Computer-Aided Lipase Engineering for Improving Their Stability and Activity in the Food Industry: State of the Art
Abstract
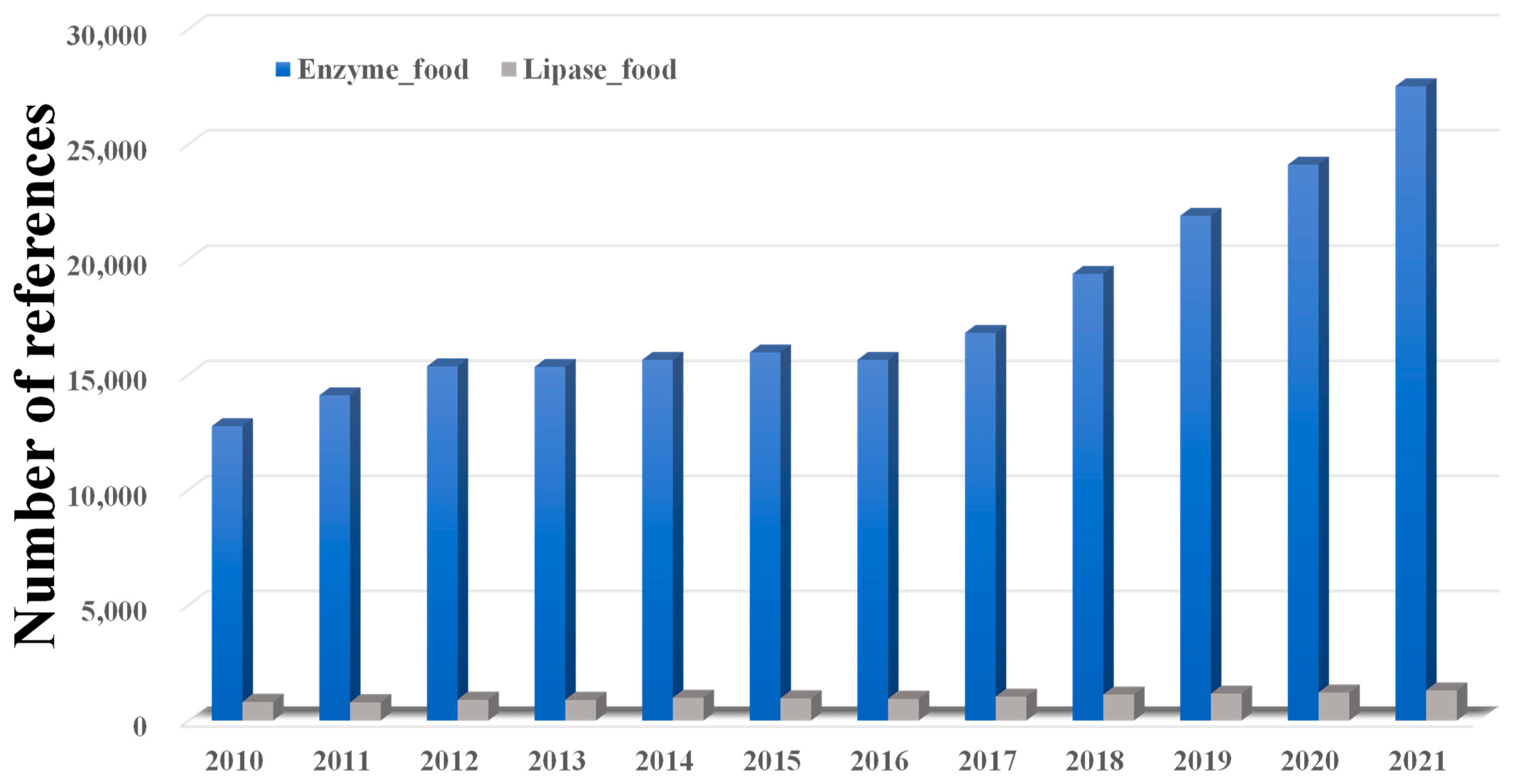
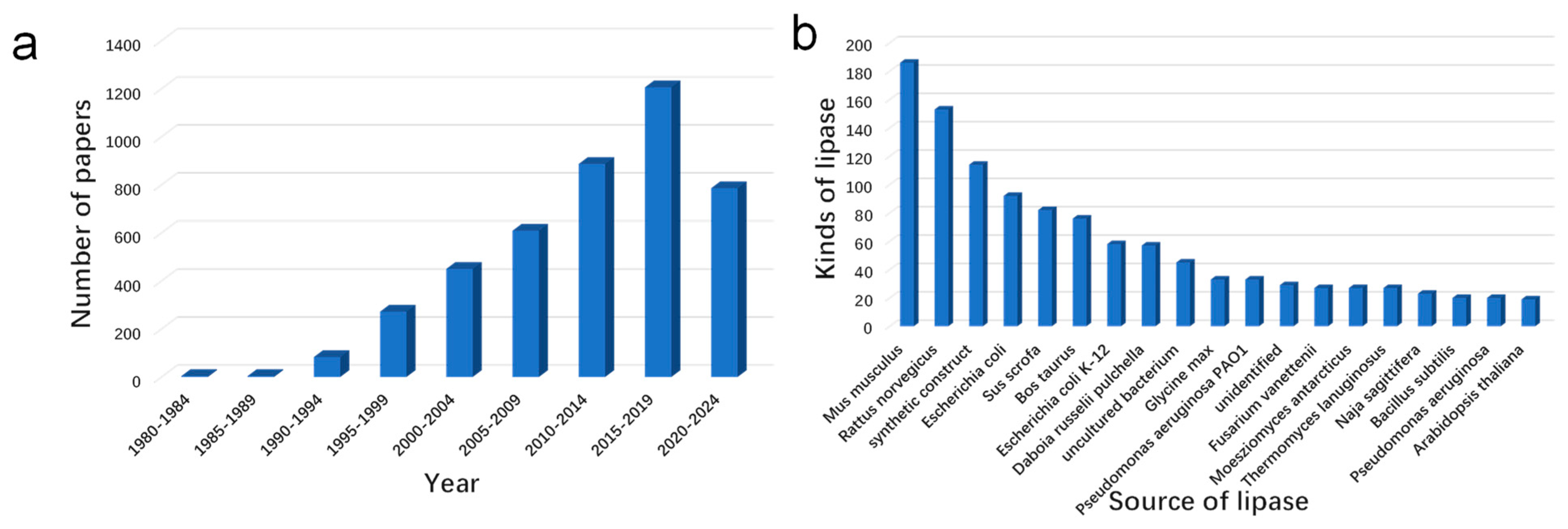
1. Introduction
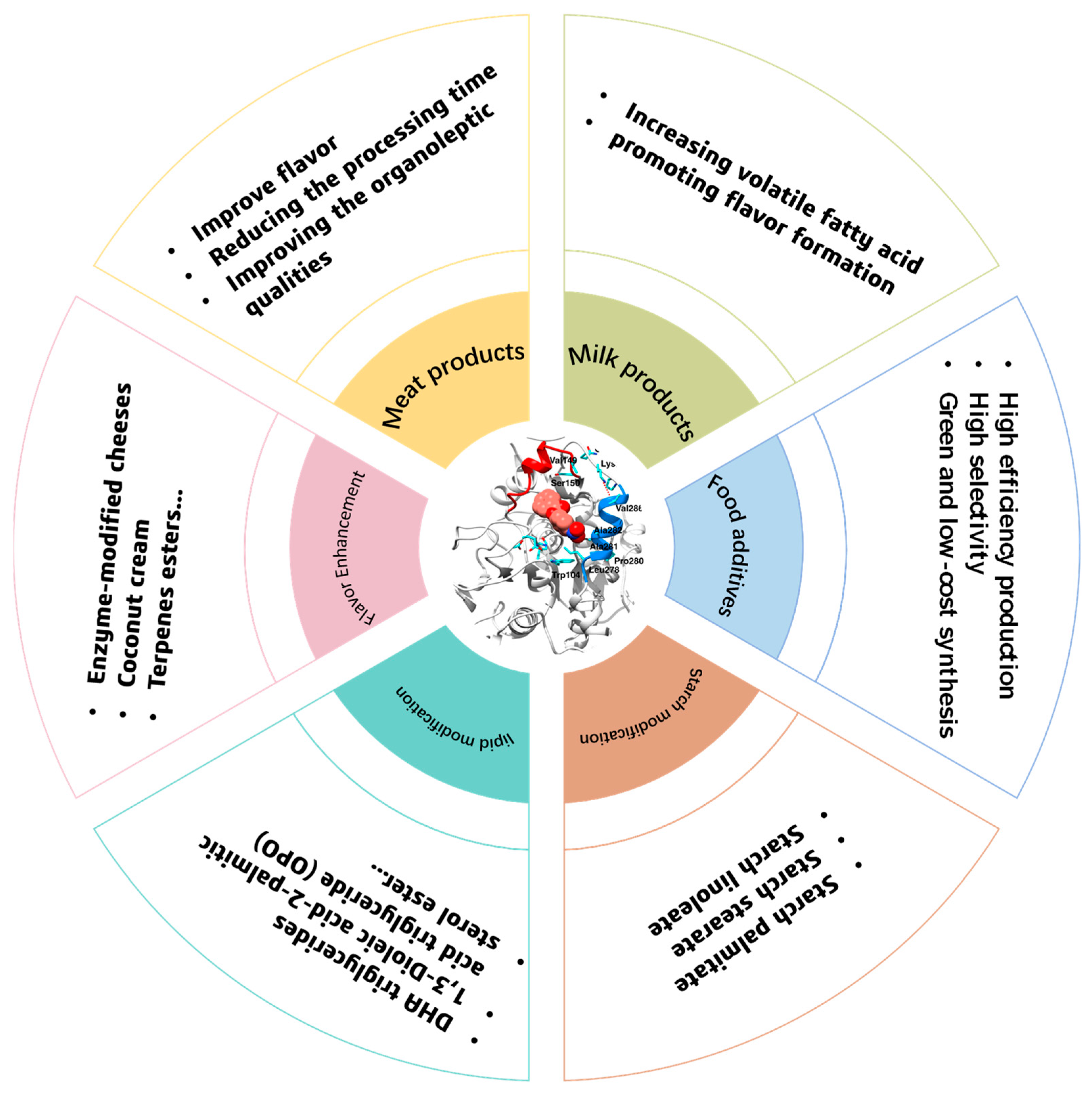
2. Lipases as a Powerful Catalyst in the Food Field
| Substrates | Enzymes | Solvents | Time (h) | Products | Yield | References | |
|---|---|---|---|---|---|---|---|
| Acyl Aceptor | Acyl Donor | ||||||
| Rapeseed oil | / | Non-GMO lipase came from Takabio | Water | 24 | Mono- and diglycerides of fatty acids | 56% | [49] |
| Vegetable oils | / | Mycelium-Bound lipase from filamentous fungus | Sodium phosphate buffer | 9 | polyunsaturated fatty acids | 96.06% | [50] |
| Sesame oil fatty acids | / | Lipozyme RM IM | Water | 2 | sn-2 PA | 67.70% | [65] |
| Phenolic acid ethyl esters | Glycerin | Novozym 435 | Glycerin | 4 | monoferuloyl glycerol | 91.60% | [66] |
| Triolein | Olive oil | Yarrowia lipolytica lipase | Solvent-free | 0.25 | Structured lipids | 33% | [67] |
| Solid β-sitostanol | Lauric or oleic acid | Ophiostoma piceae lipase | Isooctane/water biphasic systems | 3 | β-sitostanol esters | 90% | [68] |
| Xylobiose | Vinyl laurate | Lipase N435 | 2-Methyl-2-butanol | 72 | 4′-O-laurylxylobiose | 86% | [69] |
| Hexyl alcohol | Octanoic acid | Candida rugosa lipase | Isooctane | 0.67 | hexyl octoate | 50% | [70] |
| Glycerol | Conjugated linoleic acid | Lipases B from Candida antarctica | Acetone | 3 | partial glycerides of conjugated linoleic acids | 54% | [71] |
| Cyanidin-3-O-galactoside | Saturated fatty acids | Novozyme 435 | Tert-butanol | 72 | cyanidin-3-O-(6″-dodecanoyl) galactoside | 73% | [72] |
| d-glucose | Vinyl hexanoate, vinyl octanoate | Novozyme 435 | THF/pyridine (4:1 v/v) | 48 | Sugar fatty acid esters | 82–95% | [51] |
| d-xylose, L-arabinose | Vinyl laurate | Novozyme 435 | 2-Methyl-2-butanol | 0.17 | d-xylose laurate esters, l-arabinose laurate esters | 53%, 48.6% | [52] |
| A. tequilana fructans | Vinyl laurate | Novozyme 435 | Hexane | 96 | Carbohydrate fatty acid esters | 80% | [73] |
| Lysine | lauric acid | Lipases B from Candida antarctica | Deep eutectic solvents | 120 | Lipoamino acids | 59.64% | [1] |
3. The Applications of Computer-Aided Methods in Designing and Engineering Lipases
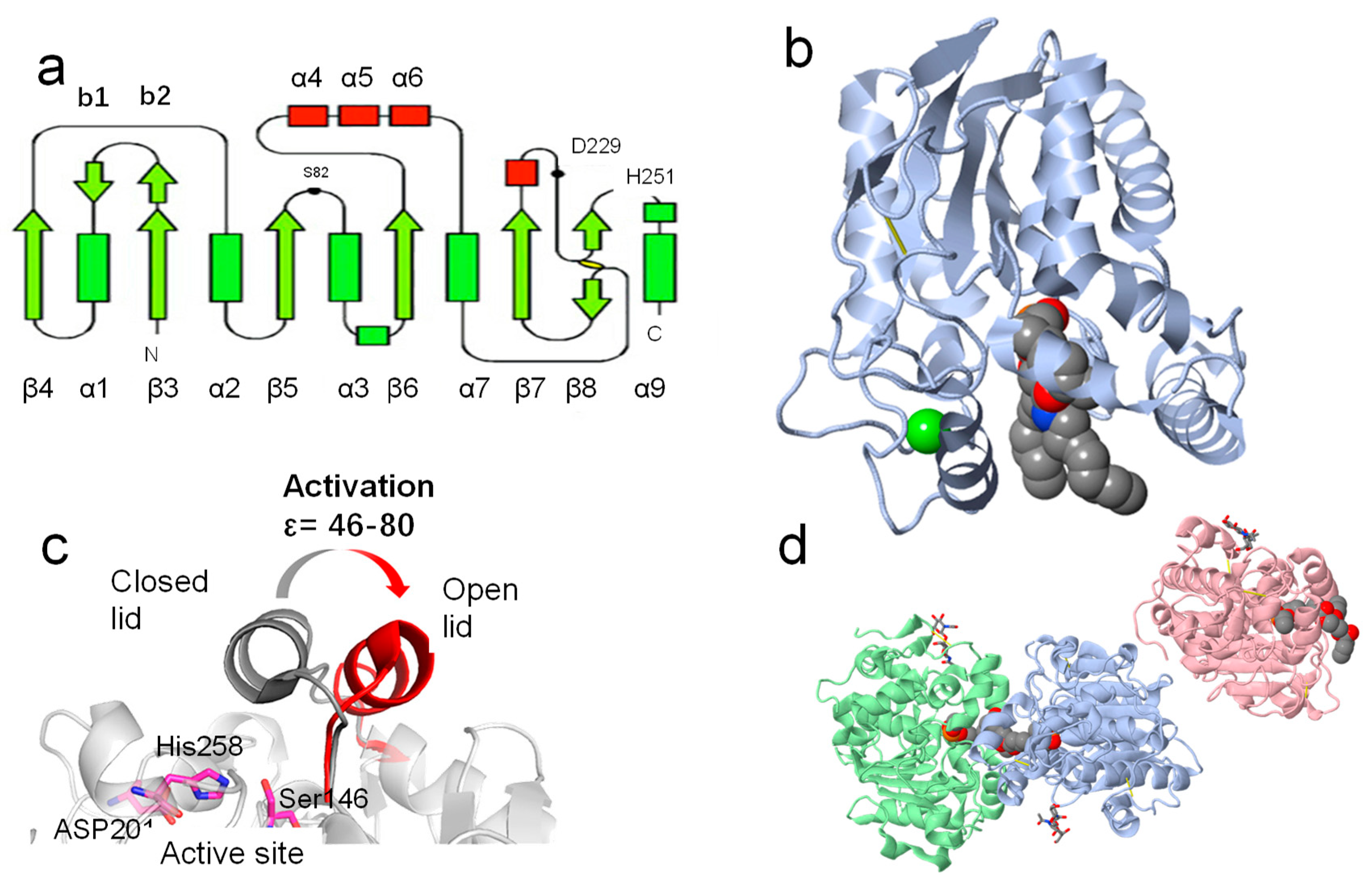
3.1. General Structure of Lipases
3.2. Computer-Aided Methods for the Prediction of Lipases Structures
3.3. Protein Engineering Strategies
3.4. Computer-Aided Methods for the Thermostability Engineering of Lipases
3.5. Computer-Aided Methods for Solvent Tolerance Engineering
4. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nian, B.; Cao, C.; Liu, Y. Synergistic Catalytic Synthesis of Gemini Lipoamino Acids Based on Multiple Hydrogen-Bonding Interactions in Natural Deep Eutectic Solvents-Enzyme System. J. Agric. Food Chem. 2020, 68, 989–997. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Luo, Q.; Song, F.; Liu, G.; Chen, S.; Li, Y.; Li, X.; Lu, Y. Effects of oxidative torrefaction on the physicochemical properties and pyrolysis products of hemicellulose in bamboo processing residues. Ind. Crops Prod. 2023, 191, 115986. [Google Scholar] [CrossRef]
- Itoh, T. Ionic Liquids as Tool to Improve Enzymatic Organic Synthesis. Chem. Rev. 2017, 117, 10567–10607. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, L.; Yildiz, C.B.; Eltoukhy, L.; Cheng, L.; Jaeger, K.-E.; Schwaneberg, U.; Davari, M.D. Enzyme Hydration: How to Retain Resistance in Ionic Liquids. ACS Sustain. Chem. Eng. 2022, 10, 15104–15114. [Google Scholar] [CrossRef]
- Mustafa, A.; Niikura, F.; Pastore, C.; Allam, H.A.; Hassan, O.B.; Mustafa, M.; Inayat, A.; Salah, S.A.; Salam, A.A.; Mohsen, R. Selective synthesis of alpha monoglycerides by a clean method: Techno-economic and environmental assessment. Sustain. Chem. Pharm. 2022, 27, 100690. [Google Scholar] [CrossRef]
- Rafiee, F.; Rezaee, M. Different strategies for the lipase immobilization on the chitosan based supports and their applications. Int. J. Biol. Macromol. 2021, 179, 170–195. [Google Scholar] [CrossRef]
- Ali, S.; Khan, S.A.; Hamayun, M.; Lee, I.-J. The Recent Advances in the Utility of Microbial Lipases: A Review. Microorganisms 2023, 11, 510. [Google Scholar] [CrossRef]
- Cavalcante, F.T.T.; Cavalcante, A.L.G.; de Sousa, I.G.; Neto, F.S.; dos Santos, J.C.S. Current Status and Future Perspectives of Supports and Protocols for Enzyme Immobilization. Catalysts 2021, 11, 1222. [Google Scholar] [CrossRef]
- Cavalcante, F.T.T.; Neto, F.S.; Rafael de Aguiar Falcão, I.; Erick da Silva Souza, J.; de Moura Junior, L.S.; da Silva Sousa, P.; Rocha, T.G.; de Sousa, I.G.; de Lima Gomes, P.H.; de Souza, M.C.M.; et al. Opportunities for improving biodiesel production via lipase catalysis. Fuel 2021, 288, 119577. [Google Scholar] [CrossRef]
- Tan, Y.; Henehan, G.T.; Kinsella, G.K.; Ryan, B.J. An extracellular lipase from Amycolatopsis mediterannei is a cutinase with plastic degrading activity. Comput. Struct. Biotechnol. J. 2021, 19, 869–879. [Google Scholar] [CrossRef]
- Choudhury, P. Industrial application of lipase: A review. BioPharm 2015, 1, 41–47. [Google Scholar]
- Singh, A.K.; Mukhopadhyay, M. Overview of Fungal Lipase: A Review. Appl. Biochem. Biotechnol. 2012, 166, 486–520. [Google Scholar] [CrossRef]
- Monteiro, R.R.C.; Arana-Peña, S.; da Rocha, T.N.; Miranda, L.P.; Berenguer-Murcia, Á.; Tardioli, P.W.; dos Santos, J.C.S.; Fernandez-Lafuente, R. Liquid lipase preparations designed for industrial production of biodiesel. Is it really an optimal solution? Renew. Energy 2021, 164, 1566–1587. [Google Scholar] [CrossRef]
- Phukon, L.C.; Chourasia, R.; Kumari, M.; Godan, T.K.; Sahoo, D.; Parameswaran, B.; Rai, A.K. Production and characterisation of lipase for application in detergent industry from a novel Pseudomonas helmanticensis HS6. Bioresour. Technol. 2020, 309, 123352. [Google Scholar] [CrossRef]
- Kersten, S. Physiological regulation of lipoprotein lipase. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2014, 1841, 919–933. [Google Scholar] [CrossRef]
- Tan, T.; Lu, J.; Nie, K.; Deng, L.; Wang, F. Biodiesel production with immobilized lipase: A review. Biotechnol. Adv. 2010, 28, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Troncoso, E.; Aguilera, J.M.; McClements, D.J. Fabrication, characterization and lipase digestibility of food-grade nanoemulsions. Food Hydrocoll. 2012, 27, 355–363. [Google Scholar] [CrossRef]
- Jeganathan, J.; Bassi, A.; Nakhla, G. Pre-treatment of high oil and grease pet food industrial wastewaters using immobilized lipase hydrolyzation. J. Hazard. Mater. 2006, 137, 121–128. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, W.; Zhou, L.; Zhang, R. Study on the influences of ultrasound on the flavor profile of unsmoked bacon and its underlying metabolic mechanism by using HS-GC-IMS. Ultrason. Sonochem. 2021, 80, 105807. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, Y.; Pan, D.; Wang, Y.; Cao, J. Effects of high pressure treatment on lipolysis-oxidation and volatiles of marinated pork meat in soy sauce. Meat Sci. 2018, 145, 186–194. [Google Scholar] [CrossRef]
- Zhang, D.; Palmer, J.; Teh, K.H.; Flint, S. Identification and selection of heat-stable protease and lipase-producing psychrotrophic bacteria from fresh and chilled raw milk during up to five days storage. LWT 2020, 134, 110165. [Google Scholar] [CrossRef]
- Salgado, C.A.; Baglinière, F.; Vanetti, M.C.D. Spoilage potential of a heat-stable lipase produced by Serratia liquefaciens isolated from cold raw milk. LWT 2020, 126, 109289. [Google Scholar] [CrossRef]
- Zhang, X.-J.; Qi, F.-Y.; Qi, J.-M.; Yang, F.; Shen, J.-W.; Cai, X.; Liu, Z.-Q.; Zheng, Y.-G. Efficient enzymatic synthesis of L-ascorbyl palmitate using Candida antarctica lipase B-embedded metal-organic framework. Biotechnol. Progr. 2022, 38, e3218. [Google Scholar] [CrossRef]
- Yadav, M.G.; Kavadia, M.R.; Vadgama, R.N.; Odaneth, A.A.; Lali, A.M. Production of 6-O-l-Ascorbyl Palmitate by Immobilized Candida antarctica Lipase B. Appl. Biochem. Biotechnol. 2018, 184, 1168–1186. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wang, J.; Huang, F.; Zheng, M. An efficient and robust continuous-flow bioreactor for the enzymatic preparation of phytosterol esters based on hollow lipase microarray. Food Chem. 2022, 372, 131256. [Google Scholar] [CrossRef]
- Ghide, M.K.; Li, K.; Wang, J.; Abdulmalek, S.A.; Yan, Y. Immobilization of Rhizomucor miehei lipase on magnetic multiwalled carbon nanotubes towards the synthesis of structured lipids rich in sn-2 palmitic acid and sn-1,3 oleic acid (OPO) for infant formula use. Food Chem. 2022, 390, 133171. [Google Scholar] [CrossRef]
- Cai, Z.; Wei, Y.; Wu, M.; Guo, Y.; Xie, Y.; Tao, R.; Li, R.; Wang, P.; Ma, A.; Zhang, H. Lipase Immobilized on Layer-by-Layer Polysaccharide-Coated Fe3O4@SiO2 Microspheres as a Reusable Biocatalyst for the Production of Structured Lipids. ACS Sustain. Chem. Eng. 2019, 7, 6685–6695. [Google Scholar] [CrossRef]
- Wang, J.-Z.; Wu, C.-K.; Yan, C.-H.; Chen, H.; You, S.; Sheng, S.; Wu, F.-A.; Wang, J. Nutritional targeting modification of silkworm pupae oil catalyzed by a smart hydrogel immobilized lipase. Food Funct. 2021, 12, 6240–6253. [Google Scholar] [CrossRef]
- Montoya, D.; Barbosa, L.O.; Méndez, J.; Murillo, W. Morphological, Structural, and Functional Evaluation of Rice Starch Acylated in a System Catalyzed by the B-Lipase of Candida antarctica. Starch-Stärke 2020, 72, 2000010. [Google Scholar] [CrossRef]
- Zarski, A.; Bajer, K.; Zarska, S.; Kapusniak, J. From high oleic vegetable oils to hydrophobic starch derivatives: I. Development and structural studies. Carbohydr. Polym. 2019, 214, 124–130. [Google Scholar] [CrossRef]
- Chowdhury, R.; Maranas, C.D. From directed evolution to computational enzyme engineering—A review. AlChE J. 2020, 66, e16847. [Google Scholar] [CrossRef]
- Chen, K.; Arnold, F.H. Enzyme Engineering for Nonaqueous Solvents: Random Mutagenesis to Enhance Activity of Subtilisin E in Polar Organic Media. Bio/Technology 1991, 9, 1073–1077. [Google Scholar] [CrossRef] [PubMed]
- Kan, S.B.J.; Lewis Russell, D.; Chen, K.; Arnold Frances, H. Directed evolution of cytochrome c for carbon–silicon bond formation: Bringing silicon to life. Science 2016, 354, 1048–1051. [Google Scholar] [CrossRef]
- Otten, L.G.; Hollmann, F.; Arends, I.W.C.E. Enzyme engineering for enantioselectivity: From trial-and-error to rational design? Trends Biotechnol. 2010, 28, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.-S.; Boyken, S.E.; Baker, D. The coming of age of de novo protein design. Nature 2016, 537, 320–327. [Google Scholar] [CrossRef]
- Cen, Y.; Singh, W.; Arkin, M.; Moody, T.S.; Huang, M.; Zhou, J.; Wu, Q.; Reetz, M.T. Artificial cysteine-lipases with high activity and altered catalytic mechanism created by laboratory evolution. Nat. Commun. 2019, 10, 3198. [Google Scholar] [CrossRef]
- Bornscheuer, U.T.; Hauer, B.; Jaeger, K.E.; Schwaneberg, U. Directed Evolution Empowered Redesign of Natural Proteins for the Sustainable Production of Chemicals and Pharmaceuticals. Angew. Chem. Int. Ed. 2019, 58, 36–40. [Google Scholar] [CrossRef]
- Li, D.-Y.; Lou, Y.-J.; Xu, J.; Chen, X.-Y.; Lin, X.-F.; Wu, Q. Electronic Effect-Guided Rational Design of Candida antarctica Lipase B for Kinetic Resolution Towards Diarylmethanols. Adv. Synth. Catal. 2021, 363, 1867–1872. [Google Scholar] [CrossRef]
- Hwang, H.T.; Qi, F.; Yuan, C.; Zhao, X.; Ramkrishna, D.; Liu, D.; Varma, A. Lipase-catalyzed process for biodiesel production: Protein engineering and lipase production. Biotechnol. Bioeng. 2014, 111, 639–653. [Google Scholar] [CrossRef]
- Kumar, R.; Goomber, S.; Kaur, J. Engineering lipases for temperature adaptation: Structure function correlation. Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2019, 1867, 140261. [Google Scholar] [CrossRef]
- Skjold-Jørgensen, J.; Vind, J.; Svendsen, A.; Bjerrum, M.J. Understanding the activation mechanism of Thermomyces lanuginosus lipase using rational design and tryptophan-induced fluorescence quenching. Eur. J. Lipid Sci. Technol. 2016, 118, 1644–1660. [Google Scholar] [CrossRef]
- Uppada, S.R.; Akula, M.; Bhattacharya, A.; Dutta, J.R. Immobilized lipase from Lactobacillus plantarum in meat degradation and synthesis of flavor esters. J. Genet. Eng. Biotechnol. 2017, 15, 331–334. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Sang, S.; Qian, D.; Jian, Y.; He, X.; Li, X.; Li, C.; Liu, Y.; Chen, S. Effects of the addition of exogenous lipase on lipolysis and lipid oxidation during wet-curing and dry-ripening of silver carp inoculated with mixed starter cultures. Int. J. Food Sci. Technol. 2021, 56, 4568–4575. [Google Scholar] [CrossRef]
- Chen, X.; Luo, J.; Lou, A.; Wang, Y.; Yang, D.; Shen, Q.W. Duck breast muscle proteins, free fatty acids and volatile compounds as affected by curing methods. Food Chem. 2021, 338, 128138. [Google Scholar] [CrossRef]
- Zhao, B.; Zhou, H.; Zhang, S.; Pan, X.; Li, S.; Zhu, N.; Wu, Q.; Wang, S.; Qiao, X.; Chen, W. Changes of protein oxidation, lipid oxidation and lipolysis in Chinese dry sausage with different sodium chloride curing salt content. Food Sci. Hum. Wellness 2020, 9, 328–337. [Google Scholar] [CrossRef]
- Neta, N.S.; Teixeira, J.A.; Rodrigues, L.R. Sugar Ester Surfactants: Enzymatic Synthesis and Applications in Food Industry. Crit. Rev. Food Sci. Nutr. 2015, 55, 595–610. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Luo, Z.; Yu, S.; Fu, X. Lipase-catalyzed Synthesis of Starch Palmitate in Mixed Ionic Liquids. J. Agric. Food. Chem. 2012, 60, 9273–9279. [Google Scholar] [CrossRef]
- Ma, H.; Xin, J.; Zheng, L.; Wang, Y.; Cui, T. Microwave-assistant lipase-catalyzed synthesis of corn starch ester in solvent free system. Food Res. Dev. 2019, 40, 167–172. [Google Scholar]
- Monié, A.; David, A.; Clemens, K.; Malet-Martino, M.; Balayssac, S.; Perez, E.; Franceschi, S.; Crepin, M.; Delample, M. Enzymatic hydrolysis of rapeseed oil with a non-GMO lipase: A strategy to substitute mono- and diglycerides of fatty acids and improve the softness of sponge cakes. LWT 2021, 137, 110405. [Google Scholar] [CrossRef]
- Lima, R.T.; Alves, A.M.; de Paula, A.V.; de Castro, H.F.; Andrade, G.S.S. Mycelium-bound lipase from Penicillium citrinum as biocatalyst for the hydrolysis of vegetable oils. Biocatal. Agric. Biotechnol. 2019, 22, 101410. [Google Scholar] [CrossRef]
- Liang, M.-Y.; Chen, Y.; Banwell, M.G.; Wang, Y.; Lan, P. Enzymatic Preparation of a Homologous Series of Long-Chain 6-O-Acylglucose Esters and Their Evaluation as Emulsifiers. J. Agric. Food. Chem. 2018, 66, 3949–3956. [Google Scholar] [CrossRef] [PubMed]
- Méline, T.; Muzard, M.; Deleu, M.; Rakotoarivonina, H.; Plantier-Royon, R.; Rémond, C. d-Xylose and l-arabinose laurate esters: Enzymatic synthesis, characterization and physico-chemical properties. Enzyme Microb. Technol. 2018, 112, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Bernal, C.; Guzman, F.; Illanes, A.; Wilson, L. Selective and eco-friendly synthesis of lipoaminoacid-based surfactants for food, using immobilized lipase and protease biocatalysts. Food Chem. 2018, 239, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Nian, B.; Cao, C.; Liu, Y. How Candida antarctica lipase B can be activated in natural deep eutectic solvents: Experimental and molecular dynamics studies. J. Chem. Technol. Biotechnol. 2020, 95, 86–93. [Google Scholar]
- Nian, B.; Cao, C.; Liu, Y. Activation and stabilization of Candida antarctica lipase B in choline chloride-glycerol-water binary system via tailoring the hydrogen-bonding interaction. Int. J. Biol. Macromol. 2019, 136, 1086–1095. [Google Scholar] [CrossRef]
- Nian, B.; Cao, C.; Liu, Y. Lipase and Metal Chloride Hydrate-Natural Deep Eutectic Solvents Synergistically Catalyze Amidation Reaction via Multiple Noncovalent Bond Interactions. ACS Sustain. Chem. Eng. 2019, 7, 18174–18184. [Google Scholar] [CrossRef]
- Nian, B.; Li, X. Can deep eutectic solvents be the best alternatives to ionic liquids and organic solvents: A perspective in enzyme catalytic reactions. Int. J. Biol. Macromol. 2022, 217, 255–269. [Google Scholar] [CrossRef]
- Otache, M.A.; Duru, R.U.; Achugasim, O.; Abayeh, O.J. Advances in the Modification of Starch via Esterification for Enhanced Properties. J. Polym. Environ. 2021, 29, 1365–1379. [Google Scholar] [CrossRef]
- Cao, X.; Pan, Y.; Qiao, M.; Yuan, Y. Synthesis of human milk fat substitutes based on enzymatic preparation of low erucic acid acyl-donors from rapeseed oil. Food Chem. 2022, 387, 132907. [Google Scholar] [CrossRef]
- Wei, W.; Sun, C.; Wang, X.; Jin, Q.; Xu, X.; Akoh, C.C.; Wang, X. Lipase-Catalyzed Synthesis of Sn-2 Palmitate: A Review. Engineering 2020, 6, 406–414. [Google Scholar] [CrossRef]
- Nagachinta, S.; Akoh, C.C. Synthesis of Structured Lipid Enriched with Omega Fatty Acids and sn-2 Palmitic Acid by Enzymatic Esterification and Its Incorporation in Powdered Infant Formula. J. Agric. Food. Chem. 2013, 61, 4455–4463. [Google Scholar] [CrossRef]
- Yang, H.; Mu, Y.; Chen, H.; Su, C.; Yang, T.; Xiu, Z. Sn-1,3-specific Interesterification of Soybean Oil with Medium-chain Triacylglycerol Catalyzed by Lipozyme TL IM. Chin. J. Chem. Eng. 2014, 22, 1016–1020. [Google Scholar] [CrossRef]
- Rivero-Pino, F.; Padial-Dominguez, M.; Guadix, E.M.; Morales-Medina, R. Novozyme 435 and Lipozyme RM IM Preferably Esterify Polyunsaturated Fatty Acids at the sn-2 Position. Eur. J. Lipid Sci. Technol. 2020, 122, 2000115. [Google Scholar] [CrossRef]
- Yang, P.; Zhang, H.; Wan, J.; Hu, J.; Liu, J.; Wang, J.; Zhang, Y.; Yu, L. Dietary sn-2 palmitic triacylglycerols reduced faecal lipids, calcium contents and altered lipid metabolism in Sprague–Dawley rats. Int. J. Food Sci. Nutr. 2019, 70, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Jin, Q.; Guo, Z.; Xu, X.; Wang, X. Preparation of human milk fat substitutes from basa catfish oil: Combination of enzymatic acidolysis and modeled blending. Eur. J. Lipid Sci. Technol. 2016, 118, 1702–1711. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, H.; Shi, J.; Zheng, M.; Xiang, X.; Huang, F.; Xiao, J. Ultrasound irradiation promoted enzymatic alcoholysis for synthesis of monoglyceryl phenolic acids in a solvent-free system. Ultrason. Sonochem. 2018, 41, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Akil, E.; Pereira, A.d.S.; El-Bacha, T.; Amaral, P.F.F.; Torres, A.G. Efficient production of bioactive structured lipids by fast acidolysis catalyzed by Yarrowia lipolytica lipase, free and immobilized in chitosan-alginate beads, in solvent-free medium. Int. J. Biol. Macromol. 2020, 163, 910–918. [Google Scholar] [CrossRef]
- Molina-Gutiérrez, M.; Hakalin, N.L.S.; Rodríguez-Sanchez, L.; Prieto, A.; Martínez, M.J. Green synthesis of β-sitostanol esters catalyzed by the versatile lipase/sterol esterase from Ophiostoma piceae. Food Chem. 2017, 221, 1458–1465. [Google Scholar] [CrossRef]
- Gérard, D.; Méline, T.; Muzard, M.; Deleu, M.; Plantier-Royon, R.; Rémond, C. Enzymatically-synthesized xylo-oligosaccharides laurate esters as surfactants of interest. Carbohydr. Res. 2020, 495, 108090. [Google Scholar] [CrossRef]
- Chen, G.; Du, H.; Jiang, B.; Miao, M.; Feng, B. Activity of Candida rugosa lipase for synthesis of hexyl octoate under high hydrostatic pressure and the mechanism of this reaction. J. Mol. Catal. B Enzym. 2016, 133, S439–S444. [Google Scholar] [CrossRef]
- Verdasco-Martín, C.M.; Garcia-Verdugo, E.; Porcar, R.; Fernandez-Lafuente, R.; Otero, C. Selective synthesis of partial glycerides of conjugated linoleic acids via modulation of the catalytic properties of lipases by immobilization on different supports. Food Chem. 2018, 245, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Kortesniemi, M.; Yang, B.; Zheng, J. Enzymatic Acylation of Anthocyanins Isolated from Alpine Bearberry (Arctostaphylos alpina) and Lipophilic Properties, Thermostability, and Antioxidant Capacity of the Derivatives. J. Agric. Food. Chem. 2018, 66, 2909–2916. [Google Scholar] [CrossRef] [PubMed]
- Casas-Godoy, L.; Arrizon, J.; Arrieta-Baez, D.; Plou, F.J.; Sandoval, G. Synthesis and emulsifying properties of carbohydrate fatty acid esters produced from Agave tequilana fructans by enzymatic acylation. Food Chem. 2016, 204, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Khan, I.M.; He, W.; Li, Y.; Jin, P.; Campanella, O.H.; Zhang, H.; Huo, Y.; Chen, Y.; Yang, H.; et al. Rebuilding the lid region from conformational and dynamic features to engineering applications of lipase in foods: Current status and future prospects. Compr. Rev. Food Sci. Food Saf. 2022, 21, 2688–2714. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Cen, Y.; Singh, W.; Fan, J.; Wu, L.; Lin, X.; Zhou, J.; Huang, M.; Reetz, M.T.; Wu, Q. Stereodivergent Protein Engineering of a Lipase To Access All Possible Stereoisomers of Chiral Esters with Two Stereocenters. J. Am. Chem. Soc. 2019, 141, 7934–7945. [Google Scholar] [CrossRef]
- Wang, Y.; Xue, P.; Cao, M.; Yu, T.; Lane, S.T.; Zhao, H. Directed Evolution: Methodologies and Applications. Chem. Rev. 2021, 121, 12384–12444. [Google Scholar] [CrossRef]
- Cui, H.; Stadtmüller, T.H.J.; Jiang, Q.; Jaeger, K.-E.; Schwaneberg, U.; Davari, M.D. How to Engineer Organic Solvent Resistant Enzymes: Insights from Combined Molecular Dynamics and Directed Evolution Study. ChemCatChem 2020, 12, 4073–4083. [Google Scholar] [CrossRef]
- Nardini, M.; Lang, D.A.; Liebeton, K.; Jaeger, K.E.; Dijkstra, B.W. Crystal structure of pseudomonas aeruginosa lipase in the open conformation. The prototype for family I.1 of bacterial lipases. J. Biol. Chem. 2000, 275, 31219–31225. [Google Scholar] [CrossRef]
- Skjold-Jørgensen, J.; Vind, J.; Svendsen, A.; Bjerrum, M.J. Lipases That Activate at High Solvent Polarities. Biochemistry 2016, 55, 146–156. [Google Scholar] [CrossRef]
- Khan, F.I.; Lan, D.; Durrani, R.; Huan, W.; Zhao, Z.; Wang, Y. The Lid Domain in Lipases: Structural and Functional Determinant of Enzymatic Properties. Front. Bioeng. Biotechnol. 2017, 5, 16. [Google Scholar] [CrossRef]
- Willems, N.; Lelimousin, M.; Koldsø, H.; Sansom, M.S. Interfacial activation of M37 lipase: A multi-scale simulation study. Biochim. Biophys. Acta Biomembr. 2017, 1859, 340–349. [Google Scholar] [CrossRef]
- Wang, W.; Dasetty, S.; Sarupria, S.; Blenner, M. Rational engineering of low temperature activity in thermoalkalophilic Geobacillus thermocatenulatus lipase. Biochem. Eng. J. 2021, 174, 108093. [Google Scholar] [CrossRef]
- Petřek, M.; Otyepka, M.; Banáš, P.; Košinová, P.; Koča, J.; Damborský, J. CAVER: A new tool to explore routes from protein clefts, pockets and cavities. BMC Bioinf. 2006, 7, 316. [Google Scholar] [CrossRef] [PubMed]
- Maity, K.; Bajaj, P.; Surolia, N.; Surolia, A.; Suguna, K. Insights into the substrate specificity of a thioesterase Rv0098 of mycobacterium tuberculosis through X-ray crystallographic and molecular dynamics studies. J. Biomol. Struct. Dyn. 2012, 29, 973–983. [Google Scholar] [CrossRef]
- de Melo, J.J.C.; Gonçalves, J.R.; Brandão, L.M.d.S.; Souza, R.L.; Pereira, M.M.; Lima, Á.S.; Soares, C.M.F. Evaluation of lipase access tunnels and analysis of substance transport in comparison with experimental data. Bioprocess. Biosyst. Eng. 2022, 45, 1149–1162. [Google Scholar] [CrossRef]
- Šrejber, M.; Navrátilová, V.; Paloncýová, M.; Bazgier, V.; Berka, K.; Anzenbacher, P.; Otyepka, M. Membrane-attached mammalian cytochromes P450: An overview of the membrane’s effects on structure, drug binding, and interactions with redox partners. J. Inorg. Biochem. 2018, 183, 117–136. [Google Scholar] [CrossRef] [PubMed]
- Urban, P.; Lautier, T.; Pompon, D.; Truan, G. Ligand access channels in cytochrome P450 enzymes: A review. Int. J. Mol. Sci. 2018, 19, 1617. [Google Scholar] [PubMed]
- Zwama, M.; Yamasaki, S.; Nakashima, R.; Sakurai, K.; Nishino, K.; Yamaguchi, A. Multiple entry pathways within the efflux transporter AcrB contribute to multidrug recognition. Nat. Commun. 2018, 9, 124. [Google Scholar] [CrossRef]
- Bashiri, R.; Curtis, T.P.; Ofiţeru, I.D. The limitations of the current protein classification tools in identifying lipolytic features in putative bacterial lipase sequences. J. Biotechnol. 2022, 351, 30–37. [Google Scholar] [CrossRef]
- Kulminskaya, N.; Gamez, C.F.R.; Hofer, P.; Cerk, I.K.; Dubey, N.; Viertlmayr, R.; Sagmeister, T.; Pavkov-Keller, T.; Zechner, R.; Oberer, M. Unmasking Crucial Residues in Adipose Triglyceride Lipase (ATGL) for Co-Activation with Comparative Gene Identification-58 (CGI-58). bioRxiv 2023. bioRxiv:2023.06.26.546551. [Google Scholar]
- Zhou, X.; Zheng, W.; Li, Y.; Pearce, R.; Zhang, C.; Bell, E.W.; Zhang, G.; Zhang, Y. I-TASSER-MTD: A deep-learning-based platform for multi-domain protein structure and function prediction. Nat. Protoc. 2022, 17, 2326–2353. [Google Scholar] [CrossRef] [PubMed]
- Schwede, T.; Kopp, J.R.; Guex, N.; Peitsch, M.C. SWISS-MODEL: An automated protein homology-modeling server. Nucleic Acids Res. 2003, 31, 3381–3385. [Google Scholar] [CrossRef]
- Li, J.; Wang, S.; Liu, C.; Li, Y.; Wei, Y.; Fu, G.; Liu, P.; Ma, H.; Huang, D.; Lin, J.; et al. Going Beyond the Local Catalytic Activity Space of Chitinase Using a Simulation-Based Iterative Saturation Mutagenesis Strategy. ACS Catal. 2022, 12, 10235–10244. [Google Scholar] [CrossRef]
- Peng, Z.; Mao, X.; Mu, W.; Du, G.; Chen, J.; Zhang, J. Modifying the Substrate Specificity of Keratinase for Industrial Dehairing to Replace Lime-Sulfide. ACS Sustain. Chem. Eng. 2022, 10, 6863–6870. [Google Scholar] [CrossRef]
- Zhou, S.; Wu, Y.; Xie, Z.-X.; Jia, B.; Yuan, Y.-J. Directed genome evolution driven by structural rearrangement techniques. Chem. Soc. Rev. 2021, 50, 12788–12807. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A.; Pereira, P.C. Biocatalysis engineering: The big picture. Chem. Soc. Rev. 2017, 46, 2678–2691. [Google Scholar] [CrossRef]
- Vavra, O.; Damborsky, J.; Bednar, D. Fast approximative methods for study of ligand transport and rational design of improved enzymes for biotechnologies. Biotechnol. Adv. 2022, 60, 108009. [Google Scholar] [CrossRef]
- Ruff, A.J.; Dennig, A.; Schwaneberg, U. To get what we aim for–progress in diversity generation methods. FEBS J. 2013, 280, 2961–2978. [Google Scholar] [CrossRef]
- Son, H.F.; Joo, S.; Seo, H.; Sagong, H.-Y.; Lee, S.H.; Hong, H.; Kim, K.-J. Structural bioinformatics-based protein engineering of thermo-stable PETase from Ideonella sakaiensis. Enzyme Microb. Technol. 2020, 141, 109656. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, W.; Mu, W. Molecular Dynamics Simulation for Food Enzyme Engineering: Why This Technique Should Be Encouraged To Learn. J. Agric. Food. Chem. 2021, 69, 4–6. [Google Scholar] [CrossRef]
- Mazurenko, S.; Prokop, Z.; Damborsky, J. Machine Learning in Enzyme Engineering. ACS Catal. 2020, 10, 1210–1223. [Google Scholar] [CrossRef]
- Santiago, G.; de Salas, F.; Lucas, M.F.; Monza, E.; Acebes, S.; Martinez, Á.T.; Camarero, S.; Guallar, V. Computer-Aided Laccase Engineering: Toward Biological Oxidation of Arylamines. ACS Catal. 2016, 6, 5415–5423. [Google Scholar]
- Wu, L.; Qin, L.; Nie, Y.; Xu, Y.; Zhao, Y.-L. Computer-aided understanding and engineering of enzymatic selectivity. Biotechnol. Adv. 2022, 54, 107793. [Google Scholar] [CrossRef] [PubMed]
- Quijano-Rubio, A.; Yeh, H.-W.; Park, J.; Lee, H.; Langan, R.A.; Boyken, S.E.; Lajoie, M.J.; Cao, L.; Chow, C.M.; Miranda, M.C.; et al. De novo design of modular and tunable protein biosensors. Nature 2021, 591, 482–487. [Google Scholar] [CrossRef]
- Cao, L.; Goreshnik, I.; Coventry, B.; Case, J.B.; Miller, L.; Kozodoy, L.; Chen, R.E.; Carter, L.; Walls, A.C.; Park, Y.-J.; et al. De novo design of picomolar SARS-CoV-2 miniprotein inhibitors. Science 2020, 370, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Xiao, Y.; Shakhnovich, E.I.; Zhang, W.; Mu, W. Semi-rational design and molecular dynamics simulations study of the thermostability enhancement of cellobiose 2-epimerases. Int. J. Biol. Macromol. 2020, 154, 1356–1365. [Google Scholar] [CrossRef]
- Xu, P.; Ni, Z.-F.; Zong, M.-H.; Ou, X.-Y.; Yang, J.-G.; Lou, W.-Y. Improving the thermostability and activity of Paenibacillus pasadenensis chitinase through semi-rational design. Int. J. Biol. Macromol. 2020, 150, 9–15. [Google Scholar] [CrossRef]
- Tian, M.; Yang, L.; Lv, P.; Wang, Z.; Fu, J.; Miao, C.; Li, Z.; Li, L.; Liu, T.; Du, W.; et al. Improvement of methanol tolerance and catalytic activity of Rhizomucor miehei lipase for one-step synthesis of biodiesel by semi-rational design. Bioresour. Technol. 2022, 348, 126769. [Google Scholar] [CrossRef]
- Lutz, S. Beyond directed evolution--semi-rational protein engineering and design. Curr. Opin. Biotechnol. 2010, 21, 734–743. [Google Scholar] [CrossRef]
- Meng, S.; An, R.; Li, Z.; Schwaneberg, U.; Ji, Y.; Davari, M.D.; Wang, F.; Wang, M.; Qin, M.; Nie, K.; et al. Tunnel engineering for modulating the substrate preference in cytochrome P450BsβHI. Bioresour. Bioprocess. 2021, 8, 26. [Google Scholar] [CrossRef]
- Tan, Z.; Li, J.; Wu, M.; Tang, C.; Zhang, H.; Wang, J. High-level heterologous expression of an alkaline lipase gene from Penicillium cyclopium PG37 in Pichia pastoris. World J. Microbiol. Biotechnol. 2011, 27, 2767–2774. [Google Scholar] [CrossRef]
- Bai, Q.; Cai, Y.; Yang, L.; Wu, Q.; Su, M.; Tang, L. Substitution of L133 with Methionine in GXSXG Domain Significantly Changed the Activity of Penicillium expansum Lipase. Catal. Lett. 2022, 152, 2047–2055. [Google Scholar] [CrossRef]
- Bi, Y.; Yu, M.; Zhou, H.; Zhou, H.; Wei, P. Biosynthesis of oleyl oleate in solvent-free system by Candida rugosa Lipase (CRL) immobilized in macroporous resin with cross-linking of aldehyde-dextran. J. Mol. Catal. B Enzym. 2016, 133, 1–5. [Google Scholar] [CrossRef]
- Ashraf, N.M.; Krishnagopal, A.; Hussain, A.; Kastner, D.; Sayed, A.M.M.; Mok, Y.-K.; Swaminathan, K.; Zeeshan, N. Engineering of serine protease for improved thermostability and catalytic activity using rational design. Int. J. Biol. Macromol. 2019, 126, 229–237. [Google Scholar] [CrossRef]
- Xu, Z.; Cen, Y.-K.; Zou, S.-P.; Xue, Y.-P.; Zheng, Y.-G. Recent advances in the improvement of enzyme thermostability by structure modification. Crit. Rev. Biotechnol. 2020, 40, 83–98. [Google Scholar] [CrossRef]
- Singh, B.; Bulusu, G.; Mitra, A. Understanding the Thermostability and Activity of Bacillus subtilis Lipase Mutants: Insights from Molecular Dynamics Simulations. J. Phys. Chem. B 2015, 119, 392–409. [Google Scholar] [CrossRef]
- Akbulut, N.; Tuzlakoğlu Öztürk, M.; Pijning, T.; İşsever Öztürk, S.; Gümüşel, F. Improved activity and thermostability of Bacillus pumilus lipase by directed evolution. J. Biotechnol. 2013, 164, 123–129. [Google Scholar] [CrossRef]
- Zhao, J.-F.; Wang, Z.; Gao, F.-L.; Lin, J.-P.; Yang, L.-R.; Wu, M.-B. Enhancing the thermostability of Rhizopus oryzae lipase by combined mutation of hot-spots and engineering a disulfide bond. RSC Adv. 2018, 8, 41247–41254. [Google Scholar] [CrossRef]
- Thomson, R.E.S.; Carrera-Pacheco, S.E.; Gillam, E.M.J. Engineering functional thermostable proteins using ancestral sequence reconstruction. J. Biol. Chem. 2022, 298, 102435. [Google Scholar] [CrossRef]
- Gumulya, Y.; Baek, J.-M.; Wun, S.-J.; Thomson, R.E.S.; Harris, K.L.; Hunter, D.J.B.; Behrendorff, J.B.Y.H.; Kulig, J.; Zheng, S.; Wu, X.; et al. Engineering highly functional thermostable proteins using ancestral sequence reconstruction. Nat. Catal. 2018, 1, 878–888. [Google Scholar] [CrossRef]
- Rozi, M.F.A.M.; Rahman, R.N.Z.R.A.; Leow, A.T.C.; Ali, M.S.M. Ancestral sequence reconstruction of ancient lipase from family I.3 bacterial lipolytic enzymes. Mol. Phylogenet. Evol. 2022, 168, 107381. [Google Scholar] [CrossRef]
- Spence, M.A.; Kaczmarski, J.A.; Saunders, J.W.; Jackson, C.J. Ancestral sequence reconstruction for protein engineers. Curr. Opin. Struct. Biol. 2021, 69, 131–141. [Google Scholar] [CrossRef]
- Tian, F.; Yang, C.; Wang, C.; Guo, T.; Zhou, P. Mutatomics analysis of the systematic thermostability profile of Bacillus subtilis lipase A. J. Mol. Model. 2014, 20, 2257. [Google Scholar] [CrossRef] [PubMed]
- Dehouck, Y.; Kwasigroch, J.M.; Gilis, D.; Rooman, M. PoPMuSiC 2.1: A web server for the estimation of protein stability changes upon mutation and sequence optimality. BMC Bioinf. 2011, 12, 151. [Google Scholar] [CrossRef]
- Delgado, J.; Radusky, L.G.; Cianferoni, D.; Serrano, L. FoldX 5.0: Working with RNA, small molecules and a new graphical interface. Bioinformatics 2019, 35, 4168–4169. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhu, H.; Ma, X.; Ma, Y.; Wei, D. Structure-based stabilization of an enzyme: The case of penicillin acylase from Alcaligenes faecalis. Protein Pept. Lett. 2006, 13, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Musil, M.; Stourac, J.; Bendl, J.; Brezovsky, J.; Prokop, Z.; Zendulka, J.; Martinek, T.; Bednar, D.; Damborsky, J. FireProt: Web server for automated design of thermostable proteins. Nucleic Acids Res. 2017, 45, W393–W399. [Google Scholar] [CrossRef]
- Xia, Y.; Li, X.; Yang, L.; Luo, X.; Shen, W.; Cao, Y.; Peplowski, L.; Chen, X. Development of thermostable sucrose phosphorylase by semi-rational design for efficient biosynthesis of alpha-D-glucosylglycerol. Appl. Microbiol. Biotechnol. 2021, 105, 7309–7319. [Google Scholar] [CrossRef]
- Priyanka, P.; Tan, Y.; Kinsella, G.K.; Henehan, G.T.; Ryan, B.J. Solvent stable microbial lipases: Current understanding and biotechnological applications. Biotechnol. Lett 2019, 41, 203–220. [Google Scholar] [CrossRef]
- Serdakowski, A.L.; Dordick, J.S. Enzyme activation for organic solvents made easy. Trends Biotechnol. 2008, 26, 48–54. [Google Scholar] [CrossRef]
- Dutta Banik, S.; Nordblad, M.; Woodley, J.M.; Peters, G.H. A Correlation between the Activity of Candida antarctica Lipase B and Differences in Binding Free Energies of Organic Solvent and Substrate. ACS Catal. 2016, 6, 6350–6361. [Google Scholar] [CrossRef]
- Kumar, A.; Dhar, K.; Kanwar, S.S.; Arora, P.K. Lipase catalysis in organic solvents: Advantages and applications. Biol. Proced. Online 2016, 18, 2. [Google Scholar] [CrossRef] [PubMed]
- Foresti, M.L.; Galle, M.; Ferreira, M.L.; Briand, L.E. Enantioselective esterification of ibuprofen with ethanol as reactant and solvent catalyzed by immobilized lipase: Experimental and molecular modeling aspects. J. Chem. Technol. Biotechnol. 2009, 84, 1461–1473. [Google Scholar]
- Klibanov, A.M. Why are enzymes less active in organic solvents than in water? Trends Biotechnol. 1997, 15, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Okamura, T.; Nogami, Y.; Matsumoto, T.; Yamada, R.; Ogino, H. Protein engineering to improve the stability of Thermomyces lanuginosus lipase in methanol. Biochem. Eng. J. 2022, 187, 108659. [Google Scholar] [CrossRef]
- Frauenkron-Machedjou, V.J.; Fulton, A.; Zhao, J.; Weber, L.; Jaeger, K.-E.; Schwaneberg, U.; Zhu, L. Exploring the full natural diversity of single amino acid exchange reveals that 40–60% of BSLA positions improve organic solvents resistance. Bioresour. Bioprocess. 2018, 5, 2. [Google Scholar] [CrossRef]
- Pramanik, S.; Cui, H.; Dhoke, G.V.; Yildiz, C.B.; Vedder, M.; Jaeger, K.-E.; Schwaneberg, U.; Davari, M.D. How Does Surface Charge Engineering of Bacillus subtilis Lipase A Improve Ionic Liquid Resistance? Lessons Learned from Molecular Dynamics Simulations. ACS Sustain. Chem. Eng. 2022, 10, 2689–2698. [Google Scholar] [CrossRef]
- Van Boxstael, S.; Cunin, R.; Khan, S.; Maes, D. Aspartate Transcarbamylase from the Hyperthermophilic Archaeon Pyrococcus abyssi: Thermostability and 1.8Å Resolution Crystal Structure of the Catalytic Subunit Complexed With the Bisubstrate Analogue N-Phosphonacetyl-l-aspartate. J. Mol. Biol. 2003, 326, 203–216. [Google Scholar] [CrossRef]
- Behera, R.K.; Mazumdar, S. Thermodynamic basis of the thermostability of CYP175A1 from Thermus thermophilus. Int. J. Biol. Macromol. 2010, 46, 412–418. [Google Scholar] [CrossRef]
- Cui, H.; Eltoukhy, L.; Zhang, L.; Markel, U.; Jaeger, K.-E.; Davari, M.D.; Schwaneberg, U. Less Unfavorable Salt Bridges on the Enzyme Surface Result in More Organic Cosolvent Resistance. Angew. Chem. Int. Ed. 2021, 60, 11448–11456. [Google Scholar] [CrossRef]
- Frauenkron-Machedjou, V.J.; Fulton, A.; Zhu, L.; Anker, C.; Bocola, M.; Jaeger, K.-E.; Schwaneberg, U. Towards Understanding Directed Evolution: More than Half of All Amino Acid Positions Contribute to Ionic Liquid Resistance of Bacillus subtilis Lipase A. ChemBioChem 2015, 16, 937–945. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Frauenkron-Machedjou, V.J.; Fulton, A.; Zhu, L.; Davari, M.D.; Jaeger, K.-E.; Schwaneberg, U.; Bocola, M. Unraveling the effects of amino acid substitutions enhancing lipase resistance to an ionic liquid: A molecular dynamics study. Phys. Chem. Chem. Phys. 2018, 20, 9600–9609. [Google Scholar] [CrossRef]
- Zhao, J.; Jia, N.; Jaeger, K.-E.; Bocola, M.; Schwaneberg, U. Ionic liquid activated Bacillus subtilis lipase A variants through cooperative surface substitutions. Biotechnol. Bioeng. 2015, 112, 1997–2004. [Google Scholar] [CrossRef] [PubMed]
- El Harrar, T.; Frieg, B.; Davari, M.D.; Jaeger, K.-E.; Schwaneberg, U.; Gohlke, H. Aqueous ionic liquids redistribute local enzyme stability via long-range perturbation pathways. Comput. Struct. Biotechnol. J. 2021, 19, 4248–4264. [Google Scholar] [CrossRef] [PubMed]
- El Harrar, T.; Davari, M.D.; Jaeger, K.-E.; Schwaneberg, U.; Gohlke, H. Critical assessment of structure-based approaches to improve protein resistance in aqueous ionic liquids by enzyme-wide saturation mutagenesis. Comput. Struct. Biotechnol. J. 2022, 20, 399–409. [Google Scholar] [CrossRef]



| Enzymes | Substrate | Solvents | Substitutions/Modifictaion Region | Protein Engineering Strategy | Yields/Conclusion | References |
|---|---|---|---|---|---|---|
| Bacillus subtilis Lipase A | p-Nitrophenyl butyrate | Ionic liquids | Aromatic/aliphatic/polar/charged amino acids | Multiple site saturation mutagenesis | 6–13% of substitutions of these sites can improve resistance | [141] |
| Bacillus subtilis Lipase A | p-Nitrophenyl butyrate | Ionic liquids-water | M134N, N138S, L140S | Multiple site saturation mutagenesis | Variant M2 (M134R/L140S) showed almost double resistance (233% vs. 111%) of ILs co-solvent | [142] |
| Bacillus subtilis Lipase A | / | Ionic liquids | Perturbation pathways | Molecular dynamics simulations | Identifying these perturbation pathways and specific IL ion-residue interactions there effectively predicts focused variant libraries with improved ILs tolerance. | [144] |
| Bacillus subtilis Lipase A | / | Ionic liquids | / | Molecular dynamics simulations | The combination of five simple-to-compute physicochemical and evolutionary properties (P9-P12) substantially increases the precision of predicting relevant variants and positions of BsLipA for increasing aIL resistance | [145] |
| Thermomyces lanuginosus lipase | BAL-tributyrate | 50% (v/v) methanol | A28S | Site-directed mutagenesis | Half-life was 6.7-fold longer than wide-type in 50% (v/v) methanol at 40 °C | [135] |
| Bacillus subtilis Lipase A | p-Nitrophenyl butyrate | Trifluoroethanol/1,4-dioxane/dimethyl sulfoxide | / | site saturation mutagenesis | Charged substitutions on the surface predominantly improved resistance while polar ones were preferred in buried “regions” | [136] |
| Bacillus subtilis Lipase A | p-Nitrophenyl butyrate | 1,4-dioxane/ dimethyl sulfoxide | Salt bridge | Molecular dynamics simulations + site saturation mutagenesis | The variants of organic solvents can be improved 7.6-fold | [140] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, W.; Nian, B. Computer-Aided Lipase Engineering for Improving Their Stability and Activity in the Food Industry: State of the Art. Molecules 2023, 28, 5848. https://doi.org/10.3390/molecules28155848
Cheng W, Nian B. Computer-Aided Lipase Engineering for Improving Their Stability and Activity in the Food Industry: State of the Art. Molecules. 2023; 28(15):5848. https://doi.org/10.3390/molecules28155848
Chicago/Turabian StyleCheng, Wenjun, and Binbin Nian. 2023. "Computer-Aided Lipase Engineering for Improving Their Stability and Activity in the Food Industry: State of the Art" Molecules 28, no. 15: 5848. https://doi.org/10.3390/molecules28155848
APA StyleCheng, W., & Nian, B. (2023). Computer-Aided Lipase Engineering for Improving Their Stability and Activity in the Food Industry: State of the Art. Molecules, 28(15), 5848. https://doi.org/10.3390/molecules28155848






