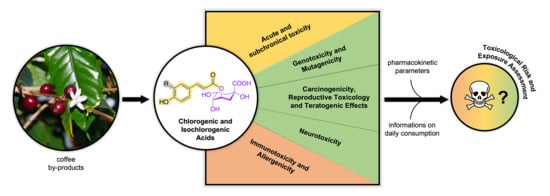
Risk Assessment of Chlorogenic and Isochlorogenic Acids in Coffee By-Products
Abstract
:1. Introduction
2. Literature Research
3. Chlorogenic Acids
3.1. Structures, Properties, and Natural Occurrence
3.2. Biosynthetic Pathways and Totally Synthetic Approaches
4. Amounts of CQA in Coffee By-Products
5. Absorption, Distribution, Metabolism, and Excretion
6. Toxicological Information
6.1. Acute and Subchronic Toxicity
6.2. Genotoxicity and Mutagenicity
6.3. Carcinogenicity, Reproductive Toxicity, and Teratogenic Effects
6.4. Neurotoxicity
6.5. Immunotoxicity and Allergenicity
6.6. Other Adverse Effects
7. Regulatory and Nutritional Information
8. Exposure and Risk Assessment
8.1. Theoretical Maximum Daily Intake (TMDI) and Limitations
8.2. Acute Oral Exposure
8.3. Chronic Oral Exposure
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Lachenmeier, D.W.; Teipel, J.; Scharinger, A.; Kuballa, T.; Walch, S.G.; Grosch, F.; Brunzel, M.; Okaru, A.O.; Schwarz, S. Fully automated identification of coffee species and simultaneous quantification of furfuryl alcohol using NMR spectroscopy. J. AOAC Int. 2020, 103, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Rimbach, G.; Nagursky, J.; Erbersdobler, H.F. Lebensmittel-Warenkunde für Einsteiger, 2nd ed.; Springer: Berlin, Germany, 2015. [Google Scholar]
- Rösemann, U. Coffea—Für viele müde Menschen die erste Pflanze des Tages. Biol. Unserer Zeit 2017, 47, 336–337. [Google Scholar] [CrossRef]
- Tritsch, N.; Steger, M.C.; Segatz, V.; Blumenthal, P.; Rigling, M.; Schwarz, S.; Zhang, Y.; Franke, H.; Lachenmeier, D.W. Risk Assessment of Caffeine and Epigallocatechin Gallate in Coffee Leaf Tea. Foods 2022, 11, 263. [Google Scholar] [CrossRef]
- Hegnauer, R. Chemotaxonomie der Pflanzen: Eine Übersicht über die Verbreitung und die Systematische Bedeutung der Pflanzenstoffe; Band 21; Birkhäuser: Basel, Switerland, 1973; p. 169. [Google Scholar]
- Baltes, W. Lebensmittelchemie; Springer: Berlin/Heidelberg, Germany, 2007; pp. 398–413. [Google Scholar]
- Esquivel, P.; Jiménez, V.M. Functional properties of coffee and coffee by-products. Food Res. Int. 2012, 46, 488–495. [Google Scholar] [CrossRef]
- Lai, W.T.; Khong, N.M.H.; Lim, S.S.; Hee, Y.Y.; Sim, B.I.; Lau, K.Y. A review: Modified agricultural by-products for the development and fortification of food products and nutraceuticals. Trends Food Sci. Technol. 2017, 59, 148–160. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Sillanpää, M.; Witek-Krowiak, A. Agricultural waste peels as versatile biomass for water purification—A review. Chem. Eng. J. 2015, 270, 244–271. [Google Scholar] [CrossRef]
- Klingel, T.; Kremer, J.I.; Gottsein, V.; Rajcic de Rezende, T.; Schwarz, S.; Lachenmeier, D.W. A Review of coffee by-products including Leaf, Flower, Cherry, Husk, Silver Skin, and Spent Grounds as Novel Foods within the European Union. Foods 2020, 9, 665. [Google Scholar] [CrossRef]
- European Union. European Union Regulation (EU) 2015/2283 of the European parliament and of the council of 25 November 2015 on novel foods, amending Regulation (EU) No 1169/2011 of the European Parliament and of the Council and repealing Regulation (EC) No 258/97 of the European Parliament and of the Council and Commission Regulation (EC) No 1852/2001. Off. J. Eur. Union 2015, L327, 1–22. [Google Scholar]
- EFSA. Novel Food. Available online: http://www.efsa.europa.eu/en/topics/topic/novel-food (accessed on 27 September 2022).
- Blinová, L.; Sirotiak, M.; Pastierova, A.; Soldán, M. Review: Utilization of Waste from Coffee Production. Res. Pap. Fac. Mater. Sci. Technol. Slovak Univ. Technol. 2017, 25, 91–101. [Google Scholar] [CrossRef] [Green Version]
- Lachenmeier, D.W.; Schwarz, S.; Rieke-Zapp, J.; Cantergiani, E.; Rawel, H.; Martín-Cabrejas, M.A.; Martuscelli, M.; Gottstein, V.; Angeloni, S. Coffee By-Products as Sustainable Novel Foods: Report of the 2nd International Electronic Conference on Foods—“Future Foods and Food Technologies for a Sustainable World”. Foods 2021, 11, 3. [Google Scholar] [CrossRef]
- Lachenmeier, D.W.; Rajcic de Rezende, T.; Schwarz, S. An Update on sustainable valorization of coffee by-products as novel foods within the European Union. Biol. Life Sci. Forum 2021, 6, 37. [Google Scholar] [CrossRef]
- Payen, S. Comptes Rendus Hebdomadaires des Séances de l’Académie des Sciences; Bachelier: Paris, France, 1846; Volume 23, pp. 244–251. [Google Scholar]
- Payen, S. Untersuchung des Kaffees. Ann. Chem. Pharm. 1846, 60, 286–294. [Google Scholar]
- Rochleder, F. Untersuchung der Kaffeebohnen. Justus Liebigs Ann. Chem. 1846, 59, 300–310. [Google Scholar] [CrossRef]
- Gorter, K. Beiträge zur Kenntnis des Kaffees (Erste Abhandlung). Justus Liebigs Ann. Chem. 1908, 358, 327–348. [Google Scholar] [CrossRef]
- Freudenberg, K. Über Gerbstoffe. III. Chlorogensäure, der gerbstoff-artige Bestandteil der Kaffeebohnen. Ber. Dtsch. Chem. Ges. A 1920, 53, 232–239. [Google Scholar] [CrossRef]
- Clifford, M.N.; Johnston, K.L.; Knigh, S.; Kuhnert, N. Hierarchical Scheme for LC-MSn Identification of Chlorogenic Acids. J. Agric. Food Chem. 2003, 51, 2900–2911. [Google Scholar] [CrossRef]
- Clifford, M.N.; Jaganath, I.B.; Ludwig, I.A.; Crozier, A. Chlorogenic acids and the acyl-quinic acids: Discovery, biosynthesis, bioavailability and bioactivity. Nat. Prod. Rep. 2017, 34, 1391–1421. [Google Scholar] [CrossRef] [Green Version]
- Farah, A.; Donangelo, C.M. Phenolic compounds in coffee. Braz. J. Plant. Physiol. 2006, 18, 23–36. [Google Scholar] [CrossRef]
- Cartwright, R.A.; Roberts, E.A.H.; Flood, E.A. Chem. Indust. 1955, 1062–1063.
- Corse, J.; Sondheimer, E.; Lundin, R. 3-feruloylquinic acid: A 3′-methyl ether of chlorogenic acid. Tetrahedron 1962, 18, 2107–2108. [Google Scholar] [CrossRef]
- Awwad, S.; Issa, R.; Alnsour, L.; Albals, D.; Al-Momani, I. Quantification of Caffeine and Chlorogenic acid in Green and Roasted Coffee Samples Using HPLC-DAD and Evaluation of the Effect of Degree of Roasting on Their Levels. Molecules 2021, 26, 7502. [Google Scholar] [CrossRef] [PubMed]
- Silva, N.; Mazzafera, P.; Cesarino, I. Should I stay or should I go: Are chlorogenic acids mobilized towards lignin biosynthesis? Phytochemistry 2019, 166, 112063. [Google Scholar] [CrossRef] [PubMed]
- Boerjan, W.; Ralph, J.; Baucher, M. Lignin biosynthesis. Ann. Rev. Plant. Biol. 2003, 54, 519–546. [Google Scholar] [CrossRef] [PubMed]
- Fischer, H.O.L.; Dangschat, G. Konstitution der Chlorogensäure (3. Mitteil. Über Chinasäure Derivate). Ber. Dtsch. Chem. Ges. A 1932, 65, 1037–1040. [Google Scholar] [CrossRef]
- Narita, Y.; Inouye, K. Chapter 21—Chlorogenic acids from coffee. In Coffee in Health and Disease Prevention; Preedy, V.R., Ed.; Academic Press: San Diego, CA, USA, 2015; pp. 189–199. [Google Scholar]
- Clifford, M.N. Researchgate. 2017. Available online: https://www.researchgate.net/publication/312591202 (accessed on 10 January 2023).
- IUPAC. IUPAC Commission on the Nomenclature of Organic Chemistry (CNOC) and IUPAC-IUB Commission on Biochemical Nomenclature (CBN). Nomenclature of cyclitols. Recommendations (1973). Biochem. J. 1976, 153, 23–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abrankó, L.; Clifford, M.N. An Unambiguous Nomenclature for the Acyl-quinic Acids Commonly known as Chlorogenic Acids. J. Agric. Food Chem. 2017, 65, 3602–3608. [Google Scholar] [CrossRef] [PubMed]
- Corse, J.; Patterson, D.C. 3-O-Sinapolyquinic acid. Phytochemistry 1969, 8, 203–205. [Google Scholar] [CrossRef]
- Lu, H.; Tian, Z.; Cui, Y.; Liu, Z.; Ma, X. Chlorogenic acid: A comprehensive review of the dietary sources, processing effects, bioavailability, beneficial properties, mechanisms of action, and future directions. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3130–3158. [Google Scholar] [CrossRef]
- Parejo, I.; Jauregui, O.; Sánchez-Rabaneda, F.; Viladomat, F.; Bastida, J.; Codina, C. Separation and characterization of phenolic compounds in fennel (Foeniculum vulgare) using liquid chromatography–negative electrospray ionization tandem mass spectrometry. J. Agric. Food Chem. 2004, 52, 3679–3687. [Google Scholar] [CrossRef]
- Clifford, M.N.; Kellard, B.; Birch, G.G. Characterisation of chlorogenic acids by simultaneous isomerisation and transesterification with tetramethylammonium hydroxide. Food Chem. 1989, 33, 115–123. [Google Scholar] [CrossRef]
- Clifford, M.N.; Kellard, B.; Birch, G.G. Characterisation of caffeoylferuloylquinic acids by simultaneous isomerisation and transesterification with tetramethylammonium hydroxide. Food Chem. 1989, 34, 81–88. [Google Scholar] [CrossRef]
- Dawidowicz, A.L.; Typek, R. Transformation of chlorogenic acids during the coffee beans roasting process. Eur. Food Res. Technol. 2017, 243, 379–390. [Google Scholar] [CrossRef]
- Macheiner, L.; Schmidt, A.; Schreiner, M.; Mayer, H.K. Green coffee infusion as a source of caffeine and chlorogenic acid. J. Food Compos. Anal. 2019, 84, 103307. [Google Scholar] [CrossRef]
- Liang, N.; Xue, W.; Kennepohl, P.; Kitts, D.D. Interaction between major chlorogenic acid isomers and chemical changes in coffee brew that affect antioxidant activities. Food Chem. 2016, 213, 251–259. [Google Scholar] [CrossRef] [Green Version]
- Bobková, A.; Jakabová, S.; Belej, L.; Jurcaga, L.; Capla, J.; Bobko, M.; Demianová, A. Analysis of caffeine and chlorogenic acids content regarding the preparation method of coffee beverage. Int. J. Food. Eng. 2021, 17, 403–410. [Google Scholar] [CrossRef]
- Ludwig, I.A.; Mena, P.; Calani, L.; Concepción, C.; Del Rio, D.; Lean, M.E.J.; Crozier, A. Variations in caffeine and chlorogenic contents of coffee: What are we drinking? Food Funct. 2014, 5, 1718. [Google Scholar] [CrossRef] [Green Version]
- Farah, A.; De Paulis, T.; Moreira, D.P.; Trugo, L.C.; Martin, P.R. Chlorogenic acids and lactones in regular and water-decaffeinated arabica coffees. J. Agric. Food Chem. 2006, 54, 374–381. [Google Scholar] [CrossRef]
- Jiang, D.; Peterson, D.G. Role of hydroxycinnamic acids in food flavor: A brief overview. Phytochem. Rev. 2010, 9, 187–193. [Google Scholar] [CrossRef]
- Kuhnert, N. Analysis of chlorogenic acid lactones and caffeoyl shikimic acids in roasted coffee. Eng. Food Sci. 2013, 17, 568–576. [Google Scholar]
- Duke, J.A. List of Plants for Chlorogenic Acid; Dr. Duke’s Phytochemical and Ethnobotanical Database; U.S. Department of Agriculture: Washington, DC, USA, 1992. [Google Scholar]
- Daraee, A.; Ghoreishi, S.M.; Hedayati, A. Supercritical CO2 extraction of chlorogenic acid from sunflower (Helianthus annuus) seed kernels: Modeling and optimization by response surface methodology. J. Supercrit. Fluids 2019, 144, 19–27. [Google Scholar] [CrossRef]
- Kweon, M.-H.; Hwang, H.-J.; Sung, H.-C. Identification and Antioxidant Activity of Novel Chlorogenic Acid Derivatives from Bamboo (Phyllostachys edulis). J. Agri. Food Chem. 2001, 49, 4646–4652. [Google Scholar] [CrossRef] [PubMed]
- Jalal, M.A.F.; Read, D.J.; Haslam, E. Phenolic composition and its seasonal variation in Calluna vulgaris. Phytochemistry 1982, 21, 1397–1401. [Google Scholar] [CrossRef]
- Petrisor, G.; Motelica, L.; Craciun, L.N.; Oprea, O.C.; Ficai, D.; Ficai, A. Melissa officinalis: Composition, Pharmacological Effects and Derived Release Systems—A Review. Int. J. Mol. Sci. 2022, 23, 3591. [Google Scholar] [CrossRef] [PubMed]
- Von Bruchhausen, F. Hagers Handbuch der Pharmazeutischen Praxis; Springer: Berlin/Heidelberg, Germany, 1949; ISBN 3-540-52688-9. [Google Scholar]
- Leung, A.Y.; Foster, S. Encyclopedia of Common Natural Ingredients, 2nd ed.; John Wiley & Sons: New York, NY, USA, 1995; p. 649. [Google Scholar]
- Kim, S.M.; Shang, Y.F.; Um, B.H. Preparative separation of chlorogenic acid by centrifugal partition chromatography from highbush blueberry leaves (Vaccinium corymbosum L.). Phytochem. Anal. 2010, 21, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Mei, Y.; Sun, H.; Du, G.; Wang, X.; Lyu, D. Exogenous chlorogenic acid alleviates oxidative stress in apple leaves by enhancing antioxidant capacity. Sci. Hortic. 2020, 274, 109676. [Google Scholar] [CrossRef]
- Kliewer, W.M. Sugars and Organic Acids of Vitis vinifera. Plant Physiol. 1966, 41, 923–931. [Google Scholar] [CrossRef] [Green Version]
- Luthria, D.L.; Mukhopadhyay, S. Influence of Sample Preparation on Assay of Phenolic Acids from Eggplant. J. Agric. Food Chem. 2006, 54, 41–47. [Google Scholar] [CrossRef]
- Cheng, G.W.; Crisosto, C.H. Browning Potential, Phenolic Composition, and Polyphenoloxidase Activity of Buffer Extracts of Peach and Nectarine Skin Tissue. J. Am. Soc. Hortic. Sci. 1995, 120, 835–838. [Google Scholar] [CrossRef] [Green Version]
- Stacewicz-Sapuntzakis, M.; Bowen, P.E.; Hussain, E.A.; Damayanti-Wood, B.I.; Farnsworth, N.R. Chemical composition and potential health effects of prunes: A functional food? Crit. Rev. Food Sci. Nutr. 2001, 41, 251–286. [Google Scholar] [CrossRef]
- Clifford, M.N.; Shutler, S.; Thomas, G.A.; Ohiokpehai, O. The chlorogenic acids content of coffee substitutes. Food Chem. 1987, 24, 99–107. [Google Scholar] [CrossRef]
- Zhen, J.; Villani, T.S.; Guo, Y.; Qi, Y.; Chin, K.; Pan, M.-H.; Ho, C.-T.; Simon, J.E.; Wu, Q. Phytochemistry, antioxidant capacity, total phenolic content and anti-inflammatory activity of Hibiscus sabdariffa leaves. Food Chem. 2016, 190, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez Ortiz, A.L.; Berti, F.; Navarini, L.; Crisafulli, P.; Colomban, S.; Forzato, C. Aqueous extracts of walnut (Juglans regia L.) leaves: Quantitative analyses of hydroxycinnamic and chlorogenic acids. J. Chromatogr. Sci. 2018, 56, 753–760. [Google Scholar] [CrossRef]
- Mijangos-Ramos, I.F.; Zapata-Estrella, H.E.; Ruiz-Vargas, J.A.; Escalante-Erosa, F.; Gómez-Ojeda, N.; García-Sosa, K.; Cechinel-Filho, V.; Meira-Quintão, N.L.; Peña-Rodríguez, L.M. Bioactive dicaffeoylquinic acid derivatives from the root extract of Calea urticifolia. Rev. Bras. Farmacogn. 2018, 28, 339–343. [Google Scholar] [CrossRef]
- Yin, X.-L.; Xu, B.-Q.; Zhang, Y.-Q. Gynura divaricata rich in 3, 5-/4, 5-dicaffeoylquinic acid and chlorogenic acid reduces islet cell apoptosis and improves pancreatic function in type 2 diabetic mice. Nutr. Metab. 2018, 15, 73. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-J.; Jang, Y.-N.; Han, Y.-M.; Kim, H.-M.; Jeong, J.-M.; Son, M.J.; Jin, C.B.; Kim, H.J.; Seo, H.S. Caffeoylquinic acid-rich extract of Aster glehni F. Schmidt ameliorates nonalcoholic fatty liver through the regulation of PPARδ and adiponectin in ApoE KO mice. PPAR Res. 2017, 2017, 3912567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Topal, M.; Gocer, H.; Topal, F.; Kalin, P.; Köse, L.P.; Gülçin, İ.; Çakmak, K.C.; Küçük, M.; Durmaz, L.; Gören, A.C.; et al. Antioxidant, antiradical, and anticholinergic properties of cynarin purified from the Illyrian thistle (Onopordum illyricum L.). J. Enzym. Inhib. Med. Chem. 2016, 31, 266–275. [Google Scholar] [CrossRef] [Green Version]
- Meinhart, A.D.; Damin, F.M.; Caldeirão, L.; da Silveira, T.F.F.; Filho, J.T.; Godoy, H.T. Chlorogenic acid isomer contents in 100 plants commercialized in Brazil. Food Res. Int. 2017, 99, 522–530. [Google Scholar] [CrossRef]
- Li, X.; Li, K.; Xie, H.; Xie, Y.; Li, Y.; Zhao, X.; Jiang, X.; Chen, D. Antioxidant and Cytoprotective Effects of the Di-O- Caffeoylquinic Acid Family: The Mechanism, Structure−Activity Relationship, and Conformational Effect. Molecules 2018, 23, 222. [Google Scholar] [CrossRef] [Green Version]
- Mossi, A.; Echeverrigaray, S. Identification and characterization of antimicrobial components in leaf extracts of globe artichoke (Cynara scolymus L.). Acta Hortic. 1999, 501, 111–114. [Google Scholar] [CrossRef]
- Mahmood, N.; Moore, P.; De Tommasi, N.; De Simone, F.; Colman, S.; Hay, A.; Pizza, C. Inhibition of HIV infection by caffeoylquinic acid derivatives. Antivir. Chem. Chemother. 1993, 4, 235–240. [Google Scholar] [CrossRef]
- Meng, S.; Cao, J.; Feng, Q.; Peng, J.; Hu, Y. Roles of Chlorogenic Acid in Regulating Glucose and Lipid Metabolism: A Review. Evid. Based Complement. Alternat. Med. 2013, 2013, 801457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hur, J.Y.; Soh, Y.; Kim, B.-H.; Suk, K.; Sohn, N.W.; Kim, H.C.; Kwon, H.C.; Lee, K.R.; Kim, S.Y. Neuroprotective and neurotrophic effects of quinic acids from Aster scaber in PC12 cells. Biol. Pharm. Bull. 2001, 24, 921–924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.S.; Park, R.Y.; Jeon, H.J.; Kwon, Y.S.; Chun, W. Neuroprotective effects of 3,5-dicaffeoylquinic acid on hydrogen peroxide-induced cell death in SH-SY5Y cells. Phytother. Res. 2005, 19, 243–245. [Google Scholar] [CrossRef]
- Dos Santos, M.D.; Chen, G.; Almeida, M.C.; Soares, D.M.; de Souza, G.E.P.; Lopes, N.P.; Lantz, R.C. Effects of caffeoylquinic acid derivatives and C-flavonoid from Lychnophora ericoides on in vitro inflammatory mediator production. Nat. Prod. Commun. 2010, 5, 733–740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Islam, M.S.; Yoshimoto, M.; Yahara, S.; Okuno, S.; Ishiguro, K.; Yamakawa, O. Identification and characterization of foliar polyphenolic composition in sweet potato (Ipomoea batatas L.) genotypes. J. Agric. Food Chem. 2002, 50, 3718–3722. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, M.; Yahara, S.; Okuno, S.; Islam, M.S.; Ishiguro, K.; Yamakawa, O. Antimutagenicity of mono-, di-, and tricaffeoylquinic acid derivatives isolated from sweet potato (Ipomoea batatas L.) leaf. Biosci. Biotechnol. Biochem. 2002, 66, 2336–2341. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Su, C.; Chen, X.; Wang, Q.; Jiao, W.; Luo, H.; Tang, J.; Wang, W.; Li, S.; Guo, S. Chlorogenic acids in cardiovascular disease: A review of dietary consumption, pharmacology, and pharmacokinetics. J. Agric. Food Chem. 2020, 68, 6464–6484. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, J.; Ballevre, O.; Luo, H.; Zhang, W. Antihypertensive effects and mechanisms of chlorogenic acids. Hypertens. Res. 2012, 35, 370–374. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.-N.; Wang, W.; Hattori, M.; Daneshtalab, M.; Ma, C.-M. Synthesis, Anti-HCV, Antioxidant and Reduction of Intracellular Reactive Oxygen Species Generation of a Chlorogenic Acid Analogue with an Amide Bond Replacing the Ester Bond. Molecules 2016, 21, 737. [Google Scholar] [CrossRef] [Green Version]
- Ma, C.-M.; Kully, M.; Khan, J.K.; Hattori, M.; Daneshtalab, M. Synthesis of chlorogenic acid derivatives with promising antifungal activity. Bioorg. Med. Chem. 2007, 15, 6830–6833. [Google Scholar] [CrossRef]
- Timmermann, B.N.; Hoffmann, J.J.; Jolad, S.D.; Schram, K.H.; Klenck, R.E.; Bates, R.B. Constituents of Chrysothamnus paniculatus 3: 3,4,5-tricaffeoylquinic acid (a new shikimate prearomatic) and 3,4-, 3,5-and 4,5-dicaffeoylquinic acids. J. Nat. Prod. 1983, 46, 365–368. [Google Scholar] [CrossRef]
- Agata, I.; Goto, S.; Hatano, T.; Nishibe, S.; Okuda, T. 1,3,5-Tri-O-caffeoylquinic acid from Xanthium strumarium. Phytochemistry 1993, 33, 508–509. [Google Scholar] [CrossRef]
- Merfort, I. Caffeoylquinic acids from flowers of Arnica montana and Arnica chamissonis. Phytochemistry 1992, 31, 2111–2113. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, J.; Zheng, B.; Guan, Y.; Wang, L.; Chen, L.; Cai, W. Rapid characterization of chlorogenic acids in Duhaldea nervosa based on ultra-high-performance liquid chromatography-linear trap quadrupole-Orbitrap-mess spectroscopy and mass spectral trees similarity filter technique. J. Sep. Sci. 2018, 41, 1764–1774. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Jeon, J.S.; Kang, S.W.; Jung, Y.J.; Ly, L.N.; Um, B.H. Content of antioxidative caffeoylquinic acid derivatives in field-grown Ligularia fischeri (Ledeb.) Turcz and responses to sunlight. J. Agric. Food Chem. 2012, 60, 5597–5603. [Google Scholar] [CrossRef] [PubMed]
- Matsui, T.; Ebuchi, S.; Fujise, T.; Abesundara, K.J.; Doi, S.; Yamada, H.; Matsumoto, K. Strong antihyperglycemic effects of water-soluble fraction of Brazilian propolis and its bioactive constituent, 3,4,5-tri-O-caffeoylquinic acid. Biol. Pharm. Bull. 2004, 27, 1797–1803. [Google Scholar] [CrossRef] [Green Version]
- Scholz, E.; Heinrich, M.; Hunkler, D. Caffeoylquinic acids and some biological activities of Pluchea symphytifolia. Planta Med. 1994, 60, 360–364. [Google Scholar] [CrossRef]
- Liu, W.; Li, J.; Zhang, X.; Zu, Y.; Yang, Y.; Liu, W.; Xu, Z.; Gao, H.; Sun, X.; Jiang, X.; et al. Current Advances in Naturally Occurring Caffeoylquinic Acids: Structure, Bioactivity, and Synthesis. J. Agric. Food Chem. 2020, 68, 10489–10516. [Google Scholar] [CrossRef]
- Smith, P.; Ho, C.K.; Takagi, Y.; Djaballah, H.; Shuman, S. Nanomolar Inhibitors of Trypanosoma brucei RNA Triphosphatase. mBio 2016, 7, e0058-16. [Google Scholar] [CrossRef] [Green Version]
- Comino, C.; Hehn, A.; Moglia, A.; Menin, B.; Bourgaud, F.; Lanteri, S.; Portis, E. The isolation and mapping of a novel hydroxycinnamoyltransferase in the globe artichoke chlorogenic acid pathway. BMC Plant Biol. 2009, 9, 30. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Yang, Y.; Zheng, K.; Xie, M.; Feng, K.; Jawdy, S.S.; Gunter, L.E.; Ranjan, P.; Singan, V.R.; Engle, N.; et al. Genome-wide association studies and expression-based quantitative trait loci analyses reveal roles of HCT2 in caffeoylquinic acid biosynthesis and its regulation by defense-responsive transcription factors in Populus. New Phytol. 2018, 220, 502–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menin, B.; Comino, C.; Moglia, A.; Dolzhenko, Y.; Portis, E.; Lanteri, S. Identification and mapping of genes related to caffeoylquinic acid synthesis in Cynara cardunculus L. Plant Sci. 2010, 179, 338–347. [Google Scholar] [CrossRef]
- Moglia, A.; Acquadro, A.; Eljounaidi, K.; Milani, A.M.; Cagliero, C.; Rubiolo, P.; Genre, A.; Cankar, K.; Beekwilder, J.; Comino, C. Genome-wide identification of BAHD acyltransferases and in vivo characterization of HQT-like enzymes involved in caffeoylquinic acid synthesis in globe artichoke. Front. Plant Sci. 2016, 7, 1424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villegas, R.; Kojima, M. Purification and characterization of hydroxycinnamoyl D-glucose. Quinate hydroxycinnamoyl transferase in the root of sweet potato, Ipomoea batatas Lam. J. Biol. Chem. 1986, 261, 8729–8733. [Google Scholar] [CrossRef] [PubMed]
- Lallemand, L.A.; Zubieta, C.; Lee, S.G.; Wang, Y.; Acajjaoui, S.; Timmins, J.; McSweeney, S.; Jez, J.M.; McCarthy, J.G.; McCarthy, A.A. A structural basis for the biosynthesis of the major chlorogenic acids found in coffee. Plant Physiol. 2012, 160, 249–260. [Google Scholar] [CrossRef] [Green Version]
- Moglia, A.; Lanteri, S.; Comino, C.; Hill, L.; Knevitt, D.; Cagliero, C.; Rubiolo, P.; Bornemann, S.; Martin, C. Dual catalytic activity of hydroxycinnamoyl-Coenzyme A quinate transferase from tomato allows it to moonlight in the synthesis of both mono-and dicaffeoylquinic acids. Plant Physiol. 2014, 166, 1777–1787. [Google Scholar] [CrossRef]
- Xue, M.; Shi, H.; Zhang, J.; Liu, Q.-Q.; Guan, J.; Zhang, J.-Y.; Ma, Q. Stability and Degradation of Caffeoylquinic acids under different storage conditions studied by high-performance liquid chromatography with Photo Diode Array Detection and High-performance liquid chromatography with electrospray ionization collision-induced dissociation tandem mass spectrometry. Molecules 2016, 21, 948. [Google Scholar] [CrossRef] [Green Version]
- Kaiser, N.; Birkholz, D.; Colomban, S.; Navarini, L.; Engelhardt, U.H. A New Method for the preparative Isolation of chlorogenic acid lactones from coffee and model roasts of 5-caffeoylquinic acid. J. Agric. Food Chem. 2013, 61, 6937–6941. [Google Scholar] [CrossRef]
- Jaiswal, R.; Matei, M.F.; Golon, A.; Witt, M.; Kuhnert, N. Understanding the fate of chlorogenic acids in coffee roasting using mass spectrometry based targeted and non-targeted analytical strategies. Food Funct. 2012, 3, 976–984. [Google Scholar] [CrossRef]
- Hemmerle, H.; Burger, H.-J.; Below, P.; Schubert, G.; Rippel, R.; Schindler, P.W.; Paulus, E.; Herling, A.W. Chlorogenic acid and synthetic chlorogenic acid derivatives: Novel inhibitors of hepatic glucose-6-phosphate translocase. J. Med. Chem. 1997, 40, 137–145. [Google Scholar] [CrossRef]
- Sefkow, M. First efficient synthesis of chlorogenic acid. Eur. J. Org. Chem. 2001, 2001, 1137–1141. [Google Scholar] [CrossRef]
- Kadidae, L.O.; Usami, A.; Koyama, T.; Honda, M.; Kunimoto, K.-K. New route for synthesis of 3- and 5-caffeoylquinic acids via protected quinic acids. Eur. J. Chem. 2015, 6, 367–373. [Google Scholar] [CrossRef] [Green Version]
- Panizzi, L.; Scarpati, M.L.; Oriente, G. Sintesi dell’acido clorogenico. Experientia 1955, 11, 383–384. [Google Scholar] [CrossRef]
- Panizzi, L.; Scarpati, M.L.; Oriente, G. Synthesis of Chlorogenic acid. Gazz. Chim. Ital. 1956, 86, 913–922. [Google Scholar]
- Sefkow, M.; Kelling, A.; Schilde, U. First Efficient Syntheses of 1-, 4-, and 5-Caffeoylquinic Acid. Eur. J. Org. Chem. 2001, 2001, 2735–2742. [Google Scholar] [CrossRef]
- Oyama, K.; Watanabe, N.; Yamada, T.; Suzuki, M.; Sekiguchi, Y.; Kondo, T.; Yoshida, K. Efficient and versatile synthesis of 5-O-acylquinic acids with a direct esterification using a p-methoxybenzyl quinate as a key intermediate. Tetrahedron 2015, 71, 3120–3130. [Google Scholar] [CrossRef]
- Nagaoka, T.; Banskota, A.H.; Xiong, Q.; Tezuka, Y.; Kadota, S. Synthesis and antihepatotoxic and antiproliferative activities of di-and tri-O-caffeoylquinic acid derivatives. J. Trad. Med. 2001, 18, 183–190. [Google Scholar]
- Miyamae, Y.; Kurisu, M.; Han, J.; Isoda, H.; Shigemori, H. Structure−activity relationship of caffeoylquinic acids on the accelerating activity on ATP production. Chem. Pharm. Bull. 2011, 59, 502–507. [Google Scholar] [CrossRef] [Green Version]
- Raheem, K.S.; Botting, N.P.; Williamson, G.; Barron, D. Total synthesis of 3, 5-O-dicaffeoylquinic acid and its derivatives. Tetrahedron Lett. 2011, 52, 7175–7177. [Google Scholar] [CrossRef]
- Brummond, K.M.; DeForrest, J.E. Synthesis of the Naturally Occurring (−)-1, 3, 5-Tri-O-Caffeoylquinic Acid. Synlett 2009, 2009, 1517–1519. [Google Scholar] [CrossRef] [Green Version]
- Fujioka, K.; Shibamoto, T. Chlorogenic acid and caffeine contents in various commercial brewed coffees. Food Chem. 2008, 106, 217–221. [Google Scholar] [CrossRef]
- Misto, M.; Lestari, N.P.; Purwandan, E. Chlorogenic acid content of local robusta coffee at variations of roasting temperature. J. Pendidik. Fis. Indon. 2022, 18, 25–32. [Google Scholar] [CrossRef]
- Viencz, T.; Acre, L.B.; Rocha, R.B.; Alves, E.A.; Ramalho, A.R.; Benassi, M.T. Caffeine, trigonelline, chlorogenic acids, metlanoidins, and diterpenes contents of coffea canephora coffees produced in the amazon. J. Food. Comp. Anal. 2023, 117, 105140. [Google Scholar] [CrossRef]
- Budavari, S.; Angelica, E. Caffeic Acid. Chlorogenic Acid. Coffee, Green. Crataegus. Maté. In The Merck Index, 12th ed.; Merck & Co., Inc.: Whitehall, NJ, USA, 1996; p. 109ff. [Google Scholar]
- Wasserman, G.; Stahl, H.D.; Rehman, W.; Whitman, P. Coffee. In Kirk-Othmer Encyclopedia of Chemical Technology, 4th ed.; John Wiley and Sons: New York, NY, USA, 1993; Volume 6, pp. 793–811. [Google Scholar]
- Friedman, M. Chemistry, biochemistry, and dietary role of potato polyphenols. A review. J. Agric. Food Chem. 1997, 45, 1523–1540. [Google Scholar] [CrossRef]
- Shahrzad, S.; Bitsch, I. Determination of some pharmacologically active phenolic acids in juice by high-performance liquid chromatography. J. Chromatogr. A 1996, 741, 223–231. [Google Scholar] [CrossRef]
- Wirz, K.; Schwarz, S.; Richling, E.; Walch, S.G.; Lachenmeier, D.W. Coffee Flower as a Promising Novel Food—Chemical Characterization and Sensory Evaluation. Biol. Life Sci. Forum 2022, 18, 53. [Google Scholar] [CrossRef]
- De Abreu Pinheiro, F.; Elias, L.F.; de Jesus Filho, M.; Modol, M.U.; de Cassia Gomas Rocha, J.; Lemos, M.F.; Scherer, R.; Carodos, W.S. Arabica and Conilon coffee flowers: Bioactive compounds and antioxidant capacity under different processes. Food Chem. 2021, 336, 127701. [Google Scholar] [CrossRef]
- Monteiro, Â.; Colomban, S.; Azinheira, H.G.; Guerra-Guimarães, L.; Do Céu Silva, M.; Navarini, L.; Resmini, M. Dietary Antioxidants in Coffee Leaves: Impact of Botanical Origin and Maturity on Chlorogenic Acids and Xanthones. Antioxidants 2020, 9, 6. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Gomez, R.; Vanheuverzwjin, J.; Souard, F.; Delporte, C.; Stevingy, C.; Stoffelen, P.; De Braekeleer, K.; Kauffmann, J.-M. Determination of Three Main Chlorogenic Acids in Water Extracts of Coffee Leaves by Liquid Chromatography Coupled to an Electrochemical Detector. Antioxidants 2018, 7, 143. [Google Scholar] [CrossRef] [Green Version]
- Torres-Mancera, M.T.; Baqueiro-Peña, I.; Figueroa-Montero, A.; Rodríguez-Serrano, G.; González-Zamora, E.; Favela-Torres, E.; Saucedo-Castañeda, G. Biotransformation and improved enzymatic extraction of chlorogenic acid from coffee pulp by filamentous fungi. Biotechnol. Prog. 2013, 29, 337–345. [Google Scholar] [CrossRef]
- Torres-Mancera, M.T.; Cόrdova Loόpez, J.; Rodríguez Serrano, G.; Roussos, S.; Ramírez-Coronel, M.A.; Favela-Torres, E.; Saucedo-Castañeda, G. Enzymatic extraction of hydroxycinnamic acids from coffee pulp. Food Technol. Biotechnol. 2011, 49, 369–373. [Google Scholar]
- Palomino García, L.R.; Biasetto, C.R.; Araujo, A.R.; Del Bianchi, V.L. Enhanced extraction of phenolic compounds from coffee industry’s residues through solid state fermentation by Penicillium purpurogenum. Food Sci. Technol. 2015, 35, 704–711. [Google Scholar] [CrossRef] [Green Version]
- Esquivel, P.; Viñas, M.; Steingass, C.B.; Gruschwitz, M.; Guevara, E.; Carle, R.; Schweiggert, R.M.; Jiménez, V.M. Coffee (Coffea arabica L.) by-Products as a Source of Carotenoids and Phenolic Compounds—Evaluation of Varieties with Different Peel Color. Front. Sustain. Food Syst. 2020, 4, 590–597. [Google Scholar] [CrossRef]
- Rodríguez-Durán, L.V.; Ramírez-Coronel, M.A.; Aranda-Delgado, E.; Nampoothiri, K.M.; Favela-Torres, E.; Aguilar, C.N.; Saucedo-Castañeda, G. Soluble and Bound Hydroxycinnamates in Coffee Pulp (Coffea arabica) from Seven Cultivars at Three Ripening Stages. J. Agric. Food Chem. 2014, 62, 7869–7876. [Google Scholar] [CrossRef]
- Murthy, P.S.; Madhava-Naidu, M. Sustainable Management of Coffee Industry by-Products and Value Addition—A Review. Resour. Conserv. Recycl. 2012, 66, 45–58. [Google Scholar] [CrossRef]
- Cangussu, L.B.; Melo, J.C.; Franca, A.S.; Oliveira, L.S. Chemical Characterization of Coffee Husks, a By-Product of Coffea arabica Production. Foods 2021, 10, 3125. [Google Scholar] [CrossRef]
- Iriondo-DeHond, A.; Aparicio García, N.; Fernandez-Gomez, B.; Guisantes-Batan, E.; Velázquez Escobar, F.; Blanch, G.P.; San Andres, M.I.; Sanchez-Fortun, S.; del Castillo, M.D. Validation of coffee by-products as novel food ingredients. Innov. Food Sci. Emerg. Technol. 2019, 51, 194–204. [Google Scholar] [CrossRef] [Green Version]
- Heeger, A.; Kosińska-Cagnazzo, A.; Cantergiani, E.; Andlauer, W. Bioactives of coffee cherry pulp and its utilisation for production of Cascara beverage. Food Chem. 2017, 221, 969–975. [Google Scholar] [CrossRef]
- Regazzoni, L.; Saligari, F.; Marinello, C.; Rossoni, G.; Aldini, G.; Carini, M.; Orioli, M. Coffee silver skin as a source of polyphenols: High resolution mass spectrometric profiling of components and antioxidant activity. J. Funct. Foods 2016, 20, 472–485. [Google Scholar] [CrossRef]
- Bresciani, L.; Calani, L.; Bruni, R.; Brighenti, F.; Del Rio, D. Phenolic composition, caffeine content and antioxidant capacity of coffee silverskin. Food. Res. Int. 2014, 61, 196–201. [Google Scholar] [CrossRef]
- Beltrán-Medina, E.A.; Guatemala-Morales, G.M.; Corona-González, R.I.; Padilla-Camberos, E.; Mondragón-Cortez, P.M.; Ruiz-Palomino, P.; Arriola-Guevara, E. Evaluation of the Analytical Conditions for the Determination of Chlorogenic Acid in Coffee Silverskin. Preprints.org 2019, 1, 2019090213. [Google Scholar] [CrossRef] [Green Version]
- Martuscelli, M.; Esposito, L.; Di Mattia, C.D.; Ricci, A.; Mastrocola, D. Characterization of Coffee Silver Skin as Potential Food-Safe Ingredient. Foods 2021, 10, 1367. [Google Scholar] [CrossRef] [PubMed]
- Husniati, H.; Oktiani, D. Chlorogenic Acid Isolation from Coffee as Affected by the Homogeneity of Cherry Maturity. Pelita Perkebunan 2019, 35, 119–124. [Google Scholar] [CrossRef] [Green Version]
- Bravo, J.; Juániz, I.; Monente, C.; Caemmerer, B.; Kroh, L.W.; Paz De Peña, M.; Cid, C. Evaluation of Spent Coffee Obtained from the Most Common Coffeemakers as a Source of Hydrophilic Bioactive Compounds. J. Agri. Food Chem. 2012, 60, 12565–12573. [Google Scholar] [CrossRef]
- Murthy, P.S.; Naidu, M.M. Protease production by Aspergillus oryzae in solid state fermentation utilizing coffee by-products. World Appl. Sci. J. 2010, 8, 199–205. [Google Scholar]
- Belitz, H.-D.; Grosch, W.; Schieberle, P. Lehrbuch der Lebensmittelchemie, 6. Vollständig Überarbeitete Auflage; Springer: Berlin/Heidelberg, Germany, 2008; ISBN 9783540732020. [Google Scholar]
- Perrone, D.; Donangelo, R.; Donangelo, C.M.; Farah, A. Modeling weight loss and chlorogenic acids content in coffee during roasting. J. Agri. Food Chem. 2010, 58, 12238–12243. [Google Scholar] [CrossRef]
- Available online: https://pubchem.ncbi.nlm.nih.gov (accessed on 2 May 2023).
- Hörter, D.; Dressman, J.B. Influence if Physicochemical Properties on Dissolution of Drugs. Adv. Drug Deliv. Rev. 1997, 25, 3–14. [Google Scholar] [CrossRef]
- Simeonova, R.; Vitcheva, V.; Zheleva-Dimitrova, D.; Balabanova, V.; Savov, I.; Yagi, S.; Dimitrova, B.; Voynikov, Y.; Gevrenova, R. Trans-3,5-dicaffeoylquinic acid from Geigeria alata Benth. & Hook.f. ex Oliv. & Hiern with beneficial effects on experimental diabetes in animal model of essential hypertension. Food Chem. Toxicol. 2019, 132, 110678. [Google Scholar] [CrossRef]
- Veras, K.S.; Fachel, F.N.S.; de Araújo, B.V.; Teixeira, H.F.; Koester, L.S. Oral Pharmacokinetics of Hydroxycinnamic Acids: An Updated Review. Pharmaceutics 2022, 14, 2663. [Google Scholar] [CrossRef]
- D’Antuono, I.; Garbetta, A.; Linsalata, V.; Minverini, F.; Cardinali, A. Polyphenols from Artichoke Heads (Cyanara cardunculus (L.) subsp. scolymus Hayek): In Vitro Bio-Accessibility, Intestinal Uptake and Bioavailability. Food Funct. 2015, 6, 1268–1277. [Google Scholar] [CrossRef]
- Ren, J.; Jiang, X.; Li, C.; Li, K.; Chen, Z.; Ma, G. Absorptive Profile of Chlorogenic Acid in Rats. Pharmazie 2007, 62, 689–692. [Google Scholar] [CrossRef] [PubMed]
- Di, L.; Kerns, E.H.; Hong, Y.; Chen, H. Development and Application of High Throughput Plasma Stability Assay for Drug Discovery. Int. J. Pharm. 2005, 297, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Diana, A.; Michielin, O.; Zoete, V. SwissADME: A Free Web Tool to Evaluate Pharmacokinetics, Drug-Likeness and Medicinal Chemistry Friendliness of Small Molecules; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Karlsson, J.; Ungell, A.L.; Grasjö, J.; Artursson, P. Paracellular Drug Transport across Intestinal Epithelia: Influence of Charge and Induced Water Flux. Eur. J. Pharm. Sci. 1999, 9, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Gonthier, M.P.; Remesy, C.; Scalbert, A.; Cheynier, V.; Souquet, J.M.; Poutanen, K.; Aura, A.M. Microbial Metabolism of Caffeic Acid and Its Esters Chlorogenic and Cafetaric Acids by Human Fecal Microbiota in Vitro. Biomed. Pharmacother. 2006, 60, 536–540. [Google Scholar] [CrossRef]
- Ludwig, I.A.; Paz de Pena, M.; Concepción, C.; Alan, C. Catabolism of Coffee Chlorogenic Acids by Human Colonic Microbiota. BioFactors 2013, 39, 623–632. [Google Scholar] [CrossRef]
- Garrait, G.; Jarrige, J.F.; Blanquet, S.; Beyssac, E.; Cardot, J.M.; Alric, M. Gastrointestinal Absorption and Urinary Excretion of Trans-Cinnamic and p-Coumaric Acids in Rats. J. Agri. Food Chem. 2006, 54, 2944–2950. [Google Scholar] [CrossRef]
- Lafay, S.; Gil-Izquierdo, A.; Manach, C.; Besson, C.; Scalbert, A. Chlorogenic Acid Is Absorbed in Its Intact Form in the Stomach of Rats. J. Nutr. 2006, 136, 1192–1197. [Google Scholar] [CrossRef] [Green Version]
- Qi, W.; Zhao, T.; Yang, W.W.; Wang, G.H.; Yu, H.; Zhao, H.X.; Yang, C.; Sun, L.X. Comparative Pharmacokinetics of Chlorogenic Acids after Oral Administration in Rats. J. Pharm. Anal. 2011, 1, 270–274. [Google Scholar] [CrossRef] [Green Version]
- Jung, J.W.; Kim, J.M.; Jeong, J.S.; Son, M.; Lee, H.S.; Lee, M.G.; Kang, H.E. Pharmacokinetics of Chlorogenic Acid und Corydaline in DA.9701, a new Botanical Gastroprokinetic Agent, in Rats. Xenobiotica 2014, 44, 635–643. [Google Scholar] [CrossRef]
- Kurlbaum, M.; Högger, P. Plasma Protein Bindung of Polyphenols from Maritime Pine Bark Extract (USP). J. Pharma. Biomed. Anal. 2011, 54, 127–132. [Google Scholar] [CrossRef]
- De Oliveira, D.M.; Samaio, G.R.; Pinto, C.B.; Catharino, R.R.; Bastos, D.H.M. Bioavailability of Chlorogenic Acids in Rats after acute Ingestion of Maté Tea (Ilex paraguariensis) or 5-Caffeoylquinic Acid. Eur. J. Nutr. 2017, 56, 2541–2556. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, C.S.; Chen, Q.Z.; Wang, S.; Xiong, Y.A.; Jing, J.; Lv, J.J. Characterization, Pharmacokinetics and Tissue Distribution of Chlorogenic Acid-Loaded Self-Microemulsifying Drug Delivery System. Eur. J. Pharm. Sci. 2017, 100, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Kishida, K.; Masumoto, H. Urinary Excretion Rate and Bioavailability of Chlorogenic Acid, Caffeic Acid, p-Coumaric Acid, and Ferulic Acid in Non-Fasted Rats Maintained under Physiological Conditions. Heliyon 2019, 5, e02708. [Google Scholar] [CrossRef] [Green Version]
- Omar, M.H.; Mulle, W.; Stalmach, A.; Auger, C.; Rounat, J.M.; Teissedre, P.L.; Caldwell, S.T.; Hartley, R.C.; Crozier, A. Absorption, Disposition, Metabolism, and Excretion of [3-14C]Caffeic Acid in Rats. J. Agri. Food Chem. 2012, 60, 5205–5214. [Google Scholar] [CrossRef]
- Choudhury, R.; Srai, S.K.; Debnam, E.; Rice-Evans, C.A. Urinary Excretion of Hydroxycinnamates and Flavonoids after Oral and Intravenous Administration. Free Rad. Biol. Med. 1999, 27, 278–286. [Google Scholar] [CrossRef]
- Camarasa, J.; Escubedo, E.; Adzet, T. Pharmacokinetics of Caffeic Acid in Rats by a High-Performance Liquid Chromatography Method. J. Pharm. Biomed. Anal. 1988, 6, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Hao, H.P.; Wang, G.J.; Cui, N.; Zheng, C.N.; Wang, Y.X. Simultaneous Quantification of Dicaffeoylquinic Acids in Rat Plasma After an Intranvenous Administration of Mailuoning Injection Using Liquid Chromtography-Mass Spectrometry. J. Chromat. Sci. 2009, 47, 216–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Wang, G.J.; Hao, H.P.; Cui, N.; Sun, X.R. Pharmacokinetics and excretion of phenolic acids from mailuoning injection in rats. Chin. J. Clin. Pharmacol. Ther. 2008, 13, 776–781. [Google Scholar]
- Mehta, P.; Dhapte, V. A Comprehensive Review on Pharmacokinetic Profile of Some Traditional Chinese Medicines. New, J. Sci. 2016, 2016, 7830367. [Google Scholar] [CrossRef] [Green Version]
- Su, D.; Huang, J.; Song, Y.; Feng, Y. Comparative pharmacokinetics and tissue distribution study of mono-, and dicaffeoylquinic acids isomers of Ainsliaea fragrans Champ by a fast UHPLC-MS/MS method. Fitoterapia 2014, 99, 139–152. [Google Scholar] [CrossRef]
- Schafer, E.W.; Bowles, W.A.; Hurlbut, J. The Acute Oral Toxicity, Repellency, and Hazard Potential of 998 Chemicals to One or More Species of Wild and Domestic Birds. Arch. Environ. Contam. Toxicol. 1983, 12, 355–382. [Google Scholar] [CrossRef] [PubMed]
- Venkatakirshna, K.; Sudeep, H.V.; Shyamprasad, K. Acute and sub-chronic toxicity of a standardized green coffee bean extract (CQA-7TM) in Wistar albino rats. SAGE Open Med. 2021, 9, 2050312120984885. [Google Scholar] [CrossRef]
- Costa Silva Faria, W.; Almeida da Silva, A.; Veggi, N.; Honda Kawashita, N.; de Franca Lemes, S.A.; Miguel de Barros, W.; da Conceicao Cardoso, E.; Converti, A.; de Melo Moura, W.; Bragagnolo, N. Acute and subacute oral toxicity assessment of dry encapsulated and non-encapsulated green coffee fruit extracts. J. Food Drug Anal. 2020, 28, 143–161. [Google Scholar] [CrossRef]
- Kato, M.; Ochiai, R.; Kozuma, K.; Sato, H.; Katsuragi, Y. Effect of Chlorogenic Acid Intake on Cognitive Function in the Elderly: A Pilot Study. Evid. Based Complement. Alternat. Med. 2018, 2018, 8608497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitts, D.D.; Wijewickreme, A.N. Effect of dietary caffeic and chlorogenic acids on in vivo xenobiotic systems. Plant Foods Hum. Nutr. 1994, 45, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Eklund, A. Effect of chlorogenic acid in a casein diet for rats. Nutritional and pathological observations. Nutr. Metab. 1975, 18, 258–264. [Google Scholar] [CrossRef]
- Hirose, M.; Masuda, A.; Imaida, K.; Kagawa, M.; Tsuda, H.; Ito, N. Induction of forestomach lesions in rats by oral administration of naturally occurring antioxidants for 4 weeks. Jpn. J. Cancer Res. 1987, 78, 317–321. [Google Scholar] [CrossRef]
- Chaube, S.; Swinyard, C.A. Teratological and toxicological studies of alkaloidal and phenolic compounds from Solanum tuberosum L. Toxicol. Appl. Pharmacol. 1976, 36, 227–237. [Google Scholar] [CrossRef]
- Thom, E. The effect of chlorogenic acid enriched coffee on glucose absorption in healthy volunteers and its effect on body mass when used long-term in overweight and obese people. J. Int. Med. Res. 2007, 35, 900–908. [Google Scholar] [CrossRef]
- Dellalibera, O.; Lemaire, B.; Lafay, S. Svetol®, green coffee extract, induces weight loss and increases lean to fat ratio in volunteers with overweight problems. Phytotherapie 2006, 4, 194–197. [Google Scholar] [CrossRef]
- Derelanko, M.J.; Hollinger, M.A. Toxicity Classification in Handbook of Toxicology, 2nd ed.; Taylor and Francis: New York, NY, USA, 2002. [Google Scholar]
- Seo, H.S.; Lee, Y.J.; Kim, H.J.; Jin, C.B. Composition for Improving Skin Barrier Damage and/or Alleviating Skin Inflammation, Containing 3,5-Dicaffeoylquinic Acid as Active Ingredient. US Patent Application US 2021/0393722 A1, 23 December 2021. [Google Scholar]
- Tice, R. Chlorogenic Acid [327-97-9] and Caffeic Acid [331-39-5]: Review of Toxicological Literature, Prepared for National Institute of Environmental Health Sciences, Research Triangle Park, NC, USA. 1998. Available online: https://ntp.niehs.nih.gov/sites/default/files/ntp/htdocs/chem_background/exsumpdf/chlorogenicacid_508.pdf (accessed on 24 April 2023).
- Yamada, K.; Shirahata, S.; Mirakami, H.; Nishiyama, K.; Shinohara, K.; Omura, H. DNA breakage by phenyl compounds. Agric. Biol. Chem. 1985, 49, 1423–1428. [Google Scholar] [CrossRef] [Green Version]
- Yoshie, Y.; Ohshima, H. Nitric Oxide synergistically enhances DNA strand breakage induced by polyhoydroxyaromatic compounds, but inhibits that induced by the Fenton reaction. Arch. Biochem. Biophys. 1997, 342, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Trush, M.A. Reactive oxygen-dependent DNA damage resulting from the oxidation of phenolic compounds by a copper-redox cycle mechanism. Cancer Res. 1994, 7, 1895s–1898s. [Google Scholar]
- MacGregor, J.T.; Jurd, L. Mutagenicity of plant flavonoids: Structural requirements for mutagenic activity in Salmonella typhumurium. Mutat. Res. 1978, 54, 297–309. [Google Scholar] [CrossRef]
- San, R.H.C.; Chan, R.I.M. Inhibitory effect of phenolic compounds on aflatoxin B1 metabolism and induced mutagenesis. Mutat. Res. 1987, 177, 229–239. [Google Scholar] [CrossRef]
- Stich, H.F.; Rosin, M.P.; Wu, C.H.; Powrie, W.D. A comparative genotoxicity study of chlorogenic acid (3-O-caffeoylquinic acid). Mutat. Res. 1981, 90, 201–212. [Google Scholar] [CrossRef]
- Fung, V.A.; Cameron, T.P.; Hughes, T.J.; Kirby, P.E.; Dunkel, V.C. Mutagenic activity of some coffee flavor ingredients. Mutat. Res. 1988, 204, 219–228. [Google Scholar] [CrossRef]
- Ariza, R.R.; Dorado, G.; Barbancho, M.; Pueyo, C. Study of the causes of direct-acting mutagenicity in coffee and tea using the Ara test in Salmonella typhimurium. Mutat. Res. 1988, 201, 89–96. [Google Scholar] [CrossRef]
- Rosin, M.P. The influence of pH on the convertogenic activity of plant phenolics. Mutat. Res. 1984, 135, 109–113. [Google Scholar] [CrossRef]
- Wood, A.W.; Huang, M.-T.; Chang, R.L.; Newmark, H.L.; Lehr, R.E.; Yagi, H.; Sayer, D.; Jerina, M.; Conney, A.H. Inhibition of the mutagenicity of bay-region diol epoxides of polycyclic aromatic hydrocarbons by naturally occurring plant phenols: Exceptional activity of ellagic acid. Proc. Natl. Acad. Sci. USA 1982, 79, 5513–5517. [Google Scholar] [CrossRef]
- Damiani de Mendonca Pereira, E.; da Silva, J.; da Silva Carvalho, P.; Grivicich, I.; Nascimento Picada, J.; Batista Salgado Junior, I.; Jouglard Vesques, G.; da Silva Pereira, M.A.; Reginatto, F.H.; de Barros Falcao Ferraz, A. In vivo and in vitro toxicological evaluations of aqueous extract from Cecropia pachystachya leaves. J. Toxicol. Environ. Health Part A 2020, 83, 659–671. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, F.W.; San, R.H.C.; Stich, H.F. An intestinal cell-mediated chromosome aberration test for the detection of genotoxic agents. Mutat. Res. 1983, 111, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Hernandes, L.C.; Machado, A.R.T.; Tuttis, K.; Ribeiro, D.L.; Aissa, A.F.; Devoz, P.P.; Antunes, L.M.G. Caffeic acid and chlorogenic acid cytotoxicity, genotoxicity and impact on global DNA methylation in human leukemic cell lines. Genet. Mol. Biol. 2020, 43, e2090347. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.M.; Huisman, J.W.; Diehl, J.F. Mutagenicity studies on irradiated potatoes and chlorogenic acid; micronucleus test in rats. Toxicology 1976, 6, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Alarcón-Herrea, N.; Flores-Maya, S.; Bellido, B.; Garcia-Bores, A.M.; Mendoza, E.; Ávila-Acevedo, G.; Hernández-Echeagaray, E. Protective effects of chlorogenic acid in 3-nitropropionic acid induced toxicity and genotoxicity. Food Chem. Toxicol. 2017, 109, 1018–1025. [Google Scholar] [CrossRef]
- Banerjee, P.; Eckert, A.O.; Schrey, A.K.; Preissner, R. ProTox-II: Prediction of Toxicity of Chemicals. Available online: https://tox-new.charite.de/protox_II/index.php?site=home (accessed on 26 April 2023).
- Fukuhara, Y.; Yoshida, D.; Goto, F. Reduction of mutagenic products in the presence of polyphenols during pyrolysis of protein. Agric. Biol. Chem. 1981, 45, 1061–1066. [Google Scholar] [CrossRef]
- Yamada, J.; Tomita, Y. Antimutagenic activity of caffeic acid and related compounds. Biosci. Biotechnol. Biochem. 1996, 60, 328–329. [Google Scholar] [CrossRef]
- Xu, J.-G.; Hu, Q.-P.; Liu, Y. Antioxidant and DNA-Protective Activities of Chlorogenic Acid Isomers. J. Agric. Food Chem. 2012, 60, 11625–11630. [Google Scholar] [CrossRef]
- Mori, H.; Tanaka, T.; Shima, H.; Kuniyasu, T.; Takahashi, M. Inhibitory effect of chlorogenic acid on methylazoxymethanol acetate-induced carcinogenesis in large intestine and liver of hamsters. Cancer Lett. 1986, 30, 49–54. [Google Scholar] [CrossRef]
- Luo, J.; Wang, K.; Li, G.S.; Lei, D.Q.; Huang, Y.J.; Li, W.D.; Chen, Y.Q. 3,5-Dicaffeoylquinic Acid Disperses Aspergillus Fumigatus Biofilm and Enhances Fungicidal Efficacy of Voriconazole and Amphotericin B. Med. Sci. Monit. 2018, 24, 427–437. [Google Scholar] [CrossRef] [Green Version]
- Mentese, A.; Demir, S.; Alemdar, N.T.; Aliyazicioglu, Y.; Deger, O. The Effect of Chlorogenic Acid on 5-Fluorouracil-Induced Oxidative Damage in Rat Ovarian Tissue. Farabi Med. J. 2022, 1, 1–7. [Google Scholar]
- El-Khadragy, M.F.; Al-Megrin, W.; Alomar, S.; Alkhuiji, A.F.; Metwally, D.M.; Mahgoub, S.; Amin, H.K.; Habotta, O.A.; Abdel Moneim, A.E.; Albeltagy, R.S. Chlorogenic acid abates male reproductive dysfunction in arsenic-exposed mice via attenuation of testicular oxido-inflammatory stress and apoptotic responses. Chem. Biol. Interact. 2021, 333, 109333. [Google Scholar] [CrossRef]
- Yi, Y.Y.; Wan, S.X.; Hou, Y.X.; Cheng, J.; Guo, J.H.; Wang, S.; Khan, A.; Sun, N.; Li, H. Chlorogenic acid rescues zearalenone induced injury to mouse ovarian granulosa cells. Ecotoxicol. Environ. Saf. 2020, 194, 110401. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Chiu, C.W.; Pamukcu, A.M.; Bryan, G.T. Identification of carcinogenic tannin isolated from bracken fern (Pteridium aquilinum). J. Natl. Cancer Inst. 1976, 56, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Bartolomeu, A.R.; Romualdo, G.R.; Lisόn, C.G.; Besharat, Z.M.; Corrales, J.A.M.; Chaves, M.Á.G.; Barbisan, L.F. Caffeine and Chlorogenic Acid Combination Attenuate Early-Stage Chemically Induced Colon Carcinogenesis in Mice: Involvement of oncomiR miR-21a-5p. Int. J. Mol. Sci. 2022, 23, 6292. [Google Scholar] [CrossRef]
- Mikami, Y.; Yamazawa, T. Chlorogenic acid, a polyphenol in coffee, protects neurons against glutamate neurotoxicity. Life Sci. 2015, 139, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xi, Y.; Zeng, X.; Zhao, H.; Cao, J.; Jiang, W. Effects of chlorogenic acid against aluminium neurotoxicity in ICR mice through chelation and antioxidant actions. J. Funct. Food 2018, 40, 365–376. [Google Scholar] [CrossRef]
- Skalny, A.V.; Aschner, M.; Jiang, Y.; Gluhcheva, Y.G.; Tizabi, Y.; Lobinski, R.; Tinkov, A.A. Molecular mechanism of aluminium neurotoxicity: Update on adverse effects and therapeutic strategies. Adv. Neurotoxicol. 2021, 5, 1–34. [Google Scholar] [CrossRef]
- Nirmal, M.; Om Praba, G.; Verlmurugan, D. Modeling studies on phospholipase A2-inhibitor complexes. Indian J. Biochem. Biophys. 2008, 45, 256–262. [Google Scholar]
- Tribuiani, N.; da Silva, A.M.; Ferraz, M.C.; Silva, M.G.; Bentes, A.P.; Graziano, T.S.; dos Santos, M.G.; Cogo, J.C.; Varanda, E.A.; Groppo, F.C.; et al. Vellozia flavicans Mart. ex Schult. hydroalcoholic extract inhibits the neuromuscular blockade induced by Bothrops jararacussu venom. BMC Complement. Altern. Med. 2014, 14, 48. [Google Scholar] [CrossRef] [Green Version]
- Yao, J.; Peng, S.; Fang, J. Reversing ROS-mediated neurotoxicity by chlorogenic acid involves its direct antioxidant activity and activation of Nrf2-ARE signaling pathway. BioFactors 2019, 45, 616–626. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, M.Y.D.; Dobrachinski, F.; Silva, H.B.; Lopes, J.P.; Goncalves, F.Q.; Soares, F.A.A.; Porciúncula, L.O.; Andrade, G.M.; Cunha, R.A.; Tomé, A.R. Neuromodulation and neuroprotective effects of chlorogenic acids in excitatory synapses of mouse hippocampal slices. Sci. Rep. 2021, 11, 10488. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Wang, H.; Zhang, Y.; Zhang, Z. Protective Effects of Chlorogenic Acid on Cerebral Ischemia/Reperfusion Injury Rats by Regulating Oxidative Stress-Related Nrf2 Pathway. Drug Des. Devel. Ther. 2020, 14, 51–60. [Google Scholar] [CrossRef] [Green Version]
- Matsuda, R.; Sakagami, H.; Amano, S.; Iijima, Y.; Sano, M.; Uesawa, Y.; Tanura, N.; Oishi, Y.; Takeshima, H. Inhibition of Neurotoxicity/Anticancer Activity of Bortezomib by Caffeic Acid and Chlorogenic Acid. Anticancer Res. 2022, 42, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Liberato, J.L.; Rosa, M.N.; Miranda, M.C.R.; Lopes, J.L.C.; Lopes, N.P.; Gobbo-Neto, L.; Fontana, A.C.K.; Dos Santos, W.F. Neuroprotective Properties of Chlorogenic Acid and 4,5-Caffeoylquinic Acid from Brazilian arnica (Lychnophora ericoides) after Acute Retinal Ischemia. Planta Med. 2023, 89, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Li, J. Chlorogenic acid prevents alcohol-induced brain damage in neonatal rat. Transl. Neurosci. 2017, 8, 176–181. [Google Scholar] [CrossRef] [Green Version]
- Xi, Y.; Li, H.; Yu, M.; Li, X.; Li, Y.; Hui, B.; Zeng, X.; Wang, J.; Li, J. Protective effects of chlorogenic acid on trimethyltin chloride-induced neurobehavioral dysfunction in mice relying on the gut microbiota. Food Funct. 2022, 13, 1535–1550. [Google Scholar] [CrossRef]
- Rebai, O.; Amri, M. Chlorogenic Acid prevents AMPA-mediated Excitotoxicity in Optic Nerve Oligodendrocytes through a PKC and Caspase-dependent Pathways. Neurotox. Res. 2018, 34, 559–573. [Google Scholar] [CrossRef]
- Lim, D.W.; Park, J.; Jung, J.; Kim, S.-H.; Um, M.Y.; Yoon, M.; Kim, Y.T.; Han, D.; Lee, C.; Lee, J. Dicaffeoylquinic acids alleviate memory loss via reduction of oxidative stress in stress-hormone-induced depressive mice. Pharmacol. Res. 2020, 161, 105252. [Google Scholar] [CrossRef]
- Kaye, M.; Freedman, S.O. Allergy to raw coffee—An occupational disease. Can. Med. Assoc. J. 1961, 84, 469–471. [Google Scholar]
- Freedman, S.O.; Shulman, R.; Krupey, J.; Sehon, A.H. Antigenic properties of chlorogenic acid. J. Allergy 1964, 35, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Freedman, S.O.; Shulman, R.; Krupey, J. Loss of allergenicity of chlorogenic acid in the gastrointestinal tract. J. Allergy 1964, 35, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Freedman, S.O.; Siddiqi, A.I.; Krupey, J.H. Identification of a simple chemical compound (chlorogenic acid) as an allergen in plant materials causing human atopic disease. Am. J. Med. Sci. 1962, 244, 548–555. [Google Scholar] [PubMed]
- Freedman, S.O.; Krupey, J.; Sehon, A.H. Chlorogenic acid: An allergen in green coffee bean. Nature 1961, 192, 241–243. [Google Scholar] [CrossRef]
- Bariana, D.S.; Krupey, J.; Scarpati, L.M.; Freedman, S.O.; Sehon, A.H. Chlorogenic acid: Further evidence for its antigenic and allergenic activity. Nature 1965, 207, 1155–1157. [Google Scholar] [CrossRef]
- Layton, L.L.; Greene, F.C.; Panzani, R. Allergy to green coffee. J. Allergy 1965, 36, 84–91. [Google Scholar] [CrossRef]
- Layton, L.L.; Panzani, R.; Corse, J.W. Nondiffusible allergenic contaminant isolated from samples of chlorogenic acid causing allergic reactions: Pure chlorogenic acid not an allergen. J. Allergy 1966, 38, 268–275. [Google Scholar] [CrossRef]
- Freedman, S.O.; Sechon, A.H. Allergenic activity of chlorogenic acid. J. Allergy 1965, 36, 493–495. [Google Scholar] [CrossRef]
- De Zotti, R.; Patussi, V.; Fiorito, A.; Larese, F. Sensitization to green coffee bean (GCB) and castor bean (CB) allergens among dock workers. Int. Arch. Occup. Environ. Health 1988, 61, 7–12. [Google Scholar] [CrossRef]
- Liu, Z.; Shi, Y.; Zhou, G. Evaluation of the immunosensitizing potential of chlorogenic acid using a popliteal lymph node assay in BALB/c mice. Food Chem. Toxicol. 2012, 48, 1059–1065. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, X.; Chen, G. Adverse effect and mechanism of chlorogenic acid in clearing heat and detoxication traditional Chinese medicine injections. Chin. J. Mod. Appl. Pharm. 2009, 7, 555–558. [Google Scholar]
- Lin, M.; Gong, W.; Wang, Y.; Sun, L.; Fan, X. Structure-Activity Differences of Chlorogenic Acid and Its Isomers on Sensitization via Intravenous Exposure. Int. J. Toxicol. 2012, 31, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Unterhauser, J.; Ahammer, L.; Rainer, T.; Eidelpes, R.; Führer, S.; Nothegger, B.; Covaciu, C.E.; Cova, V.; Kamenik, A.S.; Leidl, K.R.; et al. Covalent polyphenol modification of a reactive cysteine in the major apple allergen Mal d 1. Food Chem. 2023, 410, 135374. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Li, S.; Xu, H.; Zhang, T.; Lin, X.; Wu, X. Effect of Covalent Interaction with Chlorogenic Acid on the Allergenic Capacity of Ovalbumin. J. Agric. Food Chem. 2018, 66, 9794–9800. [Google Scholar] [CrossRef] [PubMed]
- Karefyllakis, D.; Salakou, S.; Bitter, J.H.; van der Goot, A.J.; Nikiforidis, C.V. Covalent Bonding of Chlorogenic Acid Induces Structural Modifications on Sunflower Proteins. ChemPhysChem 2018, 19, 459. [Google Scholar] [CrossRef]
- Zamani-Garmsiri, F.; Ghasempour, G.; Aliabadi, M.; Hashemnia, S.M.R.; Emamgholipour, S.; Meshkani, R. Combination of metformin and chlorogenic acid attenuates hepatic steatosis and inflammation in high-fat diet fed mice. IUBMB Life 2021, 73, 252–263. [Google Scholar] [CrossRef]
- Namvarjah, F.; Shokri-Afra, H.; Moradi-Sardareh, H.; Khorzoughi, R.B.; Pasalar, P.; Panahi, G.; Meshkani, R. Chlorogenic acid improves anti-lipogenic activity of metformin by positive regulating of AMPK signaling in HepG2 cells. Cell Biochem. Biophys. 2022, 80, 537–545. [Google Scholar] [CrossRef]
- European Commission. Commission Implementing Regulation (EU) 2020/917 of 1 July 2020 authorising the placing on the market of infusion from coffee leaves of Coffea arabica L. and/or Coffea canephora Pierre ex A. Froehner as a traditional foodfrom a third country under Regulation (EU) 2015/2283 of the European Parliament and of the Council and amending Implementing Regulation (EU) 2017/2470. Off. J. Eur. Union 2020, L209, 10–13. [Google Scholar]
- Steger, M.C.; Rigling, M.; Blumenthal, P.; Segatz, V.; Quintanilla-Belucci, A.; Beisel, J.M.; Rieke-Zapp, J.; Schwarz, S.; Lachenmeier, D.W.; Zhang, Y. Coffee Leaf Tea from El Salvador: On-Site Production Considering Influences of Processing on Chemical Composition. Foods 2022, 11, 2553. [Google Scholar] [CrossRef]
- Konstantinidis, N.; Franke, H.; Schwarz, S.; Lachenmeier, D.W. Risk Assessment of Trigonelline in Coffee and Coffee By-Products. Molecules 2023, 28, 3460. [Google Scholar] [CrossRef]
- Max Rubner-Institut. Nationale Verzehrsstudie II: Ergebnisbericht, Teil 1; Max Rubner-Institut: Karlsruhe, Germany, 2008; Available online: https://www.bmel.de/SharedDocs/Downloads/DE/_Ernaehrung/NVS_Ergebnisbericht.pdf?__blob=publicationFile&v=2 (accessed on 6 May 2023).
- Gold, L.S.; Gaylor, D.W.; Slone, T.H. Comparison of cancer risk estimates based on a variety of risk assessment methodologies. Regul. Toxicol. Pharmacol. 2003, 37, 45–53. [Google Scholar] [CrossRef] [PubMed]






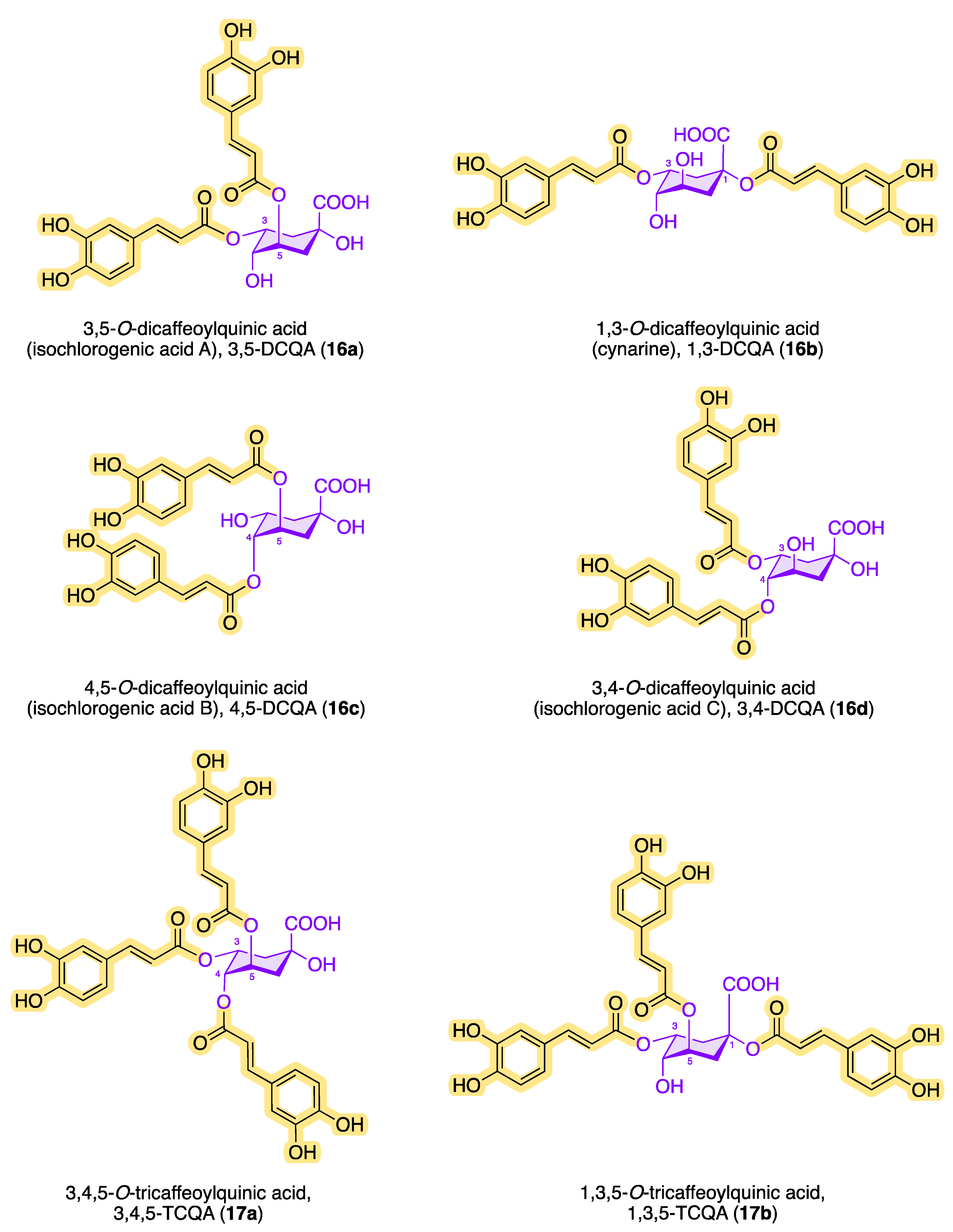

| Coffee By-Product | Chlorogenic Acid Content, Expressed in [g/100 g] (unless Otherwise Stated) * | Ref. | |||||
|---|---|---|---|---|---|---|---|
| 3-CQA (10a) | 4-CQA (12a) | 5-CQA (11a) | 3,4-DCQA (16d) | 3,5-DCQA (16a) | 4,5-DCQA (16c) | ||
| coffee flowers (blossoms) | n.r. | n.r. | 0.13–2.64 | 0.01–0.25 | 0.19–5.84 | n.r. | [118] |
| 0.01–0.07 (in total) | [119] | ||||||
| coffee leaves | 0.04–0.30 | 0.09–0.84 | 1.39–5.58 | 0.03–0.18 | 0.01–1.13 | 0.01–0.12 | [120] |
| 1.1–18.0 mg/L | 4.2–30.0 mg/L | 1.0–190.0 mg/L ** | n.r. | n.r. | n.r. | [121] | |
| coffee pulp | n.r. | n.r. | 0.03–0.13 | n.r. | n.r. | n.r. | [122,123] |
| n.r. | n.r. | 2.3 | n.r. | n.r. | n.r. | [124] | |
| 0.03 | 0.04 | 0.22 | 0.05 | 0.06 | 0.03 | [125] | |
| n.r. | n.r. | 0.13–2.44 | n.r. | n.r. | n.r. | [126] | |
| n.r. | n.r. | 2.40 | n.r. | n.r. | n.r. | [127] | |
| coffee husk | n.r. | n.r. | 13.3 | n.r. | n.r. | n.r. | [124] |
| 0.01 | 0.02 | 0.57 | 0.08 | 0.15 | 0.04 | [125] | |
| n.r. | n.r. | 2.50 | n.r. | n.r. | n.r. | [127] | |
| n.r. | n.r. | 0.04–0.17 | n.r. | n.r. | n.r. | [128] | |
| n.r. | n.r. | 0.17 | n.r. | n.r. | n.r. | [129] | |
| n.r. | n.r. | 0.02–0.17 | n.r. | n.r. | n.r. | [128] | |
| n.r. | n.r. | 69.9 mg/L ** | n.r. | n.r. | n.r. | [130] | |
| silver skin | 1.80–5.16 | 2.38–6.02 | 7.33–8.98 | 0.93–1.04 | 0.55–1.00 | 0.79–1.06 | [131] |
| 0.15 | 0.09 | 0.20 | n.r. | n.r. | n.r. | [132] | |
| n.r. | n.r. | 2.2 | n.r. | n.r. | n.r. | [133] | |
| n.r. | n.r. | 0.94–2.13 | n.r. | n.r. | n.r. | [129] | |
| n.r. | n.r. | 0.39 | n.r. | n.r. | n.r. | [134] | |
| n.r. | n.r. | 20–30 mg/L ** | n.r. | n.r. | n.r. | [130] | |
| n.r. | n.r. | 0.02–0.04 | n.r. | n.r. | n.r. | [134] | |
| n.r. | n.r. | 3.00 | n.r. | n.r. | n.r. | [127] | |
| parchment | n.r. | n.r. | 0.61 | n.r. | n.r. | n.r. | [129] |
| n.r. | n.r. | 0.40 | n.r. | n.r. | n.r. | [135] | |
| spent coffee grounds | 1.50 | 1.62 | 1.87 | 0.29 | 1.00 | 1.70 | [131] |
| n.r. | n.r. | 2.30 | n.r. | n.r. | n.r. | [127] | |
| 0.62–1.32 (in total) | 3.31–5.79 (in total) | [136] | |||||
| green unroasted beans | 1.25 | 1.94 | 13.4 | 0.66 | 1.10 | 0.63 | [131] |
| n.r. | n.r. | 3.6–4.4 | n.r. | n.r. | n.r. | [135] | |
| Coffee By-Product | Min (%) | Max (%) | Average (%) |
|---|---|---|---|
| coffee flowers (blossoms) | 0.01 | 2.60 | 1.31 |
| coffee leaves | 1.39 | 5.60 | 3.50 |
| coffee pulp | 0.03 | 2.40 | 1.22 |
| coffee husk | 0.02 | 13.3 | 6.66 |
| cascara | 0.01 | 0.01 | 0.01 |
| silver skin | 0.02 | 8.98 | 4.50 |
| parchment | 0.40 | 0.61 | 0.51 |
| spent coffee grounds | 1.32 | 2.30 | 1.81 |
| green unroasted beans | 3.60 | 13.4 | 8.50 |
| total | 0.76 | 5.47 | 3.11 |
| Parameter | 5-Caffeoylquinic Acid (5-CQA) | 3,5-Dicaffeoylquinic Acid (3,5-DCQA) |
|---|---|---|
| CAS | 327-97-9 | 2450-53-5 |
| chemical formula | C16H18O9 | C25H24O12 |
| molecular weight | 354.31 g/mol | 516.45 g/mol |
| solubility | soluble in hot water (40 mg/mL at 25 °C), ethanol and acetone | soluble in DMSO (slightly) and methanol (slightly) |
| form | solid, white crystals | hygroscopic solid, off-white to pale yellow |
| pKa | 3.33 [141] | 3.54 (predicted) |
| logP | −0.4 | 1.0 |
| MP | 210 °C | 170–172 °C |
| LD50 | 4000 mg/kg (rat, i.p.) | 2154 mg/kg (rat, p.o.) [142] |
| Test System | Strains | Metabolic Activation with S9 Mix | Dose of CQA | Result | Ref. |
|---|---|---|---|---|---|
| isolated λ DNA | - | - | 250 µM (89 µg/mL) | positive | [179] |
| isolated plasmid DNA (pBR322) | - | - | 100 µM (35 µg/mL) | positive (in the presence of NO-releasing compounds) | [180] |
| phage DNA (øX174 RF I) | - | - | n.p. | positive (in the presence of Cu2+) | [181] |
| S. typhimurium assay | TA98 | +/− | 0.17 or 1.7 µmol/plate (58.8 or 588 µg/plate) | negative | [182] |
| S. typhimurium assay | TA98 | + | 1, 3, 6 or 9 mg/mL (3, 9, 20 or 30 mM) | negative | [183] |
| S. typhimurium assay | TA98, TA100 | +/− | 19 or 28 mg/plate (53 or 79 µmol/plate) | positive (in the presence of Mn2+); negative (in the presence and absence of S9 mix and in the presence of Cu2+ only) | [184] |
| S. typhimurium assay | TA98, TA100, TA1535, TA1537, TA1538 | +/− | 0.33–10 mg/plate (0.94–28 µmol/plate) | negative | [185] |
| S. typhimurium assay | BA13 | - | 0.3–28 µmol/plate (0.1–9.9 mg/plate) | weakly positive | [186] |
| S. cerevisiae assay | D7 | +/− | 20, 40 or 80 mg/mL (56, 110 or 230 mM) | positive (w/o S9 mix); negative (w/S9 mix) | [184] |
| S. cerevisiae assay | D7 | - | 1 mg/mL (3 mM) | positive | [187] |
| Chinese hamster V79-6 cells | - | - | 500 nmol/mL (177 µg/mL) | negative | [188] |
| Chinese hamster V79 cells | - | - | 0.07 mg/mL | positive | [189] |
| Mouse lymphoma L5178Y cells | - | +/− | 6.5–10 mg/mL (18.5–28 mM) | positive (w/S9 mix); negative (w/o S9 mix) | [185] |
| CHO cells (chromosomal aberration test) | - | +/− | 10–40 µg/mL (29–110 µM) | positive (w/o S9 mix, but in the presence of Mn2+ and Cu2+); negative (w/S9 mix) | [184] |
| CHO cells (chromosomal aberration test) | - | +/− | 125, 150 or 250 µg/mL (353, 420 or 706 µM) | positive (w/o S9 mix); negative (w/S9 mix) | [190] |
| HL-60 or Jurkat cells | - | n.a. | 1–100 µM | negative | [191] |
| Micronucleus test in bone marrow (rats) | - | n.a. | 150 mg/kg (420 µmol/kg, p.o., 24 h) | negative | [192] |
| Micronucleus test in bone marrow (mice) | - | n.a. | 100 mg/kg (i.p.) | negative | [193] |
| Coffee By-Product | Consumption of Infusions (mL/day) | 5-CQA | 3,5-DCQA | ||
|---|---|---|---|---|---|
| Content Per Serving (g/200 mL) * | TMDI (g/day) | Content Per Serving (g/200 mL) * | TMDI (g/day) | ||
| coffee flowers (blossoms) | 1300 | 0.052 | 0.338 | 0.117 | 0.759 |
| coffee leaves | 1300 | 0.112 | 0.728 | 0.023 | 0.147 |
| coffee pulp | 1300 | 0.048 | 0.312 | 0.001 | 0.008 |
| coffee husk | 1300 | 0.266 | 1.729 | 0.003 | 0.020 |
| Cascara beverage | 1300 | 0.000 | 0.001 | n.d. | n.d. |
| silver skin | 1300 | 0.180 | 1.167 | 0.020 | 0.130 |
| coffee parchment | 1300 | 0.012 | 0.079 | n.d. | n.d. |
| spent coffee grounds | 1300 | 0.046 | 0.299 | 0.020 | 0.130 |
| green unroasted beans | 1300 | 0.268 | 1.742 | 0.022 | 0.143 |
| total average | 0.109 | 0.711 | 0.029 | 0.191 | |
| Coffee By-Product | 5-CQA | 3,5-DCQA |
|---|---|---|
| Beverage Volume (in L/day) for Reaching Oral BMDL10 of 196 mg/kg bw | Beverage Volume (in L/day) for Reaching Oral BMDL10 of 211 mg/kg bw | |
| coffee flowers (blossoms) | 53 | 25 |
| coffee leaves | 24 | 131 |
| coffee pulp | 57 | 2467 |
| coffee husk | 10 | 987 |
| Cascara beverage | 13,700 | n.d. |
| silver skin | 15 | 148 |
| coffee parchment | 225 | n.d. |
| spent coffee grounds | 60 | 148 |
| green unroasted beans | 10 | 135 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Behne, S.; Franke, H.; Schwarz, S.; Lachenmeier, D.W. Risk Assessment of Chlorogenic and Isochlorogenic Acids in Coffee By-Products. Molecules 2023, 28, 5540. https://doi.org/10.3390/molecules28145540
Behne S, Franke H, Schwarz S, Lachenmeier DW. Risk Assessment of Chlorogenic and Isochlorogenic Acids in Coffee By-Products. Molecules. 2023; 28(14):5540. https://doi.org/10.3390/molecules28145540
Chicago/Turabian StyleBehne, Sascha, Heike Franke, Steffen Schwarz, and Dirk W. Lachenmeier. 2023. "Risk Assessment of Chlorogenic and Isochlorogenic Acids in Coffee By-Products" Molecules 28, no. 14: 5540. https://doi.org/10.3390/molecules28145540
APA StyleBehne, S., Franke, H., Schwarz, S., & Lachenmeier, D. W. (2023). Risk Assessment of Chlorogenic and Isochlorogenic Acids in Coffee By-Products. Molecules, 28(14), 5540. https://doi.org/10.3390/molecules28145540