Recent Progress in 1,2-cis glycosylation for Glucan Synthesis
Abstract
1. Introduction
α-D-glucans
| Linkage | Name | Source | Ref. | |
|---|---|---|---|---|
| Linear | Side Chain | |||
| (1→4)-α | - | Amylose | Mycobacterium tuberculosis | [85,86] |
| (1→4)-α | - | Amylose | Streptomyces venezuelae | [87] |
| (1→4)-α | - | Amylose | Fusicoccum amygdale | [88] |
| (1→4)-α | - | Amylose | Agaricus blazei | [89] |
| (1→4)-α | - | Amylose | Pleurotus ostreatus | [84] |
| (1→4)-α | - | Starch | Rice bran | [90] |
| (1→4)-α | (1→6)-α | Glycogen | Saccharomyces cerevisiae | [91] |
| (1→4)-α | (1→6)-α | Glycogen | Agaricus bisporus | [92] |
| (1→4)-α | (1→6)-α | Glycogen | Cordyceps sinensis | [93] |
| (1→4)-α | (1→6)-α | Glycogen | Coprinus comatus | [94] |
| (1→4)-α | (1→6)-α | Glycogen | Flammulina velutipes | [95] |
| (1→4)-α | (1→6)-α | Glycogen | Gastrodia elata Bl | [96] |
| (1→4)-α | (1→6)-α | Glycogen | Lonicera japonica Thunb | [97] |
| (1→4)-α | (1→6)-α | Glycogen | Actinidia chinensis | [98] |
| (1→4)-α | (1→2); (1→6)-α | Glycogen | Tricholoma matsutake | [71] |
| (1→4)(1→6)-α | - | Reuteran | Lactobacillus reuteri | [99] |
| (1→4)(1→6)-α | - | Pullulan | Aureobasidium pullulans Cyttaria harioti Tremella mesenterica | [100] |
| (1→4)(1→6)-α | - | Pullulan | Tremella mesenterica | [101] |
| (1→3)-α | - | Pseudonigeran | Aspergillus flavipes Aspergillus flavus Aspergillus fumigatus Aspergillus ochraceus | [102] |
| (1→3)-α | - | - | Aspergillus fumigatus | [103,104,105,106] |
| (1→3)-α | - | Pseudonigeran | Aspergillus nidulans | [107,108,109] |
| (1→3)-α | - | - | Aspergillus niger | [110,111] |
| (1→3)-α | - | Pseudonigeran | Aspergillus niger NNRL 326 | [111] |
| (1→3)-α | - | - | Aspergillus wentii | [112] |
| (1→3)-α | - | Pseudonigeran | Blastomyces dermatiditis (yeast form) | [113,114] |
| (1→3)-α | - | Pseudonigeran | Eupenicillium crustaceum | [111] |
| (1→3)-α | - | Pseudonigeran | Fusarium oxysporum | [111] |
| (1→3)-α | - | Pseudonigeran | Fusicoccum amygdale | [88] |
| (1→3)-α | - | Pseudonigeran | Histoplasma capsulatum | [115] |
| (1→3)-α | - | Pseudonigeran | Histoplasma farciminosum | [116] |
| (1→3)-α | - | Pseudonigeran | Paracoccidioides brasiliensis | [117] |
| (1→3)-α | - | Pseudonigeran | Penicillium brevi-compactum Penicillium decumbens | [102] |
| (1→3)-α | - | Pseudonigeran | Penicillium expansum | [118] |
| (1→3)-α | - | Pseudonigeran | Penicillium chrysogenum | [119] |
| (1→3)-α | - | Pseudonigeran | Poria cocos | [120] |
| (1→3)-α | - | Pseudonigeran | Agrocybe cylindracea | [121] |
| (1→3)-α | - | - | Amanita muscaria | [122] |
| (1→3)-α | - | Pseudonigeran | Armillaria mellea | [123] |
| (1→3)-α | - | Pseudonigeran | Cryptococcus albidus | [124] |
| (1→3)-α | - | Pseudonigeran | Cryptococcus terreus | [124] |
| (1→3)-α | - | Pseudonigeran | Ganoderma lucidum | [125] |
| (1→3)-α | - | Pseudonigeran | Ganoderma tsugae | [126] |
| (1→3)-α | - | - | Laetiporus sulphureus | [127] |
| (1→3)-α | - | Pseudonigeran | Lentinus edodes | [128] |
| (1→3)-α | - | Pseudonigeran | Piptoporus betulinus | [127] |
| (1→3)-α | - | Pseudonigeran | Pleurotus ostreatus | [36] |
| (1→3)-α | - | Pseudonigeran | Pleurotus eryngii | [88] |
| (1→3)-α | - | Pseudonigeran | Polyporus tumulosus | [129] |
| (1→3)-α | - | Pseudonigeran | Schizophyllum commune | [130] |
| (1→3)-α | - | Pseudonigeran | Tremella mesenterica | [101] |
| (1→3)-α | (1→6)-α | Mutan | Lactobacillus reuteri Streptococcus mutans Streptococcus salivarius Streptococcus sownei | [131,132] |
| (1→3)(1→4)-α | - | Nigeran | Aspergillus niger var.awamori Aspergillus niger var.unknowy some Aspergillus species | [133] |
| (1→3)(1→4)-α | - | - | Aspergillus wentii | [112] |
| (1→3)(1→4)-α | - | - | Cladosporium herbarum | [134] |
| (1→3)(1→4)-α | - | Elsinan | Elsinoe leucospila | [135] |
| (1→3)(1→4)-α | - | - | Neurospora crassa | [136] |
| (1→3)(1→4)-α | - | Nigeran | Few other Penicillium species | [137] |
| (1→3)(1→4)-α | - | - | Schizosaccharomyces pombe | [124] |
| (1→3)(1→4)-α | - | Nigeran | Armillaria mellea | [123] |
| (1→3)(1→4)-α | - | - | Coriolus versicolor | [138] |
| (1→3)(1→4)-α | - | Pseudonigeran | Cryptococcus neoformans | [139] |
| (1→3)(1→4)-α | - | Pseudonigeran | Laetiporus sulphureus | [127] |
| (1→3)(1→4)-α | - | Pseudonigeran | Lentinus edodes | [128] |
| (1→3)(1→4)-α | - | Isolichenin | Cetraria richardsonii | [140] |
| (1→3)(1→4)-α | - | Isolichenin | Cetraria islandica | [140] |
| (1→3)(1→4)-α | - | Isolichenin | Letharia vulpine | [140] |
| (1→3)(1→4)-α | - | Everniin | Evernia prunastri | [141,142] |
| (1→3)(1→4)-α | - | Nigeran | Parmelia carperata Parmelia cetrarioides Ramalina species, Cladonia species | [140] |
| (1→3)(1→4)-α | - | Isolichenin | Alectoria sarmentosa Alectoria sulcate Cetraria species Usnea species Parmelia species | [141,142] |
| (1→3)(1→6)-α | (1→3)-α | Alternan | Leuconostoc mesenteroides Streptococcus salivarius | [131,132] |
| (1→3)(1→6)-α | - | - | Termitomyces eurhizus | [45] |
| (1→3)(1→4)(1→6)-α | - | Acroscyphan | Acroscyphus sphaerophoroides | [141,142] |
| (1→6)-α | - | - | Coriolus versicolor | [138] |
| (1→6)-α | - | - | Sarcodon aspratus | [143] |
| (1→6)-α | - | - | Termitomyces eurhizus | [45] |
| (1→6)-α | - | Starch | Banana | [144] |
| (1→6)-α | - | Starch | Dimocarpus longan Lour cv Shixia | [145] |
| (1→6)-α | - | Starch | Pueraria lobata (willed) ohwi | [146] |
| (1→6)-α | - | Starch | Ipomea batatus | [147] |
| (1→6)-α 1 | - | - | Chlorella vulgaris | [148] |
| (1→6)-α | (1→3)-α | - | Lobelia chinensis | [149] |
| (1→6)-α | (1→2); (1→3); (1→4)-α | Dextran | Lactobacillus species Leuconostoc dextranicum Leuconostoc mesenteroides Streptococcus mutans Weissella species | [131,132] |
| (1→2)-α | - | - | - | |
2. 1,2-cis glycosylation
2.1. Bimodal Glycosylation Approach
2.1.1. Bimodal Glycosylation Approach for 1,2-cis α-glucosylation
2.1.2. Bimodal Glycosyl Donor Approach for Application to 1,2-cis α-galactosylation and 1,2-cis-β-mannosylation
2.2. ZnI2-mediated Stereoselective Glycosylation Approach
2.2.1. ZnI2-mediated 1,2-cis α-glucosylation
2.2.2. ZnI2-mediated 1,2-cis β-mannosylation, and cis β-galactosylation
3. Recent Progress on the Synthesis of α-glucans
3.1. Application of the Bimodal Glycosylation Approach for Stereoselective 1,2-cis α-glucosylation toward the Synthesis of α-glucans
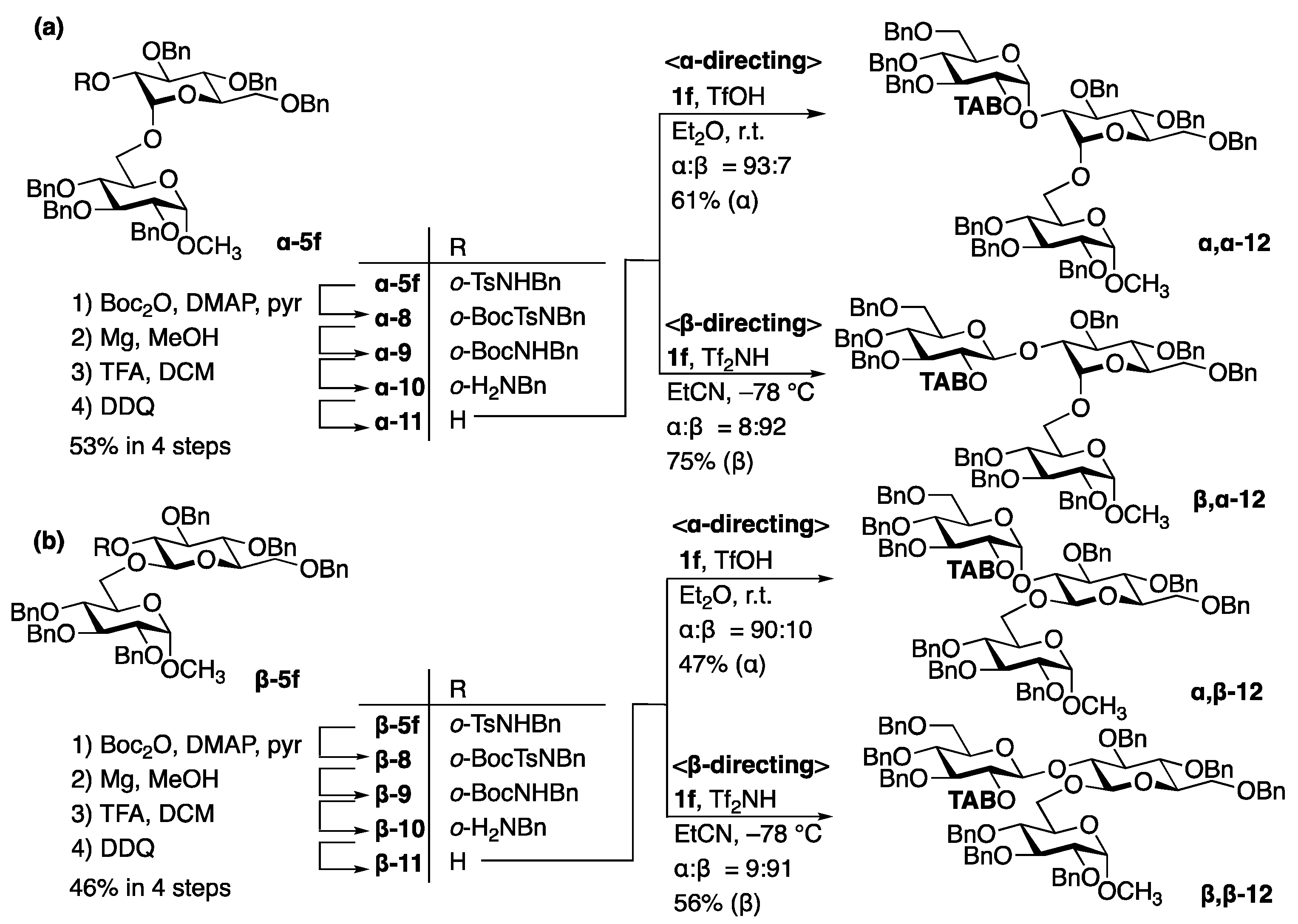
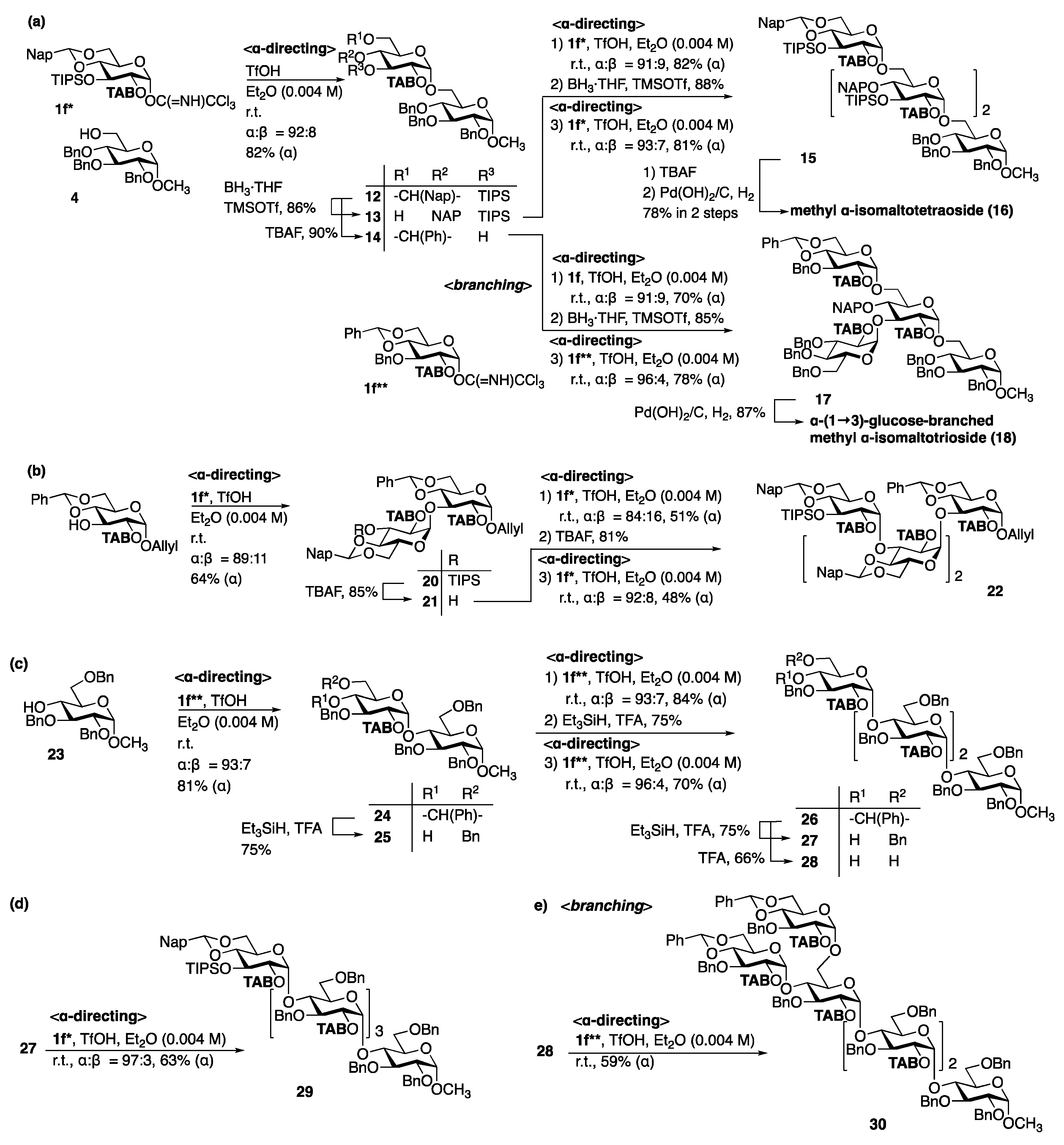
3.1.1. Bimodal Glycosylation Approach for the Synthesis of Linear α-glucans
3.1.2. Bimodal Glycosylation Approach for the Synthesis of Branched α-glucans
3.2. Application of ZnI2-mediated Stereoselective 1,2-cis α-glucosylation toward the Synthesis of α-glucans
3.2.1. ZnI2-mediated Glycosylation Approach for the Synthesis of Linear α-glucans
3.2.2. ZnI2-mediated Glycosylation Approach for the Synthesis of Branched α-glucans
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andreana, P.R.; Crich, D. Guidelines for O-Glycoside Formation from First Principles. ACS Cent. Sci. 2021, 7, 1454–1462. [Google Scholar] [CrossRef]
- Gangoiti, J.; Corwin, S.F.; Lamothe, L.M.; Vafiadi, C.; Hamaker, B.R.; Dijkhuizen, L. Synthesis of novel α-glucans with potential health benefits through controlled glucose release in the human gastrointestinal tract. Crit. Rev. Food Sci. Nutr. 2020, 60, 123–146. [Google Scholar] [CrossRef]
- Shivatare, S.S.; Wong, C.-H. Synthetic Carbohydrate Chemistry and Translational Medicine. J. Org. Chem. 2020, 85, 15780–15800. [Google Scholar] [CrossRef]
- Loh, C.C.J. Exploiting non-covalent interactions in selective carbohydrate synthesis. Nat. Rev. Chem. 2021, 5, 792–815. [Google Scholar] [CrossRef]
- Wang, L.; Overkleeft, H.S.; van der Marel, G.A.; Codée, J.D.C. Reagent Controlled Stereoselective Synthesis of α-Glucans. J. Am. Chem. Soc. 2018, 140, 4632–4638. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Y.; Overkleeft, H.S.; van der Marel, G.A.; Codée, J.D.C. Reagent Controlled Glycosylations for the Assembly of Well-Defined Pel Oligosaccharides. J. Org. Chem. 2020, 85, 15872–15884. [Google Scholar] [CrossRef]
- Inuki, S.; Tabuchi, H.; Matsuzaki, C.; Yonejima, Y.; Hisa, K.; Kimura, I.; Yamamoto, K.; Ohno, H. Chemical Synthesis and Evaluation of Exopolysaccharide Fragments Produced by Leuconostoc mesenteroides Strain NTM048. Chem. Pharm. Bull. 2022, 70, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Shetty, P.R.; Batchu, U.R.; Buddana, S.K.; Sambasiva Rao, K.; Penna, S. A comprehensive review on α-D-Glucans: Structural and functional diversity, derivatization and bioapplications. Carbohydr. Res. 2021, 503, 108297. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.-L.; Li, J.-Y.; Wang, Y.; Chen, Y.; Wen, Q.-L. Extraction, Structure and Bioactivity of Polysaccharides from Tricholoma matsutake (S. Ito et Imai) Singer (Review). Appl. Biochem. Microbiol. 2022, 58, 375–381. [Google Scholar] [CrossRef]
- Stephens, Z.; Wilson, L.F.L.; Zimmer, J. Diverse mechanisms of polysaccharide biosynthesis, assembly and secretion across kingdoms. Curr. Opin. Struct. Biol. 2023, 79, 102564. [Google Scholar] [CrossRef] [PubMed]
- Thitipraphunkul, K.; Uttapap, D.; Piyachomkwan, K.; Takeda, Y. A comparative study of edible canna (Canna edulis) starch from different cultivars. Part II. Molecular structure of amylose and amylopectin. Carbohydr. Polym. 2003, 54, 489–498. [Google Scholar] [CrossRef]
- Sarko, A.; Wu, H.-C.H. The Crystal Structures of A-, B- and C-Polymorphs of Amylose and Starch. Starch 1978, 30, 73–78. [Google Scholar] [CrossRef]
- Helbert, W.; Chanzy, H. Single crystals of V amylose complexed with n-butanol or n-pentanol: Structural features and properties. Int. J. Biol. Macromol. 1994, 16, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Bail, P.L.; Rondeau, C.; Buleon, A. Structural investigation of amylose complexes with small ligands: Helical conformation, crystalline structure and thermostability. Int. J. Biol. Macromol. 2005, 35, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Rappenecker, G.; Zugenmaier, P. Detailed refinement of the crystal structure of Vh-amylose. Carbonhydr. Res. 1981, 89, 11–19. [Google Scholar] [CrossRef]
- Zhang, Q.; Lu, Z.; Hu, H.; Yang, W.; Marszalek, P.E. Direct detection of the formation of V-amylose helix by single molecule force spectroscopy. J. Am. Chem. Soc. 2006, 128, 9387–9393. [Google Scholar] [CrossRef]
- Sivak, M.N.; Preiss, J. (Eds.) Starch: Basic Science to Biotechnology. In Advances in Food and Nutrition Research; Academic Press: Cambridge, MA, USA, 1998; Volume 41. [Google Scholar]
- Buléon, A.; Colonna, P.; Planchot, V.; Ball, S. Starch granules: Structure and biosynthesis. Int. J. Biol. Macromol. 1998, 23, 85–112. [Google Scholar] [CrossRef]
- Wang, T.L.; Bogracheva, T.Y.; Hedley, C.L. Starch: As simple as A, B, C? J. Exp. Bot. 1998, 49, 481–502. [Google Scholar] [CrossRef]
- James, M.G.; Robertson, D.S.; Myers, A.M. Characterization of the maize gene sugary1, a determinant of starch composition in kernels. Plant Cell 1995, 7, 417–429. [Google Scholar]
- Hehre, E.J.; Hamilton, D.M.; Carlson, A.S. Synthesis of a polsaccharide of the starch glycogen class from sucrose by a cell-free, bacterial enzyme system (amylosucrase). J. Biol. Chem. 1949, 177, 267–279. [Google Scholar] [CrossRef]
- Potocki de Montalk, G.; Remaud-Simeon, M.; Willemot, R.-M.; Sarçabal, P.; Planchot, V.; Monsan, P. Amylosucrase from Neisseria polysaccharea: Novel catalytic properties. FEBS Lett. 2000, 471, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-S.; Kim, H.-S.; Hong, J.-S.; Huber, K.C.; Shim, J.-H.; Yoo, S.-H. Effects of amylosucrase treatment on molecular structure and digestion resistance of pre-gelatinised rice and barley starches. Food Chem. 2013, 138, 966–975. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.-S.; Hong, M.-G.; Park, S.-H.; Lee, B.-H.; Yoo, S.-H. Biocatalytic Fabrication of α-Glucan-Coated Porous Starch Granules by Amylolytic and Glucan-Synthesizing Enzymes as a Target-Specific Delivery Carrier. Biomacromolecules 2019, 20, 4143–4149. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ren, J.; Liu, J.; Sun, L.; Wang, Y.; Liu, B.; Li, C.; Li, Z. Modification by α-D-glucan branching enzyme lowers the in vitro digestibility of starch from different sources. Int. J. Biol. Macromol. 2018, 107, 1758–1764. [Google Scholar] [CrossRef]
- Park, I.; Park, M.; Yoon, N.; Cha, J. Comparison of the Structural Properties and Nutritional Fraction of Corn Starch Treated with Thermophilic GH13 and GH57 α-Glucan Branching Enzymes. Foods 2019, 8, 452. [Google Scholar] [CrossRef]
- Ban, X.; Dhoble, A.S.; Li, C.; Gu, Z.; Hong, Y.; Cheng, L.; Holler, T.P.; Kaustubh, B.; Li, Z. Bacterial 1,4-α-glucan branching enzymes: Characteristics, preparation and commercial applications. Crit. Rev. Biotechnol. 2020, 40, 380–396. [Google Scholar] [CrossRef]
- Yu, L.; Kong, H.; Gu, Z.; Li, C.; Ban, X.; Cheng, L.; Hong, Y.; Li, Z. Two 1,4-α-glucan branching enzymes successively rearrange glycosidic bonds: A novel synergistic approach for reducing starch digestibility. Carbohydr. Polym. 2021, 262, 117968. [Google Scholar] [CrossRef]
- Xu, T.; Li, Z.; Gu, Z.; Li, C.; Cheng, L.; Hong, Y.; Ban, X. The N-terminus of 1,4-α-glucan branching enzyme plays an important role in its non-classical secretion in Bacillus subtilis. Food Biosci. 2023, 52, 102491. [Google Scholar] [CrossRef]
- Lambré, C.; Baviera, J.M.B.; Bolognesi, C.; Cocconcelli, P.S.; Crebelli, R.; Gott, D.M.; Grob, K.; Lampi, E.; Mengelers, M.; Mortensen, A.; et al. Safety evaluation of the food enzyme 1,4-α-glucan branching enzyme from the non-genetically modified Geobacillus thermodenitrificans strain TRBE14. EFSA J. 2023, 21, e07834. [Google Scholar]
- Carbonero, E.R.; Montai, A.V.; Woranovicz-Barreira, S.; Gorin, P.A.J.; Lacomini, M. Polysaccharides of lichenized fungi of three Cladina spp.: Significance as chemotypes. Phytochemistry 2002, 61, 681–686. [Google Scholar] [CrossRef]
- Synytsya, A.; Novak, M. Structural analysis of glucans. Ann. Transl. Med. 2014, 2, 17–31. [Google Scholar]
- Naessens, M.; Cerdobbel, A.; Soetaert, W.; Vandamme, E.J. Leuconostoc dextransucrase and dextran: Production, properties and applications. J. Chem. Technol. Biotechnol. 2005, 80, 845–860. [Google Scholar] [CrossRef]
- Zhong, X.; Wang, G.; Fang, S.; Zhou, S.; Ishiwata, A.; Cai, H.; Ding, F. Immunomodulatory Effect and Biological Significance of β-Glucans. Pharmaceutics 2023, 15, 1615. [Google Scholar] [CrossRef]
- Okuyama, M.; Saburi, W.; Mori, H.; Kimura, A. α-Glucosidases and α-1,4-Glucan Lyases: Structures, Functions, and Physiological Actions. Cell. Mol. Life Sci. 2016, 73, 2727–2751. [Google Scholar] [CrossRef]
- Synytsya, A.; Novák, M. Structural Diversity of Fungal Glucans. Carbohydr. Polym. 2013, 92, 792–809. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Xu, X.; Yu, M.; Yang, Z.; Zheng, L. Characterisation and immunostimulatory activity of an α-(1→6)-D-glucan from the cultured Armillariella tabescens mycelia. Food Chem. 2008, 111, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Han, X.Q.; Wu, X.M.; Chai, X.Y.; Chen, D.; Dai, H.; Dong, H.L.; Ma, Z.Z.; Gao, X.M.; Tu, P.F. Isolation, characterization and immunological activity of a polysaccharide from the fruit bodies of an edible mushroom, Sarcodon aspratus (Berk.) S. Ito. Food. Res. Int. 2011, 44, 489–493. [Google Scholar] [CrossRef]
- Painter, T.J. Details of the fine structure of nigeran revealed by the kinetics of its oxidation by periodate. Carbohydr. Res. 1990, 200, 403–408. [Google Scholar] [CrossRef]
- Pasteur, L. On the viscous fermentation and the butyrous fermentation. Bull. Soc. Chim. Paris 1861, 11, 30–31. [Google Scholar]
- Leemhuis, H.; Pijning, T.; Dobruchowska, J.M.; van Leeuwen, S.S.; Kralj, S.; Dijkstra, B.W.; Dijkhuizen, L. Glucansucrases: Three-dimensional structures, reactions, mechanism, α-glucan analysis and their implications in biotechnology and food applications. J. Biotechnol. 2013, 163, 250–272. [Google Scholar] [CrossRef] [PubMed]
- van Hijum, S.A.F.T.; Kralj, S.; Ozimek, L.K.; Dijkhuizen, L.; van Geel-Schutten, I.G.H. Structure-function relationships of glucansucrase and fructansucrase enzymes from lactic acid bacteria. Microbiol. Mol. Biol. Rev. 2006, 70, 157–176. [Google Scholar] [CrossRef] [PubMed]
- Simpson, C.L.; Cheetham, N.W.H.; Jacques, N.A. Four glucosyltransferases, gtfJ, gtfK, gtfL and gtfM, from Streptococcus salivarius ATCC 25975. Microbiology 1995, 141, 1451–1460. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.-K.; Oh, J.-S.; Kim, D. Molecular characterization and expression analysis of the glucansucrase DSRWC from Weissella cibaria synthesizing a α(1→6) glucan. FEMS Microbiol. Lett. 2009, 292, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Chakraborty, I.; Pramanik, M.; Rout, D.; Islam, S.S. Structural studies of water-soluble polysaccharides of an edible mushroom, Termitomyces eurhizus. A reinvestigation. Carbohydr. Res. 2004, 339, 1135–1140. [Google Scholar] [CrossRef] [PubMed]
- Purama, R.K.; Goswami, P.; Khan, A.T.; Goyal, A. Structural analysis and properties of dextran produced by Leuconostoc mesenteroides NRRL B-640. Carbohydr. Polym. 2009, 76, 30–35. [Google Scholar] [CrossRef]
- Loesche, W.J. Role of Streptococcus mutans in human dental decay. Microbiol. Rev. 1986, 50, 353. [Google Scholar] [CrossRef]
- He, Q.; Kobayashi, K.; Kusumi, R.; Kimura, S.; Enomoto, Y.; Yoshida, M.; Kim, U.-J.; Wada, M. In vitro Synthesis of Branchless Linear (1→6)-α-D-Glucan by Glucosyltransferase K: Mechanical and Swelling Properties of Its Hydrogels Crosslinked with Diglycidyl Ethers. ACS Omega 2020, 5, 31272–31280. [Google Scholar] [CrossRef]
- Rosenfeld, E.L.; Lukomskaya, I.S. The splitting of dextran and isomaltose by animal tissues. Clin. Chim. Acta 1957, 2, 105–114. [Google Scholar] [CrossRef]
- Wang, R.; Dijkstra, P.J.; Karperien, M. Dextran. Biomaterials from Nature for Advanced Devices and Therapies; Wiley: Hoboken, NJ, USA, 2016; pp. 307–319. [Google Scholar]
- Hong, M.-G.; Yoo, S.-H.; Lee, B.-H. Effect of highly branched α-glucans synthesized by dual glycosyltransferases on the glucose release rate. Carbohydr. Polymer 2022, 278, 119016. [Google Scholar] [CrossRef]
- Banerjee, A.; Bandopadhyay, R. Use of dextran nanoparticle: A paradigm shift in bacterial exopolysaccharide based biomedical applications. Int. J. Biol. Macromol. 2016, 87, 295–301. [Google Scholar] [CrossRef]
- Lamothe, L.M.; Francey, C.; Lerea-Antes, J.S.; Rytz, A.; D’Urzo, C.; Delodder, F.; Piccardi, N.; Curti, D.; Murciano Martinez, P.; Darimont, C.; et al. Effects of α-D-glucans with alternating 1,3/1,6 α-D-glucopyranosyl linkages on postprandial glycemic response in healthy subjects. Carbohydr. Polym. Technol. Appl. 2022, 4, 100256. [Google Scholar] [CrossRef]
- Zonneveld, B.J.M. The Significance of α-1,3-glucan of the cell wall and α-1,3-glucanase for cleistothecium development. Biochim. Biophys. Acta. 1972, 273, 174–187. [Google Scholar] [CrossRef] [PubMed]
- Johnston, I.R. The composition of the cell wall of Aspergillus niger. Biochem. J. 1965, 96, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Zonneveld, B.J.M. Biochemical analysis of the cell wall of Aspergillus nidulans. Biochim. Biophys. Acta. 1971, 249, 506–514. [Google Scholar] [CrossRef]
- Yoshimi, A.; Miyazawa, K.; Abe, K. Function and Biosynthesis of Cell Wall α-1,3-Glucan in Fungi. J. Fungi 2017, 3, 63. [Google Scholar] [CrossRef] [PubMed]
- Van der Kaaij, R.M.; Janecek, S.; van der Maarel, M.J.E.C.; Dijkhuizen, L. Phylogenetic and biochemical characterization of a novel cluster of intracellular fungal α-amylase enzymes. Microbiology 2007, 153, 4003–4015. [Google Scholar] [CrossRef]
- Marion, C.L.; Rappleye, C.A.; Engle, J.T.; Goldman, W.E. An α-(1,4)-amylase is essential for α-(1,3)-glucan production and virulence in Histoplasma capsulatum. Mol. Microbiol. 2006, 62, 970–983. [Google Scholar] [CrossRef]
- Camacho, E.; Sepulveda, V.E.; Goldman, W.E.; San-Blas, G.; Niño-Vega, G.A. Expression of Paracoccidioides brasiliensis AMY1 in a Histoplasma capsulatum amy1 mutant, relates an α-(1,4)-amylase to cell wall α-(1,3)-glucan synthesis. PLoS ONE 2012, 7, e50201. [Google Scholar] [CrossRef]
- Koizumi, A.; Miyazawa, K.; Ogata, M.; Takahashi, Y.; Yano, S.; Yoshimi, A.; Sano, M.; Hidaka, M.; Nihira, T.; Nakai, H.; et al. Cleavage of α-1,4-glycosidic linkages by the glycosylphosphatidylinositol-anchored α-amylase AgtA decreases the molecular weight of cell wall α-1,3-glucan in Aspergillus oryzae. Front. Fungal Biol. 2023, 3, 1061841. [Google Scholar] [CrossRef]
- Jelsma, J.; Kreger, D.R. Polymorphism in crystalline (1→3)-α-D-glucan from fungal cell-walls. Carbohydr. Res. 1979, 71, 51–64. [Google Scholar] [CrossRef]
- Złotko, K.; Wiater, A.; Waśko, A.; Pleszczyńska, M.; Paduch, R.; Jaroszuk-Ściseł, J.; Bieganowski, A. A Report on Fungal (1→3)-α-D-glucans: Properties, Functions and Application. Molecules 2019, 24, 3972. [Google Scholar] [CrossRef]
- Moreno-Mendieta, S.; Guillén, D.; Hernández-Pando, R.; Sánchez, S.; Rodríguez-Sanoja, R. Potential of glucans as vaccine adjuvants: A review of the α-glucans case. Carbohydr. Polym. 2017, 165, 103–114. [Google Scholar] [CrossRef]
- Patra, S.; Maity, P.; Chakraborty, I.; Sen, I.K.; Ghosh, D.; Rout, D.; Bhanja, S.K. Structural studies of immunomodulatory (1→3)-, (1→4)-α glucan from an edible mushroom Polyporus grammocephalus. Int. J. Biol. Macromol. 2021, 168, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Xu, J.; Wu, W.; Wen, Y.; Lu, S.; El-Seedi, H.R.; Zhao, C. Structure–immunomodulatory activity relationships of dietary polysaccharides. Curr. Res. Food Sci. 2022, 5, 1330–1341. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Kong, H.; Fang, Y.; Nishinari, K.; Phillips, G.O. Schizophyllan: A review on its structure, properties, bioactivities and recent developments. Bioact. Carbohydr. Diet. Fibre 2013, 1, 53–71. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Agafonova, S.V.; Rokhin, A.V.; Penzina, T.A.; Borovskii, G.B. Branched glucan from the fruiting bodies of Piptoporus betulinus (Bull.:Fr) Karst. Appl. Biochem. Microbiol. 2012, 48, 65–70. [Google Scholar] [CrossRef]
- Pozsgay, V.; Nánási, P.; Neszmélyi, A. Utilisation of the d-glucopyranosyl group as a non-participating group in stereoselective glycosylation: Synthesis of O-α-D-glucopyranosyl-(1→2)-O-α-D-glucopyranosyl-(1→6)-D-glucose. Carbohydr. Res. 1979, 75, 310–313. [Google Scholar] [CrossRef]
- Rychener, M.; Bigler, P.; Pfander, H. Synthese und 1H-NMR-Studie der vier unverzweigten peracetylierten β-D-Glucopyranosyl-β-gentiobiosen. Helv. Chim. Acta 1984, 67, 378–385. [Google Scholar] [CrossRef]
- Gómez de Segura, A.; Alcalde, M.; Bernabé, M.; Ballesteros, A.; Plou, F.J. Synthesis of methyl α-D-glucooligosaccharides by entrapped dextransucrase from Leuconostoc mesenteroides B-1299. J. Biotechnol. 2006, 124, 439–445. [Google Scholar] [CrossRef]
- Brissonnet, Y.; Ladevèze, S.; Tezé, D.; Fabre, E.; Deniaud, D.; Daligault, F.; Tellier, C.; Šesták, S.; Remaud-Simeon, M.; Potocki-Veronese, G.; et al. Polymeric Iminosugars Improve the Activity of Carbohydrate-Processing Enzymes. Bioconjugate Chem. 2015, 26, 766–772. [Google Scholar] [CrossRef]
- Ahrazem, O.; Rubio-Moraga, A.; Jimeno, M.; Gómez-Gómez, L. Structural characterization of highly glucosylated crocins and regulation of their biosynthesis during flower development in Crocus. Front. Plant Sci. 2015, 6, 971–985. [Google Scholar] [CrossRef] [PubMed]
- Hoshi, H.; Yagi, Y.; Iijima, H.; Matsunaga, K.; Ishihara, Y.; Yasunara, T. Isolation and Characterization of a Novel Immunomodulatory α-Glucan-Protein Complex from the Mycelium of Tricholoma matsutake in Basidiomycetes. J. Agric. Food Chem. 2005, 53, 8948–8956. [Google Scholar] [CrossRef]
- Kroon-Batenburg, L.M.; Kroon, J. The crystal and molecular structures of cellulose I and II. Glycoconj. J. 1997, 14, 677–690. [Google Scholar] [CrossRef]
- Chawla, P.R.; Bajaj, I.B.; Survase, S.A.; Singhal, R.S. Microbial Cellulose: Fermentative Production and Applications. Food Technol. Biotechnol. 2009, 47, 107–124. [Google Scholar]
- Brown, G.D.; Gordon, S. Immune recognition. A new receptor for β-glucans. Nature 2001, 413, 36–37. [Google Scholar] [CrossRef]
- Brown, G.D.; Herre, J.; Williams, D.L.; Willment, J.A.; Marshall, A.S.; Gordon, S. Dectin-1 mediates the biological effects of β-glucans. J. Exp. Med. 2003, 197, 1119–1124. [Google Scholar] [CrossRef] [PubMed]
- Zipfel, C.; Robatzek, S. Pathogen-Associated Molecular Pattern-Triggered Immunity: Veni, Vidi…? Plant Physiol. 2010, 154, 551–554. [Google Scholar] [CrossRef] [PubMed]
- Legentil, L.; Paris, F.; Ballet, C.; Trouvelot, S.; Daire, X.; Vetvicka, V.; Ferrières, V. Molecular Interactions of β-(1→3)-Glucans with Their Receptors. Molecules 2015, 20, 9745–9766. [Google Scholar] [CrossRef]
- Adachi, Y. Role of the 1,3-β-D-Glucan Receptor Dectin-1 in Fungal Infection and Activation of Innate and Anti-Tumor Immunity. Trends Glycosci. Glycotechnol. 2007, 19, 195–207. [Google Scholar] [CrossRef]
- Fesel, P.H.; Zuccaro, A. β-Glucan: Crucial Component of the Fungal Cell Wall and Elusive MAMP in Plants. Fungal Genet. Biol. 2016, 90, 53–60. [Google Scholar] [CrossRef]
- Vetvicka, V.; Vannucci, L.; Sima, P.; Richter, J. Beta Glucan: Supplement or Drug? From Laboratory to Clinical Trials. Molecules 2019, 24, 1251–1268. [Google Scholar] [CrossRef] [PubMed]
- Miyagawa, A. Chemical Synthesis of β-(1,3)-Glucan Oligosaccharide and Its Application. Trends Glycosci. Glycotechnol. 2018, 30, E117–E127. [Google Scholar] [CrossRef]
- Lemassu, A.; Ortalo-Magne, A.; Bardou, F.; Silve, G.; Laneelle, M.A.; Daffe, M. Extracellular and surface-exposed polysaccharides of non-tuberculous mycobacteria. Microbiol. 1996, 142, 1513–1520. [Google Scholar] [CrossRef] [PubMed]
- Ortalo-Magne, A.; Dupont, M.A.; Lemassu, A.; Andersen, A.B.; Gounon, P.; Daffe, M. Molecular composition of the outermost capsular material of the tubercle bacillus. Microbiol. 1995, 141, 1609–1620. [Google Scholar] [CrossRef]
- Miah, F.; Bibb, M.J.; Barclay, J.E.; Findlay, K.C.; Bornemann, S. Developmental delay in a Streptomyces venezuelae glgE null mutant is associated with the accumulation of α-maltose 1-phosphate. Microbiol. 2016, 162, 1208–1219. [Google Scholar] [CrossRef]
- Buck, K.W.; Obaidah, M.A. The composition of cell wall of Fusicoccum amygdali. Biochem. J. 1971, 125, 461–471. [Google Scholar] [CrossRef][Green Version]
- Mizuno, T.; Hagiwara, T.; Nakamura, T.; Ito, H.; Shimura, K.; Sumiya, T.; Asakura, A. Antitumor activity and some properties of water-soluble polysaccharides from “Himematsutake”, the fruiting body of Agaricus blazei Murill. Agric. Biol. Chem. 1990, 54, 2889–2896. [Google Scholar]
- Ghosh, T.; Auerochs, S.; Saha, S.; Ray, B.; Marschall, M. Anti-cytomegalovirus activity of sulfated glucans generated from a commercial preparation of rice bran. Antivir. Chem. Chemother. 2010, 21, 85–95. [Google Scholar] [CrossRef]
- Gunja Smith, Z.; Smith, E.E. Evidence for the periplasmic location of glycogen in Saccharomyces. Biochem. Biophys. Res. Commun. 1974, 56, 588–592. [Google Scholar] [CrossRef]
- Smiderle, F.R.; Sassaki, G.L.; Van Arkel, J.; Iacomini, M.; Wichers, H.J.; Van Griensven, L.J.L.D. High molecular weight glucan of the medicinal mushroom Agaricus bisporus is an α-glucan that forms complexes with low molecular weight galactan. Molecules 2010, 15, 5818–5830. [Google Scholar] [CrossRef]
- Yalin, W.; Cuirong, S.; Yuanjiang, P. Studies on isolation and structural features of a polysaccharide from the mycelium of a Chinese edible fungus (Cordyceps sinensis). Carbohydr. Polym. 2006, 63, 251–256. [Google Scholar] [CrossRef]
- Li, B.; Dobruchowska, J.M.; Gerwig, G.J.; Dijkhuizen, L.; Kamerling, J.P. Structural investigation of water-soluble polysaccharides extracted from the fruit bodies of Coprinus comatus. Carbohydr. Polym. 2013, 91, 314–321. [Google Scholar] [CrossRef]
- Pang, X.; Yao, W.; Yang, X.; Xie, C.; Liu, D.; Zhang, J.; Gao, X. Purification, characterization and biological activity on hepatocytes of a polysaccharide from Flammulina velutipes mycelium. Carbohydr. Polym. 2007, 70, 291–297. [Google Scholar] [CrossRef]
- Qiu, H.; Tang, W.; Tong, X.; Ding, K.; Zuo, J. Structure elucidation and sulfated derivatives preparation of two α-D-glucans from Gastrodia elata and their anti- dengue virus bioactivities. Carbohydr. Res. 2007, 342, 2230–2236. [Google Scholar] [CrossRef]
- Wang, P.; Liao, W.; Fang, J.; Liu, Q.; Yao, J.; Hu, M.; Ding, K. A glucan isolated from flowers of Lonicera japonica Thunb. inhibits aggregation and neurotoxicity of Aβ42. Carbohydr. Polym. 2014, 110, 142–147. [Google Scholar] [CrossRef]
- Niu, H.; Song, D.; Sun, Y.; Zhang, W.; Mu, H.; Duan, J. Preparation and sulfation of an α-D-glucan from Actinidia chinensis roots and their potential activities. Int. J. Biol. Macromol. 2016, 92, 981–987. [Google Scholar] [CrossRef]
- Kralj, S.; Stripling, E.; Sanders, P.; Van Geel-Schutten, G.H.; Dijkhu-izen, L. Highly hydrolytic reuteransucrase from probiotic Lactobacillus reuteri strain ATCC 55730. Appl. Environ. Microbiol. 2005, 71, 3942–3950. [Google Scholar] [CrossRef]
- McIntyre, D.D.; Vogel, H.J. Structural studies of pullulan by nuclear magnetic resonance spectroscopy. Starch 1993, 45, 406–410. [Google Scholar] [CrossRef]
- Reid, I.D.; Bartnicki-García, S. Cell-wall composition and structure of yeast cells and conjugation tubes of Tremella mesenterica. J. Gen. Microbiol. 1976, 96, 35–50. [Google Scholar] [CrossRef] [PubMed]
- Leal, J.A.; Guerrrero, C.; Gomez-Miranda, B.; Prieto, A.; Bernabe, M. Chemical and structural similarities in wall polysaccharides of some Penicillium, Eupenicillium and Aspergillus species. FEMS Microbiol. Lett. 1992, 69, 165–168. [Google Scholar] [CrossRef]
- Beauvais, A.; Maubon, D.; Park, S.; Morelle, W.; Tangu, M.; Huerre, M.; Perlin, D.S.; Latgé, J.P. Two α-(1-3) glucan synthases with different functions in Aspergillus fumigatus. Appl. Environ. Microbiol. 2005, 71, 1531–1538. [Google Scholar] [CrossRef] [PubMed]
- Beauvais, A.; Bozza, S.; Kniemeyer, O.; Formosa, C.; Formosa, C.; Balloy, V.; Henry, C.; Roberson, R.W.; Dague, E.; Chignard, M.; et al. Deletion of the α-(1,3)-glucan synthase genes induces a restructuring of the conidial cell wall responsible for the avirulence of Aspergillus fumigatus. PLoS Pathog. 2013, 9, e1003716. [Google Scholar] [CrossRef]
- Fontaine, T.; Beauvais, A.; Loussert, C.; Thevenard, B.; Fulgsang, C.C.; Ohno, N.; Clavaud, C.; Prevost, M.-C.; Latgé, J.-P. Cell wall α1-3glucans induce the aggregation of germinating conidia of Aspergillus fumigatus. Fungal. Genet. Biol. 2010, 47, 707–712. [Google Scholar] [CrossRef]
- Henry, C.; Latgé, J.-P.; Beauvais, A. α1,3 glucans are dispensable in Aspergillus fumigatus. Eukaryot. Cell 2012, 11, 26–29. [Google Scholar] [CrossRef]
- Borgia, P.T.; Dodge, C.L. Characterization of Aspergillus nidulans mutants deficient in cell wall chitin or glucan. J. Bacteriol. 1992, 174, 377–383. [Google Scholar] [CrossRef]
- He, X.; Li, S.; Kaminskyj, S.G.W. Characterization of Aspergillus nidulans α-glucan synthesis: Roles for two synthases and two amylases. Mol. Microbiol. 2014, 91, 579–595. [Google Scholar] [CrossRef]
- Yoshimi, A.; Sano, M.; Inaba, A.; Kokubun, Y.; Fujioka, T.; Mizutani, O.; Hagiwara, D.; Fujikawa, T.; Nishimura, M.; Yano, S.; et al. Functional analysis of the α-1,3-glucan synthase genes agsA and agsB in Aspergillus nidulans: agsB is the major α-1,3-glucan synthase in this fungus. PLoS ONE 2013, 8, e54893. [Google Scholar] [CrossRef] [PubMed]
- Damveld, R.A.; vanKuyk, P.A.; Arentshorst, M.; Klis, F.M.; van den Hondel, C.A.M.J.J.; Ram, A.F.J. Expression of agsA, one of five 1,3-α-D-glucan synthase-encoding genes in Aspergillus niger, is induced in response to cell wall stress. Fungal. Genet. Biol. 2005, 42, 165–177. [Google Scholar] [CrossRef]
- Horisberger, M.; Lewis, B.A.; Smith, F. Structure of a (1→3)-α-D-glucan (pseudonigeran) of Aspergillus niger NNRL 326 cell wall. Carbohydr. Res. 1972, 23, 183–188. [Google Scholar] [CrossRef]
- Choma, A.; Wiater, A.; Komaniecka, I.; Paduch, R.; Pleszczyńska, M.; Szczodrak, J. Chemical characterization of a water insoluble (1→3)-α-D-glucan from an alkaline extract of Aspergillus wentii. Carbohydr. Polym. 2013, 91, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Manandar, M.; Scalarone, G.M. Comparative Studies on Alpha 1-3 Glucan in Blastomyces Dermatitidis Yeast Lysate Antigens and the Use of the Lysates for the Detection of Antibodies. In Proceedings of the Pacific Division American Association for the Advancement of Science, San Francisco, CA, USA, 14–19 August 2009; Volume 28. [Google Scholar]
- Hogan, L.H.; Klein, B.S. Altered expression of surface α-1,3-glucan in genetically related strains of Blastomyces dermatitidis that differ in virulence. Infect. Immun. 1994, 62, 3543–3546. [Google Scholar] [CrossRef]
- Schoffelmeer, E.A.; Klis, F.M.; Sietsma, J.H.; Cornelissen, B.J. The cell wall of Fusarium oxysporum. Fungal Genet. Biol. 1999, 27, 275–282. [Google Scholar] [CrossRef]
- Eissenberg, L.G.; Poirier, S.; Goldman, W.E. Phenotypic variation and persistence of Histoplasma capsulatum Yeasts in host cells. Infect. Immun. 1996, 64, 5310–5314. [Google Scholar] [CrossRef] [PubMed]
- San-Blas, G.; Carbonell, L.M. Chemical and ultrastructural studies on the cell wall of the yeast like and mycelial forms of Histoplasma farcinimosum. J. Bacteriol. 1974, 119, 602–611. [Google Scholar] [CrossRef] [PubMed]
- Kanestuna, F.; Carbonell, L.M. Cell wall glucans of the yeast and mycelial forms of Paracoccidioides brasiliensis. J. Bacteriol. 1970, 101, 675–680. [Google Scholar]
- Parra, E.; Barbero, J.J.; Bernabe, M.; Leal, J.A.; Prieto, A.; Gomez-Miranda, B. Structural investigation of two cell-wall polysaccharides of Penicillium expansum strains. Carbohydr. Res. 1994, 257, 239–248. [Google Scholar] [CrossRef]
- Wang, T.; Deng, L.; Li, S.; Tan, T. Structural characterization of a water insoluble (1→3)-α-D-glucan isolated from Penicillium chrysogenum. Carbohydr. Polym. 2007, 67, 133–137. [Google Scholar] [CrossRef]
- Haung, Q.; Zhang, L.; Cheung, P.C.K.; Tan, X. Evaluation of sulfated α-glucans from Poria cocos mycelia as a potential antitumor agent. Carbohydr. Polym. 2006, 64, 337–344. [Google Scholar] [CrossRef]
- Kiho, T.; Yoshida, I.; Nagai, K.; Ukai, S.; Hara, C. (1→3)-α-D-glucan from an alkaline extract of Agrocybe cylindracea, and antitumor activity of its O-(carboxymethyl)ated derivatives. Carbohydr. Res. 1989, 189, 273–279. [Google Scholar] [CrossRef]
- Grun, C. Structure and Biosynthesis of Fungal α-glucans; Universiteit Utrecht, Faculteit Scheikunde: Utrecht, The Netherlands, 2003. [Google Scholar]
- Bacon, J.S.D.; Jones, D.; Farmer, V.C.; Webley, D.M. The occurrence of (1→3)-α-glucan in Cryptococcus, Schizosaccharomyces and Polyporus species, and its hydrolysis by a Streptomyces culture filtrate lysing cell walls of Cryptococcus. Biochim. Biophys. Acta 1968, 158, 313–315. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, L.; Nakamura, Y.; Norisuye, T. Viscosity behavior and chain Conformation of a (1→3)-α-glucan from Ganoderma lucidum. Polym. Bull. 1998, 41, 471–478. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, J.; Zhang, L.; Nakamura, Y.; Norisuye, T. Chemical structure of the water-insoluble polysaccharide isolated from the fruit body of Ganoderma lucidium. Polym. J. 1998, 10, 838–842. [Google Scholar] [CrossRef]
- Jelsma, J.; Kreger, D.R. Observations on the cell-wall compositions of the bracket fungi Laetiporus sulphureus and Piptoporus betulinus. Arch. Microbiol. 1978, 119, 249–255. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, L.; Cheng, S. Solution properties of an α-(1→3)-d-glucan from Lentinus edodes and its sulfated derivatives. Carbohydr. Res. 2002, 337, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Angyal, S.J.; Bender, J.; Ralph, B.J. Structure of polysaccharides from the Polyporus tumulosus cell wall. Biochim. Biophys. Acta 1974, 362, 175–187. [Google Scholar] [CrossRef]
- Siehr, D. Studies on the cell wall of Schizophyllum commune. Permethylation and enzymic hydrolysis. Can. J. Biochem. 1976, 54, 130–136. [Google Scholar] [CrossRef]
- Monsan, P.; Bozonnet, S.; Albenne, C.; Joucla, G.; Willemot, R.M.; Remaud-Simeon, M. Homopolysaccharides from Lactic acid bacteria. Int. Dairy J. 2001, 11, 675–685. [Google Scholar] [CrossRef]
- Torino, M.I.; de Valdez, G.F.; Mozzi, F. Biopolymers from lactic acid bacteria. Novel applications in foods and beverages. Front. Microbiol. 2015, 6, 834. [Google Scholar] [CrossRef]
- Bobbit, T.F.; Nordin, J.H.; Roux, M.; Revol, J.F.; Marchessault, R.H. Distribution and conformation of crystalline nigeranin hyphal walls of Aspergillus niger and Aspergillus awamori. J. Bacteriol. 1977, 132, 691–703. [Google Scholar] [CrossRef]
- Miyazaki, T.; Naoi, Y. Chemical structure of the water-soluble glucan from the cell wall of Cladosporium herbarum. Studies on fungal polysaccharide. XV. Chem. Pharm. Bull. 1974, 22, 2058–2063. [Google Scholar] [CrossRef][Green Version]
- Misaki, A.; Tsumuraya, Y.; Takaya, S. A New Fungal α-D-Glucan, Elsinan, Elaborated by Elsinoe Leucospila. Agric. Biol. Chem. 1978, 42, 491–493. [Google Scholar] [CrossRef]
- Cardemil, L.; Pincheira, G. Characterization of the carbohydrate component of fraction I in the Neurospora crassa cell wall. J. Bacteriol. 1979, 137, 1067–1072. [Google Scholar] [CrossRef]
- Bobbitt, T.F.; Nordin, J.H. Hyphal nigeran as a potential phylogenetic marker for Aspergillus and Penicillium species. Mycologia 1978, 70, 1201–1211. [Google Scholar] [CrossRef] [PubMed]
- Hirase, S.; Nakai, S.; Akatsu, T.; Kobayashi, A.; Ohara, M.; Matsunaga, K.; Fujii, M.; Kodaira, S.; Fujii, T.; Furusho, T.; et al. Structural studies on the antitumor active polysaccharides from Coriolus versicolor (Basidiomycetes). II. Structural of β-D-glucan moieties of fractionated polysaccharides. Yakugaku Zasshi 1976, 96, 419–424. [Google Scholar] [CrossRef]
- James, P.G.; Cherniak, R. 4-Methylmorpholine N-oxide-methyl sulfoxide soluble glucan of Piptoporus betulinus. Carbohydr. Res. 1990, 206, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Olafsdottir, E.S.; Ingolfsdottir, K. Polysaccharides from lichens: Structural characteristics and biological activity. Planta Med. 2001, 67, 199–208. [Google Scholar] [CrossRef]
- Shibata, S. Polysaccharides of lichens. J. Nat. Sci. Council. SriLanka 1973, 1, 183–188. [Google Scholar]
- Stüde, F. Ueber Everniin, Pectin und eine neue glycogene Substanz. Liebigs Ann. Chem. 1864, 131, 241–251. [Google Scholar] [CrossRef][Green Version]
- Han, X.Q.; Chai, X.Y.; Jia, Y.M.; Han, C.X.; Tu, P.F. Structure elucidation and immunological activity of a novel polysaccharide from the fruit bodies of an edible mushroom, Sarcodon aspratus (Berk. ) S. Ito. Int. J. Biol. Macromol. 2010, 47, 420–424. [Google Scholar] [CrossRef]
- Wen, L.; Shi, D.; Zhou, T.; Liu, H.; Jiang, Y.; Yang, B. Immunomodulatory mechanism of α-D-(1→6) glucan isolated from banana. RSC Adv. 2019, 9, 6995–7003. [Google Scholar] [CrossRef]
- Zhu, Q.; Jiang, Y.; Lin, S.; Wen, L.; Wu, D.; Zhao, M.; Chen, F.; Jia, Y.; Yang, B. Structural identification of (1→6)-α-D-glucan, a key responsible for the health benefits of longan, and evaluation of anticancer activity. Biomacromol. 2013, 14, 1999–2003. [Google Scholar] [CrossRef]
- Cui, H.; Liu, Q.; Tao, Y.; Zhang, H.; Zhang, L.; Ding, K. Structure and chain conformation of a (1→6)-α-D-glucan from the root of Puerarian lobata (Willd.) Ohwi and the antioxidant activity of its sulfated derivative. Carbohydr. Polym. 2008, 74, 771–778. [Google Scholar] [CrossRef]
- Zhao, G.H.; Kan, J.Q.; Li, Z.X.; Chen, Z.D. Characterization and immuno stimulatory activity of an (1→6)-α-D-glucan from the root of Ipomoea batatas. Int. Immunopharm. 2005, 5, 1436–1445. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zhao, L.; Liua, X.; Hua, F.; Cui, F.; Bi, Y.; Ma, Y.; Feng, S. Structural characterization of a sulfated glucan isolated from the aqueous extract of Hedysarum polybotrys Hand.-Mazz. Carbohydr. Polym. 2012, 87, 160–169. [Google Scholar] [CrossRef]
- Li, X.J.; Bao, W.R.; Leung, C.H.; Ma, D.L.; Zhang, G.; Lu, A.P.; Wang, S.C.; Han, Q.B. Chemical structure and immunomodulating activities of an α-glucan purified from Lobelia chinensis Lour. Molecules 2016, 21, 779. [Google Scholar] [CrossRef]
- Morelli, L.; Compostella, F.; Panza, L.; Imperio, D. Unusual Promoters and Leaving Groups in Glycosylation Reactions: The Evolution of Carbohydrate Synthesis. Carbohydr. Res. 2022, 519, 108625. [Google Scholar] [CrossRef] [PubMed]
- Singh, Y.; Geringer, S.A.; Demchenko, A.V. Synthesis and Glycosidation of Anomeric Halides: Evolution from Early Studies to Modern Methods of the 21st Century. Chem. Rev. 2022, 122, 11701–11758. [Google Scholar] [CrossRef]
- Ishiwata, A.; Tanaka, K.; Ao, J.; Ding, F.; Ito, Y. Recent advances in stereoselective 1,2-cis-O-glycosylations. Front. Chem. 2022, 10, 972429. [Google Scholar] [CrossRef]
- Takahashi, D.; Toshima, K. 1,2-cis O-glycosylation methods. In Comprehensive Glycoscience; Barchi, J., Ed.; Elsevier Science: Amsterdam, The Netherlands, 2021; Volume 2, pp. 365–412. [Google Scholar]
- Lv, Z.; Liu, H.; Hao, H.; Rahman, F.-U.; Zhang, Y. Chemical synthesis of oligosaccharides and their application in new drug research. Eur. J. Med. Chem. 2023, 249, 115164. [Google Scholar] [CrossRef]
- Shadrick, M.; Singh, Y.; Demchenko, A.V. Stereocontrolled α-galactosylation under Cooperative Catalysis. J. Org. Chem. 2020, 85, 15936–15944. [Google Scholar] [CrossRef]
- Ishiwata, A.; Lee, Y.J.; Ito, Y. Recent advances in stereoselective glycosylation through intramolecular aglycon delivery. Org. Biomol. Chem. 2010, 8, 3596–3608. [Google Scholar] [CrossRef]
- Ishiwata, A.; Ito, Y. Intramolecular Aglycon Delivery. In Selective Glycosylations—Synthetic Methods and Catalysts; Bennett, C.S., Ed.; Wiley: Weinheim, Germany, 2017; Chapter II-4; pp. 81–96. [Google Scholar]
- Ishiwata, A. Synthetic Study on Glycoconjugates Containing 1,2-cis Glycoside and Their Application. Trends Glycosci. Glycotech. 2019, 31, SE53–SE54. [Google Scholar] [CrossRef]
- Nigudkar, S.S.; Demchenko, A.V. Stereocontrolled 1,2-cis glycosylation as the driving force of progress in synthetic carbohydrate chemistry. Chem. Sci. 2015, 6, 2687–2704. [Google Scholar] [CrossRef] [PubMed]
- Leng, W.-L.; Yao, H.; He, J.-X.; Liu, X.-W. Venturing beyond Donor-Controlled Glycosylation: New Perspectives toward Anomeric Selectivity. Acc. Chem. Res. 2018, 51, 628–639. [Google Scholar] [CrossRef] [PubMed]
- van der Vorm, S.; Hansen, T.; van Hengst, J.M.A.; Overkleeft, H.S.; van der Marel, G.A.; Codée, J.D.C. Acceptor reactivity in glycosylation reactions. Chem. Soc. Rev. 2019, 48, 4688–4706. [Google Scholar] [CrossRef] [PubMed]
- Njeri, D.K.; Valenzuela, E.A.; Ragains, J.R. Leveraging Trifluoromethylated Benzyl Groups toward the Highly 1,2-cis-Selective Glucosylation of Reactive Alcohols. Org. Lett. 2021, 23, 8214–8218. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Takemoto, Y. Regio- and stereoselective glycosylation of 1,2-O-unprotected sugars using organoboron catalysts. Tetrahedron 2020, 76, 131328. [Google Scholar] [CrossRef]
- Feng, Y.; Guo, T.; Yang, H.; Liu, G.; Zhang, Q.; Zhang, S.; Chai, Y. Ni(II)-Catalyzed Regio- and Stereoselective O-Alkylation for the Construction of 1,2-cis-Glycosidic Linkages. Org. Lett. 2022, 24, 6282–6287. [Google Scholar] [CrossRef]
- Ma, Z.; Hu, Y.; Li, X.; Liu, R.; Xia, E.; Xu, P.; Yang, Y. Stereoselective synthesis of α-glucosides with glucosyl (Z)-Ynenoates as donors. Carbohydr. Res. 2023, 523, 108710. [Google Scholar] [CrossRef]
- Szarek, W.A.; Horton, D. Anomeric Effect; American Chemical Society: Washington, DC, USA, 1979. [Google Scholar]
- Deslongchamps, P. Stereoelectronic Effect in Organic Chemistry; Pergamon: Oxford, UK, 1983. [Google Scholar]
- Juaristi, E.; Cuevas, G. The Anomeric Effect; CRC: Boca Raton, FL, USA, 1995. [Google Scholar]
- Kirby, A.J. Stereoelectronic Effect; Oxford University Press: New York, NY, USA, 1996. [Google Scholar]
- Perrin, C.L. Reverse anomeric effect: Fact or fiction? Tetrahedron 1995, 51, 11901–11935. [Google Scholar] [CrossRef]
- Randell, K.D.; Johnston, B.D.; Green, D.F.; Pinto, B.M. Is there a generalized reverse anomeric effect? Substituent and solvent effects on the configurational equilibria of neutral and protonated N-Arylglucopyranosylamines and N-Aryl-5-thioglucopyranosylamines. J. Org. Chem. 2000, 65, 220–226. [Google Scholar] [CrossRef]
- Vaino, A.R.; Szarek, W.A. An examination of the purported reverse anomeric effect beyond acetylated N-xylosyl-and N-glucosylimidazoles. J. Org. Chem. 2001, 66, 1097–1102. [Google Scholar] [CrossRef] [PubMed]
- Perrin, C.L.; Kuperman, J. Anomeric effects versus steric hindrance to ionic solvation in protonated glucosylanilines and cyclohexylanilines. J. Am. Chem. Soc. 2003, 125, 8846–8851. [Google Scholar] [CrossRef]
- Reichardt, C. (Ed.) Solvents and Solvent Effects in Organic Chemistry, 3rd ed.; Wiley-VCH: Weinheim, Germany, 2003. [Google Scholar]
- Lemieux, R.U.; Pavia, A.A.; Martin, J.C.; Watanabe, K.A. Solvation effects on conformational equilibria. Studies related to the conformational properties of 2-methoxytetrahydropyran and related methyl glycopyranosides. Can. J. Chem. 1969, 47, 4427–4439. [Google Scholar] [CrossRef]
- Eby, R.; Schuerch, C. The Use of 1-O-Tosyl-D-glucopyranose Derivatives in α-D-Glucoside Synthesis. Carbohydr. Res. 1974, 34, 79–90. [Google Scholar] [CrossRef]
- Schmidt, R.R.; Rücker, E. Stereoselective glycosidations of uronic acids. Tetrahedron Lett. 1980, 21, 421–1424. [Google Scholar] [CrossRef]
- Lemieux, R.U.; Ratcliffe, R.M. The azidonitration of tri-O-acetyl-D-galactal. Can. J. Chem. 1979, 57, 1244–1251. [Google Scholar] [CrossRef]
- Ishiwata, A.; Ito, Y. High throughput screening of O-glycosylation conditions. Tetrahedron Lett. 2005, 46, 3521–3524. [Google Scholar] [CrossRef]
- Ishiwata, A.; Munemura, Y.; Ito, Y. Synergistic solvent effect in 1,2-cis-glycoside formation. Tetrahedron 2008, 64, 92–102. [Google Scholar] [CrossRef]
- Chao, C.-S.; Li, C.-W.; Chen, M.-C.; Chang, S.-S.; Mong, K.-K.T. Low-Concentration 1,2-trans β-Selective Glycosylation Strategy and Its Applications in Oligosaccharide Synthesis. Chem. Eur. J. 2009, 15, 10972–10982. [Google Scholar] [CrossRef]
- Chao, C.-S.; Lin, C.-Y.; Mulani, S.; Hung, W.-C.; Mong, K.-K.T. Neighboring-group participation by C-2 ether functions in glycosylations directed by nitrile solvents. Chem. Eur. J. 2011, 17, 12193–12202. [Google Scholar] [CrossRef]
- Demchenko, A.; Stauch, T.; Boons, G.J. Solvent and other effects on the stereoselectivity of thioglycoside glycosidations. Synlett 1997, 1997, 818–820. [Google Scholar] [CrossRef]
- Takatani, M.; Nakano, J.; Arai, M.A.; Ishiwata, A.; Ohta, H.; Ito, Y. Accelerated glycosylation under frozen conditions. Tetrahedron Lett. 2004, 45, 3929–3932. [Google Scholar] [CrossRef]
- Ishiwata, A.; Sakurai, A.; Dürr, K.; Ito, Y. Effects of frozen conditions on stereoselectivity and velocity of O-glycosylation reactions. Bioorg. Med. Chem. 2010, 18, 3687–3695. [Google Scholar] [CrossRef]
- Csávás, M.; Herczeg, M.; Bajza, I.; Borbás, A. Protecting Group Manipulations in Carbohydrate Synthesis, Comprehensive Glycoscience, 2nd ed.; Barchi, J.J., Jr., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 464–524. [Google Scholar]
- Ghosh, B.; Kulkarni, S.S. Advances in Protecting Groups for Oligosaccharide Synthesis. Chem. Asian J. 2020, 15, 450–462. [Google Scholar] [CrossRef] [PubMed]
- Meyer, A.G.; Bissember, A.C.; Hyland, C.J.T.; Williams, C.C.; Szabo, M.; Pearsall, M.A.; Hyland, I.K.; Olivier, W.J. Seven-Membered Rings. In Progress in Heterocyclic Chemistry; Gribble, G.W., Joule, J.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 579–633. [Google Scholar]
- Ma, X.; Zheng, Z.; Fu, Y.; Zhu, X.; Liu, P.; Zhang, L. A “Traceless” Directing Group Enables Catalytic SN2 Glycosylation toward 1,2-cis-Glycopyranosides. J. Am. Chem. Soc. 2021, 143, 11908–11913. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zhang, Y.; Zhu, X.; Wei, Y.; Zhang, L. Directed SN2 Glycosylation Employing an Amide-Functionalized 1-Naphthoate Platform Featuring a Selectivity-Safeguarding Mechanism. J. Am. Chem. Soc. 2023, 145, 11921–11926. [Google Scholar] [CrossRef]
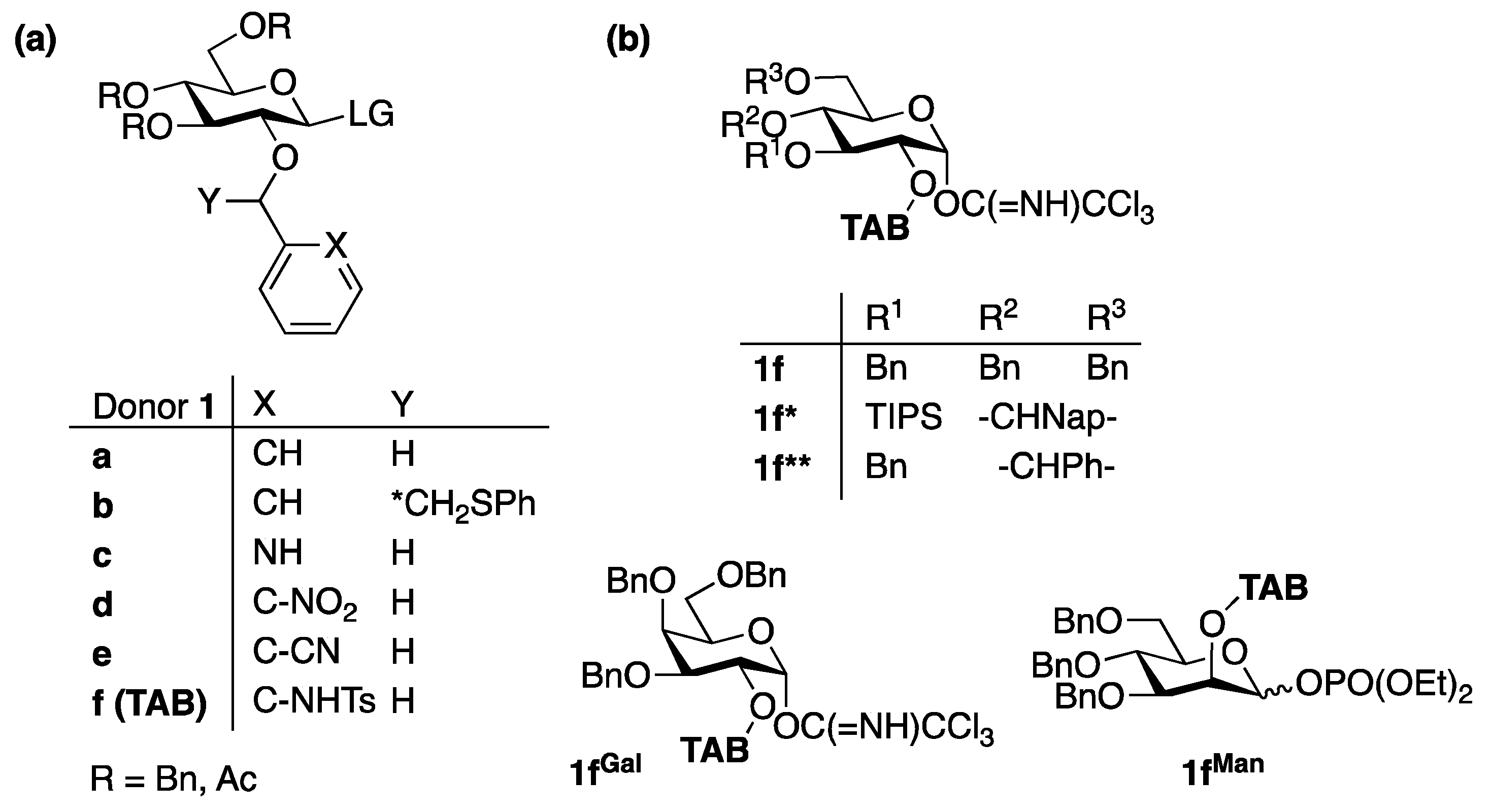
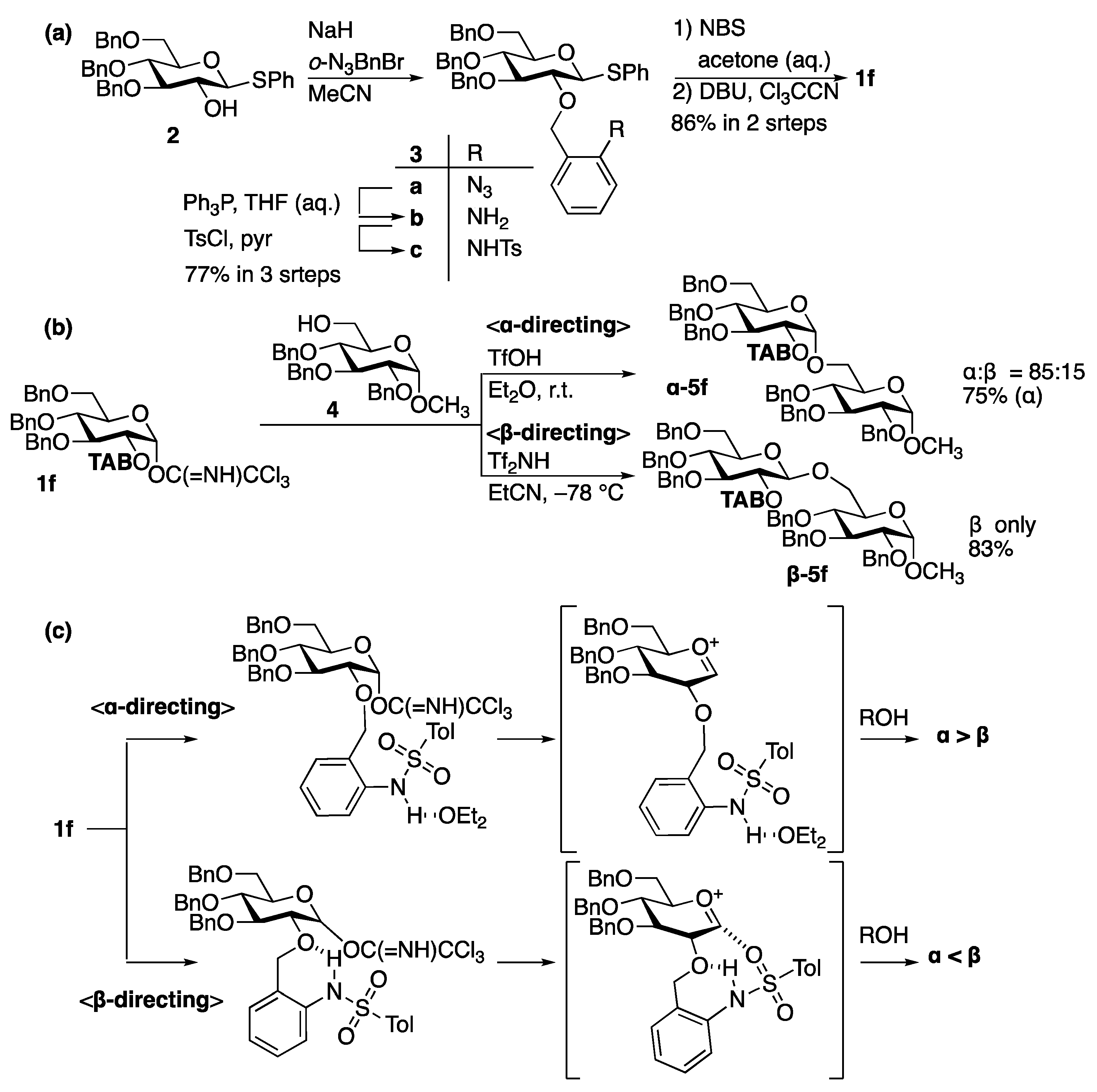
- Ding, F.; Ishiwata, A.; Ito, Y. Bimodal Glycosyl Donors Protected by 2-O-(ortho-Tosylamido)benzyl Group. Org. Lett. 2018, 20, 4384–4388. [Google Scholar] [CrossRef]
- Ding, F.; Ishiwata, A.; Ito, Y. Recent advances of the stereoselective bimodal glycosylations for the synthesis of various glucans. Stud. Nat. Prod. Chem. 2022, 74, 1–40. [Google Scholar]
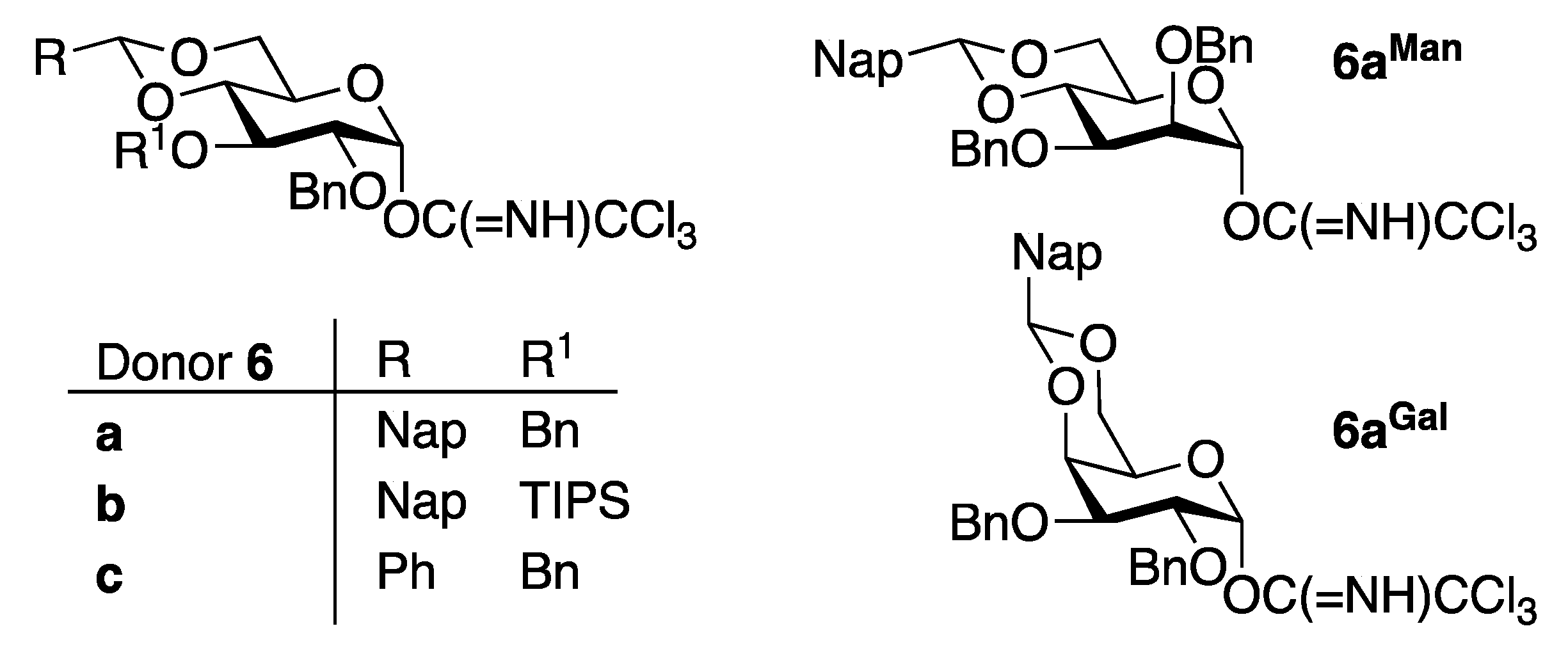
- Ding, F.; Ishiwata, A.; Zhou, S.; Zhong, X.; Ito, Y. Unified Strategy toward Stereocontrolled Assembly of Various Glucans Based on Bimodal Glycosyl Donors. J. Org. Chem. 2020, 85, 5536–5558. [Google Scholar] [CrossRef]
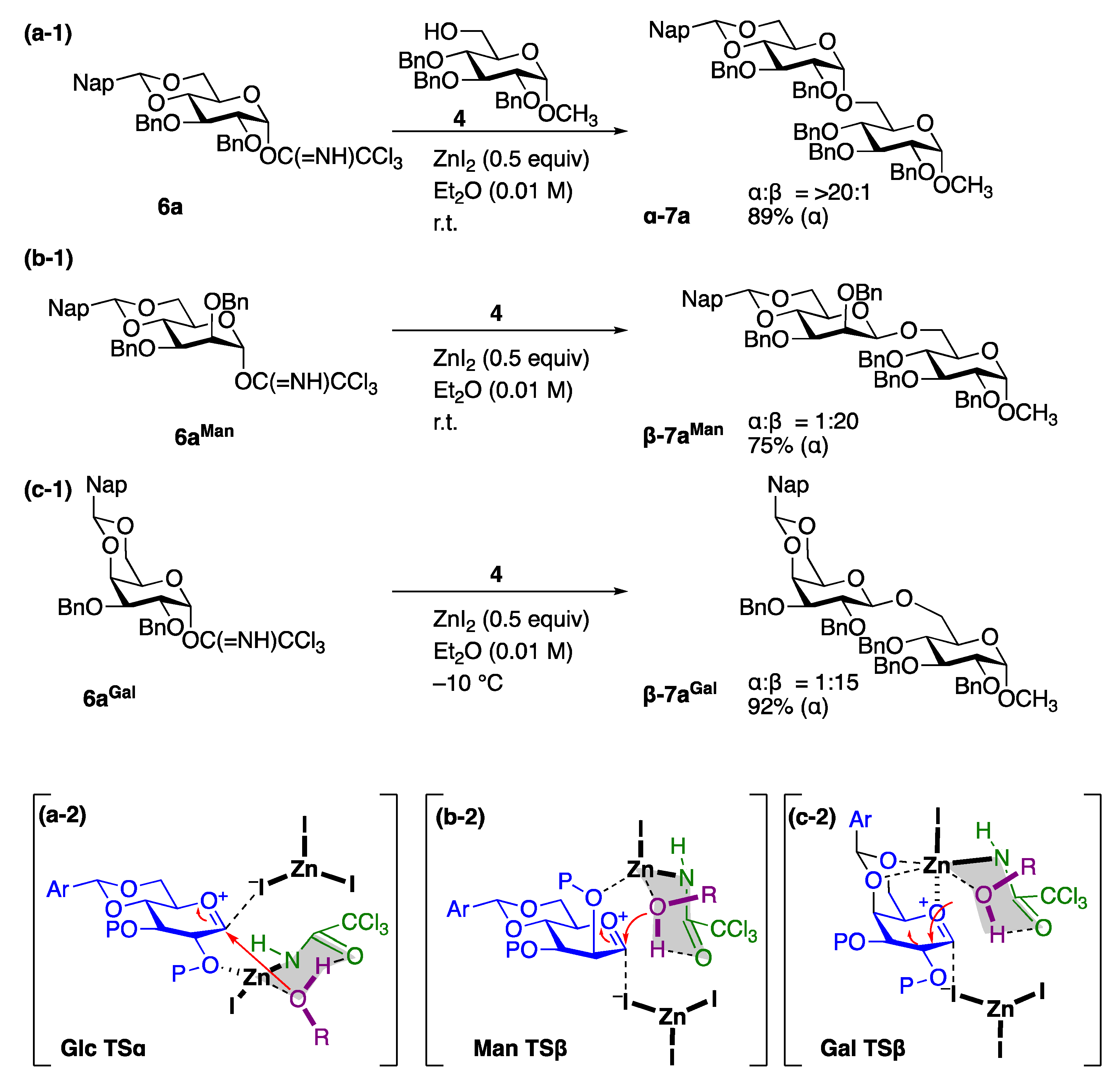
- Zhou, S.; Zhong, X.; Guo, A.; Xiao, Q.; Ao, J.; Zhu, W.; Cai, H.; Ishiwata, A.; Ito, Y.; Liu, X.-W.; et al. ZnI2-Directed Stereocontrolled α-glucosylation. Org. Lett. 2021, 23, 6841–6845. [Google Scholar] [CrossRef]
- Hoang, K.M.; Lees, N.R.; Herzon, S.B. Programmable Synthesis of 2-Deoxyglycosides. J. Am. Chem. Soc. 2019, 141, 8098–8103. [Google Scholar] [CrossRef]
- Hoang, K.L.M.; Liu, X.-W. The Intriguing Dual-directing Effect of 2-Cyanobenzyl Ether for a Highly Stereospecific Glycosylation Reaction. Nat. Commun. 2014, 5, 5051. [Google Scholar] [CrossRef]
- Kimura, T.; Eto, T.; Takahashi, D.; Toshima, K. Stereocontrolled Photoinduced Glycosylation Using an Aryl Thiourea as an Organo photoacid. Org. Lett. 2016, 18, 3190–3193. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Liu, H.; Tang, A.H.; Payne, R.J.; Li, X. A Solution to Chemical Pseudaminylation via a Bimodal Glycosyl Donor for Highly Stereocontrolled α- and β-Glycosylation. Org. Lett. 2019, 21, 3584–3588. [Google Scholar] [CrossRef]
- Yang, F.; Sun, Y.; Xu, P.; Molinaro, A.; Silipo, A.; Yu, B. Synthesis of Unprecedented α/β-Alternate (1→4)-Glucans via Stereoselective Iterative Glycosylation. Chem. Eur. J. ASAP 2023, 29, e202300659. [Google Scholar] [CrossRef] [PubMed]
- Di Bussolo, V.; Caselli, M.; Romano, M.R.; Pineschi, M.; Crotti, P. New Stereoselective β-C-Glycosidation by Uncatalyzed 1,4-Addition of Organolithium Reagents to a Glycal-Derived Vinyl Oxirane. J. Org. Chem. 2004, 69, 7383–7386. [Google Scholar] [CrossRef] [PubMed]
- Di Bussolo, V.; Romano, M.R.; Pineschi, M.; Crotti, P. Stereoselective Synthesis of 4-(N-Mesylamino)-2,3-unsaturated-α-O-glycosides via a New Glycal-Derived Vinyl α-N-(Mesyl)-aziridine. Org. Lett. 2005, 7, 1299–1302. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; William, R.; Wang, F.; Ma, J.; Ji, L.; Liu, X.-W. A Short and Highly Efficient Synthesis of L-Ristosamine and L-epi-Daunosamine Glycosides. Org. Lett. 2011, 13, 652–655. [Google Scholar] [CrossRef]
- Ding, F.; William, R.; Wang, S.; Gorityala, B.K.; Liu, X.-W. Ready access to 3-amino-2,3-dideoxysugars via regio- and stereo-selective tandem hydroamination–glycosylation of glycals. Org. Biomol. Chem. 2011, 9, 3929–3939. [Google Scholar] [CrossRef]
- Ding, F.; William, R.; Cai, S.T.; Ma, J.; Liu, X.-W. Stereoselective Synthesis of 1,3-cis-3-Arylsulphonaminodeoxydisaccharides and Oligosaccharides. J. Org. Chem. 2012, 77, 5245–5254. [Google Scholar] [CrossRef]
- Smolinsky, G. The Vapor Phase Pyrolysis of Several Subsituted Azidobenzenes. J. Org. Chem. 1961, 26, 4108–4110. [Google Scholar] [CrossRef]
- Majumdar, K.C.; Ganai, S. An expedient approach to substituted triazolo [1,5-a][1,4]benzodiazepines via Cu-catalyzed tandem Ullmann C–N coupling/azide-alkyne cycloaddition. Tetrahedron Lett. 2013, 54, 6192–6195. [Google Scholar] [CrossRef]
- Kowalska, K.; Pedersen, C.M. Catalytic stereospecific O-glycosylation. Chem. Commun. 2017, 53, 2040–2043. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; William, R.; Wang, F.; Liu, X.-W. Triflimide-catalyzed allyl–allyl cross-coupling: A metal-free allylic alkylation. Chem. Commun. 2012, 48, 8709–8711. [Google Scholar] [CrossRef] [PubMed]
- Mundal, D.A.; Avetta Jr, C.T.; Thomson, R.J. Triflimide-catalysed sigmatropic rearrangement of N-allylhydrazones as an example of a traceless bond construction. Nat. Chem. 2010, 2, 294–297. [Google Scholar] [CrossRef]
- Boxer, M.B.; Yamamoto, H. Triflimide (HNTf2)-catalyzed aldehyde cross-aldol reaction using “super silyl” enol ethers. Nat. Protoc. 2006, 1, 2434–2438. [Google Scholar] [CrossRef]
- Wang, P.; Zhu, J.; Yuan, Y.; Danishefsky, S.J. Total Synthesis of the 2,6-Sialylated Immunoglobulin G Glycopeptide Fragment in Homogeneous Form. J. Am. Chem. Soc. 2009, 131, 16669–16671. [Google Scholar] [CrossRef]
- Mootoo, D.R.; Konradsson, P.; Udodong, U.; Fraser-Reid, B. Armed and disarmed n-pentenyl glycosides in saccharide couplings leading to oligosaccharides. J. Am. Chem. Soc. 1988, 110, 5583–5584. [Google Scholar] [CrossRef]
- Deng, S.; Gangadharmath, U.; Chang, C.-W.T. Sonochemistry: A Powerful Way of Enhancing the Efficiency of Carbohydrate Synthesis. J. Org. Chem. 2006, 71, 5179–5185. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; Ishiwata, A.; Ito, Y. Stereodivergent Mannosylation Using 2-O-(ortho-Tosylamido)benzyl Group. Org. Lett. 2018, 20, 4833–4837. [Google Scholar] [CrossRef]
- Ishiwata, A.; Ito, Y. Glycoscience, Chemistry and Chemical Biology, 2nd ed.; Fraser-Reid, B.O., Tatsuta, K., Thiem, J., Eds.; Springer: Berlin, Germany, 2008; Volume II, Chapter 5.6; pp. 1279–1312. [Google Scholar]
- Crich, D.; Sun, S. Formation of β-Mannopyranosides of Primary Alcohols Using the Sulfoxide Method. J. Org. Chem. 1996, 61, 4506–4507. [Google Scholar] [CrossRef]
- Crich, D.; Sun, S. Direct Formation of β-Mannopyranosides and Other Hindered Glycosides from Thioglycosides. J. Am. Chem. Soc. 1998, 120, 435–436. [Google Scholar] [CrossRef]
- Crich, D.; Sun, S. Direct chemical synthesis of β-mannopyranosides and other glycosides via glycosyl triflates. Tetrahedron 1998, 54, 8321–8348. [Google Scholar] [CrossRef]
- Weingart, R.; Schmidt, R.R. Can preferential β-mannopyranoside formation with 4,6-O-benzylidene protected mannopyranosyl sulfoxides be reached with trichloroacetimidates? Tetrahedron Lett. 2000, 41, 8753–8758. [Google Scholar] [CrossRef]
- Crich, D.; Smith, M. 1-Benzenesulfinyl Piperidine/Trifluoromethanesulfonic Anhydride: A Potent Combination of Shelf-Stable Reagents for the Low-Temperature Conversion of Thioglycosides to Glycosyl Triflates and for the Formation of Diverse Glycosidic Linkages. J. Am. Chem. Soc. 2001, 123, 9015–9020. [Google Scholar] [CrossRef] [PubMed]
- Crich, D.; Smith, M. Solid-Phase Synthesis of β-Mannosides. J. Am. Chem. Soc. 2002, 124, 8867–8869. [Google Scholar] [CrossRef]
- Baek, J.Y.; Choi, T.J.; Jeon, H.B.; Kim, K.S. A Highly Reactive and Stereoselective β-Mannopyranosylation System: Mannosyl 4-Pentenoate/PhSeOTf. Angew. Chem., Int. Ed. 2006, 45, 7436–7440. [Google Scholar] [CrossRef]
- Cumpstey, I. Intramolecular Aglycon Delivery. Carbohydr. Res. 2008, 343, 1553–1573. [Google Scholar] [CrossRef]
- Fairbanks, A.J. Glycosylation through intramolecular aglycon delivery. In Comprehensive Glycoscience, 2nd ed.; Barchi, J.J., Jr., Ed.; Elsevier Science: Amsterdam, The Netherlands, 2021; Volume 2, pp. 413–434. [Google Scholar]
- Barresi, F.; Hindsgaul, O. Synthesis of β-mannopyranosides by intramolecular aglycon delivery. J. Am. Chem. Soc. 1991, 113, 9376–9377. [Google Scholar] [CrossRef]
- Stork, G.; Kim, G. Stereocontrolled synthesis of disaccharides via the temporary silicon connection. J. Am. Chem. Soc. 1992, 114, 1087–1088. [Google Scholar] [CrossRef]
- Ito, Y.; Ogawa, T. A Novel-Approach to the Stereoselective Synthesis of β-Mannosides. Angew. Chem. Int. Ed. 1994, 33, 1765–1767. [Google Scholar] [CrossRef]
- Ennis, S.C.; Fairbanks, A.J.; Tennant-Eyles, R.J.; Yeates, H.S. Steroselective Synthesis of α-Glucosides and β-Mannosides: Tethering and Activation with N-Iodosuccinimide. Synlett 1999, 1999, 1387–1390. [Google Scholar] [CrossRef]
- Sati, G.C.; Martin, J.L.; Xu, Y.; Malakar, T.; Zimmerman, P.M.; Montgomery, J. Fluoride Migration Catalysis Enables Simple, Stereoselective, and Iterative Glycosylation. J. Am. Chem. Soc. 2020, 142, 7235–7242. [Google Scholar] [CrossRef] [PubMed]
- Pistorio, S.G.; Yasomanee, J.P.; Demchenko, A.V. Hydrogen-Bond-Mediated Aglycone Delivery: Focus on β-mannosylation. Org. Lett. 2014, 16, 716–719. [Google Scholar] [CrossRef]
- David, S.; Malleron, A.; Dini, C. Preparation of oligosaccharides with β-D-mannopyranosyl and 2-azido-2-deoxy-β-D-mannopyranosyl residues by inversion at C-2 after coupling. Carbohydr. Res. 1989, 188, 193–200. [Google Scholar] [CrossRef]
- Matsuo, I.; Isomura, M.; Ajisaka, K. Synthesis of an asparagine-linked core pentasaccharide by means of simultaneous inversion reactions. J. Carbohydr. Chem. 1999, 18, 841–850. [Google Scholar] [CrossRef]
- Twaddle, G.W.J.; Yashunsky, D.V.; Nikolaev, A.V. The chemical synthesis of β-(1→4)-linked D-mannobiose and D-mannotriose. Org. Biomol. Chem. 2003, 1, 623–628. [Google Scholar] [CrossRef]
- Sato, K.; Akai, S.; Yoshitomo, A.; Takai, Y. An improved method for synthesizing antennary β-d-mannopyranosyl disaccharide units. Tetrahedron Lett. 2004, 45, 8199–8201. [Google Scholar] [CrossRef]
- Ishii, N.; Ogiwara, K.; Sano, K.; Kumada, J.; Yamamoto, K.; Matsuzaki, Y.; Matsuo, I. Specificity of Donor Structures for endo-β-N-Acetylglucosaminidase-Catalyzed Transglycosylation Reactions. ChemBioChem 2018, 19, 136–141. [Google Scholar] [CrossRef]
- Meng, S.; Bhetuwal, B.R.; Nguyen, H.; Qi, X.; Fang, C.; Saybolt, K.; Li, X.; Liu, P.; Zhu, J. β-mannosylation through O-Alkylation of Anomeric Cesium Alkoxides: Mechanistic Studies and Synthesis of the Hexasaccharide Core of Complex Fucosylated N-Linked Glycans. Eur. J. Org. Chem. 2020, 2020, 2291–2301. [Google Scholar] [CrossRef]
- O’Sullivan, S.; Doni, E.; Tuttle, T.; Murphy, J.A. Metal-free reductive cleavage of C–N and S–N bonds by photoactivated electron transfer from a neutral organic donor. Angew. Chem. Int. Ed. 2014, 53, 474–478. [Google Scholar] [CrossRef] [PubMed]
- Niggemann, M.; Fu, L.; Damsen, H. Taming a vinyl cation with a simple Al(OTf)3 catalyst to promote C−C bond cleavage. Chem. Eur. J. 2017, 23, 12184–12189. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, T.; Sasaki, H.; Sato, M.; Tamari, K.; Matsuda, K. A Cell Wall Proteo-Heteroglycan from Piricularia oryzae: Further Studies of the Structure. J. Biochem. 1977, 82, 1657–1662. [Google Scholar] [CrossRef] [PubMed]
- Vijay, I.K.; Perdew, G.H. Biosynthesis of mammary glycoproteins structural characterization of lipid-linked glucosyloligosaccharides. Eur. J. Biochem. 1982, 126, 167–172. [Google Scholar] [CrossRef]
- Gunnarsson, A.; Svensson, S. Structural studies on the O-glycosidically linked carbohydrate chains of glucoamylase G1 from Aspergillus niger. Eur. J. Biochem. 1984, 145, 463–467. [Google Scholar] [CrossRef]
- Trinel, P.A.; Maes, E.; Zanetta, J.P.; Delplace, F.; Coddeville, B.; Jouault, T.; Strecker, G.; Poulain, D. Candida albicans phospholipomannan, a new member of the fungal mannose inositol phosphoceramide family. J. Biol. Chem. 2002, 277, 37260. [Google Scholar] [CrossRef]
- Goto, M. Protein O-glycosylation in fungi: Diverse structures and multiple functions. Biosci. Biotechnol. Biochem. 2007, 71, 1415–1427. [Google Scholar] [CrossRef]
- Aubry, S.; Sasaki, K.; Sharma, I.; Crich, D. Influence of Protecting Groups on the Reactivity and Selectivity of Glycosylation: Chemistry of the 4,6-O-Benzylidene Protected Mannopyranosyl Donors and Related Species. Top. Curr. Chem. 2010, 301, 141–188. [Google Scholar]
- Crich, D. Mechanism of a Chemical Glycosylation. Acc. Chem. Res. 2010, 43, 1144–1153. [Google Scholar] [CrossRef]
- Zhong, X.; Zhou, S.; Ao, J.; Guo, A.; Xiao, Q.; Huang, Y.; Zhu, W.; Cai, H.; Ishiwata, A.; Ito, Y.; et al. Zinc(II) Iodide-Directed β-mannosylation: Reaction Selectivity, Mode, and Application. J. Org. Chem. 2021, 86, 16901–16915. [Google Scholar] [CrossRef]
- Zhou, S.; Ao, J.; Guo, A.; Zhao, X.; Deng, N.; Wang, G.; Yang, Q.; Ishiwata, A.; Liu, X.-W.; Li, Q.; et al. ZnI2-mediated β-galactosylation of C2-Ether-Type Donor. Org. Lett. 2022, 24, 8025–8030. [Google Scholar] [CrossRef] [PubMed]
- Pongener, I.; Pepe, D.A.; Ruddy, J.J.; McGarrigle, E.M. Stereoselective β-mannosylations and β-rhamnosylations from glycosyl hemiacetals mediated by lithium iodide. Chem. Sci. 2021, 12, 10070–10075. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Yang, J.; Cai, C.; Sun, T.; Zhang, Q.; Chai, Y. Catalytic and highly stereoselective β-mannopyranosylation using a 2,6-lactone-bridged mannopyranosyl ortho-hexynylbenzoate as donor. Chin. Chem. Lett. 2022, 33, 4878–4881. [Google Scholar] [CrossRef]
- Gucchait, A.; Ghosh, A.; Kumar Misra, A. Convergent synthesis of the pentasaccharide repeating unit of the biofilms produced by Klebsiella pneumoniae. Beilstein J. Org. Chem. 2019, 15, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Sun, B.; Cao, X.; Zhu, H.; Oluwadahunsi, O.M.; Liu, D.; Zhu, H.; Zhang, J.; Zhang, Q.; Zhang, G.; et al. Chemical Synthesis of Homogeneous Human E-Cadherin N-Linked Glycopeptides: Stereoselective Convergent Glycosylation and Chemoselective Solid-Phase Aspartylation. Org. Lett. 2020, 22, 8349–8353. [Google Scholar] [CrossRef]
- Helenius, A.; Aebi, M. Intracellular Functions of N-Linked Glycans. Science 2001, 291, 2364–2369. [Google Scholar] [CrossRef]
- Wang, Z.; Chinoy, Z.S.; Ambre, S.G.; Peng, W.; McBride, R.; de Vries, R.P.; Glushka, J.; Paulson, J.C.; Boons, G.-J. A General Strategy for the Chemoenzymatic Synthesis of Asymmetrically Branched N-Glycans. Science 2013, 341, 379–383. [Google Scholar] [CrossRef]
- Koizumi, A.; Matsuo, I.; Takatani, M.; Seko, A.; Hachisu, M.; Takeda, Y.; Ito, Y. Top-Down Chemoenzymatic Approach to a High-Mannose-Type Glycan Library: Synthesis of a Common Precursor and Its Enzymatic Trimming. Angew. Chem. Int. Ed. 2013, 52, 7426–7431. [Google Scholar] [CrossRef]
- Shivatare, S.S.; Chang, S.-H.; Tsai, T.-I.; Ren, C.-T.; Chuang, H.-Y.; Hsu, L.; Lin, C.-W.; Li, S.-T.; Wu, C.-Y.; Wong, C.-H. Efficient Convergent Synthesis of Bi-, Tri-, and Tetra-Antennary Complex Type N-Glycans and Their HIV-1 Antigenicity. J. Am. Chem. Soc. 2013, 135, 15382–15391. [Google Scholar] [CrossRef]
- Walczak, M.A.; Hayashida, J.; Danishefsky, S.J. Building Biologics by Chemical Synthesis: Practical Preparation of Di- and Triantennary N-Linked Glycoconjugates. J. Am. Chem. Soc. 2013, 135, 4700–4703. [Google Scholar] [CrossRef]
- Chao, Q.; Ding, Y.; Chen, Z.-H.; Xiang, M.-H.; Wang, N.; Gao, X.-D. Recent Progress in Chemo-Enzymatic Methods for the Synthesis of N-Glycans. Front. Chem. 2020, 8, 513. [Google Scholar] [CrossRef]
- Kashiwagi, G.A. Intrinsic Issues in the Assembly of 1,2-Linked Oligosaccharides. Asian J. Org. Chem. 2020, 9, 689–697. [Google Scholar] [CrossRef]
- Ikuta, D.; Hirata, Y.; Wakamori, S.; Shimada, H.; Tomabechi, Y.; Kawasaki, Y.; Ikeuchi, K.; Hagimori, T.; Matsumoto, S.; Yamada, H. Conformationally supple glucose monomers enable synthesis of the smallest cyclodextrins. Science 2019, 364, 674–677. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Delbianco, M.; Seeberger, P.H. Automated Assembly of Starch and Glycogen Polysaccharides. J. Am. Chem. Soc. 2021, 143, 9758–9768. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishiwata, A.; Tanaka, K.; Ito, Y.; Cai, H.; Ding, F. Recent Progress in 1,2-cis glycosylation for Glucan Synthesis. Molecules 2023, 28, 5644. https://doi.org/10.3390/molecules28155644
Ishiwata A, Tanaka K, Ito Y, Cai H, Ding F. Recent Progress in 1,2-cis glycosylation for Glucan Synthesis. Molecules. 2023; 28(15):5644. https://doi.org/10.3390/molecules28155644
Chicago/Turabian StyleIshiwata, Akihiro, Katsunori Tanaka, Yukishige Ito, Hui Cai, and Feiqing Ding. 2023. "Recent Progress in 1,2-cis glycosylation for Glucan Synthesis" Molecules 28, no. 15: 5644. https://doi.org/10.3390/molecules28155644
APA StyleIshiwata, A., Tanaka, K., Ito, Y., Cai, H., & Ding, F. (2023). Recent Progress in 1,2-cis glycosylation for Glucan Synthesis. Molecules, 28(15), 5644. https://doi.org/10.3390/molecules28155644






