Structure Characterization and Immunomodulatory Activity of Misgurnus anguillicaudatus Carbohydrates
Abstract
:1. Introduction
2. Results and Discussion
2.1. Homogeneity and Molecular Weight Analysis
2.2. Monosaccharide Composition Analysis
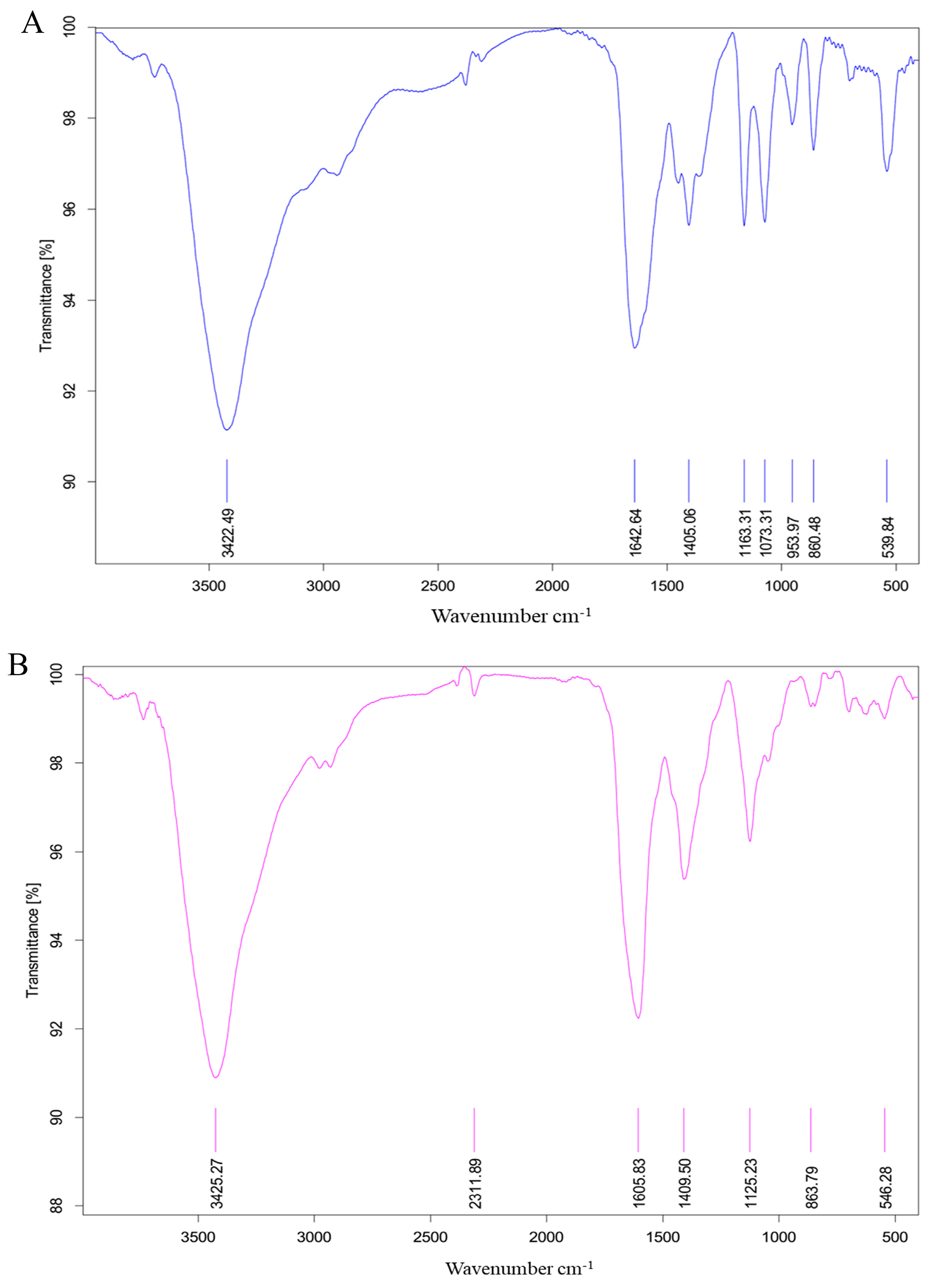
2.3. Fourier Transform Infrared (FT-IR) Spectrum
2.4. NMR Spectra (13C NMR) for MAO and MAP
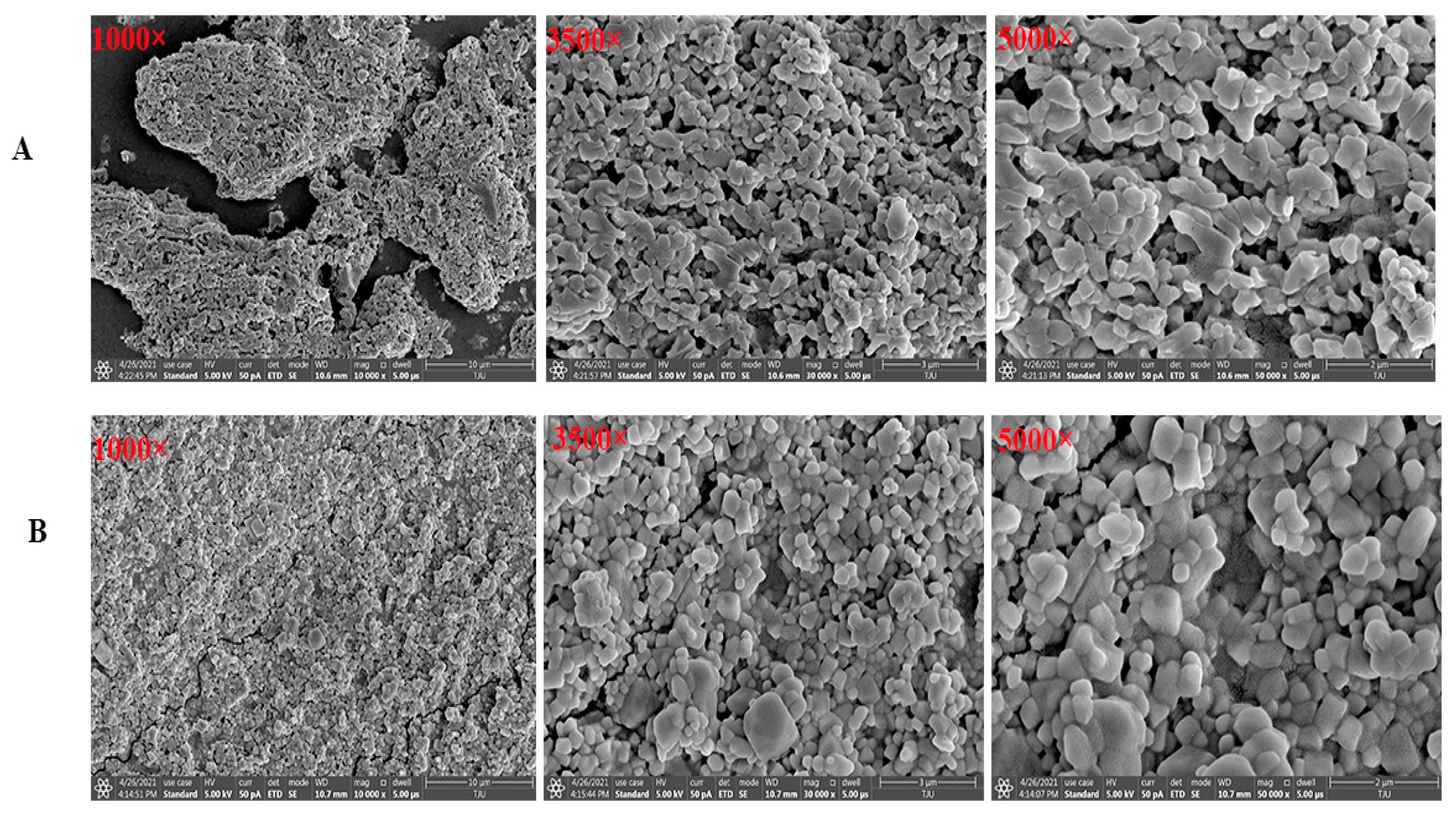
2.5. Scanning Electron Microscope (SEM) Morphology Observation
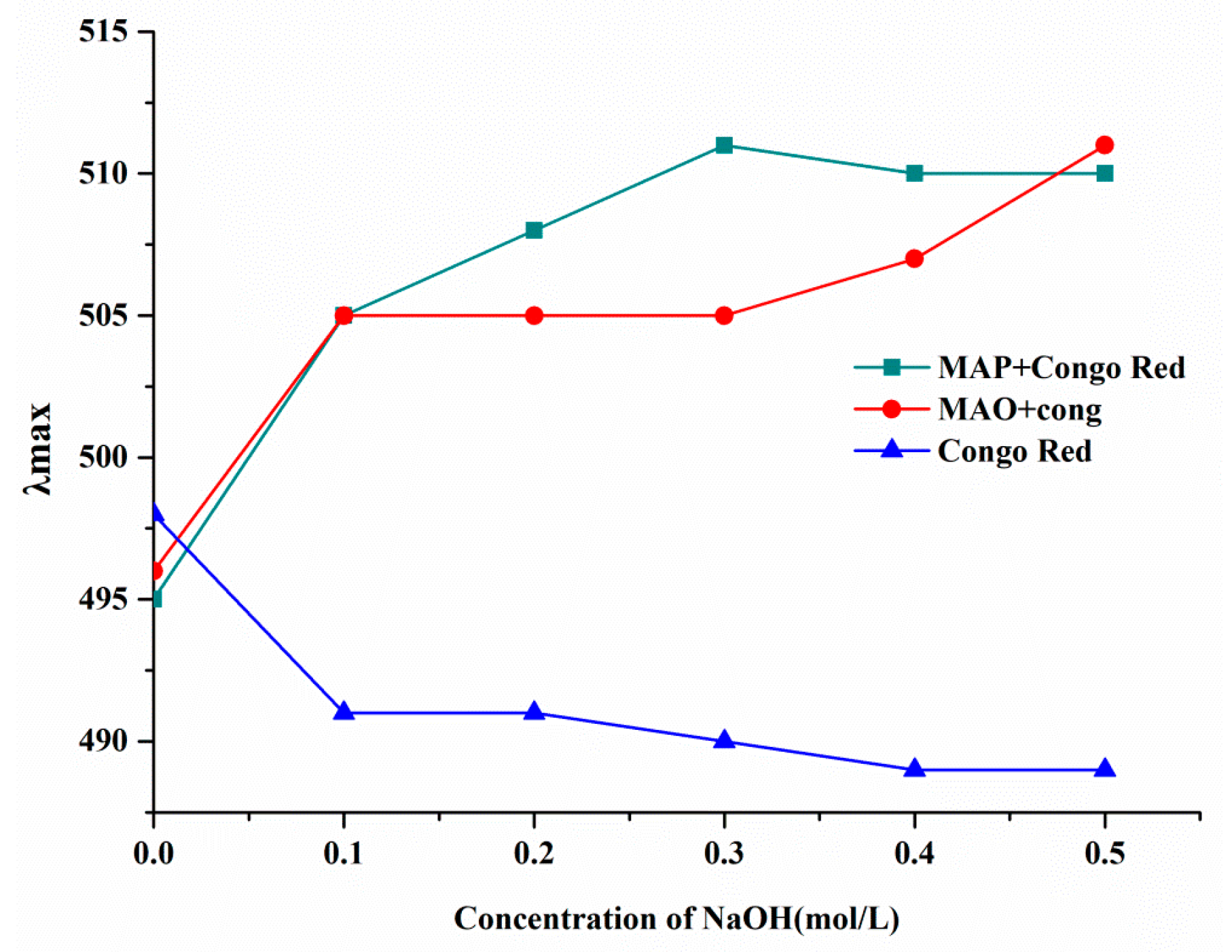
2.6. Conformational Structure of MAO and MAP
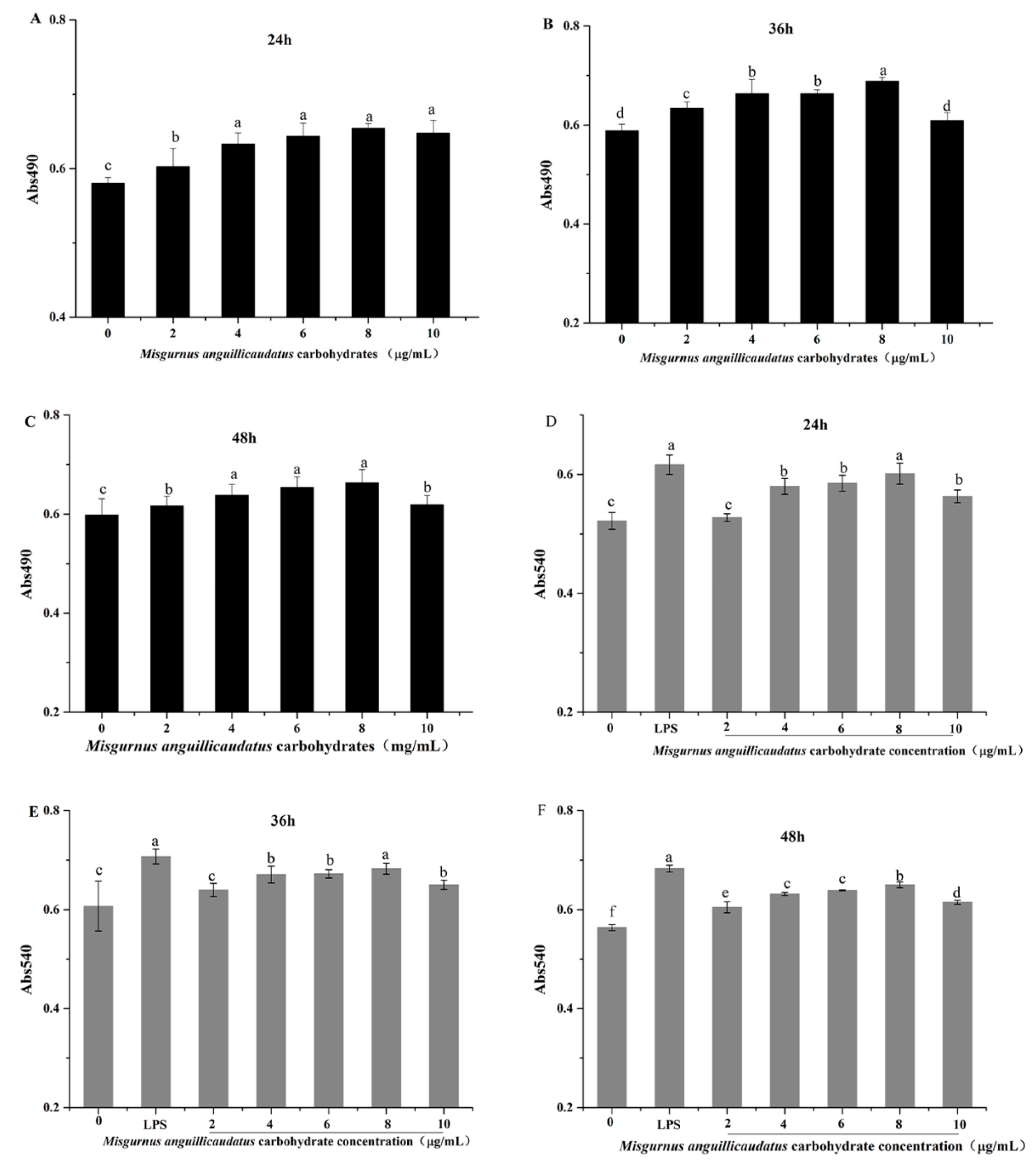
2.7. Effects of MAC on RAW264.7 Cell Viability
2.8. Effects of MAC on RAW264.7 Cells Phagocytic Activity
2.9. Effects of MAC on RAW264.7 Cells Morphology
2.10. Effects of MAC on TNF-α and IL-6 Secretion in RAW264.7 Cells
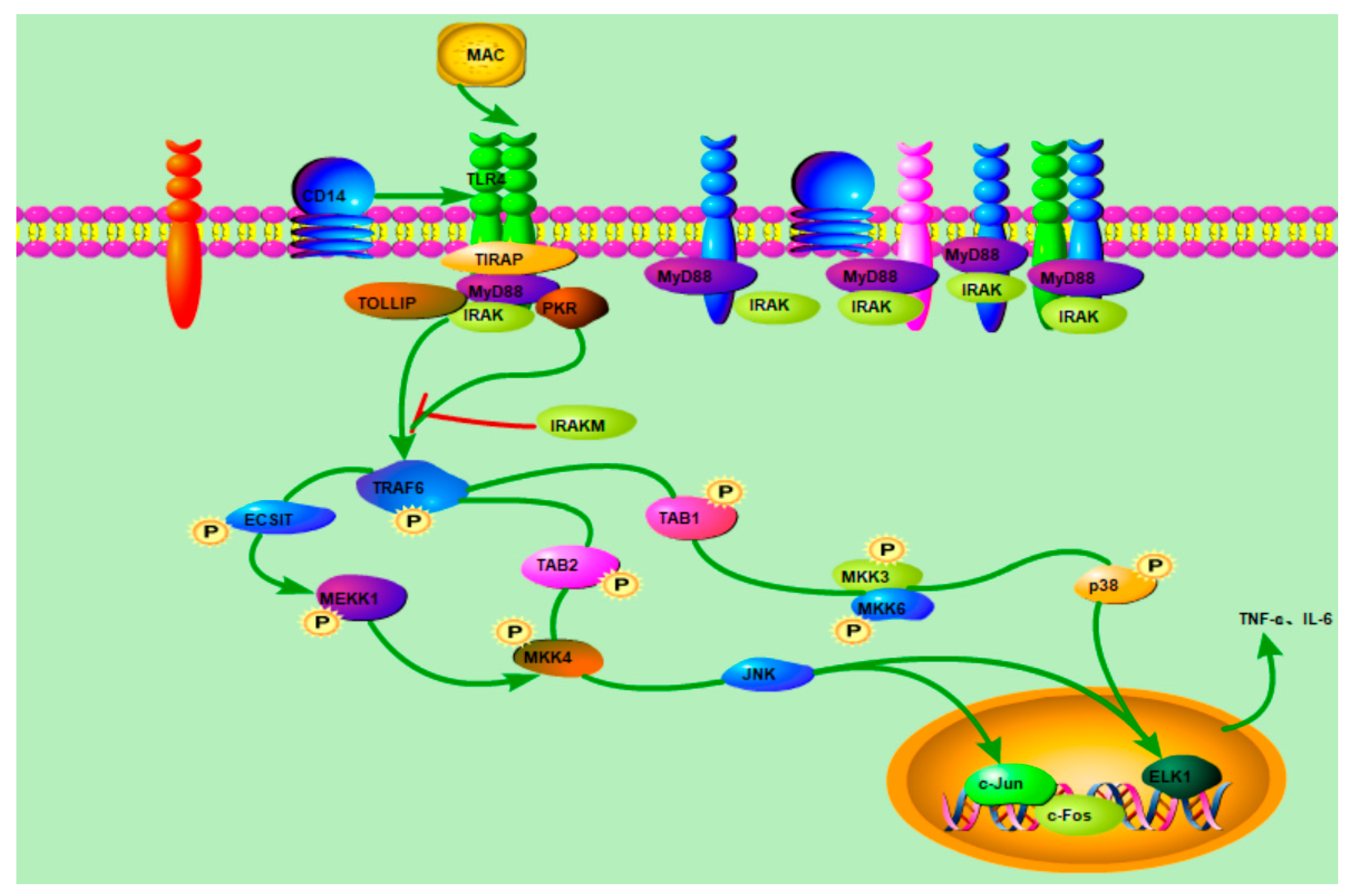
2.11. Western Blot Analysis
3. Materials and Methods
3.1. Materials
3.2. Extraction and Purification of Misgurnus anguillicaudatus Carbohydrates in Loach Mucus
3.3. Molecular Weight Determination
3.4. Monosaccharide Composition Analysis
3.5. FT-IR Analysis
3.6. Nuclear Magnetic Resonance (NMR) Detection
3.7. Congo Red Experiment
3.8. Scanning Electron Microscopy (SEM)
3.9. Immunomodulatory Activity of Misgurnus anguillicaudatus Carbohydrates (MAC)
3.9.1. Cell Culture
3.9.2. Macrophage Proliferation Assay
3.9.3. Phagocytic Assay
3.9.4. Scanning Electron Microscope Observation Assay
3.9.5. The RT-PCR Method to Measure the Expression Level of Cellular Immune Factor mRNA
3.9.6. Western Blot Assay
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Hou, C.; Liu, L.; Ren, J.; Huang, M.; Yuan, E. Structural characterization of two Hericium erinaceus polysaccharides and their protective effects on the alcohol-induced gastric mucosal injury. Food Chem. 2022, 375, 131896. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, M.; Gao, Y.; Li, H.; Fang, L.; Liu, C.; Liu, X.; Min, W. Effects of Exopolysaccharides from Lactiplantibacillus plantarum JLAU103 on Intestinal Immune Response, Oxidative Stress, and Microbial Communities in Cyclophosphamide-Induced Immunosuppressed Mice. J. Agric. Food Chem. 2022, 70, 2197–2210. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ren, Z.; Yu, R.; Chen, S.; Qin, T. Structural characterization of enzymatic modification of Hericium erinaceus polysaccharide and its immune-enhancement activity. Int. J. Biol. Macromol. 2020, 166, 1396–1408. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Zhang, J.; Zhang, T. Immunomodulatory activities of polysaccharides from Ganoderma on immune effector cells. Food Chem. 2021, 340, 127933. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, Y.; Yuan, X.; Sun, G.; Shen, B.; Xu, F.; Fan, G.; Jin, M.; Li, X.; Liu, G. Berberine inhibits lipopolysaccharide-induced expression of inflammatory cytokines by suppressing TLR4-mediated NF-kB and MAPK signaling pathways in rumen epithelial cells of Holstein calves. J. Dairy Res. 2019, 86, 171–176. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, J.; Hou, T.; An, S.; Guo, B.; Liu, C.; Hu, L.; Huang, Y.; Zhang, S.; Song, M. Extraction kinetics, physicochemical properties and immunomodulatory activity of the novel continuous phase transition extraction of polysaccharides from Ganoderma lucidum. Food Funct. 2021, 12, 9708–9718. [Google Scholar] [CrossRef]
- Yang, G.; Cui, X.; Liu, S.; Lu, J.; Hou, X.; Meng, W.; Wu, B.; Su, Y.; Zhang, H.; Zheng, W.; et al. Effects of dietary Lactobacillus helveticus on the growth rate, disease resistance and intestinal health of pond loach (Misgurnus anguillicaudatus). Aquaculture 2021, 544, 737038. [Google Scholar] [CrossRef]
- Wang, X.; Pan, J.; Chen, L.; Li, R.; Han, Y.; Di, Z.; Ling, B.; Ahmad, A.; Yang, N.; Fan, L. Prevalence, virulence-related genes and antimicrobial resistance of Aeromonas spp. from loach Misgurnus anguillicaudatus with skin ulcer and healthy controls in Southern China. Aquaculture 2022, 552, 738040. [Google Scholar] [CrossRef]
- Hussein, M.N.; Cao, X.; Li, Y.; Mei, J. Characterization of neural stem cells and proliferating cells in the brain of loach (Misgurnus anguillicaudatus). Egypt. J. Histol. 2021, 45, 1288–1295. [Google Scholar] [CrossRef]
- Zhang, L.-S.; Wang, X.; Dong, L.-L. Antioxidation and antiglycation of polysaccharides from Misgurnus anguillicaudatus. Food Chem. 2011, 124, 183–187. [Google Scholar] [CrossRef]
- Qin, C.; Huang, K.; Xu, H. Isolation and characterization of a novel polysaccharide from the mucus of the loach, Misgurnus anguillicaudatus. Carbohydr. Polym. 2002, 49, 367–371. [Google Scholar] [CrossRef]
- Hashemifesharaki, R.; Xanthakis, E.; Altintas, Z.; Guo, Y.; Gharibzahedi, S.M.T. Microwave-assisted extraction of polysaccharides from the marshmallow roots: Optimization, purification, structure, and bioactivity. Carbohydr. Polym. 2020, 240, 116301. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Wu, Y.; Jia, G.; Wang, C.; Guo, Q. Structural characterization and immunomodulatory activity of mycelium polysaccharide from liquid fermentation of Monascus purpureus (Hong Qu). Carbohydr. Polym. 2021, 262, 117945. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Chen, P.; Zhao, Y.; Zeng, Q.; Ou, S.; Zhang, Y.; Wang, P.; Chen, N.; Ou, J. Isolation, structural characterization and anti-oxidant activity of a novel polysaccharide from garlic bolt. Carbohydr. Polym. 2021, 267, 118194. [Google Scholar] [CrossRef]
- Zhou, Y.; Qian, C.; Yang, D.; Tang, C.; Zhao, Z. Purification, Structural Characterization and Immunomodulatory Effects of Polysaccharides from Amomumvillosum Lour. on RAW 264.7 Macrophages. Molecules 2021, 26, 2672. [Google Scholar] [CrossRef]
- Ye, G.; Li, J.; Zhang, J.; Liu, H.; Wang, Z. Structural characterization and antitumor activity of a polysaccharide from Dendrobium wardianum. Carbohydr. Polym. 2021, 269, 118253. [Google Scholar] [CrossRef]
- Tian, J.; Zhang, C.; Wang, X.M.; Rui, X.; Li, W. Structural characterization and immunomodulatory activity of intracellular polysaccharide from the mycelium of Paecilomyces cicadae TJJ1213. Food Res. Int. 2021, 147, 110515. [Google Scholar] [CrossRef] [PubMed]
- Abuduwaili, A.; Nuerxiati, R.; Mutailifu, P.; Gao, Y.; Lu, C.; Yili, A. Isolation, structural modification, characterization, and bioactivity of polysaccharides from Folium Isatidis. Ind. Crops Prod. 2022, 176, 114319. [Google Scholar] [CrossRef]
- Zeng, F.; Chen, W.; He, P.; Zhan, Q.; Wang, Q.; Wu, H.; Zhang, M. Structural characterization of polysaccharides with potential antioxidant and immunomodulatory activities from Chinese water chestnut peels. Carbohydr. Polym. 2020, 246, 116551. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wei, S.; Lu, X.; Qiao, X.; Simal-Gandara, J.; Capanoglu, E.; Wozniak, L.; Zou, L.; Cao, H.; Xiao, J.; et al. A neutral polysaccharide with a triple helix structure from ginger: Characterization and immunomodulatory activity. Food Chem. 2021, 350, 129261. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wei, X.; Wang, F.; Xu, J.; Tang, X.; Li, N. Structural characterization and antioxidant activity of polysaccharide from ginger. Int. J. Biol. Macromol. 2018, 111, 862–869. [Google Scholar] [CrossRef]
- He, P.; Pan, L.; Wu, H.; Zhang, L.; Zhang, Y.; Zhang, Y.; Yang, J.; Lin, Z.; Zhang, M. Isolation, Identification, and Immunomodulatory Mechanism of Peptides from Lepidium meyenii (Maca) Protein Hydrolysate. J. Agric. Food Chem. 2022, 70, 4328–4341. [Google Scholar] [CrossRef]
- Ji, C.; Zhang, Z.; Chen, J.; Song, D.; Liu, B.; Li, J.; Liu, R.; Niu, J.; Wang, D.; Ling, N. Immune-Enhancing Effects of a Novel Glucan from Purple Sweet Potato Ipomoea batatas (L.) Lam on RAW264.7 Macrophage Cells via TLR2-and TLR4-Mediated Pathways. J. Agric. Food Chem. 2021, 32, 69. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Liu, M.; Li, L.; Chen, M.; Sun, H. Cordyceps polysaccharide marker CCP modulates immune responses via highly selective TLR4/MyD88/p38 axis. Carbohydr. Polym. 2021, 271, 118443. [Google Scholar] [CrossRef]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Zhang, L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, T.; Mao, G.; Mao, R.; Zou, Y.; Zheng, D.; Feng, W.; Ren, Y.; Wang, W.; Zheng, W.; Song, J.; et al. Antitumor and immunomodulatory activity of a water-soluble low molecular weight polysaccharide from Schisandra chinensis (Turcz) Baill. Food Chem. Toxicol. 2013, 55, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Huang, K. Characteristic immunostimulation by MAP, a polysaccharide isolated from the mucus of the loach, Misgurnus anguillicaudatus. Carbohydr. Polym. 2005, 59, 75–82. [Google Scholar] [CrossRef]
- Gan, T.; Feng, C.; Lan, H.; Yang, R.; Li, W. Comparison of the structure and immunomodulatory activity of polysaccharides from fresh and dried longan. J. Funct. Foods 2021, 76, 104323. [Google Scholar] [CrossRef]
- Chen, X.; Wu, X.; Zhang, K.; Sun, F.; Zhou, W.; Wu, Z.; Li, X. Purification, characterization, and emulsification stability of high- and low-molecular-weight fractions of polysaccharide conjugates extracted from green tea. Food Hydrocoll. 2022, 129, 107667. [Google Scholar] [CrossRef]
- Wang, W.; Zou, Y.; Li, Q.; Mao, R.; Shao, X.; Jin, D.; Zheng, D.; Zhao, T.; Zhu, H.; Zhang, L.; et al. Immunomodulatory effects of a polysaccharide purified from Lepidium meyenii Walp. on macrophages. Process Biochem. 2016, 51, 542–553. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, Z.; Cheng, L.; Zhang, X.; Liu, Y.; Zhang, R.; Weng, P.; Wu, Z. Polysaccharides confer benefits in immune regulation and multiple sclerosis by interacting with gut microbiota. Food Res. Int. 2021, 149, 110675. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, L.; Zhang, X.; Wan, L.; Chen, Z. Structural characterization of polysaccharide from Centipeda minima and its hypoglycemic activity through alleviating insulin resistance of hepatic HepG2 cells. J. Funct. Foods 2021, 82, 104478. [Google Scholar] [CrossRef]
- Li, J.; Fan, Y.; Huang, G.; Huang, H. Extraction, structural characteristics and activities of Zizylphus vulgaris polysaccharides. Ind. Crops Prod. 2022, 178, 114675. [Google Scholar] [CrossRef]
- Lind, N.A.; Rael, V.E.; Pestal, K.; Liu, B.; Barton, G.M. Regulation of the nucleic acid-sensing Toll-like receptors. Nat. Rev. Immunol. 2022, 22, 224–235. [Google Scholar] [CrossRef]
- Wang, D.D.; Pan, W.J.; Shomaila, M.; Chen, X.D.; Yan, C. Polysaccharide isolated from Sarcodon aspratus induces RAW264.7 activity via TLR4-mediated NF-κB and MAPK signaling pathways. Int. J. Biol. Macromol. 2018, 120 Pt A, 1039–1047. [Google Scholar] [CrossRef]
- Li, M.; Wen, J.; Huang, X.; Nie, Q.; Wu, X.; Ma, W.; Nie, S.; Xie, M. Interaction between polysaccharides and toll-like receptor 4: Primary structural role, immune balance perspective, and 3D interaction model hypothesis. Food Chem. 2021, 374, 131586. [Google Scholar] [CrossRef]
- Ai, J.; Yang, Z.; Liu, J.; Schols, H.A.; Battino, M.; Bao, B.; Tian, L.; Bai, W. Structural Characterization and In Vitro Fermentation Characteristics of Enzymatically Extracted Black Mulberry Polysaccharides. J. Agric. Food Chem. 2022, 70, 3654–3665. [Google Scholar] [CrossRef]
- Ferreira, S.S.; Passos, C.P.; Madureira, P.; Vilanova, M.; Coimbra, M.A. Structure-function relationships of immunostimulatory polysaccharides: A review. Carbohydr. Polym. 2015, 132, 378–396. [Google Scholar] [CrossRef]
- Chao, L.; Zd, A.; Bza, C.; Qiang, H.; Gl, B.; Xiong, F. Structural characterization and immune enhancement activity of a novel polysaccharide from Moringa oleifera leaves. Carbohydr. Polym. 2020, 234, 115897. [Google Scholar]
- Yun, L.; Wu, T.; Liu, R.; Li, K.; Zhang, M. Structural Variation and Microrheological Properties of a Homogeneous Polysaccharide from Wheat Germ. J. Agric. Food Chem. 2018, 66, 2977–2987. [Google Scholar] [CrossRef]
- Yun, L.; Tao, W.; Mao, Z.; Wen, l.; Min, Z.; Xiaotao, S. A novel wheat germ polysaccharide: Structural characterization, potential antioxidant activities and mechanism. Int. J. Biol. Macromol. 2020, 165 Pt B, 1978–1987. [Google Scholar] [CrossRef]
- Chang, Y.; Guo, A.; Jing, Y.; Lin, J.; Deng, Y. Immunomodulatory activity of puerarin in RAW264.7 macrophages and cyclophosphamide-induced immunosuppression mice. Immunopharmacol. Immunotoxicol. 2021, 43, 223–229. [Google Scholar] [CrossRef] [PubMed]
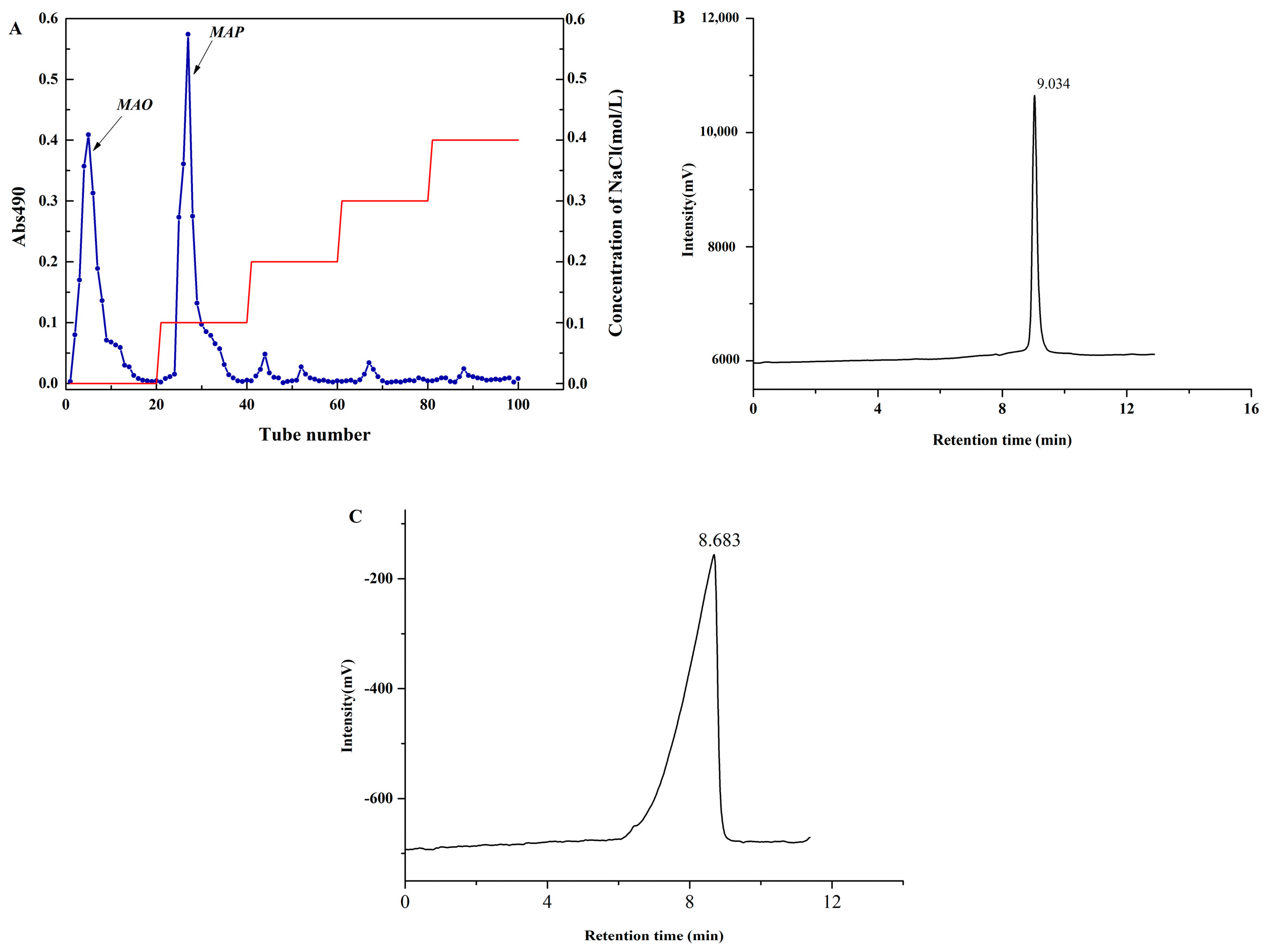




| Sample | Retention Time (min) | Mw (Da) | Mn (Da) | Mw/Mn |
|---|---|---|---|---|
| MAO | 9.034 | 2854 | 2114 | 1.35 |
| MAP | 8.683 | 3873 | 2748 | 1.41 |
| Sample | Total Sugars Content (%) | Protein Content (%) | Glucuronic Acid Content (%) |
|---|---|---|---|
| MAC | 76.31% ± 0.11% | 23.53% ± 0.12% | 0.97% ± 0.02% |
| MAO | 94.432% ± 0.45% | - | - |
| MAP | 98.80% ± 0.27% | - | 1.08% ± 0.26% |
| Forward | Reverse | Base Pair Length | |
|---|---|---|---|
| TNF-α | CCTGTAGCCCACGTCGTAG | GGGAGTAGACAAGGTACAACCC | 148 bp |
| IL-6 | CTGCAAGAGACTTCCATCCAG | AGTGGTATAGACAGGTCTGTTGG | 131 bp |
| β-actin | GCCTTCCGTGTTCCTACC | GGAGACAACCTGGTCCTCA | 156 bp |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yun, L.; Han, C.; He, X.; Li, Q.; Fersht, V.; Zhang, M. Structure Characterization and Immunomodulatory Activity of Misgurnus anguillicaudatus Carbohydrates. Molecules 2023, 28, 5771. https://doi.org/10.3390/molecules28155771
Yun L, Han C, He X, Li Q, Fersht V, Zhang M. Structure Characterization and Immunomodulatory Activity of Misgurnus anguillicaudatus Carbohydrates. Molecules. 2023; 28(15):5771. https://doi.org/10.3390/molecules28155771
Chicago/Turabian StyleYun, Liyuan, Conglin Han, Xiaoqing He, Qian Li, Viktor Fersht, and Min Zhang. 2023. "Structure Characterization and Immunomodulatory Activity of Misgurnus anguillicaudatus Carbohydrates" Molecules 28, no. 15: 5771. https://doi.org/10.3390/molecules28155771






