Study of the Combustion Mechanism of Zn/KMnO4 Pyrotechnic Composition
Abstract
1. Introduction
2. Results
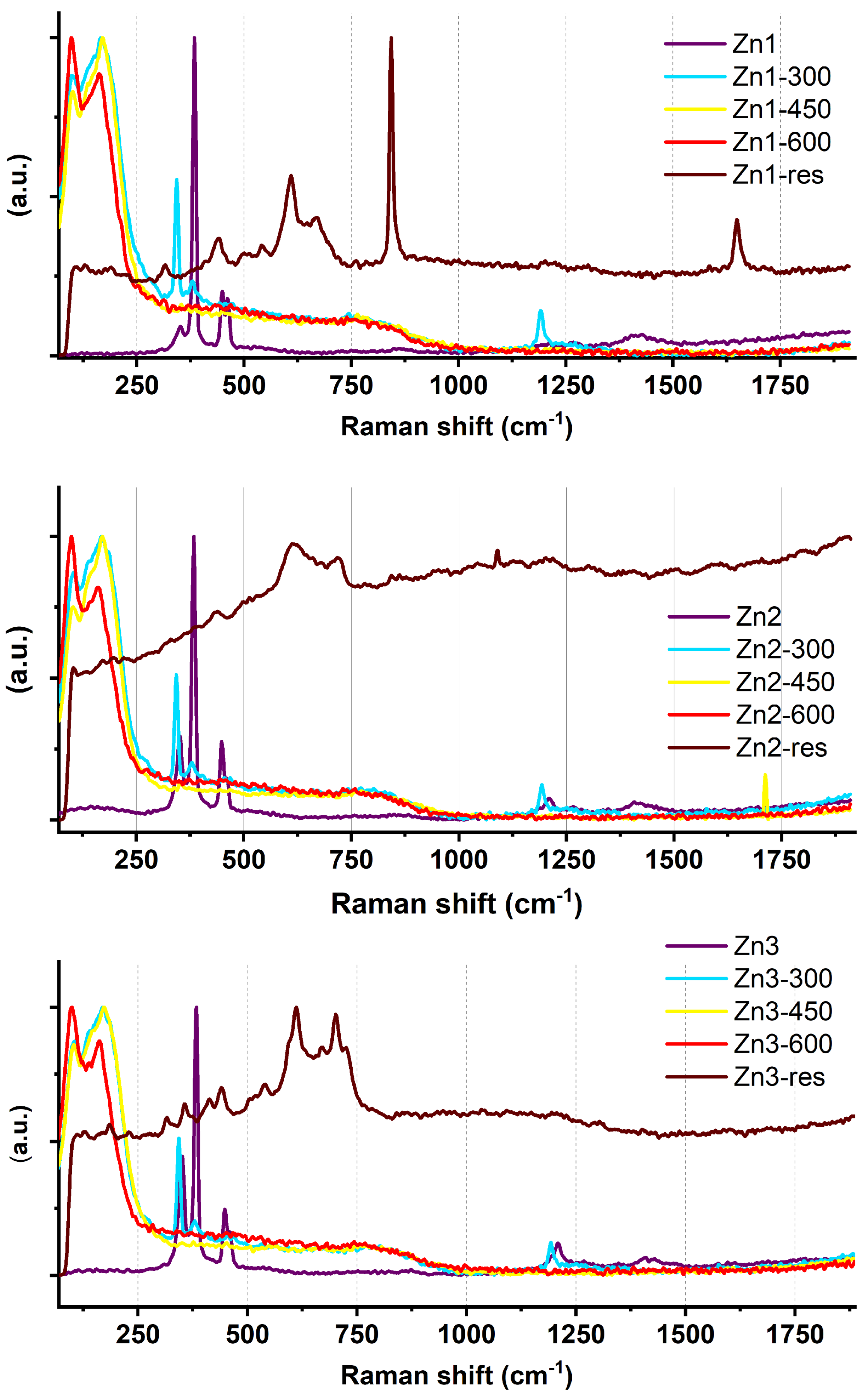
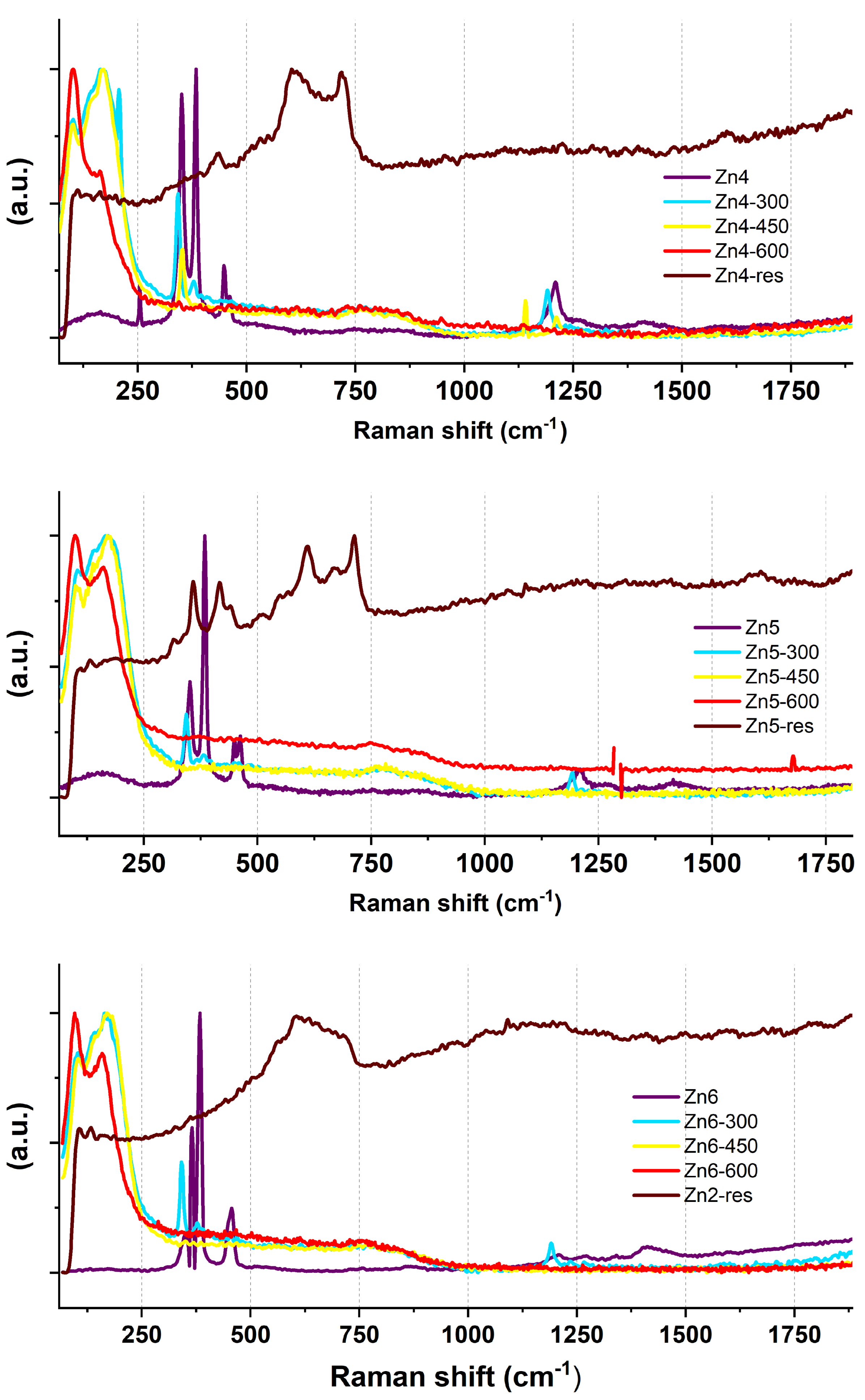
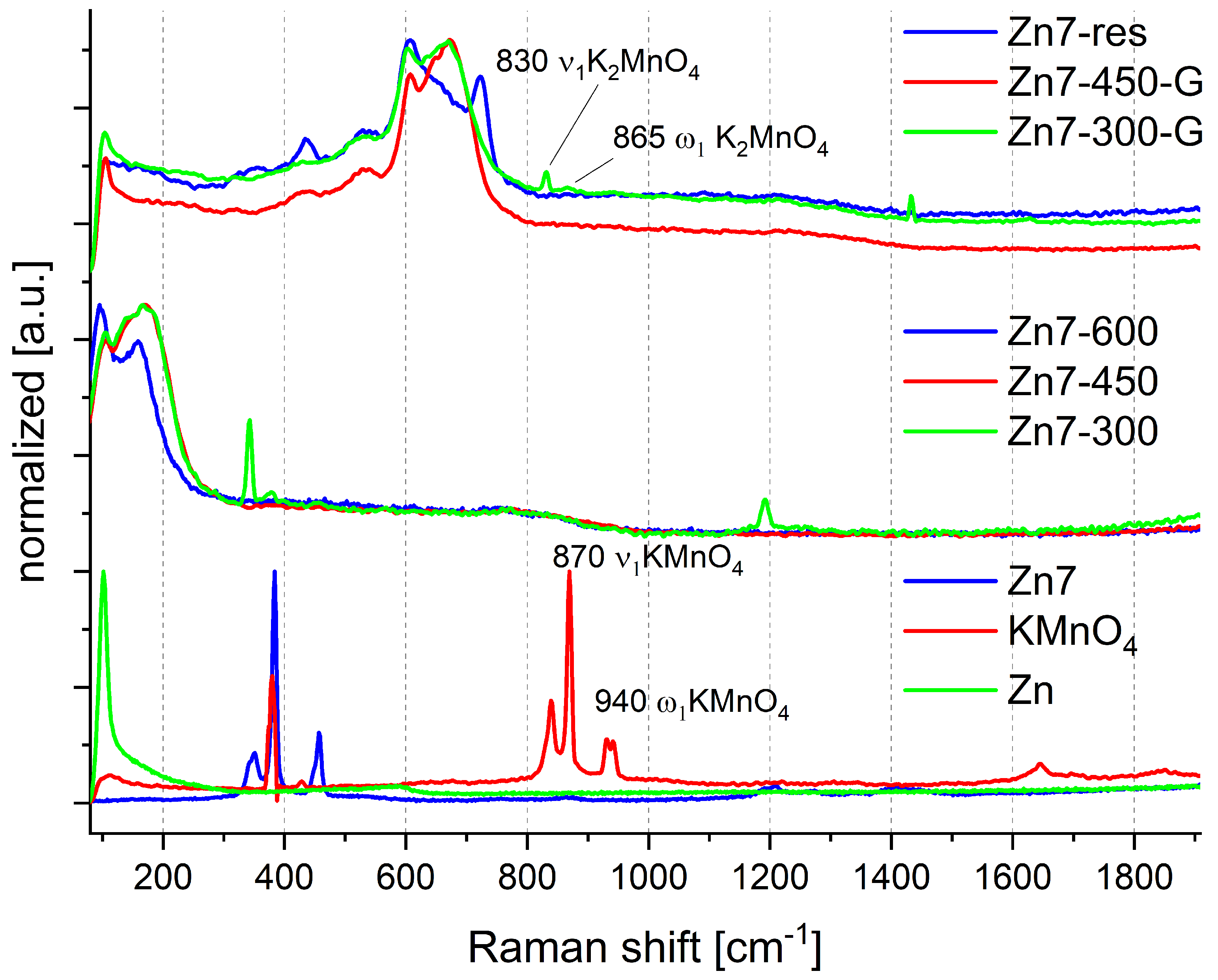
2.1. Raman Spectroscopic Investigations
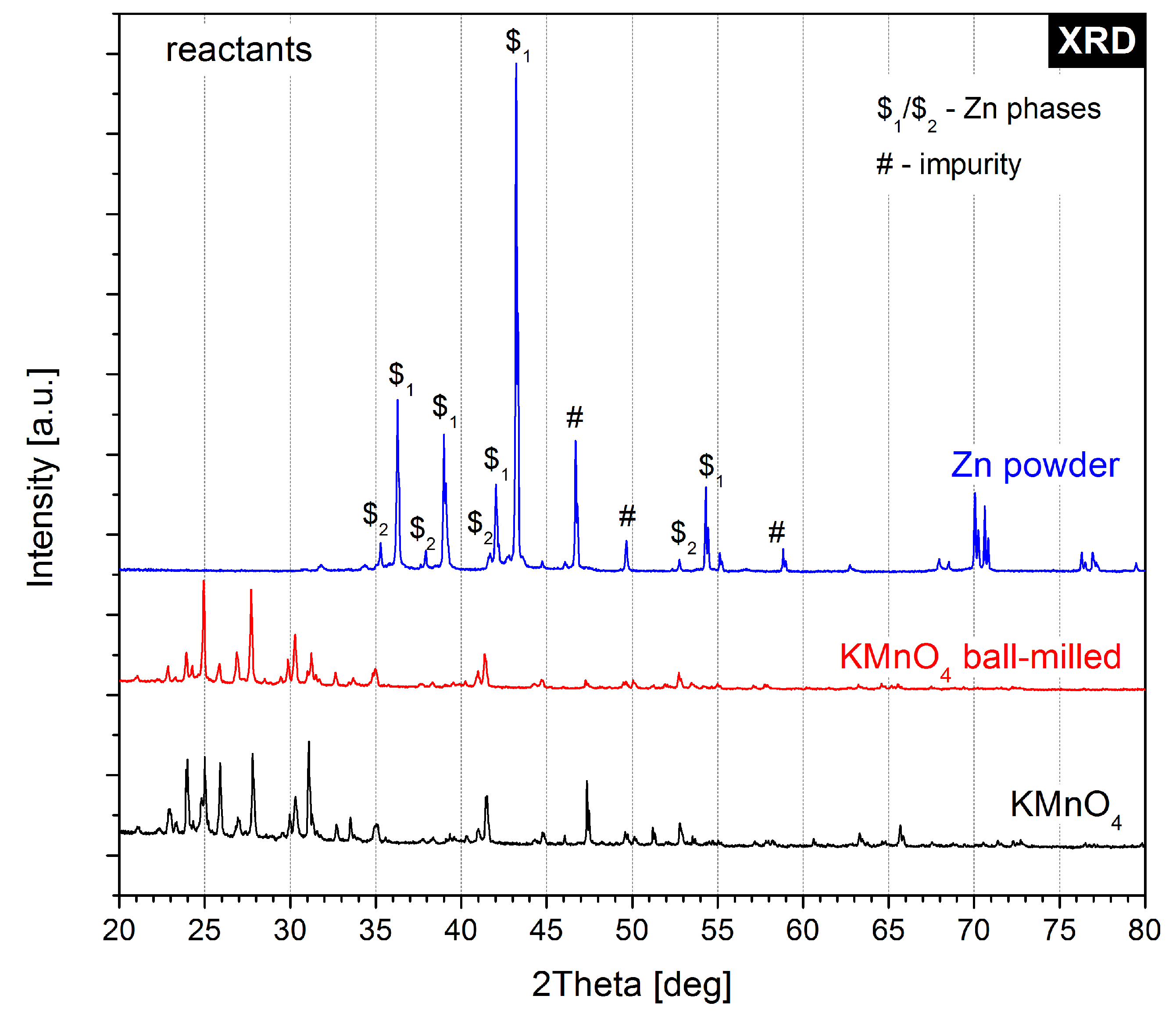
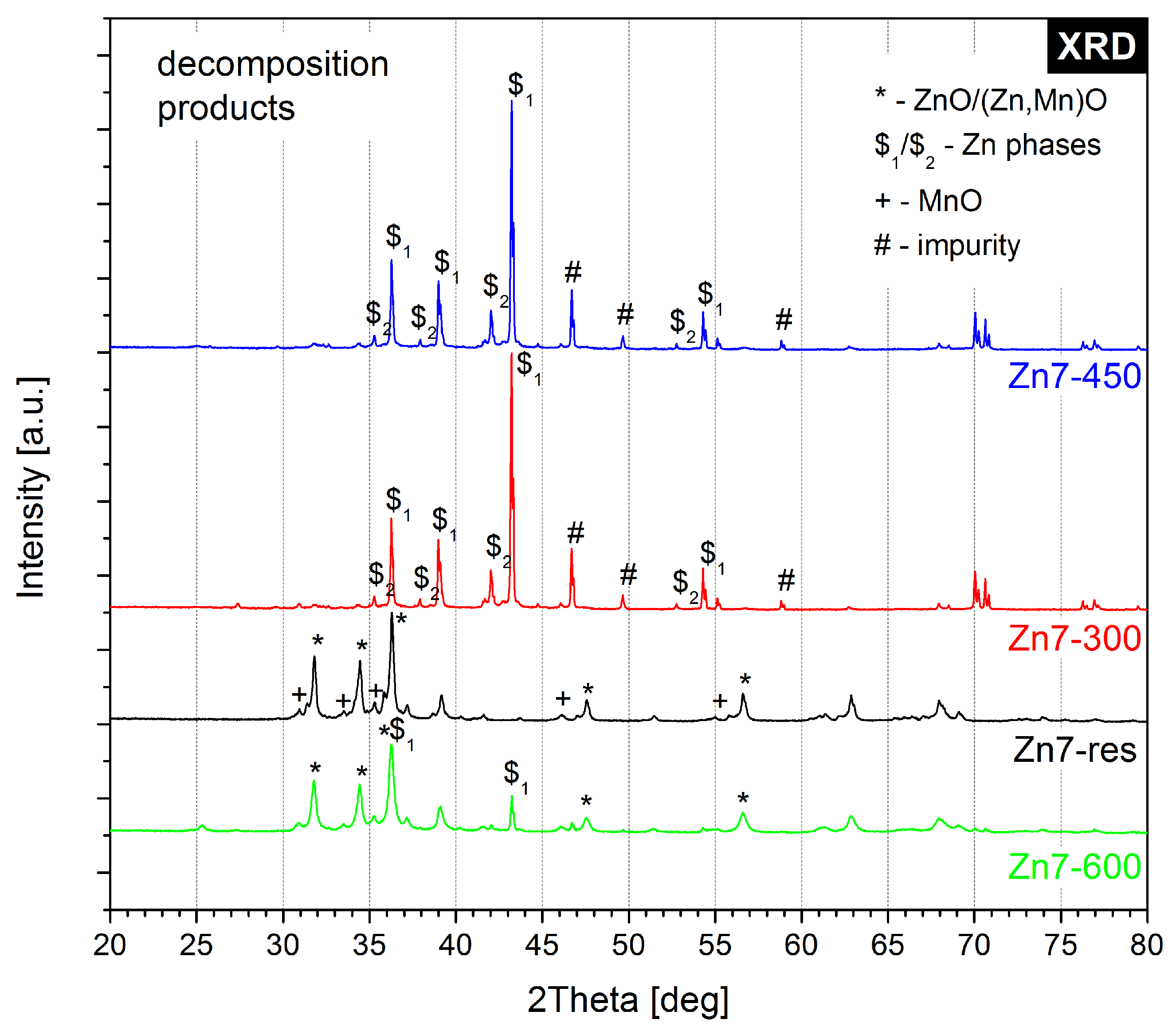
2.2. X-ray Diffractometry
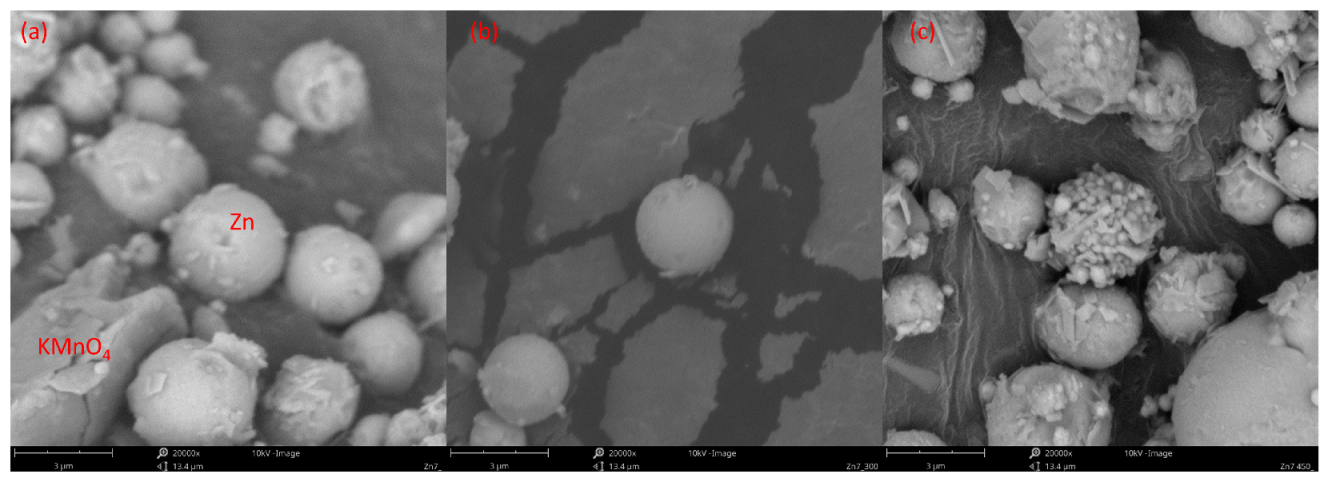
2.3. Scanning Electron Microscopy Investigations
3. Materials and Methods
3.1. Preparation of PDC Samples
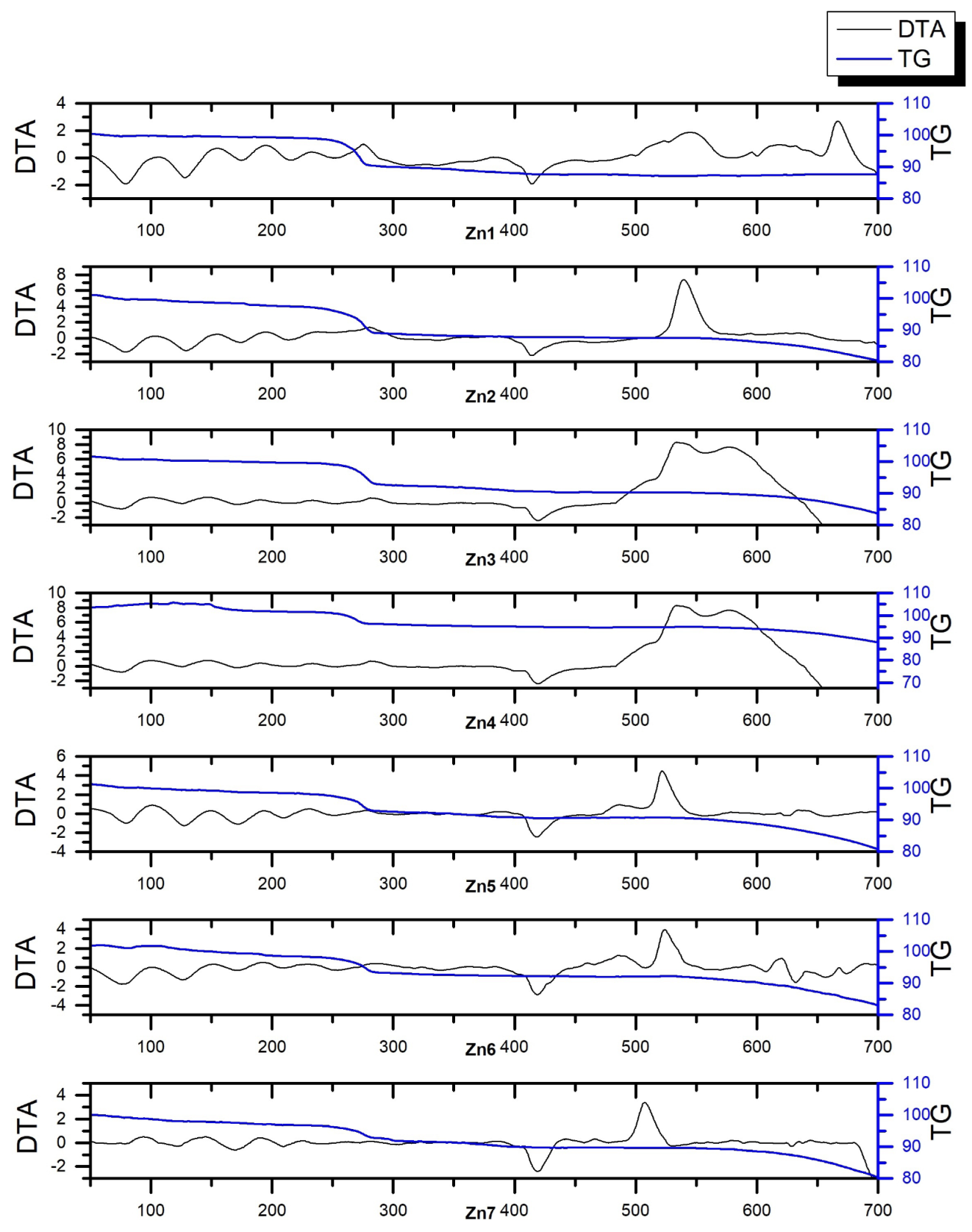
3.1.1. Thermal Conditioning of Samples
- 300 C—the samples all showed an initial mass loss step at approx. 270 C, which we attributed to the decomposition of the permanganate anion. Hence, this temperature was chosen to identify the products of the reaction underlying this mass loss step.
- 450 C—zinc is known to have a melting point of 419.5 C [30]. This temperature point was chosen to allow for the investigation of whether the fusion of zinc and, therefore, significantly increased surface of contact between the reagents would lead to the occurrence of any reactions in the condensed (solid/liquid) phase.
- 600 C—the samples typically underwent ignition at approx. 500 C, with the exact ignition temperature being dependent on the ratio of the two reagents in the sample. This temperature point was selected as a “blank sample” for comparison with the samples of post-combustion residues, for whom oxidation by atmospheric oxygen is also a factor that alters their chemical composition.
3.1.2. Post-Combustion Residues
3.2. Scanning Electron Microscopy
3.3. Raman Spectroscopy
3.4. X-ray Diffractometry
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
Appendix A
References
- Shaw, A.; Poret, J.; Groven, L.; Koenig, J.; Brusnahan, J. Pyrotechnic Delay Element Device. US Patent 11,614,313, 28 March 2023. [Google Scholar]
- Tichapondwa, S.M.; Guo, S.; Roux, W.E. Performance of Mn/Bi2O3 pyrotechnic time delay compositions. Cent. Eur. J. Energetic Mater. 2021, 18, 46–62. [Google Scholar] [CrossRef]
- Guo, S.; Focke, W.W.; Tichapondwa, S.M. Al-Ni-NiO Pyrotechnic Time-Delays. Propellants Explos. Pyrotech. 2020, 45, 665–670. [Google Scholar] [CrossRef]
- Bell, T.M.; Williamson, D.M.; Walley, S.M.; Morgan, C.G.; Kelly, C.L.; Batchelor, L. An Assessment of Printing Methods for Producing Two-Dimensional Lead-Free Functional Pyrotechnic Delay-Lines for Mining Applications. Propellants Explos. Pyrotech. 2020, 45, 53–76. [Google Scholar] [CrossRef]
- Akhtar, M.J.; Ahamed, M.; Alhadlaq, H. Bismuth Oxide (Bi2O3) Nanoparticles Cause Selective Toxicity in a Human Endothelial (HUVE) Cell Line Compared to Epithelial Cells. Toxics 2023, 11, 343. [Google Scholar] [CrossRef] [PubMed]
- Ahamed, M.; Akhtar, M.J.; Khan, M.A.M.; Alhadlaq, H.A. Co-exposure of Bi2O3 nanoparticles and bezo [a] pyrene-enhanced in vitro cytotoxicity of mouse spermatogonia cells. Environ. Sci. Pollut. Res. 2021, 28, 17109–17118. [Google Scholar] [CrossRef]
- Ren, H.; Jiao, Q.; Chen, S. Mixing Si and carbon nanotubes by a method of ball-milling and its application to pyrotechnic delay composition. J. Phys. Chem. Solids 2010, 71, 145–148. [Google Scholar] [CrossRef]
- Beck, M.W.; Brown, M.E. Modification of the burning rate of antimony/potassium permanganate pyrotechnic delay compositions. Combust. Flame 1986, 66, 67–75. [Google Scholar] [CrossRef]
- Zhang, B.; Huang, C.; Yan, S.; Li, Y.; Cheng, Y. Enhanced reactivity of boron, through adding nano-aluminum and wet ball milling. Appl. Surf. Sci. 2013, 286, 91–98. [Google Scholar] [CrossRef]
- Gabdrashova, S.; Tulepov, M.; Korchagin, M.; Sassykova, L.; Abdrakova, F.Y.; Bexultan, Z.B.; Aitenov, Y.; Toktagul, S.; Baiseitov, D. Development of pyrotechnic delay mixtures based on a composite material hardened with carbon nanotubes. Dig. J. Nanomater. Biostruct 2021, 16, 1341–1350. [Google Scholar] [CrossRef]
- Leonova, M.; Timofeeva, S.; Murzin, M. Dust load in silicon production and occupational risks. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Kazimierz Dolny, Poland, 21–23 November 2019; Volume 687, p. 066012. [Google Scholar]
- Skjold, T. An experimental investigation of flame propagation in clouds of silicon dust dispersed in air, hydrogen-air mixtures, and hybrid Si-H2-air mixtures. In Proceedings of the Twenty-Fifth International Colloquium on the Dynamics of Explosions and Reactive Systems, Taipei, China, 28 July–2 August 2013. [Google Scholar]
- Boreiko, C.J.; Rossman, T.G. Antimony and its compounds: Health impacts related to pulmonary toxicity, cancer, and genotoxicity. Toxicol. Appl. Pharmacol. 2020, 403, 115156. [Google Scholar]
- Zhang, P.; Wu, T.L.; Ata-Ul-Karim, S.T.; Ge, Y.Y.; Cui, X.; Zhou, D.M.; Wang, Y.J. Influence of soil properties and aging on antimony toxicity for barley root elongation. Bull. Environ. Contam. Toxicol. 2020, 104, 714–720. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Lee, D.G. Permanganate: A green and versatile industrial oxidant. Org. Process Res. Dev. 2001, 5, 599–603. [Google Scholar] [CrossRef]
- Polis, M.; Szydło, K.; Jarosz, T.; Procek, M.; Skóra, P.; Stolarczyk, A. Investigation of combustion of KMnO4/Zn pyrotechnic delay composition. Materials 2022, 15, 6406. [Google Scholar] [CrossRef] [PubMed]
- Agnew, U.M.; Slesinger, T.L. Zinc Toxicity; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- McGrath, S.P.; Chaudri, A.M.; Giller, K.E. Long-term effects of metals in sewage sludge on soils, microorganisms and plants. J. Ind. Microbiol. 1995, 14, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Tribelhorn, M.J.; Venables, D.S.; Brown, M.E. Combustion of some zinc-fuelled binary pyrotechnic systems. Thermochim. Acta 1995, 256, 309–324. [Google Scholar] [CrossRef][Green Version]
- Herbstein, F.; Ron, G.; Weissman, A. The thermal decomposition of potassium permanganate and related substances. Part I. Chemical aspects. J. Chem. Soc. A Inorg. Phys. Theor. 1971, 1821–1826. [Google Scholar] [CrossRef]
- Clark, R.J.; Dines, T.J.; Doherty, J.M. Resonance Raman spectroscopy of the manganate (VI) ion: Band excitation profiles and excited-state geometry. Inorg. Chem. 1985, 24, 2088–2091. [Google Scholar]
- Hu, X.; Shi, L.; Zhang, D.; Zhao, X.; Huang, L. Accelerating the decomposition of KMnO4 by photolysis and auto-catalysis: A green approach to synthesize a layered birnessite-type MnO2 assembled hierarchical nanostructure. RSC Adv. 2016, 6, 14192–14198. [Google Scholar]
- Gao, T.; Fjellvåg, H.; Norby, P. A comparison study on Raman scattering properties of α-and β-MnO2. Anal. Chim. Acta 2009, 648, 235–239. [Google Scholar] [CrossRef]
- Julien, C.; Massot, M. Raman spectroscopic studies of lithium manganates with spinel structure. J. Phys. Condens. Matter 2003, 15, 3151. [Google Scholar] [CrossRef]
- Bernardini, S.; Bellatreccia, F.; Casanova Municchia, A.; Della Ventura, G.; Sodo, A. Raman spectra of natural manganese oxides. J. Raman Spectrosc. 2019, 50, 873–888. [Google Scholar] [CrossRef]
- Zygmunt, B.; Wilk, Z. Formation of jets by shaped charges with metal powder liners. Propellants Explos. Pyrotech. Int. J. Deal. Sci. Technol. Asp. Energetic Mater. 2008, 33, 482–487. [Google Scholar] [CrossRef]
- Liberati, A.C.; Che, H.; Vo, P.; Yue, S. Influence of secondary component hardness when cold spraying mixed metal powders on carbon fiber reinforced polymers. J. Therm. Spray Technol. 2021, 30, 1239–1253. [Google Scholar] [CrossRef]
- Tribelhorn, M.J.; Venables, D.S.; Brown, M.E. A thermoanalytical study of some zinc-fuelled binary pyrotechnic systems. Thermochim. Acta 1995, 269, 649–663. [Google Scholar] [CrossRef]
- Focke, W.W.; Tichapondwa, S.M.; Montgomery, Y.C.; Grobler, J.M.; Kalombo, M.L. Review of gasless pyrotechnic time delays. Propellants Explos. Pyrotech. 2019, 44, 55–93. [Google Scholar] [CrossRef]
- Chase, M.W.; NISO. NIST-JANAF Thermochemical Tables; American Chemical Society: Washington, DC, USA, 1998; Volume 9. [Google Scholar]
- Haynes, W.M. CRC Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Herbstein, F. Crystallographic data for potassium manganate K2MnO4. Acta Crystallogr. 1960, 13, 357. [Google Scholar] [CrossRef]
- Miklaszewski, E.J.; Shaw, A.P.; Poret, J.C.; Son, S.F.; Groven, L.J. Performance and aging of Mn/MnO2 as an environmentally friendly energetic time delay composition. ACS Sustain. Chem. Eng. 2014, 2, 1312–1317. [Google Scholar] [CrossRef]



| Composition Label | Zn1 | Zn2 | Zn3 | Zn4 | Zn5 | Zn6 | Zn7 |
|---|---|---|---|---|---|---|---|
| Zn [wt.%] | 35 | 45 | 50 | 55 | 60 | 65 | 70 |
| KMnO4 [wt.%] | 65 | 55 | 50 | 45 | 40 | 35 | 30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polis, M.; Szydło, K.; Zakusylo, R.; Hawelek, L.; Stolarczyk, A.; Jarosz, T. Study of the Combustion Mechanism of Zn/KMnO4 Pyrotechnic Composition. Molecules 2023, 28, 5741. https://doi.org/10.3390/molecules28155741
Polis M, Szydło K, Zakusylo R, Hawelek L, Stolarczyk A, Jarosz T. Study of the Combustion Mechanism of Zn/KMnO4 Pyrotechnic Composition. Molecules. 2023; 28(15):5741. https://doi.org/10.3390/molecules28155741
Chicago/Turabian StylePolis, Mateusz, Konrad Szydło, Roman Zakusylo, Lukasz Hawelek, Agnieszka Stolarczyk, and Tomasz Jarosz. 2023. "Study of the Combustion Mechanism of Zn/KMnO4 Pyrotechnic Composition" Molecules 28, no. 15: 5741. https://doi.org/10.3390/molecules28155741
APA StylePolis, M., Szydło, K., Zakusylo, R., Hawelek, L., Stolarczyk, A., & Jarosz, T. (2023). Study of the Combustion Mechanism of Zn/KMnO4 Pyrotechnic Composition. Molecules, 28(15), 5741. https://doi.org/10.3390/molecules28155741






