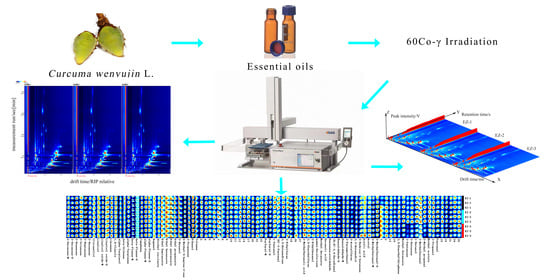
Influence of 60Co-γ Irradiation on the Components of Essential Oil of Curcuma
Abstract
:1. Introduction
2. Results
2.1. GC-IMS Analysis of EOs in Curcuma
2.2. EO Fingerprint Comparison of Samples
2.3. PCA of EOs in Samples
2.4. Chemical Composition of the EOs
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Isolation of the EOs
4.3. 60Co Irradiation
4.4. GC-IMS Analysis
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Committee for the Pharmacopoeia of P.R. China. Pharmacopoeia of P.R. China, Part I.; Beijing Chemical Industry Press: Beijing, China, 2020; p. 286. [Google Scholar]
- Liu, M.; Guo, X.H.; Sun, Q.; Chen, J.; Wu, W.H.; Zhang, X.Q. Research progress on chemical composition and pharmacological effects of Curcuma wenyujin. Mod. Drugs Clin. 2021, 36, 204–208. [Google Scholar]
- Wu, H.X.; Sun, Z.M.; Wang, Q. Overview of pharmacological research and clinical application research progress of Curcuma wenyujin essential oils. China Med. Her. 2011, 13, 79–83. [Google Scholar]
- Xiao, Y.; Yang, F.Q.; Li, S.P.; Hu, G.; Lee, S.M.; Wang, Y.T. Essential oil of Curcuma wenyujin induces apoptosis in human hepatoma cells. World J. Gastroenterol. 2008, 14, 4309–4318. [Google Scholar] [CrossRef] [PubMed]
- Yin, G.P.; Zhang, Q.Z.; An, Y.W.; Zhu, J.J.; Wang, Z.M. Advance in chemical constituents and pharmacological activity of Curcuma wenyujin. China J. Chin. Mater. Med. 2012, 37, 354–360. [Google Scholar]
- Li, J.S. Inspection of the sterilization effect of irradiation on proprietary Chinese medicines. Chin. J. Disinfect. 2002, 19, 1. [Google Scholar]
- Lin, T.; Bi, F.J.; Lv, W.S.; Zhang, L.W.; Jin, H.Y.; Ma, S.C.; Jiang, Y.Q. Current status and supervision of irradiation sterilization of traditional Chinese medicines. Chin. Pharm. J. 2019, 54, 1442–1447. [Google Scholar]
- Arroyo-Manzanares, N.; García-Nicolás, M.; Castell, A.; Campillo, N.; Viñas, P.; López-García, I.; Hernández-Córdoba, M. Untargeted headspace gas chromatography—Ion mobility spectrometry analysis for detection of adulterated honey. Talanta 2019, 205, 120123. [Google Scholar] [CrossRef]
- Yang, J.C.; Cao, S.Y.; Yang, L. Study on the combination of gas chromatography and ion migration spectrometer. Mod. Instrum. Med. Sci. 2014, 20, 20–24. [Google Scholar]
- Chen, J.; Tao, L.; Zhang, T. Effect of four types of thermal processing methods on the aroma profiles of acidity regulator-treated tilapia muscles using E-nose, HS-SPME-GC-MS, and HS-GC-IMS. LWT—Food Sci. Technol. 2021, 147, 111585. [Google Scholar] [CrossRef]
- Li, M.; Yang, R.; Zhang, H.; Wang, S.; Chen, D.; Lin, S. Development of a flavor fingerprint by HS-GC-IMS with PCA for volatile compounds of Tricholoma matsutake Singer. Food Chem. 2019, 290, 32–39. [Google Scholar] [CrossRef]
- Chen, M.G.; Lin, X.E.; Li, X.G.; Liu, X.D.; Gao, H.M.; Ming, J.H.; Dai, M.J.; Zhou, Z.X. Comprehensive Evaluation of Durian Quality Based on Principal Component Analysis and Cluster Analysis. Sci. Technol. Food Ind. 2023, 44, 278–286. [Google Scholar]
- Lu, T.L.; Yang, G.M.; Song, K.; Li, L.; Cai, B.C. Determination of the volatile oil from processed products of Rhizoma Curcumae by GC·MS. Chin. Tradit. Pat. Med. 2003, 25, 810–811. [Google Scholar]
- Zeng, J.H. Study on the Construction of GC-MS Fingerprint of Volatile Oil in Guangxi Shu and Its Spectral Activity Relationship. Master’s Thesis, Central South University of Forestry and Technology, Changsha, China, 2012. [Google Scholar]
- Wang, D.W.; Zhang, L.; Li, Y.F.; Duan, T.C. Microwave-assisted extraction of GC-MS analysis of volatile oil components of curcum. Chin. J. Anal. Chem. 2008, 36, 1. [Google Scholar]
- Tan, Y.; Wang, G.Q.; Wu, J.Z.; Chen, G.; Zhou, A.M. Analysis of Volatile Flavour Components in Four Pomelo Peel Essential Oils Based on GC-MS and GC-IMS. Sci. Technol. Food Ind. 2021, 42, 256–268. [Google Scholar]
- Yang, F.; Zhao, Q.; Wang, Y.; Ma, S.L.; Gong, Y. Curcumin inhibits the effect of STAT3 signaling pathway on proliferation of pancreatic cancer cells. World Chin. J. Dig. 2011, 19, 5. [Google Scholar]
- Shi, J.H. Experimental pharmacological study of volatile oil of Wengor: Study of the antitumor effect of β-elemicene. China J. Chin. Mater. Medica 1981, 6, 32. [Google Scholar]
- Song, S.; Lu, T.L.; Li, L. Analgesic and Anti—Inflammatory Effects of Different Kinds Rhizoma Curcumae. Chin. Arch. Tradit. Chin. Med. 2005, 23, 2. [Google Scholar]
- Wang, X.; Xia, Q.; Xu, D.J.; Chen, F.H.; Chen, X.H.; Su, Y. An experimental study on the anticoagulant and antithrombotic effects of diketone in the operation of curcum. Chin. Tradit. Pat. Med. 2012, 34, 4. [Google Scholar]
- Mao, C.Q.; Xie, H.; Lu, T.L. Studies oil Antiplatelet Aggregation and Analgestic Action of Curcmna phaeocaulis. J. Chin. Med. Mater. 2000, 023, 212–213. [Google Scholar]
- Xi, Z.T.; Shan, C.M.; Jiang, X.L.; LUAN, X.Y.; Li, K.K. Experimental Study on Rhizoma Sparganii and Radices Zedoariae Treating Hepatic Fibrosis. China J. Chin. Mater. Medica 2002, 27, 4. [Google Scholar]
- Du, L.P.; Hu, Z.Y.; Deng, Y.Y.; Chen, Y.P. Experimental Study on the Influence of Curcumae on Extracellular Matrix of Kidney. Shanghai J. Tradit. Chin. Med. 2001, 35, 3. [Google Scholar]






| Count | Compound Name | CAS | RI | Molecular Formula | Rt/s | Dt/ms (RIPrel) | Comment |
|---|---|---|---|---|---|---|---|
| 1 | gamma-Decalactone | C706149 | 1598.5 | C10H18O2 | 1209.228 | 1.47454 | - |
| 2 | Methyl decanoate | C110429 | 1508.0 | C11H22O2 | 1079.39 | 1.54878 | - |
| 3 | (E,E)-2,4-Decadienal | C25152845 | 1454.2 | C10H16O | 1002.04 | 1.42077 | - |
| 4 | Decanoic acid | C334485 | 1360.2 | C10H20O2 | 867.201 | 1.57124 | - |
| 5 | Eugenol | C97530 | 1341.6 | C10H12O2 | 840.393 | 1.29711 | - |
| 6 | 2-Decanone | C693549 | 1256.7 | C10H20O | 718.508 | 1.48226 | Monomers |
| 7 | 2-Decanone | C693549 | 1255.1 | C10H20O | 716.197 | 1.99783 | Dimers |
| 8 | alpha-Terpineol | C98555 | 1206.8 | C10H18O | 646.87 | 1.22248 | - |
| 9 | Diethyl succinate | C123251 | 1234.7 | C8H14O4 | 686.937 | 1.29422 | - |
| 10 | Citronellol | C106229 | 1197.5 | C10H20O | 633.591 | 1.34963 | - |
| 11 | Linalool | C78706 | 1106.9 | C10H18O | 503.438 | 1.2229 | - |
| 12 | Ethyl heptanoate | C106309 | 1122.5 | C9H18O2 | 525.872 | 1.9311 | - |
| 13 | 2-Nonanone | C821556 | 1091.9 | C9H18O | 481.9 | 1.88944 | - |
| 14 | Linalool oxide | C60047178 | 1080.6 | C10H18O2 | 465.66 | 1.24616 | Monomers |
| 15 | Linalool oxide | C60047178 | 1081.0 | C10H18O2 | 466.245 | 1.8138 | Dimers |
| 16 | 1,8-Cineole | C470826 | 1027.6 | C10H18O | 389.61 | 1.73145 | - |
| 17 | beta-Ocimene | C13877913 | 1054.5 | C10H16 | 428.22 | 1.21822 | - |
| 18 | Benzeneacetaldehyde | C122781 | 1045.1 | C8H8O | 414.765 | 1.2491 | - |
| 19 | Limonene | C138863 | 1025.6 | C10H16 | 386.685 | 1.20792 | - |
| 20 | alpha-Terpinene | C99865 | 1015.0 | C10H16 | 371.475 | 1.22263 | - |
| 21 | 6-Methyl-5-hepten-2-one | C110930 | 990.5 | C8H14O | 339.885 | 1.17704 | - |
| 22 | beta-Pinene | C127913 | 976.0 | C10H16 | 327.6 | 1.21969 | Monomers |
| 23 | beta-Pinene | C127913 | 976.0 | C10H16 | 327.6 | 1.64175 | Dimers |
| 24 | Camphene | C79925 | 944.9 | C10H16 | 301.275 | 1.21822 | - |
| 25 | alpha-Pinene | C80568 | 931.8 | C10H16 | 290.16 | 1.22116 | Monomers |
| 26 | alpha-Pinene | C80568 | 931.1 | C10H16 | 289.575 | 1.67557 | Dimers |
| 27 | 2-Ethylhexanol | C104767 | 1018.3 | C8H18O | 376.21 | 1.79856 | - |
| 28 | Ethyl hexanoate | C123660 | 1007.5 | C8H16O2 | 360.735 | 1.81595 | - |
| 29 | 2-Octanone | C111137 | 999.2 | C8H16O | 348.832 | 1.7651 | - |
| 30 | Benzaldehyde | C100527 | 959.8 | C7H6O | 313.914 | 1.15225 | Monomers |
| 31 | Benzaldehyde | C100527 | 959.4 | C7H6O | 313.517 | 1.47206 | Dimers |
| 32 | Ethyl pentanoate | C539822 | 901.2 | C7H14O2 | 264.315 | 1.71158 | - |
| 33 | 2-Heptanone | C110430 | 891.6 | C7H14O | 256.379 | 1.62862 | - |
| 34 | 2-Furanmethanol | C98000 | 873.3 | C5H6O2 | 246.856 | 1.1188 | - |
| 35 | 2-Acetylfuran | C1192627 | 911.1 | C6H6O2 | 272.648 | 1.45333 | - |
| 36 | 2,5-Dimethylthiophene | C638028 | 856.5 | C6H8S | 238.127 | 1.07866 | - |
| 37 | Furfural | C98011 | 828.3 | C5H4O2 | 223.446 | 1.08401 | Monomers |
| 38 | Furfural | C98011 | 826.7 | C5H4O2 | 222.652 | 1.3329 | Dimers |
| 39 | 2-Methylbutanoic acid | C116530 | 827.5 | C5H10O2 | 223.049 | 1.46938 | - |
| 40 | 2-Hexanol | C626937 | 793.1 | C6H14O | 205.193 | 1.56974 | - |
| 41 | 2-Hexanone | C591786 | 791.9 | C6H12O | 204.546 | 1.50912 | - |
| 42 | 2,3-Butanediol | C513859 | 778.3 | C4H10O2 | 198.14 | 1.3627 | - |
| 43 | 1-Pentanol | C71410 | 760.5 | C5H12O | 190.908 | 1.25055 | - |
| 44 | Acetal | C105577 | 748.9 | C6H14O2 | 186.155 | 0.96913 | - |
| 45 | (E)-2-Pentenal | C1576870 | 747.3 | C5H8O | 185.535 | 1.36062 | - |
| 46 | 3-Hydroxy-2-butanone | C513860 | 733.1 | C4H8O2 | 179.749 | 1.33778 | - |
| 47 | 2,5-Dimethylfuran | C625865 | 739.9 | C6H8O | 182.503 | 1.0218 | - |
| 48 | Methyl butanoate | C623427 | 716.5 | C5H10O2 | 172.993 | 1.15331 | - |
| 49 | Ethyl propanoate | C105373 | 695.4 | C5H10O2 | 164.398 | 1.44128 | - |
| 50 | 2-Pentanone | C107879 | 686.6 | C5H10O | 161.106 | 1.37552 | - |
| 51 | 2-Ethylfuran | C3208160 | 674.9 | C6H8O | 157.998 | 1.3211 | - |
| 52 | 2-Methylbutanal | C96173 | 663.8 | C5H10O | 155.072 | 1.40387 | - |
| 53 | 3-Methylbutanal | C590863 | 650.6 | C5H10O | 151.597 | 1.4118 | Dimers |
| 54 | 1-Butanol | C71363 | 656.2 | C4H10O | 153.06 | 1.37552 | - |
| 55 | 3-Methylbutanal | C590863 | 645.1 | C5H10O | 150.134 | 1.20319 | Monomers |
| 56 | 2-Butanone | C78933 | 591.7 | C4H8O | 136.053 | 1.24854 | - |
| 57 | 2-Methyl propanal | C78842 | 555.1 | C4H8O | 126.36 | 1.28596 | - |
| 58 | 2-Propanol | C67630 | 565.4 | C3H8O | 129.104 | 1.22814 | - |
| 59 | 2,3-Butanedione | C431038 | 582.1 | C4H6O2 | 133.493 | 1.18732 | - |
| 60 | Acetic acid | C64197 | 565.4 | C2H4O2 | 129.104 | 1.16918 | - |
| 61 | 2-Propanone | C67641 | 502.4 | C3H6O | 112.462 | 1.11816 | - |
| 62 | Ethanol | C64175 | 476.1 | C2H6O | 105.513 | 1.12723 | - |
| 63 | Methyl acetate | C79209 | 550.9 | C3H6O2 | 125.263 | 1.20319 | - |
| 64 | Toluene | C108883 | 772.7 | C7H8 | 195.852 | 1.01046 | - |
| 65 | Pentanal | C110623 | 693.6 | C5H10O | 163.667 | 1.18165 | - |
| No | Compounds | Molecular Formula | Comment | [+] EZ-1 | [+] EZ-2 | [+] EZ-3 |
|---|---|---|---|---|---|---|
| 1 | gamma-Decalactone | C10H18O2 | - | 2912.16 | 3431.98 | 3369.21 |
| 2 | Methyl decanoate | C11H22O2 | - | 3341.36 | 3892.70 | 4013.00 |
| 3 | (E,E)-2,4-Decadienal | C10H16O | - | 2973.06 | 3282.99 | 3434.15 |
| 4 | Decanoic acid | C10H20O2 | - | 547.35 | 633.02 | 734.73 |
| 5 | Eugenol | C10H12O2 | - | 1281.77 | 1241.07 | 1233.21 |
| 6 | 2-Decanone | C10H20O | Monomers | 6027.45 | 6303.08 | 6420.79 |
| 7 | 2-Decanone | C10H20O | Dimers | 5086.61 | 5864.29 | 5719.93 |
| 8 | alpha-Terpineol | C10H18O | - | 11,283.16 | 11,913.89 | 11,295.72 |
| 9 | Diethyl succinate | C8H14O4 | - | 1309.52 | 1346.32 | 1390.25 |
| 10 | Citronellol | C10H20O | - | 1096.75 | 1081.54 | 1118.81 |
| 11 | Linalool | C10H18O | - | 9174.36 | 8964.94 | 9084.55 |
| 12 | Ethyl heptanoate | C9H18O2 | - | 8358.19 | 8392.94 | 8400.00 |
| 13 | 2-Nonanone | C9H18O | - | 17,585.94 | 17,143.82 | 17,310.05 |
| 14 | Linalool oxide | C10H18O2 | Monomers | 4690.67 | 4701.49 | 4740.07 |
| 15 | Linalool oxide | C10H18O2 | Dimers | 1377.23 | 1291.99 | 1354.36 |
| 16 | 1,8-Cineole | C10H18O | - | 12,249.16 | 12,122.78 | 12,239.11 |
| 17 | beta-Ocimene | C10H16 | - | 1694.48 | 1418.96 | 1385.90 |
| 18 | Benzeneacetaldehyde | C8H8O | - | 907.73 | 843.63 | 960.16 |
| 19 | Limonene | C10H16 | - | 1129.65 | 1087.89 | 1100.73 |
| 20 | alpha-Terpinene | C10H16 | - | 918.33 | 753.93 | 883.22 |
| 21 | 6-Methyl-5-hepten-2-one | C8H14O | - | 2011.42 | 1931.01 | 1962.76 |
| 22 | beta-Pinene | C10H16 | Monomers | 2522.96 | 2519.12 | 2598.67 |
| 23 | beta-Pinene | C10H16 | Dimers | 5057.77 | 4934.82 | 4995.13 |
| 24 | Camphene | C10H16 | - | 2191.88 | 1963.45 | 1998.16 |
| 25 | alpha-Pinene | C10H16 | Monomers | 1366.07 | 1343.20 | 1351.68 |
| 26 | alpha-Pinene | C10H16 | Dimers | 3062.82 | 2762.18 | 2848.96 |
| 27 | 2-Ethylhexanol | C8H18O | - | 570.80 | 651.25 | 626.41 |
| 28 | Ethyl hexanoate | C8H16O2 | - | 6863.47 | 6978.71 | 7043.41 |
| 29 | 2-Octanone | C8H16O | - | 2915.13 | 2853.83 | 2942.25 |
| 30 | Benzaldehyde | C7H6O | Monomers | 221.68 | 219.65 | 238.90 |
| 31 | Benzaldehyde | C7H6O | Dimers | 473.44 | 415.37 | 483.78 |
| 32 | Ethyl pentanoate | C7H14O2 | - | 20,873.63 | 20,994.98 | 20,390.77 |
| 33 | 2-Heptanone | C7H14O | - | 7475.55 | 7083.04 | 7137.75 |
| 34 | 2-Furanmethanol | C5H6O2 | - | 388.76 | 373.74 | 385.81 |
| 35 | 2-Acetylfuran | C6H6O2 | - | 235.13 | 147.71 | 235.75 |
| 36 | 2,5-Dimethylthiophene | C6H8S | - | 373.28 | 428.12 | 347.52 |
| 37 | Furfural | C5H4O2 | Monomers | 179.78 | 171.41 | 169.89 |
| 38 | Furfural | C5H4O2 | Dimers | 301.64 | 223.43 | 233.77 |
| 39 | 2-Methylbutanoic acid | C5H10O2 | - | 296.69 | 266.89 | 276.09 |
| 40 | 2-Hexanol | C6H14O | - | 7430.20 | 7138.66 | 7364.58 |
| 41 | 2-Hexanone | C6H12O | - | 453.71 | 389.56 | 391.78 |
| 42 | 2,3-Butanediol | C4H10O2 | - | 847.31 | 685.55 | 654.67 |
| 43 | 1-Pentanol | C5H12O | - | 120.72 | 108.34 | 108.60 |
| 44 | Acetal | C6H14O2 | - | 113.23 | 112.71 | 135.34 |
| 45 | (E)-2-Pentenal | C5H8O | - | 79.52 | 61.97 | 58.52 |
| 46 | 3-Hydroxy-2-butanone | C4H8O2 | - | 467.65 | 377.73 | 358.13 |
| 47 | 2,5-Dimethylfuran | C6H8O | - | 45.71 | 46.97 | 51.40 |
| 48 | Methyl butanoate | C5H10O2 | - | 74.38 | 79.05 | 70.13 |
| 49 | Ethyl propanoate | C5H10O2 | - | 1540.94 | 1377.37 | 1423.45 |
| 50 | 2-Pentanone | C5H10O | - | 113.31 | 125.79 | 49.42 |
| 51 | 2-Ethylfuran | C6H8O | - | 225.15 | 186.35 | 177.51 |
| 52 | 2-Methylbutanal | C5H10O | - | 744.68 | 675.02 | 583.69 |
| 53 | 3-Methylbutanal | C5H10O | Dimers | 788.51 | 732.97 | 689.25 |
| 54 | 1-Butanol | C4H10O | - | 398.41 | 379.24 | 285.82 |
| 55 | 3-Methylbutanal | C5H10O | Monomers | 299.34 | 222.36 | 229.26 |
| 56 | 2-Butanone | C4H8O | - | 253.22 | 227.04 | 159.78 |
| 57 | 2-Methyl propanal | C4H8O | - | 161.44 | 153.49 | 88.17 |
| 58 | 2-Propanol | C3H8O | - | 1192.77 | 1087.52 | 1045.79 |
| 59 | 2,3-Butanedione | C4H6O2 | - | 391.70 | 358.15 | 347.32 |
| 60 | Acetic acid | C2H4O2 | - | 718.70 | 654.59 | 652.46 |
| 61 | 2-Propanone | C3H6O | - | 9466.58 | 8838.34 | 8918.75 |
| 62 | Ethanol | C2H6O | - | 2438.91 | 2048.25 | 2256.04 |
| 63 | Methyl acetate | C3H6O2 | - | 364.86 | 337.56 | 295.63 |
| 64 | Toluene | C7H8 | - | 498.55 | 426.60 | 460.66 |
| 65 | Pentanal | C5H10O | - | 94.88 | 126.62 | 106.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lei, C.; Liu, J.; Zhou, W.; Zhou, W.; Li, S.; Huang, D. Influence of 60Co-γ Irradiation on the Components of Essential Oil of Curcuma. Molecules 2023, 28, 5877. https://doi.org/10.3390/molecules28155877
Lei C, Liu J, Zhou W, Zhou W, Li S, Huang D. Influence of 60Co-γ Irradiation on the Components of Essential Oil of Curcuma. Molecules. 2023; 28(15):5877. https://doi.org/10.3390/molecules28155877
Chicago/Turabian StyleLei, Chang, Jianjun Liu, Wenchao Zhou, Wei Zhou, Shunxiang Li, and Dan Huang. 2023. "Influence of 60Co-γ Irradiation on the Components of Essential Oil of Curcuma" Molecules 28, no. 15: 5877. https://doi.org/10.3390/molecules28155877
APA StyleLei, C., Liu, J., Zhou, W., Zhou, W., Li, S., & Huang, D. (2023). Influence of 60Co-γ Irradiation on the Components of Essential Oil of Curcuma. Molecules, 28(15), 5877. https://doi.org/10.3390/molecules28155877