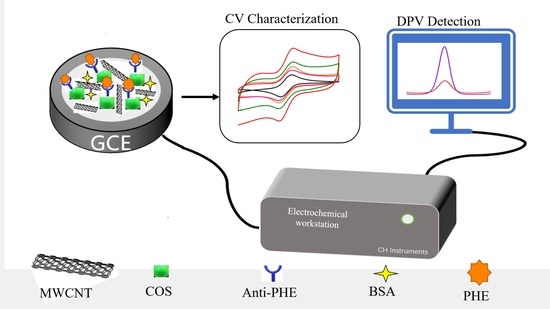
The Method and Study of Detecting Phenanthrene in Seawater Based on a Carbon Nanotube–Chitosan Oligosaccharide Modified Electrode Immunosensor
Abstract
1. Introduction
2. Results and Discussion
2.1. Electrochemical Characterization of Immunosensors
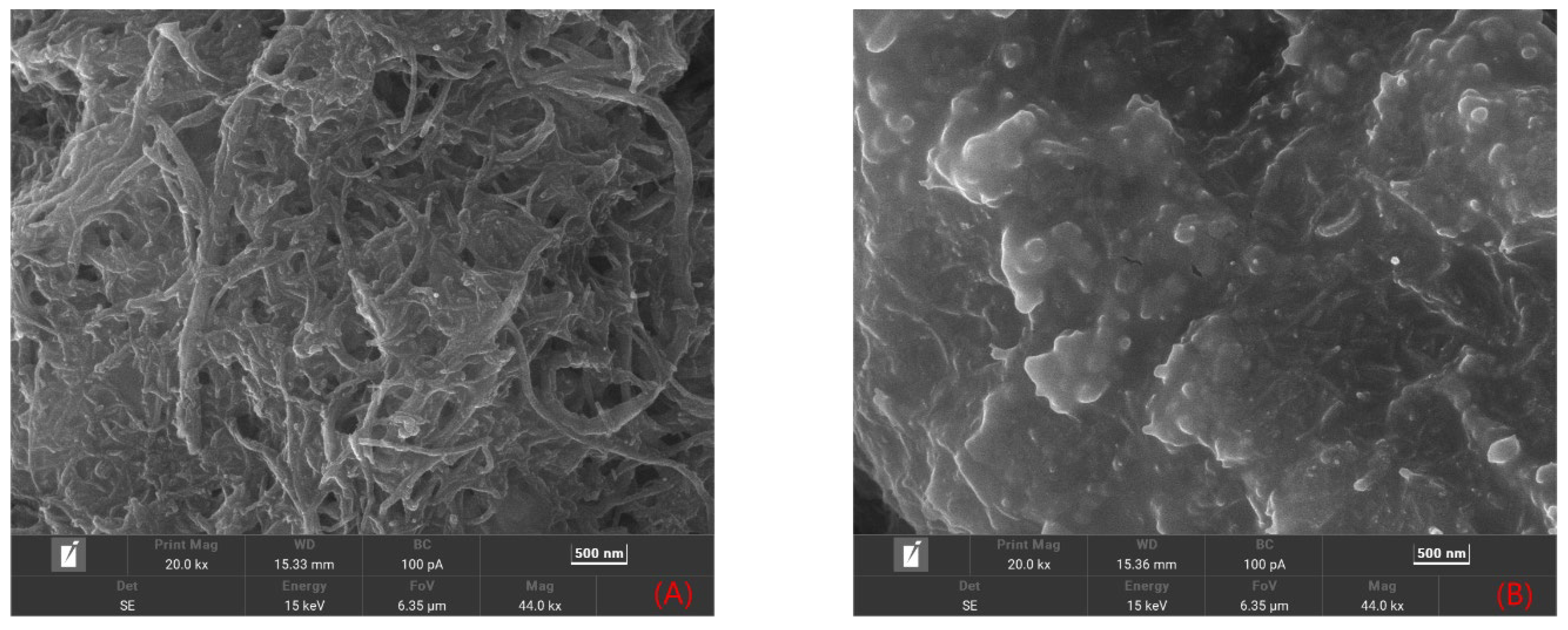
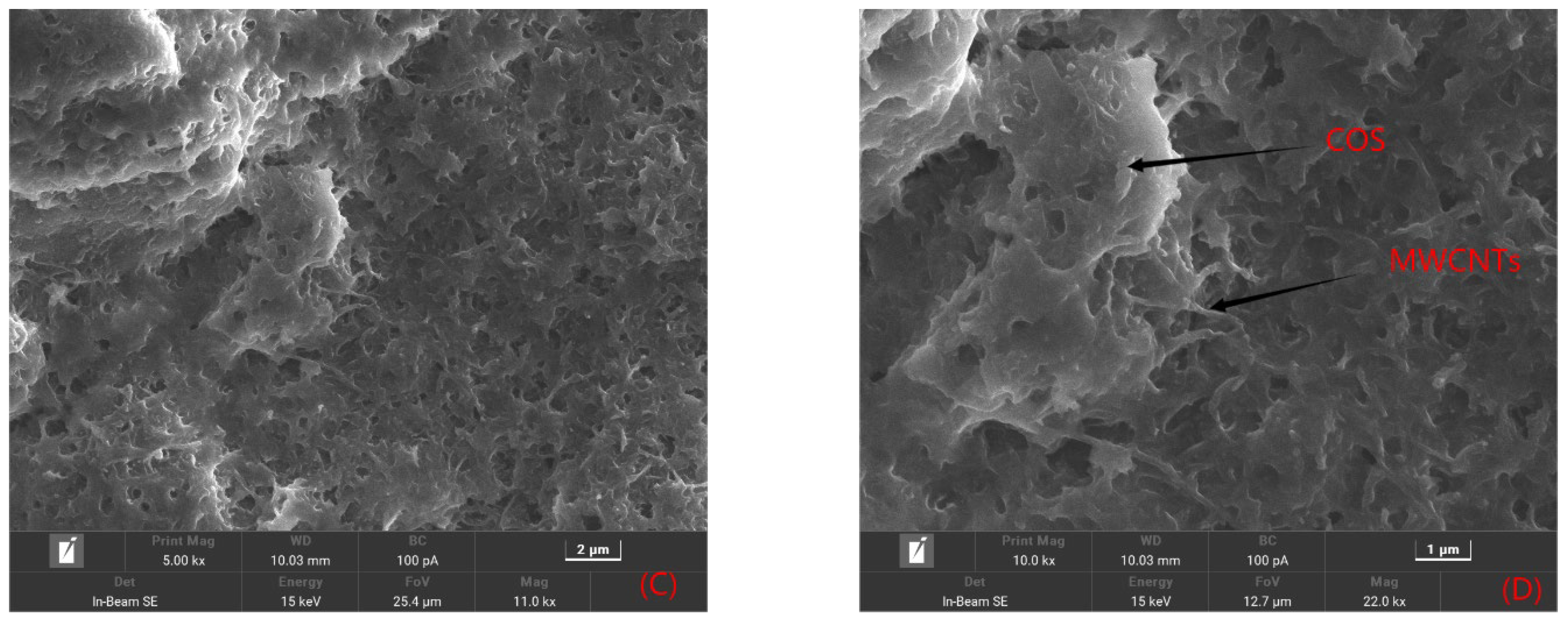
2.1.1. Morphological Features of the Sensor Platform
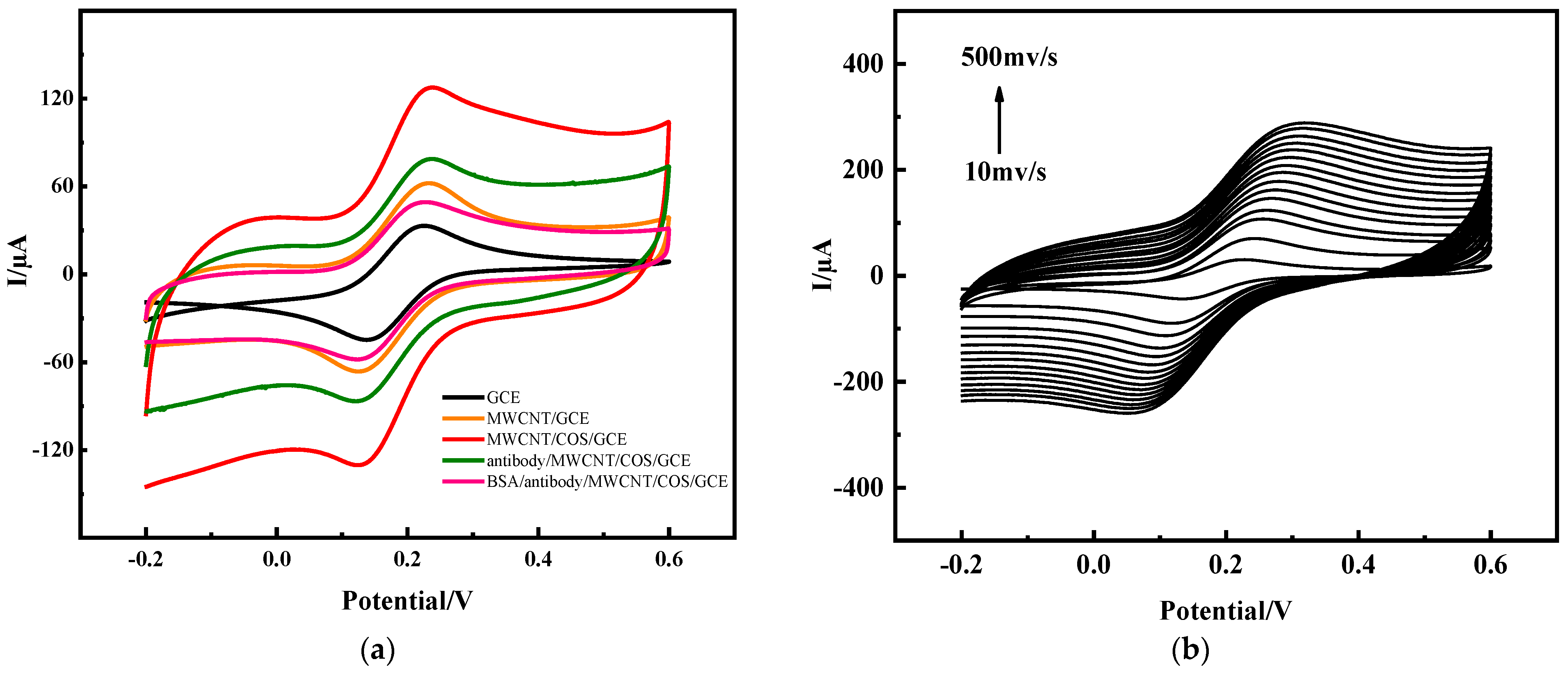
2.1.2. Performance Characterization of Electrochemical Immunosensors
2.2. Optimization of Experimental Conditions
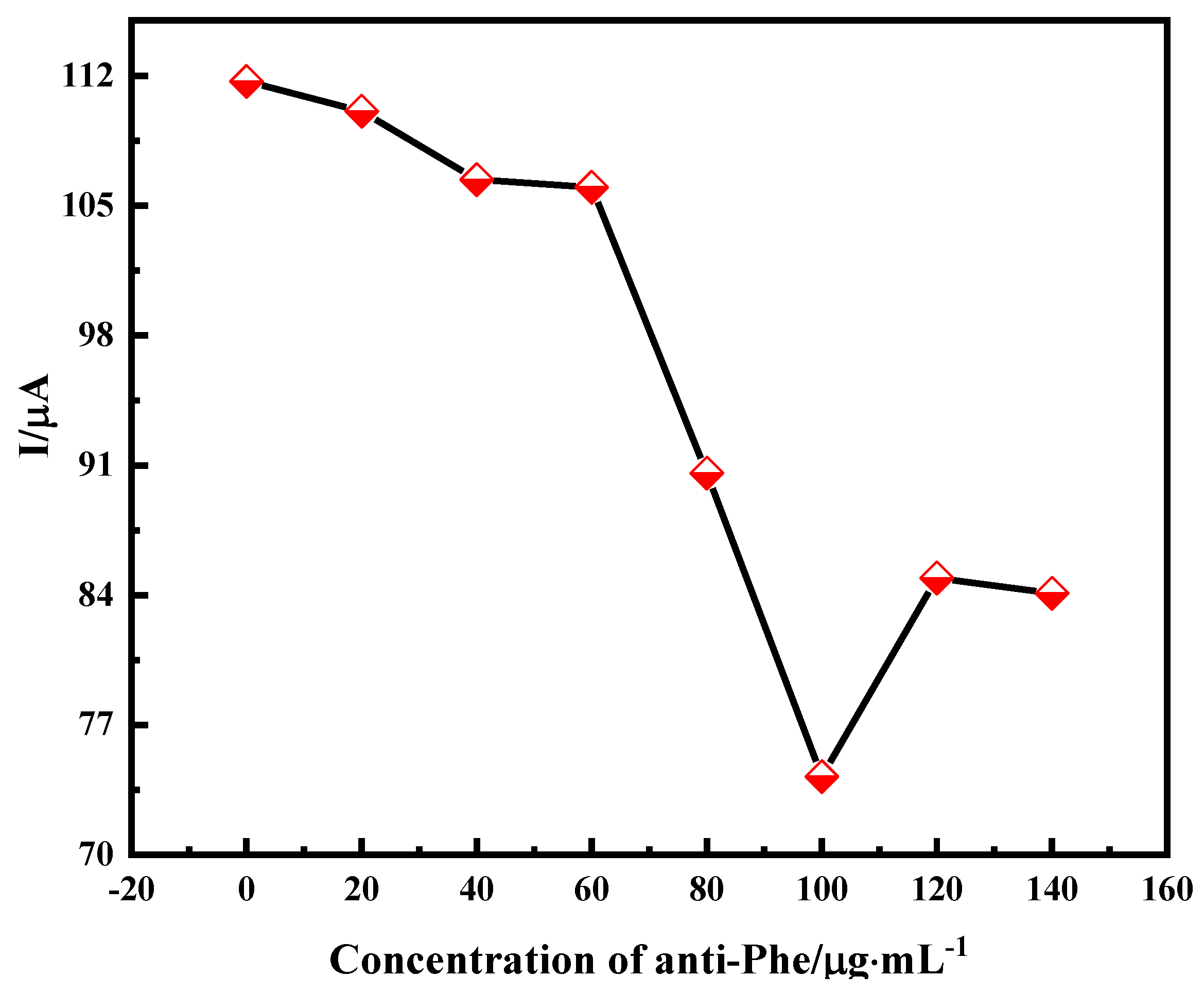
2.2.1. Effect of Antibody Concentration on Immune Sensors
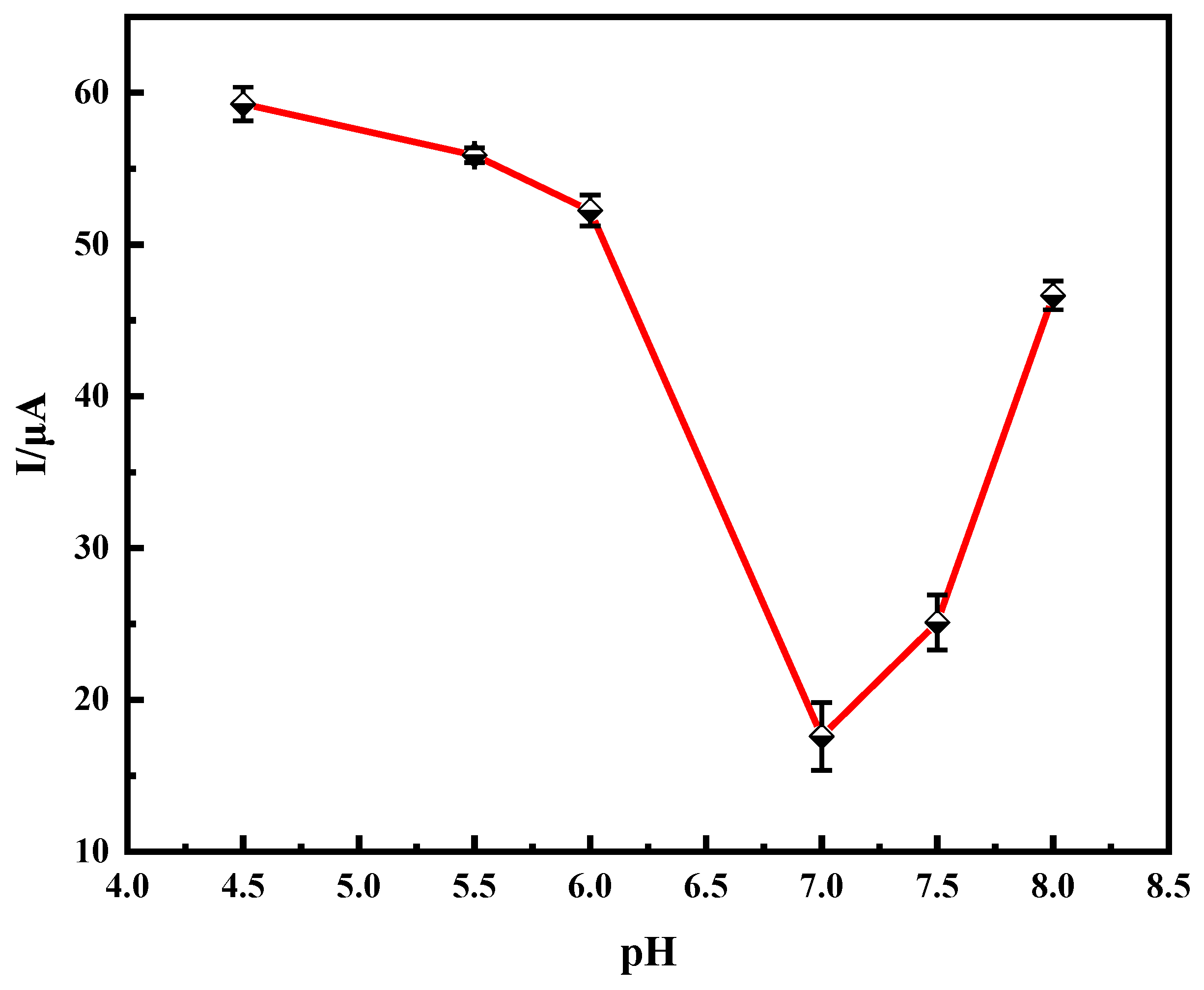
2.2.2. Effect of pH on Immune Sensors
2.2.3. Probe Concentration on the Immunosensor
2.3. Performance of Immunosensors
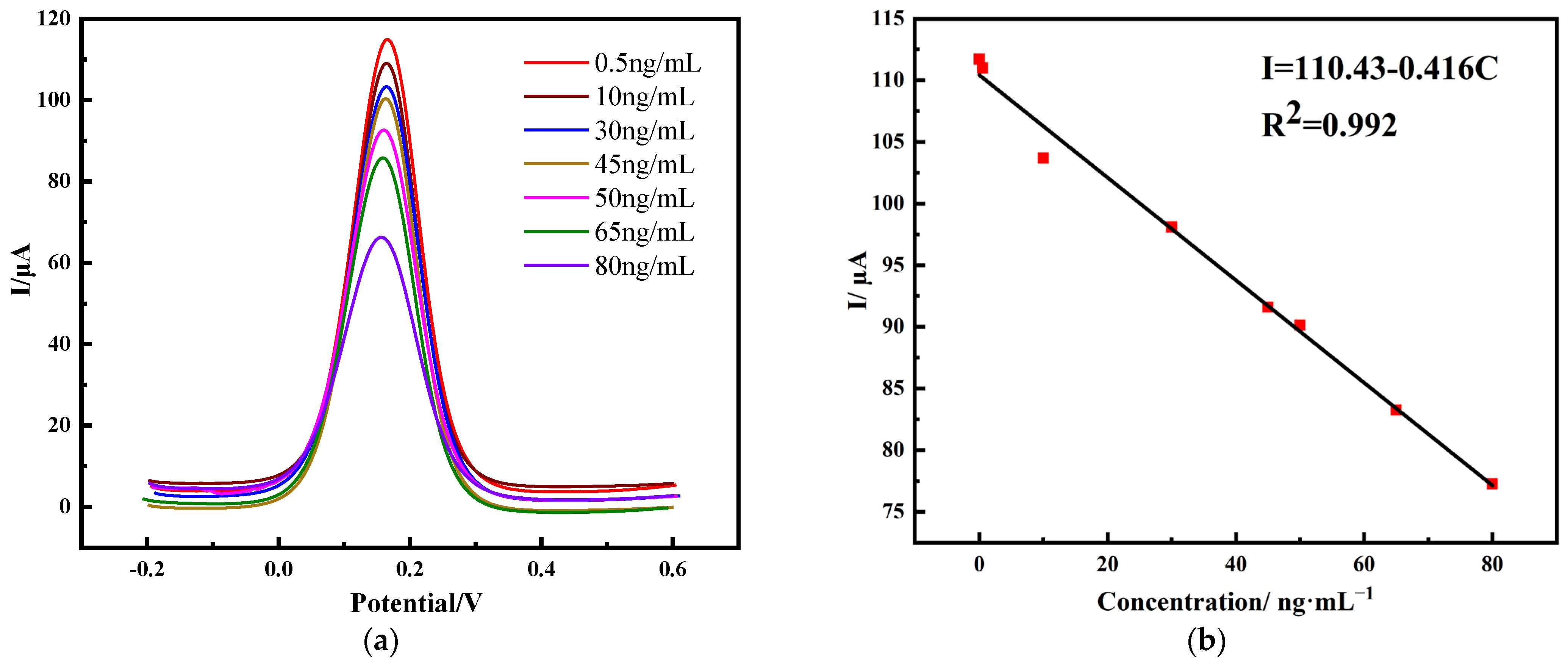
2.3.1. Detection of PHE Using Differential Pulsed Voltammetry (DPV)
2.3.2. Specificity, Reproducibility and Stability of Immune Sensors
2.4. Actual Sample Determination
3. Materials and Methods
3.1. Chemicals
3.2. Apparatus
3.3. Experimental Processes
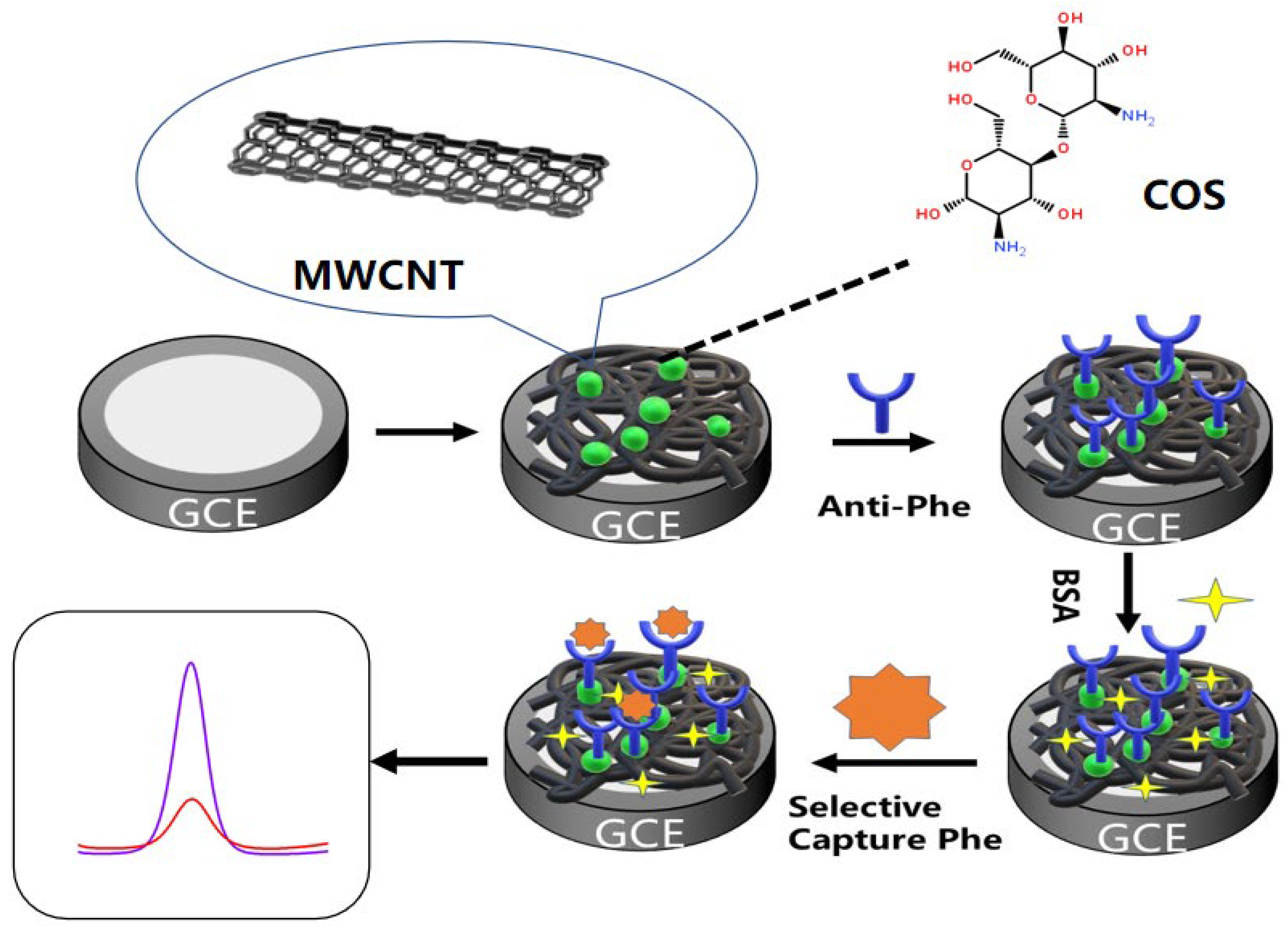
3.3.1. Preparation of Multi-Walled Carbon Nanotube–Chitosan Oligosaccharide Composite Membranes
3.3.2. Preparation of Electrochemical Immunosensors
3.3.3. Immunoassay for Phenanthrene Detection
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Abaroa-Perez, B.; Sanchez-Almeida, G.; Hernandez-Brito, J.J.; Vega-Moreno, D. In Situ Miniaturised Solid Phase Extraction (m-SPE) for Organic Pollutants in Seawater Samples. J. Anal. Methods Chem. 2018, 2018, 7437031. [Google Scholar] [CrossRef]
- Keshavarzifard, M.; Zakaria, M.P.; Hwai, T.S.; Yusuff, F.F.; Mustafa, S.; Vaezzadeh, V.; Magam, S.M.; Masood, N.; Alkhadher, S.A.; Abootalebi-Jahromi, F. Baseline distributions and sources of Polycyclic Aromatic Hydrocarbons (PAHs) in the surface sediments from the Prai and Malacca Rivers, Peninsular Malaysia. Mar. Pollut. Bull. 2014, 88, 366–372. [Google Scholar] [CrossRef]
- Augusto, S.; Pereira, M.J.; Maguas, C.; Branquinho, C. A step towards the use of biomonitors as estimators of atmospheric PAHs for regulatory purposes. Chemosphere 2013, 92, 626–632. [Google Scholar] [CrossRef]
- Burritt, D.J. The polycyclic aromatic hydrocarbon phenanthrene causes oxidative stress and alters polyamine metabolism in the aquatic liverwort Riccia fluitans L. Plant Cell Environ. 2008, 31, 1416–1431. [Google Scholar] [CrossRef]
- Abdel-Shafy, H.I.; Mansour, M.S.M. A review on polycyclic aromatic hydrocarbons: Source, environmental impact, effect on human health and remediation. Egypt. J. Pet. 2016, 25, 107–123. [Google Scholar]
- Lehr, R.E.; Kumar, S.; Levin, W.; Wood, A.W.; Chang, R.L.; Conney, A.H.; Yagi, H.; Sayer, J.M.; Jerina, D.M. The Bay Region Theory of Polycyclic Aromatic Hydrocarbon Carcinogenesis. In Polycyclic Hydrocarbons and Carcinogenesis; American Chemical Society: Washington, DC, USA, 1985; pp. 63–84. [Google Scholar]
- Cheng, G.; Zarth, A.T.; Upadhyaya, P.; Villalta, P.W.; Balbo, S.; Hecht, S.S. Investigation of the presence in human urine of mercapturic acids derived from phenanthrene, a representative polycyclic aromatic hydrocarbon. Chem. Biol. Interact. 2017, 274, 80–88. [Google Scholar] [CrossRef]
- Patel, A.B.; Shaikh, S.; Jain, K.R.; Desai, C.; Madamwar, D. Polycyclic Aromatic Hydrocarbons: Sources, Toxicity, and Remediation Approaches. Front. Microbiol. 2020, 11, 562813. [Google Scholar]
- Al-Moubarak, E.; Shiels, H.A.; Zhang, Y.; Du, C.; Hanington, O.; Harmer, S.C.; Dempsey, C.E.; Hancox, J.C. Inhibition of the hERG potassium channel by phenanthrene: A polycyclic aromatic hydrocarbon pollutant. Cell. Mol. Life Sci. 2021, 78, 7899–7914. [Google Scholar]
- Gruber, B.; Schneider, J.; Fohlinger, M.; Buters, J.; Zimmermann, R.; Matuschek, G. A minimal-invasive method for systemic bio-monitoring of the environmental pollutant phenanthrene in humans: Thermal extraction and gas chromatography—Mass spectrometry from 1 mL capillary blood. J. Chromatogr. A 2017, 1487, 254–257. [Google Scholar]
- Loughery, J.R.; Kidd, K.A.; Mercer, A.; Martyniuk, C.J. Part A: Temporal and dose-dependent transcriptional responses in the liver of fathead minnows following short term exposure to the polycyclic aromatic hydrocarbon phenanthrene. Aquat. Toxicol. 2018, 199, 90–102. [Google Scholar]
- Qazi, F.; Shahsavari, E.; Prawer, S.; Ball, A.S.; Tomljenovic-Hanic, S. Detection and identification of polyaromatic hydrocarbons (PAHs) contamination in soil using intrinsic fluorescence. Environ. Pollut. 2021, 272, 116010. [Google Scholar] [CrossRef]
- Yu, Z.; Grasso, M.F.; Sorensen, H.H.; Zhang, P. Ratiometric SERS detection of polycyclic aromatic hydrocarbons assisted by beta-cyclodextrin-modified gold nanoparticles. Mikrochim. Acta 2019, 186, 391. [Google Scholar] [CrossRef]
- Gazioglu, I.; Zengin, O.S.; Tartaglia, A.; Locatelli, M.; Furton, K.G.; Kabir, A. Determination of Polycyclic Aromatic Hydrocarbons in Nutritional Supplements by Fabric Phase Sorptive Extraction (FPSE) with High-Performance Liquid Chromatography (HPLC) with Fluorescence Detection. Anal. Lett. 2020, 54, 1683–1696. [Google Scholar] [CrossRef]
- Zhou, H.; Wu, X.; Chen, D.; Zhu, H.; Fang, C.; Zhang, H.; Chen, W.; Zhan, B.; Song, C. Simultaneous Determination of 24 Polycyclic Aromatic Hydrocarbons in Oils by Gas Chromatography-Mass Spectrometry Using an Improved Clean-up Procedure. Food Anal. Methods 2019, 12, 1957–1963. [Google Scholar] [CrossRef]
- Nording, M.; Frech, K.; Persson, Y.; Forsman, M.; Haglund, P. On the semi-quantification of polycyclic aromatic hydrocarbons in contaminated soil by an enzyme-linked immunosorbent assay kit. Anal. Chim. Acta 2006, 555, 107–113. [Google Scholar] [CrossRef]
- Felix, F.S.; Angnes, L. Electrochemical immunosensors—A powerful tool for analytical applications. Biosens. Bioelectron. 2018, 102, 470–478. [Google Scholar] [CrossRef]
- Pollap, A.; Kochana, J. Electrochemical Immunosensors for Antibiotic Detection. Biosensors 2019, 9, 61. [Google Scholar] [CrossRef]
- Cho, I.-H.; Lee, J.; Kim, J.; Kang, M.-s.; Paik, J.; Ku, S.; Cho, H.-M.; Irudayaraj, J.; Kim, D.-H. Current Technologies of Electrochemical Immunosensors: Perspective on Signal Amplification. Sensors 2018, 18, 207. [Google Scholar] [CrossRef]
- Mohd Nurazzi, N.; Muhammad Asyraf, M.R.; Khalina, A.; Abdullah, N.; Sabaruddin, F.A.; Kamarudin, S.H.; Ahmad, S.; Mahat, A.M.; Lee, C.L.; Aisyah, H.A.; et al. Fabrication, Functionalization, and Application of Carbon Nanotube-Reinforced Polymer Composite: An Overview. Polymers 2021, 13, 1047. [Google Scholar]
- Zhang, Z.; Cong, Y.; Huang, Y.; Du, X. Nanomaterials-based Electrochemical Immunosensors. Micromachines 2019, 10, 397. [Google Scholar] [CrossRef]
- Piro, B.; Reisberg, S. Recent Advances in Electrochemical Immunosensors. Sensors 2017, 17, 794. [Google Scholar] [CrossRef]
- Xue, S.-M.; Xu, Z.-L.; Tang, Y.-J.; Ji, C.-H. Polypiperazine-amide Nanofiltration Membrane Modified by Different Functionalized Multiwalled Carbon Nanotubes (MWCNTs). ACS Appl. Mater. Interfaces 2016, 8, 19135–19144. [Google Scholar] [CrossRef]
- Chapelle, C.; David, G.; Caillol, S.; Negrell, C.; Desroches Le Foll, M. Advances in chitosan oligosaccharides chemical modifications. Biopolymers 2021, 112, e23461. [Google Scholar] [CrossRef]
- Anil, S. Potential Medical Applications of Chitosan oligosaccharides. Polymers 2022, 14, 3558. [Google Scholar] [CrossRef]
- Mohan, K.; Rajan, D.K.; Rajarajeswaran, J.; Divya, D.; Ganesan, A.R. Recent trends on chitosan based hybrid materials for wastewater treatment: A review. Curr. Opin. Environ. Sci. Health 2023, 33, 100473. [Google Scholar] [CrossRef]
- Zouaoui, F.; Bourouina-Bacha, S.; Bourouina, M.; Jaffrezic-Renault, N.; Zine, N.; Errachid, A. Electrochemical sensors based on molecularly imprinted chitosan: A review. TrAC Trends Anal. Chem. 2020, 130, 115982. [Google Scholar] [CrossRef]
- Liaqat, F.; Eltem, R. Chitooligosaccharides and their biological activities: A comprehensive review. Carbohydr Polym. 2018, 184, 243–259. [Google Scholar] [CrossRef]
- Wang, B.; Akiba, U.; Anzai, J.I. Recent Progress in Nanomaterial-Based Electrochemical Biosensors for Cancer Biomarkers: A Review. Molecules 2017, 22, 1048. [Google Scholar] [CrossRef]
- Yan, Y.; Qiu, C.; Qu, W.; Zhuang, Y.; Chen, K.; Wang, C.; Zhang, R.; Wang, P.; Wu, Y.; Gao, J. Detection of BaP in seawater based on multi-walled carbon nanotubes composites immunosenor. Front. Chem. 2022, 10, 950854. [Google Scholar] [CrossRef]
- Sun, Y.; Li, Y.; Meng, X.; Meng, X.; Qiao, B.; Si, Z.; Hu, P.; Lu, S.; Ren, H.; Liu, Z.; et al. Magnetic nanoparticles-phenanthrene conjugates (MNPs-Phe) probe based competitive chemiluminescence enzyme immunoassay (MNPs-icCLEIA) for phenanthrene and other homologous polycyclic aromatic hydrocarbons. Sens. Actuators B Chem. 2017, 252, 633–640. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, B.; Wahid, F.; Feng, W.; Chen, X. Intelligent Detection and Analysis of Polycyclic Aromatic Hydrocarbons Based on Surface-Enhanced Raman Scattering Spectroscopy. Comput. Intell. Neurosci. 2022, 2022, 8330702. [Google Scholar] [CrossRef]
- Tran, V.T.; Seok, J.S.; Yoon, I.; Ju, H. Ultraviolet Fluorescence-Based Quantitative Detection of Polycyclic Aromatic Hydrocarbons. BioChip J. 2021, 15, 356–361. [Google Scholar] [CrossRef]



| k3 Fe(CN)6 | Ipa | I’pa | ΔIpa | Ipc | I’pc | ΔIpc |
|---|---|---|---|---|---|---|
| c/(mmol·L−1) | (μA) | (μA) | (%) | (μA) | (μA) | (%) |
| 1 | −24.4 | −23.99 | 1.68% | 17.76 | 17.59 | 0.95% |
| 5 | −57.75 | −46.17 | 20.05% | 47.19 | 34.54 | 26.80% |
| 10 | −125.4 | −118.5 | 5.50% | 100.1 | 97.96 | 2.14% |
| 15 | −289.9 | −275.0 | 5.13% | 295.1 | 293.8 | 0.44% |
| Method | Linear Range (ng·mL−1) | LOD (ng·mL−1) | Reference |
|---|---|---|---|
| MNPs-Phe-McAb/CLEIA | 1.85–71.51 | 0.85 | [31] |
| Cyclodextrin/Au NPs/SERS | - | 0.1–0.9 | [32] |
| aluminum film/ultraviolet (UV) fluorescence | 0–250 | 0.49 | [33] |
| MWCNTs/COS/anti-PHE/GCE | 0.5–80 | 0.30 | this work |
| Initial (ng·mL−1) | Add (ng·mL−1) | Found (ng·mL−1) | Recovery (%) |
|---|---|---|---|
| 97.31 | 10 | 106.92 | 96.1 |
| - | 20 | 116.95 | 98.2 |
| - | 30 | 127.04 | 99.1 |
| - | 40 | 137.93 | 101.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Qu, W.; Qiu, C.; Chen, K.; Zhuang, Y.; Zeng, Z.; Yan, Y.; Gu, Y.; Tao, W.; Gao, J.; et al. The Method and Study of Detecting Phenanthrene in Seawater Based on a Carbon Nanotube–Chitosan Oligosaccharide Modified Electrode Immunosensor. Molecules 2023, 28, 5701. https://doi.org/10.3390/molecules28155701
Wu Y, Qu W, Qiu C, Chen K, Zhuang Y, Zeng Z, Yan Y, Gu Y, Tao W, Gao J, et al. The Method and Study of Detecting Phenanthrene in Seawater Based on a Carbon Nanotube–Chitosan Oligosaccharide Modified Electrode Immunosensor. Molecules. 2023; 28(15):5701. https://doi.org/10.3390/molecules28155701
Chicago/Turabian StyleWu, Yuxuan, Wei Qu, Chengjun Qiu, Kaixuan Chen, Yuan Zhuang, Zexi Zeng, Yirou Yan, Yang Gu, Wei Tao, Jiaqi Gao, and et al. 2023. "The Method and Study of Detecting Phenanthrene in Seawater Based on a Carbon Nanotube–Chitosan Oligosaccharide Modified Electrode Immunosensor" Molecules 28, no. 15: 5701. https://doi.org/10.3390/molecules28155701
APA StyleWu, Y., Qu, W., Qiu, C., Chen, K., Zhuang, Y., Zeng, Z., Yan, Y., Gu, Y., Tao, W., Gao, J., & Li, K. (2023). The Method and Study of Detecting Phenanthrene in Seawater Based on a Carbon Nanotube–Chitosan Oligosaccharide Modified Electrode Immunosensor. Molecules, 28(15), 5701. https://doi.org/10.3390/molecules28155701