Untargeted Metabolomics Approach Using UHPLC-HRMS to Unravel the Impact of Fermentation on Color and Phenolic Composition of Rosé Wines
Abstract
:1. Introduction
2. Results and Discussion
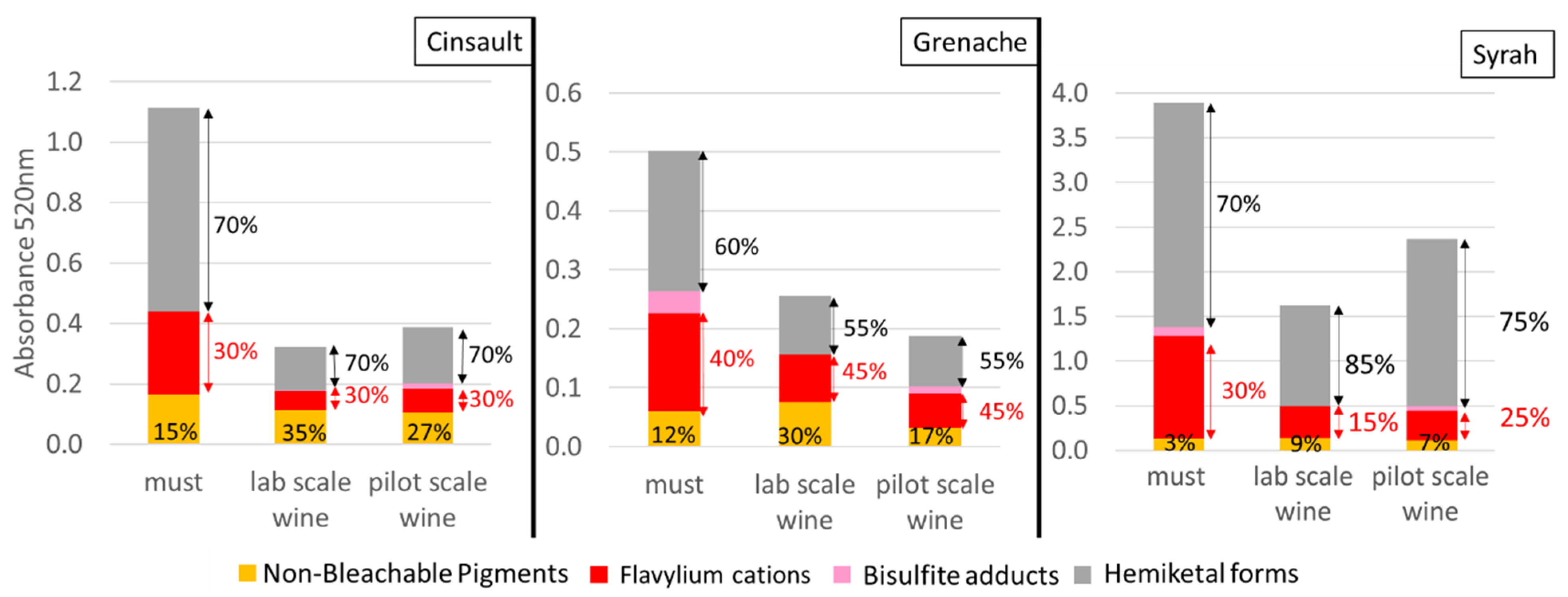
2.1. Spectrophotometric Analysis
UV-Visible Absorbance Characteristics
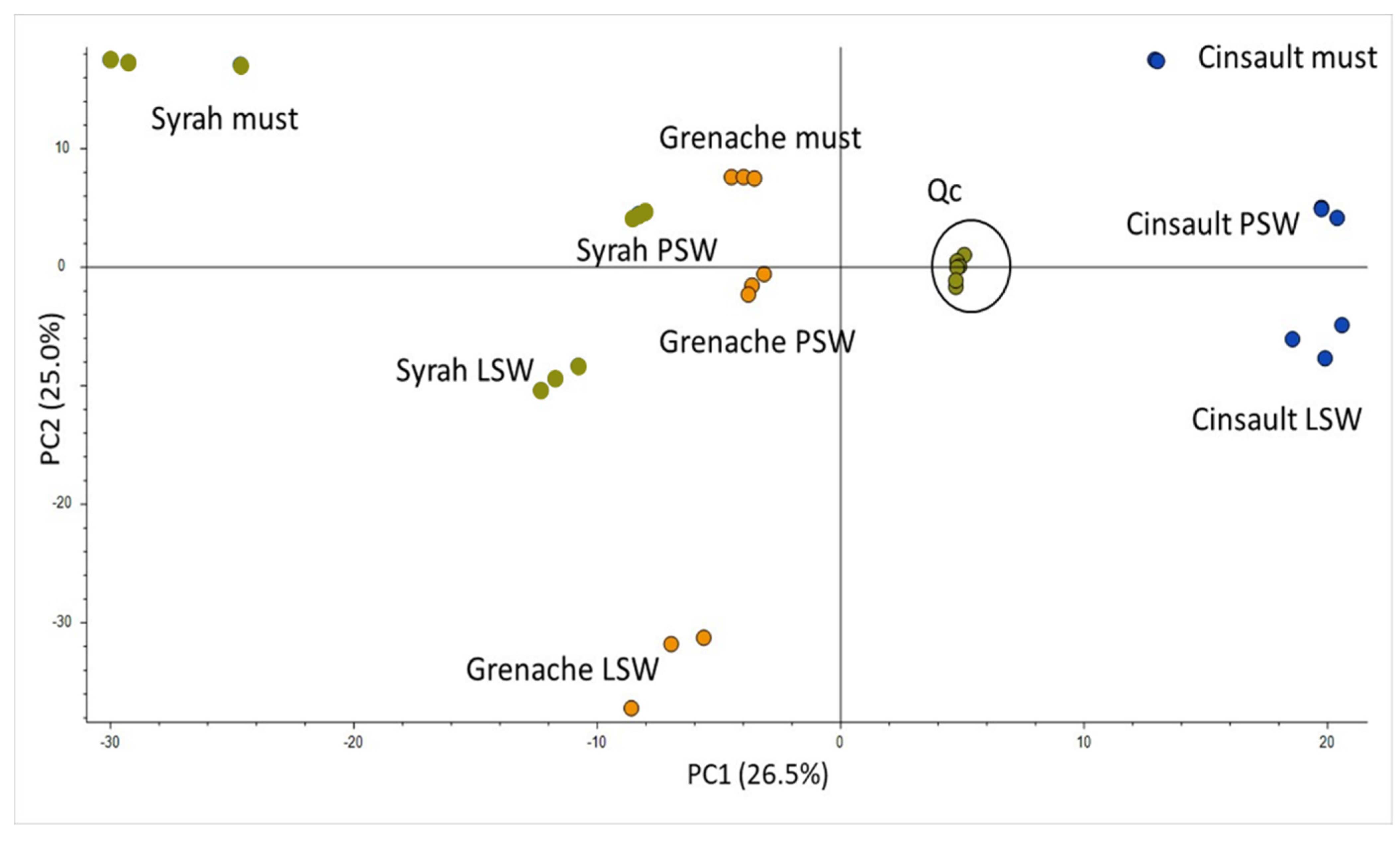
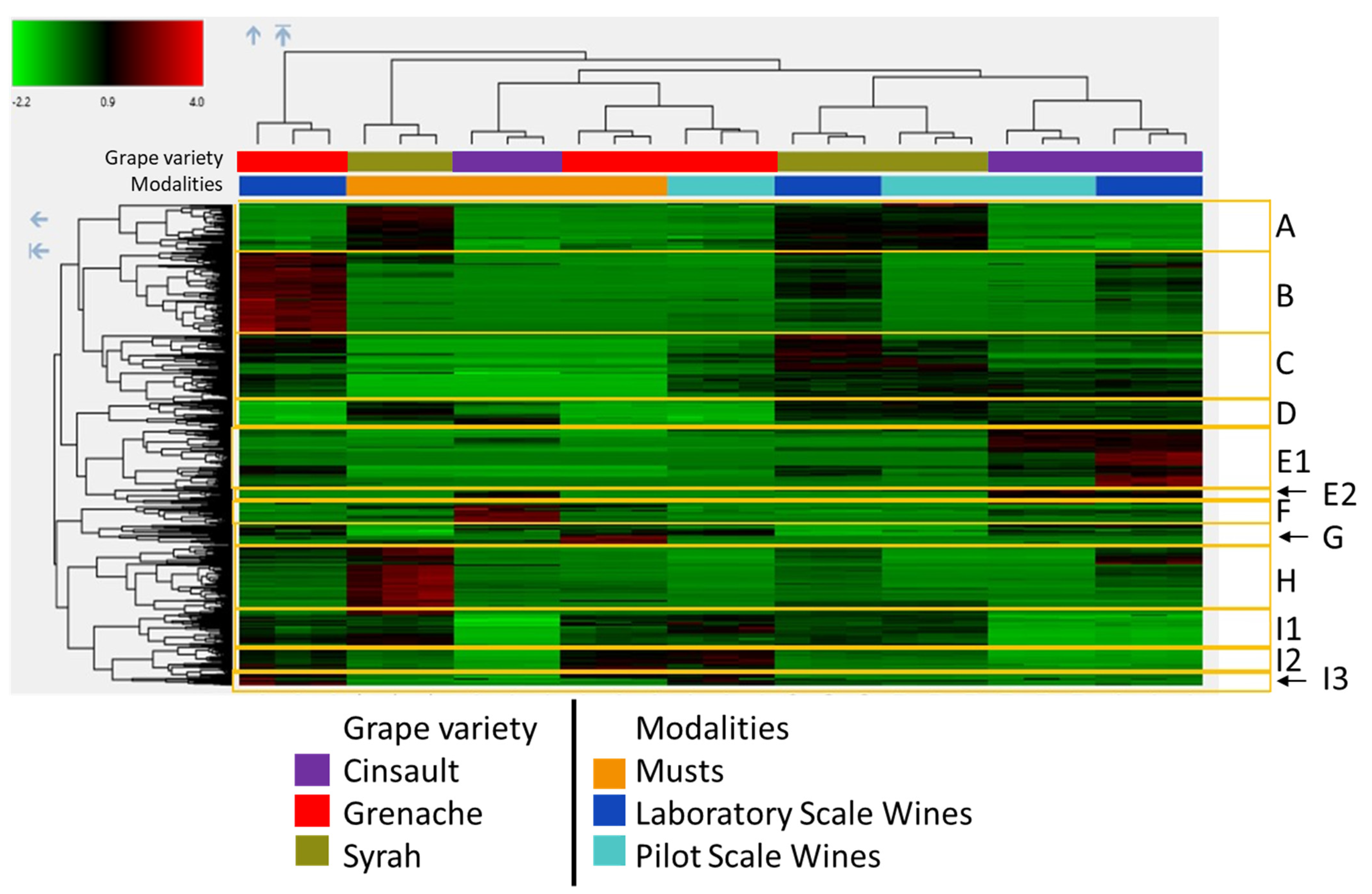
2.2. Untargeted Metabolomics by High Resolution Mass Spectrometry
3. Materials and Methods
3.1. Chemicals
3.2. Must Preparation
3.3. Fermentation
3.4. Spectrophotometric Analysis
3.5. Untargeted Analysis
3.5.1. LC-HRMS Analysis
3.5.2. Data Analysis
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Peres, S.; Giraud-Heraud, E.; Masure, A.-S.; Tempere, S. Rose Wine Market: Anything but Colour? Foods 2020, 9, 1850. [Google Scholar] [CrossRef] [PubMed]
- Leborgne, C.; Lambert, M.; Ducasse, M.-A.; Meudec, E.; Verbaere, A.; Sommerer, N.; Boulet, J.-C.; Masson, G.; Mouret, J.-R.; Cheynier, V. Elucidating the Color of Rosé Wines Using Polyphenol-Targeted Metabolomics. Molecules 2022, 27, 1359. [Google Scholar] [CrossRef] [PubMed]
- Wirth, J.; Caillé, S.; Souquet, J.; Samson, A.; Dieval, J.; Vidal, S.; Fulcrand, H.; Cheynier, V. Impact of post-bottling oxygen exposure on the sensory characteristics and phenolic composition of Grenache rosé wines. Food Chem. 2012, 132, 1861–1871. [Google Scholar] [CrossRef]
- de Freitas, V.A.P.; Fernandes, A.; Oliveira, J.; Teixeira, N.; Mateus, N. A review of the current knowledge of red wine colour. OENO One 2017, 51, 1001–1021. [Google Scholar] [CrossRef]
- Cheynier, V.; Rigaud, J.; Souquet, J.M.; Barillère, J.M.; Moutounet, M. Effect of pomace contact and hyperoxidation on the phenolic composition and quality of grenache and chardonnay wines. Am. J. Enol. Vitic. 1989, 40, 36–42. [Google Scholar] [CrossRef]
- Lambert, M.; Meudec, E.; Verbaere, A.; Mazerolles, G.; Wirth, J.; Masson, G.; Cheynier, V.; Sommerer, N. A High-Throughput UHPLC-QqQ-MS Method for Polyphenol Profiling in Rosé Wines. Molecules 2015, 20, 7890–7914. [Google Scholar] [CrossRef]
- Leborgne, C.; Ducasse, M.-A.; Meudec, E.; Carrillo, S.; Verbaere, A.; Sommerer, N.; Bougreau, M.; Masson, G.; Vernhet, A.; Mouret, J.-R.; et al. Multi-method study of the impact of fermentation on the polyphenol composition and color of Grenache, Cinsault, and Syrah rosé wines. Food Chem. 2023, 403, 134396. [Google Scholar] [CrossRef]
- Cayla, L.; Pouzalgues, N.; Masson, G. Connaissance et Maîtrise de la Couleur des vins Rosés. February 2011; p. 8. Available online: https://centredurose.fr/ (accessed on 26 July 2023).
- Romero-Cascales, I.; Ortega-Regules, A.; López-Roca, J.M.; Fernández-Fernández, J.I.; Gómez-Plaza, E. Differences in Anthocyanin Extractability from Grapes to Wines According to Variety. Am. J. Enol. Vitic. 2005, 56, 212–219. [Google Scholar] [CrossRef]
- Alañón, M.E.; Pérez-Coello, M.S.; Marina, M.L. Wine science in the metabolomics era. TrAC Trends Anal. Chem. 2015, 74, 1–20. [Google Scholar] [CrossRef]
- Arapitsas, P.; Mattivi, F. LC-MS Untargeted Protocol for the Analysis of Wine. In Metabolic Profiling: Methods and Protocols; Theodoridis, G.A., Gika, H.G., Wilson, I.D., Eds.; in Methods in Molecular Biology; Springer: New York, NY, USA, 2018; pp. 225–235. [Google Scholar] [CrossRef]
- Sáez, V.; Schober, D.; González, Á.; Arapitsas, P. LC–MS-Based Metabolomics Discriminates Premium from Standard Chilean cv. Cabernet Sauvignon Wines from Different Valleys. Metabolites 2021, 11, 829. [Google Scholar] [CrossRef]
- Somers, T.C.; Vérette, E.; Pocock, K.F. Hydroxycinnamate esters of Vitis vinifera: Changes during white vinification, and effects of exogenous enzymic hydrolysis. J. Sci. Food Agric. 1987, 40, 67–78. [Google Scholar] [CrossRef]
- Boulet, J.-C.; Trarieux, C.; Souquet, J.-M.; Ducasse, M.-A.; Caillé, S.; Samson, A.; Williams, P.; Doco, T.; Cheynier, V. Models based on ultraviolet spectroscopy, polyphenols, oligosaccharides and polysaccharides for prediction of wine astringency. Food Chem. 2016, 190, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Cheynier, V.; Souquet, J.M.; Moutounet, M. Glutathione Content and Glutathione to Hydroxycinnamic Acid Ratio in Vitis vinifera Grapes and Musts. Am. J. Enol. Vitic. 1989, 40, 320–324. [Google Scholar] [CrossRef]
- Brouillard, R.; Dubois, J.-E. Mechanism of the structural transformations of anthocyanins in acidic media. J. Am. Chem. Soc. 1977, 99, 1359–1364. [Google Scholar] [CrossRef]
- Zara, G.; Nardi, T. Yeast Metabolism and Its Exploitation in Emerging Winemaking Trends: From Sulfite Tolerance to Sulfite Reduction. Fermentation 2021, 7, 57. [Google Scholar] [CrossRef]
- Ochando, T.; Mouret, J.-R.; Humbert-Goffard, A.; Aguera, E.; Sablayrolles, J.-M.; Farines, V. Comprehensive study of the dynamic interaction between SO2 and acetaldehyde during alcoholic fermentation. Food Res. Int. 2020, 136, 109607. [Google Scholar] [CrossRef]
- Brouillard, R.; Chahine, J.M.E.H. Chemistry of anthocyanin pigments. 6. Kinetic and thermodynamic study of hydrogen sulfite addition to cyanin. Formation of a highly stable Meisenheimer-type adduct derived from a 2-phenylbenzopyrylium salt. J. Am. Chem. Soc. 1980, 102, 5375–5378. [Google Scholar] [CrossRef]
- Timberlake, C.F.; Bridle, P. Interactions between Anthocyanins, Phenolic Compounds, and Acetaldehyde and Their Significance in Red Wines. Am. J. Enol. Vitic. 1976, 27, 97–105. [Google Scholar] [CrossRef]
- Lee, D.F.; Swinny, E.E.; Jones, G.P. NMR identification of ethyl-linked anthocyanin-flavanol pigments formed in model wine ferments. Tetrahedron Lett. 2004, 45, 1671–1674. [Google Scholar] [CrossRef]
- Trouillas, P.; Sancho-García, J.C.; De Freitas, V.; Gierschner, J.; Otyepka, M.; Dangles, O. Stabilizing and Modulating Color by Copigmentation: Insights from Theory and Experiment. Chem. Rev. 2016, 116, 4937–4982. [Google Scholar] [CrossRef] [Green Version]
- Dueñas, M.; Salas, E.; Cheynier, V.; Dangles, O.; Fulcrand, H. UV−Visible Spectroscopic Investigation of the 8,8-Methylmethine Catechin-malvidin 3-Glucoside Pigments in Aqueous Solution: Structural Transformations and Molecular Complexation with Chlorogenic Acid. J. Agric. Food Chem. 2006, 54, 189–196. [Google Scholar] [CrossRef]
- Arapitsas, P.; Ugliano, M.; Marangon, M.; Piombino, P.; Rolle, L.; Gerbi, V.; Versari, A.; Mattivi, F. Use of Untargeted Liquid Chromatography–Mass Spectrometry Metabolome To Discriminate Italian Monovarietal Red Wines, Produced in Their Different Terroirs. J. Agric. Food Chem. 2020, 68, 13353–13366. [Google Scholar] [CrossRef] [PubMed]
- Ontañón, I.; Sánchez, D.; Sáez, V.; Mattivi, F.; Ferreira, V.; Arapitsas, P. Liquid chromatography–mass spectrometry-based metabolomics for understanding the compositional changes induced by oxidative or anoxic storage of red wines. J. Agric. Food Chem. 2020, 68, 13367–13379. [Google Scholar] [CrossRef] [PubMed]
- Roullier-Gall, C.; Hemmler, D.; Gonsior, M.; Li, Y.; Nikolantonaki, M.; Aron, A.; Coelho, C.; Gougeon, R.D.; Schmitt-Kopplin, P. Sulfites and the wine metabolome. Food Chem. 2017, 237, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Vallverdú-Queralt, A.; Meudec, E.; Eder, M.; Lamuela-Raventos, R.M.; Sommerer, N.; Cheynier, V. The Hidden Face of Wine Polyphenol Polymerization Highlighted by High-Resolution Mass Spectrometry. ChemistryOpen 2017, 6, 336–339. [Google Scholar] [CrossRef]
- White, W.H.; Gunyuzlu, P.L.; Toyn, J.H. Saccharomyces cerevisiae Is Capable of de Novo Pantothenic Acid Biosynthesis Involving a Novel Pathway of β-Alanine Production from Spermine. J. Biol. Chem. 2001, 276, 10794–10800. [Google Scholar] [CrossRef] [Green Version]
- Iriti, M.; Vigentini, I. Tryptophan-Ethylester, the False (Unveiled) Melatonin Isomer in Red Wine. Int. J. Tryptophan Res. 2015, 8, 27–29. [Google Scholar] [CrossRef] [Green Version]
- Jeandet, P.; Bessis, R.; Sbaghi, M.; Meunier, P.; Trollat, P. Resveratrol Content of Wines of Different Ages: Relationship With Fungal Disease Pressure in the Vineyard. Am. J. Enol. Vitic. 1995, 46, 1–4. [Google Scholar] [CrossRef]
- Es-Safi, N.-E.; Meudec, E.; Bouchut, C.; Fulcrand, H.; Ducrot, P.-H.; Herbette, G.; Cheynier, V. New Compounds Obtained by Evolution and Oxidation of Malvidin 3-O-Glucoside in Ethanolic Medium. J. Agric. Food Chem. 2008, 56, 4584–4591. [Google Scholar] [CrossRef]
- Ruiz-Rodríguez, A.; Carrera, C.A.; Setyaningsih, W.; Barbero, G.F.; Ferreiro-González, M.; Palma, M.; Barroso, C.G. Tryptophan Levels during Grape Ripening: Effects of Cultural Practices. Molecules 2017, 22, 941. [Google Scholar] [CrossRef] [Green Version]
- Cheynier, V.F.; Van Hulst, M.W.J. Oxidation of trans-caftaric acid and 2-S-glutathionylcaftaric acid in model solutions. J. Agric. Food Chem. 1988, 36, 10–15. [Google Scholar] [CrossRef]
- Fulcrand, H.; Cheminat, A.; Brouillard, R.; Cheynier, V. Characterization of compounds obtained by chemical oxidation of caffeic acid in acidic conditions. Phytochemistry 1994, 35, 499–505. [Google Scholar] [CrossRef]
- Poupard, P.; Sanoner, P.; Baron, A.; Renard, C.M.G.C.; Guyot, S. Characterization of procyanidin B2 oxidation products in an apple juice model solution and confirmation of their presence in apple juice by high-performance liquid chromatography coupled to electrospray ion trap mass spectrometry: LC-ESI-MS/MS of procyanidin B2 oxidation products. J. Mass Spectrom. 2011, 46, 1186–1197. [Google Scholar] [CrossRef] [PubMed]
- Cheynier, V.; Osse, C.; Rigaud, J. Oxidation of Grape Juice Phenolic Compounds in Model Solutions. J. Food Sci. 1988, 53, 1729–1732. [Google Scholar] [CrossRef]
- Becerra-Rodríguez, C.; Taghouti, G.; Portier, P.; Dequin, S.; Casal, M.; Paiva, S.; Galeote, V. Yeast Plasma Membrane Fungal Oligopeptide Transporters Display Distinct Substrate Preferences despite Their High Sequence Identity. J. Fungi 2021, 7, 963. [Google Scholar] [CrossRef]
- Cordente, A.G.; Capone, D.L.; Curtin, C.D. Unravelling glutathione conjugate catabolism in Saccharomyces cerevisiae: The role of glutathione/dipeptide transporters and vacuolar function in the release of volatile sulfur compounds 3-mercaptohexan-1-ol and 4-mercapto-4-methylpentan-2-one. Appl. Microbiol. Biotechnol. 2015, 99, 9709–9722. [Google Scholar] [CrossRef]
- Martínez-Lüscher, J.; Brillante, L.; Kurtural, S.K. Flavonol Profile Is a Reliable Indicator to Assess Canopy Architecture and the Exposure of Red Wine Grapes to Solar Radiation. Front. Plant Sci. 2019, 10, 10. [Google Scholar] [CrossRef] [Green Version]
- Boursiquot, J.M.; Sapis, J.C.; Macheix, J.J. Les esters hydroxycinnamiques chez le genre Vitis. Essai d’application taxonomique. Premiers resultats. Note presentee par Roger Buvat. Comptes Rendus L’Académie Sci. Sér. III Sci. Vie 1986, 302, 177. [Google Scholar]
- Duc, C.; Maçna, F.; Sanchez, I.; Galeote, V.; Delpech, S.; Silvano, A.; Mouret, J.-R. Large-Scale Screening of Thiol and Fermentative Aroma Production during Wine Alcoholic Fermentation: Exploring the Effects of Assimilable Nitrogen and Peptides. Fermentation 2020, 6, 98. [Google Scholar] [CrossRef]
- Atanasova, V.; Fulcrand, H.; Cheynier, V.; Moutounet, M. Effect of oxygenation on polyphenol changes occurring in the course of wine-making. Anal. Chim. Acta 2002, 458, 15–27. [Google Scholar] [CrossRef]
- OIV. Recueil des Methodes Internationales d’Analyses—OIV Caractéristiques Chromatiques. 2019. Available online: https://www.oiv.int/fr (accessed on 26 July 2023).
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.-M.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed minimum reporting standards for chemical analysis. Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Grape Variety | Stage | L* | a* | b* | Hue |
|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | ||
| Cinsault | must | 75.10 ± 0.64 d | 16.29 ± 0.92 c | 17.49 ± 1.06 a | 0.82 ± 0.06 c |
| Cinsault | lab-scale wine (LSW) | 88.82 ± 0.59 b | 6.05 ± 0.51 f | 13.20 ± 0.64 b | 1.14 ± 0.05 a |
| Cinsault | pilot-scale wine (PSW) | 89.29 ± 0.53 b | 8.18 ± 1.14 e | 15.72 ± 1.22 ab | 1.09 ± 0.07 a |
| Grenache | must | 87.43 ± 0.90 b | 11.25 ± 0.52 d | 14.01 ± 0.50 b | 0.89 ± 0.01 c |
| Grenache | lab-scale wine (LSW) | 89.83 ± 2.90 b | 4.87 ± 0.24 f | 10.40 ± 2.89 c | 1.12 ± 0.10 b |
| Grenache | pilot-scale wine (PSW) | 93.57 ± 0.19 a | 4.94 ± 0.26 f | 6.69 ± 0.48 de | 0.97 ± 0.02 c |
| Syrah | must | 53.48 ± 2.83 e | 47.65 ± 1.16 a | 8.52 ± 0.56 cd | 0.18 ± 0.01 e |
| Syrah | lab-scale wine (LSW) | 75.87 ± 1.81 d | 28.55 ± 0.47 b | 7.06 ± 1.46 de | 0.24 ± 0.05 d |
| Syrah | pilot-scale wine (PSW) | 78.98 ± 0.74 c | 28.71 ± 0.58 b | 4.97 ± 0.40 e | 0.17 ± 0.01 d |
| Cluster (Number of Features) | Compounds | Variety | Process Stage | Marker of/Origin |
|---|---|---|---|---|
| A (73) | Anthocyanins | Syrah | Variety | |
| B (126) | Unknown N compounds | Higher in Grenache | Lab-scale wines | Fermentation |
| C (103) | Yeast metabolites | wines | Fermentation | |
| D (43) | Stilbenes Rearrangement product of mv3glc? | Lower in Grenache | Fungal pressure in the vineyard? | |
| E1 (93) | Norisoprenoids flavonoids N compounds higher Mw N and S compounds | Higher in Cinsault | Higher in wine | Variety, Environmental conditions revealed by fermentation |
| E2 (24) | Higher molecular weight compounds | Higher in Cinsault | Higher in wine | Reactions in wine |
| F (32) | N compounds | Cin > Gre > Syr | Higher in musts | Grape |
| G (16) | Tryptophan derivatives Caftaric–GRP adducts, GRP dimer | Higher in Grenache | Higher in musts | Grape variety/vine growing Grape variety PPO oxidation Degradation in wine |
| H (107) | N compounds (Phe) peonidin 3-glucoside flavonol derivatives GRP isomer, GRP2 | Syrah | Higher in musts | Variety, Anthocyanin profile GSH/AH ratio PPO oxidation Degradation in wine |
| I1 (51) | GRP, coutaric acid, flavan-3-ols | Lower in Cinsault | Variety | |
| I2 (49) | Caftaric, fertaric N compounds | Lower in Cinsault Higher in Grenache | Variety | |
| I3 (12) | Tryptophol Ethyl caffeic coumarin | Lower in Cinsault Higher in grenache | Higher in wine | Fermentation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leborgne, C.; Meudec, E.; Sommerer, N.; Masson, G.; Mouret, J.-R.; Cheynier, V. Untargeted Metabolomics Approach Using UHPLC-HRMS to Unravel the Impact of Fermentation on Color and Phenolic Composition of Rosé Wines. Molecules 2023, 28, 5748. https://doi.org/10.3390/molecules28155748
Leborgne C, Meudec E, Sommerer N, Masson G, Mouret J-R, Cheynier V. Untargeted Metabolomics Approach Using UHPLC-HRMS to Unravel the Impact of Fermentation on Color and Phenolic Composition of Rosé Wines. Molecules. 2023; 28(15):5748. https://doi.org/10.3390/molecules28155748
Chicago/Turabian StyleLeborgne, Cécile, Emmanuelle Meudec, Nicolas Sommerer, Gilles Masson, Jean-Roch Mouret, and Véronique Cheynier. 2023. "Untargeted Metabolomics Approach Using UHPLC-HRMS to Unravel the Impact of Fermentation on Color and Phenolic Composition of Rosé Wines" Molecules 28, no. 15: 5748. https://doi.org/10.3390/molecules28155748
APA StyleLeborgne, C., Meudec, E., Sommerer, N., Masson, G., Mouret, J.-R., & Cheynier, V. (2023). Untargeted Metabolomics Approach Using UHPLC-HRMS to Unravel the Impact of Fermentation on Color and Phenolic Composition of Rosé Wines. Molecules, 28(15), 5748. https://doi.org/10.3390/molecules28155748






