Agricultural Solid Wastes Based Adsorbent Materials in the Remediation of Heavy Metal Ions from Water and Wastewater by Adsorption: A Review
Abstract
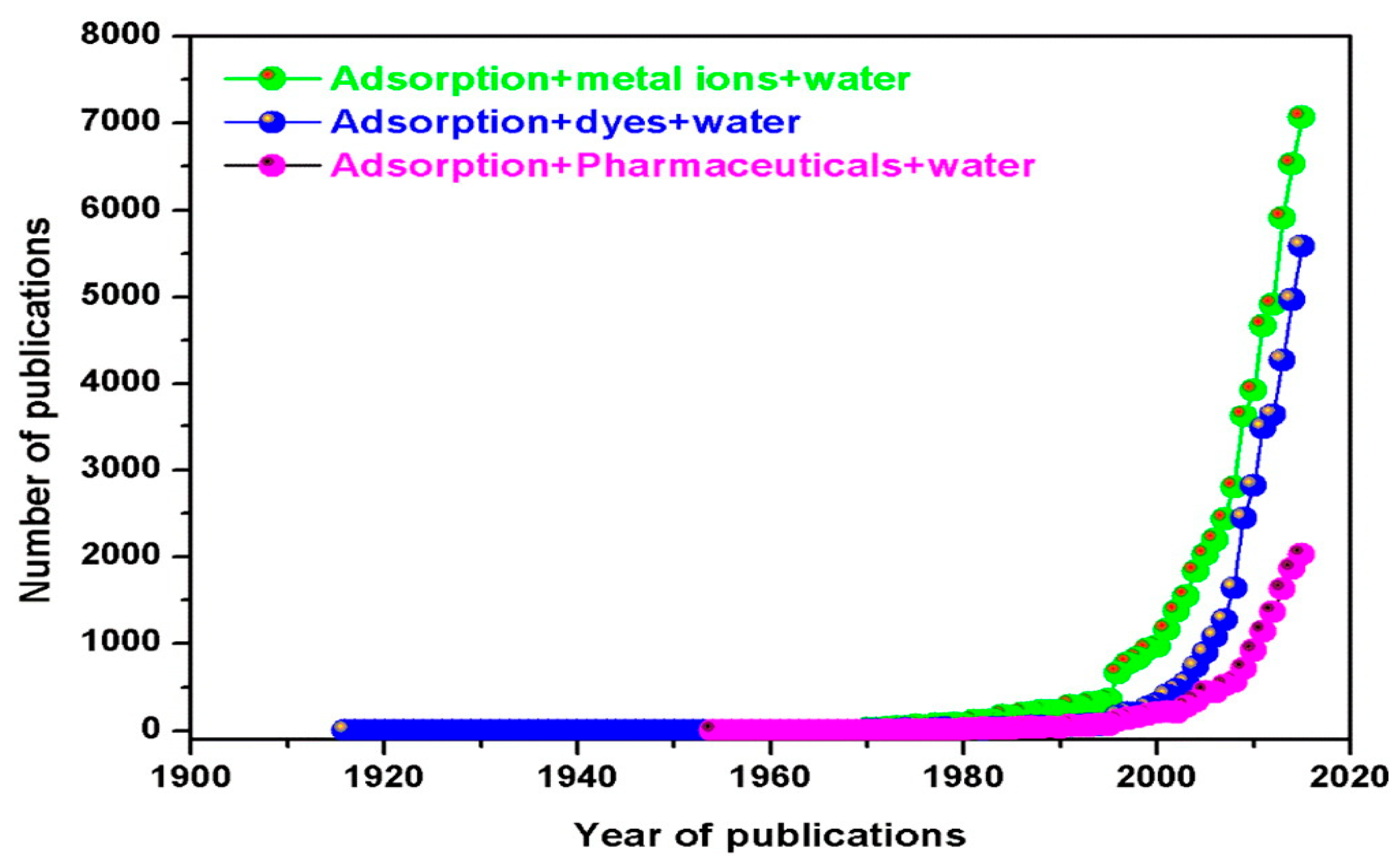
:1. Introduction
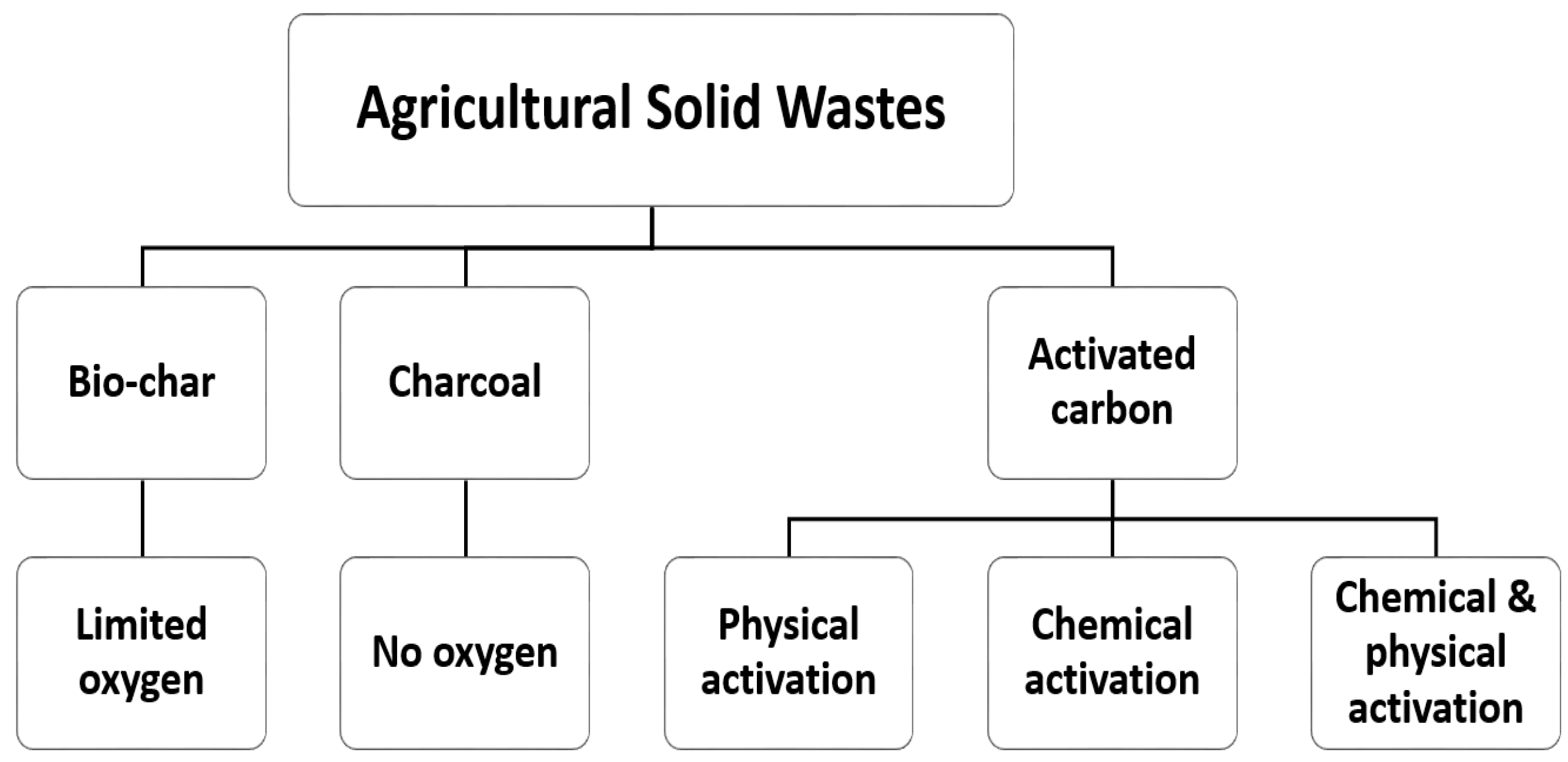
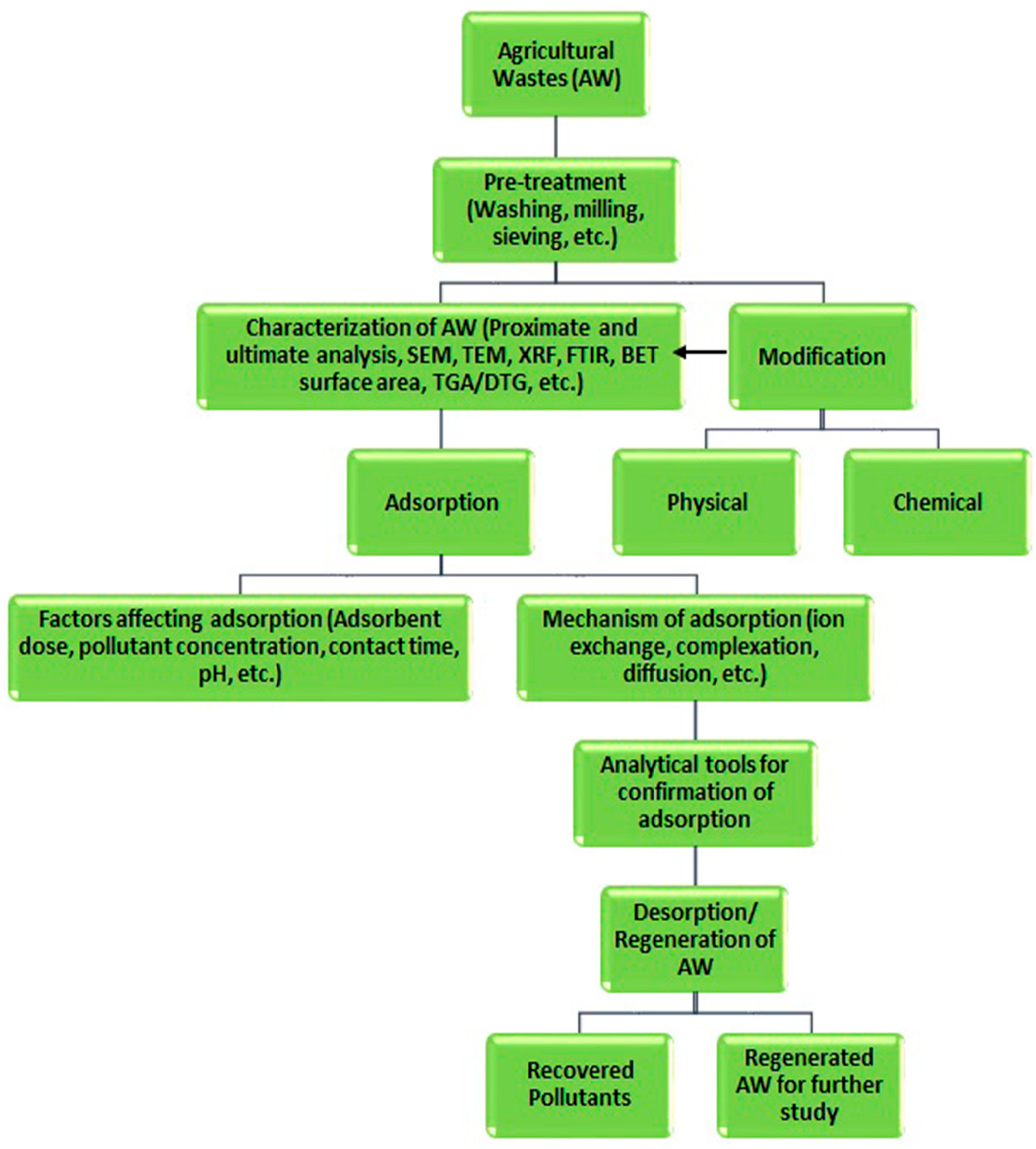
2. Characteristics of the Role of Adsorbents and Agricultural Waste-Based Adsorbents in Heavy Metal Adsorption
3. Batch Metal Ion Adsorption by Agricultural Solid Waste Biomass Adsorbents under Various Physicochemical Process Parameters
3.1. The Effects of the Initial Metal Ion Concentration and the Contact Time
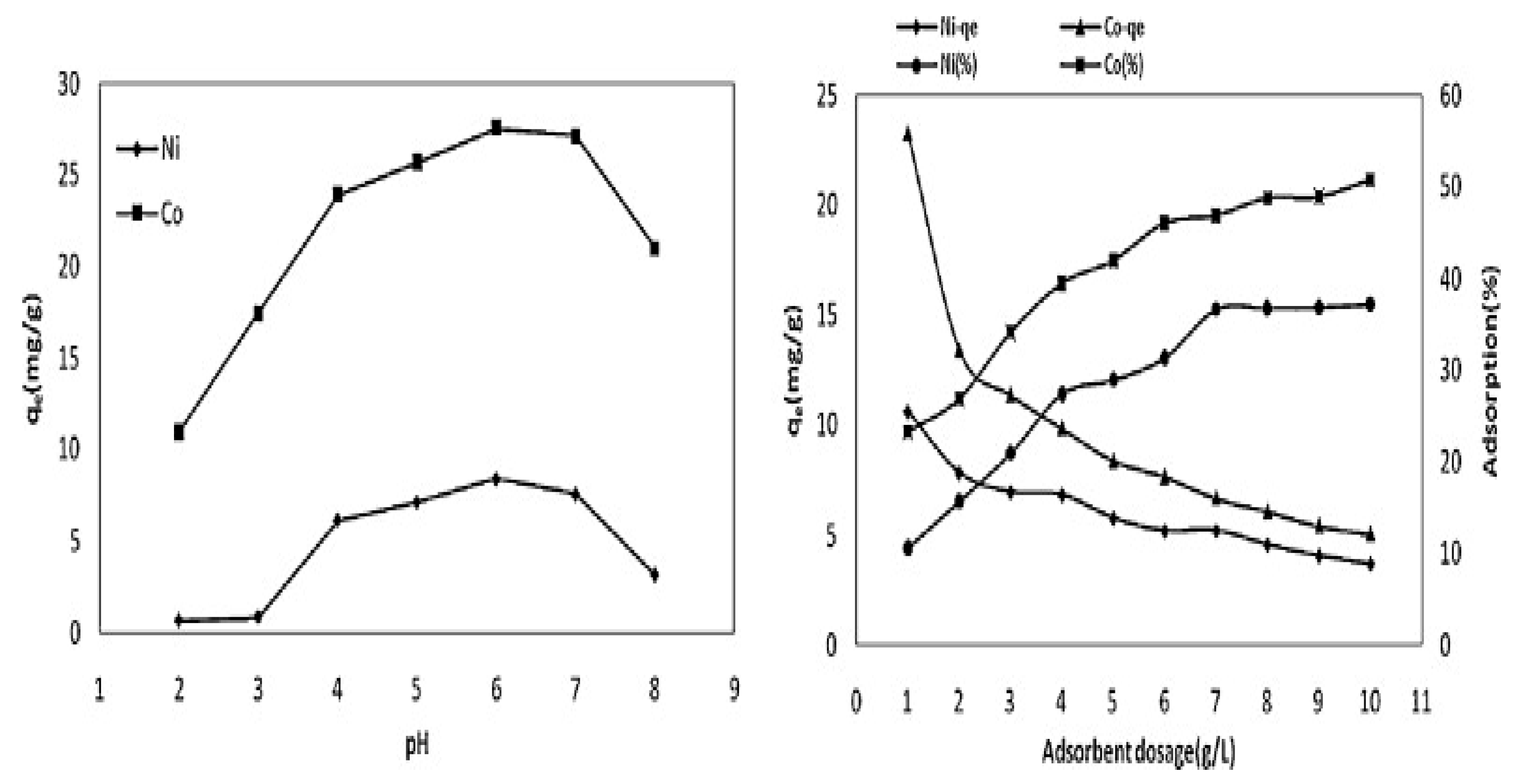
3.2. Effects of the Adsorbent Dose
3.3. Influential Effect of the Solution pH
3.4. Effects of the Temperature and Thermodynamics of Adsorption
4. Future Perspectives and Future Challenges
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- UNWWDR. United Nations World Water Development Report 2020. Available online: https://www.unwater.org/publications/un-world-water-development-report-2020 (accessed on 22 November 2022).
- Tee, G.T.; Gok, X.Y.; Yong, W.F. Adsorption of pollutants in wastewater via biosorbents, nanoparticles and magnetic biosorbents: A review. Environ. Res. 2022, 212, 113248. [Google Scholar] [CrossRef] [PubMed]
- Afroze, S.; Sen, T.K. A Review on Heavy Metal Ions and Dye Adsorption from Water by Agricultural Solid Waste Adsorbents. Water Air Soil Pollut. 2018, 229, 225. [Google Scholar] [CrossRef]
- Dawood, S.; Sen, T.K.; Phan, C. Synthesis and characterization of slow pyrolysis pinecone biochar in the removal of organic and inorganic pollutants from aqueous solution by adsorption: Kinetic, equilibrium, mechanism and thermodynamic. Bioresour. Technol. 2017, 246, 76–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gisi, S.D.; Lofrano, G.; Grassi, M.; Notarnicola, M. Characteristics and adsorption capacities of low-cost sorbents for wastewater treatment: A review. Sustain. Mater. Technol. 2016, 9, 10–40. [Google Scholar]
- Liu, C.; Zhang, H.X. Modified biochar adsorbents (MBAs) for heavy metal ions adsorption: A critical review. J. Environ. Chem. Eng. 2022, 10, 107393. [Google Scholar] [CrossRef]
- Dawood, S. Synthesis and Characterization of Biomass and Clay Minerals-Based Adsorbents for the Removal of Cationic Dye and Metal Ion from Wastewater by Adsorption. Ph.D. Thesis, Curtin University Library, Bentley, WA, Australia, 2018. [Google Scholar]
- Chatterjee, S.; Bhattacharjee, I.; Chandra, G. Biosorption of heavy metals from industrial wastewater by Geobacillus thermodenitrificans. J. Hazard. Mater. 2010, 175, 117–125. [Google Scholar] [CrossRef]
- Rafatullah, M.; Sulaiman, O.; Hashim, R.; Ahmad, A. Adsorption of copper (II), chromium (III), nickel (II) and lead (II) ions from aqueous solutions by meranti sawdust. J. Hazard. Mater. 2009, 170, 969–977. [Google Scholar] [CrossRef]
- Duruibe, J.; Ogwuegbu, M.; Egwurugwu, J. Heavy metal pollution and human biotoxic effects. Int. J. Phys. Sci. 2007, 2, 112–118. [Google Scholar]
- Garg, R.; Garg, R.; Sillanpää, M.; Alimuddin Khan, M.A.; Mubarak, N.M.; Tan, Y.H. Rapid adsorptive removal of chromium from wastewater using walnut-derived biosorbents. Sci. Rep. 2023, 13, 6859. [Google Scholar] [CrossRef]
- Rajendran, S.; Priya, A.K.; Kumar, P.S.; Hoang, T.K.; Sekar, K.; Chong, K.Y.; Khoo, K.S.; Ng, H.S.; Show, P.L. A critical and recent developments on adsorption technique for removal of heavy metals from wastewater—A review. Chemosphere 2022, 303, 135146. [Google Scholar] [CrossRef]
- Jiang, Q.; Han, Z.; Li, W.; Ji, T.; Yuan, Y.; Zhang, J.; Zhao, C.; Cheng, Z.; Wang, S. Adsorption properties of heavy metals and antibiotics by chitosan from larvae and adulty trypoxylus dichotonus. Carbohydr. Polym. 2021, 276, 118735. [Google Scholar] [CrossRef] [PubMed]
- Sen, T.K. Air, Gas and Water Pollution Control Using Industrial and Agricultural Solid Wastes Adsorbents, 1st ed.; CRC-Press/Taylor & Francis: New York, NY, USA, 2017; ISBN 13:978-1-138-19673-5. [Google Scholar]
- Qu, J.; Song, T.; Liang, J.; Bai, X.; Li, Y.; Wei, Y.; Huang, S.; Dong, L.; Jin, Y. Adsorption of lead (Ⅱ) from aqueous solution by modified Auricularia matrix waste: A fixed-bed column study. Ecotoxicol. Environ. Saf. 2019, 169, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Feng, N.; Guo, X.; Liang, S.; Zhu, Y.; Liu, J. Biosorption of heavy metals from aqueous solutions by chemically modified orange peel. J. Hazard. Mater. 2011, 185, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Rana, K.; Shah, M.; Limbachiya, N. Adsorption of Copper Cu (2+) Metal Ion From Wastewater Using Sulphuric Acid Treated Sugarcane Bagasse as Adsorbent. Int. J. Adv. Eng. Res. Sci. 2014, 1, 55–59. [Google Scholar]
- Al-Sahari, M.; Al-Gheethi, A.; Mohamed, R.M.S.R.; Noman, E.; Naushad, M.; Rizuan, M.B.; Vo, D.-V.N.; Ismail, N. Green approach and strategies for wastewater treatment using bioelectrochemical systems: A critical review of fundamental concepts, applications, mechanism, and future trends. Chemosphere 2021, 285, 131373. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, S.M.; Natasha; Mosa, A.; El-Naggar, A.; Hossain, F.; Abdelrahman, H.; Niazi, N.K.; Shahid, M.; Zhang, T.; Tsang, Y.F.; et al. Manganese oxide modified biochar: Production, characterization and application for the removal of pollutants from aqueous environments: A review. Bioresour. Technol. 2022, 346, 126581. [Google Scholar] [CrossRef]
- Chen, X.; Hossain, F.; Duan, C.; Lu, J.; Tsang, Y.F.; Islam, S.; Zhou, Y. Isotherm models for adsorption of heavy metals from water—A review. Chemosphere 2022, 307, 135545. [Google Scholar] [CrossRef]
- Hossain, N.; Bhuiyan, M.A.; Pramanik, B.K.; Nizamuddin, S.; Griffin, G. Waste Materials for Wastewater Treatment and Waste Adsorbents for Biofuel and Cement Supplement Applications: A Critical Review. J. Clean. Prod. 2020, 255, 120261. [Google Scholar] [CrossRef]
- Naef, A.; Qasem, A.; Ramy, H.M.; Dahiru, U.L. Removal of heavy metal ions from wastewater: A comprehensive and critical review. Clean Water 2021, 4, 36. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhao, X.; Cao, J. Advanced nanomaterials for degrading persistent organic pollutants. In Advanced Nanomaterials for Pollutant Sensing and Environmental Catalysis; Elsevier: Amsterdam, The Netherlands, 2020; pp. 249–305. [Google Scholar] [CrossRef]
- Khan, S.H.; Pathak, B. Zinc oxide based photocatalytic degradation of persistent pesticides: A comprehensive review. Environ. Nanotechnol. Monit. Manag. 2020, 13, 100290. [Google Scholar] [CrossRef]
- Richardson, S.D.; Kimura, S.Y. Emerging environmental contaminants: Challenges facing our next generation and potential engineering solutions. Environ. Technol. Innov. 2017, 8, 40–56. [Google Scholar] [CrossRef]
- Divyapriya, G.; Singh, S.; Martínez-Huitle, C.A.; Scaria, J.; Karim, A.V.; Nidheesh, P.V. Treatment of real wastewater by photoelectrochemical methods: An overview. Chemosphere 2021, 276, 130188. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Meikap, B.C.; Sen, T.K. Adsorptive Removal of Aqueous Phase Copper (Cu2+) and Nickel (Ni2+) Metal Ions by Synthesized Biochar–Biopolymeric Hybrid Adsorbents and Process Optimization by Response Surface Methodology (RSM). Water Air Soil Pollut. 2019, 230, 197. [Google Scholar] [CrossRef]
- Wu, C.-H.; Kuo, C.-Y.; Guan, S.-S. Adsorption Kinetics of Lead and Zinc Ions by Coffee Residues. Pol. J. Environ. Stud. 2015, 24, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Sud, D.; Mahajan, G.; Kaur, M.P. Agricultural waste material as potential adsorbent for sequestering heavy metal ions from aqueous solutions—A review. Bioresour. Technol. 2008, 99, 6017–6027. [Google Scholar] [CrossRef]
- Tan, K.L.; Hameed, B.H. Insight into the adsorption kinetics models for the removal of contaminants from aqueous solutions. J. Taiwan Inst. Chem. Eng. 2017, 74, 25–48. [Google Scholar] [CrossRef]
- Ogunlalu, O.; Oyekunle, I.P.; Iwuozor, K.O.; Aderibigbe, A.D.; Emenike, E.C. Trends in the mitigration of heavy metal ions from aqueous solutions using unmodified and chemically modified agricultural waste adsorbents. Curr. Res. Green Sustain. Chem. 2021, 4, 100188. [Google Scholar] [CrossRef]
- Ho, Y.S. Review of second-order models for adsorption systems. J. Hazard. Mater. 2006, 136, 681–689. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Liu, Y.J. Biosorption isotherms, kinetics and thermodynamics. Sep. Purif. Technol. 2008, 61, 229–242. [Google Scholar] [CrossRef]
- Plazinski, W.; Rudzinski, W. A novel two-resistance model for description of the adsorption kinetics onto porous particles. Langmuir 2010, 26, 802–808. [Google Scholar] [CrossRef]
- Alberti, G.; Amendola, V.; Pesavento, M.; Biesuz, R. Beyond the synthesis of novel solid phases: Review on modelling of sorption phenomena. Coord. Chem. Rev. 2012, 256, 28–45. [Google Scholar] [CrossRef]
- Freundlich, H. Over the adsorption in solution. J. Phys. Chem. 1906, 57, 385–470. [Google Scholar]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica, platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef] [Green Version]
- Gopinath, K.P.; Vo, D.-V.; Prakash, D.G.; Joseph, A.; Viswanathan, S.; Arun, J. Environmental applications of carbon-based materials: A review. Environ. Chem. Lett. 2021, 19, 557–582. [Google Scholar] [CrossRef]
- Dutta, B.K. Principles of Mass Transfer and Separation Processes, 1st ed.; Prentice Hall of India: New Delhi, India, 2007; Chapter 12; pp. 609–677. [Google Scholar]
- Galamboš, M.; Suchánek, P.; Rosskopfová, O. Sorption of anthropogenic radionuclides on natural and synthetic inorganic sorbents. J. Radioanal. Nucl. Chem. 2012, 293, 613–633. [Google Scholar] [CrossRef]
- Chakraborty, R.; Asthana, A.; Singh, A.K.; Jain, B.; Susan, A.B.H. Adsorption of heavy metal ions by various low-cost adsorbents: A review. Int. J. Environ. Anal. Chem. 2022, 102, 342–379. [Google Scholar] [CrossRef]
- Shaukat, M.I.; Wahi, R.; Ngaini, Z. The application of agricultural wastes for heavy metals adsorption: A meta-analysis of recent studies. Bioresour. Technol. Rep. 2022, 17, 100902. [Google Scholar] [CrossRef]
- Gümüş, D.; Gümüş, F. Modeling heavy metal removal by retention on Laurusnobilis leaves biomass: Linear and nonlinear isotherms and design. Int. J. Phytoremediat. 2020, 22, 755–763. [Google Scholar] [CrossRef]
- Rambabu, K.; Banat, F.; Pham, Q.M.; Ho, S.H.; Ren, N.Q.; Show, P.L. Biological remediation of acid mine drainage: Review of past trends and current outlook. Environ. Sci. Ecotechnol. 2020, 2, 100024. [Google Scholar] [CrossRef]
- Singha, B.; Das, S.K. Adsorptive removal of Cu (II) from aqueous solution and industrial effluent using natural/agricultural wastes. Colloids Surf. B Biointerfaces 2013, 107, 97–106. [Google Scholar] [CrossRef]
- Ahmad, A.; Ghazi, Z.A.; Saeed, M.; Ilyas, M.; Ahmad, R.; Muqsit Khattak, A.; Iqbal, A. A comparative study of the removal of Cr(vi) from synthetic solution using natural biosorbents. New J. Chem. 2017, 41, 10799–10807. [Google Scholar] [CrossRef]
- Taşar, Ş.; Kaya, F.; Özer, A. Biosorption of lead (II) ions from aqueous solution by peanut shells: Equilibrium, thermodynamic and kinetic studies. J. Environ. Chem. Eng. 2014, 2, 1018–1026. [Google Scholar] [CrossRef]
- Li, Q.; Zhai, J.; Zhang, W.; Wang, M.; Zhou, J. Kinetic studies of adsorption of Pb(II), Cr(III) and Cu(II) from aqueous solution by sawdust and modified peanut husk. J. Hazard. Mater. 2007, 141, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Afroze, S.; Sen, T.K.; Ang, H.M. Adsorption removal of zinc (II) from aqueous phase by raw and base modified Eucalyptus sheathiana bark: Kinetics, mechanism and equilibrium study. Process Saf. Environ. Prot. 2016, 102, 336–352. [Google Scholar] [CrossRef]
- Ahmad, T.; Danish, M. A review of avocado waste-derived adsorbents: Characterizations, adsorption characteristics, and surface mechanism. Chemosphere 2022, 296, 134036. [Google Scholar] [CrossRef]
- Anastopoulos, I.; Bhatnagar, A.; Hameed, B.H.; Ok, Y.S.; Omirou, M. A review on waste-derived adsorbents from sugar industry for pollutant removal in water and wastewater. J. Mol. Liq. 2017, 240, 179–188. [Google Scholar] [CrossRef] [Green Version]
- Nahar, K.; Chowdhury, M.A.K.; Chowdhury, M.A.H.; Rahman, A.; Mohiuddin, K.M. Heavy metals in handloom-dyeing effluents and their biosorption by agricultural by products. Environ. Sci. Pollut. Res. 2018, 25, 7954–7967. [Google Scholar] [CrossRef]
- Ngabura, M.; Hussain, S.A.; Ghani, W.A.; Jami, M.S.; Tan, Y.P. Utilization of renewable durian peels for biosorption of zinc from wastewater. J. Environ. Chem. Eng. 2018, 6, 2528–2539. [Google Scholar] [CrossRef]
- Al-Qahtani, K.M. Water purification using different waste fruit cortexes for the removal of heavy metals. J. Taibah Univ. Sci. 2016, 10, 700–708. [Google Scholar] [CrossRef] [Green Version]
- Ben-Ali, S.; Jaouali, I.; Souissi-Najar, S.; Ouederni, A. Characterization and adsorption capacity of raw pomegranate peel biosorbent for copper removal. J. Clean. Prod. 2017, 142, 3809–3821. [Google Scholar] [CrossRef]
- Gupta, H.; Gogate, P.R. Intensified removal of copper from wastewater using activated watermelon based biosorbent in the presence of ultrasound. Ultrason. Sonochem. 2016, 30, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Asim, N.; Amin, M.H.; Samsudin, N.A.; Badiei, M.; Razali, H.; Akhtaruzzaman, M.; Sopian, K. Development of effective and sustainable adsorbent biomaterial from an agricultural waste material: Cu (II) removal. Mater. Chem. Phys. 2020, 249, 123128. [Google Scholar] [CrossRef]
- Zaidi, N.A.H.M.; Lim, L.B.L.; Usman, A. Enhancing adsorption of Pb (II) from aqueous solution by NaOH and EDTA modified Artocarpus odoratissimus leaves. J. Environ. Chem. Eng. 2018, 6, 7172–7184. [Google Scholar] [CrossRef]
- Nakkeeran, E.; Saranya, N.; Giri, M.; Nandagopal, S.; Santhiagu, A.; Selvaraju, N. Hexavalent chromium removal from aqueous solutions by a novel powder prepared from Colocasia esculenta leaves. Int. J. Phytoremediat. 2016, 18, 812–821. [Google Scholar] [CrossRef] [PubMed]
- Sulyman, M.; Namiesnik, J.; Gierak, A. Low-cost Adsorbents Derived from Agricultural By-products/Wastes for Enhancing Contaminant Uptakes from Wastewater: A Review. Pol. J. Environ. Stud. 2017, 26, 479–510. [Google Scholar] [CrossRef]
- Rangel, A.V.; Becerra, M.G.; Guerrero-Amaya, H.; Ballesteros, L.M.; Mercado, D.F. Sulfate radical anion activated agro-industrial residues for Cr (VI) adsorption: Is this activation process technically and economically feasible? J. Clean. Prod. 2021, 289, 125793. [Google Scholar] [CrossRef]
- Ibrahim, R.I. Optimization process for removing of copper ions from groundwater of Iraq. Using watermelon shell as natural adsorbent. IOP Conf. Ser. Mater. Sci. Eng. 2020, 737, 012195. [Google Scholar] [CrossRef]
- Ayob, S.; Othman, N.; Ali, W.; Altowayti, H.; Khalid, F.S.; Bakar, N.A. A Review on Adsorption of Heavy Metals from Wood-Industrial Wastewater by Oil Palm. J. Ecol. Eng. 2021, 22, 249–265. [Google Scholar] [CrossRef]
- Shakya, A.; Agarwal, T. Removal of Cr(VI) from water using pineapple peel derived biochars: Adsorption potential and re-usability assessment. J. Mol. Liq. 2019, 293, 111497. [Google Scholar] [CrossRef]
- Yousef, R.; Qiblawey, H.; EI-Naas, M.H. Adsorption as a Process for Produced Water Treatment: A Review. Process 2020, 8, 1657. [Google Scholar] [CrossRef]
- Gonçalves, A.C., Jr.; da Paz Schiller, A.; Conradi, E., Jr.; Manfrin, J.; Schwantes, D.; Zimmermann, J.; Klassen, G.J.; Campagnolo, M.A. Removal of Pb2+ and Cd2+ from Contaminated Water using Activated Carbon from Canola Seed Wastes. In Proceedings of the 5th World Congress on New Technologies (NewTech’19), Lisbon, Portugal, 18–20 August 2019. [Google Scholar] [CrossRef]
- Naseem, K.; Huma, R.; Shahbaz, A.; Jamal, J.; Rehman, M.Z.U.; Sharif, A.; Ahmed, E.; Begum, R.; Irfan, A.; Al-Sehemi, A.G.; et al. Extraction of Heavy Metals from Aqueous Medium by Husk Biomass: Adsorption Isotherm, Kinetic and Thermodynamic study. Z. Phys. Chem. 2018, 233, 201–223. [Google Scholar] [CrossRef]
- Devani, M.A.; Oubagaranadin, J.U.K.; Munshi, B.; Lal, B.B.; Mandal, S. BP-ANN Approach for Modeling Cd (II) Bio-Sorption from Aqueous Solutions Using Cajanus cajan Husk. Iran. J. Chem. Eng. 2019, 38, 110–124. [Google Scholar]
- Sazali, N.; Harun, Z. A Review on Batch and Column Adsorption of Various Adsorbent Towards the Removal of Heavy Metal. J. Adv. Res. Fluid Mech. Therm. Sci. 2020, 67, 66–88. [Google Scholar]
- Chen, Y.; Wang, H.; Zhao, W.; Huang, S. Four different kinds of peels as adsorbents for the removal of Cd (II) from aqueous solution: Kinetics, isotherm, and mechanism. J. Taiwan Inst. Chem. Eng. 2018, 88, 146–151. [Google Scholar] [CrossRef]
- Mahdi, Z.; Yu, Q.J.; El Hanandeh, A. Competitive adsorption of heavy metal ions (Pb2+, Cu2+, and Ni2+) onto date seed biochar: Batch and fixed bed experiments. Sep. Sci. Technol. 2019, 54, 888–901. [Google Scholar] [CrossRef]
- Mallampati, R.; Xuanjun, L.; Adin, A.; Valiyaveettil, S. Fruit peels as efficient renewable adsorbents for removal of dissolved heavy metals and dyes from water. ACS Sustain. Chem. Eng. 2015, 3, 1117–1124. [Google Scholar] [CrossRef]
- Malik, R.; Dahiya, S. An Experimental and Quantum Chemical Study of Removal of Utmostly Quantified Heavy Metals in Wastewater Using Coconut Husk: A Novel Approach to Mechanism. Int. J. Biol. Macromol. 2017, 98, 139–149. [Google Scholar] [CrossRef]
- Thuan, T.V.; Phuong, B.B.; Nguyen, D. Response surface methodology approach for optimization of Cu2+, Ni2+ and Pb2+ adsorption using KOH-activated carbon from banana peel. Surf. Interfaces 2017, 6, 209–217. [Google Scholar] [CrossRef]
- Chi, T.; Zuo, J.; Liu, F. Performance and mechanism for cadmium and lead adsorption from water and soil by corn straw biochar. Front. Environ. Sci. Eng. 2017, 11, 15. [Google Scholar] [CrossRef]
- Yan, S.; Yu, W.; Yang, T.; Li, Q.; Guo, J. The Adsorption of Corn Stalk Biochar for Pb and Cd: Preparation, Characterization, and Batch Adsorption Study. Separations 2022, 9, 22. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, H.; Cai, J.; Zhang, X.; Zhang, J.; Shao, J. Evaluation and Prediction of Cadmium Removal from Aqueous Solution by Phosphate-Modified Activated Bamboo Biochar. Energy Fuels 2018, 32, 4469–4477. [Google Scholar] [CrossRef]
- Guiza, S. Biosorption of heavy metal from aqueous solution using cellulosic waste orange peel. Ecol. Eng. 2017, 99, 134–140. [Google Scholar] [CrossRef]
- Abbar, B.; Alem, A.; Pantet, A.; Marcotte, S.; Ahfir, N.D.; Duriatti, D. Removal of dissolved and particulate contaminants from aqueous solution using natural flex fibres. Int. J. Civ. Eng. 2017, 35, 656–661. [Google Scholar]
- Asuquo, E.D.; Martin, A.D. Sorption of cadmium (II) ion from aqueous solution onto sweet potato (Ipomoea batatas L.) peel adsorbent: Characterisation, kinetic and isotherm studies. J. Environ. Chem. Eng. 2016, 4, 4207–4228. [Google Scholar] [CrossRef] [Green Version]
- Abdelfattah, A.; Fathy, I.; Sayed, A.; Almedolab, A.; Aboelghait, K.M. Biosorption of heavy metals ions in real industrial wastewater using peanut husk as efficient and cost-effective adsorbent. Environ. Nanotechnol. Monit. Manag. 2016, 6, 176–183. [Google Scholar] [CrossRef]
- Chinyelu, E. Use of unmodified orange peel for the adsorption of Cd (II), Pb (II) and Hg(II) ions in aqueous solutions. Am. J. Phys. Chem. 2015, 4, 21–29. [Google Scholar] [CrossRef] [Green Version]
- Gutha, Y.; Munagapati, V.S.; Naushad, M.; Abburi, K. Removal of Ni (II) from aqueous solution by Lycopersicum esculentum (Tomato) leaf powder as a low-cost biosorbent. Desalination Water Treat. 2015, 54, 200–208. [Google Scholar] [CrossRef]
- Paduraru, C.; Tofan, L.; Teodosiu, C.; Bunia, I.; Tudorachi, N.; Toma, O. Biosorption of zinc (II) on rapeseed waste: Equilibrium studies and thermogravimetric investigations. Process Saf. Environ. Prot. 2015, 94, 18–28. [Google Scholar] [CrossRef]
- Boruah, P.; Sarma, A.; Bhattacharyya, K.G. Removal of Ni (II) ions from aqueous solution by using low cost biosorbent prepared from jackfruit (Artocarpus heterophyllus) leaf powder. Indian J. Chem. Technol. 2015, 22, 322–327. [Google Scholar]
- Imaga, C.; Abia, A.; Igwe, J. Adsorption Isotherm Studies of Ni (II), Cu (II) and Zn (II) Ions on Unmodified and Mercapto-Acetic Acid (MAA) Modified Sorghum Hulls. Int. Res. J. Pure Appl. Chem. 2015, 5, 318–330. [Google Scholar] [CrossRef]
- Utomo, H.D.; Hunter, K.A. Adsorption of divalent copper, zinc, cadmium, and lead ions from aqueous solution by waste tea and coffee adsorbents. Environ. Technol. 2006, 27, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Singha, A.S.; Guleria, A. Utility of chemically modified agricultural waste okra biomass for removal of toxic heavy metal ions from aqueous solution. Eng. Agric. Environ. Food 2015, 8, 52–60. [Google Scholar] [CrossRef]
- Moubarik, A.; Grimi, N. Valorization of olive stone and sugar cane bagasse by-products as biosorbents for the removal of cadmium from aqueous solution. Food Res. Int. 2015, 73, 169–175. [Google Scholar] [CrossRef]
- Rajamohan, N. Biosorption of Mercury onto Protonated Pistachio Hull Wastes–Effect of Variables and Kinetic Experiments. Int. J. Chem. Eng. Appl. 2014, 5, 415–419. [Google Scholar] [CrossRef] [Green Version]
- Putra, W.P.; Kamari, A.; Yusoff, S.N.M.; Ishak, C.F.; Mohamed, A.; Hashim, N.; Isa, I.M. Biosorption of Cu (II), Pb (II) and Zn (II) ions from aqueous solutions using selected waste materials. J. Encapsulation Adsorpt. Sci. 2014, 4, 741. [Google Scholar] [CrossRef] [Green Version]
- Song, S.-T.; Saman, N.; Johari, K.; Mat, H. Surface chemistry modifications of rice husk toward enhancement of Hg (II) adsorption from aqueous solution. Clean Technol. Environ. Policy 2014, 16, 1747–1755. [Google Scholar] [CrossRef]
- Gautam, R.K.; Mudhoo, A.; Lofrano, G.; Chattopadhyaya, M.C. Biomass-derived biosorbents for metal ions sequestration: Adsorbent modification and activation methods and adsorbent regeneration. J. Environ. Chem. Eng. 2014, 2, 239–259. [Google Scholar] [CrossRef]
- Husein, D.Z. Adsorption and removal of mercury ions from aqueous solution using raw and chemically modified Egyptian mandarin peel. Desalination Water Treat. 2013, 51, 6761–6769. [Google Scholar] [CrossRef]
- Khoramzadeh, E.; Nasernejad, B.; Halladj, R. Mercury biosorption from aqueous solution by Sugarcane Bagasse. J. Taiwan Inst. Chem. Eng. 2013, 44, 266–269. [Google Scholar] [CrossRef]
- Gomez-Aguilar, D.L.; Miranda, J.P.-R.; Salcedo-Parra, O.J. Fruit Peels as a Sustainable Waste for the Biosorption of Heavy Metals in Wastewater: A Review. Molecules 2022, 27, 2124. [Google Scholar] [CrossRef]
- Dawood, S.; Sen, T.K.; Phan, C. Synthesis and characterisation of novel-activated carbon from waste biomass pine cone and its application in the removal of Congo red dye from aqueous solution by adsorption. Water Air Soil Pollut. 2014, 225, 1818. [Google Scholar] [CrossRef]
- Marwa, B.A.; Khaled, W.; Victoria, S. Valorisation of Pine Cone as an Efficient Biosorbent for the Removal of Pb (II), Cd (II), Cu(II), and Cr(VI). Adsorpt. Sci. Technol. 2021, 2021, 6678530. [Google Scholar] [CrossRef]
- Bazzo, A.; Adebayo, M.A.; Dias, S.L.P.; Lima, E.C.; Vaghetti, J.C.P.; de Oliveira, E.R.; Leite, A.J.B.; Pavan, F.A. Avocado seed powder: Characterization and its application for crystal violet dye removal from aqueous solutions. Desalination Water Treat. 2016, 57, 15873–15888. [Google Scholar] [CrossRef]
- Leite, A.J.B.; Carmalin, S.A.; Thue, P.S.; Reis, G.; Dias, S.; Lima, E.C.; Vaghetti, J.C.; Pavan, F.A.; De Alencar, W.S. Activated carbon from avocado seeds for the removal of phenolic compounds from aqueous solutions. Desalination Water Treat. 2017, 71, 168–181. [Google Scholar] [CrossRef] [Green Version]
- Garcia, E.-A.; Cristiani-Urbina, E. Effect of pH on hexavalent and total chromium removal from aqueous solutions by avocado shell using batch and continuous systems. Environ. Sci. Pollut. Res. 2019, 26, 3157–3173. [Google Scholar] [CrossRef]
- Krishnan, K.A.; Sreejalekshmi, K.; Vimexen, V.; Dev, V.V. Evaluation of adsorption properties of sulphurised activated carbon for the effective and economically viable removal of Zn (II) from aqueous solutions. Ecotoxicol. Environ. Saf. 2016, 124, 418–425. [Google Scholar] [CrossRef]
- Wahby, A.; Abdelouahab-Reddam, Z.; El Mail, R.; Stitou, M.; Silvestre-Albero, J.; Sepúlveda-Escribano, A.; Rodríguez-Reinoso, F. Mercury removal from aqueous solution by adsorption on activated carbons prepared from olive stones. Adsorption 2011, 17, 603–609. [Google Scholar] [CrossRef]
- Yagub, M.T.; Sen, T.K.; Ang, M. Removal of cationic dye methylene blue (MB) from aqueous solution by ground raw and base modified pine cone powder. Environ. Earth Sci. 2014, 71, 1507–1519. [Google Scholar] [CrossRef]
- Hayati, B.; Maleki, A.; Najafi, F.; Daraei, H.; Gharibi, F.; McKay, G. Super High Removal Capacities of Heavy Metals (Pb2+ and Cu2+) Using CNT Dendrimer. J. Hazard. Mater. 2017, 336, 146–157. [Google Scholar] [CrossRef]
- Biswas, S.; Mohapatra, S.S.; Kumari, U.; Meikap, B.C.; Sen, T.K. Batch and continuous closed circuit semi-fluidized bed operation: Removal of MB dye using sugarcane bagasse biochar and alginate composite adsorbents. J. Environ. Chem. Eng. 2020, 8, 103637. [Google Scholar] [CrossRef]
- Kamari, S.; Shahbazi, A. Biocompatible Fe3O4@SiO2-NH2 nanocomposite as a green nanofiller embedded in PES–nanofiltration membrane matrix for salts, heavy metal ion and dye removal: Long–term operation and reusability tests. Chemosphere 2020, 243, 125282. [Google Scholar] [CrossRef] [PubMed]
- Hameed, B.H.; Rahman, A.A. Removal of phenol from aqueous solutions by adsorption onto activated carbon prepared from biomass material. J. Hazard. Mater. 2008, 160, 576–581. [Google Scholar] [CrossRef] [PubMed]
- Priya, A.; Yogeshwaran, V.; Rajendran, S.; Hoang, T.K.; Soto-Moscoso, M.; Ghfar, A.A.; Bathula, C. Investigation of mechanism of heavy metals (Cr6+, Pb2+& Zn2+) adsorption from aqueous medium using rice husk ash: Kinetic and thermodynamic approach. Chemosphere 2022, 286, 131796. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Hu, X.; Wan, Y.; Wang, S.; Gao, B. Removal of lead, copper, cadmium, zinc, and nickel from aqueous solutions by alkali-modified biochar: Batch and column tests. J. Ind. Eng. Chem. 2016, 33, 239–245. [Google Scholar] [CrossRef] [Green Version]
- Yargic, A.S.; Yarbay, R.Z.; Sahin, N.; Onal, O.E. Assessment of toxic copper (II) biosorption from aqueous solution by chemically treated tomato waste. J. Clean. Prod. 2015, 88, 152–159. [Google Scholar] [CrossRef]
- Kılıç, M.; Kırbıyık, Ç.; Çepelioğullar, Ö.; Pütün, A.E. Adsorption of heavy metal ions from aqueous solutions by bio-char, a by-product of pyrolysis. Appl. Surf. Sci. 2013, 283, 856–862. [Google Scholar] [CrossRef]
- Salleh, M.A.M.; Mahmoud, D.K.; Karim, W.A.; Idris, A. Cationic and anionic dye adsorption by agricultural solid wastes: A comprehensive review. Desalination 2011, 280, 1–13. [Google Scholar] [CrossRef]
- Ngulube, T.; Gumbo, J.R.; Masindi, V.; Maity, A. An update on synthetic dyes adsorption onto clay-based minerals. A state-of-art review. J. Environ. Manag. 2017, 191, 35–57. [Google Scholar] [CrossRef]
- Senthil Kumar, P.; Palaniyappan, M.; Priyadharshini, M.; Vignesh, A.M.; Thanjiappan, A.; Sebastina Anne Fernando, P.; Tanvir Ahmed, R.; Srinath, R. Adsorption of basic dye onto raw and surface-modified agricultural waste. Environ. Prog. Sustain. Energy 2014, 33, 87–98. [Google Scholar] [CrossRef]
- Shaikh, R.B.; Saifullah, B.; Rehman, F. Greener Method for the Removal of Toxic Metal Ions from the Wastewater by Application of Agricultural Waste as an Adsorbent. Water 2018, 10, 1316. [Google Scholar] [CrossRef] [Green Version]
- Akram, M.; Bhatti, H.N.; Iqbal, M.; Noreen, S.; Sadaf, S. Biocomposite efficiency for Cr (VI) adsorption: Kinetic, equilibrium and thermodynamics studies. J. Environ. Chem. Eng. 2017, 5, 400–411. [Google Scholar] [CrossRef]
- Olayebi, O.O.; Olagboye, S.A.; Olatoye, R.A.; Olufemi, A.S. Agricultural Waste Adsorbents for Heavy Metals Removal from Wastewater. J. Phys. Chem. Sci. 2017, 5, 5. [Google Scholar] [CrossRef]
- Zhou, N.; Chen, H.; Xi, J.; Yao, D.; Zhou, Z.; Tian, Y. Biochars with excellent Pb (II) adsorption property produced from fresh and dehydrated banana peels via hydrothermal carbonization. Bioresour. Technol. 2017, 232, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Torab-Mostaedi, M.; Asadollahzadeh, M.; Hemmati, A.; Khosravi, A. Equilibrium, kinetic, and thermodynamic studies for biosorption of cadmium and nickel on grapefruit peel. J. Taiwan Inst. Chem. Eng. 2013, 44, 295–302. [Google Scholar] [CrossRef]
- Pandharipande, S.; Kalnake, R.P. Tamarind fruit shell adsorbent synthesis, characterization and adsorption studies for Cr (VI) & Ni(II) ions from aqueous solution. Int. J. Eng. Sci. Emerg. Technol. 2013, 4, 83–89. [Google Scholar]
- Hegazi, H.A. Removal of heavy metals from wastewater using agricultural and industrial wastes as adsorbents. HBRC J. 2013, 9, 276–282. [Google Scholar] [CrossRef] [Green Version]
- Kyzas, G.Z.; Siafaka, P.I.; Pavlidou, E.G.; Chrissafis, K.J.; Bikiaris, D.N. Synthesis and adsorption application of succinyl-grafted chitosan for the simultaneous removal of zinc and cationic dye from binary hazardous mixtures. Chem. Eng. J. 2015, 259, 438–448. [Google Scholar] [CrossRef]
- Ahalya, N.; Chandraprabha, M.N.; Kanamli, R.D.; Ramchandran, T.P. Adsorption of fast green onto coffee husk. J. Chem. Eng. Res. 2014, 2, 201–207. [Google Scholar]
- Karmaker, S.; Uddin, M.N.; Ichikawa, H.; Fukumori, Y.; Saha, T.K. Adsorption of reactive orange 13 onto jackfruit seed flakes in aqueous solution. J. Environ. Chem. Eng. 2015, 3, 583–592. [Google Scholar] [CrossRef]
- Jain, S.; Jayaram, R.V. Removal of basic dyes from aqueous solution by low-cost adsorbent: Wood apple shell (Feronia acidissima). Desalination 2010, 250, 921–927. [Google Scholar] [CrossRef]
- Saeed, A.; Sharif, M.; Iqbal, M. Application potential of grapefruit peel as dye sorbent: Kinetics, equilibrium, and mechanism of crystal violet adsorption. J. Hazard. Mater. 2010, 179, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Pavan, F.A.; Camacho, E.S.; Lima, E.C.; Dotto, G.L.; Branco, V.T.; Dias, S.L. Formosa papaya seed powder (FPSP): Preparation, characterization, and application as an alternative adsorbent for the removal of crystal violet from aqueous phase. J. Environ. Chem. Eng. 2014, 2, 230–238. [Google Scholar] [CrossRef]
- Xu, M.; Mckay, G. Removal of Heavy Metals, Lead, Cadmium, and Zinc, Using Adsorption Processes by Cost-Effective Adsorbents. In Adsorption Processes for Water Treatment and Purification, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 109–138. [Google Scholar] [CrossRef]
- Lin, Z.; Yang, Y.; Liang, Z.; Zeng, L.; Zhang, A. Preparation of Chitosan/Calcium Alginate/Bentonite Composite Hydrogel and Its Heavy Metal Ions Adsorption Properties. Polymers 2021, 13, 1891. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Lopes, L.; Macena, M.; Esteves, B.; Guine, R.P. Ideal pH for the adsorption of metal ions Cr6+, Ni2+, Pb2+ in aqueous solution with different adsorbent materials. Open Agric. 2021, 6, 115–123. [Google Scholar] [CrossRef]
- Dawood, S.; Sen, T.K. Removal of anionic dye Congo red from aqueous solution by raw pine and acid-treated pine cone powder as adsorbent: Equilibrium, thermodynamic, kinetics, mechanism and process design. Water Res. 2012, 46, 1933–1946. [Google Scholar] [CrossRef]
- Gupta, V.K.; Rastogi, A.; Nayak, A. Biosorption of nickel onto treated alga (Oedogonium hatei): Application of isotherm and kinetic models. J. Colloid Interface Sci. 2010, 342, 533–539. [Google Scholar] [CrossRef]
- Sen, T.K. Adsorptive Removal of Dye (Methylene Blue) Organic Pollutant from Water by Pine Tree Leaf Biomass Adsorbent. Processes 2023, 11, 1877. [Google Scholar] [CrossRef]
- Mondal, M.; Mukherjee, R.; Sinha, A.; Sarkar, S.; De, S. Removal of cyanide from steel plant effluent using coke breeze, a waste product of steel industry. J. Water Process Eng. 2019, 28, 135–143. [Google Scholar] [CrossRef]
- Hameed, B.H.; Krishni, R.R.; Sata, S.A. A novel agricultural waste adsorbent for the removal of cationic dye from aqueous solutions. J. Hazard. Mater. 2009, 162, 305–311. [Google Scholar] [CrossRef]
- Tran, H.N.; You, S.J.; Hosseini-Bandegharaei, A.; Chao, H.P. Mistakes and inconsistencies regarding adsorption of contaminants from aqueous solutions: A critical review. Water Res. 2017, 120, 88–116. [Google Scholar] [CrossRef]
- Khatoon, H.; Rai, J.P.N. Agricultural waste materials as biosorbents for the removal of heavy metals and synthetic dyes—A review. Octa J. Environ. Res. 2016, 4, 208–229. [Google Scholar]
- Kamsonlian, S.; Suresh, S.; Ramanaiah, V.; Majumder, C.; Chand, S.; Kumar, A. Biosorptive behaviour of mango leaf powder and rice husk for arsenic (III) from aqueoussolutions. Int. J. Environ. Sci. Technol. 2012, 9, 565–578. [Google Scholar] [CrossRef] [Green Version]
- Kamsonlian, S.; Balomajumder, C.; Chand, S.; Suresh, S. Biosorption of Cd (II)andAs (III) ions from aqueous solution by teawaste biomass. Afr. J. Environ. Sci. Technol. 2011, 5, 1–7. [Google Scholar]
- Okafor, P.C.; Okon, P.U.; Daniel, E.F.; Ebenso, E.E. Adsorption Capacity of Coconut (Cocos nucifera L.) Shell for Lead, Copper, Cadmium and Arsenic from aqueous solutions. Int. J. Electrochem. Sci. 2012, 7, 12354–12369. [Google Scholar] [CrossRef]
- Tuzen, M.; Sari, A.; Mendil, D.; Uluozlu, O.D.; Soylak, M.; Dogan, M. Characterization of biosorption process of As (III) on green algae Ulothrix cylindricum. J. Hazard. Mater. 2009, 165, 566–572. [Google Scholar] [CrossRef]


| Agricultural By-Products Raw and Modified/Treated Adsorbents | Adsorbate Heavy Metal Ions | Maximum Monolayer Adsorption Capacity, qmax (mg/g), at Optimum Process Conditions | References |
|---|---|---|---|
| Avocado seed | Cr (VI) | 35.5 | Ahmet and Danish [50]; Rangel et al. [61] |
| Jackfruit peels | Cu2+ Pb2+ Cd2+ Mn2+ | 17.5 10.1 20 76.9 | Ibrahim et al. [62]; Ayob et al. [63] |
| Data palm empty fruit bunch | Cr6+ | 70.49 | Rambabu et al. [44] |
| Pineapple peel | Cr6+ | 40 | Shakya et al. [64], Yousef et al. [65] |
| Canola seeds | Pb2+ Cd2+ | 44.25 52.36 | Affonso et al. [66]; Ayob et al. [63] |
| Laurus nobilis leaves | Cu2+ Pb2+ Cd2+ Zn2+ | 6.04 96.15 8.6 8.74 | Gumus et al. [43]; Ogunlalu et al. [31] |
| Vigna radiata husk biomass | Cu2+ Co2+ Ni2+ | 11.05 15.04 19.88 | Naseem et al. [67] |
| Coffee pulp | Cr6+ | 13.48 | Ayob et al. [63] |
| Cajanus cajan Husk | Cd2+ | 42.16 | Devani et al. [68]; Sazali et al. [69] |
| Orange peel | Cd2+ | 170.3 | Chen et al. [70] |
| Litchi peel | Cd2+ | 230.5 | Chen et al. [70] |
| Date seed biochar | Ni2+ | 19.54 | Mahdi et al. [71] |
| Avocado peel | Pb (II) Ni (II) | 4.93 9.82 | Ahmet and Danish [50]; Mallampati, [72] |
| Modified peanut shell | Hg(II) | 30.72 | Sulyman et al. [60] |
| Coconut husk | Cu2+ Ni2+ Pb2+ Zn2+ | 443.0 404.5 362.2 338.0 | Malik and Dahiya [73] |
| Orange peel | Pb (II) | 204 | Sulyman et al. [60] |
| Banana peels | Cu2+ Ni2+ Pb2+ | 14.3 27.4 34.5 | Thuan et al. [74]; Ayob et al. [63] |
| Corn straw | Cd2+ Pb2+ | 38.91 28.99 | Chi et al. [75], Yousef et al. [65], Yan et al. [76] |
| Pomegranate peel | Cu2+ | 30.12 | Ben-Ali et al. [55] |
| Modified activated bamboo | Cd2+ | 202.55 | Zhang et al. [77]; Sazali et al. [69] |
| Orange peel | Cu2+ | 63.3 | Guiza [78] |
| Flax fiber tows | Cu2+ Pb2+ Zn2+ | 9.92 10.74 8.4 | Abbar et al. [79] |
| Eucalyptus bark | Zn (II) | 131.6 | Afroze et al. [49] |
| Banana peel | Cd2+ Pb2+ | 5.71 2.18 | Gisi et al. [5] |
| Sweet potato peel | Pb2+ | 18 | Asuquo et al. [80] |
| Peanut husk | Ni2+ | 56.82 | Abdelfattah et al. [81] |
| Orange peel | Hg2+ | 7.46 | Chinyelu [82] |
| Tomato leaf | Ni (II) | 58.8 | Gutha et al. [83] |
| Rapeseed waste | Zn (II) | 13.9 | Paduraru et al. [84] |
| Jackfruit leaf | Ni (II) | 11.5 | Boruah et al. [85] |
| Sorghum hulls | Cu2+ | 148.93 | Imaga, Abia et al. [86] |
| Coffee residues | Pb2+, Zn2+ | 9.7 (Pb2+), 4.4 (Zn2+) | Wu, Kuo et al. [28], Utomo and Hunter [87] |
| Modified Okra biomass | Cu2+, Zn2+, Cd2+, Pb2+ | 72.72 (Cu2+), 57.11 (Zn2+), 121.51 (Cd2+), 273.97 (Pb2+) | Singha and Guleria [88] |
| Sugarcane bagasse | Mn2+ | 0.423 | Anastopoulos et al. [51] |
| Sugarcane bagasse | Cd2+ | 0.955 | Moubarik and Grimi [89], Anastopoulos et al. [51] |
| Peanut shell | Pb2+ | 39 | Tasar et al. [47] |
| Pistachio hull waste | Hg2+ | 48.78 | Rajamohan [90] |
| Coconut tree sawdust | Cu (II) Pb (II) Zn (II) | 3.9 25.0 23.8 | Putra et al. [91] |
| Modified rice husk | Hg2+ | 89 | Song et al. [92], Yousef et al. [65] |
| Modified Sugarcane bagasse | Cu2+ | 30.9 | Rana et al. [17] |
| Garcinia cambogia plants | As | 704.11 | Gautam et al. [93] |
| Oryza sativa plants | Cd2+ | 20.70 | Gautam et al. [93] |
| Corn stover | Cr2+ | 84 | Gautam et al. [93] |
| Palm tree branches | Cr+4 | 157 | Guat et al. [2] |
| Egyptian mandarin peel (raw) | Hg2+ | 19.01 | Husein et al. [94]; Gisi et al. [5] |
| Raw sugarcane bagasse | Hg2+ | 35.71 | Khovamzadeh et al. [95]; Anastopoulos et al. [51] |
| Orange peel | Cu2+, Pb2+, Zn2+ | 70.73 (Cu2+), 209.8 (Pb2+) and 56.18 (Zn2+) | Feng and Guo [16] Gomez-Al [96] |
| Barley straw (raw) | Cu2+ | 4.64 | Gisi et al. [5] |
| Garden grass (raw) | Pb2+ | 58.34 | Gisi et al. [5] |
| Adsorbents | Contaminants (Heavy Metals and Dyes) | Characterisation Properties | References | ||||
|---|---|---|---|---|---|---|---|
| Specific Surface Area/BET(m2/g) | Particle Size Distribution | Elemental Analysis (%) | FTIR Analysis | pHpzc | |||
| Pinecone | Cd2+, Cu2+, Pb2+ | 0.2536 | 50 µm | - | O-H, C-H, -CH2, C=O | 6.2 | Dawood et al. [97] Marawa et al. [98] |
| Avocado seed | Cr6+ | 1.75 | 0.1–1.5 mm | O-H group -CH2 stretching | 6.4 | Bazzo et al. [99]; Leite et al. [100] | |
| HAS avocado shell? | Ni2+ | - | 43.13 (carbon), 7.17 (hydrogen), 48.35 (oxygen), 0.66 (nitrogen) and 0.89 (sulphur) | C==O, O-H, -CH2 stretching | 6.8 | Garcia and Cristiani-Urbina, [101] | |
| Raw pomegranate peel | Cu2+ | 598.78 | 205 µm, 850 µm and 2375 µm | C=O in carboxylic acid, acetate groups -COO, ketone, C–O groups of carboxylic acid, alcoholic, phenolic, ether and ester groups. | Ben-Ali et al. [55] | ||
| Sugarcane bagasse pith (sulphurised activated carbon) | Zn2+ | 500 | - | 9.10 (sulphur) and 5.20 (ash) | S==O, and C-S vibrations | 4.3 | Krishnan et al. [102] |
| Jack fruit leaf powder | Ni2+ | 246.9 | - | - | -OH groups, -CH2 group, and CO bonds and C=S bonds. | - | Boruah et al. [85] |
| Coffee residues | Pb2+, Zn2+ | 0.19 | - | - | - | 3.9 | Wu et al. [28] |
| Guava leaves (activated) | Cd2+ | 100.76 | Pore volume 0.415 cm3/g and pore diameter 47.091 Å | - | O–H, C–H, C=C and –SO3 bonds | - | Abdelwahab, Fouad et al. [81] |
| DateStones Pd2+Cd2+ | 950 950 | Sulyman et al. [60] | |||||
| Olive stone Hg2+ | 400–850 | - | Wahby et al. [103] | ||||
| Adsorbents | Adsorbates (Heavy Metals) | Adsorbent Dosage | Trend on Percentage (%) Removal Range | References |
|---|---|---|---|---|
| Brassica campestris agricultural waste | Ni2+, Pb2+ Cr6+ 0.2–1 g/L | Increase | Shaikh et al. [116] | |
| Mango kernel (bio-composite) | Cr (VI) | 0.05–0.3 g/L | Decrease | Akram et al. [117] |
| Bagasse (activated) | Cr | 0.5–1.5 g/L | Increase | Olayebi et al. [118] |
| Croncob (activate) | Cr | 0.5–2.4 g/L | Increase | Olayebi et al. [118] |
| Bagasse (activated) | Fe3+ | 0.5–2.5 g/L | Increase | Olayebi et al. [118] |
| Croncob (activated) | Fe3+ | Increase | Olayebi et al. [118] | |
| Banana peel biochar | Pb2+ | 0.5–3.0 g/L 0.01–0.2 g/L | Increase | Zhou et al. [119] |
| Eucalyptus sheathiana bark | Zn2+ | 0.01–0.03 g | Decrease | Afroze et al. [49] |
| Bagasse pith (sulphurised activated carbon) | Zn2+ | 0.5–8 g L−1 | Increase | Krishnan et al. [102] |
| Jackfruit leaf powder | Ni2+ | 1–5 g L−1 | Decrease | Boruah et al. [85] |
| Sugarcane bagasse (sulphuric acid-treated) | Cu2+ | 0.5–2 gm/100 mL | Increase | Rana et al. [17] |
| Grapefruit peel | Cd2+, Ni2+ | 1–4 g L−1 | Increase | Torab-mostaedi et al. [120] |
| Tamarind fruit shell | Ni2+ | 0.01–0.08 g/10 mL | 20–90 | Pandharipande and kalnaka [121] |
| Almond shell biocar | Ni2+ Cd2+ | 0.1–10 g/L 0.1–10 g/L | Increase | Kilic et al. [112 |
| Rice husk | Pb2+, Cd2+ Cu2+, Ni2+ | 0.02–0.06 g/L | Increase Increase | Hegazi [122] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sen, T.K. Agricultural Solid Wastes Based Adsorbent Materials in the Remediation of Heavy Metal Ions from Water and Wastewater by Adsorption: A Review. Molecules 2023, 28, 5575. https://doi.org/10.3390/molecules28145575
Sen TK. Agricultural Solid Wastes Based Adsorbent Materials in the Remediation of Heavy Metal Ions from Water and Wastewater by Adsorption: A Review. Molecules. 2023; 28(14):5575. https://doi.org/10.3390/molecules28145575
Chicago/Turabian StyleSen, Tushar Kanti. 2023. "Agricultural Solid Wastes Based Adsorbent Materials in the Remediation of Heavy Metal Ions from Water and Wastewater by Adsorption: A Review" Molecules 28, no. 14: 5575. https://doi.org/10.3390/molecules28145575
APA StyleSen, T. K. (2023). Agricultural Solid Wastes Based Adsorbent Materials in the Remediation of Heavy Metal Ions from Water and Wastewater by Adsorption: A Review. Molecules, 28(14), 5575. https://doi.org/10.3390/molecules28145575






