A Photosensitizer-Loaded Polydopamine Nanomedicine Agent for Synergistic Photodynamic and Photothermal Therapy
Abstract
:1. Introduction
2. Results
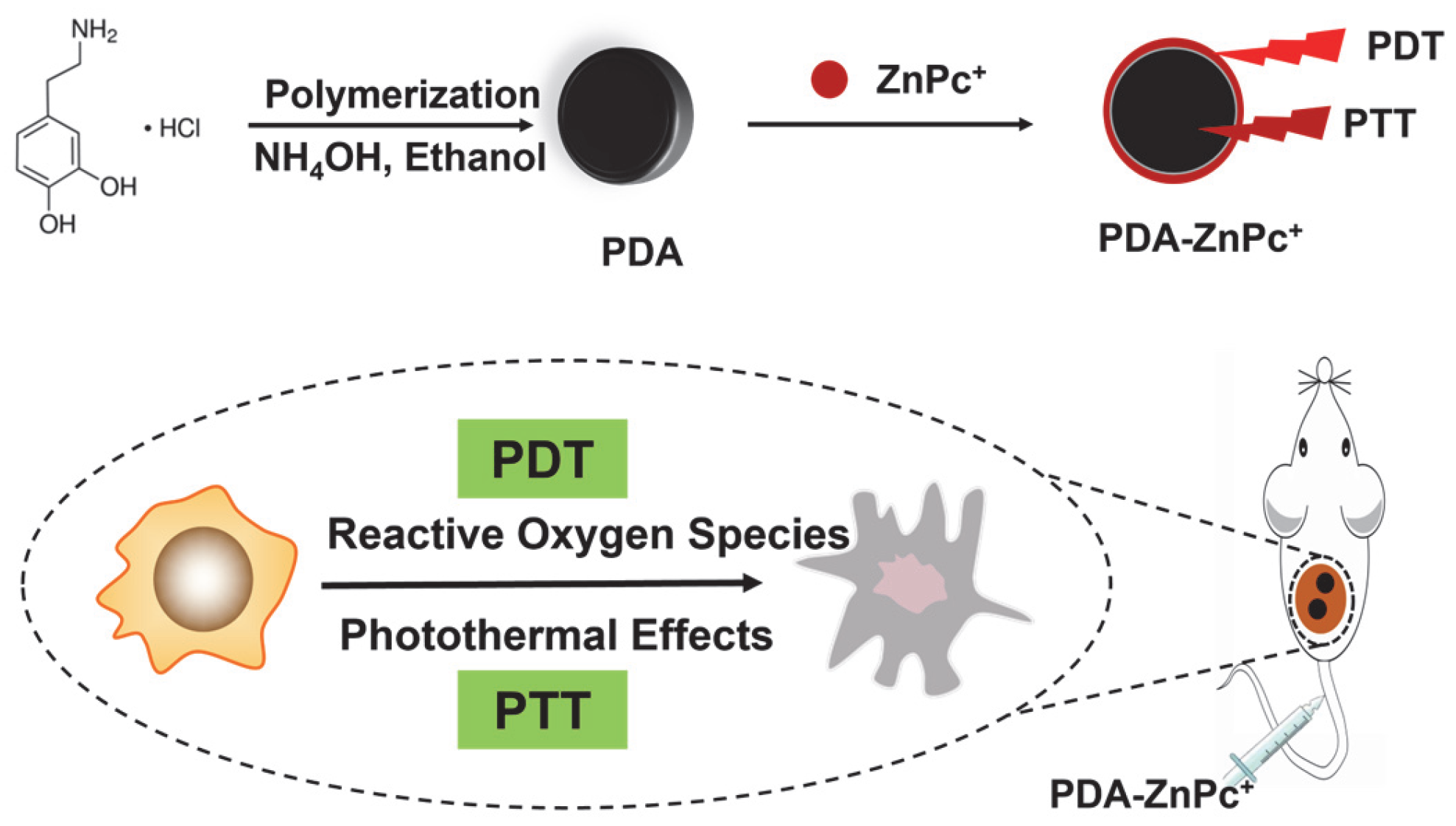
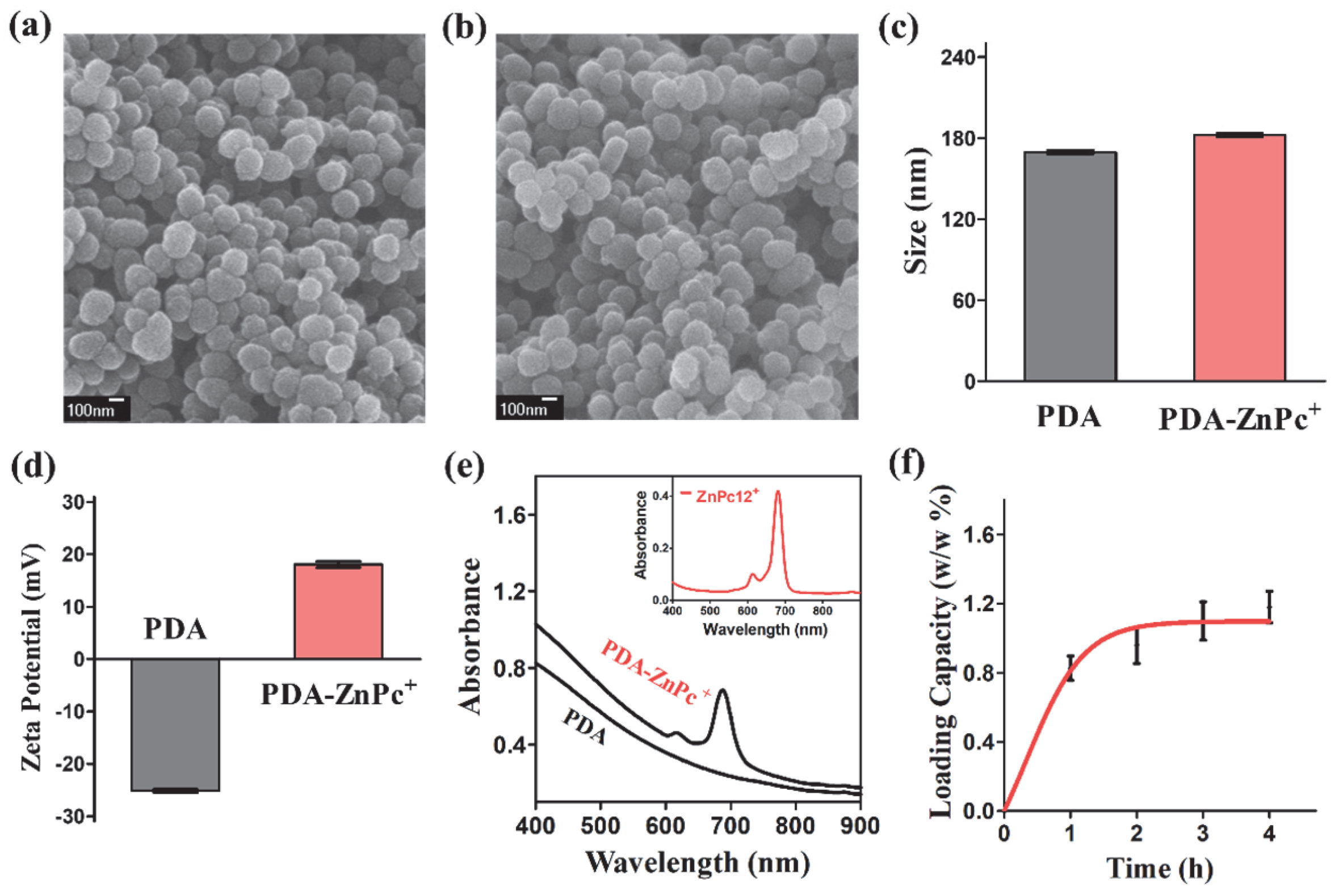
2.1. Synthesis and Characterization of PDA-ZnPc+ Nps
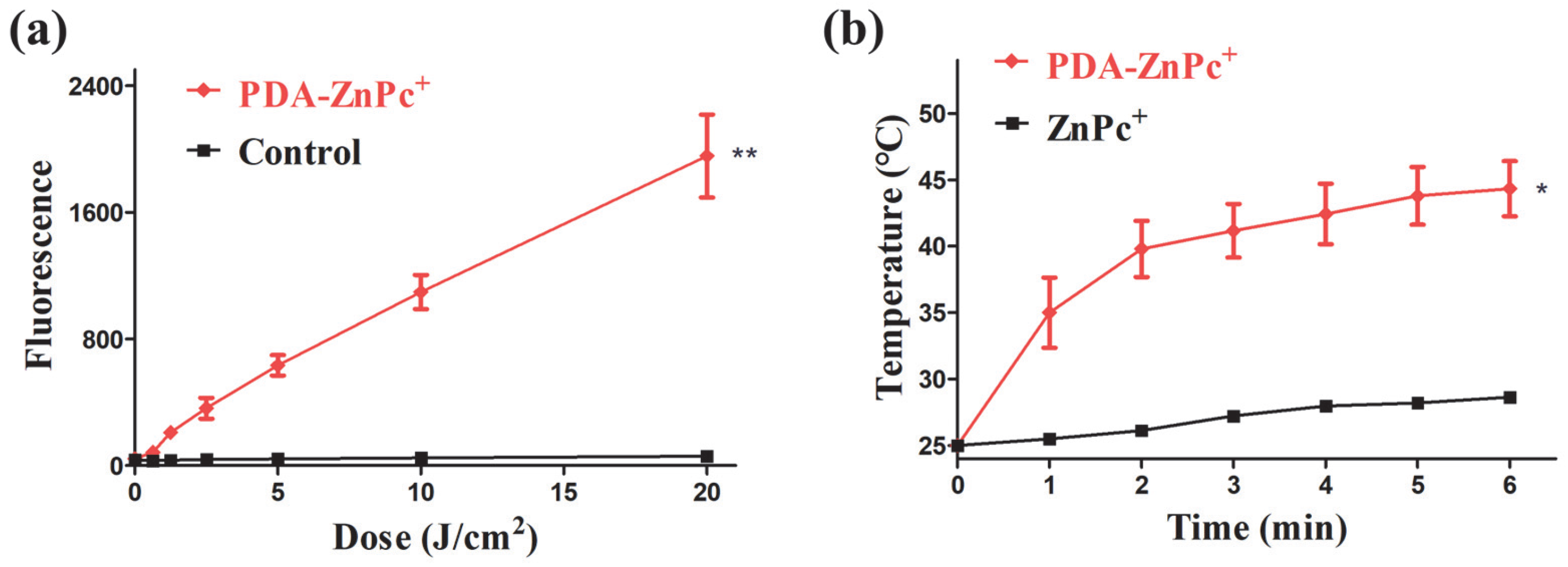
2.2. ROS and Photothermal Effect of PDA-ZnPc+ Nps
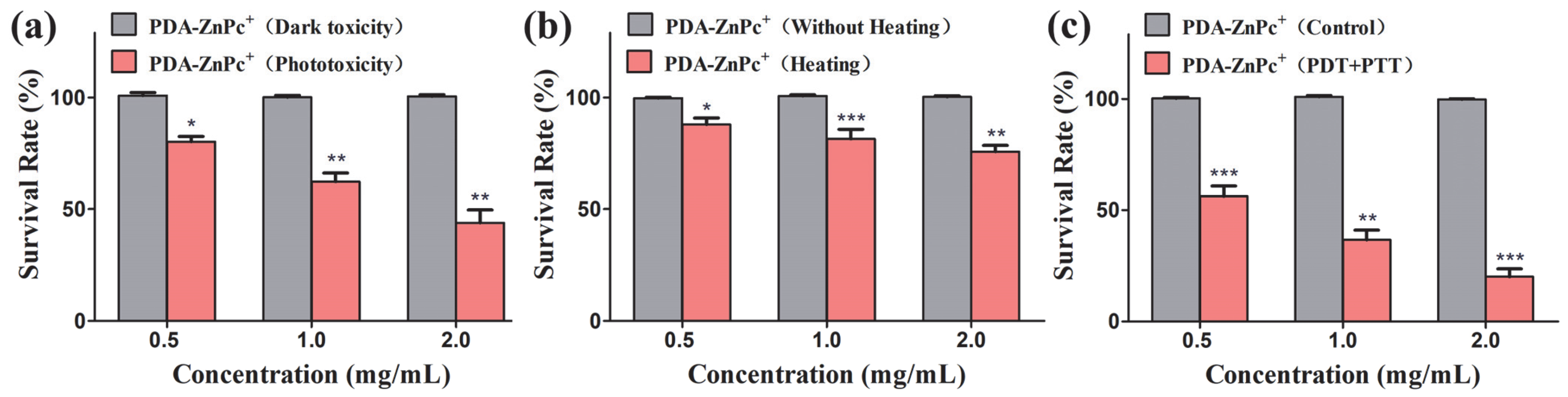
2.3. In Vitro Cytotoxicity of PDA-ZnPc+ Nps
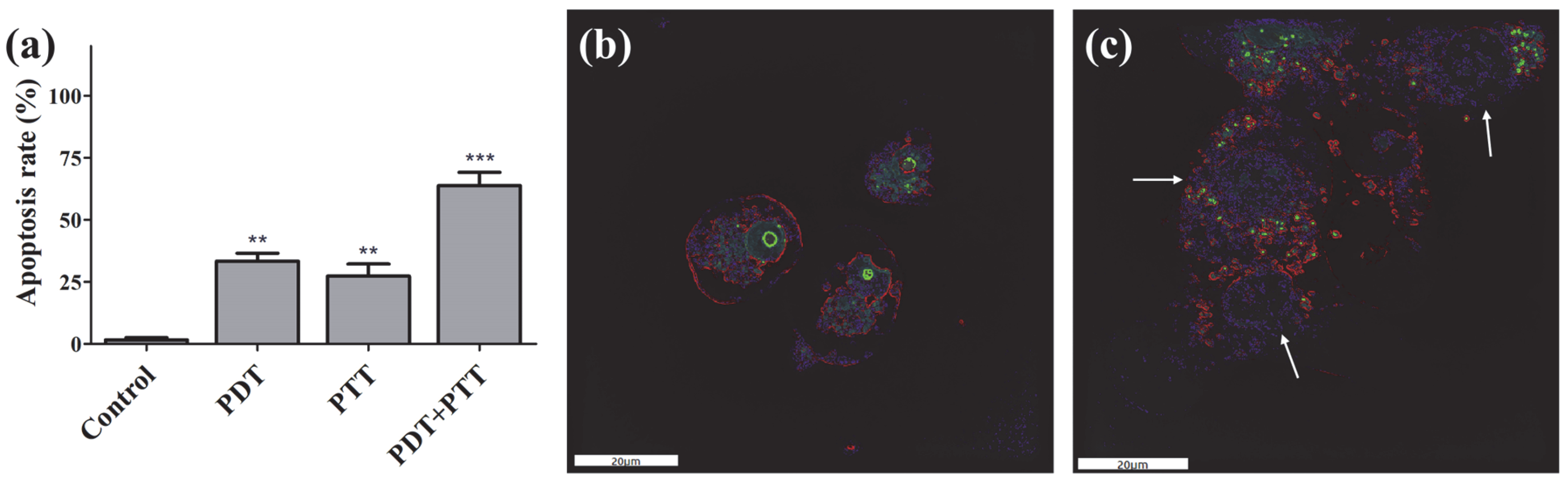
2.4. Apoptosis
2.5. Fluorescence Imaging
2.6. In Vivo Combinational PTT/PDT
2.7. In Vivo Safety Analysis of PDA-ZnPc+ Nps
3. Discussion
4. Materials and Methods
4.1. Chemicals and Cell Lines
4.2. Preparation of ZnPc(4TAP)12+ (ZnPc+)
4.3. Synthesis of PDA
4.4. Synthesis of PDA-ZnPc(4TAP)12+ Nanoparticles (PDA-ZnPc+ Nps)
4.5. Characterization of PDA-ZnPc+ Nps
4.6. ROS and Photothermal Effect of PDA-ZnPc+ Nps
4.7. In Vitro PDT, PTT, and Synergistic PDT/PTT
4.8. Apoptosis Detection
4.9. Fluorescence Imaging
4.10. Establishment of MCF-7 Tumor-Bearing Mouse Model
4.11. In Vivo Combinational PTT/PDT
4.12. H&E Staining Assay
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Tsimberidou, A.M.; Fountzilas, E.; Nikanjam, M.; Kurzrock, R. Review of precision cancer medicine: Evolution of the treatment paradigm. Cancer Treat. Rev. 2020, 86, 102019. [Google Scholar]
- Mainini, F.; Eccles, M.R. Lipid and Polymer-Based Nanoparticle siRNA Delivery Systems for Cancer Therapy. Molecules 2020, 25, 2692. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Yang, N.; Ji, W.; Zhang, H.; Dong, X.; Zhou, Z.; Li, L.; Shen, H.M.; Yao, S.Q.; Huang, W. Mito-Bomb: Targeting Mitochondria for Cancer Therapy. Adv. Mater. 2021, 33, e2007778. [Google Scholar]
- Miller, K.D.; Nogueira, L.; Devasia, T.; Mariotto, A.B.; Yabroff, K.R.; Jemal, A.; Kramer, J.; Siegel, R.L. Cancer treatment and survivorship statistics, 2022. CA A Cancer J. Clin. 2022, 72, 409–436. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA A Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Gustalik, J.; Aebisher, D.; Bartusik-Aebisher, D. Photodynamic therapy in breast cancer treatment. J. Appl. Biomed. 2022, 20, 98–105. [Google Scholar] [CrossRef]
- Li, X.; Lovell, J.F.; Yoon, J.; Chen, X. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat. Rev. Clin. Oncol. 2020, 17, 657–674. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, Y.; Chen, Y.; Liu, L.; Mo, A.; Peng, Q. Nanomaterials-based photothermal therapy and its potentials in antibacterial treatment. J. Control. Release 2020, 328, 251–262. [Google Scholar]
- Hwang, E.; Jung, H.S. Organelle-targeted photothermal agents for cancer therapy. Chem. Commun. 2021, 57, 7731–7742. [Google Scholar] [CrossRef]
- Ji, B.; Wei, M.; Yang, B. Recent advances in nanomedicines for photodynamic therapy (PDT)-driven cancer immunotherapy. Theranostics 2022, 12, 434–458. [Google Scholar] [CrossRef]
- Mundekkad, D.; Cho, W.C. Nanoparticles in Clinical Translation for Cancer Therapy. Int. J. Mol. Sci. 2022, 23, 1685. [Google Scholar] [CrossRef]
- Hou, Y.J.; Yang, X.X.; Liu, R.Q.; Zhao, D.; Guo, C.X.; Zhu, A.C.; Wen, M.N.; Liu, Z.; Qu, G.F.; Meng, H.X. Pathological Mechanism of Photodynamic Therapy and Photothermal Therapy Based on Nanoparticles. Int. J. Nanomed. 2020, 15, 6827–6838. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Li, M.; Dey, R.; Chen, Y. Nanomaterials for cancer therapy: Current progress and perspectives. J. Hematol. Oncol. 2021, 14, 85. [Google Scholar]
- Wu, S.Y.; Wu, F.G.; Chen, X. Antibody-Incorporated Nanomedicines for Cancer Therapy. Adv. Mater. 2022, 34, e2109210. [Google Scholar] [CrossRef]
- Shekhar, S.; Chauhan, M.; Sonali; Yadav, B.; Dutt, R.; Hu, L.; Muthu, M.S.; Singh, R.P. Enhanced permeability and retention effect-focused tumor-targeted nanomedicines: Latest trends, obstacles and future perspective. Nanomedicine 2022, 17, 1213–1216. [Google Scholar] [CrossRef]
- Huang, D.; Sun, L.; Huang, L.; Chen, Y. Nanodrug Delivery Systems Modulate Tumor Vessels to Increase the Enhanced Permeability and Retention Effect. J. Pers. Med. 2021, 11, 124. [Google Scholar] [CrossRef]
- Lee, K.K.; Lee, J.H.; Lee, S.C.; Lee, C.S. MnCO(3)-mineralized polydopamine nanoparticles as an activatable theranostic agent for dual-modality imaging-guided photothermal therapy of cancers. Theranostics 2022, 12, 6762–6778. [Google Scholar] [CrossRef]
- Zhang, P.; Li, X.; Xu, Q.; Wang, Y.; Ji, J. Polydopamine nanoparticles with different sizes for NIR-promoted gene delivery and synergistic photothermal therapy. Colloids Surf. B Biointerfaces 2021, 208, 112125. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Song, X.; Liu, Y.; Dai, T.; Huang, M.; Chen, X.; Chen, Z. An efficient synergistic cancer therapy by integrating cell cycle inhibitor and photosensitizer into polydopamine nanoparticles. J. Mater. Chem. B 2018, 6, 2620–2629. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Cai, L.; Dai, Y.; Liu, Y.; Zuo, F.; Ni, C.; Shi, M.; Li, J. Polydopamine-Based Nanoparticles for Photothermal Therapy/Chemotherapy and their Synergistic Therapy with Autophagy Inhibitor to Promote Antitumor Treatment. Chem. Rec. 2021, 21, 781–796. [Google Scholar] [CrossRef]
- Zhong, W.; Wong, K.H.; Xu, F.; Zhao, N.; Chen, M. NIR-responsive polydopamine-based calcium carbonate hybrid nanoparticles delivering artesunate for cancer chemo-photothermal therapy. Acta Biomater. 2022, 145, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Gao, T.; Lu, Z.; Yin, J.; Zhang, Y.; Pei, R. Aptamer-Targeted Photodynamic Platforms for Tumor Therapy. ACS Appl. Mater. Interfaces 2021, 13, 27749–27773. [Google Scholar] [CrossRef] [PubMed]
- El-Hussein, A.; Manoto, S.L.; Ombinda-Lemboumba, S.; Alrowaili, Z.A.; Mthunzi-Kufa, P. A Review of Chemotherapy and Photodynamic Therapy for Lung Cancer Treatment. Anti-Cancer Agents Med. Chem. 2021, 21, 149–161. [Google Scholar] [CrossRef]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kędzierska, E.; Knap-Czop, K.; Kotlińska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic therapy—Mechanisms, photosensitizers and combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.J.; Wang, K.P.; Mo, J.G.; Xiong, L.; Wen, Y. Photodynamic therapy regulates fate of cancer stem cells through reactive oxygen species. World J. Stem Cells 2020, 12, 562–584. [Google Scholar] [CrossRef]
- Lan, M.; Zhao, S.; Liu, W.; Lee, C.S.; Zhang, W.; Wang, P. Photosensitizers for Photodynamic Therapy. Adv. Healthc. Mater. 2019, 8, e1900132. [Google Scholar] [CrossRef]
- Wysocki, M.; Czarczynska-Goslinska, B.; Ziental, D.; Michalak, M.; Güzel, E.; Sobotta, L. Excited State and Reactive Oxygen Species against Cancer and Pathogens: A Review on Sonodynamic and Sono-Photodynamic Therapy. ChemMedChem 2022, 17, e202200185. [Google Scholar] [CrossRef]
- Roguin, L.P.; Chiarante, N.; García Vior, M.C.; Marino, J. Zinc(II) phthalocyanines as photosensitizers for antitumor photodynamic therapy. Int. J. Biochem. Cell Biol. 2019, 114, 105575. [Google Scholar] [CrossRef]
- Zheng, B.D.; Ye, J.; Huang, Y.Y.; Xiao, M.T. Phthalocyanine-based photoacoustic contrast agents for imaging and theranostics. Biomater. Sci. 2021, 9, 7811–7825. [Google Scholar] [CrossRef]
- Rennie, C.C.; Edkins, R.M. Targeted cancer phototherapy using phthalocyanine-anticancer drug conjugates. Dalton Trans. 2022, 51, 13157–13175. [Google Scholar] [CrossRef]
- Gunaydin, G.; Gedik, M.E.; Ayan, S. Photodynamic Therapy-Current Limitations and Novel Approaches. Front. Chem. 2021, 9, 691697. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Gao, J.; Ding, Y.; Lu, Y.; Wei, Q.; Cui, D.; Fan, J.; Li, X.; Zhu, E.; Lu, Y.; et al. Multi-Functional Liposome: A Powerful Theranostic Nano-Platform Enhancing Photodynamic Therapy. Adv. Sci. 2021, 8, e2100876. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Chen, J.; Cai, L.; Xu, P.; Zhang, Y.; Li, S.; Hu, P.; Chen, X.; Huang, M.; Chen, Z. Phthalocyanine-based photosensitizer with tumor-pH-responsive properties for cancer theranostics. J. Mater. Chem. B 2018, 6, 6080–6088. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, K.; Chen, Z.; Chen, J.; Hu, P.; Cai, L.; Iqbal, Z.; Huang, M. Rapid killing of bacteria by a new type of photosensitizer. Appl. Microbiol. Biotechnol. 2017, 101, 4691–4700. [Google Scholar] [CrossRef]
- Kang, E.S.; Lee, T.H.; Liu, Y.; Han, K.H.; Lee, W.K.; Yoon, I. Graphene Oxide Nanoparticles Having Long Wavelength Absorbing Chlorins for Highly-Enhanced Photodynamic Therapy with Reduced Dark Toxicity. Int. J. Mol. Sci. 2019, 20, 4344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Y.; van der Meel, R.; Chen, X.; Lammers, T. The EPR effect and beyond: Strategies to improve tumor targeting and cancer nanomedicine treatment efficacy. Theranostics 2020, 10, 7921–7924. [Google Scholar] [CrossRef]
- Yang, M.; Li, J.; Gu, P.; Fan, X. The application of nanoparticles in cancer immunotherapy: Targeting tumor microenvironment. Bioact. Mater. 2021, 6, 1973–1987. [Google Scholar] [CrossRef]
- Ma, X.; Wang, C.; Dong, Z.; Hu, C.; Feng, L. Lipid-coated CaCO(3)-PDA nanoparticles as a versatile nanocarrier to enable pH-responsive dual modal imaging-guided combination cancer therapy. J. Mater. Chem. B 2022, 10, 4096–4104. [Google Scholar] [CrossRef]
- Xiong, Y.; Xu, Z.; Li, Z. Polydopamine-Based Nanocarriers for Photosensitizer Delivery. Front. Chem. 2019, 7, 471. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Gong, F.; Yang, Z.; Zhao, X.; Li, Y.; Zeng, C.; Li, J.; Guo, S. Core-Shell Structured HMX@Polydopamine Energetic Microspheres: Synergistically Enhanced Mechanical, Thermal, and Safety Performances. Polymers 2019, 11, 568. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, S.; Dong, L.; Hu, Z.; Zhang, Y.; Xu, W.; Xing, J.; Zhang, J. A Photosensitizer-Loaded Polydopamine Nanomedicine Agent for Synergistic Photodynamic and Photothermal Therapy. Molecules 2023, 28, 5874. https://doi.org/10.3390/molecules28155874
Yan S, Dong L, Hu Z, Zhang Y, Xu W, Xing J, Zhang J. A Photosensitizer-Loaded Polydopamine Nanomedicine Agent for Synergistic Photodynamic and Photothermal Therapy. Molecules. 2023; 28(15):5874. https://doi.org/10.3390/molecules28155874
Chicago/Turabian StyleYan, Shufeng, Luying Dong, Ziyun Hu, Yucheng Zhang, Wei Xu, Jianhong Xing, and Juncheng Zhang. 2023. "A Photosensitizer-Loaded Polydopamine Nanomedicine Agent for Synergistic Photodynamic and Photothermal Therapy" Molecules 28, no. 15: 5874. https://doi.org/10.3390/molecules28155874
APA StyleYan, S., Dong, L., Hu, Z., Zhang, Y., Xu, W., Xing, J., & Zhang, J. (2023). A Photosensitizer-Loaded Polydopamine Nanomedicine Agent for Synergistic Photodynamic and Photothermal Therapy. Molecules, 28(15), 5874. https://doi.org/10.3390/molecules28155874






