Epsom Salt-Based Natural Deep Eutectic Solvent as a Drilling Fluid Additive: A Game-Changer for Shale Swelling Inhibition
Abstract
:1. Introduction
2. Results
2.1. In-House Preparation
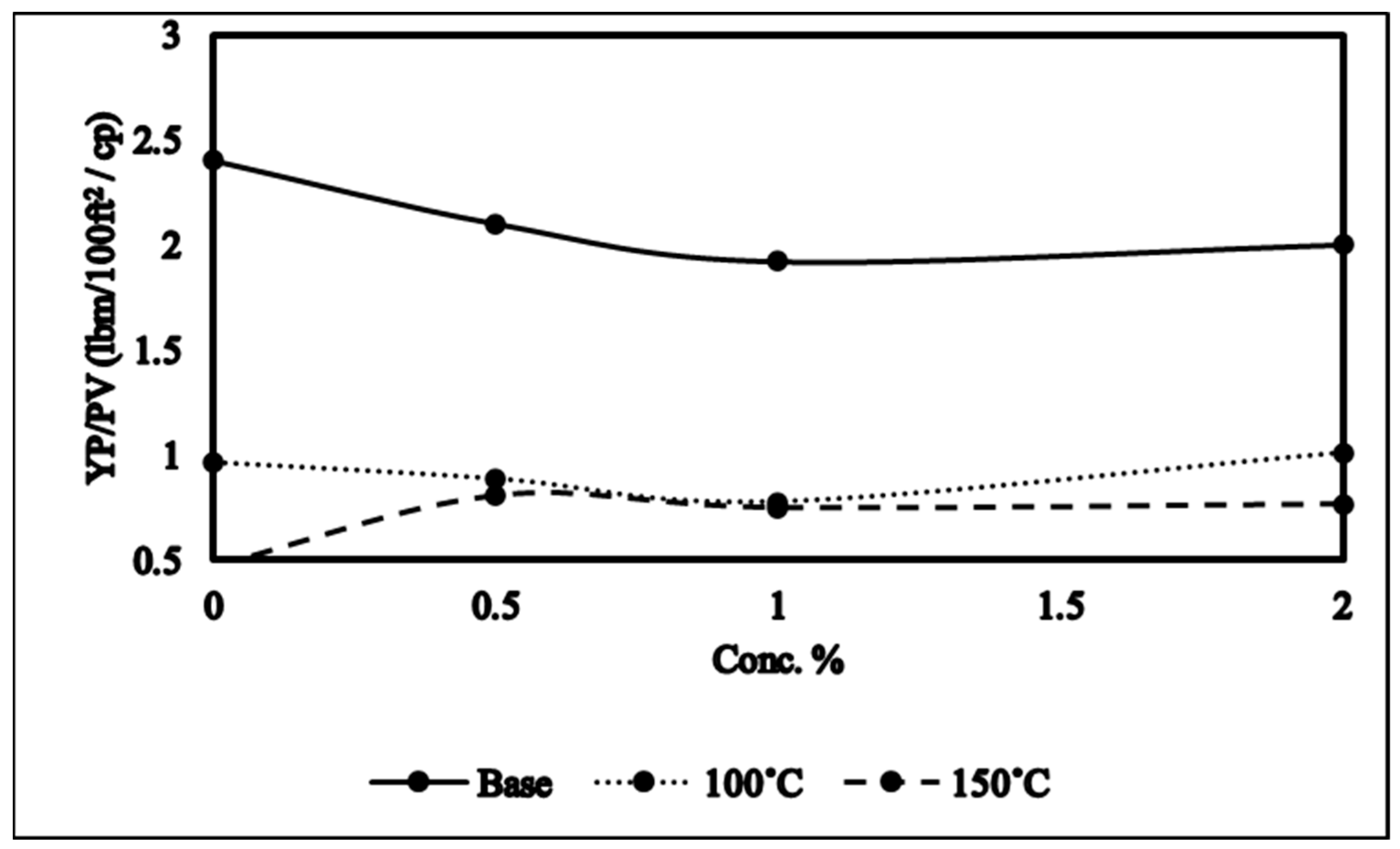
2.2. Yield Points (YP) and Plastic Viscosity (PV) Ratio (YP/PV)
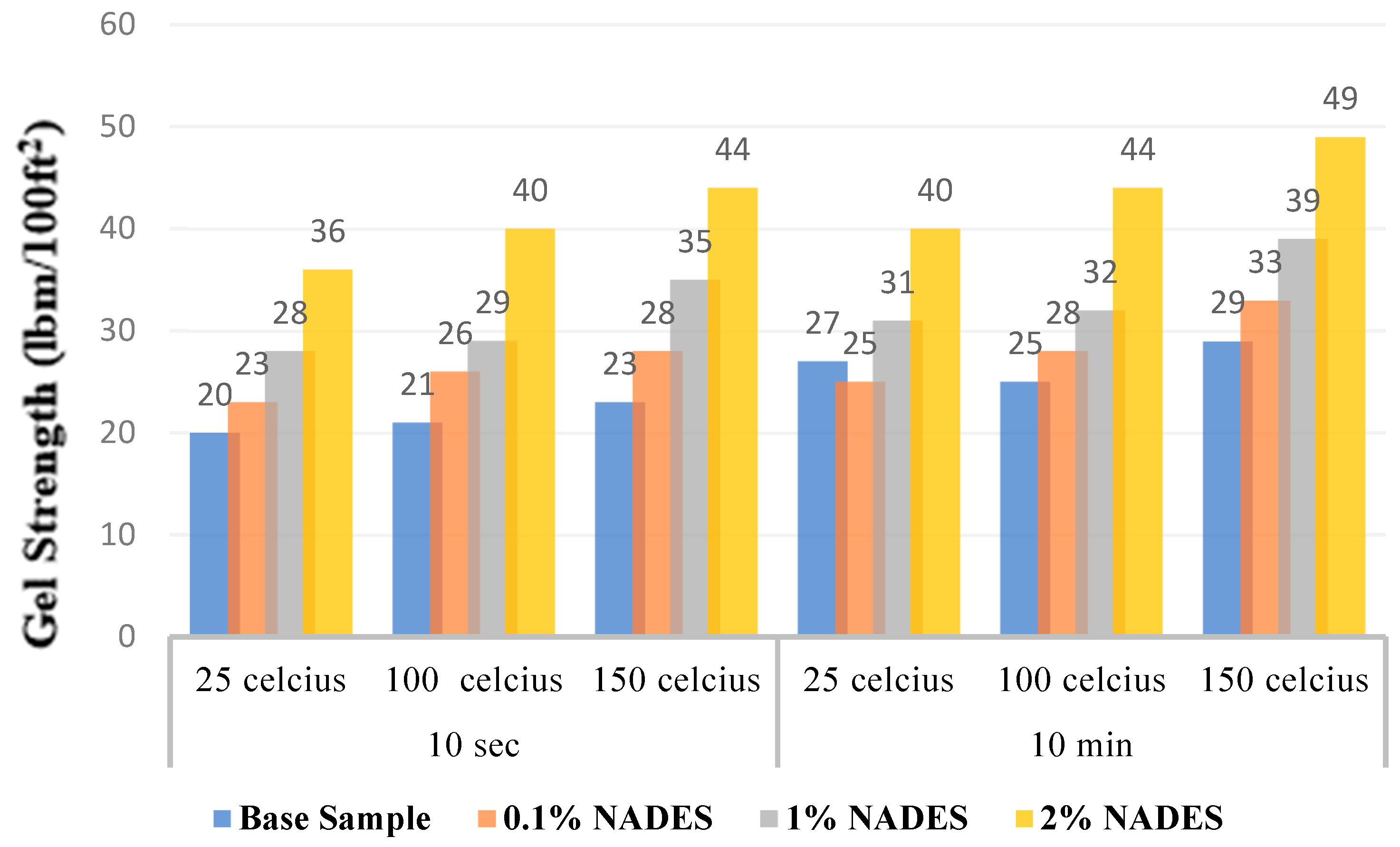
2.3. Gel Strength
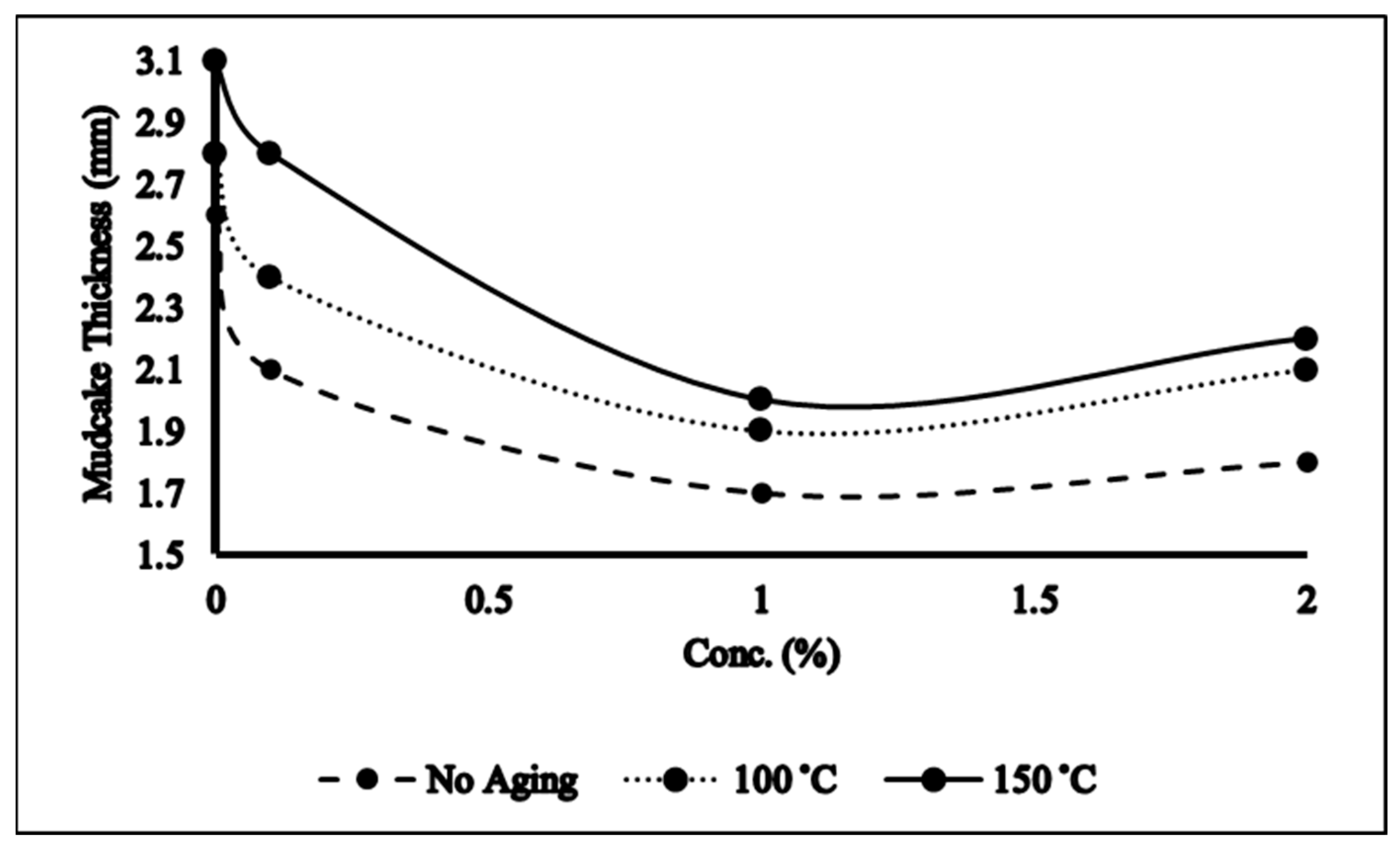
2.4. Filtration Properties
2.5. Shale Swelling Inhibition
2.6. Underlying Mechanism
2.6.1. FTIR of NADES-Based Mud
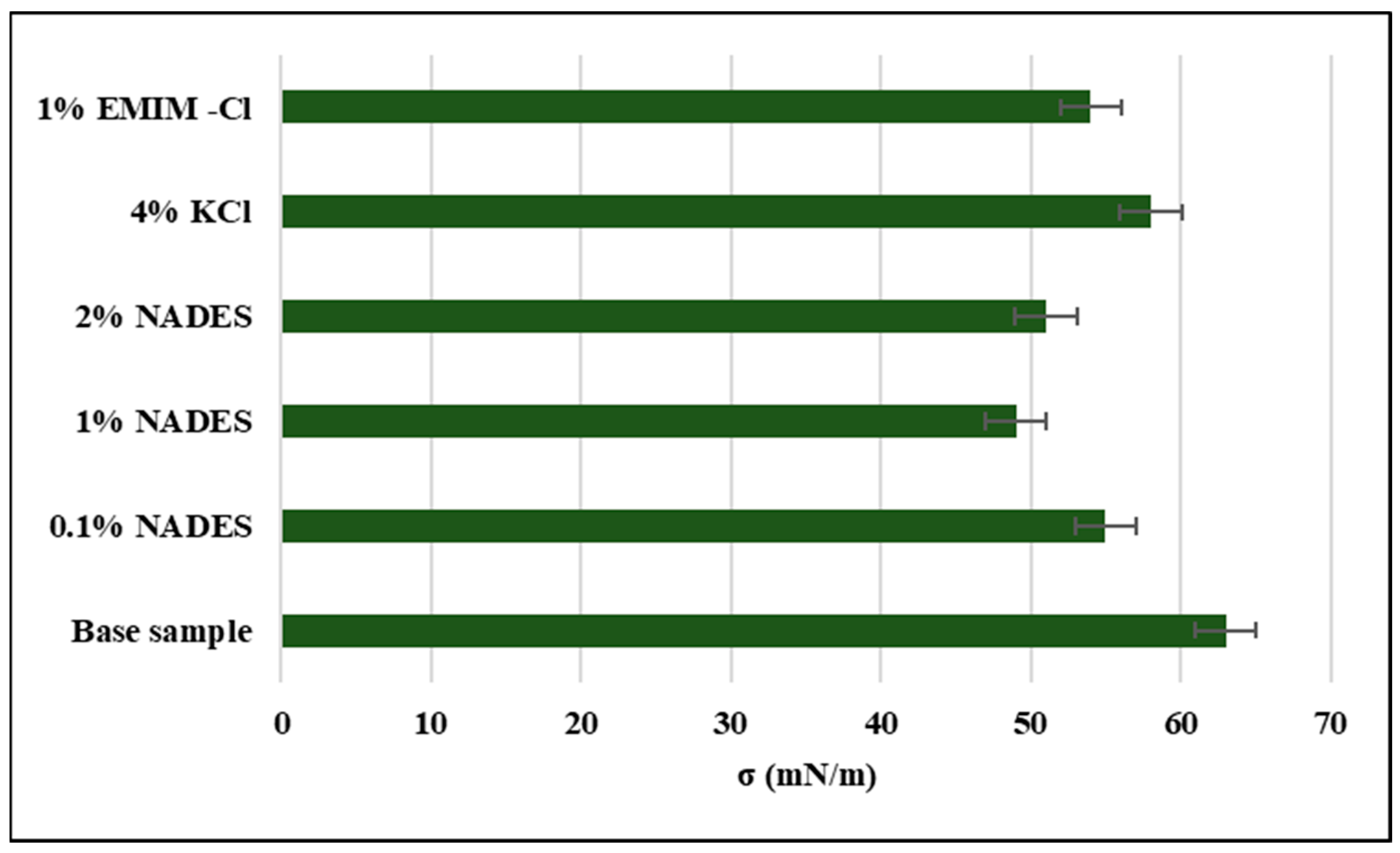
2.6.2. Surface Tension
2.6.3. Zeta Potential
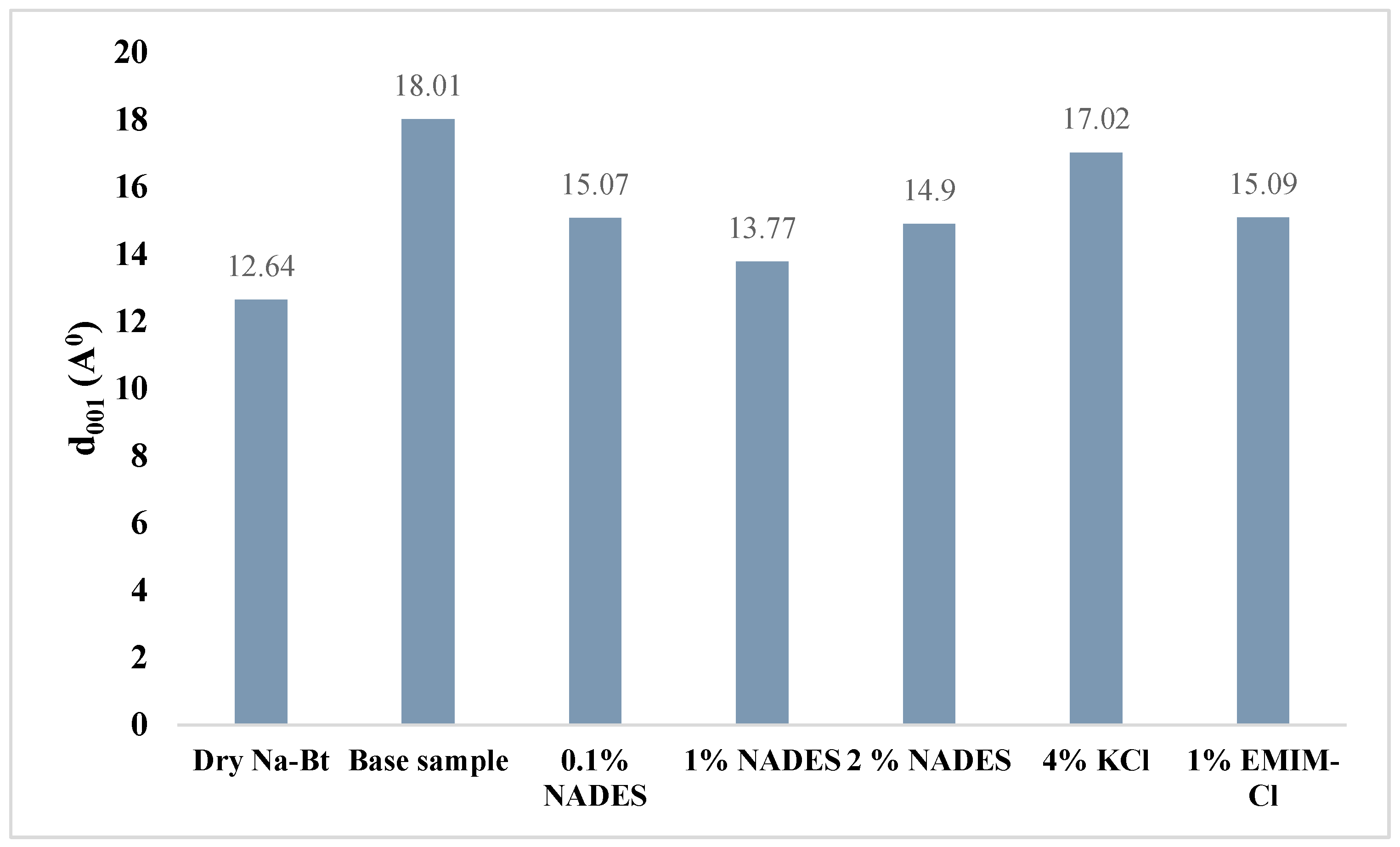
2.6.4. d-Spacing (XRD)
3. Materials and Methods
3.1. In-House Preparation of Epsom Salt-Based NADES
3.2. Drilling Fluid Composition
3.3. Bentonite Wafers Preparation for Linear Swelling Test
3.4. Rheological and Filtration Properties
3.5. Linear Swell Meter (LSM)
3.6. Surface Tension
3.7. d-Spacing
3.8. Zeta Potential
3.9. FTIR
4. Conclusions
- Different inhibitors inhibit the shale formation by neutralizing the charge on the clay surface, thus making it more stable against hydration.
- NADES-based mud improved YP/PV and filtration properties of the mud which is mainly because of hydrogen bonding between the clay and NADES that modifies the clay structure and thus rheological properties.
- Among KCl, NADES, and ILs, NADES gave the best performance as a shale inhibitor which is attributed to its ability to form hydrogen bonds with clay and thus neutralizing the charge on the clay surface which will hinder the permeation of water cations into the clay layers.
- FTIR shows that NADES has successfully bonded with the silica present in clay. NADES has also decreased surface tension on clay surface which in turn reduced the capillary action and thus reduced the permeation of water cations into clay layers. Moreover, the decline in d-spacing of NADES-based mud shows that NADES possesses more affinity than water toward clay which makes it a potential shale inhibitor against hydration.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Green, H.; Šegvić, B.; Zanoni, G.; Omodeo-Salé, S.; Adatte, T. Evaluation of shale source rocks and clay mineral diagenesis in the permian basin, USA: Inferences on basin thermal maturity and source rock potential. Geosciences 2020, 10, 381. [Google Scholar] [CrossRef]
- Rana, A.; Khan, I.; Ali, S.; Saleh, T.A.; Khan, S.A. Controlling shale swelling and fluid loss properties of water-based drilling mud via ultrasonic impregnated SWCNTs/PVP nanocomposites. Energy Fuels 2020, 34, 9515–9523. [Google Scholar] [CrossRef]
- Wang, G.; Du, H.; Jiang, S. Synergistic inhibition effect of organic salt and polyamine on water-sensitive shale swelling and dispersion. J. Energy Resour. Technol. 2019, 141, 082901. [Google Scholar] [CrossRef]
- Seedsman, R. The behaviour of clay shales in water. Can. Geotech. J. 1986, 23, 18–22. [Google Scholar] [CrossRef]
- Al-Arfaj, M.K.; Amanullah, M.; Sultan, A.S.; Hossain, E.; Abdulraheem, A. Chemical and mechanical aspects of wellbore stability in shale formations: A literature review. In Proceedings of the Abu Dhabi International Petroleum Exhibition and Conference, Abu Dhabi, United Arab Emirates, 10–13 November 2014. [Google Scholar]
- Ghasemi, A.; Jalalifar, H.; Apourvari, S.N.; Sakebi, M.R. Mechanistic study of improvement of wellbore stability in shale formations using a natural inhibitor. J. Pet. Sci. Eng. 2019, 181, 106222. [Google Scholar] [CrossRef]
- Ma, B.; Wang, R.; Ni, H.; Wang, K. Experimental study on harmless disposal of waste oil based mud using supercritical carbon dioxide extraction. Fuel 2019, 252, 722–729. [Google Scholar] [CrossRef]
- Ali, J.A.; Hamadamin, A.B.; Ahmed, S.M.; Mahmood, B.S.; Sajadi, S.M.; Manshad, A.K. Synergistic Effect of Nanoinhibitive Drilling Fluid on the Shale Swelling Performance at High Temperature and High Pressure. Energy Fuels 2022, 36, 1996–2006. [Google Scholar] [CrossRef]
- Pereira, L.B.; Sad, C.M.; Castro, E.V.; Filgueiras, P.R.; Lacerda, V., Jr. Environmental impacts related to drilling fluid waste and treatment methods: A critical review. Fuel 2022, 310, 122301. [Google Scholar] [CrossRef]
- Razali, S.; Yunus, R.; Rashid, S.A.; Lim, H.; Jan, B.M. Review of biodegradable synthetic-based drilling fluid: Progression, performance and future prospect. Renew. Sustain. Energy Rev. 2018, 90, 171–186. [Google Scholar] [CrossRef]
- Naeimavi, M.; Khazali, F.; Abdideh, M.; Saadati, Z. Potassium sorbate as substitute for KCl to shale inhibition in water-base drilling fluids. Energy Sources Part A Recovery Util. Environ. Eff. 2021, 43, 1691–1705. [Google Scholar] [CrossRef]
- Murtaza, M.; Kamal, M.S.; Mahmoud, M. Application of a novel and sustainable silicate solution as an alternative to sodium silicate for clay swelling inhibition. ACS Omega 2020, 5, 17405–17415. [Google Scholar] [CrossRef] [PubMed]
- Abbas, M.A.; Zamir, A.; Elraies, K.A.; Mahmood, S.M.; Rasool, M.H. A critical parametric review of polymers as shale inhibitors in water-based drilling fluids. J. Pet. Sci. Eng. 2021, 204, 108745. [Google Scholar]
- Zamir, A.; Elraies, K.A.; Rasool, M.H.; Ahmad, M.; Ayoub, M.; Abbas, M.A.; Ali, I. Influence of alkyl chain length in ionic liquid based drilling mud for rheology modification: A review. J. Pet. Explor. Prod. Technol. 2021, 12, 485–492. [Google Scholar] [CrossRef]
- Flieger, J.; Flieger, M. Ionic liquids toxicity—Benefits and threats. Int. J. Mol. Sci. 2020, 21, 6267. [Google Scholar] [CrossRef]
- Bakshi, K.; Mitra, S.; Sharma, V.K.; Jayadev, M.S.K.; Sakai, V.G.; Mukhopadhyay, R.; Gupta, A.; Ghosh, S.K. Imidazolium-based ionic liquids cause mammalian cell death due to modulated structures and dynamics of cellular membrane. Biochim. Et Biophys. Acta (BBA)-Biomembr. 2020, 1862, 183103. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, J.; Du, Z.; Li, B.; Juhasz, A.; Tan, M.; Zhu, L.; Wang, J. Toxicity evaluation of three imidazolium-based ionic liquids ([C6mim] R) on Vicia faba seedlings using an integrated biomarker response (IBR) index. Chemosphere 2020, 240, 124919. [Google Scholar] [CrossRef]
- Rasool, M.H.; Zamir, A.; Elraies, K.A.; Ahmad, M.; Ayoub, M.; Abbas, M.A.; Ali, I. Rheological characterization of potassium carbonate deep eutectic solvent (DES) based drilling mud. J. Pet. Explor. Prod. Technol. 2022, 12, 1785–1795. [Google Scholar] [CrossRef]
- Zahn, S.; Kirchner, B.; Mollenhauer, D. Charge spreading in deep eutectic solvents. ChemPhysChem 2016, 17, 3354–3358. [Google Scholar] [CrossRef] [PubMed]
- García, G.; Atilhan, M.; Aparicio, S. An approach for the rationalization of melting temperature for deep eutectic solvents from DFT. Chem. Phys. Lett. 2015, 634, 151–155. [Google Scholar] [CrossRef]
- Ofei, T.N.; Bavoh, C.B.; Rashidi, A.B. Insight into ionic liquid as potential drilling mud additive for high temperature wells. J. Mol. Liq. 2017, 242, 931–939. [Google Scholar] [CrossRef]
- Jia, H.; Huang, P.; Wang, Q.; Han, Y.; Wang, S.; Zhang, F.; Pan, W.; Lv, K. Investigation of inhibition mechanism of three deep eutectic solvents as potential shale inhibitors in water-based drilling fluids. Fuel 2019, 244, 403–411. [Google Scholar] [CrossRef]
- Huang, P.; Jia, H.; Han, Y.; Wang, Q.; Wei, X.; Luo, Q.; Dai, J.; Song, J.; Yan, H.; Liu, D. Designing novel high-performance shale inhibitors by optimizing the spacer length of imidazolium-based bola-form ionic liquids. Energy Fuels 2020, 34, 5838–5845. [Google Scholar] [CrossRef]
- Yang, L.; Jiang, G.; Shi, Y.; Yang, X. Application of ionic liquid and polymeric ionic liquid as shale hydration inhibitors. Energy Fuels 2017, 31, 4308–4317. [Google Scholar] [CrossRef]
- Luo, Z.; Wang, L.; Yu, P.; Chen, Z. Experimental study on the application of an ionic liquid as a shale inhibitor and inhibitive mechanism. Appl. Clay Sci. 2017, 150, 267–274. [Google Scholar] [CrossRef]
- Rasool, M.H.; Zamir, A.; Elraies, K.A.; Ahmad, M.; Ayoub, M.; Abbas, M.A. Potassium carbonate based deep eutectic solvent (DES) as a potential drilling fluid additive in deep water drilling applications. Pet. Sci. Technol. 2021, 39, 612–631. [Google Scholar] [CrossRef]
- Paiva, A.; Craveiro, R.; Aroso, I.; Martins, M.; Reis, R.L.; Duarte, A.R.C. Natural deep eutectic solvents–solvents for the 21st century. ACS Sustain. Chem. Eng. 2014, 2, 1063–1071. [Google Scholar] [CrossRef]
- Dai, Y.; van Spronsen, J.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents as new potential media for green technology. Anal. Chim. Acta 2013, 766, 61–68. [Google Scholar] [CrossRef]
- Vanda, H.; Dai, Y.; Wilson, E.G.; Verpoorte, R.; Choi, Y.H. Green solvents from ionic liquids and deep eutectic solvents to natural deep eutectic solvents. Comptes Rendus Chim. 2018, 21, 628–638. [Google Scholar] [CrossRef]
- Dai, Y.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Tailoring properties of natural deep eutectic solvents with water to facilitate their applications. Food Chem. 2015, 187, 14–19. [Google Scholar] [CrossRef]
- Yang, Z. Natural deep eutectic solvents and their applications in biotechnology. Appl. Ion. Liq. Biotechnol. 2018, 168, 31–59. [Google Scholar]
- Choi, Y.H.; van Spronsen, J.; Dai, Y.; Verberne, M.; Hollmann, F.; Arends, I.W.; Witkamp, G.-J.; Verpoorte, R. Are natural deep eutectic solvents the missing link in understanding cellular metabolism and physiology? Plant Physiol. 2011, 156, 1701–1705. [Google Scholar] [PubMed] [Green Version]
- Mehariya, S.; Fratini, F.; Lavecchia, R.; Zuorro, A. Green extraction of value-added compounds form microalgae: A short review on natural deep eutectic solvents (NaDES) and related pre-treatments. J. Environ. Chem. Eng. 2021, 9, 105989. [Google Scholar] [CrossRef]
- Santana, A.P.; Mora-Vargas, J.A.; Guimaraes, T.G.; Amaral, C.D.; Oliveira, A.; Gonzalez, M.H. Sustainable synthesis of natural deep eutectic solvents (NADES) by different methods. J. Mol. Liq. 2019, 293, 111452. [Google Scholar] [CrossRef]
- Rasool, M.; Ahmad, M.; Hashmi, S. A Novel Potassium Chloride Based Natural Deep Eutectic Solvent: In-House Synthesis and Characterization. Moroc. J. Chem. 2023, 11, 11–13. [Google Scholar]
- Naser, J.; Mjalli, F.; Jibril, B.; Al-Hatmi, S.; Gano, Z. Potassium carbonate as a salt for deep eutectic solvents. Int. J. Chem. Eng. Appl. 2013, 4, 114. [Google Scholar] [CrossRef] [Green Version]
- Suopajärvi, T.; Ricci, P.; Karvonen, V.; Ottolina, G.; Liimatainen, H. Acidic and alkaline deep eutectic solvents in delignification and nanofibrillation of corn stalk, wheat straw, and rapeseed stem residues. Ind. Crops Prod. 2020, 145, 111956. [Google Scholar] [CrossRef]
- Ling, J.K.U.; Chan, Y.S.; Nandong, J.; Chin, S.F.; Ho, B.K. Formulation of choline chloride/ascorbic acid natural deep eutectic solvent: Characterization, solubilization capacity and antioxidant property. LWT 2020, 133, 110096. [Google Scholar] [CrossRef]
- Grønlien, K.G.; Pedersen, M.E.; Tønnesen, H.H. A natural deep eutectic solvent (NADES) as potential excipient in collagen-based products. Int. J. Biol. Macromol. 2020, 156, 394–402. [Google Scholar] [CrossRef]
- Liu, Y.; Friesen, J.B.; McAlpine, J.B.; Lankin, D.C.; Chen, S.-N.; Pauli, G.F. Natural deep eutectic solvents: Properties, applications, and perspectives. J. Nat. Prod. 2018, 81, 679–690. [Google Scholar]
- Mišan, A.; Nađpal, J.; Stupar, A.; Pojić, M.; Mandić, A.; Verpoorte, R.; Choi, Y.H. The perspectives of natural deep eutectic solvents in agri-food sector. Crit. Rev. Food Sci. Nutr. 2020, 60, 2564–2592. [Google Scholar] [CrossRef]
- Rasool, M.H.; Ahmad, M.; Ayoub, M.; Abbas, M.A. A Novel Ascorbic Acid Based Natural Deep Eutectic Solvent as a Drilling Mud Additive for Shale Stabilization. Processes 2023, 11, 1135. [Google Scholar] [CrossRef]
- Hammad Rasool, M.; Ahmad, M.; Ayoub, M.; Zamir, A.; Adeem Abbas, M. A review of the usage of deep eutectic solvents as shale inhibitors in drilling mud. J. Mol. Liq. 2022, 361, 119673. [Google Scholar] [CrossRef]
- Rasool, M.H.; Ahmad, M. Synthesis and physico-chemical characterization of novel Epsom salt based natural deep Eutectic solvent. Chem. Data Collect. 2023, 44, 101004. [Google Scholar] [CrossRef]
- Pericet-Cámara, R.; Best, A.; Butt, H.-J.; Bonaccurso, E. Effect of capillary pressure and surface tension on the deformation of elastic surfaces by sessile liquid microdrops: An experimental investigation. Langmuir 2008, 24, 10565–10568. [Google Scholar] [CrossRef]
- Beg, M.; Haider, M.B.; Thakur, N.K.; Husein, M.; Sharma, S.; Kumar, R. Clay-water interaction inhibition using amine and glycol-based deep eutectic solvents for efficient drilling of shale formations. J. Mol. Liq. 2021, 340, 117134. [Google Scholar] [CrossRef]
- Rahman, M.T.; Negash, B.M.; Danso, D.K.; Idris, A.; Elryes, A.A.; Umar, I.A. Effects of imidazolium- and ammonium-based ionic liquids on clay swelling: Experimental and simulation approach. J. Pet. Explor. Prod. Technol. 2021. [Google Scholar] [CrossRef]



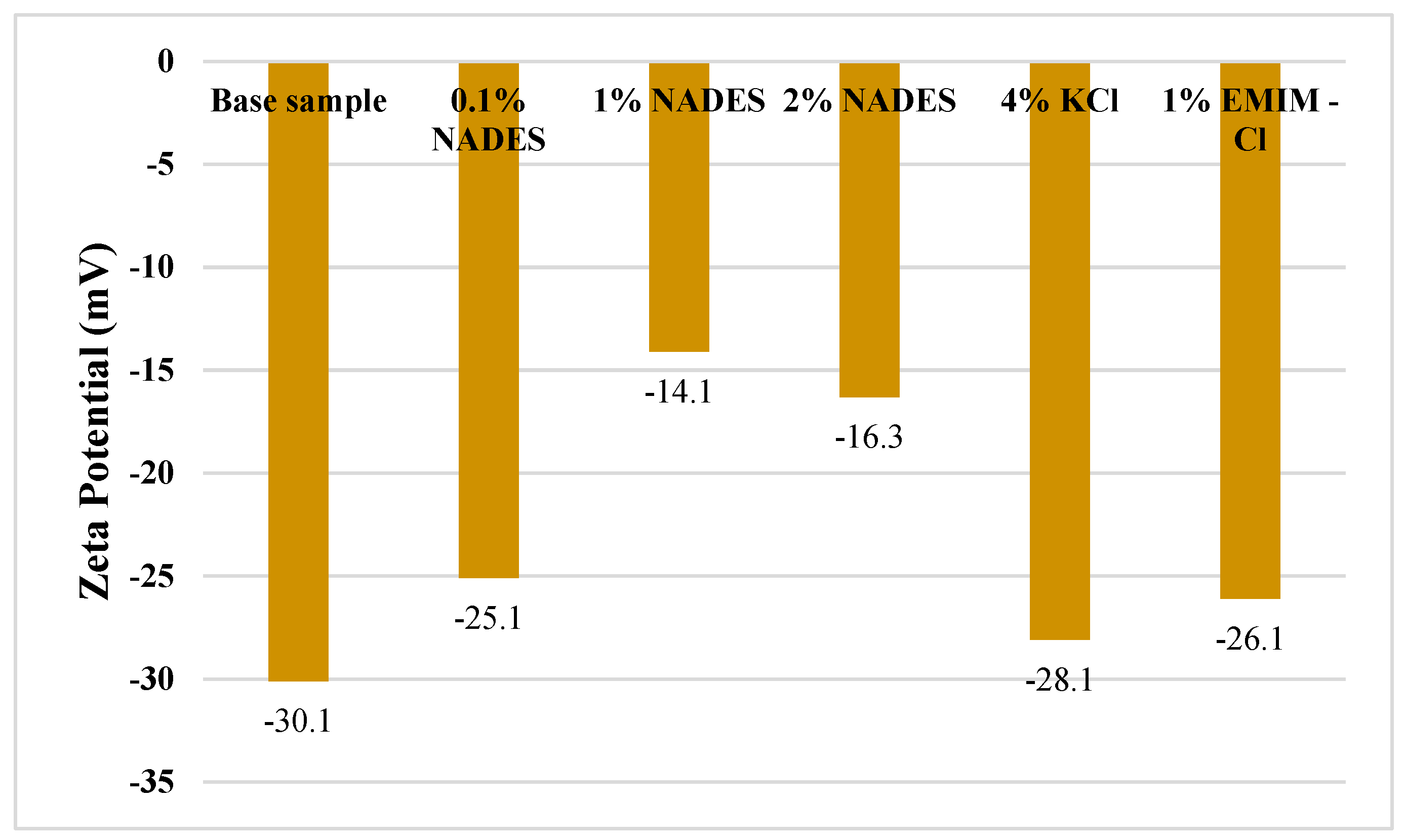
| Component | Weight/Conc/Vol |
|---|---|
| Na-Bentonite | 22.5 g |
| Soda Carobonate | 0.25 g |
| NaOH | 0.25 g |
| Water | 350 mL |
| NADES (Epsom salt: Gly) | 0.1%, 1%, 2% Volume of water |
| KCl | 4% volume of water |
| 1-ethyl-3-methylimidazolium Chloride | 1% volume of water |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rasool, M.H.; Ahmad, M. Epsom Salt-Based Natural Deep Eutectic Solvent as a Drilling Fluid Additive: A Game-Changer for Shale Swelling Inhibition. Molecules 2023, 28, 5784. https://doi.org/10.3390/molecules28155784
Rasool MH, Ahmad M. Epsom Salt-Based Natural Deep Eutectic Solvent as a Drilling Fluid Additive: A Game-Changer for Shale Swelling Inhibition. Molecules. 2023; 28(15):5784. https://doi.org/10.3390/molecules28155784
Chicago/Turabian StyleRasool, Muhammad Hammad, and Maqsood Ahmad. 2023. "Epsom Salt-Based Natural Deep Eutectic Solvent as a Drilling Fluid Additive: A Game-Changer for Shale Swelling Inhibition" Molecules 28, no. 15: 5784. https://doi.org/10.3390/molecules28155784
APA StyleRasool, M. H., & Ahmad, M. (2023). Epsom Salt-Based Natural Deep Eutectic Solvent as a Drilling Fluid Additive: A Game-Changer for Shale Swelling Inhibition. Molecules, 28(15), 5784. https://doi.org/10.3390/molecules28155784








