Characterization of Portulaca oleracea Whole Plant: Evaluating Antioxidant, Anticancer, Antibacterial, and Antiviral Activities and Application as Quality Enhancer in Yogurt
Abstract
:1. Introduction
2. Results and Discussion
2.1. Proximate Analysis of Purslane Plant
2.2. Detection of Phenolic Compounds in Purslane Extract by LC/MS
2.3. Volatile Compounds in Purslane Extract
2.4. Biological Activity of PuE
2.4.1. Scavenging Ability
2.4.2. Cytotoxic Effect
2.4.3. Antimicrobial and Antiviral Activity
2.5. Safety Experiment
2.5.1. Body Weight Gain and Final Weight
2.5.2. Serum Biochemical Parameters
2.5.3. Protective Effect of PuE against Lipid Oxidation in Rat Brain
2.6. The Experiment of Yogurt Supplemented with P. oleracea Extract at Different Concentrations (50, 150, and 250 µg/g)
2.6.1. Proximate Composition of Yogurt Supplemented with P. oleracea Extract
2.6.2. Physiochemical Parameters
2.6.3. Antioxidant Content in Yogurt Samples Supplemented with Purslane Extract
2.6.4. Lipid Oxidation of Yogurt Samples during the Storage Period
2.6.5. Color Properties
2.6.6. Texture Properties
2.6.7. Sensorial Properties of Yogurt Drink Samples
2.6.8. Lactic Acid Bacteria (Starter bacteria) Count
3. Materials and Methods
3.1. Experimental Materials
3.2. Chemical Composition of Purslane
3.3. Preparation of Purslane Extract (PuE)
3.4. Polyphenolic Content in Purslane Extract
3.4.1. Total Phenolics (TPs) and Flavonoids (TFs)
3.4.2. Phenolic Compounds Profile by LC-MS/MS
3.5. Volatile Compounds in Purslane
3.6. Purslane Extract Activity
3.6.1. Scavenging Activity of PuE
3.6.2. Cytotoxicity
3.6.3. Antiviral Activity
3.6.4. Antimicrobial Activity
3.7. Experimental Safety Layout
3.7.1. Ethical Statement
3.7.2. Estimation of Serum Biochemical Parameters
3.7.3. Protective Effect of Purslane Extract against Fe2+—Induced Brain Lipid Peroxidation
3.8. Preparation of Functional Yogurt Supplemented with Purslane Extract
3.9. Chemical Composition of Yogurt
3.10. Fluctutaion of Purslane Extract—Yogyrt Properties during Four Weeks of Cold Storage
3.10.1. Physicochemical Properties
pH Determination
Titratable Acidity (TTA)
Total Soluble Solids (TSS)
Malondialdehyde (MDA) Determination
Fat Content
Syneresis
Viscosity
Water Holding Capacity (WHC)
Texture Profile Analysis
3.10.2. Determination of Antioxidant Activity, Phenolic Content in Yogurt Samples
3.10.3. Sensorial Traits and Color Measurements
3.10.4. Total Viable and Lactic Acid Bacteria Count
3.11. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
References
- Adinepour, F.; Pouramin, S.; Rashidinejad, A.; Jafari, S.M. Fortification/enrichment of milk and dairy products by encapsulated bioactive ingredients. Food Res. Int. 2022, 157, 111212. [Google Scholar] [CrossRef]
- Minj, J.; Dogra, S. Significance of Fortification of Beneficial Natural Ingredients in Milk and Milk Products. In Dairy Processing: Advanced Research to Applications; Minj, J., Sudhakaran, V.A., Kumari, A., Eds.; Springer: Singapore, 2020; pp. 87–118. [Google Scholar]
- Paswan, V.K.; Rose, H.; Singh, C.S.; Yamini, S.; Rathaur, A. Herbs and spices fortified functional dairy products. In Herbs and Spices—New Processing Technologies; IntechOpen: London, UK, 2021; pp. 1–22. [Google Scholar]
- Anbudhasan, P.; Surendraraj, A.; Karkuzhali, S.; Sathishkumaran, S. Natural antioxidants and its benefits. Int. J. Food Nutr. Sci. 2014, 3, 225–232. [Google Scholar]
- Atta, E.M.; Mohamed, N.H.; Silaev, A.A.A. Antioxidants: An overview on the natural and synthetic types. Eur. Chem. Bull. 2017, 6, 365–375. [Google Scholar] [CrossRef]
- Venkatesh, R.; Sood, D. A Review of the Physiological Implications of Antioxidants in Food; Bachelor of Science Interactive Qualifying Project; Worcester Polytechnic Institute: Worcester, MA, USA, 2011. [Google Scholar]
- Kandylis, P. Phytochemicals and antioxidant properties of edible flowers. Appl. Sci. 2022, 12, 9937. [Google Scholar] [CrossRef]
- de Carvalho, A.P.A.; Conte-Junior, C.A. Health benefits of phytochemicals from Brazilian native foods and plants: Antioxidant, antimicrobial, anti-cancer, and risk factors of metabolic/endocrine disorders control. Trends Food Sci. Technol. 2021, 111, 534–548. [Google Scholar] [CrossRef]
- Adebooye, O.C.; Vijayalakshmi, R.; Singh, V. Peroxidase activity, chlorophylls and antioxidant profile of two leaf vegetables (Solanum nigrum L. and Amaranthus cruentus L.) under six pretreatment methods before cooking. Int. J. Food Sci. Technol. 2008, 43, 173–178. [Google Scholar] [CrossRef]
- Saad, A.M.; Mohamed, A.S.; Ramadan, M.F. Storage and heat processing affect flavors of cucumber juice enriched with plant extracts. Int. J. Veg. Sci. 2021, 27, 277–287. [Google Scholar] [CrossRef]
- Lee, Y.H.; Choo, C.; Watawana, M.I.; Jayawardena, N.; Waisundara, V.Y. An appraisal of eighteen commonly consumed edible plants as functional food based on their antioxidant and starch hydrolase inhibitory activities. J. Sci. Food Agric. 2015, 95, 2956–2964. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Elbestawy, A.R.; Gado, A.R.; Nader, M.M.; Saad, A.M.; El-Tahan, A.M.; Taha, A.E.; Salem, H.M.; El-Tarabily, K.A. Hot red pepper powder as a safe alternative to antibiotics in organic poultry feed: An updated overview. Poult. Sci. 2022, 101, 101684. [Google Scholar] [CrossRef]
- Côté, J.; Caillet, S.; Doyon, G.; Sylvain, J.-F.; Lacroix, M. Bioactive compounds in cranberries and their biological properties. Crit. Rev. Food Sci. Nutr. 2010, 50, 666–679. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; Abuljadayel, D.A.; Shafi, M.E.; Albaqami, N.M.; Desoky, E.-S.M.; El-Tahan, A.M.; Mesiha, P.K.; Elnahal, A.S.; Almakas, A.; Taha, A.E. Control of foliar phytoparasitic nematodes through sustainable natural materials: Current progress and challenges. Saudi J. Biol. Sci. 2021, 28, 7314–7326. [Google Scholar] [CrossRef]
- Cosme, P.; Rodríguez, A.B.; Espino, J.; Garrido, M. Plant phenolics: Bioavailability as a key determinant of their potential health-promoting applications. Antioxidants 2020, 9, 1263. [Google Scholar] [CrossRef] [PubMed]
- Abd Elkader, A.M.; Labib, S.; Taha, T.F.; Althobaiti, F.; Aldhahrani, A.; Salem, H.M.; Saad, A.; Ibrahim, F.M. Phytogenic compounds from avocado (Persea americana L.) extracts; antioxidant activity, amylase inhibitory activity, therapeutic potential of type 2 diabetes. Saudi J. Biol. Sci. 2022, 29, 1428–1433. [Google Scholar] [CrossRef] [PubMed]
- Saad, A.M.; Salem, H.M.; El-Tahan, A.M.; El-Saadony, M.T.; Alotaibi, S.S.; El-Shehawi, A.M.; Abd El-Mageed, T.A.; Taha, A.E.; Alkahtani, M.A.; Ahmed, A.E. Biological control: An effective approach against nematodes using black pepper plants (Piper nigrum L.). Saudi J. Biol. Sci. 2022, 29, 2047–2055. [Google Scholar] [CrossRef] [PubMed]
- Salman, K.; Mahmoud, E.A.; Abd-Alla, A. Preparing untraditional kishk formula with purslane as natural source of bioactive compounds. J. Food Dairy Sci. 2020, 11, 299–305. [Google Scholar] [CrossRef]
- Salehi, M.; Sadeghi Mahoonak, A.; Khomeiri, M. Fortification of yogurt with common purslane (Portulaca oleracea): Evalution of its fatty acid profile and antioxidant properties. J. Food Process. Preserv. 2021, 13, 79–94. [Google Scholar]
- Kumar, A.; Sreedharan, S.; Kashyap, A.K.; Singh, P.; Ramchiary, N. A review on bioactive phytochemicals and ethnopharmacological potential of purslane (Portulaca oleracea L.). Heliyon 2021, 8, e08669. [Google Scholar] [CrossRef]
- Silva, R.; Carvalho, I.S. In vitro antioxidant activity, phenolic compounds and protective effect against DNA damage provided by leaves, stems and flowers of Portulaca oleracea (Purslane). Nat. Prod. Commun. 2014, 9, 45–50. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, R.; Srivastava, V.; Singh, A. Multipurpose benefits of an underexplored species purslane (Portulaca oleracea L.): A critical review. Environ. Manag. 2023, 72, 309–320. [Google Scholar] [CrossRef]
- Hussien, H.A.; Salem, E.M. Development of gluten free snacks fortified with purslane (Portulaca oleracea) powder. Int. J. Food Sci. Nutr. 2016, 4, 136–144. [Google Scholar]
- Melilli, M.G.; Pagliaro, A.; Scandurra, S.; Gentile, C.; Di Stefano, V. Omega-3 rich foods: Durum wheat spaghetti fortified with Portulaca oleracea. Food Biosci. 2020, 37, 100730. [Google Scholar] [CrossRef]
- Abd El-Azime, A.S.; Hussein, E.M.; Ashry, O.M. Synergestic effect of aqueous purslane (Portulaca oleracea L.) extract and fish oil on radiation-induced damage in rats. Int. J. Radiat. Biol. 2014, 90, 1184–1190. [Google Scholar] [CrossRef]
- Fontana, L.; Bermudez-Brito, M.; Plaza-Diaz, J.; Munoz-Quezada, S.; Gil, A. Sources, isolation, characterisation and evaluation of probiotics. Br. J. Nutr. 2013, 109, 35–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashaolu, T.J. Immune boosting functional foods and their mechanisms: A critical evaluation of probiotics and prebiotics. Biomed. Pharmacother. 2020, 130, 110625. [Google Scholar] [CrossRef] [PubMed]
- El-Saadony, M.T.; Sitohy, M.Z.; Ramadan, M.F.; Saad, A.M. Green nanotechnology for preserving and enriching yogurt with biologically available iron (II). Innov. Food Sci. Emerg. Technol. 2021, 69, 102645. [Google Scholar] [CrossRef]
- Rezende, E.S.V.; Lima, G.C.; Naves, M.M.V. Dietary fibers as beneficial microbiota modulators: A proposed classification by prebiotic categories. Nutrition 2021, 89, 111217. [Google Scholar] [CrossRef]
- Salehi, M.; Ghorbani, M.; Sadeghi Mahoonk, A.; Khomeiri, M. Physicochemical, antioxidant and sensory properties of yogurt fortified with common purslane (Portulaca oleracea) extract. J. Food Meas. Charact. 2021, 15, 4288–4296. [Google Scholar] [CrossRef]
- El-Sayed, M.; Awad, S.; Ibrahim, A. Impact of purslane (Portulaca oleracea L.) extract as antioxidant and antimicrobial agent on overall quality and shelf life of Greek-style yoghurt. Egypt J. Food Sci. 2019, 47, 51–64. [Google Scholar] [CrossRef]
- Obied, W.; Mohamoud, E.; Mohamed, O. Portulaca oleracea (purslane): Nutritive composition and clinico-pathological effects on Nubian goats. Small Rumin. Res. 2003, 48, 31–36. [Google Scholar] [CrossRef]
- Uddin, M.; Juraimi, A.S.; Hossain, M.S.; Nahar, M.; Un, A.; Ali, M.; Rahman, M. Purslane weed (Portulaca oleracea): A prospective plant source of nutrition, omega-3 fatty acid, and antioxidant attributes. Sci. World J. 2014, 2014, 951019. [Google Scholar] [CrossRef] [Green Version]
- de Souza, P.G.; Rosenthal, A.; Ayres, E.M.M.; Teodoro, A.J. Potential functional food products and molecular mechanisms of Portulaca oleracea L. on Anticancer Activity: A Review. Oxidative Med. Cell. Longev. 2022, 2022, 7235412. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Fernandes, Â.; Dias, M.I.; Vasilakoglou, I.B.; Petrotos, K.; Barros, L.; Ferreira, I.C. Nutritional value, chemical composition and cytotoxic properties of common purslane (Portulaca oleracea L.) in relation to harvesting stage and plant part. Antioxidants 2019, 8, 293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández-Poyatos, M.d.P.; Llorent-Martínez, E.J.; Ruiz-Medina, A. Phytochemical composition and antioxidant activity of Portulaca oleracea: Influence of the steaming cooking process. Foods 2021, 10, 94. [Google Scholar] [CrossRef]
- Karoune, S.; Kechebar, M.S.A.; Douffi, H.; Djellouli, A. Phenolic compounds and their antioxidant activities in Portulaca oleracea L. related to solvent extraction. Int. J. Biosci. 2017, 11, 147–155. [Google Scholar]
- Ranjah, M.A. Lemongrass: A useful ingredient for functional foods. Int. J. Food Allied Sci. 2019, 4, 11–19. [Google Scholar]
- Sicari, V.; Loizzo, M.R.; Tundis, R.; Mincione, A.; Pellicano, T.M. Portulaca oleracea L.(Purslane) extracts display antioxidant and hypoglycaemic effects. J. Appl. Bot. Food Qual. 2018, 91, 39–46. [Google Scholar]
- Montoya-García, C.O.; García-Mateos, R.; Becerra-Martínez, E.; Toledo-Aguilar, R.; Volke-Haller, V.H.; Magdaleno-Villar, J.J. Bioactive compounds of purslane (Portulaca oleracea L.) according to the production system: A review. Sci. Hortic. 2023, 308, 111584. [Google Scholar] [CrossRef]
- Fukalova Fukalova, T.; García-Martínez, M.D.; Raigón, M.D. Nutritional composition, bioactive compounds, and volatiles profile characterization of two edible undervalued plants: Portulaca oleracea L. and Porophyllum ruderale (Jacq.) Cass. Plants 2022, 11, 377. [Google Scholar] [CrossRef] [PubMed]
- Almashad, A.A.; Ibrahim Ramadan, G.E.; Abdelrazek, R.H. Phytochemicals, antioxidant and volatile compounds evaluation of Egyptian purslane leaves. Arab. Univ. J. Agric. Sci. 2019, 27, 2573–2582. [Google Scholar] [CrossRef]
- Carrasco, F.R.; Schmidt, G.; Romero, A.L.; Sartoretto, J.L.; Caparroz-Assef, S.M.; Bersani-Amado, C.A.; Cuman, R.K.N. Immunomodulatory activity of Zingiber officinale Roscoe, Salvia officinalis L. and Syzygium aromaticum L. essential oils: Evidence for humor-and cell-mediated responses. J. Pharm. Pharmacol. 2009, 61, 961–967. [Google Scholar] [CrossRef]
- Melilli, M.G.; Pagliaro, A.; Bognanni, R.; Scandurra, S.; Di Stefano, V. Antioxidant activity and fatty acids quantification in Sicilian purslane germplasm. Nat. Prod. Res. 2020, 34, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; He, Z.; Zhang, D.; Li, H. Antioxidant activity of purslane extract and its inhibitory effect on the lipid and protein oxidation of rabbit meat patties during chilled storage. J. Sci. Food Agric. 2021, 101, 1953–1962. [Google Scholar] [CrossRef] [PubMed]
- Farshori, N.N.; Al-Sheddi, E.S.; Al-Oqail, M.M.; Musarrat, J.; Al-Khedhairy, A.A.; Siddiqui, M.A. Cytotoxicity assessments of Portulaca oleracea and Petroselinum sativum seed extracts on human hepatocellular carcinoma cells (HepG2). Asian Pac. J. Cancer Prev. 2014, 15, 6633–6638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keser, F.; Karatepe, M.; Keser, S.; Tekİn, S.; Türkoğlu, İ.; Kaygİlİ, O.; Kirbag, S. In vitro biological activities and phytochemical contents of Portulaca oleracea L.(Purslane). J. Phys. Chem. Funct. Mat. 2021, 4, 1–7. [Google Scholar]
- Seyedi, Z.; Amiri, M.S.; Mohammadzadeh, V.; Hashemzadeh, A.; Haddad-Mashadrizeh, A.; Mashreghi, M.; Qayoomian, M.; Hashemzadeh, M.R.; Simal-Gandara, J.; Taghavizadeh Yazdi, M.E. Icariin: A promising natural product in biomedicine and tissue engineering. J. Funct. Biomater. 2023, 14, 44. [Google Scholar] [CrossRef]
- Mousavi-Kouhi, S.M.; Beyk-Khormizi, A.; Amiri, M.S.; Mashreghi, M.; Hashemzadeh, A.; Mohammadzadeh, V.; Alavi, F.; Mottaghipisheh, J.; Sarafraz Ardakani, M.R.; Taghavizadeh Yazdi, M.E. Plant gel-mediated synthesis of gold-coated nanoceria using ferula gummosa: Characterization and estimation of its cellular toxicity toward breast cancer cell lines. J. Funct. Biomater. 2023, 14, 332. [Google Scholar]
- Ghorani-Azam, A.; Mottaghipisheh, J.; Amiri, M.S.; Mashreghi, M.; Hashemzadeh, A.; Haddad-Mashadrizeh, A.; Nourbakhsh, F.; Nadaf, M.; Qayoomian, M.; Yazdi, M.E.T.; et al. Resveratrol-mediated gold-nanoceria synthesis as green nanomedicine for phytotherapy of hepatocellular carcinoma. Front. Biosci. 2022, 27, 227. [Google Scholar] [CrossRef]
- Nichani, K.; Li, J.; Suzuki, M.; Houston, J.P. Evaluation of Caspase-3 activity during apoptosis with fluorescence lifetime-based cytometry measurements and phasor analyses. Cytom. Part A J. Int. Soc. Anal. Cytol. 2020, 97, 1265–1275. [Google Scholar] [CrossRef]
- Yadav, P.; Yadav, R.; Jain, S.; Vaidya, A. Caspase-3: A primary target for natural and synthetic compounds for cancer therapy. Chem. Biol. Drug Des. 2021, 98, 144–165. [Google Scholar] [CrossRef]
- Tleubayeva, M.I.; Datkhayev, U.M.; Alimzhanova, M.; Ishmuratova, M.Y.; Korotetskaya, N.V.; Abdullabekova, R.M.; Flisyuk, E.V.; Gemejiyeva, N.G. Component composition and antimicrobial activity of CO2 extract of Portulaca oleracea, growing in the Territory of Kazakhstan. Sci. World J. 2021, 2021, 5434525. [Google Scholar] [CrossRef]
- Seif, M.M.; Madboli, A.-N.; Marrez, D.A.; Aboulthana, W.M.K. Hepato-Renal protective effects of egyptian purslane extract against experimental cadmium toxicity in rats with special emphasis on the functional and histopathological changes. Toxicol. Rep. 2019, 6, 625–631. [Google Scholar] [CrossRef]
- Wang, C.; Liu, Q.; Ye, F.; Tang, H.; Xiong, Y.; Wu, Y.; Wang, L.; Feng, X.; Zhang, S.; Wan, Y.; et al. Dietary purslane (Portulaca oleracea L.) promotes the growth performance of broilers by modulation of gut microbiota. AMB Express 2021, 11, 31. [Google Scholar] [CrossRef] [PubMed]
- Saleh, S.R.; Manaa, A.; Sheta, E.; Ghareeb, D.A.; Abd-Elmonem, N.M. The synergetic effect of Egyptian Portulaca oleracea L. (Purslane) and Cichorium intybus L.(Chicory) extracts against glucocorticoid-induced testicular toxicity in rats through Attenuation of Oxidative Reactions and Autophagy. Antioxidants 2022, 11, 1272. [Google Scholar] [CrossRef]
- Mousa, A.; Taha, M.; ELdeighdye, S.; Kamal, A. The role of purslane in modulating diverse effects of high fat diet on biochemical, histological, and molecular parameters of rats’ liver. Braz. J. Biol. 2023, 83, e248755. [Google Scholar] [CrossRef] [PubMed]
- Nair, G.G.; Nair, C.K.K. Radioprotective effects of gallic acid in mice. BioMed Res. Int. 2013, 2013, 953079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saenthaweesuk, S.; Munkong, N.; Parklak, W.; Thaeomor, A.; Chaisakul, J.; Somparn, N. Hepatoprotective and antioxidant effects of Cymbopogon citratus Stapf (Lemongrass) extract in paracetamolinduced hepatotoxicity in rats. Trop. J. Pharm. Res. 2017, 16, 101–107. [Google Scholar] [CrossRef] [Green Version]
- Said, A.M.; Atwa, S.A.; Khalifa, O.A. Ameliorating effect of gum arabic and lemongrass on chronic kidney disease induced experimentally in rats. Bull. Natl. Res. Cent. 2019, 43, 47. [Google Scholar] [CrossRef] [Green Version]
- Burri, S.C.; Ekholm, A.; Håkansson, Å.; Tornberg, E.; Rumpunen, K. Antioxidant capacity and major phenol compounds of horticultural plant materials not usually used. J. Funct. Foods 2017, 38, 119–127. [Google Scholar] [CrossRef]
- Oboh, G.; Puntel, R.; Rocha, J. Hot pepper (Capsicum annuum, Tepin and Capsicum chinese, Habanero) prevents Fe2+-induced lipid peroxidation in brain–in vitro. Food Chem. 2007, 102, 178–185. [Google Scholar] [CrossRef]
- Fouda, F. Cytotoxicity assessments of Portulaca oleracea plant extracts on some cancer cells. Ann. Agric. Sci. Moshtohor 2022, 60, 835–844. [Google Scholar] [CrossRef]
- Valencia-Avilés, E.; García-Pérez, M.E.; Garnica-Romo, M.G.; de Dios Figueroa-Cárdenas, J.; Paciulli, M.; Martinez-Flores, H.E. Chemical composition, physicochemical evaluation and sensory analysis of yogurt added with extract of polyphenolic compounds from Quercus crassifolia oak bark. Funct. Foods Health Dis. 2022, 12, 502–517. [Google Scholar] [CrossRef]
- Nasser, S. The addition of lemon peel powder affects the properties of yogurt. J. Food Dairy Sci. 2022, 13, 65–70. [Google Scholar] [CrossRef]
- Sivakumar, G. Effect of tulsi leaf extract on Physico-chemical and microbial quality of raw milk. Int. J. Sci. Environ. Technol. 2017, 6, 1626–1631. [Google Scholar]
- Saad, A.M.; Mohamed, A.S.; El-Saadony, M.T.; Sitohy, M.Z. Palatable functional cucumber juices supplemented with polyphenols-rich herbal extracts. LWT-Food Sci. Technol. 2021, 148, 111668. [Google Scholar] [CrossRef]
- Fu, Q.; Zhou, S.; Yu, M.; Lu, Y.; He, G.; Huang, X.; Huang, Y. Portulaca oleracea polysaccharides modulate intestinal microflora in aged rats in vitro. Front. Microbiol. 2022, 13, 147. [Google Scholar] [CrossRef]
- Wang, X.; Kristo, E.; LaPointe, G. The effect of apple pomace on the texture, rheology and microstructure of set type yogurt. Food Hydrocoll. 2019, 91, 83–91. [Google Scholar] [CrossRef]
- Dello Staffolo, M.; Sato, A.C.; Cunha, R. Utilization of plant dietary fibers to reinforce low-calorie dairy dessert structure. Food Bioprocess Technol. 2017, 10, 914–925. [Google Scholar] [CrossRef]
- Osman, D.M.; Noureldin, H.A.; El-Gazzar, F.E.; Salman, K.H. Fortification of ice milk with purslane (Portulaca oleracea) bioactive compounds. Assiut J. Agric. Sci. 2023, 54, 34–49. [Google Scholar] [CrossRef]
- Ahmed, I.A.M.; Alqah, H.A.; Saleh, A.; Al-Juhaimi, F.Y.; Babiker, E.E.; Ghafoor, K.; Hassan, A.B.; Osman, M.A.; Fickak, A. Physicochemical quality attributes and antioxidant properties of set-type yogurt fortified with argel (Solenostemma argel Hayne) leaf extract. LWT-Food Sci. Technol. 2021, 137, 110389. [Google Scholar] [CrossRef]
- Guemidi, C.; Saada, D.A.; Chabane, O.A.; Elmastas, M.; Erenler, R.; Yilmaz, M.A.; Tarhan, A.; Akkal, S.; Khelifi, H. Antioxidant potential, lipid profile and sensory attributes of a functional yogurt formulated with hydroethanolic extract of Mentha Piperita L. Res. Sq. 2023, preprint. [Google Scholar] [CrossRef]
- Cho, W.-Y.; Kim, D.-H.; Lee, H.-J.; Yeon, S.-J.; Lee, C.-H. Journal of food quality evaluation of effect of extraction solvent on selected properties of olive leaf extract. J. Food Qual. 2020, 2020, 3013649. [Google Scholar] [CrossRef]
- Semeniuc, C.A.; Mandrioli, M.; Rodriguez-Estrada, M.T.; Muste, S.; Lercker, G. Thiobarbituric acid reactive substances in flavored phytosterol-enriched drinking yogurts during storage: Formation and matrix interferences. Eur. Food Res. Technol. 2016, 242, 431–439. [Google Scholar] [CrossRef]
- Alappat, B.; Alappat, J. Anthocyanin pigments: Beyond aesthetics. Molecules 2020, 25, 5500. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Liang, J.; Lin, X.; Xu, H.; Tadda, M.A.; Lan, L.; Liu, D. Fast start-up strategies of MBBR for mariculture wastewater treatment. J. Environ. Manag. 2019, 248, 109267. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis of AOAC International, 19th ed.; AOAC: Washington, DC, USA, 2012. [Google Scholar]
- Moczkowska, M.; Karp, S.; Niu, Y.; Kurek, M.A. Enzymatic, enzymatic-ultrasonic and alkaline extraction of soluble dietary fibre from flaxseed–A physicochemical approach. Food Hydrocoll. 2019, 90, 105–112. [Google Scholar] [CrossRef]
- Alamoudi, S.A.; Saad, A.M.; Alsubhi, N.H.; Alrefaei, G.I.; Al-Quwaie, D.A.; Binothman, N.; Aljadani, M.; Alharbi, M.; Alanazi, H.; Babalghith, A.O. Upgrading the physiochemical and sensory quality of yogurt by incorporating polyphenol-enriched citrus pomaces with antioxidant, antimicrobial, and antitumor activities. Front. Nutr. 2022, 9, 999581. [Google Scholar] [CrossRef]
- Attard, E. A rapid microtitre plate Folin-Ciocalteu method for the assessment of polyphenols. Open Life Sci. 2013, 8, 48–53. [Google Scholar] [CrossRef]
- Herald, T.J.; Gadgil, P.; Tilley, M. High-throughput micro plate assays for screening flavonoid content and DPPH-scavenging activity in Sorghum bran and flour. J. Sci. Food Agric. 2012, 92, 2326–2331. [Google Scholar] [CrossRef]
- Politeo, O.; Jukić, M.; Miloš, M. Chemical composition and antioxidant activity of essential oils of twelve spice plants. Croat. Chem. Acta 2006, 79, 545–552. [Google Scholar]
- Li, H.; Wu, X.; Li, X.; Cao, X.; Li, Y.; Cao, H.; Men, Y. Multistage extraction of star anise and black pepper derivatives for antibacterial, antioxidant, and anticancer activity. Front. Chem. 2021, 9, 660138. [Google Scholar] [CrossRef]
- Pino, J.A.; Barzola-Miranda, S.E. Characterization of odor-active compounds in pechiche (Vitex cymosa Berteo ex Speng) fruit. J. Raw Mater. Proc. Foods 2020, 1, 33–39. [Google Scholar]
- Jia, N.; Kong, B.; Liu, Q.; Diao, X.; Xia, X. Antioxidant activity of black currant (Ribes nigrum L.) extract and its inhibitory effect on lipid and protein oxidation of pork patties during chilled storage. Meat Sci. 2012, 91, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Gao, X.; Cai, Y.; Shao, X.; Jia, G.; Huang, Y.; Qin, X.; Wang, J.; Zheng, X. Antitumor activity of Portulaca oleracea L. polysaccharides against cervical carcinoma in vitro and in vivo. Carbohydr. Polym. 2013, 96, 376–383. [Google Scholar] [CrossRef]
- Dai, Z.; Nair, V.; Khan, M.; Ciolino, H.P. Pomegranate extract inhibits the proliferation and viability of MMTV-Wnt-1 mouse mammary cancer stem cells in vitro. Oncol. Rep. 2010, 24, 1087–1091. [Google Scholar] [PubMed]
- Ashour, E.A.; El-Hack, M.E.A.; Shafi, M.E.; Alghamdi, W.Y.; Taha, A.E.; Swelum, A.A.; Tufarelli, V.; Mulla, Z.S.; El-Ghareeb, W.R.; El-Saadony, M.T. Impacts of green coffee powder supplementation on growth performance, carcass characteristics, blood indices, meat quality and gut microbial load in broilers. Agriculture 2020, 10, 457. [Google Scholar] [CrossRef]
- Schumann, G.; Klauke, R. New IFCC reference procedures for the determination of catalytic activity concentrations of five enzymes in serum: Preliminary upper reference limits obtained in hospitalized subjects. Clin. Chim. Acta 2003, 327, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Beutler, E. G6PD deficiency. Blood 1994, 84, 3613–3636. [Google Scholar] [CrossRef] [Green Version]
- Bulut, M.; Selek, S.; Bez, Y.; Kaya, M.C.; Gunes, M.; Karababa, F.; Celik, H.; Savas, H.A. Lipid peroxidation markers in adult attention deficit hyperactivity disorder: New findings for oxidative stress. Psychiatry Res. 2013, 209, 638–642. [Google Scholar] [CrossRef]
- Armbruster, D.A.; Lambert, P.A. Direct assay of LDL cholesterol: Comparing measurement and calculation. Lab. Med. 1996, 27, 613–617. [Google Scholar] [CrossRef] [Green Version]
- Devi, R.; Sharma, D. Hypolipidemic effect of different extracts of Clerodendron colebrookianum Walp in normal and high-fat diet fed rats. J. Ethnopharmacol. 2004, 90, 63–68. [Google Scholar] [CrossRef]
- Dahlan, H.A.; Sani, N.A. The interaction effect of mixing starter cultures on homemade natural yogurt’s pH and viscosity. Int. J. Food Stud. 2017, 6, 152–158. [Google Scholar] [CrossRef]
- Korkmaz, I.O.; Bilici, C.; Korkmaz, S. Sensory, pH, synaeresis, water-holding capacity, and microbiological changes in homemade yogurt prepared with maca (Lepidium meyenii) powder and propolis extract. Int. J. Gastron. Food Sci. 2021, 23, 100291. [Google Scholar] [CrossRef]
- Nguyen, P.T.; Kravchuk, O.; Bhandari, B.; Prakash, S. Effect of different hydrocolloids on texture, rheology, tribology and sensory perception of texture and mouthfeel of low-fat pot-set yoghurt. Food Hydrocoll. 2017, 72, 90–104. [Google Scholar] [CrossRef] [Green Version]
- Namir, M.; Iskander, A.; Alyamani, A.; Sayed-Ahmed, E.T.A.; Saad, A.M.; Elsahy, K.; El-Tarabily, K.A.; Conte-Junior, C.A. Upgrading common wheat pasta by fiber-rich fraction of potato peel byproduct at different particle sizes: Effects on physicochemical, thermal, and sensory properties. Molecules 2022, 27, 2868. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; Khalil, O.S.F.; Osman, A.; Alshilawi, M.S.; Taha, A.E.; Aboelenin, S.M.; Shukry, M.; Saad, A.M. Bioactive peptides supplemented raw buffalo milk: Biological activity, shelf life and quality properties during cold preservation. Saudi J. Biol. Sci. 2021, 28, 4581–4591. [Google Scholar] [CrossRef]

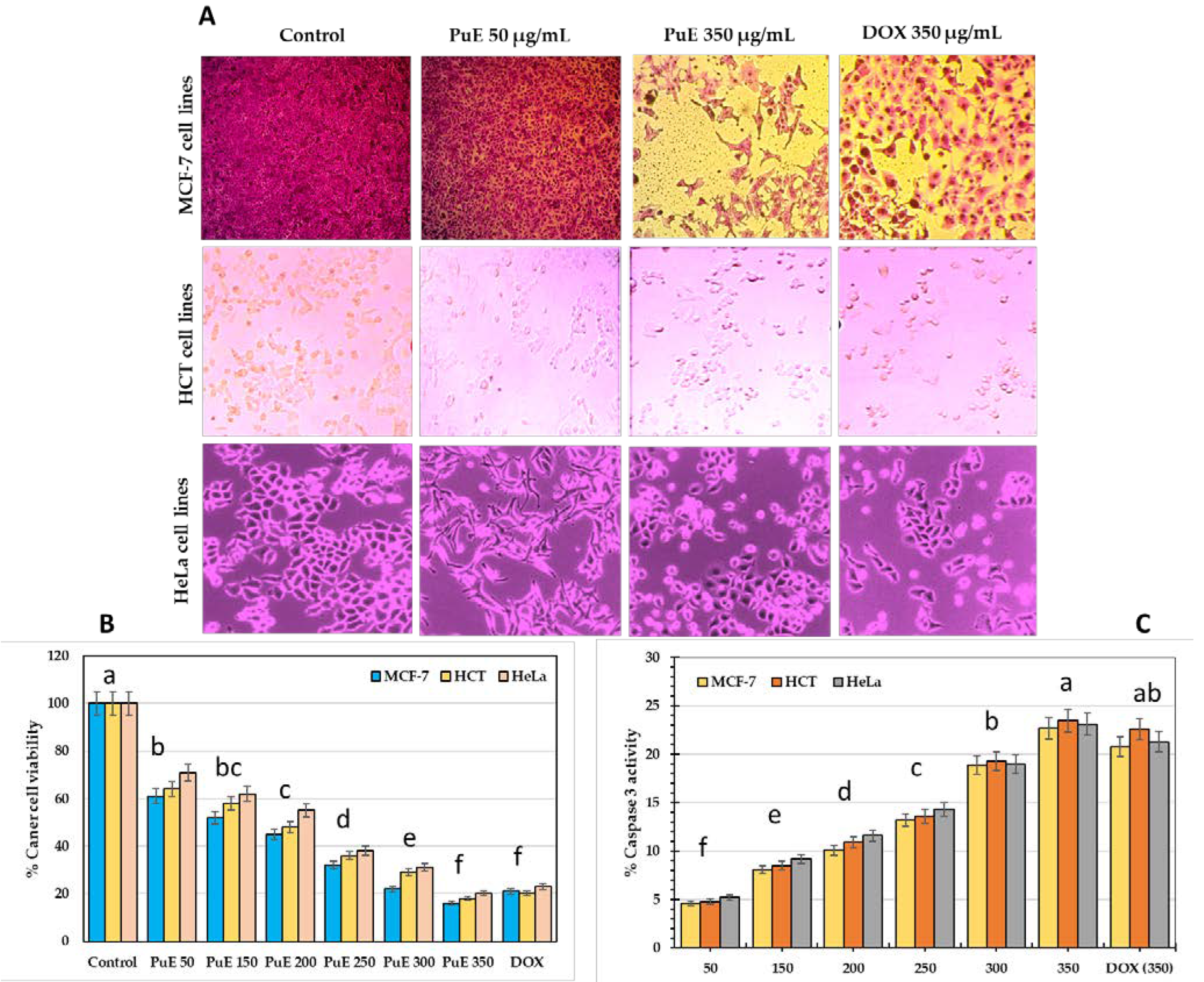

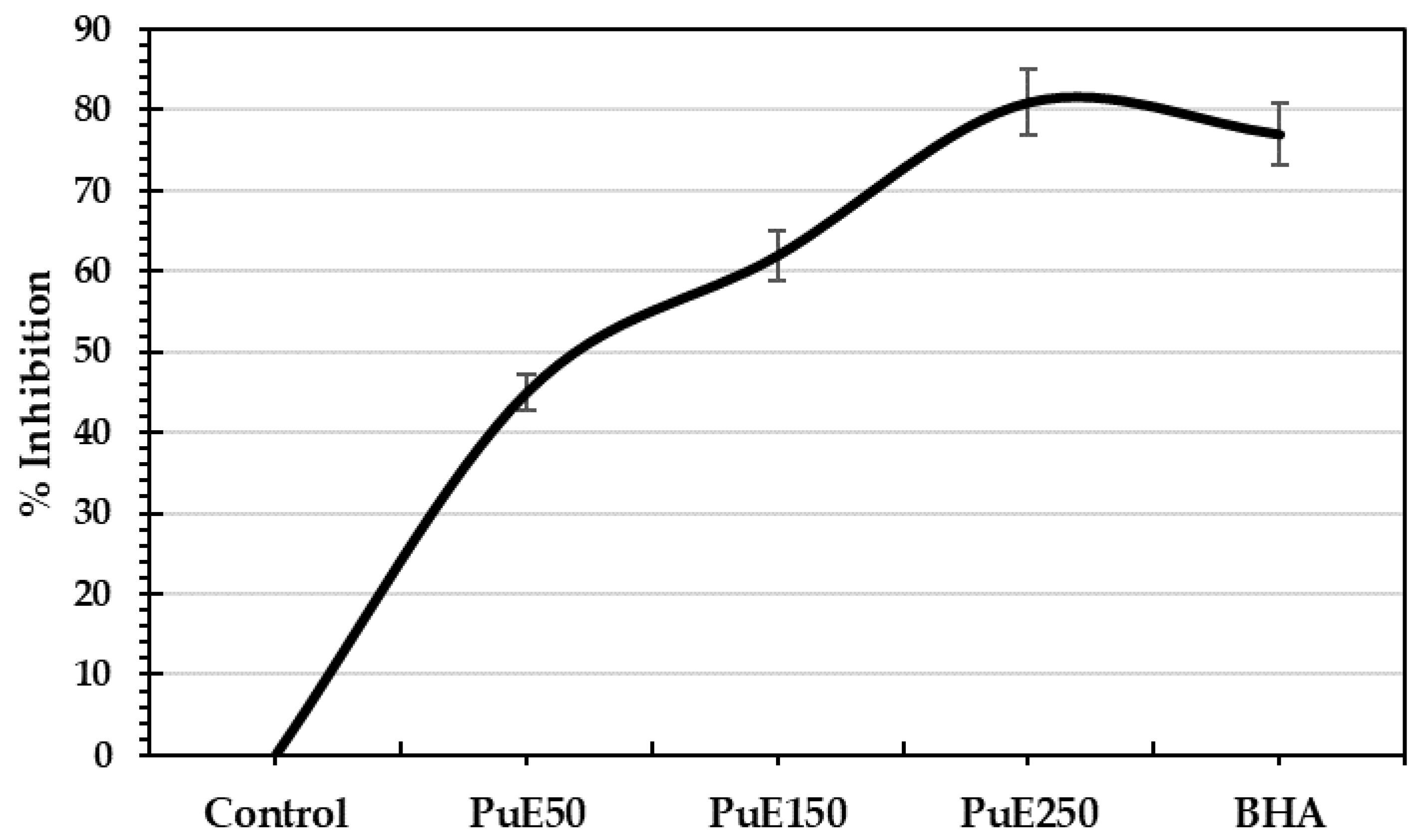

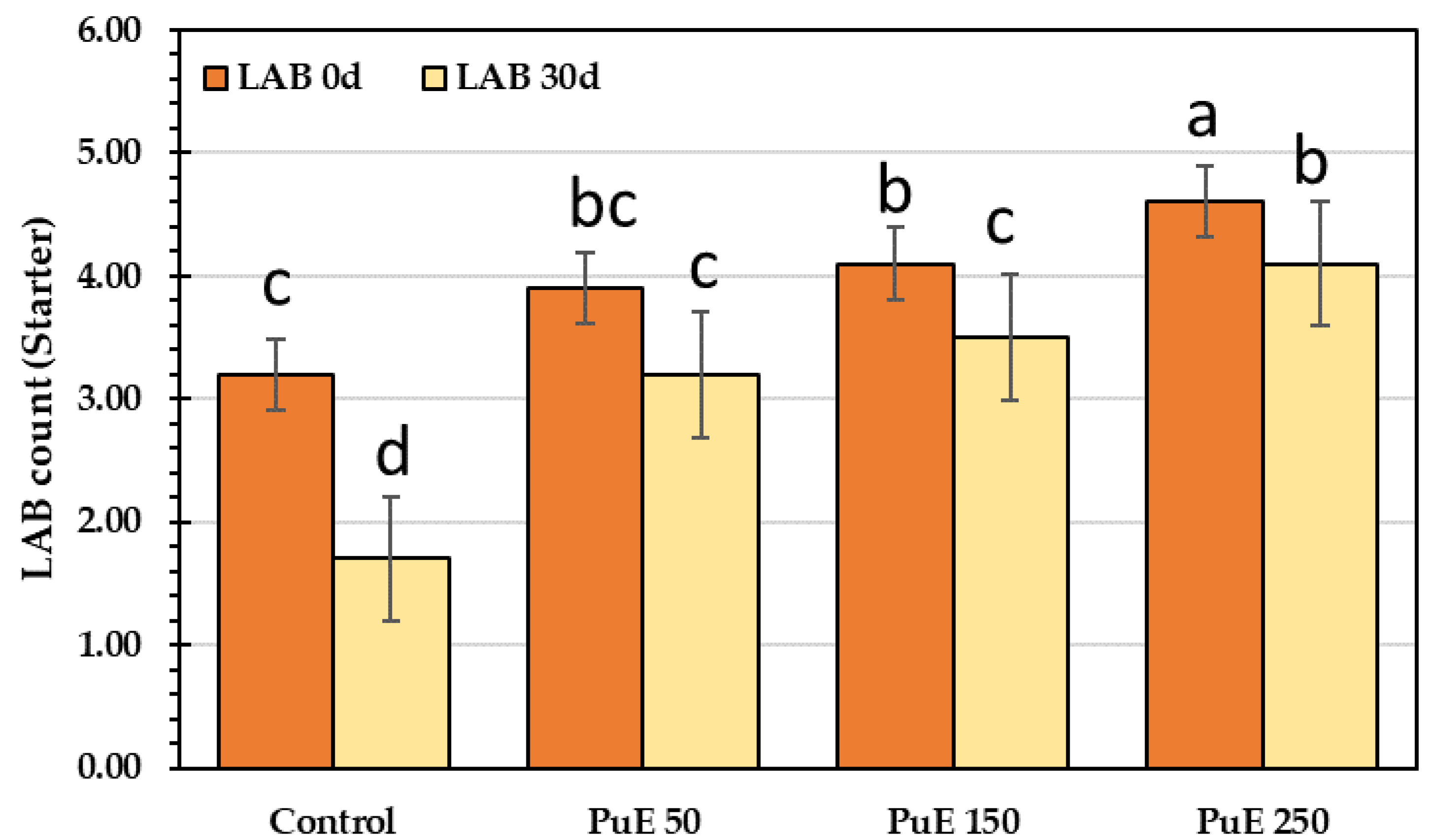
| Parameters | Concentration (%) |
|---|---|
| Moisture | 10.9 ± 0.2 |
| Total ash | 2.45 ± 0.5 |
| Crude fiber | 21.63 ± 0.8 |
| Crude protein | 18.2 ± 0.1 |
| Crude fat | 5.2 ± 0.4 |
| Carbohydrates | 44.3 ± 0.6 |
| Minerals | Concentration (ppm) |
| Mg | 695 ± 0.9 b |
| Ca | 671 ± 1.1 b,c |
| P | 450 ± 0.8 c |
| K | 5033 ± 2.1 a |
| Fe | 20 ± 0.5 d |
| Phytochemicals | Concentration (mg/mL) |
| Phenolic compounds | 250 ± 1.2 |
| Flavonoids | 56 ± 0.9 |
| RT | [M-H]−1 Ions at m/z | % Base Peak (MS2) | Identified Compounds | Contents (mg/100 g) |
|---|---|---|---|---|
| Phenolic acids | ||||
| 3.25 | 311 | 151 | protocatechuic acid glu | 8.55 ± 0.1 |
| 5.00 | 472 | 132, 151, 312 | protocatechuic acid caffeoyl-glu | 11.36 ± 0.3 |
| 9.00 | 135 | 115 | p-hydroxybenzoic acid | 12.89 ± 0.9 |
| 10.44 | 351 | 190 | chlorogenic acid | 16.56 ± 0.5 |
| 10.55 | 339 | 133, 177 | caffeic acid glu | 30.69 ± 0.8 |
| 11.30 | 322 | 165 | coumaric acid glu | 18.21 ± 0.2 |
| 14.18 | 381 | 222 | sinapic acid glu | 10.35 ± 0.3 |
| 16.30 | 350 | 190 | neochlorogenic acid | 21.64 ± 0.4 |
| 18.55 | 335 | 165 | p-coumaroylquinic acid | 14.33 ± 0.6 |
| 20.22 | 195 | 165 | syringic acid | 12.69 ± 0.1 |
| p-value | 0.025 | |||
| Flavonoids | ||||
| 13.51 | 302 | 195 | gallo-catechin | 25.63 ± 0.2 |
| 22.79 | 287 | 243 | catechin | 29.65 ± 0.6 |
| 28.52 | 440 | 303 | quercetin rham | 15.23 ± 0.1 |
| 28.62 | 444 | 286 | kaempferol glu | 16.54 ± 0.2 |
| 31.22 | 459 | 282 | kaempferol glca | 15.33 ± 0.9 |
| 31.78 | 415 | 266 | naringenin rham | 10.68 ± 0.3 |
| p-value | 0.029 | |||
| Tannins | ||||
| 3.71 | 329 | 122, 168 | galloyl glucose | 11.2 ± 0.2 |
| 7.50 | 338 | 133, 177 | caffeoyl glucose | 9.6 ± 0.6 |
| 13.88 | 351 | 192 | feruloyl glucose | 3.5 ± 0.5 |
| 15.79 | 428 | 312 | coumaric galloyl-malate | 3.1 ± 0.3 |
| 16.01 | 423 | 311 | benzyl-o-galloyl glucose | 8.9 ± 0.9 |
| p-value | 0.031 | |||
| Phenolic derivatives | ||||
| 23.95 | 395 | 195 | syringic acid derivatives | 5.6 ± 0.0 |
| 24.25 | 445 | 265 | apigenin derivatives | 10.7 ± 0.2 |
| p-value | 0.019 | |||
| Organic acids | ||||
| 1.36 | 190 | 110 | quinic acid | 510 ± 1.1 |
| 1.51 | 132 | – | malic acid | 550 ± 2.3 |
| 2.62 | 190 | 111 | citric acid | 590 ± 0.9 |
| p-value | 0.05 | |||
| VOCs | LRI | % DM |
|---|---|---|
| Oxygenated compounds | ||
| hexanal | 810 | 0.43 |
| heptanal | 903 | 0.56 |
| benzaldehyde | 960 | 1.35 |
| nonanal | 1100 | 1.2 |
| (E)-2-octenal | 1060 | 1.33 |
| safranal | 1205 | 1.22 |
| cuminal | 1240 | 2.2 |
| (E,Z)-3,5-octadien-2-one | 1070 | 0.65 |
| camphor | 1150 | 1.02 |
| (E)-2-octen-1-ol | 1066 | 1.12 |
| Nitrogen compounds | ||
| 2,3-dimethyl pyrazine | 915 | 0.5 |
| 2,3,5-trimethyl pyrazine | 917 | 0.5 |
| Terpenoids | ||
| β-cyclocitral | 1224 | 2.25 |
| limonene | 1032 | 30.12 |
| linalool | 1101 | 2.15 |
| menthone | 1155 | 1.1 |
| menthol | 1175 | 1.2 |
| carvone | 1244 | 41.63 |
| β-caryophyllene | 1420 | 2.1 |
| (E)-β-ionone | 1487 | 0.65 |
| Hydrocarbons | ||
| 2-pentyl furan | 995 | 1.3 |
| 2,6-dimethylcyclohexanol | 1110 | 5.2 |
| Oxygenated compounds | 11.8% | |
| Nitrogen compounds | 1% | |
| Terpenoids | 81.2% | |
| Hydrocarbons | 6.5% | |
| Microorganisms | Purslane Extract Concentration (µg/mL)/Inhibition Zones (cm) | Levofloxacin | MIC | |||||
|---|---|---|---|---|---|---|---|---|
| Bacteria | 50 | 150 | 200 | 250 | 300 | 350 | 350 µg/mL | |
| LM | 1.2 ± 0.0 c | 1.4 ± 0.2 d | 1.7 ± 0.2 d | 2.1 ± 0.2 d | 2.7 ± 0.3 c | 3.9 ± 0.2 b | 3.7 ± 0.2 b | 30 c |
| SA | 1.6 ± 0.1 a | 2.0 ± 0.6 a | 2.4 ± 0.1 a | 2.9 ± 0.2 a | 3.2 ± 0.1 a | 4.2 ± 0.0 a | 3.9 ± 0.1 a | 20 e |
| EC | 1.1 ± 0.2 c,d | 1.3 ± 0.1 d,e | 1.6 ± 0.3 d,e | 1.9 ± 0.3 e | 2.4 ± 0.2 d | 3.0 ± 0.1 d | 2.9 ± 0.4 d | 35 b |
| KP | 1.0 ± 0.3 c | 1.2 ± 0.6 e | 1.5 ± 0.1 e | 1.8 ± 0.2 e | 2.3 ± 0.6 d | 2.8 ± 0.2 e | 2.7 ± 0.7 e | 40 a |
| Fungi | 50 | 150 | 200 | 250 | 300 | 350 | Canesten | MIC |
| CG | 1.2 ± 0.1 c | 1.6 ± 0.3 c | 2.0 ± 0.5 c | 2.3 ± 0.4 c | 2.6 ± 0.1 cd | 3.1 ± 0.2 d | 2.9 ± 0.2 d | 35 b |
| CA | 1.4 ± 0.2 b | 1.8 ± 0.5 b | 2.3 ± 0.6 b | 2.5 ± 0.8 b | 3.0 ± 0.0 b | 3.3 ± 0.3 c | 3.1 ± 0.1 c | 25 d |
| Blood Parameters | Control | BHA | PuE (µg/g) | ||
|---|---|---|---|---|---|
| Liver Parameters | 250 µg/g | 50 | 150 | 250 | |
| ALT | 43.8 ± 0.2 c | 88.2 ± 0.2 a | 53.6 ± 0.2 b | 50.6 ± 0.1 b | 52 ± 0.2 b |
| AST | 35.6 ± 0.6 c | 81.6 ± 0.1 a | 45.1 ± 0.3 b | 40.2 ± 0.6 b,c | 43 ± 0.3 b,c |
| MDA | 46.36 ± 0.9 c | 67.9 ± 0.3 a | 51.52 ± 0.1 b | 50.51 ± 0.1 b | 50.9 ± 0.1 b |
| GSH | 57.5 ± 0.1 a | 40.2 ± 0.4 c | 51.2 ± 0.4 b | 56.3 ± 0.2 a | 53 ± 0.6 a |
| TP | 6.14 ± 0.7 a | 5.61 ± 0.9 b | 5.98 ± 0.7 b | 6.22 ± 0.8 a | 6.02 ± 0.6 a,b |
| Kidney parameters | |||||
| Urea | 16.6 ± 0.2 c | 27 ± 0.3 a | 19.2 ± 0.1 b | 17.4 ± 0.2 c | 18.1 ± 0.2 b,c |
| Creatinine | 0.39 ± 0.001 d | 0.82 ± 0.04 a | 0.55 ± 0.02 b | 0.46 ± 0.01 c | 0.51 ± 0.1 b,c |
| Lipid profile | |||||
| LDL | 16.7 ± 0.3 c | 85.4 ± 0.5 a | 24.9 ± 0.3 b | 23.5 ± 0.1 b | 24.0 ± 0.3 b,c |
| HDL | 36.2 ± 0.4 a,b | 25.4 ± 0.2 c | 37.3 ± 0.4 a | 34.2 ± 0.5 b | 37.9 ± 0.5 a |
| TG | 77.5 ± 0.9 c | 119.2 ± 0.9 a | 84 ± 0.1 b | 79.6 ± 0.6 c | 81.3 ± 0.6 b,c |
| TC | 67.4 ± 0.1 c | 135.2 ± 0.8 a | 77.1 ± 0.9 b | 72.6 ± 0.9 c | 75.4 ± 0.7 b,c |
| Yogurt | Period (Days) | pH | Acidity (mg/10 mL) | Fat (%) | TSS (%) | Syneresis (%) | Viscosity (cP) | WHC (mL/g) | |
|---|---|---|---|---|---|---|---|---|---|
| PuE concentration (µg/g) | Control (0) | 0 | 4.52 ± 0.0 a,b | 80.15 ± 0.2 f | 1.33 ± 0.1 f | 11.99 ± 0.3 g | 9.2 ± 0.2 d,e | 154.3 ± 0.6 i | 7.2 ± 0.1 d |
| 7 | 4.42 ± 0.2 c | 82.66 ± 0.1 e | 1.45 ± 0.1 e,f | 12.11 ± 0.1 f | 9.4 ± 0.1 d | 150.2 ± 0.8 i | 7.0 ± 0.5 d,e | ||
| 14 | 4.35 ± 0.3 d | 86.25 ± 0.9 c | 1.62 ± 0.0 d | 12.46 ± 0.4 e | 9.9 ± 0.6 c | 145.7 ± 0.7 j | 6.4 ± 0.3 e | ||
| 21 | 4.22 ± 0.1 f | 88.47 ± 0.4 b | 1.77 ± 0.2 c | 12.69 ± 0.6 d | 10.4 ± 0.8 b | 142.6 ± 0.5 j | 6.2 ± 0.1 e,f | ||
| 30 | 4.11 ± 0.2 g | 90.25 ± 0.8 a | 1.89 ± 0.1 c | 12.78 ± 0.1 c | 11.0 ± 0.4 a | 139.4 ± 0.8 k | 5.9 ± 0.8 f | ||
| 50 | 0 | 4.50 ± 0.1 b | 81.33 ± 0.7 e | 1.45 ± 0.5 e,f | 12.00 ± 0.2 f,g | 8.9 ± 0.7 e | 198.2 ± 0.1 g | 7.7 ± 0.6 d | |
| 7 | 4.45 ± 0.0 b,c | 82.25 ± 0.3 e | 1.67 ± 0.4 d | 12.35 ± 0.1 f | 9.2 ± 0.5 de | 189.1 ± 0.8 g | 7.5 ± 0.4 d | ||
| 14 | 4.36 ± 0.2 d | 84.99 ± 0.9 c,d | 1.88 ± 0.3 c | 12.56 ± 0.4 e | 9.4 ± 0.6 d | 180.3 ± 0.9 h | 7.3 ± 0.2 d | ||
| 21 | 4.29 ± 0.1 e | 86.78 ± 0.4 c | 1.92 ± 0.9 b | 12.75 ± 0.9 c | 9.7 ± 0.5 c | 177.6 ± 08 h | 7.0 ± 0.3 d,e | ||
| 30 | 4.21 ± 0.1 f | 89.14 ± 0.5 a,b | 1.99 ± 0.1 b | 12.89 ± 0.4 b,c | 10.2 ± 0.3 b | 165.5 ± 0.3 i | 6.8 ± 0.0 e | ||
| 150 | 0 | 4.55 ± 0.0 a | 80.92 ± 0.2 f | 1.51 ± 0.0 e | 12.22 ± 0.2 f | 8.1 ± 0.1 f | 275.3 ± 1.2 d | 8.4 ± 0.1 b,c | |
| 7 | 4.50 ± 0.2 b | 81.05 ± 0.3 e | 1.79 ± 0.3 c | 12.51 ± 0.3 e | 8.3 ± 0.8 f | 262.1 ± 1.1 d,e | 8.2 ± 0.2 c | ||
| 14 | 4.41 ± 0.3 c | 83.98 ± 0.1 d | 1.96 ± 0.2 b | 12.79 ± 0.1 c | 8.6 ± 0.3 e | 259.3 ± 1.1 e | 8.0 ± 0.3 c | ||
| 21 | 4.36 ± 0.0 d | 85.24 ± 0.2 c,d | 2.07 ± 0.1 a,b | 12.98 ± 0.2 b | 9.1 ± 0.3 d,e | 241.6 ± 0.9 e,f | 7.5 ± 0.1 d | ||
| 30 | 4.30 ± 0.2 e | 88.21 ± 0.1 b | 2.11 ± 0.2 a | 13.25 ± 0.3 a | 9.4 ± 0.6 d | 239.5 ± 0.5 f | 7.2 ± 0.7 d | ||
| 250 | 0 | 4.53 ± 0.1 a,b | 81.21 ± 0.7 e,f | 1.49 ± 0.5 e | 12.11 ± 0.5 f | 7.5 ± 0.7 g | 345.2 ± 0.9 a | 9.3 ± 0.6 a | |
| 7 | 4.49 ± 0.3 b | 82.00 ± 0.8 e | 1.78 ± 0.6 c | 12.46 ± 0.2 e | 7.8 ± 0.1 g | 331.4 ± 0.8 a,b | 9.1 ± 0.1 a,b | ||
| 14 | 4.40 ± 0.0 c | 84.51 ± 0.7 d | 1.92 ± 0.4 b | 12.62 ± 0.1 d | 8.1 ± 0.2 f | 320.7 ± 0.4 b | 8.8 ± 0.6 b | ||
| 21 | 4.32 ± 0.2 e | 86.00 ± 0.9 c | 1.99 ± 0.5 b | 12.87 ± 0.3 b,c | 8.5 ± 0.3 e,f | 310.9 ± 0.6 c | 8.6 ± 0.3 b | ||
| 30 | 4.23 ± 0.1 f | 88.95 ± 0.0 b | 2.05 ± 0.4 a,b | 13.00 ± 0.1 a,b | 8.8 ± 0.4 e | 305.3 ± 0.5 c | 8.5 ± 0.4 b,c | ||
| Yogurt Samples | Storage Period | ||||
|---|---|---|---|---|---|
| 0 d | 7 d | 14 d | 21 d | 30 d | |
| Control | 0.92 ± 0.01 a | 1.01 ± 0.03 a | 1.23 ± 0.02 a | 1.31 ± 0.05 a | 1.77 ± 0.01 a |
| PuE 50 | 0.88 ± 0.00 b | 0.93 ± 0.06 b | 1.05 ± 0.04 b | 1.21 ± 0.01 b | 1.35 ± 0.06 b |
| PuE 150 | 0.81 ± 0.01 c | 0.89 ± 0.03 c | 0.93 ± 0.08 c | 0.97 ± 0.02 c | 1.05 ± 0.04 c |
| PuE 250 | 0.83 ± 0.02 c | 0.91 ± 0.03 c | 1.02 ± 0.01 d | 1.19 ± 0.09 d | 1.26 ± 0.01 d |
| Yogurt Samples | Storage (Days) | Color | Flavor | Texture | Taste | Overall Acceptability | |
|---|---|---|---|---|---|---|---|
| PuE concentration (µg/g) | Control (0) | 0 | 9.0 ± 0.0 a | 9.0 ± 0.0 a | 8.2 ± 0.0 c,d | 8.4 ± 0.2 a | 8.7 ± 0.0 b,c |
| 7 | 8.7 ± 0.2 b,c | 8.4 ± 0.1 c | 8.2 ± 0.1 c,d | 8.1 ± 0.1 b,c | 8.4 ± 0.1 c,d | ||
| 14 | 8.5 ± 0.3 cd | 8.1 ± 0.0 d | 8.0 ± 0.2 d | 7.9 ± 0.0 c | 8.2 ± 0.2 d | ||
| 21 | 8.0 ± 0.2 e | 7.6 ± 0.2 e | 7.5 ± 0.1 e | 7.4 ± 0.1 e | 7.7 ± 0.3 e | ||
| 30 | 7.5 ± 0.7 f | 7.5 ± 0.3 e | 7.0 ± 0.0 f | 6.9 ± 0.2 f | 7.3 ± 0.4 f | ||
| 50 | 0 | 9.0 ± 0.0 a | 9.0 ± 0.0 a | 8.6 ± 0.0 b,c | 8.4 ± 0.1 a | 8.9 ± 0.0 a,b | |
| 7 | 8.8 ± 0.1 b | 8.7 ± 0.2 b,c | 8.5 ± 0.1 c | 8.2 ± 0.2 b | 8.7 ± 0.1 b,c | ||
| 14 | 8.7 ± 0.2 b,c | 8.5 ± 0.1 c | 8.3 ± 0.2 c,d | 8.1 ± 0.1 b,c | 8.5 ± 0.0 c,d | ||
| 21 | 8.5 ± 0.1 c,d | 8.4 ± 0.2 c | 8.0 ± 0.2 d | 7.9 ± 0.2 c | 8.3 ± 0.2 d | ||
| 30 | 8.3 ± 0.1 d | 8.2 ± 0.1 c,d | 7.7 ± 0.1 f | 7.7 ± 0.1 d | 8.1 ± 0.1 d,e | ||
| 150 | 0 | 9.0 ± 0.0 a | 9.0 ± 0.0 a | 8.9 ± 0.0 a | 8.4 ± 0.0 a | 9.0 ± 0.0 a | |
| 7 | 8.8 ± 0.0 b | 8.8 ± 0.1 b | 8.7 ± 0.1 b | 8.2 ± 0.2 b | 8.8 ± 0.1 b | ||
| 14 | 8.6 ± 0.1 c | 8.7 ± 0.2 b,c | 8.5 ± 0.2 c | 8.1 ± 0.1 b,c | 8.6 ± 0.2 c | ||
| 21 | 8.6 ± 0.1 c | 8.5 ± 0.1 c | 8.3 ± 0.1 c,d | 8.0 ± 0.1 c | 8.5 ± 0.1 c,d | ||
| 30 | 8.5 ± 0.2 c,d | 8.3 ± 0.2 c,d | 8.0 ± 0.0 d | 7.9 ± 0.1 c,d | 8.3 ± 0.2 d | ||
| 250 | 0 | 9.0 ± 0.0 a | 9.0 ± 0.0 a | 8.5 ± 0.2 c | 8.4 ± 0.4 a | 8.8 ± 0.1 b | |
| 7 | 8.7 ± 0.1 b,c | 8.5 ± 0.2 c | 8.4 ± 0.1 c | 8.1 ± 0.2 b,c | 8.5 ± 0.2 c,d | ||
| 14 | 8.6 ± 0.2 c | 8.3 ± 0.3 c,d | 8.2 ± 0.3 c,d | 8.0 ± 0.1 c | 8.4 ± 0.1 d | ||
| 21 | 8.2 ± 0.3 d | 8.1 ± 0.2 d | 8.0 ± 0.2 d | 7.6 ± 0.1 d | 8.1 ± 0.0 d,e | ||
| 30 | 8.1 ± 0.2 d | 8.0 ± 0.1 d | 7.6 ± 0.1 f | 7.5 ± 0.2 d,e | 7.9 ± 0.2 e | ||
| Treatments | Basel Diet (kg) | PuE (mg/kg) | BHA (mg/kg) |
|---|---|---|---|
| 1 | 1 | – | – |
| 2 | 1 | – | 350 |
| 3 | 1 | 100 | – |
| 4 | 1 | 200 | – |
| 5 | 1 | 350 | – |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Quwaie, D.A.; Allohibi, A.; Aljadani, M.; Alghamdi, A.M.; Alharbi, A.A.; Baty, R.S.; Qahl, S.H.; Saleh, O.; Shakak, A.O.; Alqahtani, F.S.; et al. Characterization of Portulaca oleracea Whole Plant: Evaluating Antioxidant, Anticancer, Antibacterial, and Antiviral Activities and Application as Quality Enhancer in Yogurt. Molecules 2023, 28, 5859. https://doi.org/10.3390/molecules28155859
Al-Quwaie DA, Allohibi A, Aljadani M, Alghamdi AM, Alharbi AA, Baty RS, Qahl SH, Saleh O, Shakak AO, Alqahtani FS, et al. Characterization of Portulaca oleracea Whole Plant: Evaluating Antioxidant, Anticancer, Antibacterial, and Antiviral Activities and Application as Quality Enhancer in Yogurt. Molecules. 2023; 28(15):5859. https://doi.org/10.3390/molecules28155859
Chicago/Turabian StyleAl-Quwaie, Diana A., Aminah Allohibi, Majidah Aljadani, Amira M. Alghamdi, Asmaa Ali Alharbi, Roua S. Baty, Safa H. Qahl, Ohud Saleh, Amani Osman Shakak, Fatimah S. Alqahtani, and et al. 2023. "Characterization of Portulaca oleracea Whole Plant: Evaluating Antioxidant, Anticancer, Antibacterial, and Antiviral Activities and Application as Quality Enhancer in Yogurt" Molecules 28, no. 15: 5859. https://doi.org/10.3390/molecules28155859
APA StyleAl-Quwaie, D. A., Allohibi, A., Aljadani, M., Alghamdi, A. M., Alharbi, A. A., Baty, R. S., Qahl, S. H., Saleh, O., Shakak, A. O., Alqahtani, F. S., Khalil, O. S. F., El-Saadony, M. T., & Saad, A. M. (2023). Characterization of Portulaca oleracea Whole Plant: Evaluating Antioxidant, Anticancer, Antibacterial, and Antiviral Activities and Application as Quality Enhancer in Yogurt. Molecules, 28(15), 5859. https://doi.org/10.3390/molecules28155859








