Profiling the Antidiabetic Potential of Compounds Identified from Fractionated Extracts of Entada africana toward Glucokinase Stimulation: Computational Insight
Abstract
:1. Introduction
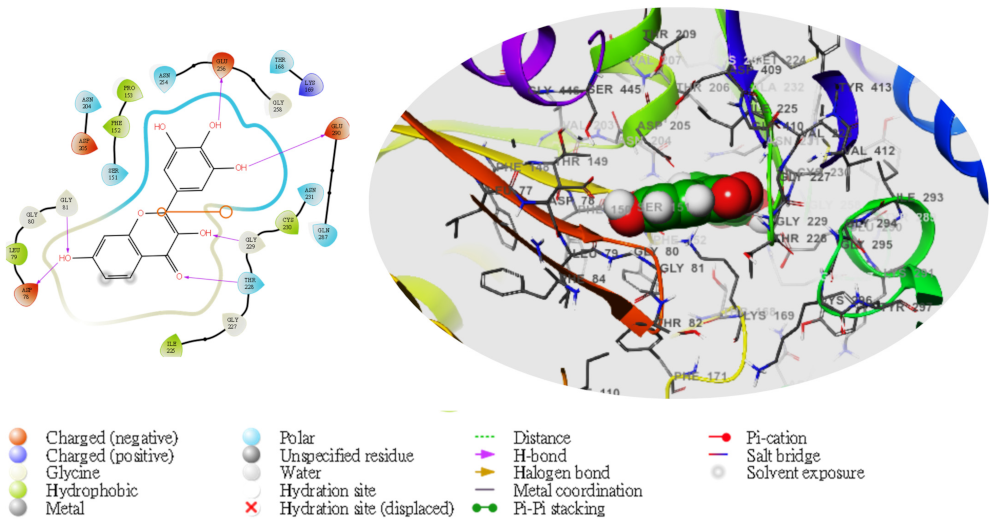
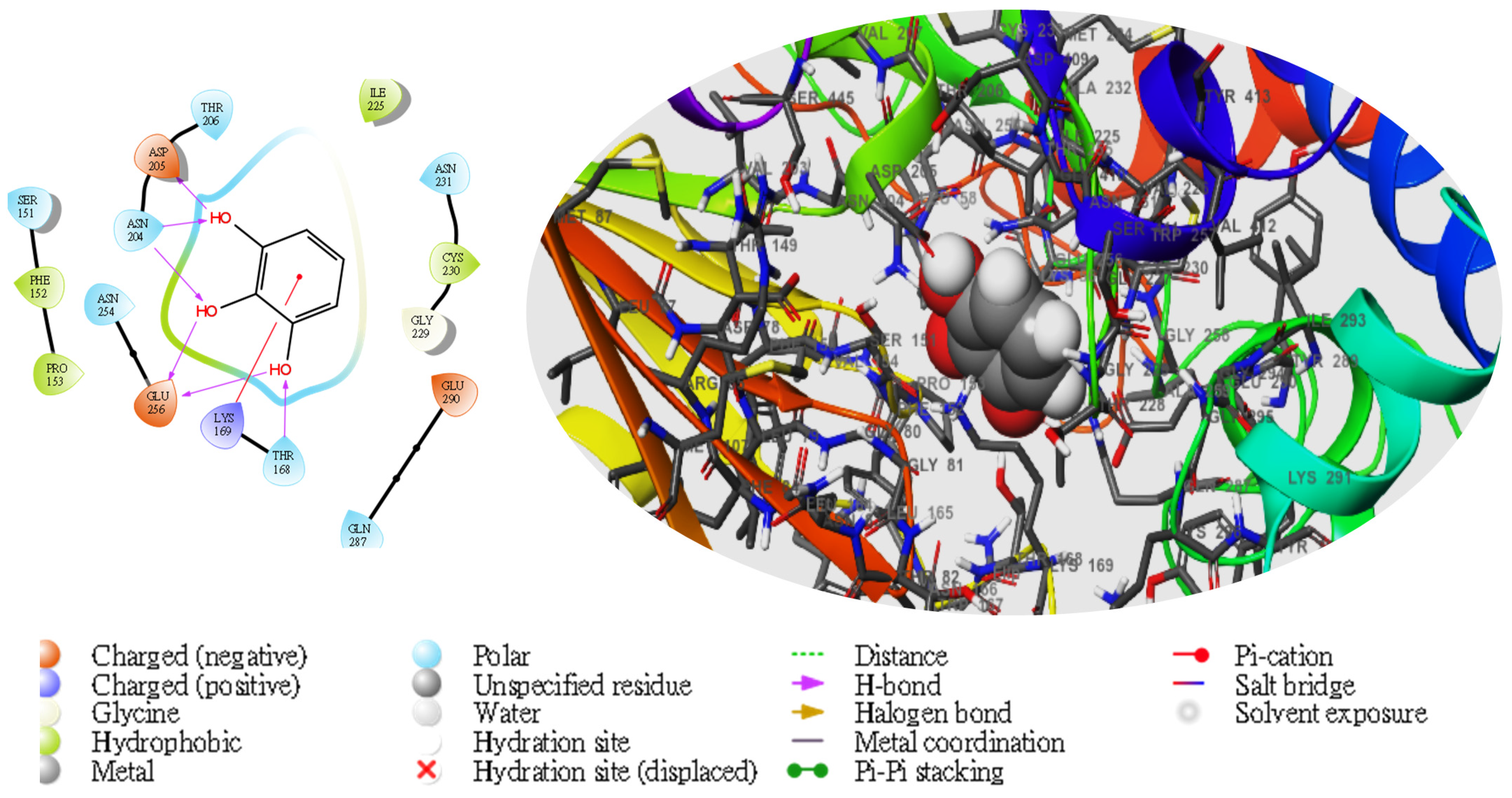
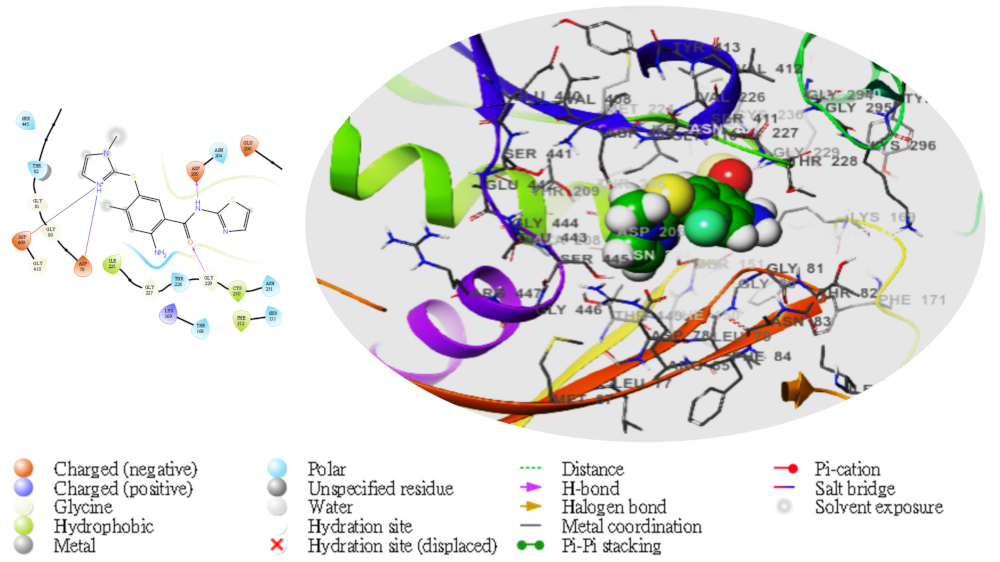
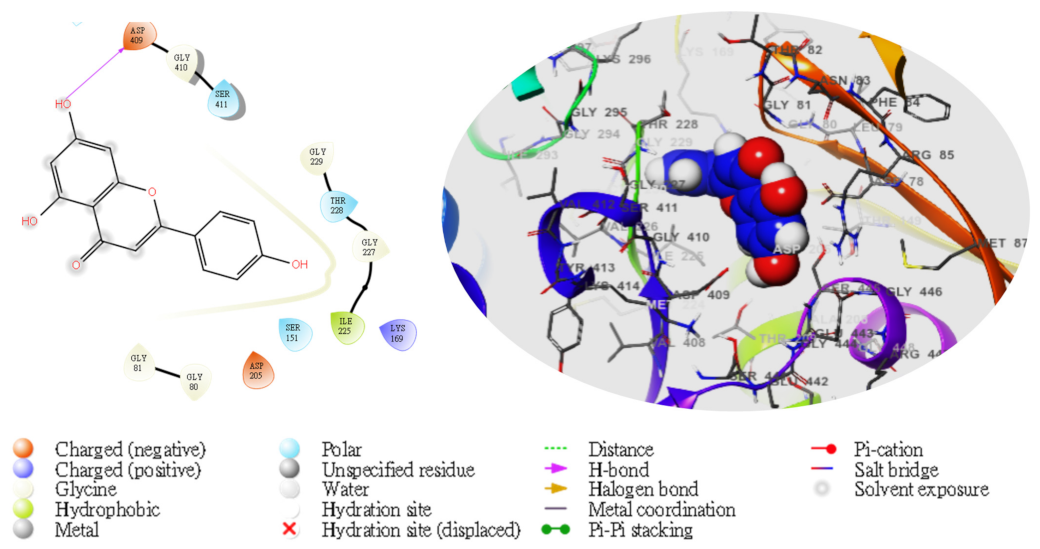
2. Results
| PubChem ID | MW | CNS | donorHB | accptHB | dip^2/V | # Acid |
|---|---|---|---|---|---|---|
| 1057 | 126.032 | −1 | 3 | 2.25 | 0.0184394 | 0 |
| 5281672 | 318.038 | −2 | 5 | 6 | 0.0087447 | 0 |
| 5281692 | 302.043 | −2 | 5 | 6.25 | 0.0242827 | 0 |
| 5280443 | 270.053 | −2 | 2 | 3.75 | 0.0556372 | 0 |
| Benzamide D | 349.047 | −1 | 2 | 2.25 | 0.0050193 | 0 |
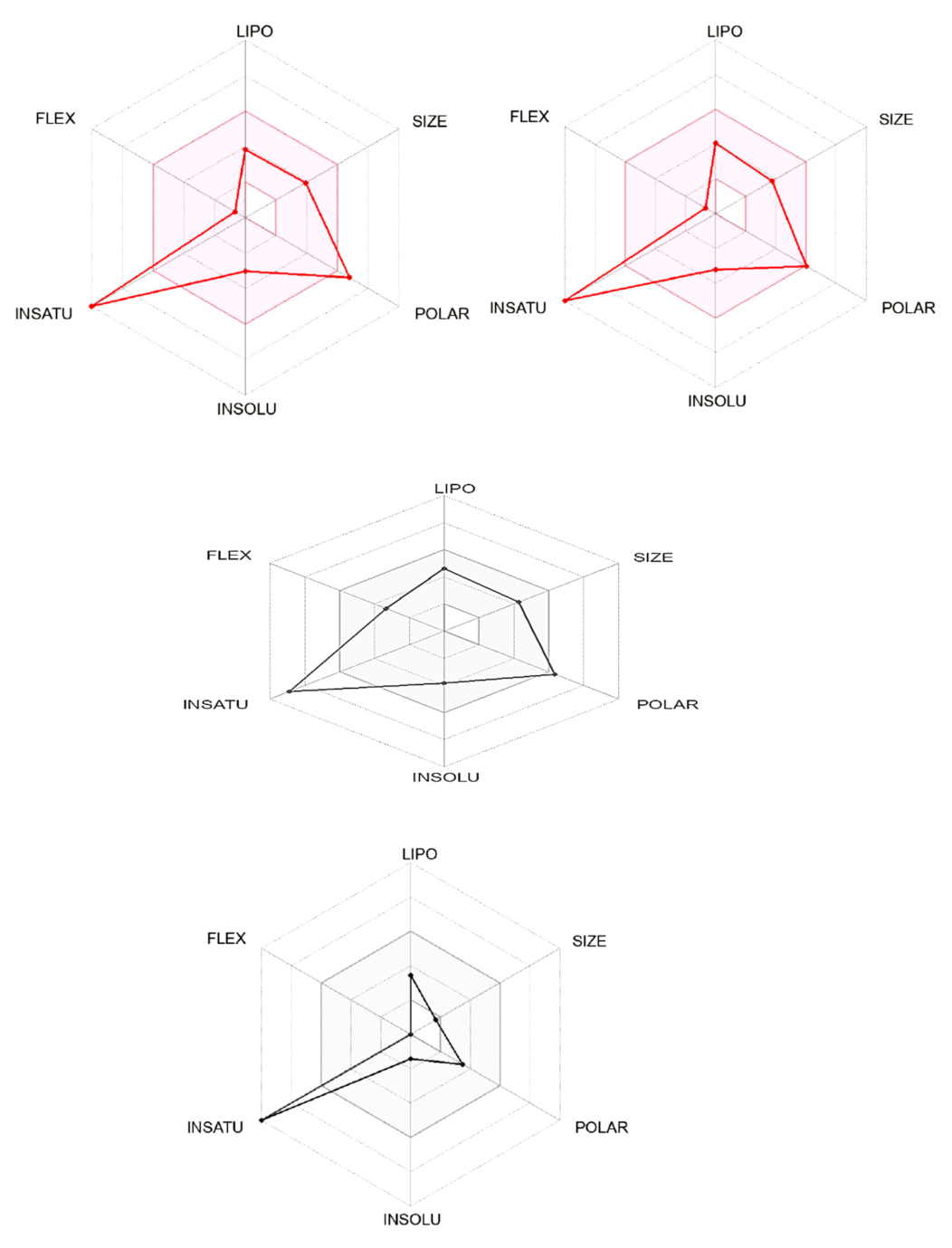
| PubChem | Lipinski # Violations | Ghose # Violations | Veber # Violations | Egan # Violations | Muegge # Violations | Bioavailability Score | Pains # Alerts |
|---|---|---|---|---|---|---|---|
| 5281672 | 1 | 0 | 1 | 1 | 2 | 0.55 | 1 |
| 5281692 | 0 | 0 | 0 | 0 | 0 | 0.55 | 1 |
| 5280443 | 0 | 0 | 0 | 0 | 0 | 0.55 | 0 |
| Benzamide D | 0 | 0 | 0 | 1 | 0 | 0.55 | 0 |
| 1057 | 0 | 3 | 0 | 0 | 1 | 0.55 | 1 |
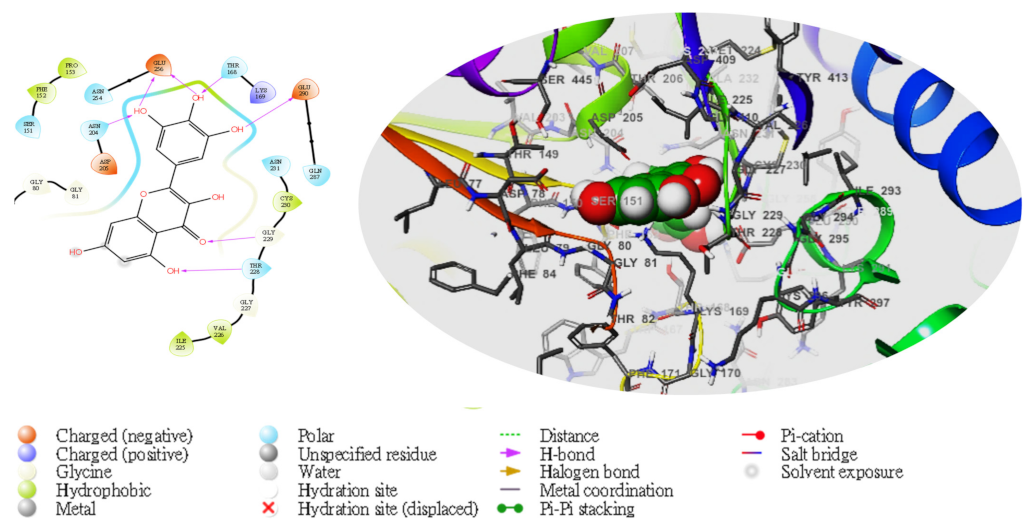
| TG | Compd | Dock Score | MMGBSA | # H-Bond | Pi-Cat | Salt Bridges |
|---|---|---|---|---|---|---|
| 4L3Q | 5281672 | −8.297 | −44.39 | 2(GLU256), GLU290, ASN204, THR168,THR228, GLY229 | 0 | 0 |
| 5281692 | −6.42 | −34.84 | ASP78, GLY81, THR228, GLY229, GLU290, GLU256 | 0 | 0 | |
| 5280443 | −1.784 | −33.09 | ASP409 | 0 | 0 | |
| Benzamide D | −5.259 | −51.55 | LYS229, ASP205 | 0 | ASP409, ASP78 | |
| 1057 | −7.215 | −34.02 | THR168, GLU256, 2(ASN204), ASP205 | LYS169 | 0 |
3. Discussion
4. Materials and Methods
4.1. Protein Preparation
4.2. Ligand Preparation
4.3. Receptor Grid Generation
4.4. Molecular Docking Analysis
4.5. Predictions of ADME Properties and Toxicological Potential
4.6. Binding Free Energy Calculation
4.7. Analysis of MD Simulations and Trajectory Point
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Alam, S.; Hasan, M.K.; Neaz, S.; Hussain, N.; Hossain, M.F.; Rahman, T. Diabetes Mellitus: Insights from epidemiology, biochemistry, risk factors, diagnosis, complications and comprehensive management. Diabetology 2021, 2, 36–50. [Google Scholar]
- Onikanni, A.S.; Lawal, B.; Olusola, A.O.; Olugbodi, J.O.; Sani, S.; Ajiboye, B.O.; Ilesanmi, O.B.; Alqarni, M.; Mostafa-Hedeab, G.; Obaidullah, A.J. Sterculia tragacantha lindl leaf extract ameliorates STZ-induced diabetes, oxidative stress, inflammation and neuronal impairment. J. Inflamm. Res. 2021, 14, 6749. [Google Scholar] [CrossRef]
- Association, A.D. Introduction: Standards of medical care in diabetes—2022. Am. Diabetes Assoc. 2022, 45, S1–S2. [Google Scholar]
- Onikanni, S.A.; Lawal, B.; Oyinloye, B.E.; Ajiboye, B.O.; Ulziijargal, S.; Wang, C.-H.; Emran, T.B.; Simal-Gandara, J. Mitochondrial defects in pancreatic beta-cell dysfunction and neurodegenerative diseases: Pathogenesis and therapeutic applications. Life Sci. 2022, 312, 121247. [Google Scholar] [CrossRef]
- Anyanwu, A.; Olopade, O.; Onung, S.; Odeniyi, I.; Coker, H.; Fasanmade, O.; Ohwovoriole, A. Serum vitamin D levels in persons with type 2 diabetes mellitus in Lagos, Nigeria. Int. J. Diabetes Clin. Res. 2020, 7, 7. [Google Scholar] [CrossRef]
- Colomer, A.; Igual, J.; Naranjo, V. Detection of early signs of diabetic retinopathy based on textural and morphological information in fundus images. Sensors 2020, 20, 1005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Yuan, G.; Zhao, X.; Peng, L.; Wang, Z.; He, Y.; Qu, C.; Peng, Z. Hard exudate detection based on deep model learned information and multi-feature joint representation for diabetic retinopathy screening. Comput. Methods Programs Biomed. 2020, 191, 105398. [Google Scholar] [CrossRef] [PubMed]
- Verhulst, M.J.; Loos, B.G.; Gerdes, V.E.; Teeuw, W.J. Evaluating all potential oral complications of diabetes mellitus. Front. Endocrinol. 2019, 10, 56. [Google Scholar] [CrossRef] [PubMed]
- Abdulrasheed-Adeleke, T.; Lawal, B.; Agwupuye, E.I.; Kuo, Y.; Eni, A.M.; Ekoh, O.F.; Lukman, H.Y.; Onikanni, A.S.; Olawale, F.; Saidu, S. Apigetrin-enriched Pulmeria alba extract prevents assault of STZ on pancreatic β-cells and neuronal oxidative stress with concomitant attenuation of tissue damage and suppression of inflammation in the brain of diabetic rats. Biomed. Pharmacother. 2023, 162, 114582. [Google Scholar] [CrossRef]
- Ajiboye, B.O.; Oyinloye, B.E.; Awurum, J.C.; Onikanni, S.A.; Adefolalu, A.; Oluba, O.M. Protective role of Sterculia tragacantha aqueous extract on pancreatic gene expression and oxidative stress parameters in streptozotocin-induced diabetic rats. J. Complement. Integr. Med. 2021, 19, 323–333. [Google Scholar] [CrossRef]
- Yang, W.-C. Botanical, pharmacological, phytochemical, and toxicological aspects of the antidiabetic plant Bidens pilosa L. Evid.-Based Complement. Altern. Med. 2014, 2014, 698617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geck, M.S.; Lecca, D.; Marchese, G.; Casu, L.; Leonti, M. Ethnomedicine and neuropsychopharmacology in Mesoamerica. J. Ethnopharmacol. 2021, 278, 114243. [Google Scholar] [CrossRef] [PubMed]
- Xuan, T.D.; Khanh, T.D. Chemistry and pharmacology of Bidens pilosa: An overview. J. Pharm. Investig. 2016, 46, 91–132. [Google Scholar] [CrossRef]
- Ansari, P.; Akther, S.; Hannan, J.; Seidel, V.; Nujat, N.J.; Abdel-Wahab, Y.H. Pharmacologically active phytomolecules isolated from traditional antidiabetic plants and their therapeutic role for the management of diabetes mellitus. Molecules 2022, 27, 4278. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, A.J.; Abdullahi, M.I. The phytochemical and pharmacological actions of Entada africana Guill. & Perr. Heliyon 2019, 5, e02332. [Google Scholar] [CrossRef]
- Adewole, E.; Yusuf, B.; Ebitimitula, O.-E.; Ojo, A.; Adewumi, D.F.; Oludoro, O.; Akinwale, H.; Adejori, A.; Oyinloye, B.E. Phytochemicals profile and in-vitro antidiabetic potentials of fractionated extracts of Entada africana and Leptadenia Hastata. Sci. Pharm. Sci. 2022, 3, 65–73. [Google Scholar] [CrossRef]
- Semwal, D.K.; Semwal, R.B.; Combrinck, S.; Viljoen, A. Myricetin: A Dietary Molecule with Diverse Biological Activities. Nutrients 2016, 8, 90. [Google Scholar] [CrossRef] [Green Version]
- Salehi, B.; Venditti, A.; Sharifi-Rad, M.; Kręgiel, D.; Sharifi-Rad, J.; Durazzo, A.; Lucarini, M.; Santini, A.; Souto, E.B.; Novellino, E.; et al. The Therapeutic Potential of Apigenin. Int. J. Mol. Sci. 2019, 20, 1305. [Google Scholar] [CrossRef] [Green Version]
- Sims, K.R., Jr.; He, B.; Koo, H.; Benoit, D.S.W. Electrostatic Interactions Enable Nanoparticle Delivery of the Flavonoid Myricetin. ACS Omega 2020, 5, 12649–12659. [Google Scholar] [CrossRef] [PubMed]
- Studies in Natural Products Chemistry. In Studies in Natural Products Chemistry; Rahman, A.-U. (Ed.) Elsevier: Amsterdam, The Netherlands, 2023; Volume 76, p. 2. [Google Scholar]
- Umar, H.I.; Josiah, S.S.; Saliu, T.P.; Jimoh, T.O.; Ajayi, A.; Danjuma, J.B. In-silico analysis of the inhibition of the SARS-CoV-2 main protease by some active compounds from selected African plants. J. Taibah Univ. Med. Sci. 2021, 16, 162–176. [Google Scholar] [CrossRef]
- Romano, J.D.; Tatonetti, N.P. Informatics and computational methods in natural product drug discovery: A review and perspectives. Front. Genet. 2019, 10, 368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zainab, B.; Ayaz, Z.; Alwahibi, M.S.; Khan, S.; Rizwana, H.; Soliman, D.W.; Alawaad, A.; Abbasi, A.M. In-silico elucidation of Moringa oleifera phytochemicals against diabetes mellitus. Saudi J. Biol. Sci. 2020, 27, 2299–2307. [Google Scholar] [CrossRef]
- Shahrajabian, M.H.; Sun, W.; Cheng, Q. The importance of flavonoids and phytochemicals of medicinal plants with antiviral activities. Mini-Rev. Org. Chem. 2022, 19, 293–318. [Google Scholar] [CrossRef]
- Ajiboye, B.O.; Iwaloye, O.; Owolabi, O.V.; Ejeje, J.N.; Okerewa, A.; Johnson, O.O.; Udebor, A.E.; Oyinloye, B.E. Screening of potential antidiabetic phytochemicals from Gongronema latifolium leaf against therapeutic targets of type 2 diabetes mellitus: Multi-targets drug design. SN Appl. Sci. 2022, 4, 14. [Google Scholar] [CrossRef]
- Oyinloye, B.E.; Iwaloye, O.; Ajiboye, B.O. Polypharmacology of Gongronema latifolium leaf secondary metabolites against protein kinases implicated in Parkinson’s disease and Alzheimer’s disease. Sci. Afr. 2021, 12, e00826. [Google Scholar] [CrossRef]
- Mekhilef, S.; Saidur, R.; Kamalisarvestani, M. Effect of dust, humidity and air velocity on efficiency of photovoltaic cells. Renew. Sustain. Energy Rev. 2012, 16, 2920–2925. [Google Scholar] [CrossRef]
- Kaushik, A.; Kaushik, M. Recent updates on glucokinase activators and glucokinase regulatory protein disrupters for the treatment of type 2 diabetes mellitus. Curr. Diabetes Rev. 2019, 15, 205–212. [Google Scholar] [CrossRef]
- Wang, H.; Tian, Q.; Zhang, J.; Liu, H.; Zhang, J.; Cao, W.; Zhang, X.; Li, X.; Wu, L.; Song, M. Blood transcriptome profiling as potential biomarkers of suboptimal health status: Potential utility of novel biomarkers for predictive, preventive, and personalized medicine strategy. EPMA J. 2021, 12, 103–115. [Google Scholar] [CrossRef]
- Chavda, V.P.; Ajabiya, J.; Teli, D.; Bojarska, J.; Apostolopoulos, V. Tirzepatide, a new era of dual-targeted treatment for diabetes and obesity: A mini-review. Molecules 2022, 27, 4315. [Google Scholar] [CrossRef]
- Chung, M.-Y.; Choi, H.-K.; Hwang, J.-T. AMPK activity: A primary target for diabetes prevention with therapeutic phytochemicals. Nutrients 2021, 13, 4050. [Google Scholar] [CrossRef]
- Mashau, M.E.; Ramatsetse, K.E.; Ramashia, S.E. Effects of adding Moringa oleifera leaves powder on the nutritional properties, lipid oxidation and microbial growth in ground beef during cold storage. Appl. Sci. 2021, 11, 2944. [Google Scholar] [CrossRef]
- Ajiboye, B.; Fagbola, T.; Folorunso, I.; Salami, A.; Aletile, O.; Akomolede, B.; Ayemoni, F.; Akinfemiwa, K.; Anwo, V.; Ojeleke, M. In silico identification of chemical compounds in Spondias mombin targeting aldose reductase and glycogen synthase kinase 3β to abate diabetes mellitus. Inform. Med. Unlocked 2023, 36, 101126. [Google Scholar] [CrossRef]
- Tibiri, A.; Rakotonandrasana, O.; Nacoulma, G.; Banzouzi, J. Radical scavenging activity, phenolic content and cytotoxicity of bark and leaves extracts of Entada africana Guill. and Perr.(Mimosaceae). J. Biol. Sci. 2007, 7, 959–963. [Google Scholar] [CrossRef] [Green Version]
- Obakiro, S.B.; Kiprop, A.; Kigondu, E.; K’owino, I.; Kiyimba, K.; Drago Kato, C.; Gavamukulya, Y. Sub-acute toxicity effects of methanolic stem bark extract of Entada abyssinica on biochemical, haematological and histopathological parameters in wistar albino rats. Front. Pharmacol. 2021, 12, 740305. [Google Scholar] [CrossRef] [PubMed]
- Kouam, A.F.; Owona, B.A.; Fifen, R.; Njayou, F.N.; Moundipa, P.F. Inhibition of CYP2E1 and activation of Nrf2 signaling pathways by a fraction from Entada africana alleviate carbon tetrachloride-induced hepatotoxicity. Heliyon 2020, 6, e04602. [Google Scholar] [CrossRef]
- Tiwari, G.; Mohanty, D. An in silico analysis of the binding modes and binding affinities of small molecule modulators of PDZ-peptide interactions. PLoS ONE 2013, 8, e71340. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.; Huang, Y.; Wang, C.; Chen, F.; Yang, L.; Ling, L.; Che, Z.; Chen, X. Recent developments in molecular docking technology applied in food science: A review. Int. J. Food Sci. Technol. 2020, 55, 33–45. [Google Scholar] [CrossRef]
- Onikanni, S.A.; Lawal, B.; Fadaka, A.O.; Bakare, O.; Adewole, E.; Taher, M.; Khotib, J.; Susanti, D.; Oyinloye, B.E.; Ajiboye, B.O. Computational and Preclinical Prediction of the Antimicrobial Properties of an Agent Isolated from Monodora myristica: A Novel DNA Gyrase Inhibitor. Molecules 2023, 28, 1593. [Google Scholar] [CrossRef] [PubMed]
- Borkotoky, S.; Banerjee, M. A computational prediction of SARS-CoV-2 structural protein inhibitors from Azadirachta indica (Neem). J. Biomol. Struct. Dyn. 2021, 39, 4111–4121. [Google Scholar] [CrossRef] [PubMed]
- Genheden, S.; Kuhn, O.; Mikulskis, P.; Hoffmann, D.; Ryde, U. The normal-mode entropy in the MM/GBSA method: Effect of system truncation, buffer region, and dielectric constant. J. Chem. Inf. Model. 2012, 52, 2079–2088. [Google Scholar] [CrossRef] [Green Version]
- Forouzesh, N.; Mishra, N. An effective MM/GBSA protocol for absolute binding free energy calculations: A case study on SARS-CoV-2 spike protein and the human ACE2 receptor. Molecules 2021, 26, 2383. [Google Scholar] [CrossRef]
- Ekins, S.; Mestres, J.; Testa, B. In silico pharmacology for drug discovery: Methods for virtual ligand screening and profiling. Br. J. Pharmacol. 2007, 152, 9–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adewole, E.; Ogola-Emma, E.; Ojo, A.; Adegbite, S. GC-MS Compound Identification in Phaseolus vulgaris-A Low-Cost Cataract Prevention Food. Food Sci. Technol. 2022, 10, 112–119. [Google Scholar]
- Johnson, T.O.; Adegboyega, A.E.; Iwaloye, O.; Eseola, O.A.; Plass, W.; Afolabi, B.; Rotimi, D.; Ahmed, E.I.; Albrakati, A.; Batiha, G.E. Computational study of the therapeutic potentials of a new series of imidazole derivatives against SARS-CoV-2. J. Pharmacol. Sci. 2021, 147, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Oselusi, S.O.; Fadaka, A.O.; Wyckoff, G.J.; Egieyeh, S.A. Computational Target-Based Screening of Anti-MRSA Natural Products Reveals Potential Multitarget Mechanisms of Action through Peptidoglycan Synthesis Proteins. ACS Omega 2022, 7, 37896–37906. [Google Scholar] [CrossRef] [PubMed]
- Adekiya, T.A.; Aruleba, R.T.; Klein, A.; Fadaka, A.O. In silico inhibition of SGTP4 as a therapeutic target for the treatment of schistosomiasis. J. Biomol. Struct. Dyn. 2022, 40, 3697–3705. [Google Scholar] [CrossRef]
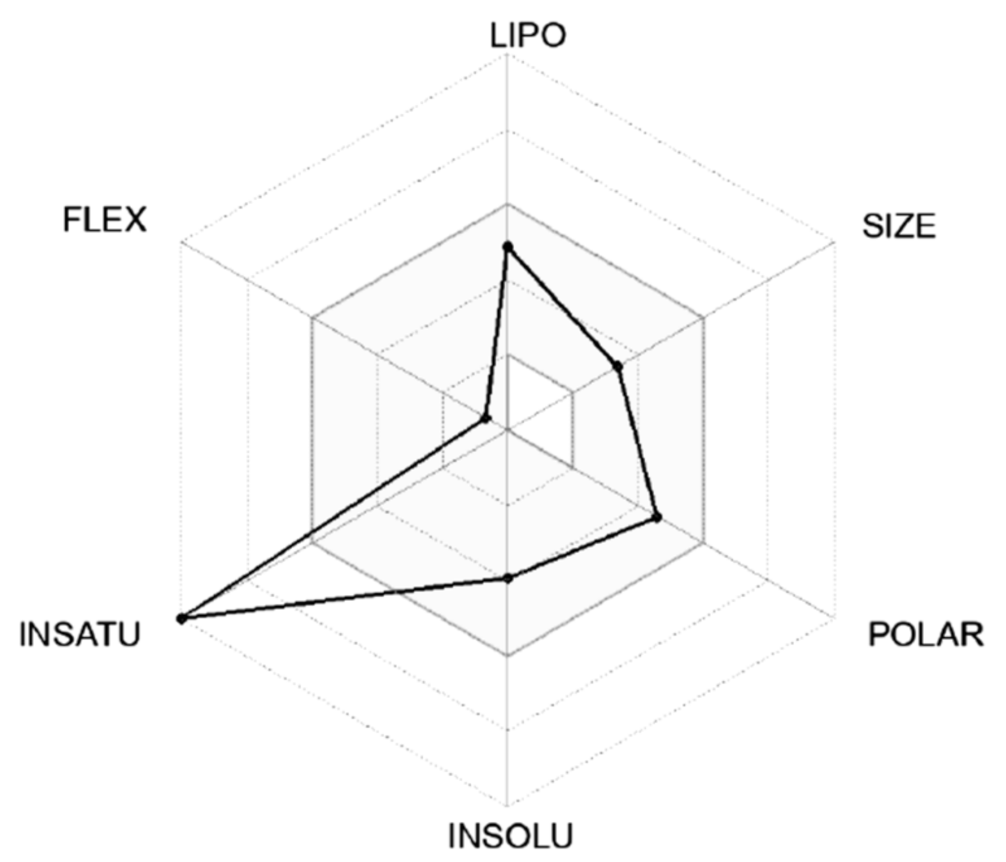
| PubChem ID | HOA | % HOA | SAfluorine | ROF | ROT | PSA |
|---|---|---|---|---|---|---|
| 1057 | 2 | 73.94 | 0 | 0 | 0 | 65.396 |
| 5281672 | 2 | 28.18 | 0 | 1 | 1 | 162.033 |
| 5281692 | 2 | 49.831 | 0 | 0 | 1 | 138.922 |
| 5280443 | 3 | 73.877 | 27.655 | 0 | 0 | 100.61 |
| Benzamide D | 3 | 93.431 | 0 | 0 | 0 | 87.867 |
| PubChem | GI Abs | BBB-p | Pgp-S | CYP1A2-I | CYP2C19-I | CYP2C9-I | CYP2D6-I | CYP3A4-I |
|---|---|---|---|---|---|---|---|---|
| 5281672 | Low | No | No | Yes | No | No | No | Yes |
| 5281692 | High | No | No | Yes | No | No | Yes | Yes |
| 5280443 | High | No | No | Yes | No | No | Yes | Yes |
| Benzamide D | Low | No | No | Yes | Yes | Yes | Yes | Yes |
| 1057 | High | Yes | No | No | No | No | No | Yes |
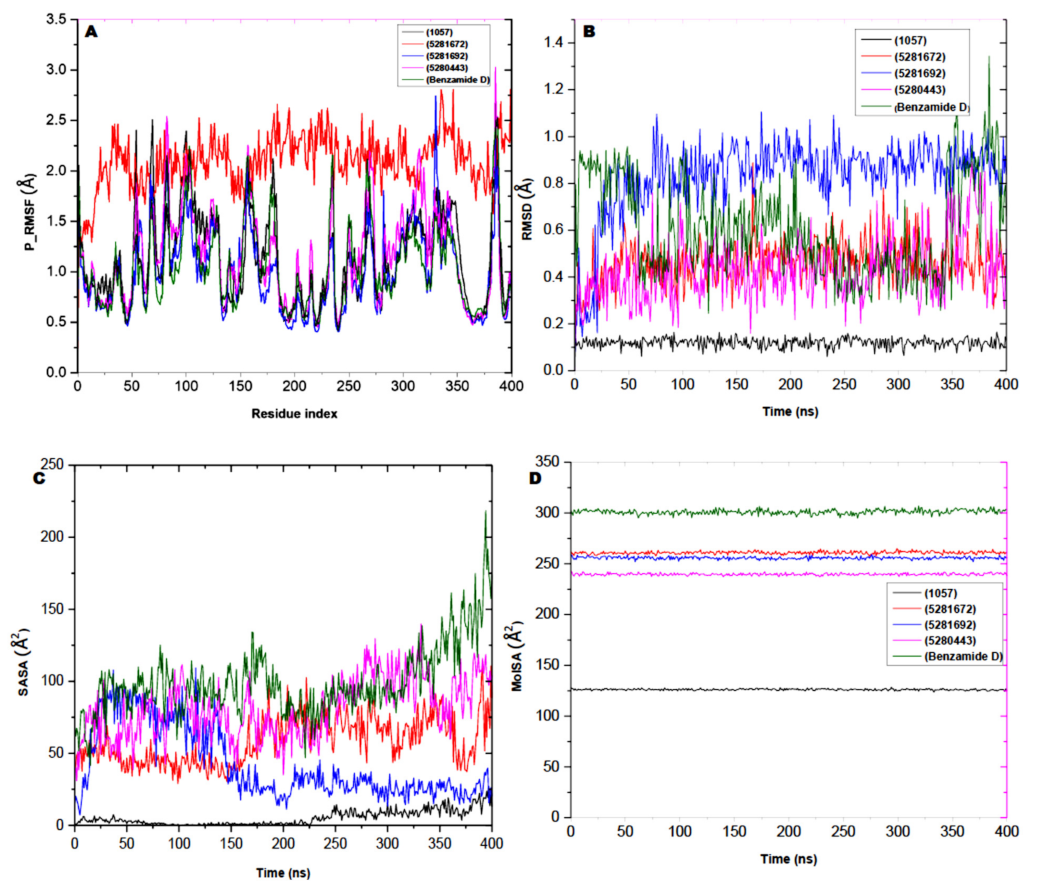
| Compound | RMSD | RMSF | MolSA | SASA |
|---|---|---|---|---|
| 5281672 | 0.45 ± 0.09 | 2.09 ± 0.28 | 260.94 ± 1.33 | 58.40 ± 16.27 |
| 5281692 | 0.82 ± 0.17 | 1.00 ± 0.40 | 255.76 ± 1.24 | 42.40 ± 24.85 |
| 5280443 | 0.43 ± 0.13 | 1.14 ± 0.46 | 239.68 ± 1.08 | 81.10 ± 20.46 |
| Benzamide D | 0.61 ± 0.20 | 1.05 ± 0.42 | 301.05 ± 2.23 | 99.18 ± 24.42 |
| 1057 | 0.11 ± 0.02 | 1.12 ± 0.44 | 126.20 ± 0.75 | 513.30 ± 5.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Onikanni, S.A.; Lawal, B.; Munyembaraga, V.; Bakare, O.S.; Taher, M.; Khotib, J.; Susanti, D.; Oyinloye, B.E.; Noriega, L.; Famuti, A.; et al. Profiling the Antidiabetic Potential of Compounds Identified from Fractionated Extracts of Entada africana toward Glucokinase Stimulation: Computational Insight. Molecules 2023, 28, 5752. https://doi.org/10.3390/molecules28155752
Onikanni SA, Lawal B, Munyembaraga V, Bakare OS, Taher M, Khotib J, Susanti D, Oyinloye BE, Noriega L, Famuti A, et al. Profiling the Antidiabetic Potential of Compounds Identified from Fractionated Extracts of Entada africana toward Glucokinase Stimulation: Computational Insight. Molecules. 2023; 28(15):5752. https://doi.org/10.3390/molecules28155752
Chicago/Turabian StyleOnikanni, Sunday Amos, Bashir Lawal, Valens Munyembaraga, Oluwafemi Shittu Bakare, Muhammad Taher, Junaidi Khotib, Deny Susanti, Babatunji Emmanuel Oyinloye, Lloyd Noriega, Ayodeji Famuti, and et al. 2023. "Profiling the Antidiabetic Potential of Compounds Identified from Fractionated Extracts of Entada africana toward Glucokinase Stimulation: Computational Insight" Molecules 28, no. 15: 5752. https://doi.org/10.3390/molecules28155752
APA StyleOnikanni, S. A., Lawal, B., Munyembaraga, V., Bakare, O. S., Taher, M., Khotib, J., Susanti, D., Oyinloye, B. E., Noriega, L., Famuti, A., Fadaka, A. O., & Ajiboye, B. O. (2023). Profiling the Antidiabetic Potential of Compounds Identified from Fractionated Extracts of Entada africana toward Glucokinase Stimulation: Computational Insight. Molecules, 28(15), 5752. https://doi.org/10.3390/molecules28155752








