Oxygen Vacancy and Interface Effect Adjusted Hollow Dodecahedrons for Efficient Oxygen Evolution Reaction
Abstract
:1. Introduction
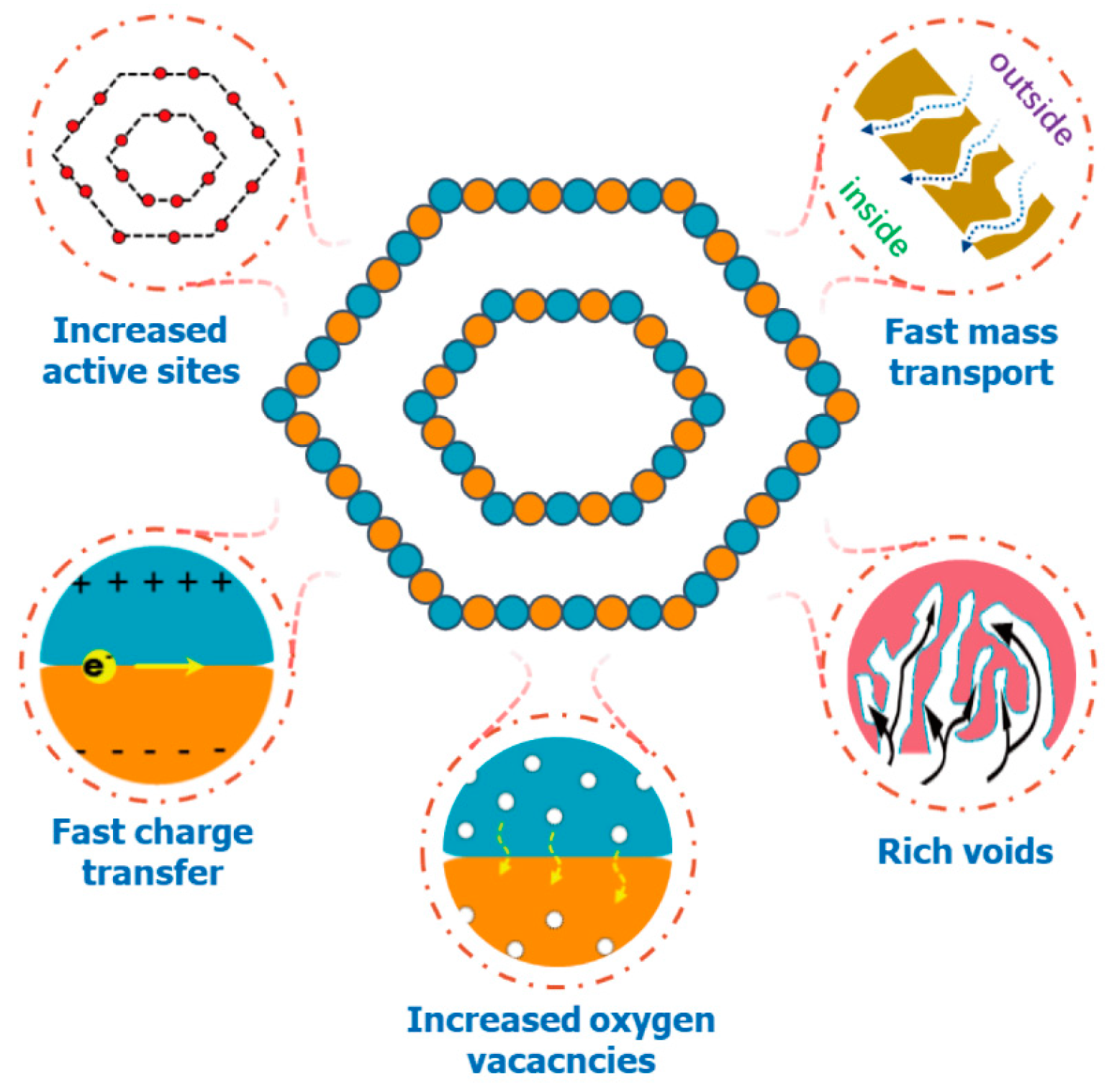
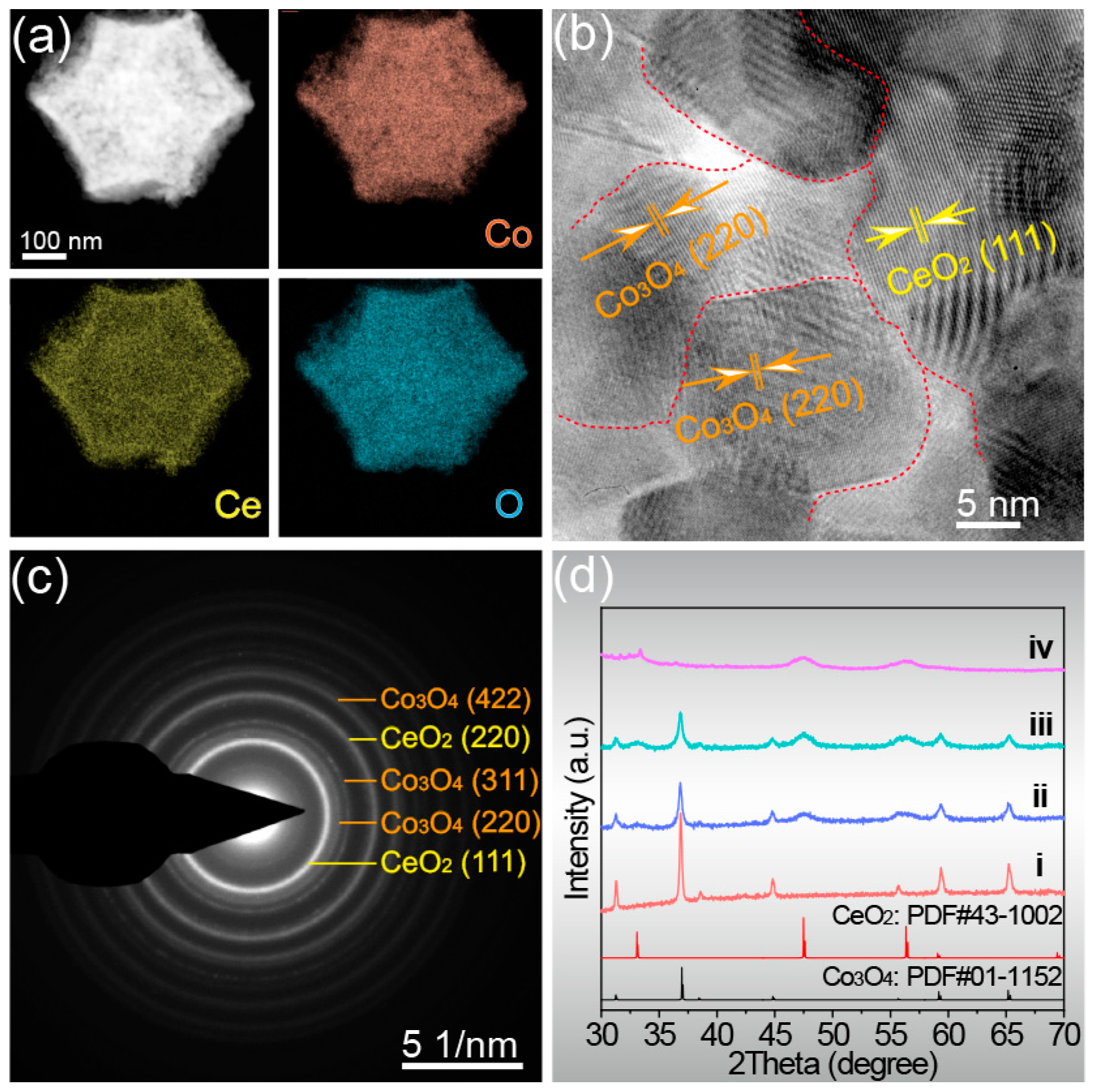
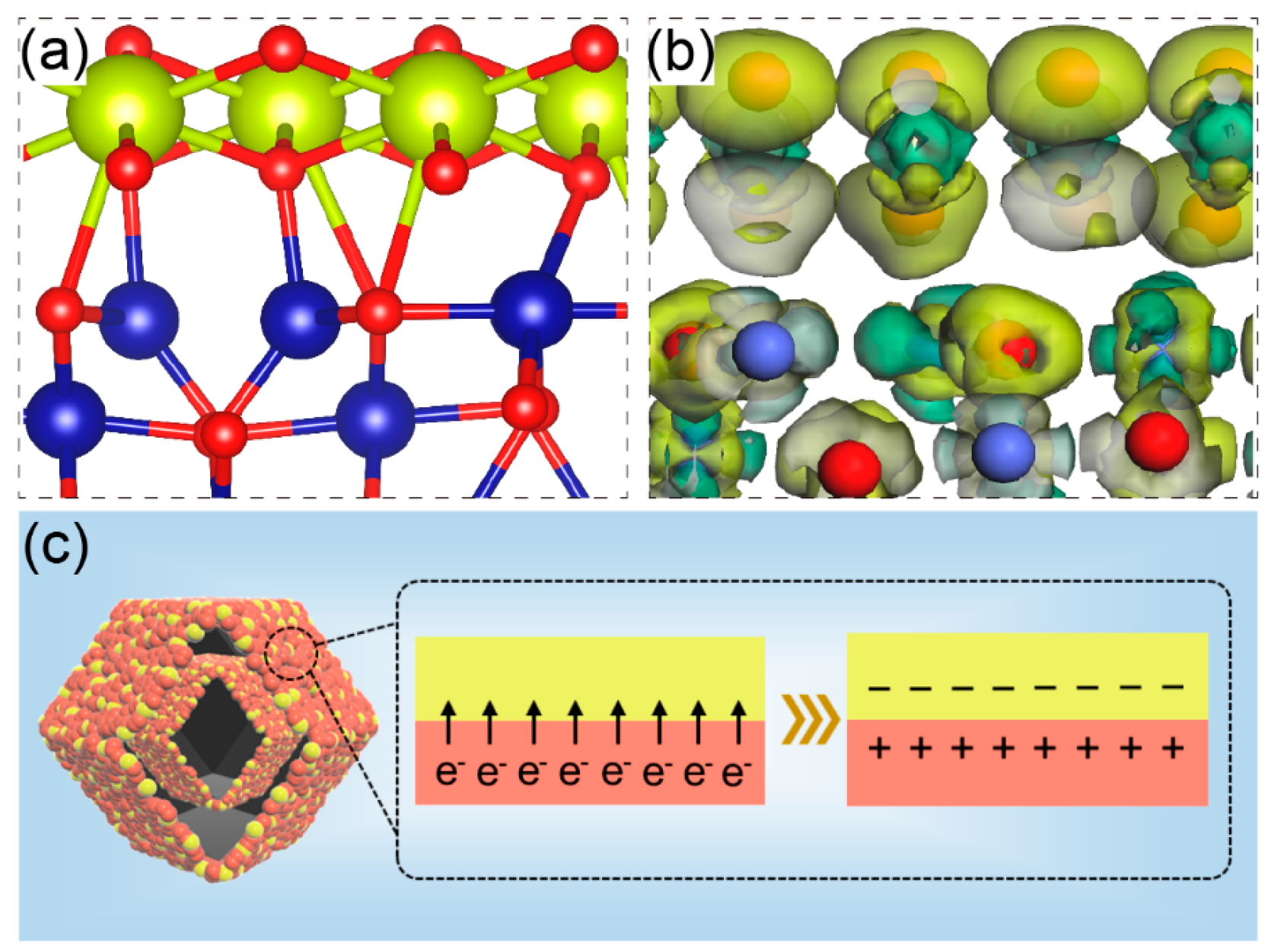
2. Results and Discussion
3. Experimental Setup
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, K.; Zou, R. Advanced transition metal-based OER electrocatalysts: Current status, opportunities, and Challenges. Small 2022, 17, 2100129. [Google Scholar] [CrossRef]
- Wang, H.; Sun, F.; Qi, J.; Zhang, D.; Sun, H.; Wang, Q.; Li, Z.; Wu, Y.A.; Hu, Z.; Wang, B. Recent progress on layered double hydroxides: Comprehensive regulation for enhanced oxygen evolution reaction. Mater. Today Energy 2022, 27, 101036. [Google Scholar] [CrossRef]
- Anantharaj, S.; Kundu, S.; Noda, S. “The Fe Effect”: A review unveiling the critical roles of Fe in enhancing OER activity of Ni and Co based catalysts. Nano Energy 2021, 80, 105514. [Google Scholar] [CrossRef]
- Song, J.; Wei, C.; Huang, Z.; Liu, C.; Zeng, L. A review on fundamentals for designing oxygen evolution electrocatalysts. Chem. Soc. Rev. 2020, 49, 2196–2214. [Google Scholar] [CrossRef] [PubMed]
- Roger, I.; Shipman, M.A.; Symes, M.D. Earth-abundant catalysts for electrochemical and photoelectrochemical water splitting. Nat. Rev. Chem. 2017, 1, 3. [Google Scholar] [CrossRef]
- Chen, F.; Wu, Z.; Adler, Z.; Wang, H. Stability challenges of electrocatalytic oxygen evolution reaction: From mechanistic understanding to reactor design. Joule 2021, 5, 1704–1731. [Google Scholar] [CrossRef]
- Lee, Y.; Suntivich, J.; May, K.J.; Perry, E.E.; Shao-horn, Y. Synthesis and activities of rutile IrO2 and RuO2 nanoparticles for oxygen evolution in acid and alkaline solutions. J. Phys. Chem. Lett. 2012, 3, 399–404. [Google Scholar] [CrossRef]
- Li, Y.; Du, X.; Huang, J.; Wu, C.; Sun, Y.; Zou, G.; Yang, C.; Xiong, J. Recent progress on surface reconstruction of earth-abundant electrocatalysts for water oxidation. Small 2019, 15, 1901980. [Google Scholar] [CrossRef]
- Kim, J.S.; Kim, B.; Kim, H.; Kang, K. Recent progress on multimetal oxide catalysts for the oxygen evolution reaction. Adv. Energy Mater. 2018, 8, 1702774. [Google Scholar] [CrossRef]
- Nozari-Asbemarz, M.; Amiri, M.; Khodayari, A.; Bezaatpour, A.; Nouhi, S.; Hosseini, P.; Wark, M.; Boukherroub, R.; Szunerits, S. In situ synthesis of Co3O4/CoFe2O4 derived from a metal-organic framework on nickel foam: High-performance electrocatalyst for water oxidation. ACS Appl. Energ. Mater. 2021, 4, 2951–2959. [Google Scholar] [CrossRef]
- Liu, N.; Guan, J. Core-shell Co3O4@FeOx catalysts for efficient oxygen evolution reaction. Mater. Today Energy 2021, 21, 100715. [Google Scholar] [CrossRef]
- Zhang, S.; Wei, N.; Yao, Z.; Zhao, X.; Du, M.; Zhou, Q. Oxygen vacancy-based ultrathin Co3O4 nanosheets as a high-efficiency electrocatalyst for oxygen evolution reaction. Int. J. Hydrogen Energy 2021, 46, 5286–5295. [Google Scholar] [CrossRef]
- Wang, J.; Cui, W.; Liu, Q.; Xing, Z.; Asiri, A.M.; Sun, X. Recent progress in cobalt-based heterogeneous catalysts for electrochemical water splitting. Adv. Mater. 2016, 28, 215–230. [Google Scholar] [CrossRef]
- Hu, C.; Zhang, L.; Gong, J. Recent progress made in the mechanism comprehension and design of electrocatalysts for alkaline water splitting. Energy Environ. Sci. 2019, 12, 2620–2645. [Google Scholar] [CrossRef]
- Dong, D.; Wu, Z.; Wang, J.; Fu, G.; Tang, Y. Recent progress in Co9S8-based materials for hydrogen and oxygen electrocatalysis. J. Mater. Chem. A 2019, 7, 16068–16088. [Google Scholar] [CrossRef]
- Lu, K.; Gu, T.; Zhang, L.; Wu, Z.; Wang, R.; Li, X. Pom-assisted coating of MOF-derived Mo-doped Co3O4 nanoparticles on carbon nanotubes for upgrading oxygen evolution reaction. Chem. Eng. J. 2021, 408, 127352. [Google Scholar] [CrossRef]
- Fabbri, E.; Nachtegaal, M.; Binninger, T.; Cheng, X.; Kim, B.J.; Durst, J.; Bozza, F.; Graule, T.; Schäublin, R.; Wiles, L.; et al. Dynamic surface self-reconstruction is the key of highly active perovskite nano-electrocatalysts for water splitting. Nat. Mater. 2017, 16, 925–931. [Google Scholar] [CrossRef]
- Singh, T.I.; Rajeshkhanna, G.; Pan, U.N.; Kshetri, T.; Lin, H.; Kim, N.H.; Lee, J.H. Alkaline water splitting enhancement by MOF-derived Fe-Co-Oxide/Co@NC-mNS heterostructure: Boosting OER and HER through defect engineering and in situ oxidation. Small 2021, 17, 2101312. [Google Scholar] [CrossRef]
- Koppisetti, H.V.S.R.M.; Ganguli, S.; Ghosh, S.; Mahalingam, V. Rejuvenating the geometric electrocatalytic OER performance of crystalline Co3O4 by microstructure engineering with sulfate. Chem-Asian J. 2021, 16, 988–998. [Google Scholar] [CrossRef]
- Yu, M.; Moon, G.; Castillo, R.G. Dual role of silver moieties coupled with ordered mesoporous cobalt oxide towards electrocatalytic oxygen evolution reaction. Angew. Chem. Int. Ed. 2020, 59, 16544–16552. [Google Scholar] [CrossRef]
- Du, X.; Ding, Y.; Zhang, X. Selectively Se-doped Co3O4@CeO2 nanoparticle-dotted nanoneedle arrays for high-efficiency overall water splitting. Appl. Surf. Sci. 2021, 562, 150227. [Google Scholar] [CrossRef]
- Song, X.Z.; Zhu, W.Y.; Wang, X.F.; Tan, Z. Recent advances of CeO2-based electrocatalysts for oxygen and hydrogen evolution as well as nitrogen reduction. ChemElectroChem 2021, 8, 996–1020. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Zheng, Z. A review of transition metal oxygen-evolving catalysts decorated by cerium-based materials: Current status and future prospects. CCS Chem. 2022, 4, 31–53. [Google Scholar] [CrossRef]
- Jamesh, M.I.; Sun, X. Recent progress on earth abundant electrocatalysts for oxygen evolution reaction (OER) in alkaline medium to achieve efficient water splitting—A review. J. Power Sources 2018, 400, 31–68. [Google Scholar] [CrossRef]
- Wang, H.; Han, X.; Zhang, L.; Wang, K.; Zhang, R.; Wang, X.; Song, S.; Zhang, H. Integrating ceria with cobalt sulfide as high-performance electrocatalysts for overall water splitting. Fundam. Res. 2021, 3, 356–361. [Google Scholar] [CrossRef]
- Jiao, C.; Wang, Z.; Zhao, X.; Wang, H.; Wang, J.; Yu, R.; Wang, D. Triple-shelled manganese-cobalt oxide hollow dodecahedra with highly enhanced performance for rechargeable alkaline batteries. Angew. Chem. Int. Edit. 2019, 58, 996–1001. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhou, T.; Wang, L.; Zhang, T. Metal-organic frameworks-derived hierarchical Co3O4 structures as efficient sensing materials for acetone detection. ACS Appl. Mater. Inter. 2018, 10, 9765–9773. [Google Scholar] [CrossRef]
- Hou, S.; Lian, Y.; Bai, Y.; Zhou, Q.; Ban, C.; Wang, Z.; Zhao, J.; Zhang, H. Hollow dodecahedral Co3S4@NiO derived from ZIF-67 for supercapacitor. Electrochim. Acta 2020, 341, 136053. [Google Scholar] [CrossRef]
- Xiao, Y.; Wang, Y.; Xiao, M.; Liu, C.; Hou, S.; Ge, J.; Xing, W. Regulating the pore structure and oxygen vacancies of cobaltosic oxide hollow dodecahedra for an enhanced oxygen evolution reaction. NPG Asia Mater. 2020, 12, 73. [Google Scholar] [CrossRef]
- Wang, X.; Liu, M.; Yu, H.; Zhang, H.; Yan, S.; Zhang, C.; Liu, S. Oxygen-deficient 3D-ordered multistage porous interfacial catalysts with enhanced water oxidation performance. J. Mater. Chem. A 2020, 8, 22886–22892. [Google Scholar] [CrossRef]
- Yan, Z.; Gong, S.; An, L.; Yue, L.; Xu, Z. Enhanced catalytic activity of graphene oxide/CeO2 supported Pt toward HCHO decomposition at room temperature. React. Kinet. Mech. Cat. 2018, 124, 293–304. [Google Scholar] [CrossRef]
- Sun, Y.; Huang, F.; Li, S.; Shen, Y.; Xie, A. Novel porous starfish-like Co3O4@nitrogen-doped carbon as an advanced anode for lithium-ion batteries. Nano Res. 2017, 10, 3457–3467. [Google Scholar] [CrossRef]
- Yan, Z.; Xu, Z.; Yu, J.; Jaroniec, M. Enhanced formaldehyde oxidation on CeO2/AlOOH-supported Pt catalyst at room temperature. Appl. Catal. B-Environ. 2016, 199, 458–465. [Google Scholar] [CrossRef]
- Yan, D.; Chen, R.; Xiao, Z.; Wang, S. Engineering the electronic structure of Co3O4 by carbon-doping for efficient overall water splitting. Electrochim. Acta 2019, 303, 316–322. [Google Scholar] [CrossRef]
- Li, T.; Li, S.; Liu, Q.; Tian, Y.; Zhang, Y.; Fu, G.; Tang, Y. Hollow Co3O4/CeO2 heterostructures in situ embedded in N-doped carbon nanofibers enable outstanding oxygen evolution. ACS Sustain. Chem. Eng. 2019, 7, 17950–17957. [Google Scholar] [CrossRef]
- Lv, C.; Yan, C.; Chen, G.; Ding, Y.; Sun, J.; Zhou, Y.; Yu, G. An amorphous noble-metal-free electrocatalyst that enables nitrogen fixation under ambient conditions. Angew. Chem. Int. Edit. 2018, 57, 6073–6076. [Google Scholar] [CrossRef]
- Low, J.; Yu, J.; Jaroniec, M.; Wageh, S.; Al-Ghamdi, A.A. Heterojunction photocatalysts. Adv. Mater. 2017, 29, 1601694. [Google Scholar] [CrossRef]
- Li, M.; Wang, X.; Liu, K.; Sun, H.; Sun, D.; Huang, K.; Tang, Y.; Xing, W.; Li, H.; Fu, G. Reinforcing Co-O covalency via Ce(4f)-O(2p)-Co(3d) gradient orbital coupling for high-efficiency oxygen evolution. Adv. Mater. 2023, 963, 2302462. [Google Scholar] [CrossRef]
- Miao, X.; Wu, L.; Lin, Y.; Yuan, X.; Zhao, J.; Yan, W.; Zhou, S.; Shi, L. The role of oxygen evolution vacancies in water oxidation for perovskite cobalt oxide electrocatalysts: Are more better? Chem. Commun. 2019, 55, 1442–1445. [Google Scholar] [CrossRef]
- Long, X.; Lin, H.; Zhou, D.; An, Y.; Yang, S. Enhancing full water-splitting performance of transition metal bifunctional electrocatalysts in alkaline solutions by tailoring CeO2-transition metal oxides-Ni nanointerfaces. ACS Energy Lett. 2018, 3, 290–296. [Google Scholar] [CrossRef]
- Xue, Z.H.; Su, H.; Yu, Q.Y.; Zhang, B.; Wang, H.H.; Li, X.H.; Chen, J.S. Janus Co/CoP nanoparticles as efficient mott-schottky electrocatalysts for overall water splitting in wide pH range. Adv. Energy Mater. 2017, 7, 1602355. [Google Scholar] [CrossRef]
- Goswami, C.; Yamada, Y.; Matus, E.V.; Ismagilov, I.Z.; Kerzhentsev, M.; Bharali, P. Elucidating the role of oxide-oxide/carbon interfaces of CuOx-CeO2/C in boosting Electrocatalytic Performance. Langmuir 2020, 36, 15141–15152. [Google Scholar] [CrossRef] [PubMed]
- Hang, L.; Sun, Y.; Men, D.; Liu, S.; Zhao, Q.; Cai, W.; Li, Y. Hierarchical micro/nanostructured C doped Co/Co3O4 hollow spheres derived from PS@Co(OH)2 for the oxygen evolution reaction. J. Mater. Chem. A 2017, 5, 11163–11170. [Google Scholar] [CrossRef]
- Gao, W.; Xia, Z.; Cao, F.; Ho, J.C.; Jiang, Z.; Qu, Y. Comprehensive understanding of the spatial configurations of CeO2 in NiO for the electrocatalytic oxygen evolution reaction: Embedded or surface-loaded. Adv. Funct. Mater. 2018, 28, 1706056. [Google Scholar] [CrossRef]
- Zhang, E.; Xie, Y.; Ci, S.; Jia, J.; Cai, P.; Yi, L.; Wen, Z. Multifunctional high-activity and robust electrocatalyst derived from metal-organic frameworks. J. Mater. Chem. A 2016, 4, 17288–17298. [Google Scholar] [CrossRef]
- Ji, D.; Liu, C.; Yao, Y.; Luo, L.; Wang, W.; Chen, Z. Cerium substitution in LaCoO3 perovskite oxide as bifunctional electrocatalysts for hydrogen and oxygen evolution reaction. Nanoscale 2021, 13, 9952–9959. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Alberto Estudillo-Wong, L.; Gao, Y.; Feng, Y.; Alonso-Vante, N. Oxygen vacancies engineering by coordinating oxygen-buffering CeO2 with CoO nanorods as efficient bifunctional oxygen electrode electrocatalyst. J. Energy Chem. 2021, 59, 615–625. [Google Scholar] [CrossRef]
- Huang, X.; Zheng, H.; Lu, G.; Wang, P.; Xing, L.; Wang, J.; Wang, G. Enhanced water splitting electrocatalysis over MnCo2O4 via introduction of suitable Ce content. ACS Sustain. Chem. Eng. 2019, 7, 1169–1177. [Google Scholar]
- Pan, L.; Wang, Q.; Li, Y.; Zhang, C. Amorphous cobalt-cerium binary metal oxides as high performance electrocatalyst for oxygen evolution reaction. J. Catal. 2020, 284, 14–21. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, D.; Hankari, S.E.; Zou, Y.; Wang, S. Recent process on layered double hydroxides and their derivatives for electrocatalytic water splitting. Adv. Sci. 2018, 5, 1800064. [Google Scholar] [CrossRef]
- Song, F.; Hu, X. Ultrathin cobalt-manganese layered double hydroxide is an efficient oxygen evolution catalyst. J. Am. Chem. Soc. 2014, 136, 16481–16484. [Google Scholar] [CrossRef] [PubMed]
- Vignesh, A.; Prabu, M.; Shanmugam, S. Porous LaCo1−xNixO3−δ nanostructures as an efficient electrocatalyst for water oxidation and for a zinc-air battery. ACS Appl. Mater. Interfaces 2016, 8, 6019–6031. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Ma, Q.; Sun, F.; Shao, Y.; Zhang, D.; Sun, H.; Li, Z.; Wang, Q.; Qi, J.; Wang, B. Oxygen Vacancy and Interface Effect Adjusted Hollow Dodecahedrons for Efficient Oxygen Evolution Reaction. Molecules 2023, 28, 5620. https://doi.org/10.3390/molecules28155620
Wang H, Ma Q, Sun F, Shao Y, Zhang D, Sun H, Li Z, Wang Q, Qi J, Wang B. Oxygen Vacancy and Interface Effect Adjusted Hollow Dodecahedrons for Efficient Oxygen Evolution Reaction. Molecules. 2023; 28(15):5620. https://doi.org/10.3390/molecules28155620
Chicago/Turabian StyleWang, Huan, Qian Ma, Fengmin Sun, Yachuan Shao, Di Zhang, Huilan Sun, Zhaojin Li, Qiujun Wang, Jian Qi, and Bo Wang. 2023. "Oxygen Vacancy and Interface Effect Adjusted Hollow Dodecahedrons for Efficient Oxygen Evolution Reaction" Molecules 28, no. 15: 5620. https://doi.org/10.3390/molecules28155620
APA StyleWang, H., Ma, Q., Sun, F., Shao, Y., Zhang, D., Sun, H., Li, Z., Wang, Q., Qi, J., & Wang, B. (2023). Oxygen Vacancy and Interface Effect Adjusted Hollow Dodecahedrons for Efficient Oxygen Evolution Reaction. Molecules, 28(15), 5620. https://doi.org/10.3390/molecules28155620






