Smart Inhibition Action of Amino Acid-Modified Layered Double Hydroxide and Its Application on Carbon Steel
Abstract
:1. Introduction
2. Results
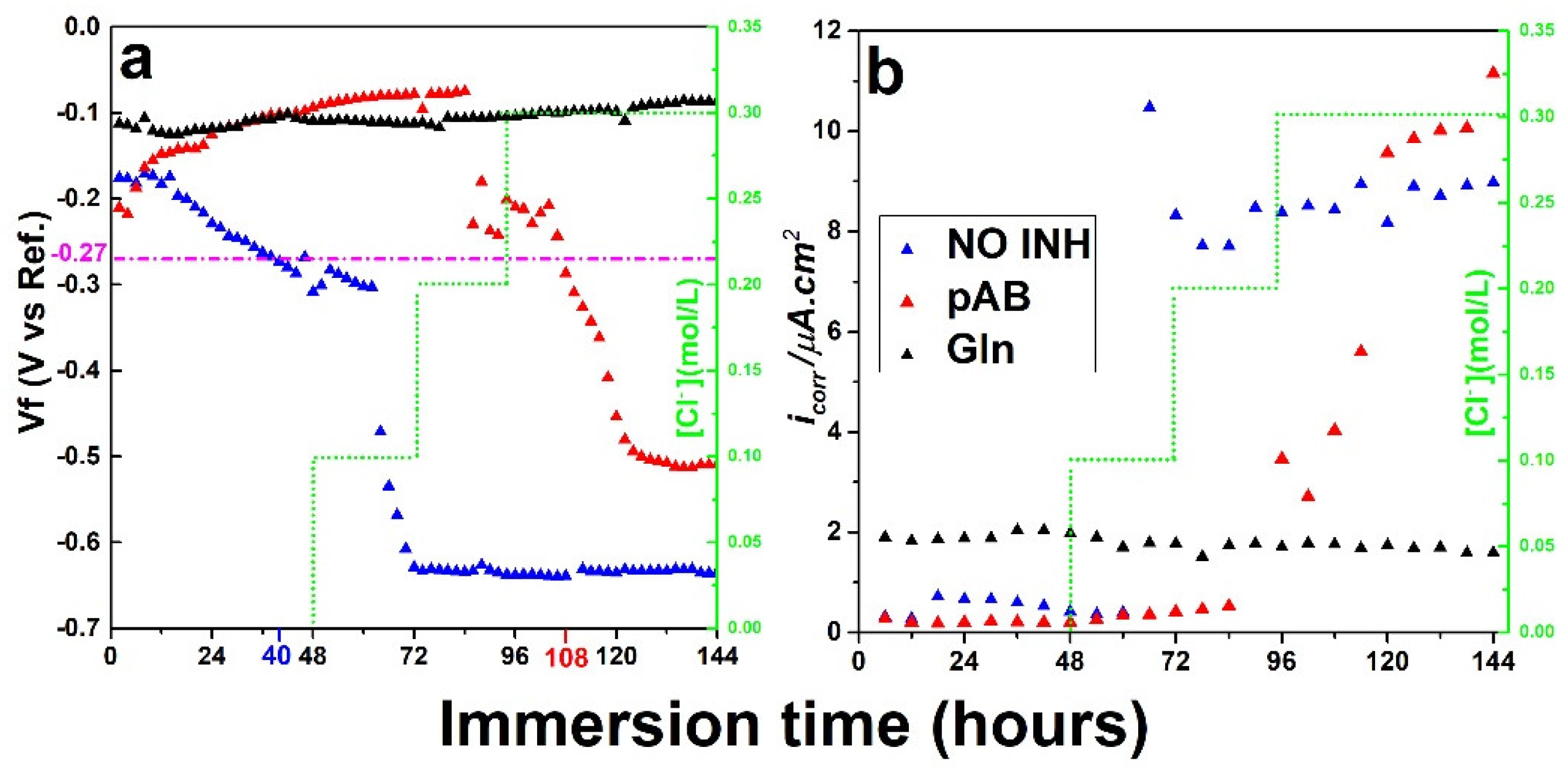
2.1. Corrosion Inhibitor Performance in Simulated Concrete Pore Solution
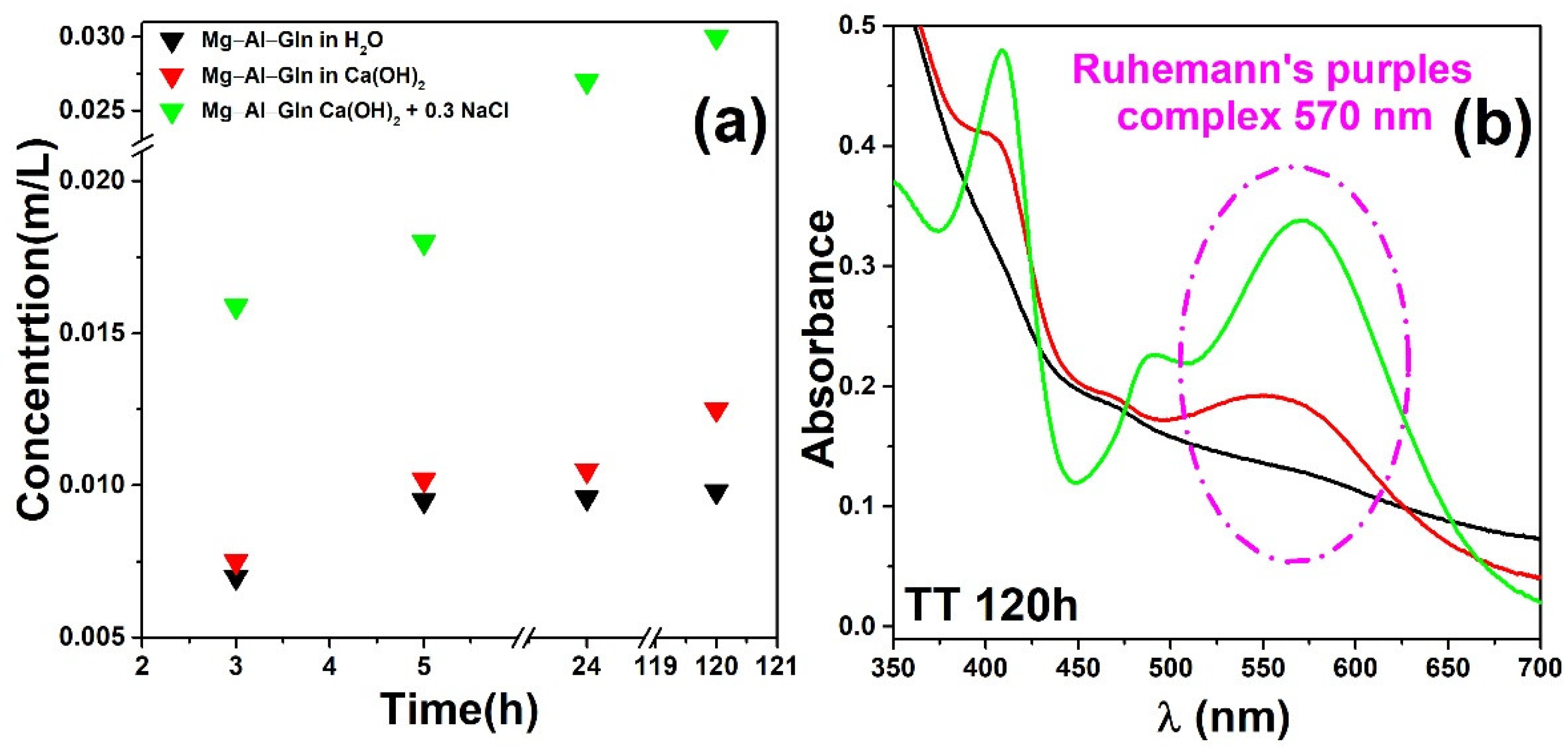
2.2. Corrosion Inhibitor Release Performance
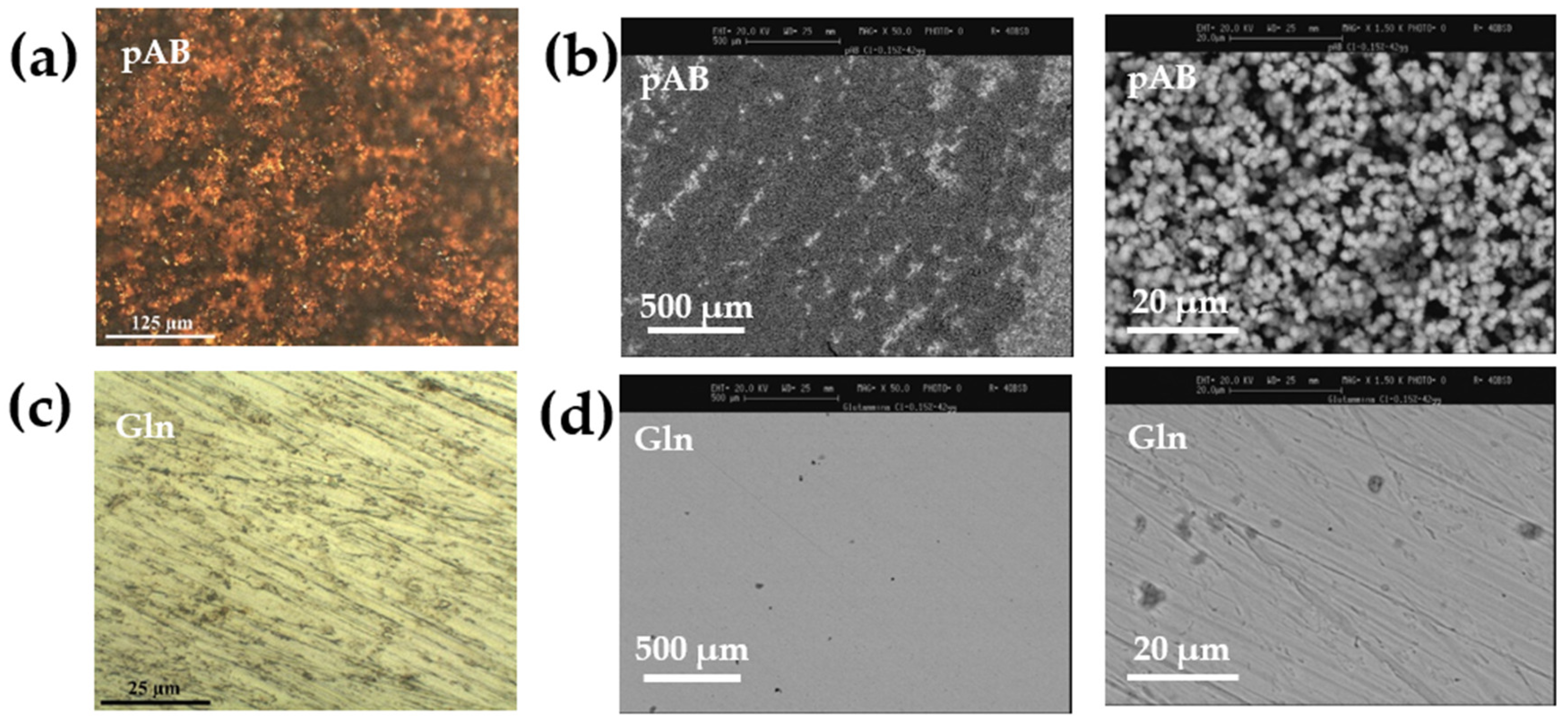
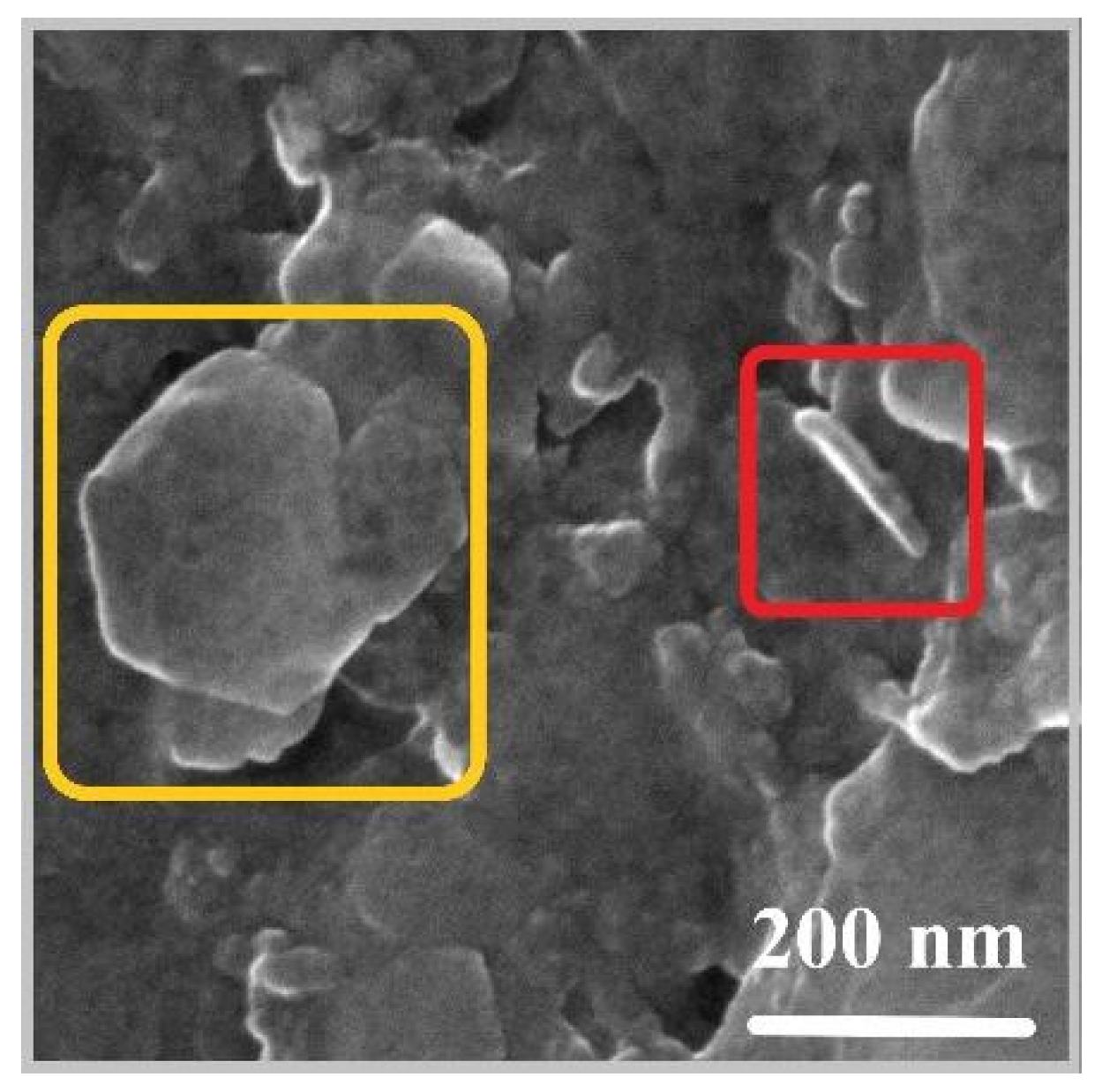
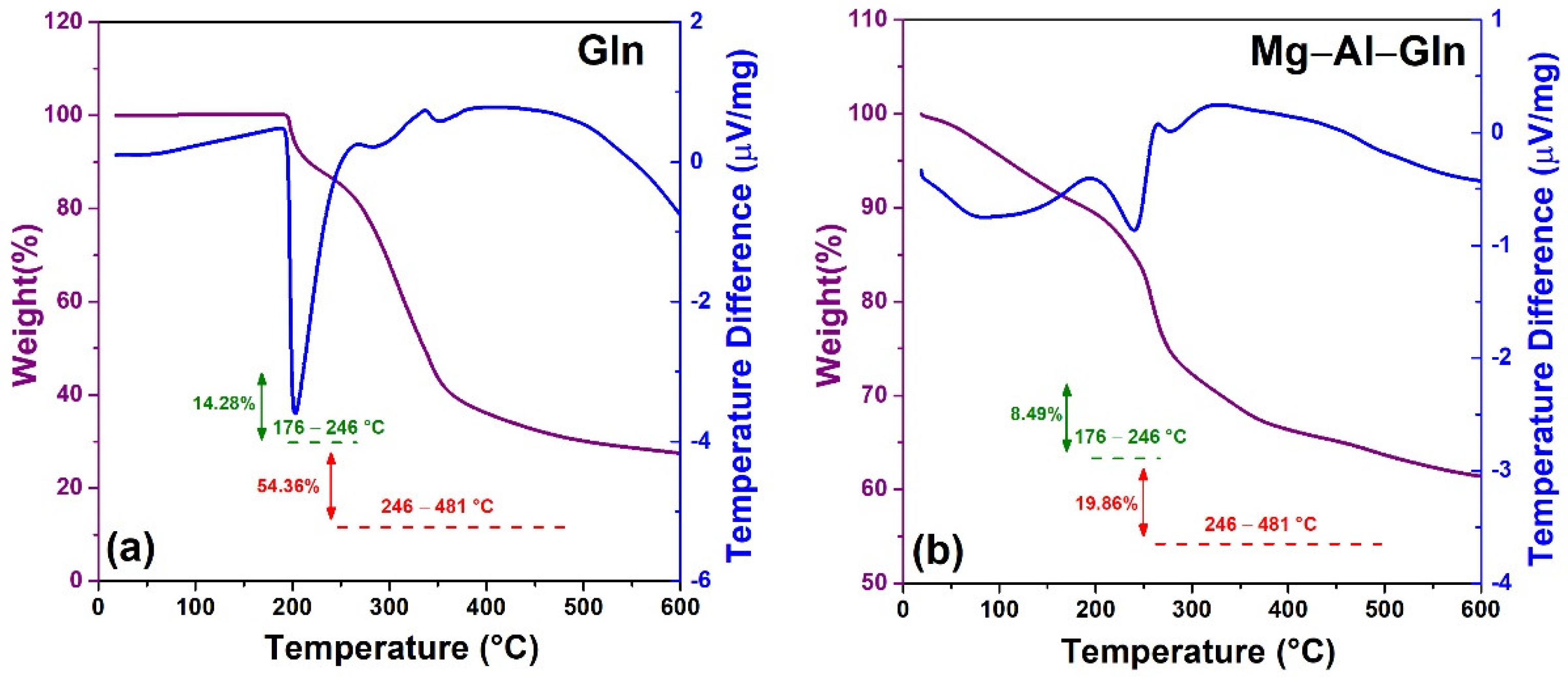
2.3. Characterization of the Synthesized LDH
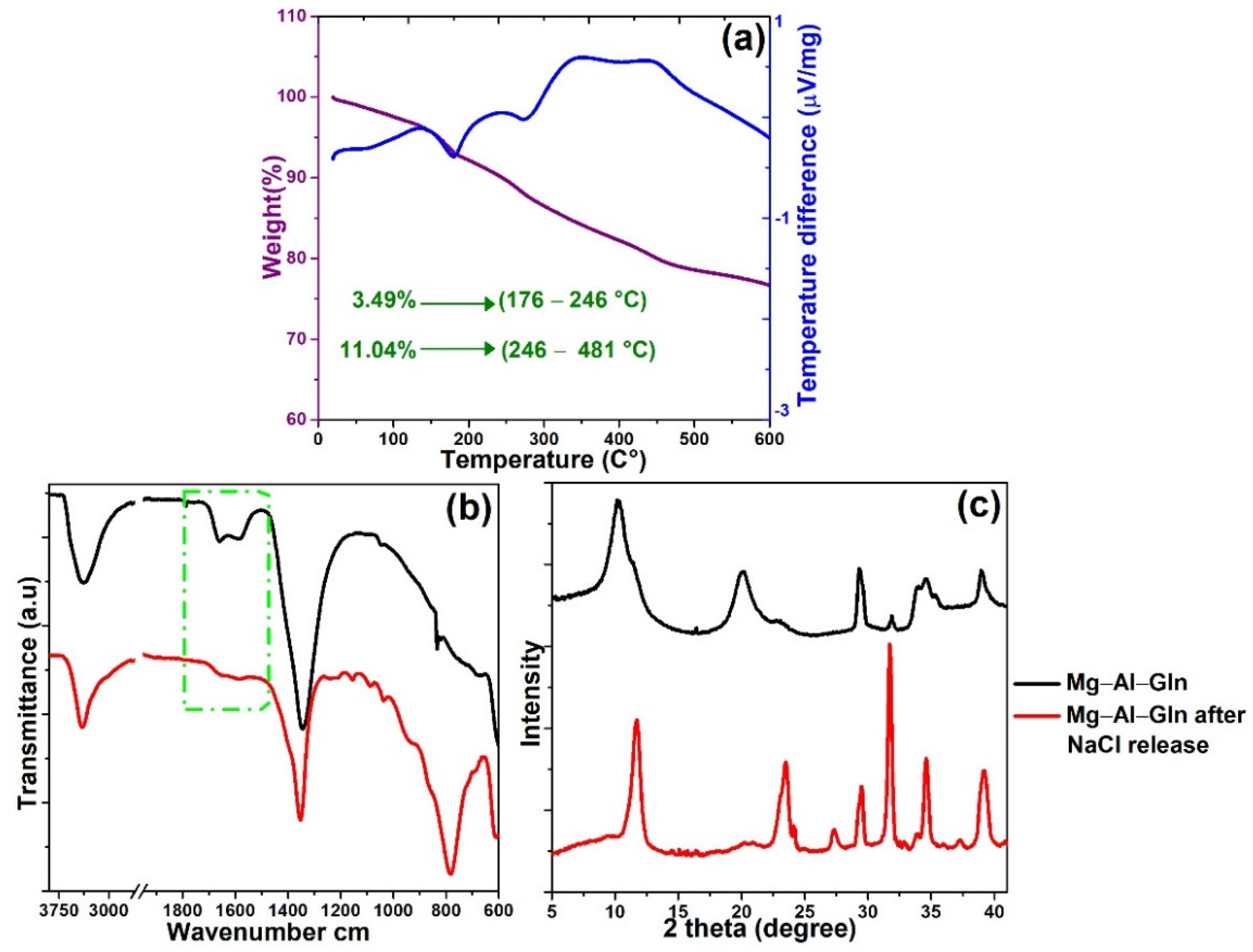
2.4. Inhibitor Release and Protective Properties of LDH
3. Experimental Section
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Bertolini, L.; Elsener, B.; Pedeferri, P.; Polder, R. Corrosion of Steel in Concrete: Prevention, Diagnosis, Repair, 1st ed.; Wiley: Weinheim, Germany, 2004. [Google Scholar]
- Elsener, B. Corrosion Inhibitors for Steel in Concrete—State of the Art Report; EFC Publications: London, UK, 2001. [Google Scholar]
- Monticelli, C.; Frignani, A.; Balbo, F.; Zucchi, F. Influence of two specific inhibitors on stell corrosion in a synthetic solution simulating a carbonated concrete with chlorides. Mater. Corros. 2011, 62, 178–186. [Google Scholar] [CrossRef]
- Bastidas, D.M.; Criado, M.; La Iglesia, V.M.; Fajardo, S.; La Iglesia, A.; Bastidas, J.M. Comparative study of three sodium phosphates as corrosion inhibitors for steel reinforcements. Cem. Concr. Compos. 2013, 43, 31–38. [Google Scholar] [CrossRef]
- Vukasovich, J.; Farr, P.G. Molybdate in corrosion inhibition—A review. Polyhedron 1986, 5, 551–559. [Google Scholar]
- Monticelli, C.; Frignani, A.; Trabanelli, G. A study on corrosion inhibitors for concrete application. Cem. Concr. Compos. 2000, 30, 635–642. [Google Scholar] [CrossRef]
- Quraishi, M.A.; Sharma, H.K. Thiazoles as corrosion inhibitors for mild steel in formic and acetic acid solutions. J. Appl. Electrochem. 2005, 35, 33–39. [Google Scholar] [CrossRef]
- Bobina, M.; Kellenberger, A.; Millet, J.P.; Muntean, C.; Vaszilcsin, N. Corrosion resistance of carbon steel in weak acid solutions in the presence of L-histidine as corrosion inhibitor. Corros. Sci. 2013, 69, 389–395. [Google Scholar] [CrossRef]
- Napundorf, T.; Seddig, T.; Ruf, E.; Ballentin, L.; Kipphardt, H.; Wolfgang, M. Alkali salts of amino acids as alkaline additives for neutralization of acidic corrosion inhibitors. Amino Acids 2023, 55, 665–678. [Google Scholar] [CrossRef] [PubMed]
- Kasprzhitskii, A.; Lazorenko, G.; Nazdracheva, T.; Yavna, V. Comparative computational study of L-Amino Acids as green corrosion inhibitors for mild steel. Computation 2021, 9, 1. [Google Scholar]
- Sedding, T.; Naundorf, T.; Kipphardt, H.; Maison, W. Glucamines as green alternatives to conventional amino alcohols in metalworking fluids. Mater. Corros. 2023, 74, 394–402. [Google Scholar] [CrossRef]
- de Luna, M.S.; Buonocore, G.; Giuliani, C.; Messina, E.; Di Carlo, G.; Lavorgna, M.; Ambrosio, L.; Ingo, G.M. Angewandte Chemie International Edition; Wiley-VCH. Publisher: Weinheim, Germany, 2018; Volume 57, pp. 7380–7384. [Google Scholar]
- Messina, E.; Giuliani, C.; Pascucci, M.; Riccucci, C.; Staccioli, M.P.; Albini, M.; Di Carlo, G. Synergistic Inhibition Effect of Chitosan and L-Cysteine for the Protection of Copper-Based Alloys against Atmospheric Chloride-Induced Indoor Corrosion. Int. J. Mol. Sci. 2022, 22, 10321. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, C.; Pascucci, M.; Riccucci, C.; Messina, E.; De Luna, M.S.; Lavorgna, M.; Ingo, G.M.; Di Carlo, G. Chitosan-based coatings for corrosion protection of copper-based alloys: A promising more sustainable approach for cultural heritage applications. Prog. Org. Coat. 2018, 122, 138. [Google Scholar]
- Zhang, D.Q.; Cai, Q.R.; He, X.M.; Gao, L.X.; Zhou, G.D. Inhibition effect of some amino acids on copper corrosion in HCl solution. Mater. Chem. Phys. 2008, 112, 353–358. [Google Scholar] [CrossRef]
- Zheludkevich, M.L.; Tedim, J.; Ferreira, M.G.S. Smart coatings for active corrosion protection based on multi-functional micro and nanocontainers. Electrochim. Acta 2012, 82, 314–323. [Google Scholar] [CrossRef]
- Giuliani, C.; Messina, E.; Staccioli, M.P.; Pascucci, M.; Riccucci, C.; Liotta, L.F.; Tortora, L.; Ingo, G.M.; Di Carlo, G. On-demand release of protective agents triggered by environmental stimuli. Front. Chem. 2020, 8, 304. [Google Scholar] [CrossRef]
- Shchukin, D.G.; Lamaka, S.V.; Yasakau, K.A.; Zheludkevich, M.L.; Ferreira, M.G.S.; Mohwald, H. Active Anticorrosion Coatings with Halloysite Nanocontainers. J. Phys. Chem. C 2008, 112, 958–964. [Google Scholar] [CrossRef]
- Leal, D.A.; Wypych, F.; Bruno Marina, C.E. Zinc-Layered Hydroxide Salt Intercalated with Molybdate Anions as a New Smart Nanocontainer for Active Corrosion Protection of Carbon Steel. Appl. Mater. Interfaces 2020, 12, 19823–19833. [Google Scholar] [CrossRef]
- Gaidis, J.M. Chemistry of corrosion inhibitors. Cem. Concr. Compos. 2004, 26, 181–189. [Google Scholar]
- Morris, W.; Vázquez, M. A migrating corrosion inhibitor evaluated in concrete containing various contents of admixed chlorides. Cem. Concr. Res. 2002, 32, 259–267. [Google Scholar] [CrossRef]
- Bertolini, L.; Gastaldi, M.; Lollini, F.; Redaelli, E. Corrosion assessment and restoration strategies of reinforced concrete buildings of the cultural heritage. Mater. Corros. 2011, 62, 146–154. [Google Scholar] [CrossRef]
- Elsener, B.; Buechler, M.; Stalger, F.; Boehni, H. Migrating corrosion inhibitor blend for reinforced concrete: Part 2. Inhibitor as repair strategy. Corrosion 2000, 56, 52–56. [Google Scholar] [CrossRef]
- Batis, G.; Pantazopoulou, P.; Routoulas, A. Corrosion protection investigation of reinforcement by inorganic coating in the presence of alkanolamine-based inhibitor. Cem. Concr. Compos. 2003, 25, 371–377. [Google Scholar] [CrossRef]
- Fedrizzi, L.; Azzolini, F.; Bonora, P.L. The use of migrating corrosion inhibitors to repair motorways’ concrete structures contaminated by chlorides. Cem. Concr. Res. 2005, 35, 551–561. [Google Scholar] [CrossRef]
- Ormelesse, M.; Berra, M.; Bolzoni, F.; Pastore, T. Corrosion inhibitors for chlorides induced corrosion in reinforced concrete structures. Cem. Concr. Res. 2006, 35, 536–547. [Google Scholar]
- Ormelesse, M.; Lazzari, L.; Goidanich, S.; Fumagalli, G.; Brenna, A. A study of organic substances as inhibitors for chloride-induced corrosion in concrete. Corros. Sci. 2009, 51, 2959–2968. [Google Scholar] [CrossRef]
- Tedim, J.; Poznyak, S.K.; Kuznetsova, A.; Raps, D.; Hack, T.; Zheludkevich, M.L.; Ferreira, M.G.S. Enhancement of Active Corrosion Protection via Combination of Inhibitor-Loaded Nanocontainers. Appl. Mater. Interfaces 2010, 2, 1528–1535. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Dong, C.; Wang, G.; Cheng, X.; Li, X. Zn–Al–NO2 layered double hydroxide as a controlled-release corrosion inhibitor for steel reinforcements. Mater. Lett. 2019, 236, 517–520. [Google Scholar]
- Zuo, J.; Wu, B.; Luo, C.; Dong, B.; Xing, F. Preparation of Mg Al Layered double hydroxides intercalated with nitride ions and corrosion protection of steel bars in simulates carbonated concrete pore solution. Corros. Sci. 2019, 152, 120–129. [Google Scholar] [CrossRef]
- Elsener, B.; Addari, D.; Coray, S.; Rossi, A. Stainless steel reinforcing bars–reason for their high pitting corrosion resistance. Mater. Corros. 2011, 62, 111–119. [Google Scholar] [CrossRef]
- Gouda, V. Corrosion and corrosion inhibition of reinforcing steel: I. Immersed in alkaline solutions. Br. Corros. J. 1970, 5, 198–203. [Google Scholar] [CrossRef]
- Sheng, S.J.; Kraft, J.J.; Schuster, S.M. A specific Quantitative Colometric Assay for L-Asparagine. Anal. Biochem. 1993, 211, 242–249. [Google Scholar] [CrossRef] [PubMed]
- ASTM STP 1065, 29; Corrosion Rates of Steel in Concrete. American Society for Testing and Materials: Philadelphia, PA, USA, 1990.
- Cavani, F.; Trifirò, F.; Vaccari, A. Hydrotalcite-type anion clays: Preparation, properties and applications. Catal. Today 1991, 11, 173–301. [Google Scholar]
- Olanrewaju, J.; Newalkar, B.L.; Mancino, C.; Komarneni, S. Simplified synthesis of nitrate form of layered double hydroxide. Mater. Lett. 2000, 45, 307–310. [Google Scholar] [CrossRef]
- Nakayama, H.; Wada, N.; Tsuhako, M. Intercalation of amino acids and peptides into Mg-Al layered double hydroxide by reconstruction method. Int. J. Pharm. 2004, 269, 469–478. [Google Scholar] [PubMed]
- Hibino, T. Delamination of Layered Double Hydroxides Containing Amino Acids. Chem. Mater. 2004, 16, 5482–5488. [Google Scholar] [CrossRef]
- Barth, A. The infrared absorption of amino acid side chains. Prog. Biophys. Mol. Biol. 2000, 74, 141–173. [Google Scholar]
- Wang, Y.; Wu, P.; Hou, Y.; Zhu, N.; Dang, Z. Intercalation of L-Alanyl-Glutamine dipeptide into Layered Double Hydroxides: Configuration Stabilization in Confined Interlayer Region. Ind. Eng. Chem. Res. 2012, 51, 11128–11136. [Google Scholar] [CrossRef]
- Rodante, F.; Marrosu, G.; Catalani, G. Thermal analysis of some l-amino acids with similar structures. Thermochim. Acta 1992, 194, 197–213. [Google Scholar] [CrossRef]
- Weiss, I.M.; Muth, C.; Drumm, R.; Kirchner, O.K. Thermal decomposition of the amino acids glycine, cysteine, aspartic acid, asparagine, glutamine acid, glutamine, arginine and histidine. BMC Biophys. 2018, 1, 2. [Google Scholar]
- Xu, J.; Song, Y.; Tan, Q.; Jiang, L. Chloride absorption by nitrate, nitrite and aminobenzoate intercalated layered double hydroxides. J. Mater. Sci. 2017, 52, 5908–5916. [Google Scholar] [CrossRef]
- Feliu, V.; Gonsalez, J.A.; Andrade, C.; Feliu, S. Equivalent circuit for modelling the steel! concrete interface. I. Experimental evidence and theoretical predictions. Corros. Sci. 1998, 40, 975–993. [Google Scholar] [CrossRef]
- Dhouibi, L.; Triki, E.; Raharinaivo, A. The application of electrochemical impedance spectroscopy to determine the long-term effectiveness of corrosion inhibitors for steel in concrete. Cem. Concr. Compos. 2002, 24, 35–43. [Google Scholar] [CrossRef]
- Andrade, C.; Keddam, M.; Novoa, X.R.; Perez, M.C.; Rangel, C.M.; Takenouti, H. Electrochemical behaviour of steel rebars in concrete: Influence of environmental factors and cement chemistry. Electrochim. Acta 2001, 46, 3905–3912. [Google Scholar]
- Meyn, M.; Benke, K.; Lagaly, G. Anion-Exchange reactions of Layered Double Hydroxides. Inorg. Chem. 1990, 29, 5201–5207. [Google Scholar] [CrossRef]




| SAMPLES | h | R0 | C | R1 | W/O | CPE | n | Rt | Χ2 |
|---|---|---|---|---|---|---|---|---|---|
| RIF. | 288 | 77.5 | 2.43 × 10−5 | 2.73 | 9.5143 × 10−3 | 8.5143 × 10−4 | 0.95 | 4.2143 × 104 | 3.0443 × 10−5 |
| Mg–Al–Gln | 288 | 68.4 | 6.5543 × 10−5 | 2.896 | 1.5143 × 10−2 | 1.8643 × 10−3 | 0.81 | 1501 | 2.0343 × 10−4 |
| Non Standard Mortar | Comprehensive Strengths (MPa) | Total Porosity (%) | Pore Size Distribution (%) | |||||
|---|---|---|---|---|---|---|---|---|
| 26.9 ± 2.03 | 14.90 | >100 µm | 100–10 µm | 10–1 | 1–0.1 | 0.1–0.01 | <0.01 | |
| 0.55 | 0.27 | 0.82 | 7.78 | 5.12 | 0.34 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Messina, E.; Pascucci, M.; Riccucci, C.; Boccaccini, F.; Blanco-Valera, M.T.; Garcia-Lodeiro, I.; Ingo, G.M.; Di Carlo, G. Smart Inhibition Action of Amino Acid-Modified Layered Double Hydroxide and Its Application on Carbon Steel. Molecules 2023, 28, 5863. https://doi.org/10.3390/molecules28155863
Messina E, Pascucci M, Riccucci C, Boccaccini F, Blanco-Valera MT, Garcia-Lodeiro I, Ingo GM, Di Carlo G. Smart Inhibition Action of Amino Acid-Modified Layered Double Hydroxide and Its Application on Carbon Steel. Molecules. 2023; 28(15):5863. https://doi.org/10.3390/molecules28155863
Chicago/Turabian StyleMessina, Elena, Marianna Pascucci, Cristina Riccucci, Francesca Boccaccini, Maria Teresa Blanco-Valera, Ines Garcia-Lodeiro, Gabriel Maria Ingo, and Gabriella Di Carlo. 2023. "Smart Inhibition Action of Amino Acid-Modified Layered Double Hydroxide and Its Application on Carbon Steel" Molecules 28, no. 15: 5863. https://doi.org/10.3390/molecules28155863
APA StyleMessina, E., Pascucci, M., Riccucci, C., Boccaccini, F., Blanco-Valera, M. T., Garcia-Lodeiro, I., Ingo, G. M., & Di Carlo, G. (2023). Smart Inhibition Action of Amino Acid-Modified Layered Double Hydroxide and Its Application on Carbon Steel. Molecules, 28(15), 5863. https://doi.org/10.3390/molecules28155863