Co-Fermentation of Agri-Food Residues Using a Co-Culture of Yeasts as a New Bioprocess to Produce 2-Phenylethanol
Abstract
:1. Introduction
2. Results
2.1. Characterization of Agri-Food Residues
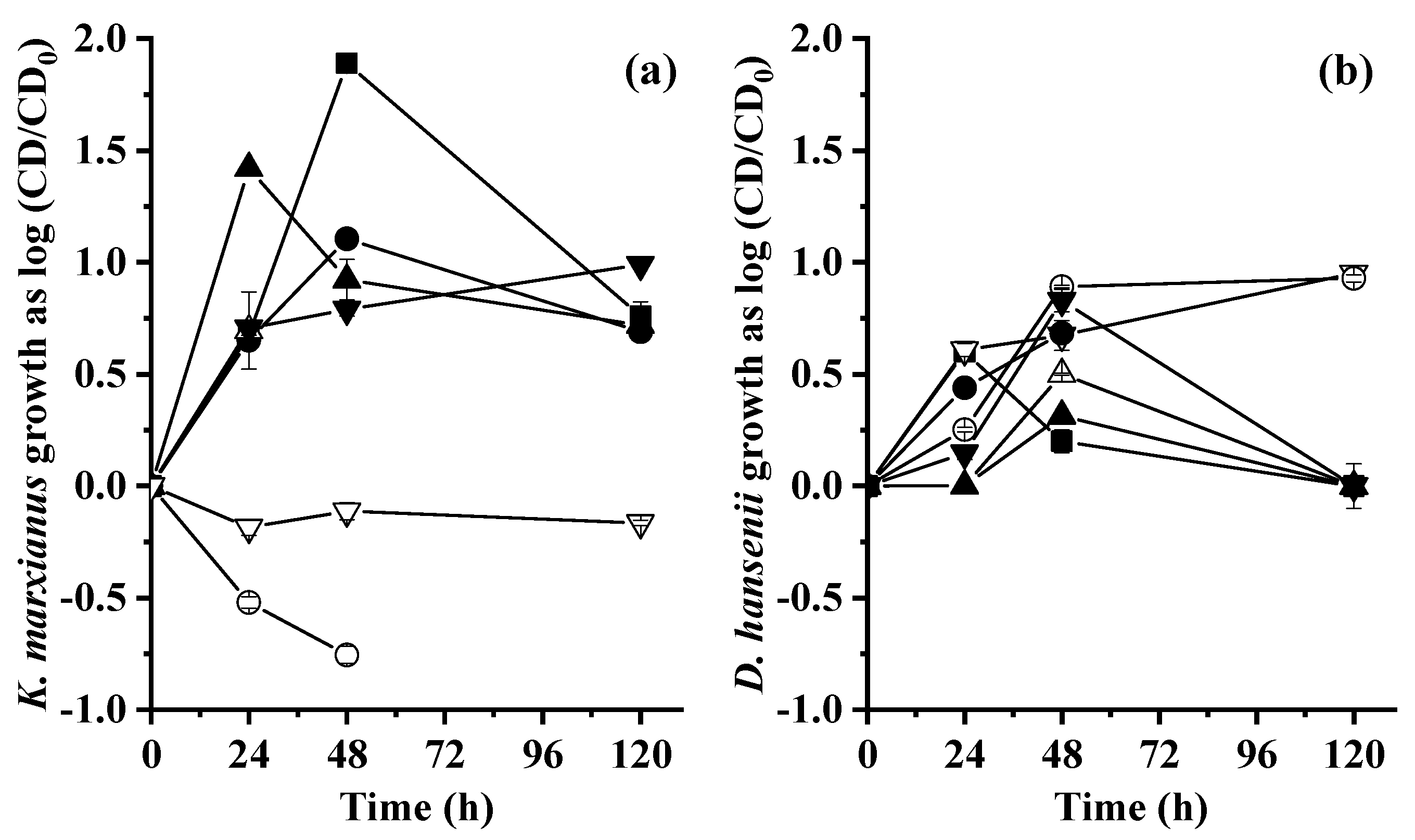
2.2. Yeast Growth
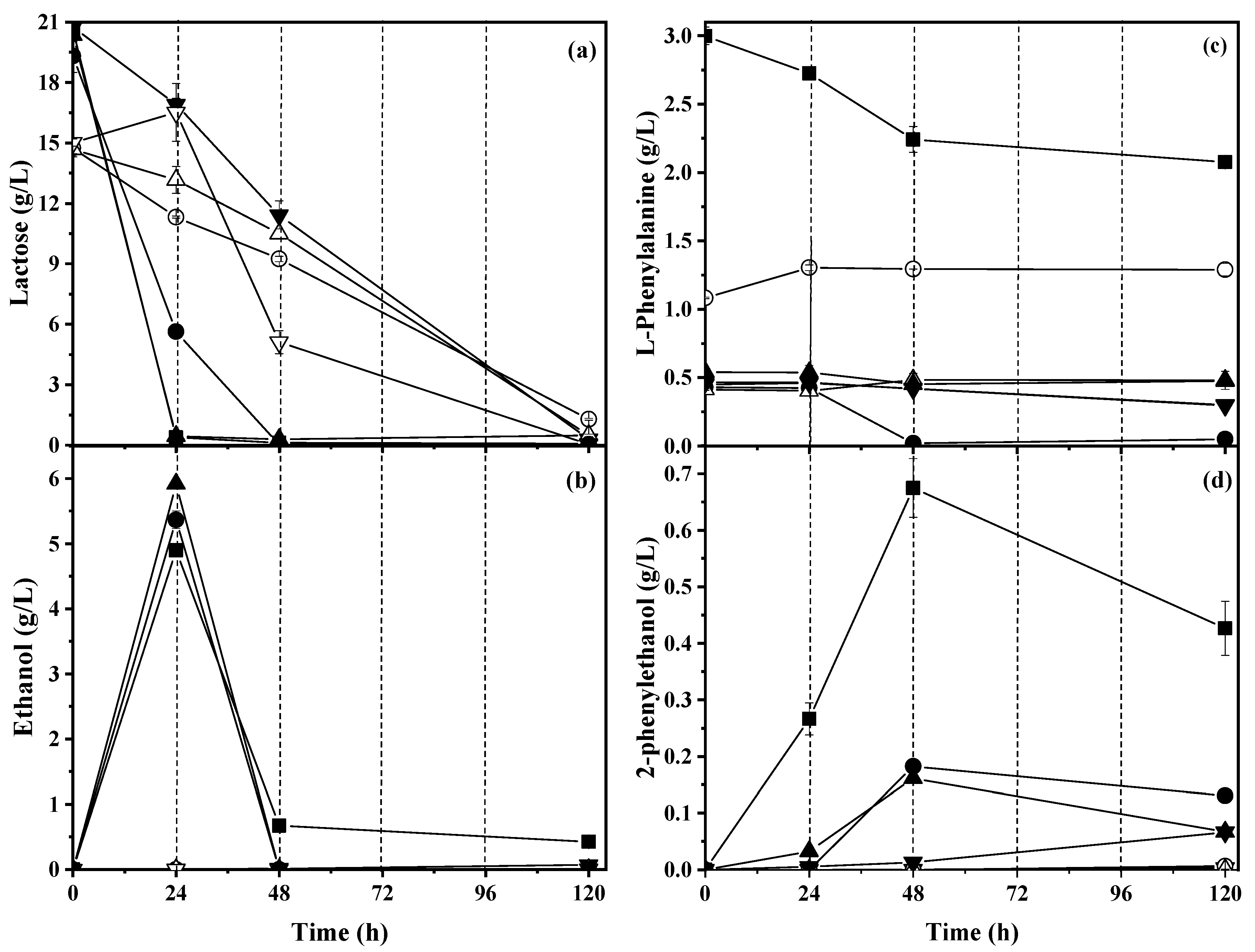
2.3. Effect of Addition of Biosourced Protein on Lactose Consumption and Ethanol Production
2.4. Effect of Addition of Biosourced Protein on L-Phenylalanine Consumption and 2-Phenylethanol Production
2.5. Co-Fermentation of Whey and Brewer’s Spent Yeast in Aerated Bioreactor
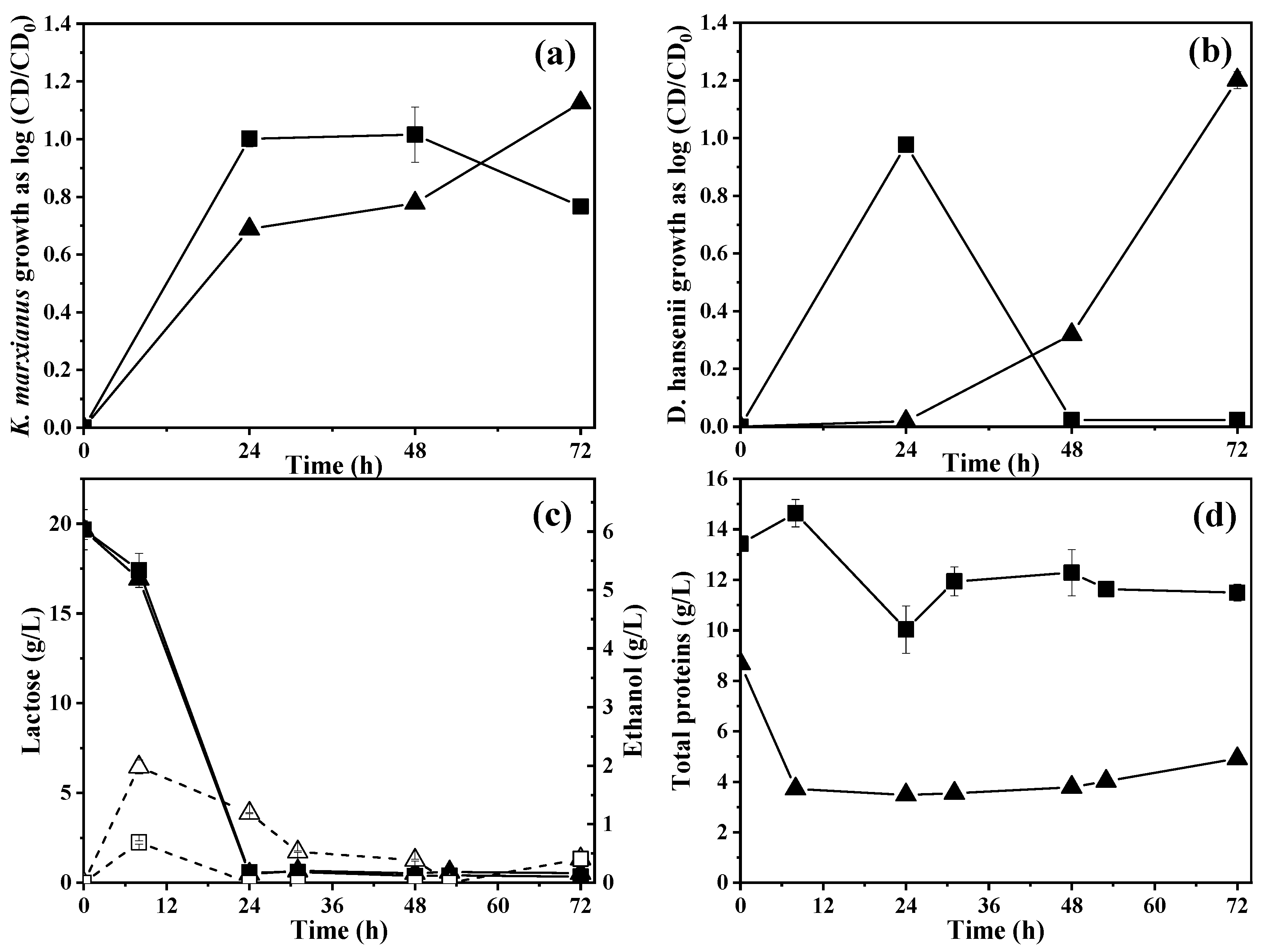
2.5.1. Yeast Growth and Protein Production under Controlled Aeration
2.5.2. Effect of Controlled Aeration on Lactose Consumption and Ethanol Production
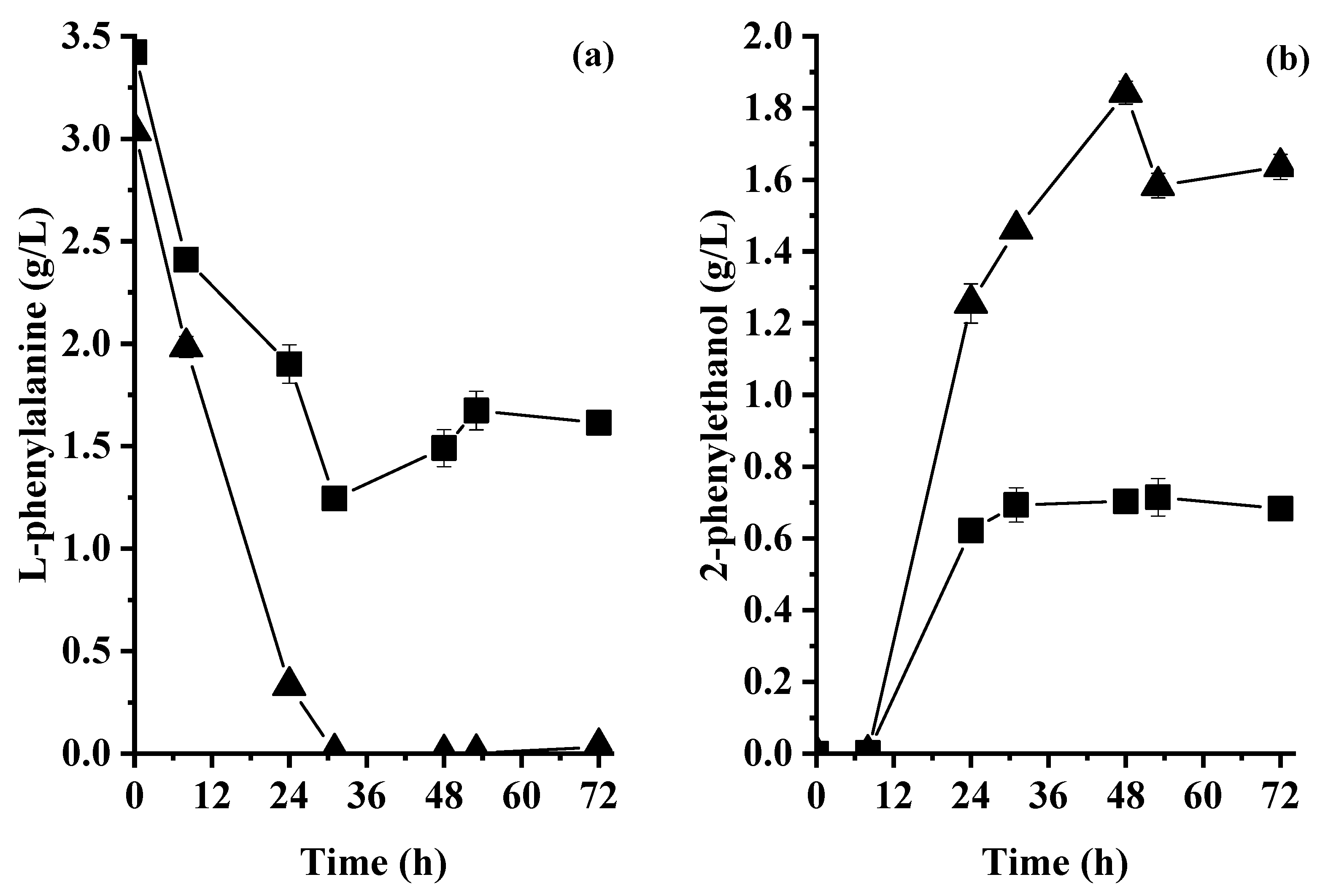
2.5.3. Effect of Controlled Aeration on the Consumption of L-Phenylalanine and Production of 2-Phenylethanol
3. Discussion
3.1. Characterization of Agri-Food Residues
3.2. Yeast Growth
3.3. Effect of Addition of Biosourced Protein on Lactose Consumption and Ethanol Production
3.4. Effect of Addition of Biosourced Proteins on L-Phenylalanine Consumption and 2-Phenylethanol Production
3.5. Co-Fermentation of Whey and Brewer’s Spent Yeast in Aerated Bioreactor
3.5.1. Yeast Growth and Protein Production under Controlled Aeration
3.5.2. Effect of Controlled Aeration on Lactose Consumption and Ethanol Production
3.5.3. Effect of Controlled Aeration on the Consumption of L-Phenylalanine and Production of 2-Phenylethanol
4. Materials and Methods
4.1. Agri-Food Residues: Handling and Characterization
4.2. Culture Media
4.3. Inoculum
4.4. Fermentation Conditions
4.5. Batch Fermentation in 2 L Bioreactor
4.6. Determination of Protein Content Using Lowry’s Method
4.7. Quantification of Amino Acids
4.8. Quantification of Lactose and Ethanol
4.9. Quantification of 2-Phenylethanol
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Mordor Intelligence Canada Whey Protein Market|2021–26|Industry Share, Size, Growth-Mordor Intelligence. Available online: https://www.mordorintelligence.com/industry-reports/canada-whey-protein-market (accessed on 20 September 2021).
- Agropur Research, Development & Innovation|Agropur. Available online: https://www.agropur.com/us/research-development-innovation (accessed on 20 September 2021).
- Bedford, E. Cheese Market in Canada-Statistics & Facts|Statista. Available online: https://www.statista.com/topics/4202/cheese-market-in-canada/ (accessed on 20 September 2021).
- BeerCanada. 2021 Industry Trends. Available online: https://industry.beercanada.com/statistics/quebec (accessed on 18 November 2021).
- Les Producteurs de lait du Quebec. Revue Des Marchés; Les Producteurs de lait du Quebec: Quebec, QC, Cananda, 2017. [Google Scholar]
- Gouvernement du Québec Production Laitière (Lait de Vache). Available online: https://www.quebec.ca/agriculture-environnement-et-ressources-naturelles/agriculture/industrie-agricole-au-quebec/productions-agricoles/production-lait-vache (accessed on 28 March 2022).
- Giroux, L.; Bury, R. Ecoworks State of Waste Management in Canada; Canadian Council of Ministers of Environment: Kanata, ON, Canada, 2014; p. 155. [Google Scholar]
- Bibeau, M.-C.; Drouin, F. Agri-Environmental Indicators. Available online: https://agriculture.canada.ca/en/agriculture-and-environment/agri-environmental-indicators (accessed on 11 August 2022).
- Das, B.; Sarkar, S.; Maiti, S.; Bhattacharjee, S. Studies on Production of Ethanol from Cheese Whey Using Kluyveromyces Marxianus. Mater. Today Proc. 2016, 3, 3253–3257. [Google Scholar] [CrossRef]
- Christensen, A.D.; Kádár, Z.; Oleskowicz-Popiel, P.; Thomsen, M.H. Production of Bioethanol from Organic Whey Using Kluyveromyces Marxianus. J. Ind. Microbiol. Biotechnol. 2011, 38, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Valdez Castillo, M.; Tahmasbi, H.; Pachapur, V.L.; Brar, S.K.; Vuckovic, D.; Sitnikov, D.; Arriaga, S.; Blais, J.; Avalos Ramirez, A. Production of Aroma and Flavor-Rich Fusel Alcohols by Cheese Whey Fermentation Using the Kluyveromyces Marxianus and Debaryomyces Hansenii Yeasts in Monoculture and Co-Culture Modes. J. Chem. Technol. Biotechnol. 2021, 96, 2354–2367. [Google Scholar] [CrossRef]
- Hazelwood, L.A.; Daran, J.M.; Van Maris, A.J.A.; Pronk, J.T.; Dickinson, J.R. The Ehrlich Pathway for Fusel Alcohol Production: A Century of Research on Saccharomyces Cerevisiae Metabolism. Appl. Environ. Microbiol. 2008, 74, 2259–2266. [Google Scholar] [CrossRef] [Green Version]
- VIGON. 2-Phenylethanol Natural. Available online: https://www.vigon.com/product/phenyl-ethyl-alcohol-natural/ (accessed on 12 July 2023).
- Díez-Antolínez, R.; Hijosa-Valsero, M.; Paniagua-García, A.I.; Garita-Cambronero, J.; Gómez, X. Yeast Screening and Cell Immobilization on Inert Supports for Ethanol Production from Cheese Whey Permeate with High Lactose Loads. PLoS ONE 2018, 13, e0210002. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, B.; Lima-Costa, M.E.; Constantino, A.; Raposo, S.; Felizardo, C.; Gonçalves, D.; Fernandes, T.; Dionísio, L.; Peinado, J.M. Growth Kinetics and Physiological Behavior of Co-Cultures of Saccharomyces Cerevisiae and Kluyveromyces Lactis, Fermenting Carob Sugars Extracted with Whey. Enzym. Microb. Technol. 2016, 92, 41–48. [Google Scholar] [CrossRef]
- Risner, D.; Tomasino, E.; Hughes, P.; Meunier-goddik, L. Volatile Aroma Composition of Distillates Produced from Fermented Sweet and Acid Whey. J. Dairy Sci. 2019, 102, 202–210. [Google Scholar] [CrossRef] [Green Version]
- Powell, I.B.; Broome, M.C.; Limsowtin, G.K.Y. Cheese: Starter Cultures: General Aspects. In Encyclopedia of Dairy Sciences: Second Edition; Fuquay, J.W., Fox, P.F., McSweeney, P.L.H., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 552–558. ISBN 9780123744029. [Google Scholar]
- Anand, S.; Khanal, S.N.; Marella, C. Whey and Whey Products. In Milk and Dairy Products in Human Nutrition: Production, Composition and Health; Park, Y.W., Haenlein, G.F.W., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2013; p. 477. ISBN 9781118534168. [Google Scholar]
- Pires, C.; Marques, A.; Carvalho, M.; Batista, I. Chemical Characterization of Cancer Pagurus, Maja Squinado, Necora Puber and Carcinus Maenas Shells. Poultry, Fish. Wildl. Sci. 2017, 5, 1. [Google Scholar] [CrossRef]
- Jun, J.Y.; Jung, M.J.; Jeong, I.H.; Kim, G.W.; Sim, J.M.; Nam, S.Y.; Kim, B.M. Effects of Crab Shell Extract as a Coagulant on the Textural and Sensorial Properties of Tofu (Soybean Curd). Food Sci. Nutr. 2019, 7, 547–553. [Google Scholar] [CrossRef]
- Stanojevic, S.P.; Barac, M.B.; Pesic, M.B.; Jankovic, V.S.; Vucelic-Radovic, B.V. Bioactive Proteins and Energy Value of Okara as a Byproduct in Hydrothermal Processing of Soy Milk. J. Agric. Food Chem. 2013, 61, 9210–9219. [Google Scholar] [CrossRef]
- Vong, W.C.; Au Yang, K.L.C.; Liu, S.Q. Okara (Soybean Residue) Biotransformation by Yeast Yarrowia Lipolytica. Int. J. Food Microbiol. 2016, 235, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Perpetuini, G.; Tittarelli, F.; Suzzi, G.; Tofalo, R. Cell Wall Surface Properties of Kluyveromyces Marxianus Strains from Dairy-Products. Front. Microbiol. 2019, 10, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, T.H.; Fleet, G.H.; Rogers, P.L. Composition of the Cell Walls of Several Yeast Species. Appl. Microbiol. Biotechnol. 1998, 50, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Vieira, E.; Cunha, S.C.; Ferreira, I.M.P.L.V.O. Characterization of a Potential Bioactive Food Ingredient from Inner Cellular Content of Brewer’s Spent Yeast. Waste Biomass Valorization 2019, 10, 3235–3242. [Google Scholar] [CrossRef]
- Tomé, D. Yeast Extracts: Nutritional and Flavoring Food Ingredients. ACS Food Sci. Technol. 2021, 1, 487–494. [Google Scholar] [CrossRef]
- Deesuth, O.; Laopaiboon, P.; Klanrit, P.; Laopaiboon, L. Improvement of Ethanol Production from Sweet Sorghum Juice under High Gravity and Very High Gravity Conditions: Effects of Nutrient Supplementation and Aeration. Ind. Crops Prod. 2015, 74, 95–102. [Google Scholar] [CrossRef]
- Illarionov, A.; Lahtvee, P.-J.; Kumar, R. Characterization of Potassium and Sodium Salt Stress in Yeasts. Appl. Environ. Microbiol. 2021, 87, 3100–3120. [Google Scholar] [CrossRef] [PubMed]
- Calahorra, M.; Sánchez, N.S.; Peña, A. Activation of Fermentation by Salts in Debaryomyces Hansenii. FEMS Yeast Res. 2009, 9, 1293–1301. [Google Scholar] [CrossRef] [Green Version]
- Kumura, H.; Takagaki, K.; Sone, T.; Tsukahara, M.; Tanaka, T.; Shimazaki, K.I. Casein Digestion by Debaryomyces Hansenii Isolated from Cheese. Biosci. Biotechnol. Biochem. 2002, 66, 1370–1373. [Google Scholar] [CrossRef] [Green Version]
- Foukis, A.; Stergiou, P.Y.; Theodorou, L.G.; Papagianni, M.; Papamichael, E.M. Purification, Kinetic Characterization and Properties of a Novel Thermo-Tolerant Extracellular Protease from Kluyveromyces Marxianus IFO 0288 with Potential Biotechnological Interest. Bioresour. Technol. 2012, 123, 214–220. [Google Scholar] [CrossRef]
- Beniwal, A.; Saini, P.; Kokkiligadda, A.; Vij, S. Physiological Growth and Galactose Utilization by Dairy Yeast Kluyveromyces Marxianus in Mixed Sugars and Whey during Fermentation. 3 Biotech 2017, 7, 349. [Google Scholar] [CrossRef] [PubMed]
- da Silveira, F.A.; Diniz, R.H.S.; Sampaio, G.M.S.; Brandão, R.L.; da Silveira, W.B.; Castro, I.M. Sugar Transport Systems in Kluyveromyces Marxianus CCT 7735. Antonie Van Leeuwenhoek Int. J. Gen. Mol. Microbiol. 2019, 112, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Vargas, M.; Téllez-Jurado, A.; Gómez-Aldapa, C.A.; Ramírez-Vargas, M.D.R.; Conde-Báez, L.; Castro-Rosas, J.; Cadena-Ramírez, A. Optimization of 2-Phenylethanol Production from Sweet Whey Fermentation Using Kluyveromyces Marxianus. Fermentation 2022, 8, 39. [Google Scholar] [CrossRef]
- Valdez Castillo, M.; Pachapur, V.L.; Brar, S.K.; Arriaga, S.; Blais, J.F.; Avalos Ramirez, A. Effect of the Concentration of L-Phenylalanine and Lactose on 2-Phenylethanol Production by Whey Fermentation Using the Yeasts Kluyveromyces Marxianus and Debaryomyces Hansenii under Co-Culture Mode. Bioresour. Technol. Rep. 2022, 18, 100994. [Google Scholar] [CrossRef]
- Miranda Júnior, M.; Batistote, M.; Cilli, E.M.; Ernandes, J.R. Sucrose Fermentation by Brazilian Ethanol Production Yeasts in Media Containing Structurally Complex Nitrogen Sources. J. Inst. Brew. 2009, 115, 191–197. [Google Scholar] [CrossRef]
- APHA. Standard Methods for Examination of Water and Wastewater; APHA: Washington, DC, USA, 1999; ISBN 9780875530130. [Google Scholar]
- Alabaraoye, E.; Achilonu, M.; Hester, R. Biopolymer (Chitin) from Various Marine Seashell Wastes: Isolation and Characterization. J. Polym. Environ. 2018, 26, 2207–2218. [Google Scholar] [CrossRef]
- Lucarini, A.C.; Kilikian, B.V. Comparative Study of Lowry and Bradford Methods: Interfering Substances. Biotechnol. Tech. 1999, 13, 149–154. [Google Scholar] [CrossRef]
- Vilasoa-Martínez, M.; López-Hernández, J.; Lage-Yusty, M.A. Protein and Amino Acid Contents in the Crab, Chionoecetes Opilio. Food Chem. 2007, 103, 1330–1336. [Google Scholar] [CrossRef]

| Parameters | Agri-Food Residues | |||
|---|---|---|---|---|
| Cheese Whey * | Crab Headshells | Residual Soy Cake | Brewer’s Spent Yeast | |
| pH | 4.88 ± 0.02 | N.A. | N.A. | 5.38 ± 0.01 |
| Lactose (g/kgresidue) | 35.44 ± 0.00 | N.A. | N.A. | N.A. |
| Total organic carbon (g/kgresidue) | 17.18 ± 0.25 | N.A. | N.A. | 63.79 ± 0.30 |
| Total nitrogen Kjeldahl (g/kgresidue) | 0.93 ± 0.05 | 52.18 ± 0.01 | 9.30 ± 0.04 | 13.12 ± 1.41 |
| Total solids (g/kgresidue) | 48.43 ± 0.07 | 592.30 ± 0.01 | 209.80 ± 0.22 | 220.25 ± 13.12 |
| Ashes (g/kgresidue) | 3.62 ± 0.08 | 407.70 ± 0.01 | 6.20 ± 0.15 | 4.20 ± 0.10 |
| Chemical oxygen demand (gO2/kgresidue) | 64.94 ± 2.93 | 430.46 ± 17.96 | 296.41 ± 14.41 | 320.50 ± 2.00 |
| Total protein (g/kgresidue) | 5.71 ± 0.37 | 180.21 ± 6.61 | 85.60 ± 5.85 | 72.65 ± 0.99 |
| L-phenylalanine (g/kgresidue) | 3.00 ± 0.01 | 10.00 ± 3.00 | 5.00 ± 0.10 | 11.01 ± 0.02 |
| Valine (g/kgresidue) | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.02 ± 0.00 | 2.00 ± 0.01 |
| Methionine (g/kgresidue) | 12.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 1.00 ± 0.01 |
| Isoleucine (g/kgresidue) | 0.00 ± 0.00 | 2.00 ± 0.10 | 4.01 ± 0.01 | 0.00 ± 0.00 |
| Leucine (g/kgresidue) | 5.02 ± 0.01 | 1.01 ± 0.02 | 1.02 ± 0.01 | 2.15 ± 0.00 |
| Tryptophan (g/kgresidue) | 4.01 ± 0.20 | 5.05 ± 0.01 | 4.01 ± 0.09 | 9.10 ± 0.03 |
| Calcium as Ca2+ (g/kgresidue) | 0.27 ± 0.01 | 129.00 ± 0.03 | 0.54 ± 0.01 | 0.32 ± 0.02 |
| Potassium as K+ (g/kgresidue) | 0.11 ± 0.01 | 3.47 ± 0.01 | 1.77 ± 0.02 | 3.10 ± 0.01 |
| Magnesium as Mg2+ (g/kgresidue) | 0.06 ± 0.00 | 8.02 ± 0.01 | 0.25 ± 0.01 | 0.58 ± 0.01 |
| Sodium as Na+ (g/kgresidue) | 0.37 ± 0.00 | 9.06 ± 0.03 | 0.05 ± 0.00 | 0.02 ± 0.00 |
| Parameter | Fermentation Conditions | ||||||
|---|---|---|---|---|---|---|---|
| WM Control | WNC | WHC | WNS | WHS | WNBSY | WHBSY | |
| Lactose consumption rate (glacrose/L·h)24 h | 0.84 ± 0.00 | 0.17 ± 0.00 | 0.12 ± 0.01 | 0.40 ± 0.00 | 0.11 ± 0.00 | 0.83 ± 0.01 | 0.12 ± 0.01 |
| Ethanol productivity (gethanol/L·h)24 h | 0.24 ± 0.01 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.22 ± 0.01 | 0.00 ± 0.00 | 0.30 ± 0.01 | 0.00 ± 0.00 |
| Ethanol yield production (gethanol/glactose)24 h | 0.23 ± 0.02 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.39 ± 0.02 | 0.00 ± 0.00 | 0.25 ± 0.01 | 0.00 ± 0.00 |
| L-phenylalanine concentration (gL-Phe/L)0 h | 2.99 ± 0.06 | 0.46 ± 0.00 | 0.45 ± 0.01 | 0.43 ± 0.01 | 1.08 ± 0.00 | 0.54 ± 0.01 | 0.41 ± 0.00 |
| L-phenylalanine concentration (gL-Phe/L)48 h | 2.24 ± 0.09 | 0.41 ± 0.01 | 0.42 ± 0.01 | 0.02 ± 0.00 | 1.29 ± 0.00 | 0.42 ± 0.00 | 0.48 ± 0.04 |
| L-phenylalanine consumption rate (mgL-Phe/L·h)48 h | 15.75 ± 0.65 | 1.70 ± 0.10 | 5.65 ± 0.55 | 8.55 ± 0.10 | 0.40 ± 0.10 | 1.85 ± 0.15 | 2.80 ± 0.20 |
| 2-phenylethanol concentration (g2PE/L)48 h | 0.67 ± 0.05 | 0.01 ± 0.00 | 3.50 × 10−4 ± 0.50 × 10−4 | 0.18 ± 0.00 | 2.00 × 10−4 ± 0.10 × 10−4 | 0.16 ± 0.00 | 0.00 ± 0.00 |
| 2-phenylethanol productivity (mg2PE/L·h)48 h | 17.00 ± 1.00 | 0.65 ± 0.04 | 0.04 ± 0.00 | 7.60 ± 0.00 | 0.08 ± 0.00 | 3.35 ± 0.05 | 0.00 ± 0.00 |
| 2-phenylethanol yield production (g2PE/gL-Phe)48 h | 0.89 ± 0.01 | 0.40 ± 0.01 | 0.34 ± 0.00 | 0.46 ± 0.01 | 0.00 ± 0.00 | 2.44 ± 0.10 * | 0.00 ± 0.00 |
| Parameter | Fermentation Conditions | |
|---|---|---|
| WM | WBSY | |
| Initial lactose concentration (g/L) | 19.7 ± 0.3 | 19.6 ± 0.1 |
| Initial L-phenylalanine concentration (g/L) | 3.4 ± 0.2 | 3.2 ± 0.5 |
| Initial brewer’s spent yeast mass (g/L) | 0.0 ± 0.0 | 61.1 ± 0.0 |
| Initial total organic carbon (g/L) | 17.6 ± 0.5 | 12.6 ± 0.2 |
| Initial total nitrogen (g/L) | 3.6 ± 0.3 | 2.2 ± 0.1 |
| Initial C/N ratio | 4.9 ± 0.3 | 5.5 ± 0.1 |
| Initial pH | 6.5 ± 0.0 | 6.5 ± 0.0 |
| Lactose consumption rate (glactose/L·h) 24 h | 0.8 ± 0.0 | 0.8 ± 0.0 |
| Ethanol productivity (gethanol/L·h)8 h | 0.1 ± 0.0 | 0.3 ± 0.0 |
| Ethanol yield production (gethanol/glactose)8 h | 0.3 ± 0.0 | 0.7 ± 0.0 |
| L-phenylalanine concentration (gL-Phe/L)31 h | 1.3 ± 0.0 | 0.0 ± 0.0 |
| L-phenylalanine consumption rate (gL-Phe/L·h)31 h | 0.06 ± 0.00 | 0.1 ± 0.0 |
| 2-phenylethanol concentration (g2PE/L) | 0.7 ± 0.131 h | 1.8 ± 0.048 h |
| 2-phenylethanol productivity (g2PE/L·h) | 0.03 ± 0.0031 h | 0.04 ± 0.048 h |
| 2-phenylethanol yield production (g2PE/gL-Phe) | 0.4 ± 0.031 h | 0.6 ± 0.048 h |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valdez Castillo, M.; Brar, S.K.; Arriaga, S.; Blais, J.-F.; Heitz, M.; Avalos Ramirez, A. Co-Fermentation of Agri-Food Residues Using a Co-Culture of Yeasts as a New Bioprocess to Produce 2-Phenylethanol. Molecules 2023, 28, 5536. https://doi.org/10.3390/molecules28145536
Valdez Castillo M, Brar SK, Arriaga S, Blais J-F, Heitz M, Avalos Ramirez A. Co-Fermentation of Agri-Food Residues Using a Co-Culture of Yeasts as a New Bioprocess to Produce 2-Phenylethanol. Molecules. 2023; 28(14):5536. https://doi.org/10.3390/molecules28145536
Chicago/Turabian StyleValdez Castillo, Mariana, Satinder Kaur Brar, Sonia Arriaga, Jean-François Blais, Michèle Heitz, and Antonio Avalos Ramirez. 2023. "Co-Fermentation of Agri-Food Residues Using a Co-Culture of Yeasts as a New Bioprocess to Produce 2-Phenylethanol" Molecules 28, no. 14: 5536. https://doi.org/10.3390/molecules28145536
APA StyleValdez Castillo, M., Brar, S. K., Arriaga, S., Blais, J.-F., Heitz, M., & Avalos Ramirez, A. (2023). Co-Fermentation of Agri-Food Residues Using a Co-Culture of Yeasts as a New Bioprocess to Produce 2-Phenylethanol. Molecules, 28(14), 5536. https://doi.org/10.3390/molecules28145536











