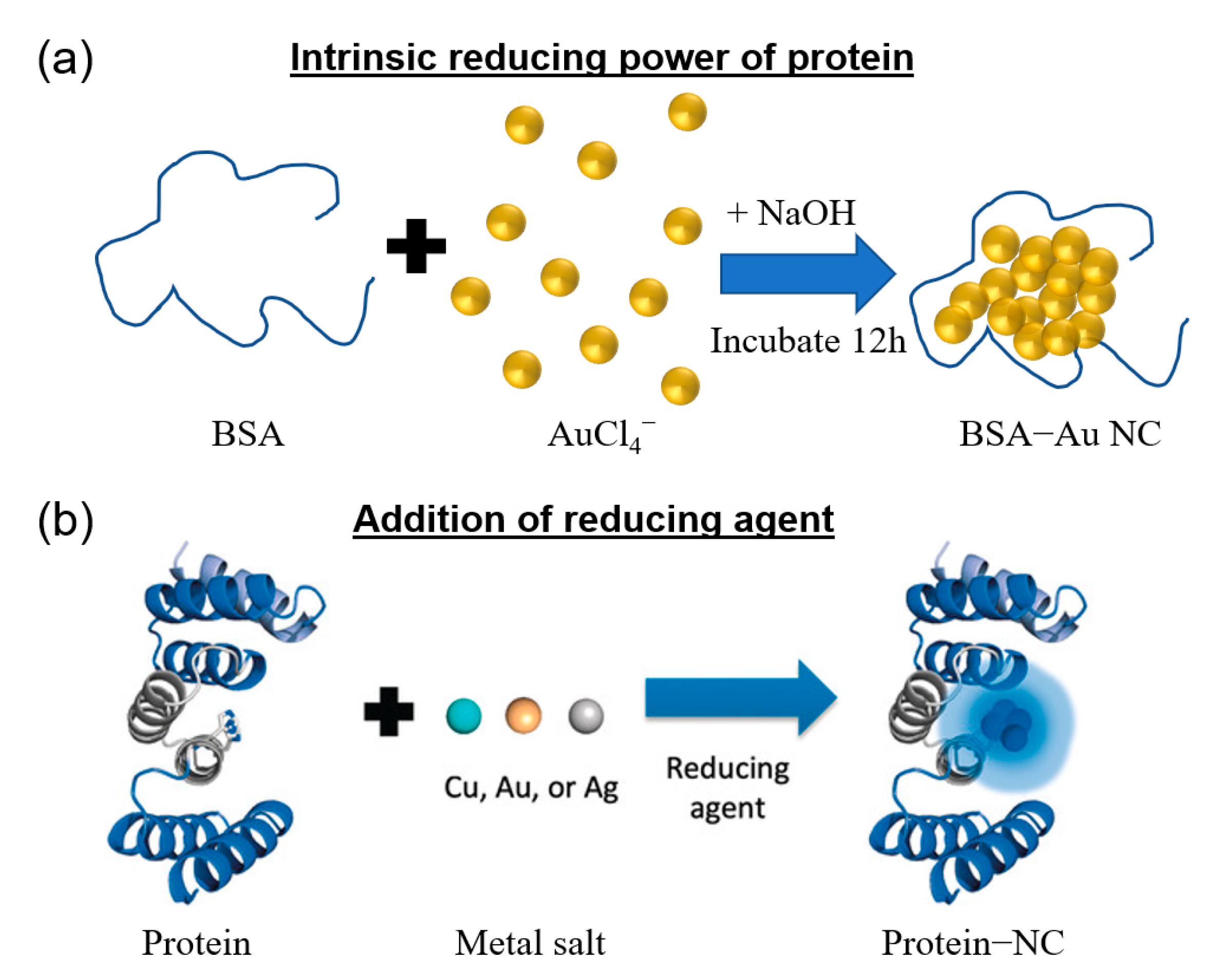




| Synthesis Approach | Protein Template | Protein Size | Metal | Photoluminescence Property (λex/λem, QY) | Size (nm) | Application | Ref. | |
|---|---|---|---|---|---|---|---|---|
| Intrinsic reducing power of protein | Triggered by alkaline pH | Human serum albumin | 66.4 kDa | Cu | 325 nm/405 nm | - | Assay for detection of human serum albumin | [53] |
| Tyrosine residues in lysozyme activated by high pH | Lysozyme | 14 kDa | Au | 300–450 nm/657 nm, 5.6% | 1 | Sensing of Hg2+ | [54] | |
| Reduced by carboxyl groups in histidine/tyrosine | Lysozyme type VI | - | Au | 380 nm/455 nm, 56% | - | Sensing of glutathione | [55] | |
| Reduced by tyrosine residues at alkaline pH; reduced by carboxyl groups in acidic conditions | Pepsin (porcine) | 3.4 kDa | Au | Au25: 360 nm/670 nm, 3.5% Au13: 330 nm/510 nm, 5.0% Au5, Au8: 330 nm/402 nm, 3.7% | 1–2 | Sensing of Pb2+ and Hg2+ | [56] | |
| Activated by alkaline conditions | Papain | 23.4 kDa | Au | 470 nm/660 nm, 4.3% | 1.2 ± 0.2 | Sensing of Cu2+ | [57] | |
| Activated by alkaline conditions | Trypsin | 5 kDa | Au | 520 nm/690 nm, 6.5% | 2.7 ± 0.4 | Sensing of heparin and in vivo cancer imaging | [58] | |
| Activated by alkaline conditions | 16-mer ferritin-like protein | <100 kDa | Au | 500 nm/650 nm | 2 | Sensing of Hg2+ and in vivo bioimaging | [59] | |
| Addition of reducing agent | NaBH4 | Bovine serum albumin | 66.5 kDa | Ag | 480 nm/625 nm, 0.4% | 1.7 | Photodynamic therapy | [45] |
| Ascorbic acid | Bovine serum albumin | 66 kDa | Cu | 330 nm/400–600 nm | 2.3 ± 0.4 | Antifouling | [60] | |
| N2H4.2H2O | Bovine serum albumin | 66.5 kDa | Cu | 525 nm/643 nm | ~2.5 | Sensing of creatinine | [61] | |
| NaBH4 | Keratin | 40–68 kDa | Ag | 400 nm/705 nm, 1.7% | 2.53 ± 0.54 | Sensing of Hg2+ | [62] | |
| N2H4 | Lysozyme | 14 kDa | Cu | 490 nm/510 nm, 18% | 3–5 | Cellular imaging | [60,63] | |
| Dithiothreitol | Lysozyme | 14.3 kDa | Cu | 360 nm/640 nm, 6.1% | 1.5 ± 0.31 | Sensing of Cu2+ and Vitamin B12 | [61,64] | |
| N2H4 | Thunnus albacares fish protein | - | Cu | 330 nm/446 nm | 1–2 | Sensing of Fe3+ | [65] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, S.C.L.; He, Z.; Wang, G.; Yu, Y.; Yang, L. Protein-Templated Metal Nanoclusters: Molecular-like Hybrids for Biosensing, Diagnostics and Pharmaceutics. Molecules 2023, 28, 5531. https://doi.org/10.3390/molecules28145531
Tan SCL, He Z, Wang G, Yu Y, Yang L. Protein-Templated Metal Nanoclusters: Molecular-like Hybrids for Biosensing, Diagnostics and Pharmaceutics. Molecules. 2023; 28(14):5531. https://doi.org/10.3390/molecules28145531
Chicago/Turabian StyleTan, Sherwin Chong Li, Zhijian He, Guan Wang, Yong Yu, and Le Yang. 2023. "Protein-Templated Metal Nanoclusters: Molecular-like Hybrids for Biosensing, Diagnostics and Pharmaceutics" Molecules 28, no. 14: 5531. https://doi.org/10.3390/molecules28145531
APA StyleTan, S. C. L., He, Z., Wang, G., Yu, Y., & Yang, L. (2023). Protein-Templated Metal Nanoclusters: Molecular-like Hybrids for Biosensing, Diagnostics and Pharmaceutics. Molecules, 28(14), 5531. https://doi.org/10.3390/molecules28145531






