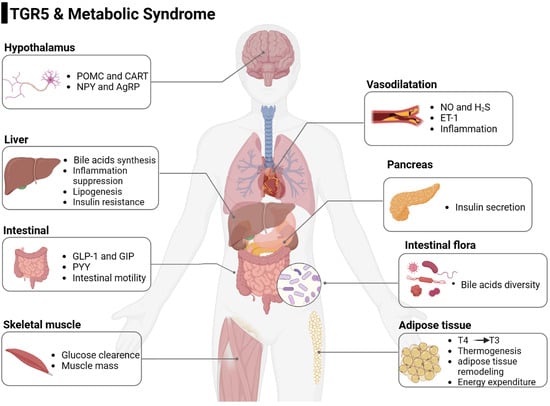
Research Progress of Takeda G Protein-Coupled Receptor 5 in Metabolic Syndrome
Abstract
:1. Introduction
2. TGR5
3. TGR5 and Bile Acids
4. TGR5 and T2DM
4.1. Promote GLP-1 Secretion
4.2. Promote GIP Secretion
4.3. Promote PYY Secretion
4.4. Increase Glucose Metabolism in Skeletal Muscle
4.5. Inhibit Inflammation
5. TGR5 and Obesity
5.1. Promote Energy Consumption and Fat Browning
5.2. Increase Intestinal Peristalsis
5.3. Regulate Hypothalamic Neurons
6. TGR5 and Atherosclerosis
6.1. Regulate Inflammation
6.2. Promote NO Release
7. TGR5 and NAFLD
7.1. Improve Insulin Resistance
7.2. Inhibit Inflammation
7.3. Ameliorate Effect on Fibrin
8. Gut Microbiota Regulates TGR5
9. TGR5 Agonists
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Hu, C.; Jia, W. Multi-omics profiling: The way toward precision medicine in metabolic diseases. J. Mol. Cell Biol. 2021, 13, 576–593. [Google Scholar] [CrossRef] [PubMed]
- Ambroselli, D.; Masciulli, F.; Romano, E.; Catanzaro, G.; Besharat, Z.M.; Massari, M.C.; Ferretti, E.; Migliaccio, S.; Izzo, L.; Ritieni, A.; et al. New Advances in Metabolic Syndrome, from Prevention to Treatment: The Role of Diet and Food. Nutrients 2023, 15, 640. [Google Scholar] [CrossRef] [PubMed]
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vrdoljak, J.; Kumric, M.; Vilovic, M.; Martinovic, D.; Rogosic, V.; Borovac, J.A.; Kurir, T.T.; Bozic, J. Can Fasting Curb the Metabolic Syndrome Epidemic? Nutrients 2022, 14, 456. [Google Scholar] [CrossRef] [PubMed]
- Chiang, J.Y.L. Bile Acid Metabolism and Signaling. Compr. Physiol. 2013, 3, 1191–1212. [Google Scholar] [CrossRef] [Green Version]
- Duboc, H.; Taché, Y.; Hofmann, A.F. The bile acid TGR5 membrane receptor: From basic research to clinical application. Dig. Liver Dis. 2014, 46, 302–312. [Google Scholar] [CrossRef] [Green Version]
- van Nierop, F.S.; Scheltema, M.J.; Eggink, H.M.; Pols, T.W.; Sonne, D.P.; Knop, F.K.; Soeters, M.R. Clinical relevance of the bile acid receptor TGR5 in metabolism. Lancet Diabetes Endocrinol. 2016, 5, 224–233. [Google Scholar] [CrossRef]
- Ferrell, J.M.; Pathak, P.; Boehme, S.; Gilliland, T.; Chiang, J.Y.L. Deficiency of Both Farnesoid X Receptor and Takeda G Protein–Coupled Receptor 5 Exacerbated Liver Fibrosis in Mice. Hepatology 2019, 70, 955–970. [Google Scholar] [CrossRef]
- Vassileva, G.; Hu, W.; Hoos, L.; Tetzloff, G.; Yang, S.; Liu, L.; Kang, L.; Davis, H.R.; Hedrick, J.A.; Lan, H.; et al. Gender-dependent effect of Gpbar1 genetic deletion on the metabolic profiles of diet-induced obese mice. J. Endocrinol. 2010, 205, 225–232. [Google Scholar] [CrossRef] [Green Version]
- Holter, M.M.; Chirikjian, M.K.; Govani, V.N.; Cummings, B.P. TGR5 Signaling in Hepatic Metabolic Health. Nutrients 2020, 12, 2598. [Google Scholar] [CrossRef]
- McGavigan, A.K.; Garibay, D.; Henseler, Z.M.; Chen, J.; Bettaieb, A.; Haj, F.G.; Ley, R.E.; Chouinard, M.L.; Cummings, B.P. TGR5 contributes to glucoregulatory improvements after vertical sleeve gastrectomy in mice. Gut 2015, 66, 226–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chevre, R.; Castaño, D.; Chua, T.; Corlianò, M.; Patankar, J.V.; Sng, L.; Sim, L.; Juin, T.L.; Carissimo, G.; Ng, L.F.P.; et al. Therapeutic modulation of the bile acid pool by Cyp8b1 knockdown protects against nonalcoholic fatty liver disease in mice. FASEB J. 2018, 32, 3792–3802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maczewsky, J.; Kaiser, J.; Gresch, A.; Gerst, F.; Düfer, M.; Krippeit-Drews, P.; Drews, G. TGR5 Activation Promotes Stimulus-Secretion Coupling of Pancreatic β-Cells via a PKA-Dependent Pathway. Diabetes 2018, 68, 324–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finn, P.D.; Rodriguez, D.; Kohler, J.; Jiang, Z.; Wan, S.; Blanco, E.; King, A.J.; Chen, T.; Bell, N.; Dragoli, D.; et al. Intestinal TGR5 agonism improves hepatic steatosis and insulin sensitivity in Western diet-fed mice. Am. J. Physiol. Liver Physiol. 2019, 316, G412–G424. [Google Scholar] [CrossRef] [Green Version]
- Castellanos-Jankiewicz, A.; Guzmán-Quevedo, O.; Fénelon, V.S.; Zizzari, P.; Quarta, C.; Bellocchio, L.; Tailleux, A.; Charton, J.; Fernandois, D.; Henricsson, M.; et al. Hypothalamic bile acid-TGR5 signaling protects from obesity. Cell Metab. 2021, 33, 1483–1492.e10. [Google Scholar] [CrossRef]
- Drigo, R.A.; Fonseca, T.L.; Werneck-De-Castro, J.P.S.; Bianco, A.C. Role of the type 2 iodothyronine deiodinase (D2) in the control of thyroid hormone signaling. Biochim. Biophys. Acta 2013, 1830, 3956–3964. [Google Scholar] [CrossRef] [Green Version]
- Sasaki, T.; Watanabe, Y.; Kuboyama, A.; Oikawa, A.; Shimizu, M.; Yamauchi, Y.; Sato, R. Muscle-specific TGR5 overexpression improves glucose clearance in glucose-intolerant mice. J. Biol. Chem. 2021, 296, 100131. [Google Scholar] [CrossRef]
- Kida, T.; Tsubosaka, Y.; Hori, M.; Ozaki, H.; Murata, T. Bile Acid Receptor TGR5 Agonism Induces NO Production and Reduces Monocyte Adhesion in Vascular Endothelial Cells. Arter. Thromb. Vasc. Biol. 2013, 33, 1663–1669. [Google Scholar] [CrossRef] [Green Version]
- Duboc, H.; Aelion, H.; Rainteau, D.; Rajca, S.; Sokol, H.; Humbert, L.; Farabos, D.; Coffin, B.; Weber, S.; Porcher, R.; et al. Crosstalk between the hepatologist and the cardiologist: A future place for the lithocholic acid as a coronary atheroma risk factor? Hepatology 2012, 56, 2426. [Google Scholar] [CrossRef]
- Tiwari, A.; Maiti, P. TGR5: An emerging bile acid G-protein-coupled receptor target for the potential treatment of metabolic disorders. Drug Discov. Today 2009, 14, 523–530. [Google Scholar] [CrossRef]
- Sasaki, T.; Kuboyama, A.; Mita, M.; Murata, S.; Shimizu, M.; Inoue, J.; Mori, K.; Sato, R. The exercise-inducible bile acid receptor Tgr5 improves skeletal muscle function in mice. J. Biol. Chem. 2018, 293, 10322–10332. [Google Scholar] [CrossRef] [Green Version]
- Hov, J.R.; Keitel, V.; Laerdahl, J.K.; Spomer, L.; Ellinghaus, E.; ElSharawy, A.; Melum, E.; Boberg, K.M.; Manke, T.; Balschun, T.; et al. Mutational Characterization of the Bile Acid Receptor TGR5 in Primary Sclerosing Cholangitis. PLoS ONE 2010, 5, e12403. [Google Scholar] [CrossRef] [Green Version]
- Kuhre, R.E.; Wewer Albrechtsen, N.J.; Larsen, O.; Jepsen, S.L.; Balk-Møller, E.; Andersen, D.B.; Deacon, C.F.; Schoonjans, K.; Reimann, F.; Gribble, F.M.; et al. Bile acids are important direct and indirect regulators of the secretion of appetite- and metabolism-regulating hormones from the gut and pancreas. Mol. Metab. 2018, 11, 84–95. [Google Scholar] [CrossRef]
- Kawamata, Y.; Fujii, R.; Hosoya, M.; Harada, M.; Yoshida, H.; Miwa, M.; Fukusumi, S.; Habata, Y.; Itoh, T.; Shintani, Y.; et al. A G Protein-coupled Receptor Responsive to Bile Acids. J. Biol. Chem. 2003, 278, 9435–9440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, Y.C.; Li, P.F. Progress in the study of TGR5 receptor-mediated signaling pathway. Chin. J. Pharmacol. 2020, 36, 153–157. [Google Scholar]
- Herbert, M.R.; Siegel, D.L.; Staszewski, L.; Cayanan, C.; Banerjee, U.; Dhamija, S.; Anderson, J.; Fan, A.; Wang, L.; Rix, P.; et al. Synthesis and SAR of 2-aryl-3-aminomethylquinolines as agonists of the bile acid receptor TGR5. Bioorg. Med. Chem. Lett. 2010, 20, 5718–5721. [Google Scholar] [CrossRef] [PubMed]
- Perino, A.; Demagny, H.; Velazquez-Villegas, L.A.; Schoonjans, K. Molecular physiology of bile acid signaling in health, disease, and aging. Physiol. Rev. 2021, 101, 683–731. [Google Scholar] [CrossRef]
- Wang, X.X.; Xie, C.; Libby, A.E.; Ranjit, S.; Levi, J.; Myakala, K.; Bhasin, K.; Jones, B.A.; Orlicky, D.J.; Takahashi, S.; et al. The role of FXR and TGR5 in reversing and preventing progression of Western diet–induced hepatic steatosis, inflammation, and fibrosis in mice. J. Biol. Chem. 2022, 298, 102530. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Francl, J.M.; Boehme, S.; Ochoa, A.; Zhang, Y.; Klaassen, C.D.; Erickson, S.K.; Chiang, J.Y. Glucose and insulin induction of bile acid synthesis: Mechanisms and implication in diabetes and obesity. J Biol Chem. 2012, 287, 1861–1873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, G.; Jiang, R.; Wang, X.; Liu, P.; Zhao, A.; Wu, Y.; Huang, F.; Liu, Z.; Rajani, C.; Zheng, X.; et al. Conjugated secondary 12α-hydroxylated bile acids promote liver fibrogenesis. Ebiomedicine 2021, 66, 103290. [Google Scholar] [CrossRef]
- Ticho, A.L.; Malhotra, P.; Dudeja, P.K.; Gill, R.K.; Alrefai, W.A. Intestinal Absorption of Bile Acids in Health and Disease. Compr. Physiol. 2019, 10, 21–56. [Google Scholar] [CrossRef]
- Pathak, P.; Liu, H.; Boehme, S.; Xie, C.; Krausz, K.W.; Gonzalez, F.; Chiang, J.Y.L. Farnesoid X receptor induces Takeda G-protein receptor 5 cross-talk to regulate bile acid synthesis and hepatic metabolism. J. Biol. Chem. 2017, 292, 11055–11069. [Google Scholar] [CrossRef] [Green Version]
- Xie, C.; Huang, W.; Young, R.L.; Jones, K.L.; Horowitz, M.; Rayner, C.K.; Wu, T. Role of Bile Acids in the Regulation of Food Intake, and Their Dysregulation in Metabolic Disease. Nutrients 2021, 13, 1104. [Google Scholar] [CrossRef]
- Donepudi, A.C.; Boehme, S.; Li, F.; Chiang, J.Y. G-protein-coupled bile acid receptor plays a key role in bile acid metabolism and fasting-induced hepatic steatosis in mice. Hepatology 2017, 65, 813–827. [Google Scholar] [CrossRef]
- Gillard, J.; Clerbaux, L.-A.; Nachit, M.; Sempoux, C.; Staels, B.; Bindels, L.B.; Tailleux, A.; Leclercq, I.A. Bile acids contribute to the development of non-alcoholic steatohepatitis in mice. JHEP Rep. 2022, 4, 100387. [Google Scholar] [CrossRef]
- Qi, Y.; Duan, G.; Wei, D.; Zhao, C.; Ma, Y. The Bile Acid Membrane Receptor TGR5 in Cancer: Friend or Foe? Molecules 2022, 27, 5292. [Google Scholar] [CrossRef]
- Scheen, A.J. Dual GIP/GLP-1 receptor agonists: New advances for treating type-2 diabetes. Ann. D’endocrinologie 2023, 84, 316–321. [Google Scholar] [CrossRef]
- Ballantyne, G.H.; Longo, W.E.; Savoca, P.E.; Adrian, T.E.; Vukasin, A.P.; Bilchik, A.J.; Sussman, J.; Modlin, I.M. Deoxycholate-stimulated release of peptide YY from the isolated perfused rabbit left colon. Am. J. Physiol. Liver Physiol. 1989, 257, G715–G724. [Google Scholar] [CrossRef]
- Saito, M.; Matsushita, M.; Yoneshiro, T.; Okamatsu-Ogura, Y. Brown Adipose Tissue, Diet-Induced Thermogenesis, and Thermogenic Food Ingredients: From Mice to Men. Front. Endocrinol. 2020, 11, 222. [Google Scholar] [CrossRef] [Green Version]
- Svensson, P.-A.; Olsson, M.; Andersson-Assarsson, J.C.; Taube, M.; Pereira, M.J.; Froguel, P.; Jacobson, P. The TGR5 gene is expressed in human subcutaneous adipose tissue and is associated with obesity, weight loss and resting metabolic rate. Biochem. Biophys. Res. Commun. 2013, 433, 563–566. [Google Scholar] [CrossRef] [Green Version]
- Depommier, C.; Everard, A.; Druart, C.; Plovier, H.; Van Hul, M.; Vieira-Silva, S.; Falony, G.; Raes, J.; Maiter, D.; Delzenne, N.M.; et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: A proof-of-concept exploratory study. Nat. Med. 2019, 25, 1096–1103. [Google Scholar] [CrossRef]
- Wu, Q.; Liang, X.; Wang, K.; Lin, J.; Wang, X.; Wang, P.; Zhang, Y.; Nie, Q.; Liu, H.; Zhang, Z.; et al. Intestinal hypoxia-inducible factor 2α regulates lactate levels to shape the gut microbiome and alter thermogenesis. Cell Metab. 2021, 33, 1988–2003.e7. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Ding, L.; Baig, M.; Tian, J.; Wang, Y.; Huang, W. Improving glucose and lipids metabolism: Drug development based on bile acid related targets. Cell Stress 2021, 5, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Bouzas, C.; Pastor, R.; Garcia, S.; Monserrat-Mesquida, M.; Martínez-González, M.; Salas-Salvadó, J.; Corella, D.; Goday, A.; Martínez, J.A.; Alonso-Gómez, M.; et al. Comparative effects of glucagon-like peptide-1 receptors agonists, 4-dipeptidyl peptidase inhibitors, and metformin on metabolic syndrome. Biomed. Pharmacother. 2023, 161, 114561. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, C.B.; Trammell, S.A.J.; Wewer Albrechtsen, N.J.; Schoonjans, K.; Albrechtsen, R.; Gillum, M.; Kuhre, R.E.; Holst, J.J. Bile acids drive colonic secretion of glucagon-like-peptide 1 and peptide-YY in rodents. Am. J. Physiol. Liver Physiol. 2019, 316, G574–G584. [Google Scholar] [CrossRef]
- Morimoto, K.; Watanabe, M.; Sugizaki, T.; Irie, J.-I.; Itoh, H. Intestinal Bile Acid Composition Modulates Prohormone Convertase 1/3 (PC1/3) Expression and Consequent GLP-1 Production in Male Mice. Endocrinology 2016, 157, 1071–1081. [Google Scholar] [CrossRef]
- Makki, K.; Brolin, H.; Petersen, N.; Henricsson, M.; Christensen, D.P.; Khan, M.T.; Wahlström, A.; Bergh, P.-O.; Tremaroli, V.; Schoonjans, K.; et al. 6α-hydroxylated bile acids mediate TGR5 signalling to improve glucose metabolism upon dietary fiber supplementation in mice. Gut 2022, 72, 314–324. [Google Scholar] [CrossRef]
- Zhai, H.; Li, Z.; Peng, M.; Huang, Z.; Qin, T.; Chen, L.; Li, H.; Zhang, H.; Zhang, W.; Xu, G. Takeda G Protein-Coupled Receptor 5-Mechanistic Target of Rapamycin Complex 1 Signaling Contributes to the Increment of Glucagon-Like Peptide-1 Production after Roux-en-Y Gastric Bypass. Ebiomedicine 2018, 32, 201–214. [Google Scholar] [CrossRef] [Green Version]
- Tian, F.; Xu, W.; Chen, L.; Chen, T.; Feng, X.; Chen, J.; Wei, D.; Huang, Q. Ginsenoside compound K increases glucagon-like peptide-1 release and L-cell abundance in db/db mice through TGR5/YAP signaling. Int. Immunopharmacol. 2022, 113, 109405. [Google Scholar] [CrossRef]
- Sorrentino, G.; Perino, A.; Yildiz, E.; El Alam, G.; Sleiman, M.B.; Gioiello, A.; Pellicciari, R.; Schoonjans, K. Bile Acids Signal via TGR5 to Activate Intestinal Stem Cells and Epithelial Regeneration. Gastroenterology 2020, 159, 956–968.e8. [Google Scholar] [CrossRef]
- Chen, M.-J.; Liu, C.; Wan, Y.; Yang, L.; Jiang, S.; Qian, D.-W.; Duan, J.-A. Enterohepatic circulation of bile acids and their emerging roles on glucolipid metabolism. Steroids 2020, 165, 108757. [Google Scholar] [CrossRef]
- Chiang, J.Y.; Pathak, P.; Liu, H.; Donepudi, A.; Ferrell, J.; Boehme, S. Intestinal Farnesoid X Receptor and Takeda G Protein Couple Receptor 5 Signaling in Metabolic Regulation. Dig. Dis. 2017, 35, 241–245. [Google Scholar] [CrossRef]
- Zhai, Z.; Niu, K.; Liu, H.; Lin, C.; Tu, Y.; Liu, Y.; Cai, L.; Ouyang, K.; Liu, J. Policosanol alleviates hepatic lipid accumulation by regulating bile acids metabolism in C57BL6/mice through AMPK–FXR–TGR5 cross-talk. J. Food Sci. 2021, 86, 5466–5478. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, K.; Chen, S.; Kang, J.; Guo, N.; Chen, H.; Liu, J.; Wu, Y.; He, P.; Tu, Y.; et al. Saponins from Camellia sinensis Seeds Stimulate GIP Secretion in Mice and STC-1 Cells via SGLT1 and TGR. Nutrients 2022, 14, 3413. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, S.; Li, P.; Chen, Q.; Li, Y.; Zhou, Y.; Wang, L.; Kang, M.; Zhang, B.; Yang, B.; et al. Pancreatic cancer-derived exosomes suppress the production of GIP and GLP-1 from STC-1 cells in vitro by down-regulating the PCSK1/3. Cancer Lett. 2018, 431, 190–200. [Google Scholar] [CrossRef]
- Wu, Y.; He, H.; Cheng, Z.; Bai, Y.; Ma, X. The Role of Neuropeptide Y and Peptide YY in the Development of Obesity via Gut-brain Axis. Curr. Protein Pept. Sci. 2019, 20, 750–758. [Google Scholar] [CrossRef]
- Yang, H.; Liu, H.; Jiao, Y.W.; Qian, J. Roux-en-Y Gastrointestinal Bypass Promotes Activation of TGR5 and Peptide YY. Endocr. Metab. Immune Disord. Drug Targets 2020, 20, 1262–1267. [Google Scholar] [CrossRef]
- Guida, C.; Stephen, S.; Watson, M.; Dempster, N.; Larraufie, P.; Marjot, T.; Cargill, T.; Rickers, L.; Pavlides, M.; Tomlinson, J.; et al. PYY plays a key role in the resolution of diabetes following bariatric surgery in humans. Ebiomedicine 2019, 40, 67–76. [Google Scholar] [CrossRef] [Green Version]
- Bala, V.; Rajagopal, S.; Kumar, D.P.; Nalli, A.D.; Mahavadi, S.; Sanyal, A.J.; Grider, J.R.; Murthy, K.S. Release of GLP-1 and PYY in response to the activation of G protein-coupled bile acid receptor TGR5 is mediated by Epac/PLC-ε pathway and modulated by endogenous H2S. Front. Physiol. 2014, 5, 420. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.; Ma, S.; Ning, M.; Yang, W.; Ye, Y.; Zhang, L.; Shen, J.; Leng, Y. TGR5 agonist ameliorates insulin resistance in skeletal muscles and improves glucose homeostasis in diabetic mice. Metabolism 2019, 99, 45–56. [Google Scholar] [CrossRef]
- Sun, L.; Li, F.; Tan, W.; Zhao, W.; Li, Y.; Zhu, X.; Gao, P.; Shu, G.; Wang, S.; Jiang, Q.; et al. Lithocholic acid promotes skeletal muscle regeneration through the TGR5 receptor. Acta Biochim. Biophys. Sin. 2023, 55, 51–61. [Google Scholar] [CrossRef]
- Sahakyan, G.; Vejux, A.; Sahakyan, N. The Role of Oxidative Stress-Mediated Inflammation in the Development of T2DM-Induced Diabetic Nephropathy: Possible Preventive Action of Tannins and Other Oligomeric Polyphenols. Molecules 2022, 27, 9035. [Google Scholar] [CrossRef]
- Xiao, H.; Sun, X.; Liu, R.; Chen, Z.; Lin, Z.; Yang, Y.; Zhang, M.; Liu, P.; Quan, S.; Huang, H. Gentiopicroside activates the bile acid receptor Gpbar1 (TGR5) to repress NF-kappaB pathway and ameliorate diabetic nephropathy. Pharmacol. Res. 2019, 151, 104559. [Google Scholar] [CrossRef]
- Perino, A.; Pols, T.W.H.; Nomura, M.; Stein, S.; Pellicciari, R.; Schoonjans, K. TGR5 reduces macrophage migration through mTOR-induced C/EBPβ differential translation. J. Clin. Investig. 2014, 124, 5424–5436. [Google Scholar] [CrossRef]
- Guo, C.; Xie, S.; Chi, Z.; Zhang, J.; Liu, Y.; Zhang, L.; Zheng, M.; Zhang, X.; Xia, D.; Ke, Y.; et al. Bile Acids Control Inflammation and Metabolic Disorder through Inhibition of NLRP3 Inflammasome. Immunity 2016, 45, 802–816. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, M.; Houten, S.M.; Mataki, C.; Christoffolete, M.A.; Kim, B.W.; Sato, H.; Messaddeq, N.; Harney, J.W.; Ezaki, O.; Kodama, T.; et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature 2006, 439, 484–489. [Google Scholar] [CrossRef]
- de Jesus, L.A.; Carvalho, S.D.; Ribeiro, M.O.; Schneider, M.; Kim, S.-W.; Harney, J.W.; Larsen, P.R.; Bianco, A.C. The type 2 iodothyronine deiodinase is essential for adaptive thermogenesis in brown adipose tissue. J. Clin. Investig. 2001, 108, 1379–1385. [Google Scholar] [CrossRef]
- Gereben, B.; Zavacki, A.M.; Ribich, S.; Kim, B.W.; Huang, S.A.; Simonides, W.S.; Zeld, A.; Bianco, A.C. Cellular and Molecular Basis of Deiodinase-Regulated Thyroid Hormone Signaling. Endocr. Rev. 2008, 29, 898–938. [Google Scholar] [CrossRef] [Green Version]
- Fonseca, T.L.; Russo, S.C.; Luongo, C.; Salvatore, D.; Bianco, A.C. Inactivation of Type 3 Deiodinase Results in Life-long Changes in the Brown Adipose Tissue Transcriptome in the Male Mouse. Endocrinology 2022, 5, 1–10. [Google Scholar] [CrossRef]
- Velazquez-Villegas, L.A.; Perino, A.; Lemos, V.; Zietak, M.; Nomura, M.; Pols, T.W.H.; Schoonjans, K. TGR5 signalling promotes mitochondrial fission and beige remodelling of white adipose tissue. Nat. Commun. 2018, 9, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Krauss, S.; Zhang, C.-Y.; Lowell, B.B. The mitochondrial uncoupling-protein homologues. Nat. Rev. Mol. Cell Biol. 2005, 6, 248–261. [Google Scholar] [CrossRef]
- Jeong, J.H.; Cho, S.; Pak, Y.K. Sterol-independent repression of low density lipoprotein receptor promoter by peroxisome proliferator activated receptor γ coactivator-1α (PGC-1α). Exp. Mol. Med. 2009, 41, 406–416. [Google Scholar] [CrossRef] [Green Version]
- Fan, M.; Wang, Y.; Jin, L.; Fang, Z.; Peng, J.; Tu, J.; Liu, Y.; Zhang, E.; Xu, S.; Liu, X.; et al. Bile Acid–Mediated Activation of Brown Fat Protects From Alcohol-Induced Steatosis and Liver Injury in Mice. Cell. Mol. Gastroenterol. Hepatol. 2021, 13, 809–826. [Google Scholar] [CrossRef]
- Lund, M.L.; Egerod, K.L.; Engelstoft, M.S.; Dmytriyeva, O.; Theodorsson, E.; Patel, B.A.; Schwartz, T.W. Enterochromaffin 5-HT cells—A major target for GLP-1 and gut microbial metabolites. Mol. Metab. 2018, 11, 70–83. [Google Scholar] [CrossRef]
- Zhan, Y.; Wen, Y.; Zhang, L.-L.; Shen, X.-L.; Chen, X.-H.; Wu, X.-H.; Tang, X.-G. Paeoniflorin Improved Constipation in the Loperamide-Induced Rat Model via TGR5/TRPA1 Signaling-Mediated 5-Hydroxytryptamine Secretion. Evid.-Based Complement Altern. Med. 2021, 2021, 6076293. [Google Scholar] [CrossRef]
- Wu, X.; Li, J.-Y.; Lee, A.; Lu, Y.-X.; Zhou, S.-Y.; Owyang, C. Satiety induced by bile acids is mediated via vagal afferent pathways. J. Clin. Investig. 2020, 5, e132400. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhou, Y.; Huang, R.; Wang, M.; Li, Y.; Li, J. Bile acids regulate anorexigenic neuropeptide through p-STAT3-SOCS3 signaling in mouse hypothalamic cells. Nan Fang Yi Ke Da Xue Xue Bao 2020, 40, 1001–1007. [Google Scholar] [PubMed]
- Perino, A.; Velázquez-Villegas, L.A.; Bresciani, N.; Sun, Y.; Huang, Q.; Fénelon, V.S.; Castellanos-Jankiewicz, A.; Zizzari, P.; Bruschetta, G.; Jin, S.; et al. Central anorexigenic actions of bile acids are mediated by TGR. Nat. Metab. 2021, 3, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Pols, T.W.; Nomura, M.; Harach, T.; Sasso, G.L.; Oosterveer, M.H.; Thomas, C.; Rizzo, G.; Gioiello, A.; Adorini, L.; Pellicciari, R.; et al. TGR5 Activation Inhibits Atherosclerosis by Reducing Macrophage Inflammation and Lipid Loading. Cell Metab. 2011, 14, 747–757. [Google Scholar] [CrossRef] [Green Version]
- Guizoni, D.M.; Vettorazzi, J.F.; Carneiro, E.M.; Davel, A.P. Modulation of endothelium-derived nitric oxide production and activity by taurine and taurine-conjugated bile acids. Nitric Oxide 2019, 94, 48–53. [Google Scholar] [CrossRef]
- Hashimoto, E.; Taniai, M.; Tokushige, K. Characteristics and diagnosis of NAFLD/NASH. J. Gastroenterol. Hepatol. 2013, 28, 64–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lambert, J.E.; Ramos-Roman, M.A.; Browning, J.D.; Parks, E.J. Increased De Novo Lipogenesis Is a Distinct Characteristic of Individuals With Nonalcoholic Fatty Liver Disease. Gastroenterology 2014, 146, 726–735. [Google Scholar] [CrossRef]
- Cegla, J. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): A multicentre, double-blind, randomized, placebo-controlled phase 2 study. Ann. Clin. Biochem. Int. J. Biochem. Lab. Med. 2016, 53, 518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Jamal, N.; Erdual, E.; Neunlist, M.; Koriche, D.; Dubuquoy, C.; Maggiotto, F.; Chevalier, J.; Berrebi, D.; Dubuquoy, L.; Boulanger, E.; et al. Glugacon-like peptide-2: Broad receptor expression, limited therapeutic effect on intestinal inflammation and novel role in liver regeneration. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 307, G274–G285. [Google Scholar] [CrossRef] [Green Version]
- Ivory, C.P.A.; Wallace, L.E.; McCafferty, D.-M.; Sigalet, D.L. Interleukin-10-independent anti-inflammatory actions of glucagon-like peptide. Am. J. Physiol. Liver Physiol. 2008, 295, G1202–G1210. [Google Scholar] [CrossRef] [Green Version]
- Baffy, G. Kupffer cells in non-alcoholic fatty liver disease: The emerging view. J. Hepatol. 2009, 51, 212–223. [Google Scholar] [CrossRef] [Green Version]
- Mobraten, K.; Haugbro, T.; Karlstrom, E.; Kleiveland, C.R.; Lea, T. Activation of the bile acid receptor TGR5 enhances LPS-induced inflammatory responses in a human monocytic cell line. J. Recept. Signal Transduct. 2015, 35, 402–409. [Google Scholar] [CrossRef]
- Yang, H.; Luo, F.; Wei, Y.; Jiao, Y.; Qian, J.; Chen, S.; Gong, Y.; Tang, L. TGR5 protects against cholestatic liver disease via suppressing the NF-κB pathway and activating the Nrf2/HO-1 pathway. Ann. Transl. Med. 2021, 9, 1158. [Google Scholar] [CrossRef]
- Shi, Y.; Su, W.; Zhang, L.; Shi, C.; Zhou, J.; Wang, P.; Wang, H.; Shi, X.; Wei, S.; Wang, Q.; et al. TGR5 Regulates Macrophage Inflammation in Nonalcoholic Steatohepatitis by Modulating NLRP3 Inflammasome Activation. Front. Immunol. 2020, 11, 609060. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, H.; Liu, X.; Xue, S.; Chen, D.; Zou, J.; Jiang, H. TGR5 deficiency activates antitumor immunity in non-small cell lung cancer via restraining M2 macrophage polarization. Acta Pharm. Sin. B 2022, 12, 787–800. [Google Scholar] [CrossRef]
- Rao, J.; Yang, C.; Yang, S.; Lu, H.; Hu, Y.; Lu, L.; Cheng, F.; Wang, X. Deficiency of TGR5 exacerbates immune-mediated cholestatic hepatic injury by stabilizing the β-catenin destruction complex. Int. Immunol. 2020, 32, 321–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, J.; Ge, T.; Tang, C.; Wang, G.; Pang, L.; Chen, Z. Synergistic anti-inflammatory effect of gut microbiota and lithocholic acid on liver fibrosis. Inflamm. Res. 2022, 71, 1389–1401. [Google Scholar] [CrossRef]
- Carino, A.; Marchianò, S.; Biagioli, M.; Bucci, M.; Vellecco, V.; Brancaleone, V.; Fiorucci, C.; Zampella, A.; Monti, M.C.; Distrutti, E.; et al. Agonism for the bile acid receptor GPBAR1 reverses liver and vascular damage in a mouse model of steatohepatitis. FASEB J. 2019, 33, 2809–2822. [Google Scholar] [CrossRef] [Green Version]
- Häussinger, D.; Keitel, V. Role of TGR5 (GPBAR1) in Liver Disease. Semin. Liver Dis. 2018, 38, 333–339. [Google Scholar] [CrossRef]
- Yang, C.; Wan, M.; Xu, D.; Pan, D.; Xia, H.; Yang, L.; Sun, G. Flaxseed Powder Attenuates Non-Alcoholic Steatohepatitis via Modulation of Gut Microbiota and Bile Acid Metabolism through Gut–Liver Axis. Int. J. Mol. Sci. 2021, 22, 10858. [Google Scholar] [CrossRef]
- Yang, J.-Y.; Lee, Y.-S.; Kim, Y.; Lee, S.-H.; Ryu, S.; Fukuda, S.; Hase, K.; Yang, C.-S.; Lim, H.; Kim, M.-S.; et al. Gut commensal Bacteroides acidifaciens prevents obesity and improves insulin sensitivity in mice. Mucosal Immunol. 2017, 10, 104–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suraweera, D.; Rahal, H.; Jimenez, M.; Viramontes, M.; Choi, G.; Saab, S. Treatment of primary biliary cholangitis ursodeoxycholic acid non-responders: A systematic review. Liver Int. 2017, 37, 1877–1886. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M.; Une, N.; Nishizawa, M.; Suzuki, S.; Ito, H.; Horiuchi, T. Incretin secretion stimulated by ursodeoxycholic acid in healthy subjects. Springerplus 2013, 2, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pellicciari, R.; Gioiello, A.; Macchiarulo, A.; Thomas, C.; Rosatelli, E.; Natalini, B.; Sardella, R.; Pruzanski, M.; Roda, A.; Pastorini, E.; et al. Discovery of 6α-Ethyl-23(S)-methylcholic Acid (S-EMCA, INT-777) as a Potent and Selective Agonist for the TGR5 Receptor, a Novel Target for Diabesity. J. Med. Chem. 2009, 52, 7958–7961. [Google Scholar] [CrossRef]
- Thomas, C.; Gioiello, A.; Noriega, L.; Strehle, A.; Oury, J.; Rizzo, G.; Macchiarulo, A.; Yamamoto, H.; Mataki, C.; Pruzanski, M.; et al. TGR5-Mediated Bile Acid Sensing Controls Glucose Homeostasis. Cell Metab. 2009, 10, 167–177. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Guan, J.; Wu, Z.; Xu, L.; Sun, C. The TGR5 Agonist INT-777 Promotes Peripheral Nerve Regeneration by Activating cAMP-dependent Protein Kinase A in Schwann Cells. Mol. Neurobiol. 2023, 60, 1901–1913. [Google Scholar] [CrossRef]
- Huang, R.; Gao, Y.; Chen, J.; Duan, Q.; He, P.; Zhang, J.; Huang, H.; Zhang, Q.; Ma, G.; Zhang, Y.; et al. TGR5 Agonist INT-777 Alleviates Inflammatory Neurodegeneration in Parkinson’s Disease Mouse Model by Modulating Mitochondrial Dynamics in Microglia. Neuroscience 2022, 490, 100–119. [Google Scholar] [CrossRef]
- de Oliveira, M.C.; Gilglioni, E.H.; de Boer, B.A.; Runge, J.H.; de Waart, D.R.; Salgueiro, C.L.; Ishii-Iwamoto, E.L.; Elferink, R.P.O.; Gaemers, I.C. Bile acid receptor agonists INT747 and INT777 decrease oestrogen deficiency-related postmenopausal obesity and hepatic steatosis in mice. Biochim. Biophys. Acta 2016, 1862, 2054–2062. [Google Scholar] [CrossRef]
- Sasaki, T.; Ikari, N.; Hashimoto, S.; Sato, R. Identification of α-ionone, nootkatone, and their derivatives as TGR5 agonists. Biochem. Biophys. Res. Commun. 2023, 653, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Li, X.; Wang, L.; Peng, W.; Ye, W.; Li, W.; Wang, Y.-D.; Chen, W.-D. Design, synthesis and evaluation of 1-benzyl-1H-imidazole-5-carboxamide derivatives as potent TGR5 agonists. Bioorganic Med. Chem. 2020, 32, 115972. [Google Scholar] [CrossRef] [PubMed]
- Vassileva, G.; Golovko, A.; Markowitz, L.; Abbondanzo, S.J.; Zeng, M.; Yang, S.; Hoos, L.; Tetzloff, G.; Levitan, D.; Murgolo, N.J.; et al. Targeted deletion of Gpbar1 protects mice from cholesterol gallstone formation. Biochem. J. 2006, 398, 423–430. [Google Scholar] [CrossRef] [Green Version]
- Piotrowski, D.W.; Futatsugi, K.; Warmus, J.S.; Orr, S.T.M.; Freeman-Cook, K.D.; Londregan, A.T.; Wei, L.; Jennings, S.M.; Herr, M.; Coffey, S.B.; et al. Identification of Tetrahydropyrido[4,3-d]pyrimidine Amides as a New Class of Orally Bioavailable TGR5 Agonists. ACS Med. Chem. Lett. 2012, 4, 63–68. [Google Scholar] [CrossRef] [Green Version]
- Alemi, F.; Kwon, E.; Poole, D.P.; Lieu, T.; Lyo, V.; Cattaruzza, F.; Cevikbas, F.; Steinhoff, M.; Nassini, R.; Materazzi, S.; et al. The TGR5 receptor mediates bile acid–induced itch and analgesia. J. Clin. Investig. 2013, 123, 1513–1530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fryer, R.M.; Ng, K.J.; Mazurek, S.G.N.; Patnaude, L.; Skow, D.J.; Muthukumarana, A.; Gilpin, K.E.; Dinallo, R.M.; Kuzmich, D.; Lord, J.; et al. G Protein–Coupled Bile Acid Receptor 1 Stimulation Mediates Arterial Vasodilation through a KCa1.1 (BKCa)–Dependent Mechanism. Experiment 2014, 348, 421–431. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gou, X.; Qin, L.; Wu, D.; Xie, J.; Lu, Y.; Zhang, Q.; He, Y. Research Progress of Takeda G Protein-Coupled Receptor 5 in Metabolic Syndrome. Molecules 2023, 28, 5870. https://doi.org/10.3390/molecules28155870
Gou X, Qin L, Wu D, Xie J, Lu Y, Zhang Q, He Y. Research Progress of Takeda G Protein-Coupled Receptor 5 in Metabolic Syndrome. Molecules. 2023; 28(15):5870. https://doi.org/10.3390/molecules28155870
Chicago/Turabian StyleGou, Xianmei, Lin Qin, Di Wu, Jian Xie, Yanliu Lu, Qianru Zhang, and Yuqi He. 2023. "Research Progress of Takeda G Protein-Coupled Receptor 5 in Metabolic Syndrome" Molecules 28, no. 15: 5870. https://doi.org/10.3390/molecules28155870
APA StyleGou, X., Qin, L., Wu, D., Xie, J., Lu, Y., Zhang, Q., & He, Y. (2023). Research Progress of Takeda G Protein-Coupled Receptor 5 in Metabolic Syndrome. Molecules, 28(15), 5870. https://doi.org/10.3390/molecules28155870