Abstract
Blueberries are fruits known for their high level of anthocyanins, which have high nutritional value and several biological properties. However, the chemical instability of anthocyanins is one of the major limitations of their application. The stability of blueberry anthocyanin extracts (BAEs) encapsulated in a ferritin nanocarrier was investigated in this study for several influencing parameters, including pH, temperature, UV–visible light, redox agents, and various metal ions. The outcomes supported the positive role of protein nanoparticles in enhancing the stability of blueberry anthocyanins by demonstrating that the stability of encapsulated BAE nanoparticles with ferritin carriers was significantly higher than that of free BAEs and a mixture of BAEs and ferritin carriers. This study provides an alternative approach for enhancing blueberry anthocyanin stability using ferritin nanocarrier encapsulation.
1. Introduction
Blueberries are one of the most popular fruits [1], containing nutrients such as sugar, vitamins, mineral elements, and trace elements, in addition to high-value functional compounds such as anthocyanins [2]. Blueberries have several biological properties, such as preventing brain nerve aging, enhancing the body’s immune system, and protecting eyesight, which might be attributed to the anthocyanins [3,4]. Anthocyanins are the most effective natural water-soluble antioxidants found to date. They have been reported to activate the intracellular antioxidant defense system, protect cells from oxidative damage, and help prevent a variety of free-radical-related diseases, including alleviating metabolic syndrome [5].
However, in addition to the chemical structure of anthocyanins, numerous factors during processing and storage can affect the stability of anthocyanins and cause their degradation, which seriously affects their bioavailability, thereby limiting the large-scale use of blueberry anthocyanins [6]. Anthocyanins, as functional pigments, are susceptible to degrading, leading to the loss of color and biological activity [7,8]. Several environmental factors can significantly affect the stability of anthocyanins, including temperature, light, oxygen, enzymes, and pH, especially a neutral or alkaline pH [9,10,11]. Therefore, improving anthocyanins’ stability is foundational to ensuring that their functional properties are exploited, and has become a crucial issue that requires an urgent solution in the field of functional food research.
In the past few years, different methods have been proposed to improve the stability and bioavailability of anthocyanins, such as microencapsulation, protein complexes, and nanoparticle coating strategies [12,13,14]. Microencapsulation methods usually employ chitosan, carboxymethyl starch, xanthan gum, gum arabic, whey protein, and cyclodextrin capsules, providing a protective effect, improving the stability of anthocyanins, and ensuring their stability and sustained release [15,16,17]. The formation of protein complexes by adding α-casein, β-casein, β-lactoglobulin, whey protein, or soy protein in food matrices can be used as an effective way to delay and reduce the degradation of blueberry anthocyanins, thereby improving their biological stability under different conditions [18,19,20]. Nevertheless, nanoencapsulation processes have shown great potential in improving the effectiveness of delivery and the stability of biological compounds, including anthocyanins [20,21]. The anthocyanin-loaded nanocomposites prepared from chitosan hydrochloride, carboxymethyl chitosan, lactoglobulin, and lecithin cholesterol nanoliposomes exhibited greater stability at different storage temperatures and pH values than free anthocyanins [22,23,24]. In addition, nanocarrier systems for blueberry anthocyanin components can also be developed through co-encapsulation methods, which can more effectively incorporate anthocyanins [25].
Ferritin is an important iron-storage protein that plays a role in regulating the balance of iron metabolism in organisms; its interior stores a large amount of non-uniformly mineralized iron nuclei composed of iron hydroxide and phosphate [26]. The reduction and removal of the internal mineralized iron core results in a protein shell. The reversible dissociation assembly process of ferritin subunits regulated by environmental pH can be used to carry and embed anthocyanins into the ferritin nanocavity reactor [27,28]. Therefore, ferritin was used in this study to construct a protein nanocarrier to encapsulate blueberry anthocyanins, and the in vitro stability of blueberry anthocyanins encapsulated with protein nanocarrier was investigated and compared with the free anthocyanins.
2. Results and Discussion
2.1. Identification of Main Anthocyanin Composition of BAEs
In our previous study [29], the components of blueberry anthocyanins were qualitatively identified as delphinidin-3-O-galactoside, delphinidin-3-O-glucoside, cyanidin-3-O-galactoside, delphinidin-3-O-arabinoside, cyanidin-3-O-glucoside, petunidin-3-O-galactoside, petunidin-3-O-glucoside, peonidin-3-O-galactoside, petunidin-3-O-arabinose, peonidin-3-O-glucoside, malvidin-3-O-galactoside, malvidin-3-O-glucoside, and malvidin-3-O-arabinose (Figure S1), which showed the same elution order with similar content ratios. These 13 anthocyanins were detected in almost all blueberries of different cultivars, but in varying amounts [30].
2.2. The Stability of Blueberry Anthocyanins
2.2.1. Effects of pH, Temperature, Light Type, and Redox Agents on the Stability of BAEs
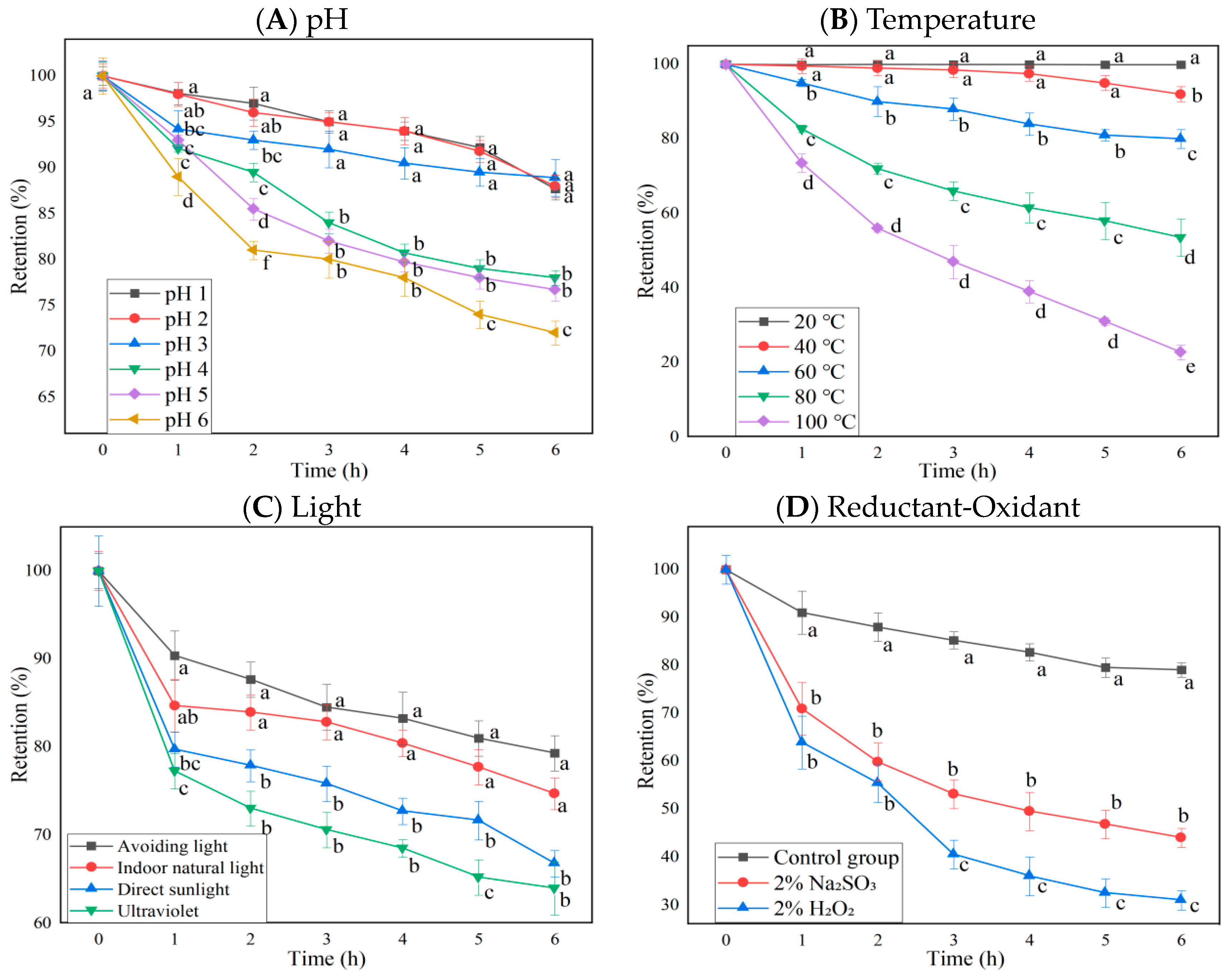
The anthocyanin retention rate changes of BAEs at different pH, temperature, light types, oxidant H2O2, and reductant Na2SO3 during six-hour storage are shown in Figure 1. The results indicated that pH value, temperature, light type, oxidant/reductant regent, and storage time were significant influencing factors on anthocyanin retention rates (p < 0.0001 for row and column factors), while their interaction was also significant (p < 0.0001). Blueberry anthocyanins were more stable in strongly acidic conditions (pH ≤ 3), since BAEs remained at 88.2 ± 1.2% retention rates after 6 h of storage. However, in the weakly acidic environment of pH 4–6, blueberry anthocyanins degrade much faster and more significantly, reaching 78.0 ± 0.8, 76.7 ± 1.2, and 72.0 ± 1.3% retention rates, respectively, after 6 h (Figure 1A). This is consistent with the findings reported by Lv et al. [31] and Zhang et al. [32], who studied the effect of pH value on the content of strawberry pigment and black bean skin anthocyanins, finding the stability of anthocyanins gradually decreased with the increase in pH values. Different pH conditions can change the molecular structure of anthocyanins, leading to color changes [12]. When pH < 2, anthocyanins exist as red to orange flavylium cations; from pH 3 to 6, anthocyanins exist as colorless chalcones or carbinal pesudobases; and when pH > 7, they change to purple to violet or blue quinonoidal bases. Due to different structural forms, the retention rate of anthocyanins varies.
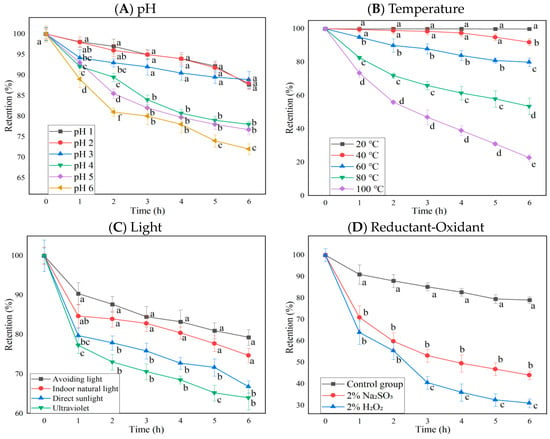
Figure 1.
The influence of pH, temperature, light, and redox agents on the retention rate of blueberry anthocyanin extracts (BAEs) during six-hour storage. (A) pH: pH 1, 2, 3, 4, 5, and 6; (B) temperature: 20, 40, 60, 80, and 100 °C; (C) light: no light, indoor natural light, direct sunlight, ultraviolet light; (D) redox agent: 2% Na2SO3 and 2% H2O2. BAEs without an added redox agent were used as a control group. Data are represented as the mean ± standard deviation (SD, n = 3). Different lowercase letters indicate significant differences in BAE retention rate with different treatments at the same storage time (p < 0.05).
Blueberry anthocyanins were more stable in low-temperature conditions (temperature ≤ 40 °C) since BAEs still maintained a retention rate of 97.5 ± 2.0% after 4 h of storage and only decreased by 8.0 ± 2.0% after 6 h. In a high-temperature environment, the degradation rate of blueberry anthocyanins was faster and the retention rates dropped, reaching 80.0 ± 2.5, 53.5 ± 5.0, and 22.7 ± 2.0% after 6 h at 60, 80, and 100 °C, respectively, with significant differences among them (p < 0.05, Figure 1B). Zhang et al. [33] found that the loss rate of mulberry anthocyanins reached 100% after treatment at 60 °C for 72 h. Changes in temperature over time resulted in anthocyanins converting to the chalcone structure, thereby decreasing the retention rate of anthocyanins [34]. Our results were consistent with the previous findings, regarding the effect of pH and temperature on strawberry and black bean skin anthocyanins [31,32]. Therefore, it is recommended that blueberry anthocyanins should be extracted, purified, processed, and preserved in an acidic and low-temperature environment.
Blueberry anthocyanins were most stable in dark conditions, with a retention rate of about 79.3 ± 2.0% after storage for 6 h, followed by indoor natural light (74.7 ± 1.8%). The anthocyanin retention rate under direct sunlight (66.7 ± 1.5%) and UV light (63.9 ± 3.0%) conditions was significantly lower than under no light or indoor natural light conditions (p < 0.05, Figure 1C). Du et al. [35] and Li et al. [36] also found anthocyanidins from Vitis amurensis and Sorbus nigricans had higher stability in the dark. This might be caused by light triggering a series of free radical reactions in singlet or triplet anthocyanins, leading to the degradation of anthocyanins [37]. Therefore, it is preferable to store blueberry anthocyanins in darkness to prevent their photodegradation.
The retention rates of blueberry anthocyanins in 2% H2O2 and 2% Na2SO3 solutions showed significant differences from the control (p < 0.05, Figure 1D). Under the action of H2O2, the BAE retention rate was only about 30% after 6 h. This might be because hydrogen peroxide can attack the C2 on the anthocyanin molecule and break its C3 bond, leading to the cleavage of the pyran ring, generating ester compounds and coumarin derivatives, destroying the structure of anthocyanins and reducing their stability [38]. Under the action of the reducing agent Na2SO3, the BAE retention rate was higher than that of the oxidant H2O2 (p < 0.05), but it also declined. After 6 h, the BAE retention rate was about 44.0 ± 2.0% (lower than the control, p < 0.05), which might be related to the bleaching property of SO32−. Na2SO3 is prone to generating SO2, bleaching free anthocyanins [39]. The retention rate of anthocyanins decreased rapidly when different concentrations of hydrogen peroxide and sodium sulfite were added, which was consistent with a previous report by Li et al. [36].
Studies show that the stability of anthocyanidin boosted the anticancer activity of blueberry anthocyanidin in HeLa cells [40]. The retention time of blueberry anthocyanidin in vivo was extended following chitosan modification, and its antioxidant activity was enhanced, according to in vitro release and digestion simulations [41].
2.2.2. Effects of Different Metal Ions and Concentrations on the Stability of BAEs
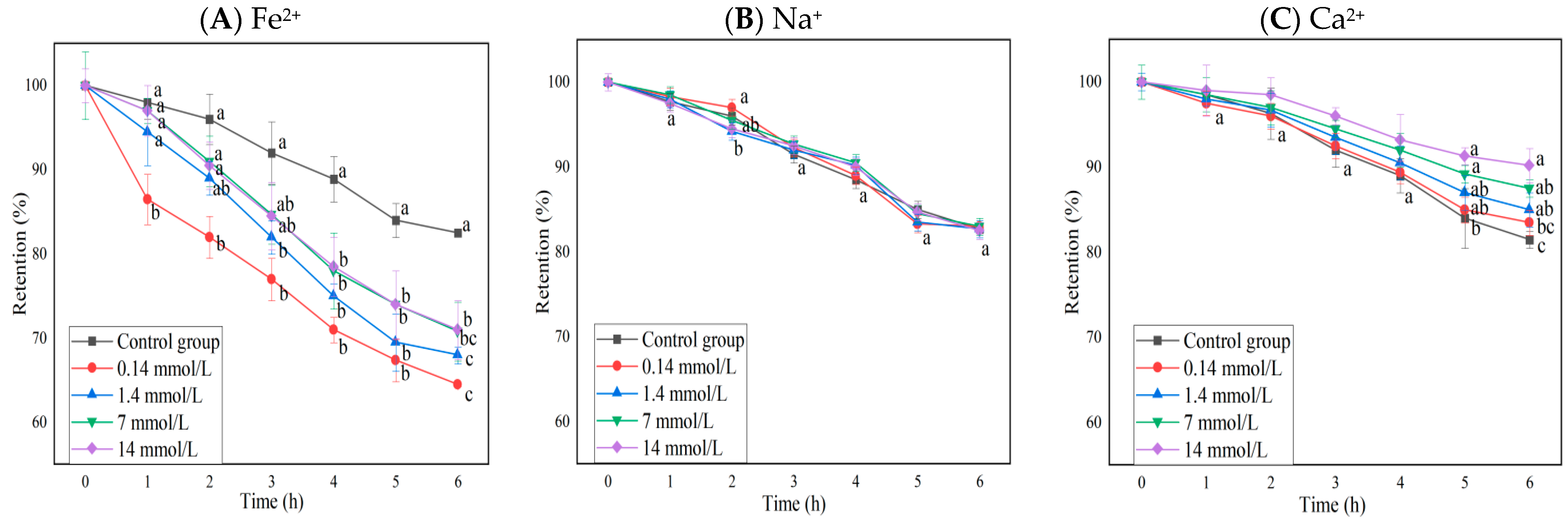
The changes in the anthocyanin retention rate of BAEs in different ion solutions at different concentrations during six-hour storage are shown in Figure 2. Fe2+ and Ca2+ had significant effects on anthocyanin retention rates (p < 0.0001), but Na+ did not (p > 0.05), and interactions between rows and columns were not significant (p > 0.05). The presence of Fe2+ decreased the retention rate of blueberry anthocyanins significantly (p < 0.05), and the retention rate of BAEs treated with a low concentration of FeSO4 decreased more rapidly than that treated with a high concentration. Furthermore, with the increase in Fe2+ concentration, the decrease in BAE retention rate became slow and weak but still greater than that of the control group (p < 0.05), whereas the presence of Ca2+ appeared to be beneficial for maintaining the stability of BAEs. Only high concentrations of CaCl2 (≥7 mmol/L) could significantly increase the retention rate of blueberry anthocyanins during long storage periods (>5 h). Guo et al. [42] also reported that Fe2+ had a significant fading effect on purple cabbage anthocyanins, while Zhang et al. [43] and Li et al. [44] found that Ca2+ had the effect of protecting and stabilizing anthocyanin extracts during the extraction process, which was consistent with our results for blueberry anthocyanins.
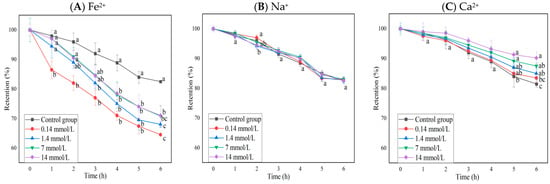
Figure 2.
The influence of different ions and concentrations (0.14, 1.4, 7, 14 mmol/L) on the retention rate of blueberry anthocyanin extracts (BAEs) during six-hour storage. (A) Fe2+; (B) Na+; (C) Ca2+. BAEs without added ions were used as a control group. Data are represented as the mean ± standard deviation (SD, n = 3). Different lowercase letters indicate significant differences in BAE retention rate with different treatments at the same storage time (p < 0.05).
2.3. The Improvement of BAE Stability using Ferritin Nanocarriers
2.3.1. Examination of Encapsulated BAE Nanoparticles with Ferritin Carriers
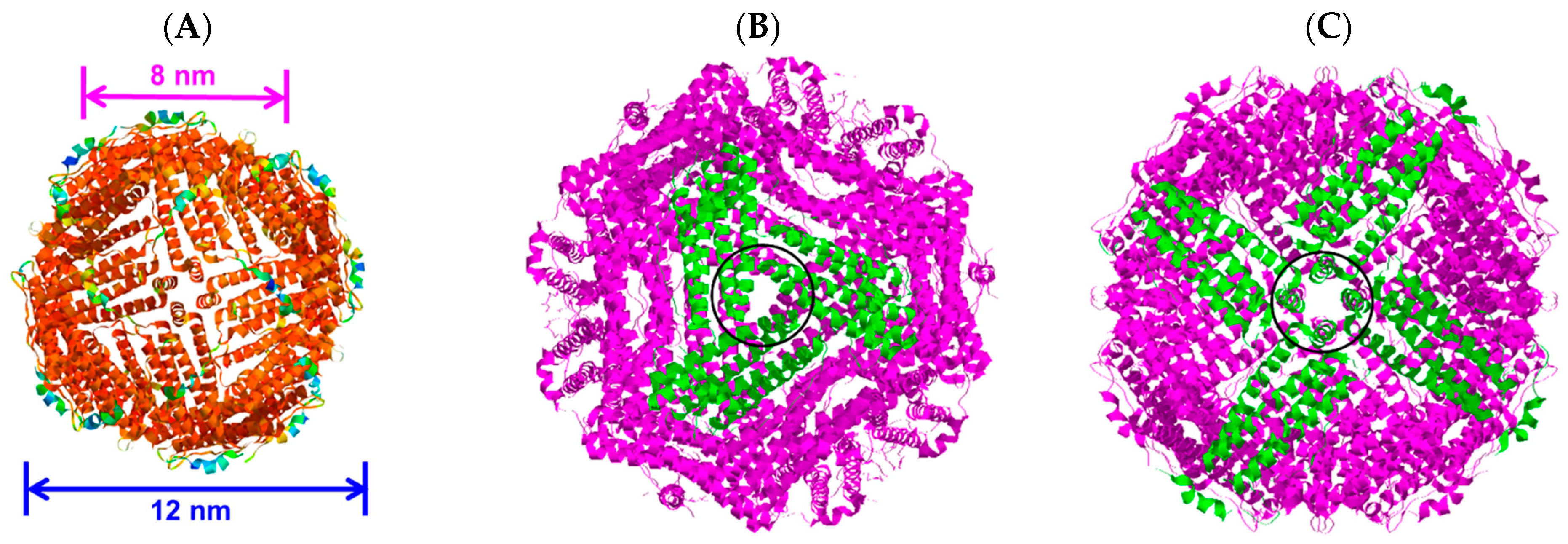
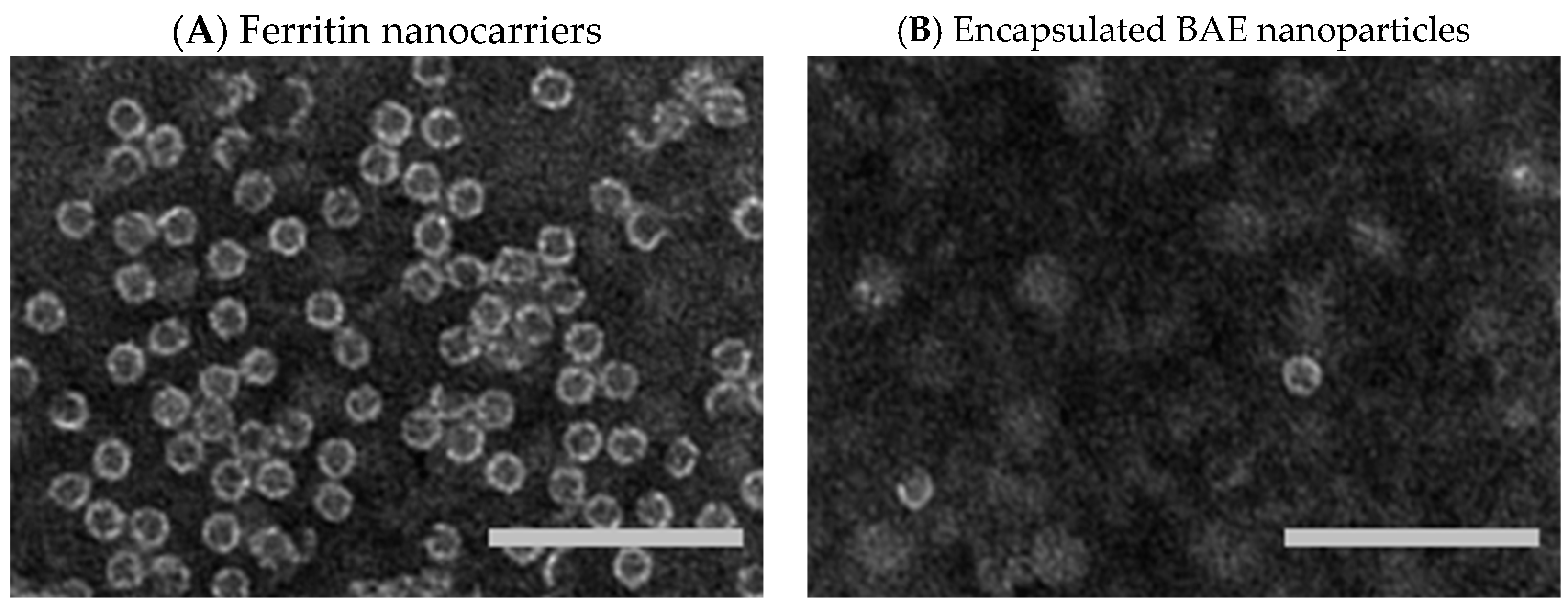
The protein ferritin is present in humans, plants, and other organisms. It oversees iron storage and elimination. Iron homeostasis can be maintained in a soluble, non-toxic, bioavailable form [45,46]. The ferritin shell is a highly symmetric dodecahedral hollow spherical molecule composed of 24 subunits with a particle size of 12 nm and an 8 nm cavity (Figure 3A, ferritin structure was drawn by RasMol 2.6. The X-ray crystallographic structure of the ferritin was obtained from the Protein Data Bank (http://www.rcsb.org/pdb, accessed on 27 July 2023)), 8 triple axis channels (Figure 3B), and 6 quadruple axis channels (Figure 3C), which provides a structural basis for the carrier to perform its embedding and delivery functions. Some nanocarriers are difficult to obtain, but ferritin nanocarriers can be produced in Escherichia coli, which has cost advantages and high feasibility and can be used to obtain heat-resistant purified products [47]. To date, ferritin has been used to encapsulate anthocyanins to maintain their stability [48]. In this study, ferritin nanocarriers were successfully obtained by removing ferrous ions. A transmission electron microscope (TEM) image showed that ferritin carriers were mostly spherical, with a hollow structure in the center of the protein shell and a protein carrier particle diameter of about 15 nm (Figure 4A), which was a nanocarrier. The ferritin shell had some channels formed by ferritin subunits and subunits spiraling along the rotation axis in the dodecahedral structure of the ferritin shell nanocavity. These channels connect the internal cavity of ferritin with the external environment, with a pore diameter of about 0.5 nm, which is necessary for metal ions, oxygen, and small molecules to enter and exit the ferritin cavity [49,50]. After embedding BAEs, the encapsulated BAE nanoparticles were observed using a TEM. It could be seen from the image that the central cavity of the protein carrier disappeared and a high number of anthocyanins were embedded in the central cavity (Figure 4B).
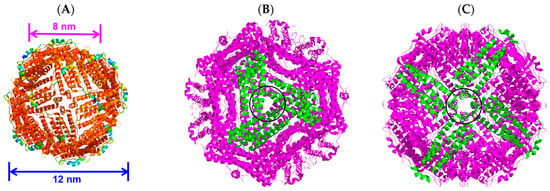
Figure 3.
The three-dimensional structure of ferritin molecules: (A) ferritin shell; (B) triple axis channel; and (C) quadruple axis channel.
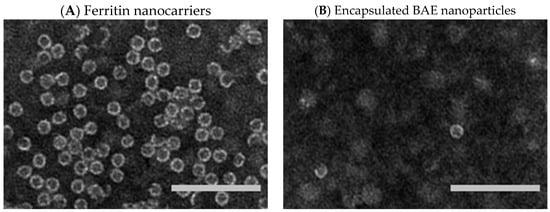
Figure 4.
Transmission electron microscope images. (A) ferritin nanocarriers; and (B) encapsulated blueberry anthocyanin extract (BAE) nanoparticles. Scale bar at 100 nm.
2.3.2. Effects of Ferritin Nanocarriers on the Stability of BAEs
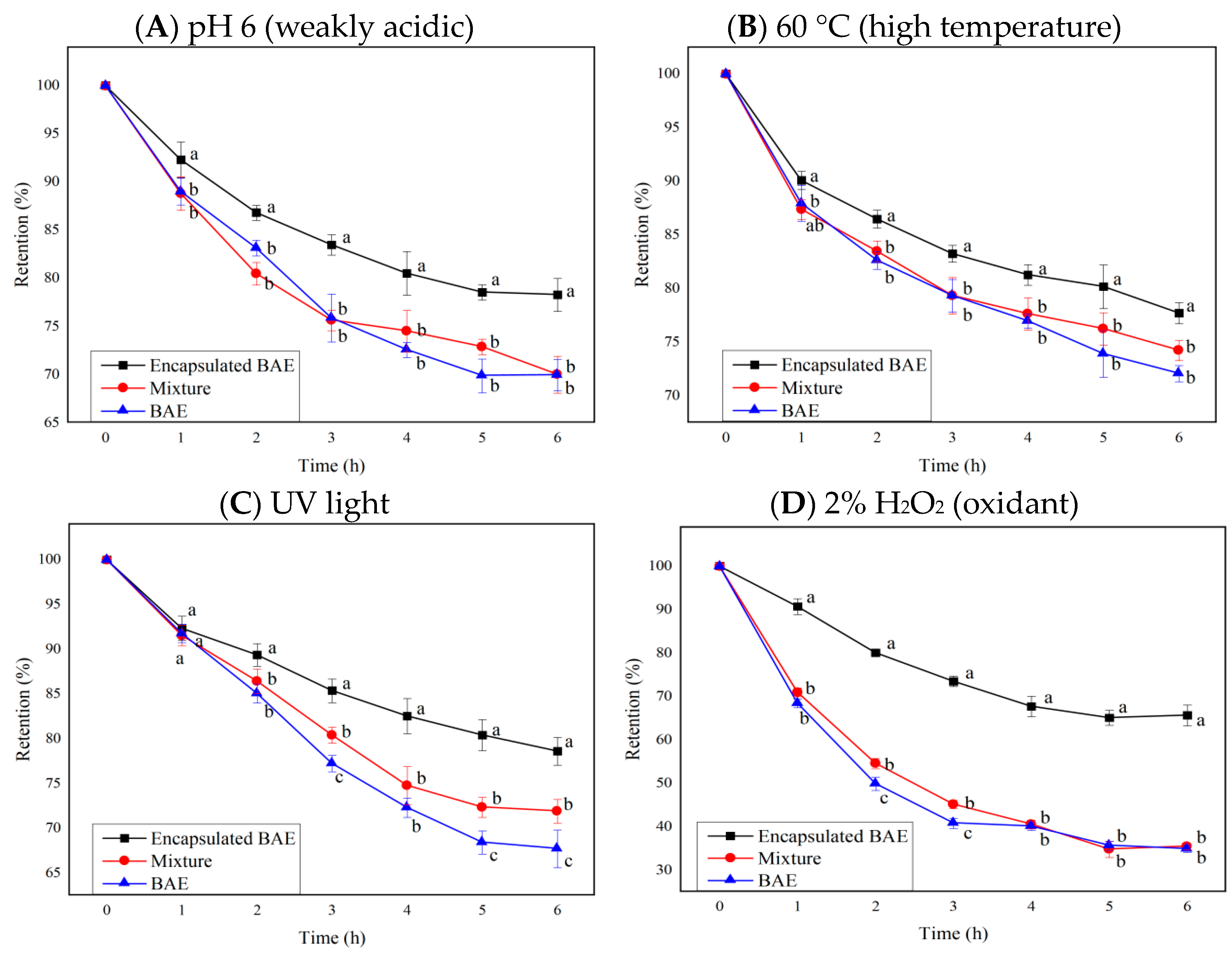
Tests on the stability of blueberry anthocyanins revealed that numerous exogenous factors, such as pH value, temperature, light radiation, ionic strength, oxidants, and reducing agents, affect the stability of anthocyanins and cause their degradation. Based on the most influential indicators in stability analysis, the stability of encapsulated BAE nanoparticles was tested and compared with that of free BAE and the unencapsulated mixture of BAEs and ferritin carriers at pH 6, 60 °C, and 2% hydrogen peroxide, and under ultraviolet light. The change curves of the retention rate of free anthocyanins, a simple mixture of carrier and anthocyanin molecules, and encapsulated BAE nanoparticles are shown in Figure 5, where results showed p < 0.0001 for interaction, row, and column factors, except interaction p > 0.05 in Figure 5B.
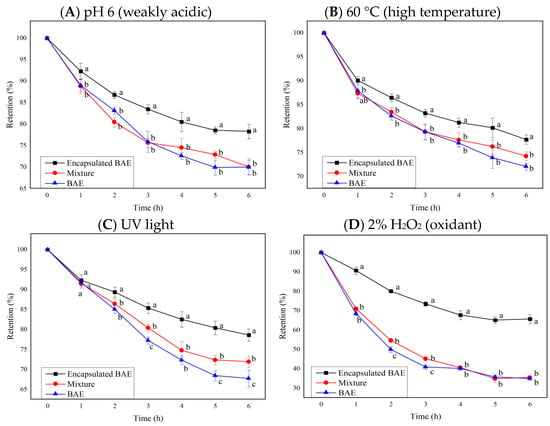
Figure 5.
The influence of ferritin nanocarriers (encapsulated BAE nanoparticles and the unencapsulated mixture) on the retention rate of blueberry anthocyanin extracts (BAEs) during six-hour storage. (A) pH 6; (B) 60 °C; (C) ultraviolet (UV) light; (D) 2% H2O2. Data are represented as the mean ± standard deviation (SD, n = 3). Different lowercase letters indicate significant differences in BAE retention rate with different treatments at the same storage time (p < 0.05).
The anthocyanin retention rate of encapsulated BAE nanoparticles was significantly greater than that of free BAEs and a simple mixture of BAEs with ferritin carriers (p < 0.05 vs. mixture and BAEs) during six-hour storage in weakly acidic, high temperature, UV light, and oxidant regent conditions. However, there was no significant difference between the mixture and BAEs under most conditions (p > 0.05). Mixing with ferritin carriers improved the stability of BAEs under UV light for some time (p < 0.05 vs. BAEs at 3, 5, 6 h). After 6 h of storage, encapsulation with ferritin nanocarriers could inhibit 27.7 ± 6.4, 20.2 ± 3.4, 33.7 ± 4.1, and 47.2 ± 1.1% degradation of BAEs under the conditions of pH 6, 60 °C, UV light, and 2% H2O2, respectively. Therefore, the encapsulated BAE nanoparticles possessed better stability, protecting anthocyanins from degradation under weakly acidic pH, high temperature, light radiation, and oxidant conditions. Previous reports also found that embedding resveratrol in ferritin nanoparticles could improve their photothermal stability and antioxidant activity [51]. The degradation rate of astaxanthin could be reduced from about 40% to 3% by preparing ferritin-astaxanthin-embedded materials, demonstrating the feasibility of using nano-ferritin to improve the stability of small molecule compounds [52]. In this study, the stability of blueberry anthocyanins was successfully improved by embedding BAEs into ferritin nanocarriers, improving the pH stability, heat resistance, light resistance, and antioxidant reduction properties. Ferritin nanocarriers had a certain protective effect on anthocyanins, which might be attributable to the protein shell of ferritin isolating the interference of adverse external environmental factors on the internal anthocyanin molecules [48]. Simultaneously, it was speculated that the amino acid residues in the cavity of ferritins could also interact with anthocyanin molecules, stabilizing and protecting them [24].
3. Materials and Methods
3.1. Materials, Chemicals, and Reagents
The blueberry anthocyanins were extracted from Rabbiteye blueberries (Lishui Town, Nanjing, China) [53] and stored at −18 °C at the Institute of Agricultural Products Processing, Chinese Academy of Agricultural Sciences. High-performance liquid chromatography (HPLC) solvents potassium chloride and acetonitrile were acquired from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China) and TEDIA (Fairfield, OH, USA), respectively. Ferritin (from horse spleen), MOPS buffer, 2,2-bipyridine, and bovine serum protein (BSA) were procured from Sigma-Aldrich (Shanghai, China). The bicinchoninic acid (BCA) and protein-assay kits were purchased from Beijing Solarbio Company (Beijing, China). The reagents used in the experiment are all of analytical grade.
3.2. HPLC Analysis
The composition of blueberry anthocyanin extracts (BAEs) was analyzed according to our previous method using HPLC analysis performed on an Agilent-1200 (Agilent Technologies, Santa Clara, CA, USA) with an XDB-C18 column (250 mm × 4.6 mm, 5μm) [29]. Mobile phase A was 1% phosphoric acid buffer, while mobile phase B was acetonitrile. Gradient elution was conducted with a 20 μL injection volume and a 0.6 mL/min flow rate. After 90 min of elution, anthocyanins were detected at 520 nm and identified by comparing them with previously reported literature [30].
3.3. Preparation of Encapsulated BAEs with the Protein Nanocarrier
The protein nanocarrier was obtained from horse ferritin. Under anaerobic conditions, a ferritin carrier (1 mM) with sodium bisulfite (3% w/v) in 50 mM Mops buffer (pH 7.0) was prepared; therefore, iron ions were reduced to ferrous ions, and ferrous ions were removed using dialysis. Furthermore, 1 mM 2,2-bipyridine was used to precipitate the residual ferrous ions and was centrifuged at 5000 rpm to remove the precipitate. The obtained supernatant was dialyzed three times with a buffer solution to remove excess 2,2-bipyridine. With BSA as the standard, the concentration of the target protein nanocarrier was calculated using an ultraviolet spectrophotometer and the Lowry method [54]. The carrier solution obtained was kept at 4 °C before use. The mixed BAE was obtained by mixing it directly with the carrier solution.
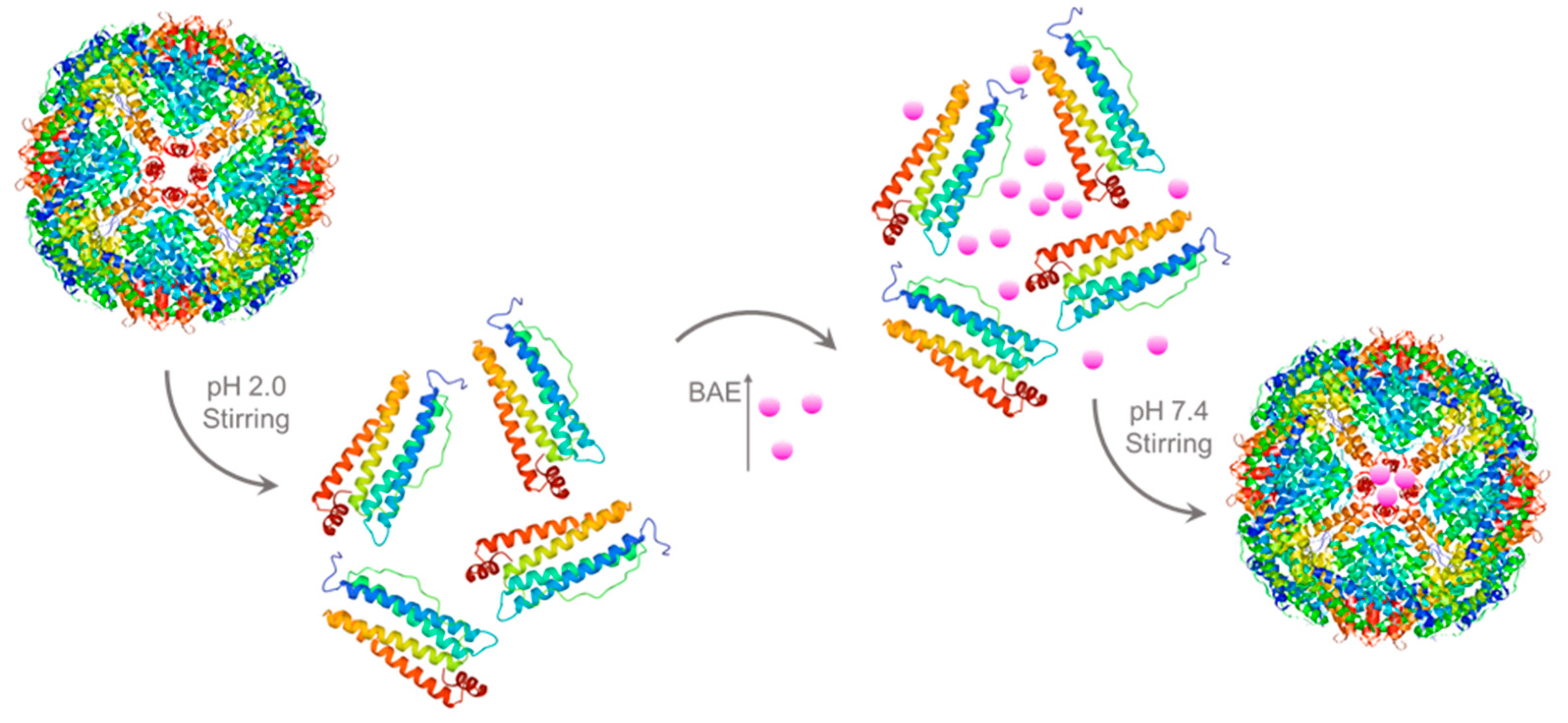
For encapsulated BAEs, the ferritin solution (2 μM) was adjusted to pH 2.0 by slowly adding 1.0 M hydrochloric acid and then incubating at 4 °C with magnetic stirring for 20 min to dissociate ferritin into free subunits. Afterward, 10 mM BAE was slowly added to a 2 μM protein carrier solution in a volume ratio of 1:11 and placed in a beaker packed with tinfoil in darkness. The pH was adjusted to neutral with 1.0 M NaOH, and the solution was stirred in the dark for 2 h. After stirring, the solution was placed into PBS buffer (pH 7.4) for dialysis for 24 h to remove free BAE (molecular weight cutoff (MWCO) = 12,000–14,000 Da). After that, BAE was encapsulated in a ferritin carrier to obtain the nanoparticle (2 μM). The preparation process of encapsulated BAEs is shown in Figure 6 (The X-ray crystallographic structure of ferritin was obtained from the Protein Data Bank (http://www.rcsb.org/pdb, accessed on 27 July 2023). The ferritin structure was drawn by RasMol 2.6.
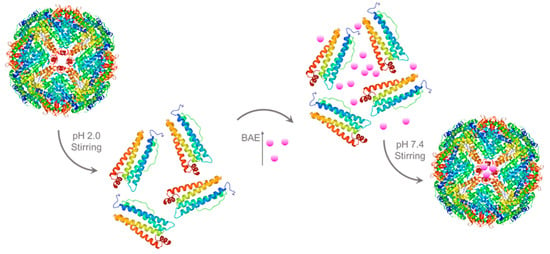
Figure 6.
Schematic illustration of preparation process of encapsulated blueberry anthocyanin extracts (BAEs) with the ferritin nanocarrier.
3.4. Transmission Electron Microscope Observation
The morphology of the protein nanocarrier and BAE nanoparticle was observed using an HT7700 Hitachi Hi-Tech Transmission Electron Microscope (Japan Hi-Tech Company, Tokyo, Japan). Briefly, an aliquot was taken and dropped onto the surface of a carbon Formvar-coated copper grid. After drying at room temperature, samples were stained negatively with 2% aqueous phosphotungstic acid for 30 s. Then, the samples were observed under a transmission electron microscope with an accelerating voltage of 80 kV. The images were presented under 60,000× magnification.
3.5. Determination of the Total Anthocyanin Content and Retention
The total anthocyanin content of free, mixed, or encapsulated BAEs during the stability test was determined by the pH differential method [55]. Before determination, the encapsulated BAE sample’s pH was adjusted to 2.0 to release the anthocyanins. Potassium chloride buffer (pH 1.0) and sodium acetate buffer (pH 4.5) were used to dilute the samples. The absorbance of each dilution was measured at 520 nm and 700 nm using a UV-6300 visible spectrophotometer (Shanghai Media Instrument Co., Ltd., Shanghai, China). The total anthocyanin content was calculated according to the following formula. The anthocyanin retention rate (%) was the percentage of total anthocyanin content after storage divided by the total anthocyanin content before storage, and is expressed as milligram per gram of dry weight basis (mg/g DW).
where A is value of absorbance, A = (A520nm − A700nm) pH 1.0 − (A520nm − A700nm) pH 4.5, MW is the molecular weight of cyanidin-3-glucoside (449.2 g/mol), DF is the dilution ratio, V is solvent volume (L), 1000 is the factor for conversion from g to mg, ε is the molar extinction coefficient (26,900 L/mol cm), L is the cuvette length (cm), and Wt is the dry weight of the sample (g).
3.6. Stability Test
The stability test was carried out on various factors, including pH, storage temperature, light conditions, oxidants, reductant agents, and salt ion type concentration. The pH stability of the free BAE was evaluated by adjusting the pH to 1, 2, 3, 4, 5, and 6, respectively, with HCl and NaOH and determining the total anthocyanin content and retention rate every hour during the storage for a total of 6 h. The stability of free BAEs was investigated for various storage temperatures (20, 40, 60, 80, and 100 °C), light conditions (no light, indoor natural light, direct sunlight, and ultraviolet (UV) light), 2% H2O2, 2% Na2SO3, and different salt ions at a range of concentrations (NaCl, CaCl2, and FeSO4, each at 0.14, 1.4, 7, and 14 mmol/L). The light conditions for these mentioned experiments were as follows: for UV light, the samples were placed 30 cm under UV light from a 40 W fluorescent lamp (Beijing Research Institute of Electric Light Source, Beijing, China) and the UV intensity was 0.375 μW/m2, detected by a UV radiation meter (Photoelectric Instrument Factory of Beijing Normal University, Beijing, China); no light meant in the dark; and indoor natural light meant the samples were placed under natural light without any fluorescent lamps. Based on the most influential indicators of stability analysis, the BAE nanoparticle was evaluated and compared to the stability of pH 6, high temperature (60 °C and 80 °C), 2% H2O2, and UV light with the free BAE and unencapsulated BAE mixture. The figures of BAE retention rate change during the stability test (Figure 1, Figure 2 and Figure 5) were obtained by using OriginLab OriginPro v8.5 SR1 (Northampton, MA, USA).
3.7. Statistical Analysis
All data were expressed as the mean ± standard deviation (SD) of three independent experiments. The statistical analysis was carried out using GraphPad Prism Version 8 (GraphPad Software, Inc., La Jolla, CA, USA). Ordinary one-way analysis of variance (ANOVA) was performed to compare the different treatment groups using Tukey’s multiple comparisons tests. An ordinary two-way ANOVA was performed to analyze the statistical significance of the row factor (storage time), column factor (different treatment), and their interaction. When the p-value was less than 0.05, it was considered to be statistically significant. Different lowercase letters indicate significant differences in BAE retention rate with different treatments at the same storage time (p < 0.05).
4. Conclusions
In this study, a ferritin nanocarrier with an approximate particle diameter of 15 nm was obtained. In addition, BAE was encapsulated with a ferritin nanocarrier, and the stability of encapsulated and free anthocyanins was compared. The results demonstrated that free BAEs are more stable at low pH (pH 3) and low temperatures (40 °C), as well as in the dark. On the other hand, encapsulated BAEs protected anthocyanins from degradation under weakly acidic pH (pH 6), high temperature (60 °C), ultraviolet light, and oxidant conditions. The results revealed that the stability of encapsulated BAE nanoparticles with ferritin carriers was considerably greater than that of free BAEs, demonstrating the advantageous role of protein nanoparticles in enhancing the stability of blueberry anthocyanins. The protein nanocarrier developed in this study would have the potential to be utilized for the delivery of BAEs as functional constituents with enhanced chemical stability; however, further efficiency experiments should be performed and limitations of the high cost should be considered and settled in the future.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28155844/s1, Figure S1: HPLC chromatogram of rabbiteye blueberry anthocyanin extracts and their identification.
Author Contributions
Conceptualization, W.H., S.L., Y.Y. and Z.T.; methodology, Z.C.; software, X.Z.; validation, S.L., W.H. and Z.T.; formal analysis, X.Z.; investigation, X.Z. and Z.C.; resources, Y.Y.; data curation, X.Z.; writing—original draft preparation, X.Z. and D.D.H.-B.; writing—review and editing, W.H., S.L. and Z.T.; visualization, B.L.; supervision, W.H. and Z.T.; project administration, W.H.; funding acquisition, W.H., S.L. and Y.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Jiangsu Provincial Key Research and Development Program, grant number BE2021705; Unveiling and Leading Projects from Science and Technology Bureau of Zhuji City, grant number 2022J11.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request.
Acknowledgments
We thank Jing Wang and Dongjie Hou of the College of Chemical Engineering in Nanjing Forestry University, Nanjing for helping in the collection of information.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Duan, Y.M.; Tarafdar, A.; Chaurasia, D.; Singh, A.; Bhargava, P.C.; Yang, J.F.; Li, Z.L.; Ni, X.H.; Tian, Y.; Li, H.K.; et al. Blueberry fruit valorization and valuable constituents: A review. Int. J. Food Microbiol. 2022, 381, 109890. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.; Feucht, W.; Schmid, M. Bioactive compounds of strawberry and blueberry and their potential health effects based on human intervention studies: A brief overview. Nutrients 2019, 11, 1510. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, I.; Marques, F.; Freitas, V.D.; Mateus, N. Antioxidant and antiproliferative properties of methylated metabolites of anthocyanins. Food. Chem. 2013, 141, 2923–2933. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.F.; Lan, W.; Wang, J.; Wang, X.Q.; Qiu, L.N. Research on the nutrition and unique health functions of blueberry. North. Hortic. 2020, 21, 138–145. [Google Scholar]
- Lee, Y.-M.; Yoon, Y.; Yoon, H.; Park, H.-M.; Song, S.; Yeum, K.-J. Dietary anthocyanins against obesity and inflammation. Nutrients 2017, 9, 1089. [Google Scholar] [CrossRef]
- Shishir, M.R.I.; Xie, L.H.; Sun, C.D.; Zheng, X.D.; Chen, W. Advances in micro and nano-encapsulation of bioactive compounds using biopolymer and lipid-based transporters. Trends Food Sci. Technol. 2018, 78, 34–60. [Google Scholar] [CrossRef]
- Fang, J. Bioavailability of anthocyanins. Drug. Metab. Rev. 2014, 46, 508–520. [Google Scholar] [CrossRef]
- Sunil, L.; Shetty, N.P. Biosynthesis and regulation of anthocyanin pathway genes. Appl. Microbiol. Biotechnol. 2022, 106, 1783–1798. [Google Scholar] [CrossRef]
- Ge, J.; Yue, P.X.; Chi, J.P.; Liang, J.; Gao, X.L. Formation and stability of anthocyanins-loaded nanocomplexes prepared with chitosan hydrochloride and carboxymethyl chitosan. Food Hydrocoll. 2018, 74, 23–31. [Google Scholar] [CrossRef]
- Sui, X.N.; Dong, X.; Zhou, W.B. Combined effect of pH and high temperature on the stability and antioxidant capacity of two anthocyanins in aqueous solution. Food Chem. 2014, 163, 163–170. [Google Scholar] [CrossRef]
- He, W.J.; Mu, H.B.; Liu, Z.M.; Lu, M.; Hang, F.; Chen, J.; Zeng, M.M.; Qin, F.; He, Z.Y. Effect of preheat treatment of milk proteins on their interactions with cyanidin-3-O-glucoside. Food Res. Int. 2018, 107, 394–405. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Balandrano, D.D.; Chai, Z.; Beta, T.; Feng, J.; Huang, W.Y. Blueberry anthocyanins: An updated review on approaches to enhancing their bioavailability. Trends Food. Sci. Technol. 2021, 118, 808–821. [Google Scholar] [CrossRef]
- Lin, Y.; Li, C.; Shi, L.J.; Wang, L.X. Anthocyanins: Modified new technologies and challenges. Foods 2023, 12, 1368. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.H.; Zhang, Y. Biopolymer-based encapsulation of anthocyanins as reinforced natural colorants for food applications. J. Agr. Food Res. 2023, 11, 100488. [Google Scholar] [CrossRef]
- Cai, X.R.; Du, X.F.; Cui, D.M.; Wang, X.N.; Yang, Z.K.; Zhu, G.L. Improvement of stability of blueberry anthocyanins by carboxymethyl starch/xantham gum combinations microencapsulation. Food Hydrocoll. 2019, 91, 238–245. [Google Scholar] [CrossRef]
- Wang, W.J.; Jung, J.; Zhao, Y.Y. Chitosan-cellulose nanocrystal microencapsulation to improve encapsulation efficiency and stability of entrapped fruit anthocyanins. Carbohydr. Polym. 2017, 157, 1246–1253. [Google Scholar] [CrossRef]
- Flores, G.; del Castillo, M.L.R.; Costabile, A.; Klee, A.; Bigetti, K.B.; Gibson, G.R. In vitro fermentation of anthocyanins encapsulated with cyclodextrins: Release, metabolism and influence on gut microbiota growth. J. Funct. Foods 2015, 16, 50–57. [Google Scholar] [CrossRef]
- Koh, J.; Xu, Z.M.; Wicker, L. Blueberry pectin and increased anthocyanins stability under in vitro digestion. Food Chem. 2020, 302, 125343. [Google Scholar] [CrossRef]
- Zang, Z.H.; Chou, S.R.; Tian, J.L.; Lang, Y.X.; Shen, Y.X.; Ran, X.L.; Gao, N.X.; Li, B. Effect of whey protein isolate on the stability and antioxidant capacity of blueberry anthocyanins: A mechanistic and in vitro simulation study. Food Chem. 2021, 336, 127700. [Google Scholar] [CrossRef]
- Ribnicky, D.M.; Roopchand, D.E.; Oren, A.; Grace, M.; Poulev, A.; Lila, M.A.; Havenaar, R.; Raskin, I. Effects of a high fat meal matrix and protein complexation on the bioaccessibility of blueberry anthocyanins using the TNO gastrointestinal model (TIM-1). Food Chem. 2014, 142, 349–357. [Google Scholar] [CrossRef]
- Aref, S.; Habiba, R.; Morsy, N.; Abdel-Daim, M.; Zayet, F. Improvement of the shelf life of grey mullet (Mugil cephalus) fish steaks using edible coatings containing chitosan, nanochitosan, and clove oil during refrigerated storage. Food Prod. Process Nutr. 2022, 4, 27. [Google Scholar] [CrossRef]
- He, B.; Ge, J.; Yue, P.X.; Yue, X.Y.; Fu, R.Y.; Liang, J.; Gao, X.L. Loading of anthocyanins on chitosan nanoparticles influences anthocyanin degradation in gastrointestinal fluids and stability in a beverage. Food Chem. 2017, 221, 1671–1677. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.S.; Temelli, F.; Chen, L.Y. Encapsulation of anthocyanin in liposomes using supercritical carbon dioxide: Effects of anthocyanin and sterol concentrations. J. Funct. Foods 2017, 34, 159–167. [Google Scholar] [CrossRef]
- Xie, C.J.; Wang, Q.; Ying, R.F.; Wang, Y.S.; Wang, Z.J.; Huang, M.G. Binding a chondroitin sulfate-based nanocomplex with kappa-carrageenan to enhance the stability of anthocyanins. Food Hydrocoll. 2020, 100, 105448. [Google Scholar] [CrossRef]
- Bamba, B.S.B.; Shi, J.; Tranchant, C.C.; Xue, J.S.; Forney, C.F.; Lim, L.; Xu, W.L.; Xu, G.H. Coencapsulation of Polyphenols and Anthocyanins from Blueberry Pomace by Double Emulsion Stabilized by Whey Proteins: Effect of Homogenization Parameters. Molecules 2018, 23, 2525. [Google Scholar] [CrossRef]
- Arosio, P.; Ingrassia, R.; Cavadini, P. Ferritins: A family of molecules for iron storage, antioxidation and more. Biochim. Biophys. Acta. 2009, 1790, 589–599. [Google Scholar] [CrossRef]
- Wang, W.M.; Wang, L.L.; Li, G.B.; Zhao, G.H.; Zhao, X.; Wang, H.F. AB loop engineered ferritin nanocages for drug loading under benign experimental conditions. Chem. Commun. 2019, 55, 12344–12347. [Google Scholar] [CrossRef]
- He, J.Y.; Fan, K.L.; Yan, X.Y. Ferritin drug carrier (FDC) for tumor targeting therapy. J. Control. Release 2019, 311, 288–300. [Google Scholar] [CrossRef]
- Hutabarat, R.P.; Xiao, Y.D.; Wu, H.; Wang, J.; Li, D.J.; Huang, W.Y. Identification of anthocyanins and optimization of their extraction from rabbiteye blueberry fruits in Nanjing. J. Food Qual. 2019, 2019, 6806790. [Google Scholar] [CrossRef]
- Chai, Z.; Herrera-Balandrano, D.D.; Yu, H.; Beta, T.; Zeng, Q.L.; Zhang, X.X.; Tian, L.L.; Niu, L.Y.; Huang, W.Y. A comparative analysis on the anthocyanin composition of 74 blueberry cultivars from China. J. Food Compos. Anal. 2021, 102, 104051. [Google Scholar] [CrossRef]
- Lv, W.Y.; Jia, D.M.; Hu, Y.F.; Chen, J.R. Effects of storage and transportation conditions on the stability of main pigment components in strawberry. J. Agron. 2021, 11, 73–77. [Google Scholar]
- Zhang, C.Y.; Xie, Y.L.; Yang, Y.H. Study on the effect of pH value of buffer system on the determination of anthocyanin content and antioxidant activity in black bean skin. J. Henan Uni. Tech. (Nat. Sci.) 2020, 41, 35–40+46. [Google Scholar]
- Zhang, G.D.; Qiu, X.D.; Hu, B.R. Optimization of microwave assisted extraction process and stability of mulberry red pigment. China Food Addit. 2014, 6, 107–111. [Google Scholar]
- Ren, Y.L.; Li, H.; Bing, G.D.; Jin, Q.H.; Lu, J.H. Natural food pigment anthocyanins. Food Sci. 1995, 7, 22–27. [Google Scholar]
- Du, Y.J.; Qin, Y.T. Study on the stability of grape procyanidins in foods. Intro. Food Saf. 2022, 34, 83–85+89. [Google Scholar]
- Li, H.F.; Yang, Y.; Qi, Y.R.; Li, J.L.; Wang, L.; Ran, M.; Yang, H.X.; Gao, H.; Huang, D.W.; Wang, Z.B. Ultrasonic-assisted extraction of anthocyanins from aronia melanocarpa with acidic natural deep eutectic solvents and its stability and antioxidant activity. Sci. Technol. Food Ind. 2023, 44, 259–269. [Google Scholar]
- Fu, X.Z.; Wang, D.; Belwal, T.; Xie, J.; Xu, Y.Q.; Li, L.; Zou, L.G.; Zhang, L.X.; Luo, Z.S. Natural deep eutectic solvent enhanced pulse-ultrasonication assisted extraction as a multi-stability protective and efficient green strategy to extract anthocyanin from blueberry pomace. LWT-Food Sci Technol. 2021, 144, 111220. [Google Scholar] [CrossRef]
- Sun, Q.Y.; Ren, S.; Lu, B.J.; Zhang, J. Study on the stability of blueberry anthocyanins. J. Nutr. 2017, 39, 400–404. [Google Scholar]
- Yan, H.G.; Hang, W.H.; Ding, Z.E. Study on the stability of anthocyanins from rabbit’s eye blueberry. Sci. Technol. Food Ind. 2013, 34, 119–124. [Google Scholar]
- Pan, F.G.; Liu, Y.J.; Liu, J.B.; Wang, E. Stability of blueberry anthocyanin, anthocyanidin and pyranoanthocyanidin pigments and their inhibitory effects and mechanisms in human cervical cancer HeLa cells. RSC. Adv. 2019, 9, 10842–10853. [Google Scholar] [CrossRef]
- Wang, L.; Wang, L.L.; Wang, X.; Lu, B.J.; Zhang, J. Preparation of blueberry anthocyanin liposomes and changes of vesicle properties, physicochemical properties, in vitro release, and antioxidant activity before and after chitosan modification. Food. Sci. Nutr. 2021, 10, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.X.; Zhang, W.; Lv, Y.P. The effect of metal ions on the color stability of anthocyanins from purple cabbage. China Condiment 2017, 42, 152–158. [Google Scholar]
- Zhang, Y.H.; Liu, N.; Zhu, W.M.; Guo, S.R. Screening of extraction conditions and stability analysis of purple tomato anthocyanins. J. Food Biotechnol. 2018, 37, 88–92. [Google Scholar]
- Li, Y.Y.; Peng, G.K.; Yang, J.S.; Ju, A.J.; Xu, Y.Q. Study on the extraction and stability of anthocyanins from the fruit of Dusi blueberry. Food. Res. Dev. 2016, 37, 46–49. [Google Scholar]
- Theil, E.C. Iron, ferritin, and nutrition. Annu. Rev. Nutr. 2004, 24, 327–343. [Google Scholar] [CrossRef]
- Arosio, P.; Levi, S. Ferritin, iron homeostasis, and oxidative damage. Free. Radic. Biol. Med. 2002, 33, 457–463. [Google Scholar] [CrossRef]
- Fan, K.; Cao, C.Q.; Pan, Y.X.; Lu, D.L.; Yang, D.; Feng, J.; Song, L.N.; Liang, M.M.; Yan, X.Y. Magnetoferritin nanoparticles for targeting and visualizing tumour tissues. Nat. Nanotechnol. 2012, 7, 459–464. [Google Scholar] [CrossRef]
- Zhang, T.; Lv, C.Y.; Chen, L.L.; Bai, G.L.; Zhao, G.H.; Xu, C.S. Encapsulation of anthocyanin molecules within a ferritin nanocage increases their stability and cell uptake efficiency. Food. Res. Int. 2014, 62, 183–192. [Google Scholar] [CrossRef]
- Yang, R.; Tian, J.; Liu, Y.Q.; Zhu, L. Interaction mechanism of ferritin protein with chlorogenic acid and iron ion: The structure, iron redox, and polymerization evaluation. Food. Chem. 2021, 349, 129144. [Google Scholar] [CrossRef]
- Chandramouli, B.; Bernacchioni, C.; Maio, D.D.; Turano, P.; Brancato, G. Electrostatic and structural bases of Fe2+ translocation through ferritin channels. J. Biol. Chem. 2016, 291, 25617–25628. [Google Scholar] [CrossRef]
- Wang, S.N.; Yang, H.Y.; He, L.X.; Guo, Y.H.; Yun, S.J.; Feng, C.P. Preparation, characterization and antioxidant properties analysis of ferritin-resveratrol composite nanoparticles. Food Sci. 2023, 44, 34–41. [Google Scholar]
- Zhang, C.X.; Zhang, X.R.; Lv, C.Y.; Zhao, G.H. Ultrasound-assisted encapsulation of astaxanthin within ferritin nanocages with enhanced efficiency. Food Sci. 2021, 42, 94–101. [Google Scholar]
- Herrera-Balandrano, D.D.; Wang, J.; Chai, Z.; Zhang, X.X.; Wang, J.L.; Wang, N.; Huang, W.Y. Impact of in vitro gastrointestinal digestion on rabbiteye blueberry anthocyanins and their absorption efficiency in Caco-2. Food Biosci. 2023, 52, 102424. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Giusti, M.M.; Wrolstad, R.E. Characterization and measurement of anthocyanins by UV-visible spectroscopy. In Current Protocols in Food Analytical Chemistry; John Wiley and Sons Inc.: Hoboken, NJ, USA, 2001; pp. F1.2.1–F1.2.13. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).