Droplet Evaporation Process of a Fluorobenzene + n-Octane + Polystyrene Mixture
Abstract
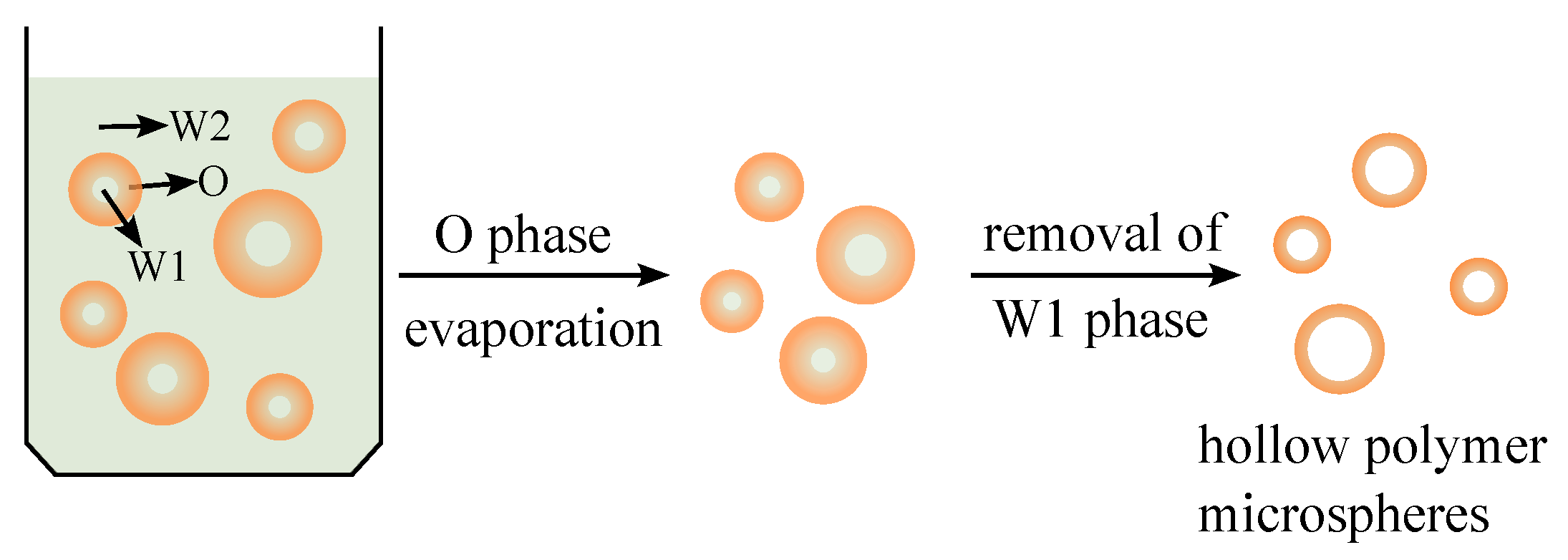
:1. Introduction
2. Results and Discussion
2.1. Vapor–Liquid Equilibrium of the Polymer Solution
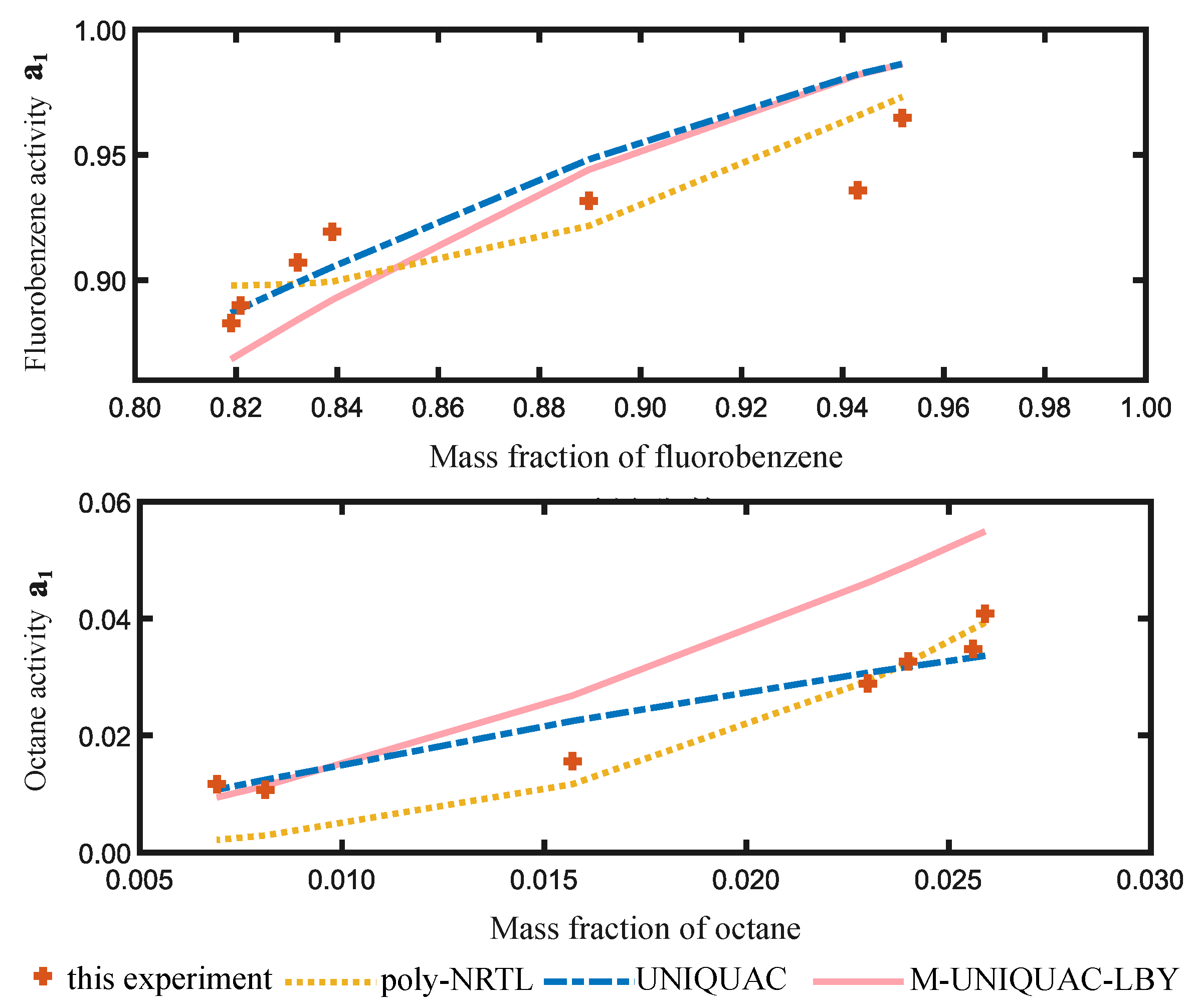
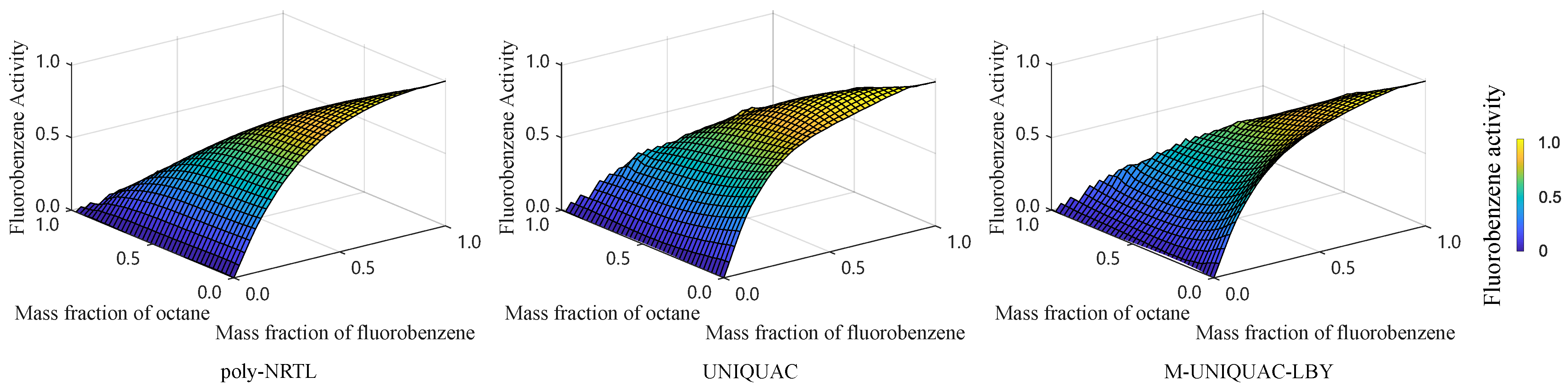
2.2. Equilibrium Data Regression and Activity Prediction
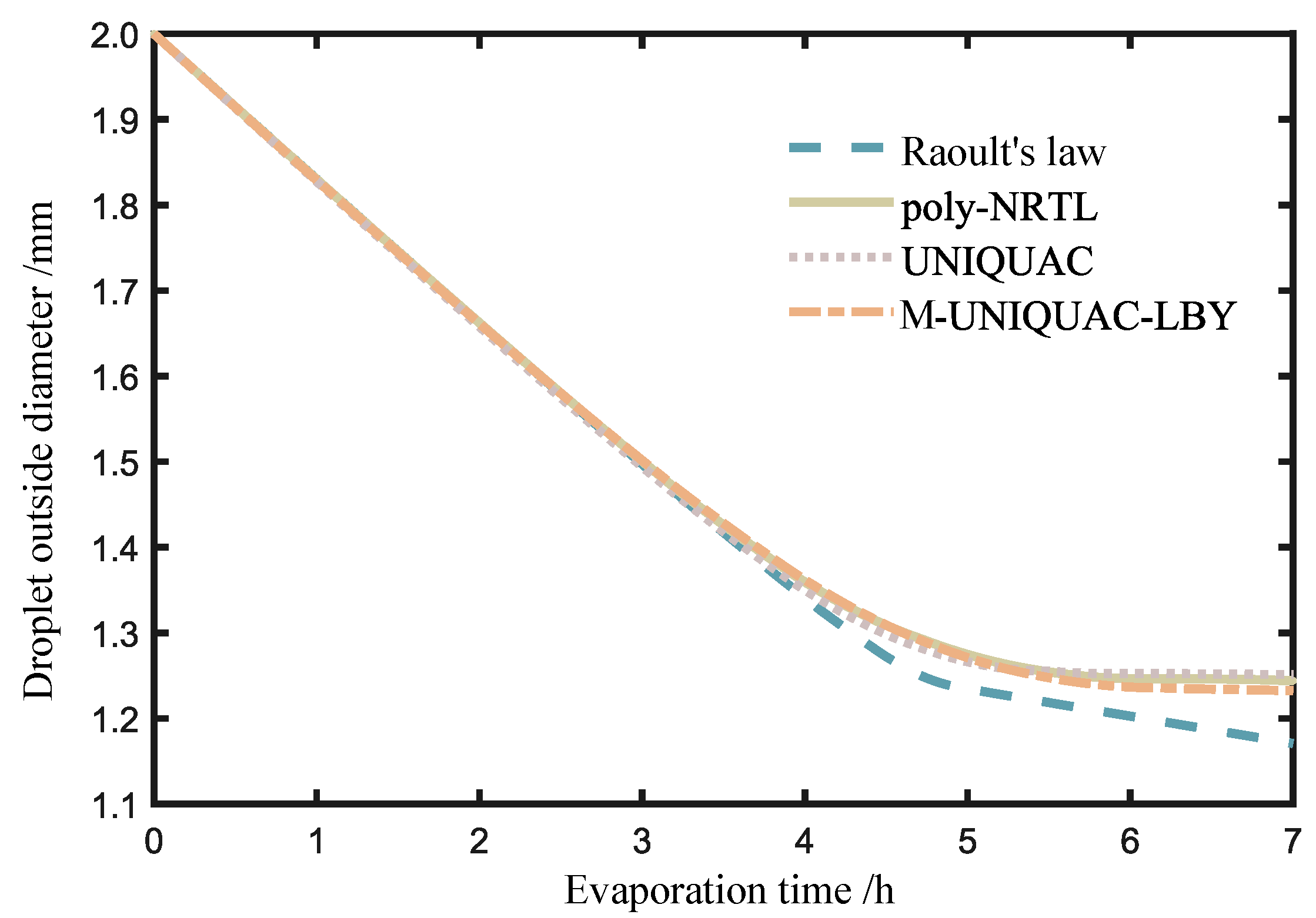
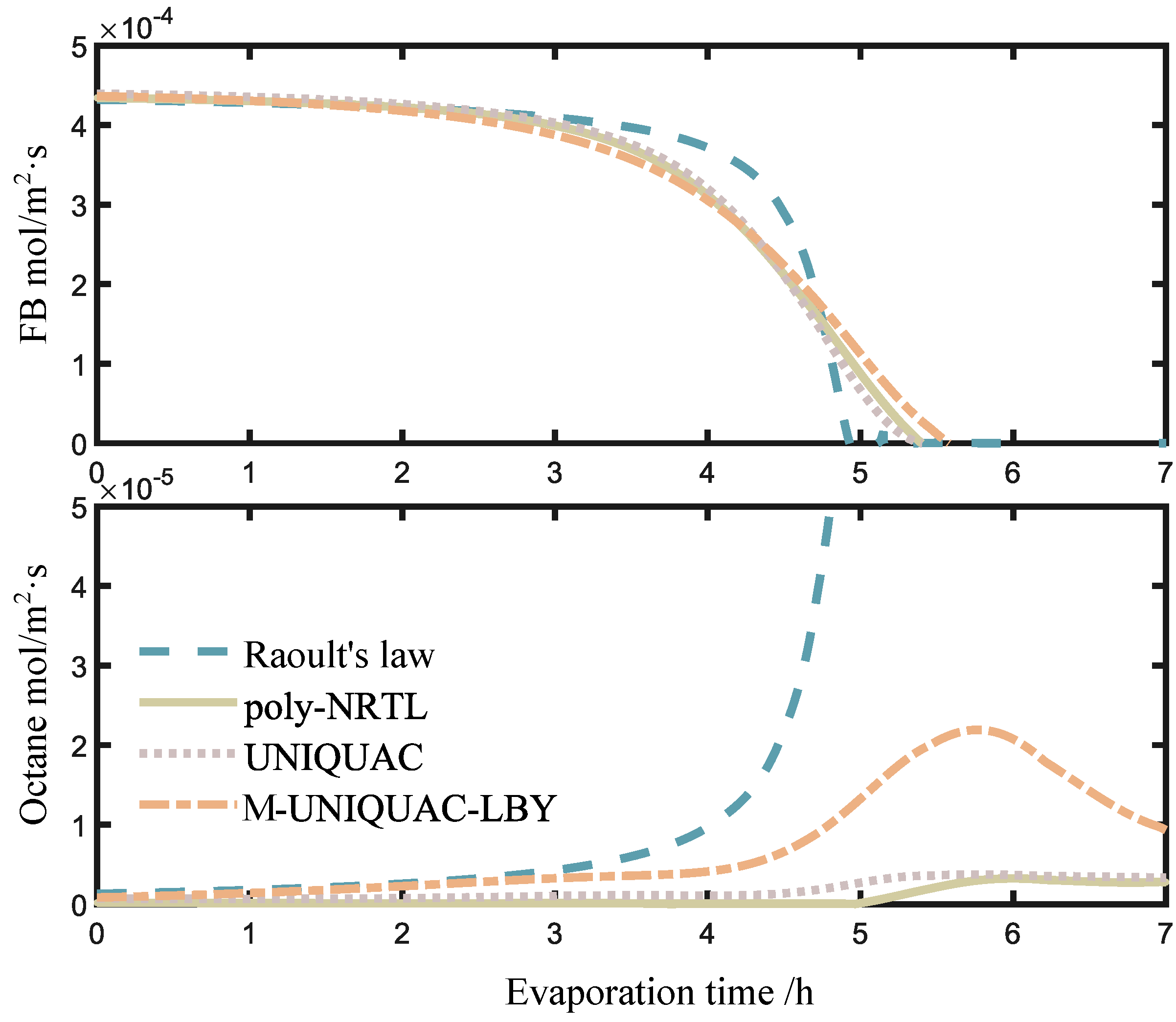
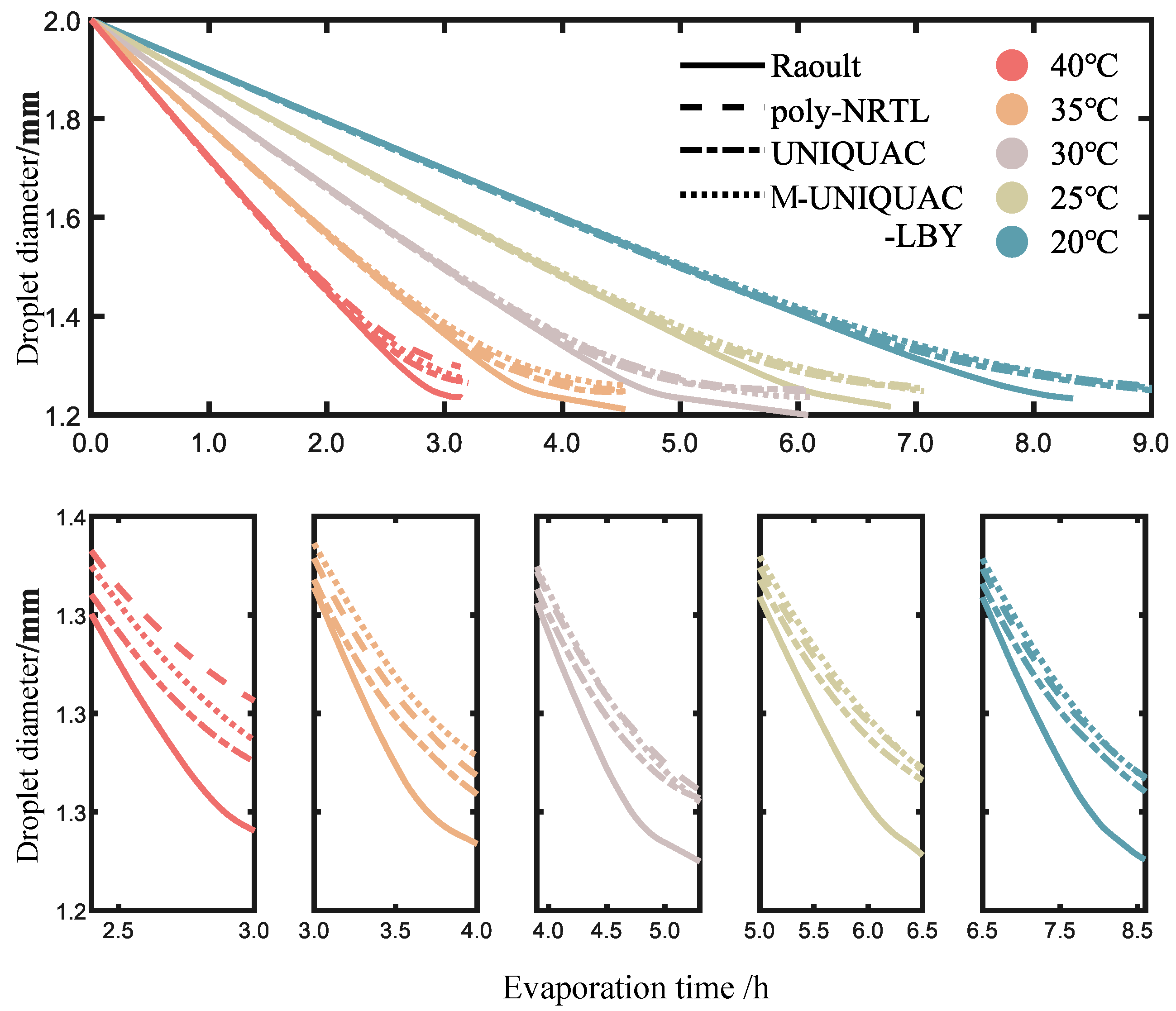
2.3. Calculation Results of Ternary Solution Droplet
3. Thermodynamic Model
3.1. Vapor–Liquid Phase Equilibrium
3.2. Activity Coefficient Models
3.3. Droplet Evaporation Model Based on Stefan Flow
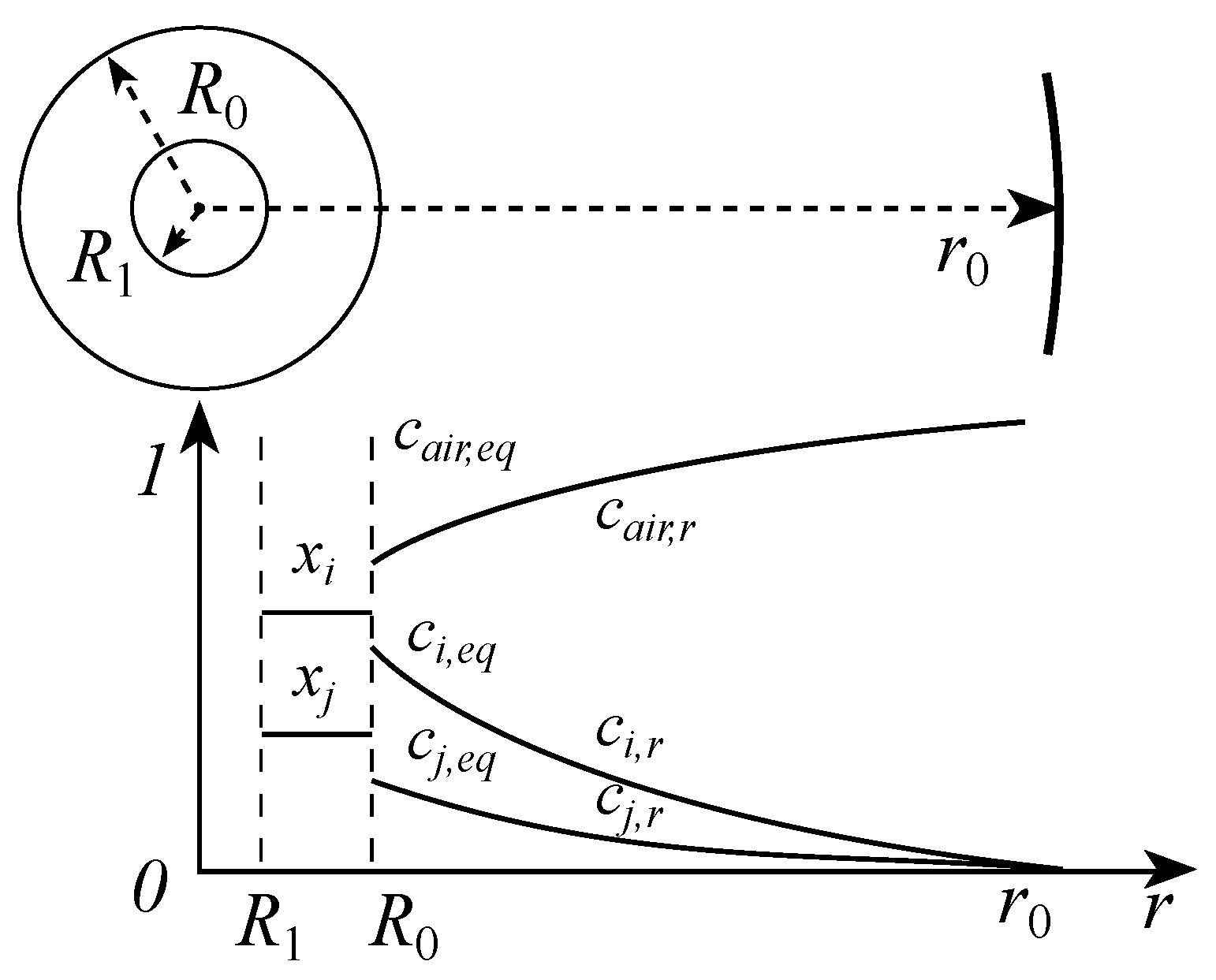
3.3.1. Convection–Diffusion Equation for the Outer Gas-Phase Environment of Multi-Component Droplets
3.3.2. Boundary Conditions for the Convection–Diffusion Equation of Multi-Component Droplets in the Outer Region
3.3.3. Control Equation for the Radius Variation of Multi-Component Droplets
3.3.4. Coupled Solution of the Governing Equations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Wei, B.; Wang, S.; Song, H.; Liu, H.; Li, J.; Liu, N. A review of recent progress in preparation of hollow polymer microspheres. Pet. Sci. 2009, 6, 306–312. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.E.; Bae, Y.C. Thermodynamic analysis of phase equilibrium and surface tension of ternary polymer solutions. AIChE J. 2019, 65, e16679. [Google Scholar] [CrossRef]
- Li, M.; Rouaud, O.; Poncelet, D. Microencapsulation by solvent evaporation: State of the art for process engineering approaches. Int. J. Pharm. 2008, 363, 26–39. [Google Scholar] [CrossRef]
- Chen, Q.; Chen, S.; Li, M.; Pan, D.; Li, B.; Zhang, Z.; Qi, X. Influence of fluorobenzene mass transfer on the qualities of poly-α-methylstyrene shells. RSC Adv. 2018, 8, 3687–3693. [Google Scholar]
- Chen, Q.; Liu, M.; Pan, D.; Chen, S.; Shi, R.; Qi, X.; Zhang, Z.; Li, B. Effects of n-hexadecane on sphericity of poly-α-methylstyrene shells. Colloids Surf. A Physicochem. Eng. Asp. 2018, 554, 1–8. [Google Scholar] [CrossRef]
- Chen, Q.; Pan, D.; Chen, S.; Liu, M.; Qi, X.; Li, B. Resisting effects of alkanes on the stability and deformation of W1-O-W2 droplets. Colloids Surf. A Physicochem. Eng. Asp. 2019, 563, 350–358. [Google Scholar] [CrossRef]
- Shao, T.; Bai, L.; Yan, B.; Jin, Y.; Cheng, Y. Modeling the solidification of O/W-emulsion droplet in solvent evaporation technique. Chem. Eng. Res. Des. 2017, 122, 233–242. [Google Scholar] [CrossRef]
- Zhou, B.; Qi, J.; Wang, W.; Xu, B.; Liu, X.; Li, B.; Chen, Y. Influence of oil-phase alkane additives on the evaporation rate of double emulsion curing process. Chem. Eng. Sci. 2022, 253, 117561. [Google Scholar] [CrossRef]
- Marin, G.B. Molecular Thermodynamic Models for Fluids of Chain-Like Molecules, Applications in Phase Equilibria and Micro-Phase Separation in Bulk and at Interface. In Advances in Chemical Engineering; Academic Press: Cambridge, MA, USA, 2011; Volume 40, pp. 153–217. [Google Scholar]
- Saeki, S.; Holste, J.C.; Bonner, D.C. Sorption of organic vapors by polystyrene. J. Polym. Sci. Part B 1981, 19, 307–320. [Google Scholar] [CrossRef]
- Pavliek, J.; Bogdani’c, G.; Wichterle, I.; Pavel, I. Vapour-liquid equilibria in water + poly(ethylene glycol) systems: New experiments and cumulative thermodynamic processing of all data. J. Chem. Thermodyn. 2020, 140, 105901. [Google Scholar] [CrossRef]
- Pavliek, J.; Rotrekl, J.; Bogdani’c, G.; Wichterle, I.; Pavel, I. Vapor-liquid and liquid-liquid equilibria in the water + poly(propylene glycol) system. J. Mol. Liq. 2021, 337, 116336. [Google Scholar] [CrossRef]
- Funabashi, A.; Sato, Y.; Inomata, H. Measurement and prediction of phase equilibria of ethylene + methyl acrylate + poly(ethylene-co-methyl acrylate) systems. Fluid Phase Equilibria 2018, 429, 98–106. [Google Scholar] [CrossRef]
- Pirdashti, M.; Heidari, Z.; Fashami, N.A.; Arzideh, S.M.; Khoiroh, I. Phase Equilibria of Aqueous Two-Phase Systems of PEG with Sulfate Salt: Effects of pH, Temperature, Type of Cation, and Polymer Molecular Weight. J. Chem. Eng. Data 2021, 66, 1425–1434. [Google Scholar] [CrossRef]
- Santos, T.; Rebelatto, E.; Chaves, B.; Lanza, M.; Oliveira, J.; Albuquerque, E.C.; Vieira de Melo, S. High pressure phase equilibrium data for carbon dioxide, methyl methacrylate and poly (dimethylsiloxane) systems. J. Supercrit. Fluids 2019, 143, 346–352. [Google Scholar] [CrossRef]
- Li, M.; Yu, X.; Huang, Q.; Zheng, H.; Wang, L.; Feng, S.; Lai, J. Phase equilibria measurements and correlation of aqueous solvent of PEG4000 with rubidium chloride at (288.15, 298.15, and 308.15) K. J. Chem. Thermodyn. 2020, 149, 106151. [Google Scholar] [CrossRef]
- Liu, H.; Huang, Y.; Wang, K.; Hu, Y. Vapor-liquid equilibria for mixed solvents-polymer systems, measurement and correlation. Fluid Phase Equilibria 2002, 194, 1067–1075. [Google Scholar] [CrossRef]
- Li, S.; Huang, X.; Huang, Q.; Guo, T.; Yun, S.; Ban, C.; Shen, G. Vapor-liquid equilibrium in the binary and ternary systems containing ethyl propionate, ethanol and alkane at 101.3 kPa. Fluid Phase Equilibria 2018, 476, 103–111. [Google Scholar] [CrossRef]
- Kang, C.H.; Sandler, S.I. Phase behavior of aqueous two-polymer systems. Fluid Phase Equilibria 1987, 38, 245–272. [Google Scholar] [CrossRef]
- Amirsoleymani, A.; Bakhshi, H.; Shabanian, S.R.; Movagharnejad, K. Phase equilibria of binary and ternary polymer solutions using modified UNIQUAC-based local composition model. J. Therm. Anal. Calorim. 2020, 142, 1493–1510. [Google Scholar] [CrossRef]
- Carey, V.P. The properties of gases & liquids: 4th Edition. Robert C. Reid, John M. Prausnitz, and Bruce E. Poling, McGraw-Hill Book Company, New York, NY, 1987, 741 pages, $49.50. Exp. Therm. Fluid Sci. 1988, 1, 409. [Google Scholar] [CrossRef]
- Haynes, W.M. CRC Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 1990. [Google Scholar]
- Larsen, B.; Rasmussen, P.; Fredenslund, A. A modified UNIFAC group-contribution model for prediction of phase equilibria and heats of mixing. Ind. Eng. Chem. Res. 1987, 26, 2274–2286. [Google Scholar] [CrossRef]
- Oishi, T.; Prausnitz, J.M. Estimation of Solvent Activities in Polymer Solutions Using a Group-Contribution Method. Ind. Eng. Chem. Process Des. Dev. 1978, 17, 333–339. [Google Scholar] [CrossRef]
- Kikic, I.; Alessi, P.; Rasmussen, P.; Fredenslund, A. On the combinatorial part of the UNIFAC and UNIQUAC models. Can. J. Chem. Eng. 1980, 58, 253–258. [Google Scholar] [CrossRef]
- Gmehling, J.; Kolbe, B.; Jorgensen, S.S.; Rasmussen, P. Vapor-Liquid Equilibria by UNIFAC Group Contribution, Revision and Extension 2. 1979. Ind. Eng. Chem. Process Des. Dev. 1979, 18, 714–722. [Google Scholar]
- Brelvi, S.W. Simple correlations for UNIQUAC structure parameters. Ind. Eng. Chem. Process Des. Dev. 1982, 21, 367–370. [Google Scholar] [CrossRef]
- Krevelen, V. Properties of Polymers: Their Correlation with Chemical Structure; Their Numerical Estimation and Prediction from Additive Group Contributions; Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Vlasov, V.A. Diffusion-kinetic model of liquid evaporation from a Stefan tube: A solution to the Stefan diffusion problem. Int. J. Heat Mass Transf. 2020, 163, 120379. [Google Scholar] [CrossRef]


| w | ||
|---|---|---|
| 0.987 | - | 0.997 |
| 0.960 | - | 0.990 |
| 0.951 | - | 0.992 |
| 0.907 | - | 0.995 |
| 0.900 | - | 0.985 |
| 0.898 | - | 0.984 |
| 0.711 | 0.043 | 0.962 |
| 0.492 | 0.038 | 0.827 |
| 0.394 | 0.032 | 0.814 |
| 0.247 | 0.030 | 0.565 |
| 0.112 | 0.020 | 0.395 |
| Temperature/°C | Liquid Mass Fraction w | Vapor-Phase Mass Fraction w | ||||
|---|---|---|---|---|---|---|
| Fluorobenzene | Octane | PS | Fluorobenzene | Octane | PS | |
| 89.3 | 0.819 | 0.026 | 0.155 | 0.990 | 0.010 | 0.000 |
| 89.1 | 0.821 | 0.026 | 0.154 | 0.987 | 0.013 | 0.000 |
| 88.5 | 0.832 | 0.024 | 0.144 | 0.989 | 0.011 | 0.000 |
| 88.1 | 0.839 | 0.023 | 0.138 | 0.991 | 0.009 | 0.000 |
| 87.8 | 0.890 | 0.016 | 0.094 | 0.997 | 0.003 | 0.000 |
| 87.7 | 0.943 | 0.008 | 0.049 | 0.995 | 0.005 | 0.000 |
| 86.7 | 0.952 | 0.007 | 0.041 | 0.997 | 0.003 | 0.000 |
| Model | Interaction Parameters | FB-PS | AARD% | |
|---|---|---|---|---|
| Poly-NRTL | 34.3086 | 17.2779 | 1.860 | |
| UNIQUAC | −174.617 | 343.576 | 1.735 | |
| M-UNIQUAC-LBY | −250.956 | 251.201 | 1.579 | |
| Model | Interaction Parameters | FB-Octane-PS | AARD% | |||||
|---|---|---|---|---|---|---|---|---|
| FB-Octane | Octane-PS | FB-PS | ||||||
| poly-NRTL | 32.680 | 43.900 | 417.540 | −916.820 | 721.100 | −383.600 | 5.778 | |
| UNIQUAC | 81.818 | −0.201 | 7.354 | −205.709 | 296.137 | −338.265 | 1.508 | |
| M-UNIQUAC-LBY | 38.909 | 2.015 | 155.028 | 163.241 | 423.125 | −420.969 | 5.675 | |
| A | B | C | |
|---|---|---|---|
| FB | 6.31155 | 1381.646 | −37.602 |
| octane | 6.92374 | 1355.126 | 209.517 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Zhou, Z.; Zhou, B. Droplet Evaporation Process of a Fluorobenzene + n-Octane + Polystyrene Mixture. Molecules 2023, 28, 5659. https://doi.org/10.3390/molecules28155659
Wang W, Zhou Z, Zhou B. Droplet Evaporation Process of a Fluorobenzene + n-Octane + Polystyrene Mixture. Molecules. 2023; 28(15):5659. https://doi.org/10.3390/molecules28155659
Chicago/Turabian StyleWang, Wei, Zhendong Zhou, and Bo Zhou. 2023. "Droplet Evaporation Process of a Fluorobenzene + n-Octane + Polystyrene Mixture" Molecules 28, no. 15: 5659. https://doi.org/10.3390/molecules28155659






