Organotin (IV) Dithiocarbamate Compounds as Anticancer Agents: A Review of Syntheses and Cytotoxicity Studies
Abstract
1. Introduction
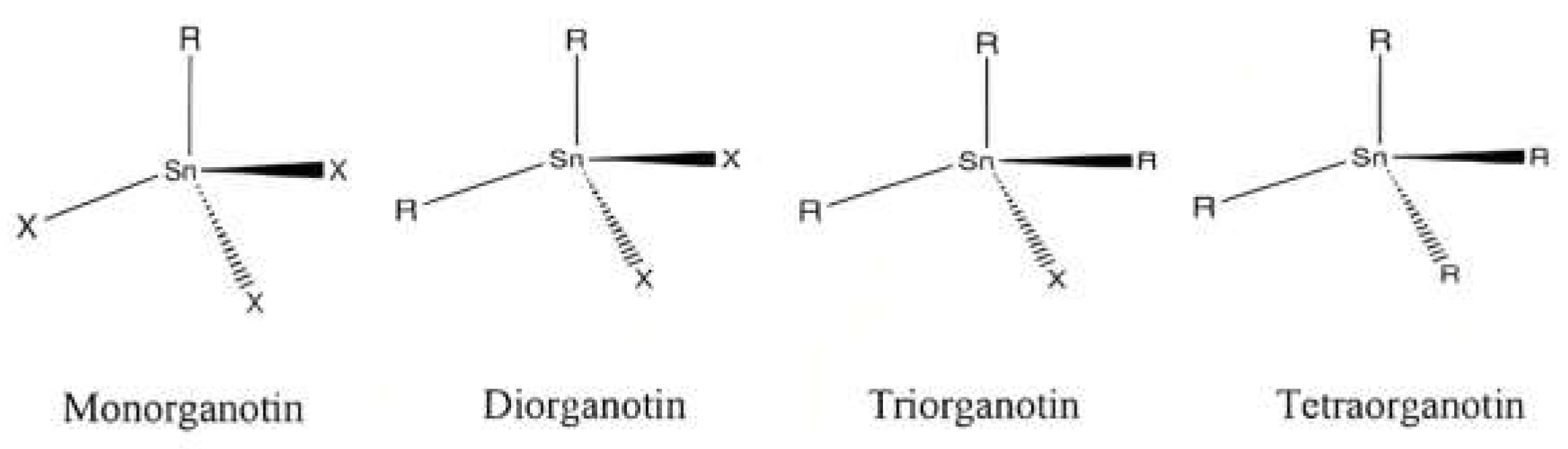
2. Background Chemistry of Organotin (IV) Dithiocarbamate
3. Synthesis of Organotin (IV) Dithiocarbamate
4. Characterization of Organotin (IV) Dithiocarbamate
4.1. Elemental Analysis of Organotin (IV) Dithiocarbamate (CHNS)
4.2. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
4.3. Nuclear Magnetic Resonance (NMR) Spectroscopy
4.3.1. 1HNMR Spectroscopy
4.3.2. 13C NMR Spectroscopy
4.3.3. 119Sn NMR Spectroscopy
- Four-coordinate compounds (δ = 200 to −60 ppm): Distorted tetrahedral structures
- Five-coordinate compounds (δ = −90 to −190 ppm): Distorted trigonal bipyramidal structure
- Six-coordinate compounds (δ = −210 to −400 ppm): Distorted octahedral structure
- Seven-coordinate compounds (δ = −338 to −446 ppm): Distorted pentagonal bipyramidal structure
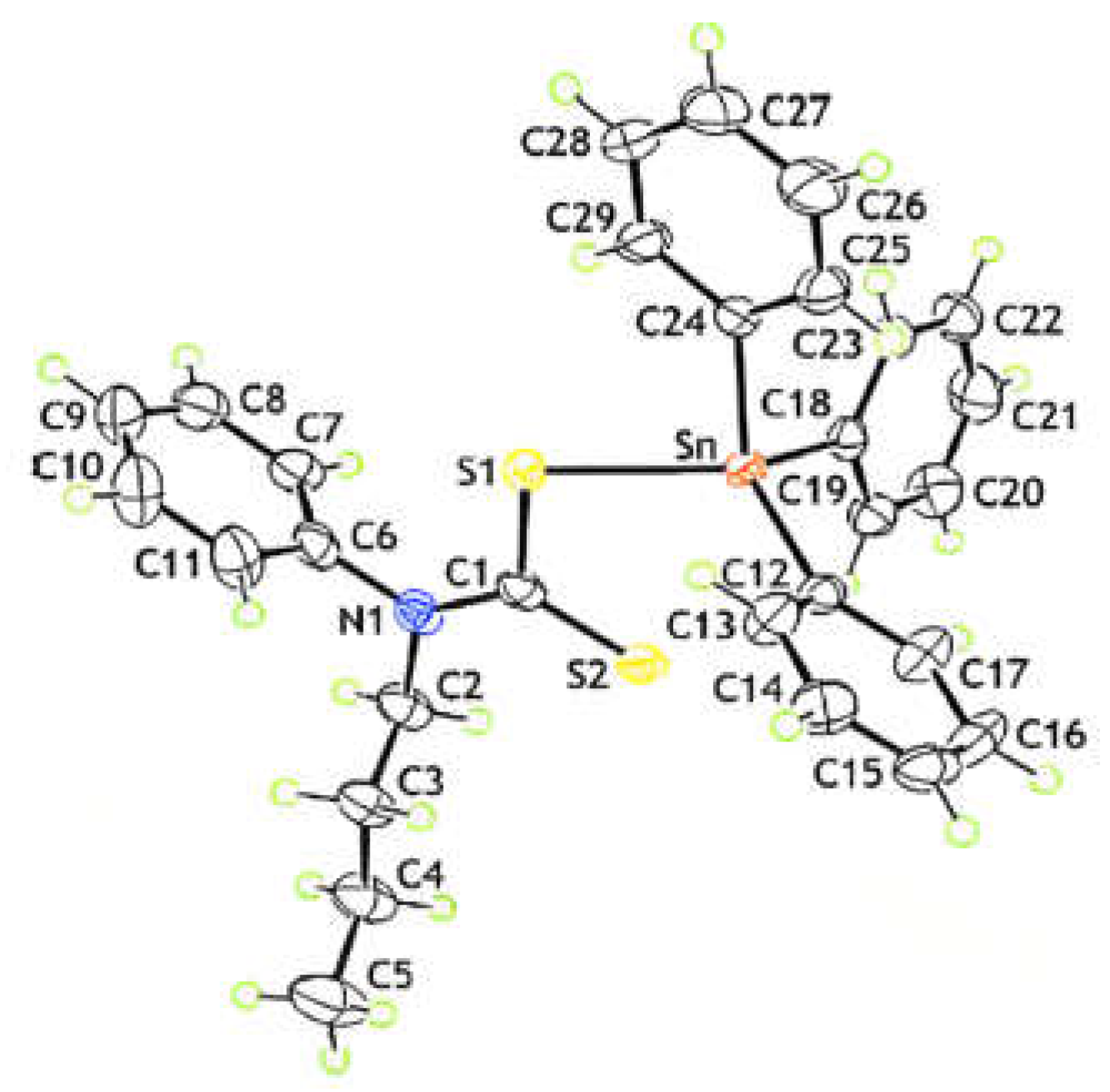
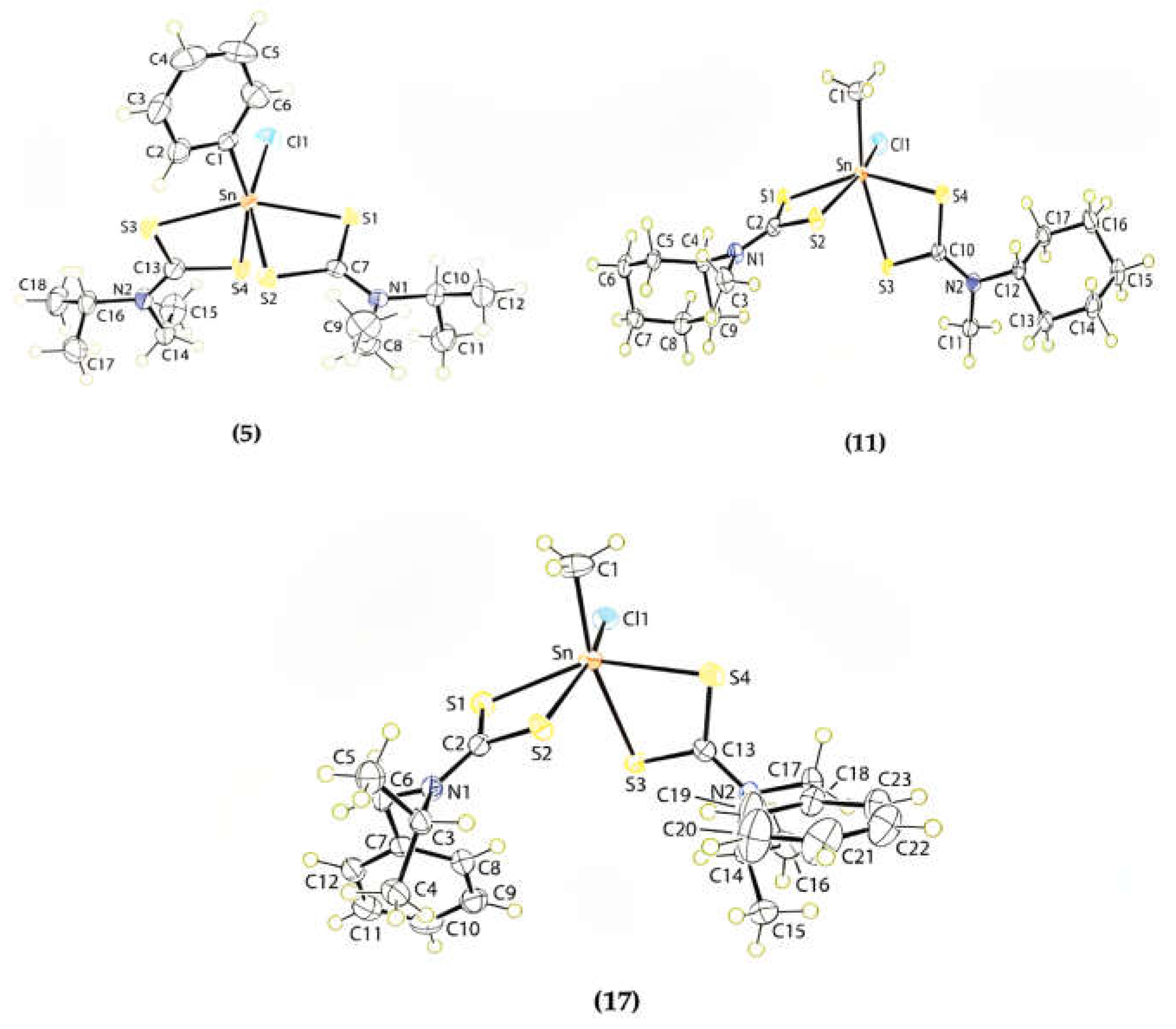
4.4. Recrystallization and Crystallography Study
5. Anticancer Effect of Organotin (IV) Dithiocarbamate
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Upadhyay, A. Cancer: An Unknown Territory; Rethinking before Going Ahead. Genes Dis. 2021, 8, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Horn, G.; Moulton, K.; Oza, A.; Byler, S.; Kokolus, S.; Longacre, M. Cancer Development, Progression, and Therapy: An Epigenetic Overview. Int. J. Mol. Sci. 2013, 14, 21087–21113. [Google Scholar] [CrossRef] [PubMed]
- Mollaei, M.; Hassan, Z.M.; Khorshidi, F.; Langroudi, L. Chemotherapeutic Drugs: Cell Death- and Resistance-Related Signaling Pathways. Are They Really as Smart as the Tumor Cells? Transl. Oncol. 2021, 14, 101056. [Google Scholar] [CrossRef]
- Debela, D.T.; Muzazu, S.G.Y.; Heraro, K.D.; Ndalama, M.T.; Mesele, B.W.; Haile, D.C.; Kitui, S.K.; Manyazewal, T. New Approaches and Procedures for Cancer Treatment: Current Perspectives. SAGE Open Med. 2021, 9, 1–10. [Google Scholar] [CrossRef]
- Kasemodel, K.; Roberts, K. Metal-Based Chemotherapy Drugs. Proc. Okla. Acad. Sci. 2019, 99, 106–113. [Google Scholar]
- Zhang, Q.; Lu, Q. Bin New Combination Chemotherapy of Cisplatin with an Electron-Donating Compound for Treatment of Multiple Cancers. Sci. Rep. 2021, 11, 788. [Google Scholar] [CrossRef]
- Ndagi, U.; Mhlongo, N.; Soliman, M.E. Metal Complexes in Cancer Therapy—An Update from Drug Design Perspective. Drug Des. Devel. Ther. 2017, 11, 599–616. [Google Scholar] [CrossRef]
- Boros, E.; Dyson, P.J.; Gasser, G. Classification of Metal-Based Drugs According to Their Mechanisms of Action. Chem 2020, 6, 41–60. [Google Scholar] [CrossRef]
- Brown, A.; Kumar, S.; Tchounwou, P.B. Cisplatin-Based Chemotherapy of Human Cancers. J. Cancer Sci. Ther. 2019, 11, 97. [Google Scholar] [PubMed]
- Dasari, S.; Bernard Tchounwou, P. Cisplatin in Cancer Therapy: Molecular Mechanisms of Action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, A.C.L.; Mohajershojai, T.; Hariri, M.; Pettersson, M.; Spiegelberg, D. Overcoming Limitations of Cisplatin Therapy by Additional Treatment with the HSP90 Inhibitor Onalespib. Front. Oncol. 2020, 10, 532285. [Google Scholar] [CrossRef] [PubMed]
- Aldossary, S.A. Review on Pharmacology of Cisplatin: Clinical Use, Toxicity and Mechanism of Resistance of Cisplatin. Biomed. Pharmacol. J. 2019, 12, 7–15. [Google Scholar] [CrossRef]
- Ranasinghe, R.; Mathai, M.L.; Zulli, A. Cisplatin for Cancer Therapy and Overcoming Chemoresistance. Heliyon 2022, 8, e10608. [Google Scholar] [CrossRef] [PubMed]
- Rabiee, N.; Safarkhani, M.; Amini, M.M. Investigating the Structural Chemistry of Organotin(IV) Compounds: Recent Advances. Rev. Inorg. Chem. 2019, 39, 13–45. [Google Scholar] [CrossRef]
- Awang, N.; Jumat, H.; Ishak, S.A.; Kamaludin, N.F. Evaluation of the Ex Vivo Antimalarial Activity of Organotin (IV) Ethylphenyldithiocarbamate on Erythrocytes Infected With Plasmodium Berghei Nk 65. Pak. J. Biol. Sci. 2014, 17, 836–842. [Google Scholar] [CrossRef]
- Awang, N.; Mokhtar, N.; Zin, N.M.; Kamaludin, N.F. Antibacterial Activity of Organotin(IV) Methyl and Ethyl Cylohexyldithiocarbamate Compounds. J. Chem. Pharm. Res. 2015, 7, 379–383. [Google Scholar]
- Javed, F.; Sirajuddin, M.; Ali, S.; Khalid, N.; Tahir, M.N.; Shah, N.A.; Rasheed, Z.; Khan, M.R. Organotin(IV) Derivatives of o-Isobutyl Carbonodithioate: Synthesis, Spectroscopic Characterization, X-Ray Structure, HOMO/LUMO and in Vitro Biological Activities. Polyhedron 2016, 104, 80–90. [Google Scholar] [CrossRef]
- Sadiq-ur-Rehman; Ali, S.; Badshah, A.; Mazhar, M.; Song, X.; Eng, G.; Khan, K.M. Synthesis, Spectroscopic Characterization: (IR, Multinuclear NMR, 119mSn Mössbauer and Mass Spectrometry), and Biological Activity (Antibacterial, Antifungal, and Cytotoxicity) of Di- and Triorganotin(IV) Complexes of (E)-3-(4-Chlorophenyl)-2-Phenylpropenoic Acid. Synth. React. Inorg. Met.-Org. Chem. 2004, 34, 1379–1399. [Google Scholar] [CrossRef]
- Saeed, A.; Channar, P.A.; Larik, F.A.; Jabeen, F.; Muqadar, U.; Saeed, S.; Flörke, U.; Ismail, H.; Dilshad, E.; Mirza, B. Design, Synthesis, Molecular Docking Studies of Organotin-Drug Derivatives as Multi-Target Agents against Antibacterial, Antifungal, α-Amylase, α-Glucosidase and Butyrylcholinesterase. Inorganica Chim. Acta 2017, 464, 204–213. [Google Scholar] [CrossRef]
- Adeyemi, J.O.; Onwudiwe, D.C. Organotin(IV) Dithiocarbamate Complexes: Chemistry and Biological Activity. Molecules 2018, 23, 2571. [Google Scholar] [CrossRef]
- Gasser, G.; Metzler-Nolte, N. The Potential of Organometallic Complexes in Medicinal Chemistry. Curr. Opin. Chem. Biol. 2012, 16, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.; Li, Y.; Li, Q. Medicinal Properties of Organotin Compounds and Their Limitations Caused by Toxicity. Inorganica Chim. Acta 2014, 423, 2–13. [Google Scholar] [CrossRef]
- Syed Annuar, S.N.; Kamaludin, N.F.; Awang, N.; Chan, K.M. Cellular Basis of Organotin(IV) Derivatives as Anticancer Metallodrugs: A Review. Front. Chem. 2021, 9, 657599. [Google Scholar] [CrossRef] [PubMed]
- Sirajuddin, M.; Ali, S.; McKee, V.; Sohail, M.; Pasha, H. Potentially Bioactive Organotin(IV) Compounds: Synthesis, Characterization, in Vitro Bioactivities and Interaction with SS-DNA. Eur. J. Med. Chem. 2014, 84, 343–363. [Google Scholar] [CrossRef]
- Hadi, A.G.; Jawad, K.; Ahmed, D.S.; Yousif, E. Synthesis and Biological Activities of Organotin (IV) Carboxylates: A Review. Syst. Rev. Pharm. 2019, 10, 26–31. [Google Scholar] [CrossRef]
- Song, X.; Zapata, A.; Eng, G. Organotins and Quantitative-Structure Activity/Property Relationships. J. Organomet. Chem. 2006, 691, 1756–1760. [Google Scholar] [CrossRef]
- Abdellah, M.A.; Hadjikakou, S.K.; Hadjiliadis, N.; Kubicki, M.; Bakas, T.; Kourkoumelis, N.; Simos, Y.V.; Karkabounas, S.; Barsan, M.M.; Butler, I.S. Synthesis, Characterization, and Biological Studies of Organotin(IV) Derivatives with o- or p-Hydroxybenzoic Acids. Bioinorg. Chem. Appl. 2009, 2009, 542979. [Google Scholar] [CrossRef]
- Sunday, A.O.; Alafara, B.A.; Oladele, O.G. Toxicity and Speciation Analysis of Organotin Compounds. Chem. Speciat. Bioavailab. 2012, 24, 216–226. [Google Scholar] [CrossRef]
- van der Kerk, G.J.M.; Luijten, J.G.A. Investigations on Organo-Tin Compounds. IV. The Preparation of a Number of Trialkyl- and Triaryltin Compounds. J. Appl. Chem. 1956, 6, 49–55. [Google Scholar] [CrossRef]
- James, B.D.; Gioskos, S.; Chandra, S.; Magee, R.J.; Cashion, J.D. Some Triphenyltin(IV) Complexes Containing Potentially Bidentate, Biologically Active Anionic Groups. J. Organomet. Chem. 1992, 436, 155–167. [Google Scholar] [CrossRef]
- Pellerito, C.; Nagy, L.; Pellerito, L.; Szorcsik, A. Biological Activity Studies on Organotin(IV)N+ Complexes and Parent Compounds. J. Organomet. Chem. 2006, 691, 1733–1747. [Google Scholar] [CrossRef]
- Mushak, P.; Krigman, M.R.; Mailman, R.B. Comparative Organotin Toxicity in the Developing Rat: Somatic and Morphological Changes and Relationship to Accumulation of Total Tin. Neurobehav. Toxicol. Teratol. 1982, 4, 209–215. [Google Scholar] [PubMed]
- Doctor, S.V.; Fox, D.A. Effects of Organotin Compounds on Maximal Electroshock Seizure (Mes) Responsiveness in Mice. i.Tri(n-Alkyl)Tin Compounds. J. Toxicol. Environ. Health 1982, 10, 43–52. [Google Scholar] [CrossRef]
- Pfeffer, C.M.; Singh, A.T.K. Apoptosis: A Target for Anticancer Therapy. Int. J. Mol. Sci. 2018, 19, 448. [Google Scholar] [CrossRef] [PubMed]
- Awang, N.; Yousof, N.S.A.M.; Rajab, N.F.; Kamaludin, N.F. In Vitro Cytotoxic Activity of New Triphenyltin (IV) Alkyl-Isopropyldi-Thiocarbamate Compounds on Human Acute T-Lymphoblastic Cell Line. J. Appl. Pharm. Sci. 2015, 5, 7–11. [Google Scholar] [CrossRef]
- Gielen, M. Organotin Compounds and Their Therapeutic Potential: A Report from the Organometallic Chemistry Department of the Free University of Brussels. Appl. Organomet. Chem. 2002, 16, 481–494. [Google Scholar] [CrossRef]
- Hamid, A.; Azmi, M.A.; Rajab, N.F.; Awang, N.; Jufri, N.F. Cytotoxic Effects of Organotin(IV) Dithiocarbamate Compounds with Different Functional Groups on Leukemic Cell Line, K-562. Sains Malays. 2020, 49, 1421–1430. [Google Scholar] [CrossRef]
- Varela-Ramirez, A.; Costanzo, M.; Carrasco, Y.P.; Pannell, K.H.; Aguilera, R.J. Cytotoxic Effects of Two Organotin Compounds and Their Mode of Inflicting Cell Death on Four Mammalian Cancer Cells. Cell Biol. Toxicol. 2011, 27, 159–168. [Google Scholar] [CrossRef]
- Awang, N.; Kamaludin, N.F.; Hamid, A.; Mokhtar, N.W.N.; Rajab, Z.F. Cytotoxicity of Triphenyltin(IV) Methyl- and Ethylisopropyldithiocarbamate Compounds in Chronic Myelogenus Leukemia Cell Line (K-562). Pak. J. Biol. Sci. 2012, 15, 833–838. [Google Scholar] [CrossRef]
- Fuertes, M.A.; Castilla, J.; Alonso, C.; Pérez, J.M. Cisplatin Biochemical Mechanism of Action: From Cytotoxicity to Induction of Cell Death Through Interconnections Between Apoptotic and Necrotic Pathways. Curr. Med. Chem. 2003, 10, 257–266. [Google Scholar] [CrossRef]
- Attanzio, A.; D’Agostino, S.; Busà, R.; Frazzitta, A.; Rubino, S.; Girasolo, M.A.; Sabatino, P.; Tesoriere, L. Cytotoxic Activity of Organotin(IV) Derivatives with Triazolopyrimidine Containing Exocyclic Oxygen Atoms. Molecules 2020, 25, 859. [Google Scholar] [CrossRef] [PubMed]
- Szorcsik, A.; Nagy, L.; Gajda-Schrantz, K.; Pellerito, L.; Nagy, E.; Edelmann, F.T. Structural Studies on Organotin(IV) Complexes Formed with Ligands Containing {S,N,O} Donor Atoms. J. Radioanal. Nucl. Chem. 2002, 252, 523–530. [Google Scholar] [CrossRef]
- Carraher, C.E.; Roner, M.R. Organotin Polyethers as Biomaterials. Materials 2009, 2, 1558–1598. [Google Scholar] [CrossRef]
- Haezam, F.N.; Awang, N.; Kamaludin, N.F.; Mohamad, R. Synthesis and Cytotoxic Activity of Organotin(IV) Diallyldithiocarbamate Compounds as Anticancer Agent towards Colon Adenocarcinoma Cells (HT-29). Saudi J. Biol. Sci. 2021, 28, 3160–3168. [Google Scholar] [CrossRef]
- Khan, N.; Farina, Y.; Mun, L.K.; Rajab, N.F.; Awang, N. Syntheses, Characterization, X-Ray Diffraction Studies and in Vitro Antitumor Activities of Diorganotin(IV) Derivatives of Bis(p-Substituted-N-Methylbenzylaminedithiocarbamates). Polyhedron 2015, 85, 754–760. [Google Scholar] [CrossRef]
- Awang, N.; Aziz, Z.A.; Kamaludin, N.F.; Chan, K.M. Cytotoxicity and Mode of Cell Death Induced by Triphenyltin (IV) Compounds in Vitro. Online J. Biol. Sci. 2014, 14, 84–93. [Google Scholar] [CrossRef][Green Version]
- Girasolo, M.A.; Tesoriere, L.; Casella, G.; Attanzio, A.; Capobianco, M.L.; Sabatino, P.; Barone, G.; Rubino, S.; Bonsignore, R. A Novel Compound of Triphenyltin(IV) with N-Tert-Butoxycarbonyl-L-Ornithine Causes Cancer Cell Death by Inducing a P53-Dependent Activation of the Mitochondrial Pathway of Apoptosis. Inorganica Chim. Acta 2017, 456, 1–8. [Google Scholar] [CrossRef]
- Adeyemi, J.O.; Onwudiwe, D.C. Antimicrobial and Cytotoxicity Studies of Some Organotin(IV) N-Ethyl-N-Phenyl Dithiocarbamate Complexes. Pol. J. Environ. Stud. 2020, 29, 2525–2532. [Google Scholar] [CrossRef]
- Baul, T.S.B. Antimicrobial Activity of Organotin(IV) Compounds: A Review. Appl. Organomet. Chem. 2008, 22, 195–204. [Google Scholar] [CrossRef]
- Awang, N.; Zakri, N.H.; Zain, N.M. Antimicrobial Activity of Organotin(IV) Alkylisopropildithiocarbamate Compounds. J. Chem. Pharm. Res. 2016, 8, 862–866. [Google Scholar]
- Kadu, R.; Roy, H.; Singh, V.K. Diphenyltin(IV) Dithiocarbamate Macrocyclic Scaffolds as Potent Apoptosis Inducers for Human Cancer HEP 3B and IMR 32 Cells: Synthesis, Spectral Characterization, Density Functional Theory Study and in Vitro Cytotoxicity. Appl. Organomet. Chem. 2015, 29, 746–755. [Google Scholar] [CrossRef]
- Awang, N.; Mohktar, S.M.; Zin, N.M.; Kamaludin, N.F. Evaluation of Antimicrobial Activities of Organotin (IV) Alkylphenyl Dithiocarbamate Compounds. Asian J. Appl. Sci. 2015, 8, 165–172. [Google Scholar] [CrossRef]
- Adeyemi, J.O.; Onwudiwe, D.C.; Nundkumar, N.; Singh, M. Diorganotin(IV) Benzyldithiocarbamate Complexes: Synthesis, Characterization, and Thermal and Cytotoxicity Study. Open Chem. 2020, 18, 453–462. [Google Scholar] [CrossRef]
- Kamaludin, N.F.; Awang, N.; Baba, I.; Hamid, A.; Meng, C.K. Synthesis, Characterization and Crystal Structure of Organotin(IV) N-Butyl-N-Phenyldithiocarbamate Compounds and Their Cytotoxicity in Human Leukemia Cell Lines. Pak. J. Biol. Sci. 2013, 16, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Syed Annuar, S.N.; Kamaludin, N.F.; Awang, N.; Chan, K.M. Triphenyltin(IV) Dithiocarbamate Compound Induces Genotoxicity and Cytotoxicity in K562 Human Erythroleukemia Cells Primarily via Mitochondria-Mediated Apoptosis. Food Chem. Toxicol. 2022, 168, 113336. [Google Scholar] [CrossRef]
- Rasli, N.R.; Hamid, A.; Awang, N.; Kamaludin, N.F. Series of Organotin(IV) Compounds with Different Dithiocarbamate Ligands Induced Cytotoxicity, Apoptosis and Cell Cycle Arrest on Jurkat E6.1, T Acute Lymphoblastic Leukemia Cells. Molecules 2023, 28, 3376. [Google Scholar] [CrossRef]
- Mamba, S.M.; Mishra, A.K.; Mamba, B.B.; Njobeh, P.B.; Dutton, M.F.; Fosso-Kankeu, E. Spectral, Thermal and in Vitro Antimicrobial Studies of Cyclohexylamine-N-Dithiocarbamate Transition Metal Complexes. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2010, 77, 579–587. [Google Scholar] [CrossRef]
- Menezes, D.C.; Vieira, F.T.; De Lima, G.M.; Porto, A.O.; Cortés, M.E.; Ardisson, J.D.; Albrecht-Schmitt, T.E. Tin(IV) Complexes of Pyrrolidinedithiocarbamate: Synthesis, Characterisation and Antifungal Activity. Eur. J. Med. Chem. 2005, 40, 1277–1282. [Google Scholar] [CrossRef]
- Cvek, B.; Dvorak, Z. Targeting of Nuclear Factor-KappaB and Proteasome by Dithiocarbamate Complexes with Metals. Curr. Pharm. Des. 2007, 13, 3155–3167. [Google Scholar] [CrossRef] [PubMed]
- Ajiboye, T.O.; Ajiboye, T.T.; Marzouki, R.; Onwudiwe, D.C. The Versatility in the Applications of Dithiocarbamates. Int. J. Mol. Sci. 2022, 23, 1317. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Yousif, E. Chemistry and Applications of Organotin(IV) Complexes: A Review. Res. J. Pharm. Biol. Chem. Sci. 2016, 7, 2611–2619. [Google Scholar]
- Iqbal, H.; Ali, S.; Shahzadi, S. Antituberculosis Study of Organotin(IV) Complexes: A Review. Cogent Chem. 2015, 1, 1029039. [Google Scholar] [CrossRef]
- Ayanda, O.S.; Fatoki, O.S.; Adekola, F.A.; Ximba, B.J. Fate and Remediation of Organotin Compounds in Seawaters and Soils. Chem. Sci. Trans. 2012, 1, 470–481. [Google Scholar] [CrossRef]
- Awang, N.; Kamaludin, N.F.; Ghazali, A.R. Cytotoxic Effect of Organotin(IV) Benzylisopropyldithiocarbamate Compounds on Chang Liver Cell and Hepatocarcinoma HepG2 Cell. Pak. J. Biol. Sci. 2011, 14, 768–774. [Google Scholar] [CrossRef] [PubMed]
- Kamaludin, N.F.; Awang, N. Synthesis and Characterisation of Organotin(IV) Nethyl- N-Phenyldithiocarbamate Compounds and the Crystal Structures of Dibutyl- And Triphenyltin(IV) Nethyl- N-Phenyldithiocarbamate. Res. J. Chem. Environ. 2014, 18, 90–98. [Google Scholar]
- Sainorudin, M.H.; Sidek, N.M.; Ismail, N.; Rozaini, M.Z.H.; Harun, N.A.; Tuan Anuar, T.N.S.; Azmi, A.A.A.R.; Yusoff, F. Synthesis, Characterization and Biological Activity of Organotin(IV) Complexes Featuring Di-2-Ethylhexyldithiocarbamate and N-Methylbutyldithiocarbamate as Ligands. GSTF J. Chem. Sci. 2015, 2, 2. [Google Scholar]
- Sharma, R.; Kaushik, N.K. Thermal Studies on Some Organotin(IV) Complexes with Piperidine and 2-Aminopyridine Dithiocarbamates. J. Therm. Anal. Calorim. 2004, 78, 953–964. [Google Scholar] [CrossRef]
- Awang, N.; Baba, I. Diorganotin(IV) Alkylcyclohexyldithiocarbamate Compounds: Synthesis, Characterization and Biological Activities. Sains Malays. 2012, 41, 977–982. [Google Scholar]
- Fanjul-Bolado, P.; Fogel, R.; Limson, J.; Purcarea, C.; Vasilescu, A. Advances in the Detection of Dithiocarbamate Fungicides: Opportunities for Biosensors. Biosensors 2020, 11, 12. [Google Scholar] [CrossRef]
- Awang, N.; Baba, I.; Yamin, B.M.; Halim, A.A. Preparation, Characterization and Antimicrobial Assay of 1,10-Phenanthroline and 2,2’-Bipyridyl Adducts of Cadmium(II) N-Sec-Butyl-N- Propyldithiocarbamate: Crystal Structure of Cd[S2CN(i-C4H9)(C3H7)]2(2,2’-Bipyridyl). World Appl. Sci. J. 2011, 12, 1568–1574. [Google Scholar]
- Nabipour, H.; Ghammamy, S.; Ashuri, S.; Aghbolagh, Z.S. Synthesis of a New Dithiocarbamate Compound and Study of Its Biological Properties. Org. Chem. J. 2010, 2, 75–80. [Google Scholar]
- Jung, O.-S.; Sohn, Y.-S. Coordination Chemistry of Organotin(IV) Dithiocarbamate Complexes. Bull. Korean Chem. Soc. 1988, 9, 365–368. [Google Scholar]
- Xu, L.Z.; Zhao, P.S.; Zhang, S.S. Crystal Structure and Characterization of Pd(II) Bis(Diisopropyldithiocarbamate) Complex. Chin. J. Chem. 2001, 19, 436–440. [Google Scholar] [CrossRef]
- Domazetis, G.; Magee, R.J.; James, B.D. Synthesis and Structure of Some Triphenyltin(IV) Dithiocarbamate Compounds. J. Organomet. Chem. 1977, 141, 57–69. [Google Scholar] [CrossRef]
- Odularu, A.T.; Ajibade, P.A. Dithiocarbamates: Challenges, Control, and Approaches to Excellent Yield, Characterization, and Their Biological Applications. Bioinorg. Chem. Appl. 2019, 2019, 826049. [Google Scholar] [CrossRef] [PubMed]
- Onwudiwe, D.C.; Ajibade, P.A. Synthesis and Characterization of Metal Complexes of N-Alkyl-N-Phenyl Dithiocarbamates. Polyhedron 2010, 29, 1431–1436. [Google Scholar] [CrossRef]
- Awang, N.; Baba, I.; Yamin, B.M.; Othman, M.S.; Kamaludin, N.F. Synthesis, Characterization and Biological Activities of Organotin (IV) Methylcyclohexyldithiocarbamate Compounds. Am. J. Appl. Sci. 2011, 8, 310–317. [Google Scholar] [CrossRef]
- Perry, D.; Geanangle, R.A. The Preparation of Tin(II) Dithiocarbamates from Ammonium Dithiocarbamate Salts. Inorganica Chim. Acta 1975, 13, 185–189. [Google Scholar] [CrossRef]
- Adeyemi, J.O.; Onwudiwe, D.C.; Ekennia, A.C.; Okafor, S.N.; Hosten, E.C. Organotin(IV)N-Butyl-N-Phenyldithiocarbamate Complexes: Synthesis, Characterization, Biological Evaluation and Molecular Docking Studies. J. Mol. Struct. 2019, 1192, 15–26. [Google Scholar] [CrossRef]
- Adeyemi, J.O.; Onwudiwe, D.C.; Hosten, E.C. Organotin(IV) Complexes Derived from N-Ethyl-N-Phenyldithiocarbamate: Synthesis, Characterization and Thermal Studies. J. Saudi Chem. Soc. 2018, 22, 427–438. [Google Scholar] [CrossRef]
- Adeyemi, J.O.; Onwudiwe, D.C.; Hosten, E.C. Synthesis, Characterization and the Use of Organotin(IV) Dithiocarbamate Complexes as Precursor to Tin Sulfide Nanoparticles by Heat up Approach. J. Mol. Struct. 2019, 1195, 395–402. [Google Scholar] [CrossRef]
- Adli, H.K.; Sidek, N.M.; Ismail, N.; Khairul, W.M. Several Organotin (IV) Complexes Featuring 1-Methylpiperazinedithiocarbamate and N-Methylcyclohexyldithiocarbamate as Ligands and Their Anti-Microbial Activity Studies. Chiang Mai J. Sci. 2013, 40, 117–125. [Google Scholar]
- Awang, N.; Baba, I.; Yamin, B.; Othman, M.; Halim, A.; Muda, J.; Aziz, A.; Lumpur, K. Malaysia Synthesis, Characterization and Crystal Structure of Triphenyltin(IV) N-Alkyl-N-Cyclohexyldithiocarbamate Compounds. World Appl. Sci. J. 2011, 12, 630–635. [Google Scholar]
- Awang, N.; Kamaludin, N.F.; Baba, I.; Chan, K.M.; Rajaajab, N.F.; Hamid, A. Synthesis, Characterization and Antitumor Activity of New Organotin(IV) Methoxyethyldithiocarbamate Complexes. Orient. J. Chem. 2016, 32, 101–107. [Google Scholar] [CrossRef]
- Mohamad, R.; Awang, N.; Farahana Kamaludin, N. Synthesis and Characterisation of New Organotin (IV)(2-Methoxyethyl)-Methyldithiocarbamate Complexes. Res. J. Pharm. Biol. Chem. Sci. 2016, 7, 1920–1925. [Google Scholar]
- Muthalib, A.F.A.; Baba, I. New Mono-Organotin (IV) Dithiocarbamate Complexes. In Proceedings of the AIP Conference Proceedings, Selangor, Malaysia, 9–11 April 2014; Volume 1614, pp. 237–243. [Google Scholar]
- Baba, I.; Raya, I. Kompleks Praseodimium Ditiokarbamat 1,10 Fenantrolin. Sains Malays. 2010, 39, 45–50. [Google Scholar]
- Fadeeva, V.P.; Tikhova, V.D.; Nikulicheva, O.N. Elemental Analysis of Organic Compounds with the Use of Automated CHNS Analyzers. J. Anal. Chem. 2008, 63, 1094–1106. [Google Scholar] [CrossRef]
- Edington, S.C.; Liu, S.; Baiz, C.R. Infrared Spectroscopy Probes Ion Binding Geometries. Methods Enzymol. 2021, 651, 157–191. [Google Scholar] [CrossRef]
- Kumar, A.; Khandelwal, M.; Gupta, S.K.; Kumar, V.; Rani, R. Fourier Transform Infrared Spectroscopy: Data Interpretation and Applications in Structure Elucidation and Analysis of Small Molecules and Nanostructures. In Data Processing Handbook for Complex Biological Data Sources; Academic Press: Cambridge, MA, USA, 2019; pp. 77–96. [Google Scholar]
- Sonia, T.A.; Sharma, C.P. Experimental Techniques Involved in the Development of Oral Insulin Carriers. In Oral Delivery of Insulin; Woodhead Publishing: Sawston, UK, 2014; pp. 169–217. [Google Scholar]
- Zia-ur-Rehman; Shahzadi, S.; Ali, S.; Badshah, A.; Jin, G.-X. Crystal Structure of 1,1-Dibutyl-1,1-Bis[(4-Methyl-1-Piperidinyl) Dithiocarbamato] Tin(IV). J. Iran. Chem. Soc. 2006, 3, 157–160. [Google Scholar] [CrossRef]
- Win, Y.F.; Teoh, S.G.; Tengku-Muhammad, T.S.; Ha, S.T.; Sivasothy, Y. Synthesis and Structural Characterization of Organotin (IV) Complexes Derived of 4-(Diethylamino) Benzoic Acid: Cytotoxic Assay on Human Liver Carcinoma Cells (HepG2). Aust. J. Basic Appl. Sci. 2010, 4, 1383–1390. [Google Scholar]
- Chaber, R.; Łach, K.; Szmuc, K.; Michalak, E.; Raciborska, A.; Mazur, D.; Machaczka, M.; Cebulski, J. Application of Infrared Spectroscopy in the Identification of Ewing Sarcoma: A Preliminary Report. Infrared Phys. Technol. 2017, 83, 200–205. [Google Scholar] [CrossRef]
- Dominguez, G.; McLeod, A.S.; Gainsforth, Z.; Kelly, P.; Bechtel, H.A.; Keilmann, F.; Westphal, A.; Thiemens, M.; Basov, D.N. Nanoscale Infrared Spectroscopy as a Non-Destructive Probe of Extraterrestrial Samples. Nat. Commun. 2014, 5, 5445. [Google Scholar] [CrossRef] [PubMed]
- Kartina, D.; Wahab, A.W.; Ahmad, A.; Irfandi, R.; Raya, I. In Vitro Antibacterial and Anticancer Activity of Zn(II)Valinedithiocarbamate Complexes. J. Phys. Conf. Ser. 2019, 1341, 032042. [Google Scholar] [CrossRef]
- Onwudiwe, D.C.; Hrubaru, M.; Ebenso, E.E. Synthesis, Structural and Optical Properties of TOPO and HDA Capped Cadmium Sulphide Nanocrystals, and the Effect of Capping Ligand Concentration. J. Nanomater. 2015, 2015, 143632. [Google Scholar] [CrossRef]
- Yin, H.D.; Xue, S.C. Synthesis and Characterization of Organotin Complexes with Dithiocarbamates and Crystal Structures of (4-NCC6H4CH2) 2Sn(S2CNEt2)2 and (2-ClC 6H4CH2)2 Sn(Cl)S 2CNBz2. Appl. Organomet. Chem. 2006, 20, 283–289. [Google Scholar] [CrossRef]
- Odola, A.J.; Woods, J.A.O. New Nickel(II) Mixed Ligand Complexes of Dithiocarbamates with Schiff Base. J. Chem. Pharm. Res. 2011, 3, 865–871. [Google Scholar]
- Alverdi, V.; Giovagnini, L.; Marzano, C.; Seraglia, R.; Bettio, F.; Sitran, S.; Graziani, R.; Fregona, D. Characterization Studies and Cytotoxicity Assays of Pt(II) and Pd(II) Dithiocarbamate Complexes by Means of FT-IR, NMR Spectroscopy and Mass Spectrometry. J. Inorg. Biochem. 2004, 98, 1117–1128. [Google Scholar] [CrossRef] [PubMed]
- Nomura, R.; Takabe, A.; Matsuda, H. Facile Synthesis of Antimony Dithiocarbamate Complexes. Polyhedron 1987, 6, 411–416. [Google Scholar] [CrossRef]
- Brown, D.A.; Glass, W.K.; Burke, M.A. The General Use of i.r. Spectral Criteria in Discussions of the Bonding and Structure of Metal Dithiocarbamates. Spectrochim. Acta A 1976, 32, 137–143. [Google Scholar] [CrossRef]
- Bonati, F.; Ugo, R. Organotin(IV) N,N-Disubstituted Dithiocarbamates. J. Organomet. Chem. 1967, 10, 257–268. [Google Scholar] [CrossRef]
- Honda, M.; Komura, M.; Kawasaki, Y.; Tanaka, T.; Okawara, R. Infra-Red and PMR Spectra of Some Organotin(IV) N,N-Dimethyldithiocarbamates. J. Inorg. Nucl. Chem. 1968, 30, 3231–3237. [Google Scholar] [CrossRef]
- Muthalib, A.F.A.; Baba, I.; Farina, Y.; Samsudin, M.W. Synthesis and Characterization of Diphenyltin(IV) Dithiocarbamate Compounds. Malays. J. Anal. Sci. 2011, 15, 106–112. [Google Scholar]
- Cotton, F.A.; McCleverty, J.A. Dimethyl- and Diethyldithiocarbamate Complexes of Some Metal Carbonyl Compounds. Inorg. Chem. 1964, 3, 1398–1402. [Google Scholar] [CrossRef]
- Adeyemi, J.O.; Onwudiwe, D.C.; Singh, M. Synthesis, Characterization, and Cytotoxicity Study of Organotin(IV) Complexes Involving Different Dithiocarbamate Groups. J. Mol. Struct. 2019, 1179, 366–375. [Google Scholar] [CrossRef]
- Khan, S.; Nami, S.A.A.; Siddiqi, K.S. Mononuclear Indolyldithiocarbamates of SnCl4 and R2SnCl2: Spectroscopic, Thermal Characterizations and Cytotoxicity Assays in Vitro. J. Organomet. Chem. 2008, 693, 1049–1057. [Google Scholar] [CrossRef]
- Awang, N.; Baba, I.; Mohd Yousof, N.S.A.; Kamaludin, N.F. Synthesis and Characterization of Organotin(IV) N-Benzyl-N-Isopropyldithiocarbamate Compounds: Cytotoxic Assay on Human Hepatocarcinoma Cells (HepG2). Am. J. Appl. Sci. 2010, 7, 1047–1052. [Google Scholar] [CrossRef]
- Adeyemi, J.O.; Saibu, G.M.; Olasunkanmi, L.O.; Fadaka, A.O.; Meyer, M.; Sibuyi, N.R.S.; Onwudiwe, D.C.; Oyedeji, A.O. Synthesis, Computational and Biological Studies of Alkyltin(IV) N-Methyl-N-Hydroxyethyl Dithiocarbamate Complexes. Heliyon 2021, 7, e07693. [Google Scholar] [CrossRef] [PubMed]
- Onwudiwe, D.C.; Ajibade, P.A. Synthesis, Characterization and Thermal Studies of Zn(II), Cd(II) and Hg(II) Complexes of N-Methyl-N-Phenyldithiocarbamate: The Single Crystal Structure of [(C(6)H(5))(CH(3))NCS(2)](4)Hg(2). Int. J. Mol. Sci. 2011, 12, 1964–1978. [Google Scholar] [CrossRef]
- Riveros, P.C.; Perilla, I.C.; Poveda, A.; Keller, H.J.; Pritzkow, H. Tris(Dialkyldithiocarbamato)Diazenido(1-) and Hydrazido(2-) Molybdenum Complexes: Synthesis and Reactivity in Acid Medium. Polyhedron 2000, 19, 2327–2335. [Google Scholar] [CrossRef]
- Prakasam, B.A.; Ramalingam, K.; Bocelli, G.; Cantoni, A. NMR and Fluorescence Spectral Studies on Bisdithiocarbamates of Divalent Zn, Cd and Their Nitrogenous Adducts: Single Crystal X-Ray Structure of (1,10-Phenanthroline)Bis(4-Methylpiperazinecarbodithioato) Zinc(II). Polyhedron 2007, 26, 4489–4493. [Google Scholar] [CrossRef]
- Yin, H.-D.; Zhai, J.; Sun, Y.-Y.; Wang, D.-Q. Synthesis, Characterizations and Crystal Structures of New Antimony (III) Complexes with Dithiocarbamate Ligands. Polyhedron 2008, 27, 663–670. [Google Scholar] [CrossRef]
- Prakasam, B.A.; Ramalingam, K.; Baskaran, R.; Bocelli, G.; Cantoni, A. Synthesis, NMR Spectral and Single Crystal X-Ray Structural Studies on Ni(II) Dithiocarbamates with NiS2PN, NiS2PC, NiS2P2 Chromophores: Crystal Structures of (4-Methylpiperazinecarbodithioato)(Thiocyanato-N) (Triphenylphosphine)Nickel(II) and Bis(Triphenylphosphine) (4-Methylpiperazinecarbodithioato)Nickel(II) Perchlorate Monohydrate. Polyhedron 2007, 26, 1133–1138. [Google Scholar] [CrossRef]
- Ajibade, P.A.; Onwudiwe, D.C.; Moloto, M.J. Synthesis of Hexadecylamine Capped Nanoparticles Using Group 12 Complexes of N-Alkyl-N-Phenyl Dithiocarbamate as Single-Source Precursors. Polyhedron 2011, 30, 246–252. [Google Scholar] [CrossRef]
- Ahmad, M.S.; Hussain, M.; Hanif, M.; Ali, S.; Qayyum, M.; Mirza, B. Di- and Triorganotin(IV) Esters of 3,4-Methylenedioxyphenylpropenoic Acid: Synthesis, Spectroscopic Characterization and Biological Screening for Antimicrobial, Cytotoxic and Antitumor Activities. Chem. Biol. Drug Des. 2008, 71, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Van Gaal, H.L.M.; Diesveld, J.W.; Pijpers, F.W.; Van der Linden, J.G.M. Carbon-13 NMR Spectra of Dithiocarbamates. Chemical Shifts, Carbon-Nitrogen Stretching Vibration Frequencies and .Pi.-Bonding in the NCS2 Fragment. Inorg. Chem. 1979, 18, 3251–3260. [Google Scholar] [CrossRef]
- Sovilj, S.P.; Vučković, G.; Babić, K.; Sabo, T.J.; Macura, S.; Juranić, N. Mixed-Ligand Complexes of Cobalt(III) with Dithiocarbamates and a Cyclic Tetradentate Secondary Amine. J. Coord. Chem. 1997, 41, 19–25. [Google Scholar] [CrossRef]
- Macomber, R.S. Proton-Carbon Chemical Shift Correlations. J. Chem. Educ. 1991, 68, 284. [Google Scholar] [CrossRef]
- Sirajuddin, M.; Ali, S.; McKee, V.; Zaib, S.; Iqbal, J. Organotin(Iv) Carboxylate Derivatives as a New Addition to Anticancer and Antileishmanial Agents: Design, Physicochemical Characterization and Interaction with Salmon Sperm DNA. RSC Adv. 2014, 4, 57505–57521. [Google Scholar] [CrossRef]
- Otera, J. 119Sn Chemical Shifts in Five- and Six-Coordinate Organotin Chelates. J. Organomet. Chem. 1981, 221, 57–61. [Google Scholar] [CrossRef]
- Jung, O.S.; Hwa Jeong, J.; Soo Sohn, Y. Preparation, Properties and Structures of Estertin(IV) Sulphides. Polyhedron 1989, 8, 2557–2563. [Google Scholar] [CrossRef]
- Ahmad, F.; Ali, S.; Parvez, M.; Munir, A.; Mazhar, M.; Khan, K.M.; Ali Shah, T. Synthesis, Characterization, and Biological Studies of Tri- and Diorganotin(Iv) Complexes with 2′, 4′-Difluoro-4-Hydroxy-[1, 1′]-Biphenyle-3-Carbolic Acid: Crystal Structure of [(CH3)3Sn(C13H7O3F2)]. Heteroat. Chem. 2002, 13, 638–649. [Google Scholar] [CrossRef]
- Sedaghat, T.; Shokohi-Pour, Z. Synthesis and Spectroscopic Studies of New Organotin(IV) Complexes with Tridentate N- and O-Donor Schiff Bases. J. Coord. Chem. 2009, 62, 3837–3844. [Google Scholar] [CrossRef]
- Sirajuddin, M.; Ali, S.; Tahir, M.N. Pharmacological Investigation of Mono-, Di- and Tri-Organotin(IV) Derivatives of Carbodithioates: Design, Spectroscopic Characterization, Interaction with SS-DNA and POM Analyses. Inorganica Chim. Acta 2016, 439, 145–158. [Google Scholar] [CrossRef]
- Shahzadi, S.; Ali, S. Structural Chemistry of Organotin(IV) Complexes. J. Iran. Chem. Soc. 2008, 5, 16–28. [Google Scholar] [CrossRef]
- Tiekink, E.R.T. Structural Chemistry of Organotin Carboxylates: A Review of the Crystallographic Literature. Appl. Organomet. Chem. 1991, 5, 1–23. [Google Scholar] [CrossRef]
- Chans, G.M.; Nieto-Camacho, A.; Ramirez-Apan, T.; Hernandez-Ortega, S.; Alvarez-Toledano, C.; Gomez, E. Synthetic, Spectroscopic, Crystallographic, and Biological Studies of Seven-Coordinated Diorganotin(IV) Complexes Derived from Schiff Bases and Pyridinic Carboxylic Acids. Aust. J. Chem. 2016, 69, 279–290. [Google Scholar] [CrossRef]
- Deschamps, J.R. X-Ray Crystallography of Chemical Compounds. Life Sci. 2010, 86, 585–589. [Google Scholar] [CrossRef]
- Francisco, M.E.Y.; Burgess, J.P.; George, C.; Bailey, G.S.; Gilliam, A.F.; Seltzman, H.H.; Thomas, B.F. Structure Elucidation of a Novel Ring-Constrained Biaryl Pyrazole CB 1 Cannabinoid Receptor Antagonist. Magn. Reson. Chem. 2003, 41, 265–268. [Google Scholar] [CrossRef]
- Kim, K.; Ibers, J.A.; Jung, O.-S.; Sohn, Y.S. Structure of Di(Tert-Butyl)Bis(N,N-Dimethyldithiocarbamato)Tin(IV). Acta Crystallogr. C 1987, 43, 2317–2319. [Google Scholar] [CrossRef]
- Abbas, S.M.; Ali, S.; Hussain, S.T.; Shahzadi, S. Review: Structural Diversity in Organotin(IV) Dithiocarboxylates and Carboxylates. J. Coord. Chem. 2013, 66, 2217–2234. [Google Scholar] [CrossRef]
- Khan, N.; Farina, Y.; Mun, L.K.; Rajab, N.F.; Awang, N. Syntheses, Spectral Characterization, X-Ray Studies and in Vitro Cytotoxic Activities of Triorganotin(IV) Derivatives of p-Substituted N-Methylbenzylaminedithiocarbamates. J. Mol. Struct. 2014, 1076, 403–410. [Google Scholar] [CrossRef]
- Sieber, K.D.; Kutschabsky, L.; Kulpe, S.S. The Molecular and Crystal Structure of Bis-Dimethyl Pentamethine Cyanine Perchlorate, C9H17N2Cl04. Cryst. Res. Technol. 1974, 9, 1111–1122. [Google Scholar] [CrossRef]
- Fuentes-Martínez, J.P.; Toledo-Martínez, I.; Román-Bravo, P.; y García, P.G.; Godoy-Alcántar, C.; López-Cardoso, M.; Morales-Rojas, H. Diorganotin(IV) Dithiocarbamate Complexes as Chromogenic Sensors of Anion Binding. Polyhedron 2009, 28, 3953–3966. [Google Scholar] [CrossRef]
- Shahzadi, S.; Ali, S.; Fettouhi, M. Synthesis, Spectroscopy, in Vitro Biological Activity and X-Ray Structure of (4-Methylpiperidine-Dithiocarbamato-S,S′)Triphenyltin(IV). J. Chem. Crystallogr. 2008, 38, 273–278. [Google Scholar] [CrossRef]
- Zia-Ur-Rehman; Shahzadi, S.; Ali, S.; Jin, G.-X. Preparation, Spectroscopy, Antimicrobial Assay, and X-Ray Structure of Dimethyl Bis-(4-Methylpiperidine Dithiocarbamato-S,S′)-Tin(IV). Turk. J. Chem. 2007, 31, 435–442. [Google Scholar]
- Yadav, R.; Trivedi, M.; Chauhan, R.; Prasad, R.; Kociok-Köhn, G.; Kumar, A. Supramolecular Architecture of Organotin(IV) 4-Hydroxypiperidine Dithiocarbamates: Crystallographic, Computational and Hirshfeld Surface Analyses. Inorg. Chim. Acta 2016, 450, 57–68. [Google Scholar] [CrossRef]
- Bondi, A. Van Der Waals Volumes and Radii. J. Phys. Chem. 1964, 68, 441–451. [Google Scholar] [CrossRef]
- Tiekink, E.R.T. Tin Dithiocarbamates: Applications and Structures. Appl. Organomet. Chem. 2008, 22, 533–550. [Google Scholar] [CrossRef]
- Anthony, E.J.; Bolitho, E.M.; Bridgewater, H.E.; Carter, O.W.L.; Donnelly, J.M.; Imberti, C.; Lant, E.C.; Lermyte, F.; Needham, R.J.; Palau, M.; et al. Metallodrugs Are Unique: Opportunities and Challenges of Discovery and Development. Chem. Sci. 2020, 11, 12888–12917. [Google Scholar] [CrossRef]
- Oun, R.; Moussa, Y.E.; Wheate, N.J. The Side Effects of Platinum-Based Chemotherapy Drugs: A Review for Chemists. Dalton Trans. 2018, 47, 6645–6653. [Google Scholar] [CrossRef]
- Gómez-Ruiz, S.; Kaluderović, G.N.; Prashar, S.; Hey-Hawkins, E.; Erić, A.; Žižak, Ž.; Juranić, Z.D. Study of the Cytotoxic Activity of Di and Triphenyltin(IV) Carboxylate Complexes. J. Inorg. Biochem. 2008, 102, 2087–2096. [Google Scholar] [CrossRef]
- Muhammad, N.; Ahmad, M.; Sirajuddin, M.; Ali, Z.; Tumanov, N.; Wouters, J.; Chafik, A.; Solak, K.; Mavi, A.; Muhammad, S.; et al. Synthesis, Characterization, Biological Activity and Molecular Docking Studies of Novel Organotin(IV) Carboxylates. Front. Pharmacol. 2022, 13, 864336. [Google Scholar] [CrossRef] [PubMed]
- Koch, B.; Basu Baul, T.S.; Chatterjee, A. P53-Dependent Antiproliferative and Antitumor Effect of Novel Alkyl Series of Diorganotin(IV) Compounds. Investig. New Drugs 2009, 27, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Ray, D.; Sarma, K.D.; Antony, A. Differential Effects of Tri-n-Butylstannyl Benzoates on Induction of Apoptosis in K562 and MCF-7 Cells. IUBMB Life 2000, 49, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Wada, O.; Manabe, S.; Iwai, H.; Arakawa, Y. Recent Progress in the Study of Analytical Methods, Toxicity, Metabolism and Health Effects of Organotin Compounds. Sangyo Igaku 1982, 24, 24–54. [Google Scholar] [CrossRef] [PubMed]
- Nath, M. Toxicity and the Cardiovascular Activity of Organotin Compounds: A Review. Appl. Organomet. Chem. 2008, 22, 598–612. [Google Scholar] [CrossRef]
- Adeyemi, J.O.; Olasunkanmi, L.O.; Fadaka, A.O.; Sibuyi, N.R.S.; Oyedeji, A.O.; Onwudiwe, D.C. Synthesis, Theoretical Calculation, and Biological Studies of Mono-and Diphenyltin(IV) Complexes of N-Methyl-N-Hydroxyethyldithiocarbamate. Molecules 2022, 27, 2947. [Google Scholar] [CrossRef]
- How, F.N.-F.; Crouse, K.A.; Tahir, M.I.M.; Tarafder, M.T.H.; Cowley, A.R. Synthesis, Characterization and Biological Studies of S-Benzyl-β-N-(Benzoyl) Dithiocarbazate and Its Metal Complexes. Polyhedron 2008, 27, 3325–3329. [Google Scholar] [CrossRef]
- Khalilov, R. A Comprehensive Review of Advanced Nano-Biomaterials in Regenerative Medicine and Drug Delivery. Adv. Biol. Earth Sci. 2023, 8, 5–18. [Google Scholar]
- Keskin, C.; Ölçekçi, A.; Baran, A.; Baran, M.F.; Eftekhari, A.; Omarova, S.; Khalilov, R.; Aliyev, E.; Sufianov, A.; Beilerli, A.; et al. Green Synthesis of Silver Nanoparticles Mediated Diospyros Kaki L. (Persimmon): Determination of Chemical Composition and Evaluation of Their Antimicrobials and Anticancer Activities. Front. Chem. 2023, 11, 1187808. [Google Scholar] [CrossRef]
- Paredes, K.O.; Díaz-García, D.; García-Almodóvar, V.; Chamizo, L.L.; Marciello, M.; Díaz-Sánchez, M.; Prashar, S.; Gómez-Ruiz, S.; Filice, M. Multifunctional Silica-Based Nanoparticles with Controlled Release of Organotin Metallodrug for Targeted Theranosis of Breast Cancer. Cancers 2020, 12, 187. [Google Scholar] [CrossRef] [PubMed]
- van der Koog, L.; Gandek, T.B.; Nagelkerke, A. Liposomes and Extracellular Vesicles as Drug Delivery Systems: A Comparison of Composition, Pharmacokinetics, and Functionalization. Adv. Healthc. Mater. 2022, 11, e2100639. [Google Scholar] [CrossRef] [PubMed]
- Corvo, M.L.; Mendo, A.S.; Figueiredo, S.; Gaspar, R.; Larguinho, M.; Guedes da Silva, M.F.C.; Baptista, P.V.; Fernandes, A.R. Liposomes as Delivery System of a Sn(IV) Complex for Cancer Therapy. Pharm. Res. 2016, 33, 1351–1358. [Google Scholar] [CrossRef]
- Galanski, M.S.; Jakupec, M.A.; Keppler, B.K. Update of the Preclinical Situation of Anticancer Platinum Complexes: Novel Design Strategies and Innovative Analytical Approaches. Curr. Med. Chem. 2005, 12, 2075–2094. [Google Scholar] [CrossRef]
- Iqbal, H.; Ali, S.; Shahzadi, S. Anti-Inflammatory and Acute Toxicity Study of Organotin (IV) Complexes: A Review. Chem. J. 2016, 6, 59–73. [Google Scholar]
- Iqbal, M.; Ali, S.; Haider, A.; Khalid, N. Therapeutic Properties of Organotin Complexes with Reference to Their Structural and Environmental Features. Rev. Inorg. Chem. 2017, 37, 51–70. [Google Scholar] [CrossRef]
- Ahmad Shah, S.S.; Ashfaq, M.; Waseem, A.; Ahmed, M.M.; Najam, T.; Shaheen, S.; Rivera, G. Synthesis and Biological Activities of Organotin(IV) Complexes as Antitumoral and Antimicrobial Agents. A Review. Mini Rev. Med. Chem. 2015, 15, 406–426. [Google Scholar] [CrossRef]
- Adokoh, C.K. Therapeutic Potential of Dithiocarbamate Supported Gold Compounds. RSC Adv. 2020, 10, 2975–2988. [Google Scholar] [CrossRef]
- Cattaruzza, L.; Fregona, D.; Mongiat, M.; Ronconi, L.; Fassina, A.; Colombatti, A.; Aldinucci, D. Antitumor Activity of Gold(III)-Dithiocarbamato Derivatives on Prostate Cancer Cells and Xenografts. Int. J. Cancer 2011, 128, 206–215. [Google Scholar] [CrossRef]
- Kamaludin, N.F.; Ismail, N.; Awang, N.; Mohamad, R.; Pim, N.U. Cytotoxicity Evaluation and the Mode of Cell Death of K562 Cells Induced by Organotin (IV) (2-Methoxyethyl) Methyldithiocarbamate Compounds. J. Appl. Pharm. Sci. 2019, 9, 10–15. [Google Scholar] [CrossRef]
- Jakšić, Ž. Mechanisms of Organotin-Induced Apoptosis. In Biochemical and Biological Effects of Organotins; Bentham Science: Sharjah, United Arab Emirates, 2012; pp. 149–163. [Google Scholar]
- Kumari, R.; Jat, P. Mechanisms of Cellular Senescence: Cell Cycle Arrest and Senescence Associated Secretory Phenotype. Front. Cell Dev. Biol. 2021, 9, 645593. [Google Scholar] [CrossRef]
- Basu, A.; Krishnamurthy, S. Cellular Responses to Cisplatin-Induced DNA Damage. J. Nucleic Acids 2010, 2010, 201367. [Google Scholar] [CrossRef]
- Yusof, E.N.M.; Latif, M.A.M.; Tahir, M.I.M.; Sakoff, J.A.; Simone, M.I.; Page, A.J.; Veerakumarasivam, A.; Tiekink, E.R.T.; Ravoof, T.B.S.A. O-Vanillin Derived Schiff Bases and Their Organotin(Iv) Compounds: Synthesis, Structural Characterisation, in-Silico Studies and Cytotoxicity. Int. J. Mol. Sci. 2019, 20, 854. [Google Scholar] [CrossRef] [PubMed]
- Arjmand, F.; Parveen, S.; Tabassum, S.; Pettinari, C. Organo-Tin Antitumor Compounds: Their Present Status in Drug Development and Future Perspectives. Inorganica Chim. Acta 2014, 423, 26–37. [Google Scholar] [CrossRef]
- Devi, J.; Pachwania, S. Recent Advancements in DNA Interaction Studies of Organotin(IV) Complexes. Inorg. Chem. Commun. 2018, 91, 44–62. [Google Scholar] [CrossRef]
- Sirajuddin, M.; Ali, S.; Haider, A.; Shah, N.A.; Shah, A.; Khan, M.R. Synthesis, Characterization, Biological Screenings and Interaction with Calf Thymus DNA as Well as Electrochemical Studies of Adducts Formed by Azomethine [2-((3,5-Dimethylphenylimino)Methyl)Phenol] and Organotin(IV) Chlorides. Polyhedron 2012, 40, 19–31. [Google Scholar] [CrossRef]
- Hussain, S.; Ali, S.; Shahzadi, S.; Tahir, M.N.; Shahid, M. Synthesis, Characterization, Single Crystal XRD and Biological Screenings of Organotin(IV) Derivatives with 4-(2-Hydroxyethyl)Piperazine-1-Carbodithioic Acid. J. Coord. Chem. 2016, 69, 687–703. [Google Scholar] [CrossRef]
- Liu, K.; Yan, H.; Chang, G.; Li, Z.; Niu, M.; Hong, M. Organotin(IV) Complexes Derived from Hydrazone Schiff Base: Synthesis, Crystal Structure, in Vitro Cytotoxicity and DNA/BSA Interactions. Inorganica Chim. Acta 2017, 464, 137–146. [Google Scholar] [CrossRef]
- Shaheen, F.; Sirajuddin, M.; Ali, S.; Zia-ur-Rehman; Dyson, P.J.; Shah, N.A.; Tahir, M.N. Organotin(IV) 4-(Benzo[d][1,3]Dioxol-5-Ylmethyl)Piperazine-1-Carbodithioates: Synthesis, Characterization and Biological Activities. J. Organomet. Chem. 2018, 856, 13–22. [Google Scholar] [CrossRef]













| Complex | Yield (%) | Melting Point (°C) | Elemental Analysis (%) (Calculated) | References | |||
|---|---|---|---|---|---|---|---|
| C | H | N | S | ||||
| Dimethyltin (IV) methylcyclohexyldithiocarbamate | 89 | 147.9–148.8 | 40.66 (41.14) | 6.46 (6.48) | 5.30 (5.33) | 26.43 (24.38) | [77] |
| Dibutyltin (IV) methylcyclohexyldithiocarbamate | 83 | 122.6–124.0 | 47.15 (47.29) | 8.08 (7.55) | 4.62 (4.60) | 22.72 (21.02) | |
| Triphenyltin (IV) methylcyclohexyldithiocarbamate | 76 | 136.8–138.2 | 57.71 (57.99) | 4.98 (5.39) | 2.57 (2.60) | 11.26 (11.90) | |
| Diphenyltin (IV) N-butyl-N-phenyldithiocarbamate | 82.1 | 102.1–104.0 | 56.15 (56.59) | 5.89 (5.31) | 3.77 (3.88) | 16.81 (17.77) | [54] |
| Triphenyltin (IV) N-butyl-N-phenyldithiocarbamate | 34.0 | 101.0–102.0 | 60.54- (60.64) | 5.08 (5.09) | 2.34 (2.44) | 11.31 (11.17) | |
| Dibutyltin (IV) N-ethyl-N-phenyldithiocarbamate | 84.5 | 126.9–128.2 | 50.72 (49.92) | 7.47 (6.12) | 4.22 (4.48) | 20.26 (20.50) | [65] |
| Di-tert-butyltin (IV) N-ethyl-N-phenyldithiocarbamate | 51.6 | 124.8–126.5 | 48.19 (49.92) | 7.34 (6.12) | 4.13 (4.48) | 20.10 (20.50) | |
| Diphenyltin (IV) N-ethyl-N-phenyldithiocarbamate | 74.8 | 70.2–72.9 | 54.13 (54.14) | 4.49 (4.54) | 4.27 (4.21) | 18.28 (19.27) | |
| Triphenyltin (IV) N-methylbutyldithiocarbamate | 78 | 88.3–90.8 | 55.70 (56.27) | 4.91 (5.31) | 2.34 (2.73) | 11.91 (12.52) | [66] |
| Dibutyltin (IV) methoxyethyldithiocarbamate | 76 | 68–69 | 41.76 (40.77) | 6.07 (7.14) | 4.91 (4.31) | 19.25 (19.75) | [84] |
| Triphenyltin (IV) methoxyethyldithiocarbamate | 89 | 93–94 | 54.38 (53.76) | 4.38 (5.24) | 2.87 (2.51) | 12.13 (11.49) | |
| Dibutyltin (IV) (2-methoxyethyl)methyl dithiocarbamates | 66.31 | 59.7–62.9 | 40.26 (38.50) | 7.33 (6.82) | 4.98 (5.04) | 23.65 (22.84) | [85] |
| Diphenyltin (IV) (2-methoxyethyl)methyl dithiocarbamates | 78 | 109.1–111.1 | 44.70 (43.93) | 5.12 (5.03) | 4.26 (4.67) | 20.24 (21.33) | |
| Tricyclohexyltin (IV) (2-methoxyethyl)methyl dithiocarbamates | 76.39 | 102.7–104.4 | 52.40 (51.89) | 8.05 (8.14) | 2.53 (2.63) | 12.47 (12.05) | |
| Dimethyltin (IV) N-ethyl-N-phenyl dithiocarbamates | 82 | 167–169 | 43.90 (44.37) | 5.01 (4.84) | 4.99 (5.17) | 23.99 (23.69) | [80] |
| Dibuthyltin (IV) N-ethyl-N-phenyl dithiocarbamates | 87 | 121–122 | 48.42 (49.92) | 5.81 (6.12) | 5.48 (4.48) | 21.50 (20.50) | |
| Compound | ν(C---N) | ν(C---S) | ν(Sn-C) | ν(Sn-S) | References |
|---|---|---|---|---|---|
| (CH3)2Sn[S2CN(CH3) (C6H11)]2 | 1475 | 974 | 554 | 357 | [77] |
| (C4H9)2Sn[S2CN(CH3) (C6H11)]2 | 1459 | 975 | 532 | 359 | |
| (C6H5)3 Sn[S2CN(CH3) (C6H11)] | 1478 | 979 | 261 | 349 | |
| (C4H9)2Sn[S2CN(CH3)(C6H11)]2 | 1478 | 979 | - | 375 | [68] |
| (C4H9)2Sn[S2CN(iC3H7)(C6H11)]2 | 1479 | 978 | - | 389 | |
| (C4H9)2Sn[S2CN(C4H9)(C6H5)]2 | 1487.42 | 951.14 | 567.97 | - | [54] |
| (C6H5)2Sn[S2CN(C4H9)(C6H5)]2 | 1457.81 | 996.86 | 258.69 | - | |
| (C6H5)ClSn[S2CN(CH3)(C2H5) ]2 | 1511 | 997, 957 | - | 318 | [86] |
| (CH3)ClSn[S2CN(CH3)(C2H5)]2 | 1519 | 995, 957 | - | 349 | |
| (C4H9)2Sn[S2CN(C2H5)(C6H5)]2 | 1488.72 | 1003.50 | 554.14 | 385.96 | [65] |
| (C6H5)2Sn[S2CN(C2H5)(C6H5)]2 | 1490.73 | 1000.62 | 256.21 | 390.23 | |
| (C6H5)3Sn[S2CN(C2H5)(C6H5)] | 1478.64 | 996.50 | 261.09 | 384.06 | |
| Bu2Sn[C16H34NCS2]2 | 1464 | 1025, 963 | 563 | 412 | [66] |
| Ph3SnS2CNC5H12 | 1497 | 983 | 605, 567 | 445 | |
| C20H26N2S4Sn | 1461 | 1006 | 551 | 450 | [80] |
| C26H38N2S4Sn | 1488 | 1020 | 553 | 447 | |
| C30H30N2S4Sn | 1462 | 1003 | 552 | 444 | |
| (CH3)2Sn(L)2 | 1503 | 981 | 507 | 451 | [53] |
| (C4H9)2Sn(L)2 | 1487 | 1008 | 512 | 440 | |
| (C6H5)2Sn(L)2 | 1478 | 996 | 510 | 443 | |
| (C6H5)2Sn[S2CN(C3H5)2]2 | 1475.54 | 977.91 | 545.85 | 441.70 | [44] |
| (C6H5)3Sn[S2CN(C3H5)2] | 1479.40 | 972.12 | 555.50 | 445.56 |
| Compound | IC50 Values (μM) | Tumor Cell Lines | References |
|---|---|---|---|
| Dibutyltin (IV) N-butyl-N-phenyldithiocarbamate | 0.8 | Jurkat E6.1 | [54] |
| Diphenyltin (IV) N-butyl-N-phenyldithiocarbamate | 1.3 | ||
| Triphenyltin (IV) N-butyl-N-phenyldithiocarbamate | 0.4 | ||
| Doxorubicin hydrochloride (control) | 0.1 | ||
| Dibutyltin (IV) N-butyl-N-phenyldithiocarbamate | 5.3 | K-562 | |
| Diphenyltin (IV) N-butyl-N-phenyldithiocarbamate | 9.2 | ||
| Triphenyltin (IV) N-butyl-N-phenyldithiocarbamate | 1.9 | ||
| Doxorubicin hydrochloride (control) | 11.0 | ||
| Triphenyltin (IV) benzylisopropyldithiocarbamate | 0.18 | Jurkat E6.1 | [35] |
| Triphenyltin (IV) methylisopropyldithiocarbamate | 0.03 | ||
| Triphenyltin (IV) ethylisopropyldithiocarbamate | 0.42 | ||
| Etoposide (control) | 0.12 | ||
| MeSnClL2 | >4000 | HeLa | [48] |
| BuSnClL2 | 8.12 | ||
| PhSnClL2 | 4.37 | ||
| Me2SnL2 | 12.30 | ||
| Bu2SnL2 | 11.75 | ||
| Ph2SnL2 | 0.01 | ||
| 5-Fluorouracil (control) | 40 | ||
| Dimethyltin (IV) benzyldithiocarbamate | 40 | Hela | [53] |
| Dibutyltin (IV) benzyldithiocarbamate | 0.019 | ||
| Diphenyltin (IV) benzyldithiocarbamate | 330 | ||
| 5-Fluorouracil (control) | 40 | ||
| Dimethyltin (IV) benzyldithiocarbamate | 185 | MCF-7 | |
| Dibutyltin (IV) benzyldithiocarbamate | 57.3 | ||
| Diphenyltin (IV) benzyldithiocarbamate | 20 | ||
| 5-Fluorouracil (control) | 56.2 | ||
| Diphenyltin (IV) diallyldithiocarbamate | 2.36 | HT-29 | [44] |
| Triphenyltin (IV) diallyldithiocarbamate | 0.39 | ||
| Ph3Sn(N,N-diisopropyldithiocarbamate) (OC2) | 0.55 | K562 | [55] |
| Ph3Sn(N,N-diallyldithiocarbamate) (OC4) | 1.1 | ||
| Imatinib mesylate (control) | 34 | ||
| Diphenytin (IV) N-methyl-N-hydroxyethyldithiocarbamate | 1.630 | PC-3 | [150] |
| 4.937 | Caco-2 | ||
| Camptothecin (control) | 24.41 | PC-3 | |
| >100 | Caco-2 | ||
| Triphenyltin (IV) diisopropyldithiocarbamate (ODTC 3) | 0.67 | Jurkat E6.1 | [56] |
| Triphenyltin (IV) diethyldithiocarbamate (ODTC 5) | 0.92 | ||
| Vincristine (control) | 0.24 |
| Category | IC50 Value (μg cm−3) |
|---|---|
| Highly toxic | <5.0 |
| Moderately toxic | 5.0 10.0 |
| Slightly toxic | 10.0–25.0 |
| Non-toxic | >25.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abd Aziz, N.A.; Awang, N.; Chan, K.M.; Kamaludin, N.F.; Mohamad Anuar, N.N. Organotin (IV) Dithiocarbamate Compounds as Anticancer Agents: A Review of Syntheses and Cytotoxicity Studies. Molecules 2023, 28, 5841. https://doi.org/10.3390/molecules28155841
Abd Aziz NA, Awang N, Chan KM, Kamaludin NF, Mohamad Anuar NN. Organotin (IV) Dithiocarbamate Compounds as Anticancer Agents: A Review of Syntheses and Cytotoxicity Studies. Molecules. 2023; 28(15):5841. https://doi.org/10.3390/molecules28155841
Chicago/Turabian StyleAbd Aziz, Nurul Amalina, Normah Awang, Kok Meng Chan, Nurul Farahana Kamaludin, and Nur Najmi Mohamad Anuar. 2023. "Organotin (IV) Dithiocarbamate Compounds as Anticancer Agents: A Review of Syntheses and Cytotoxicity Studies" Molecules 28, no. 15: 5841. https://doi.org/10.3390/molecules28155841
APA StyleAbd Aziz, N. A., Awang, N., Chan, K. M., Kamaludin, N. F., & Mohamad Anuar, N. N. (2023). Organotin (IV) Dithiocarbamate Compounds as Anticancer Agents: A Review of Syntheses and Cytotoxicity Studies. Molecules, 28(15), 5841. https://doi.org/10.3390/molecules28155841







