Carbon Materials Prepared from Invading Pelagic Sargassum for Supercapacitors’ Electrodes
Abstract
:1. Introduction
2. Results and Discussion
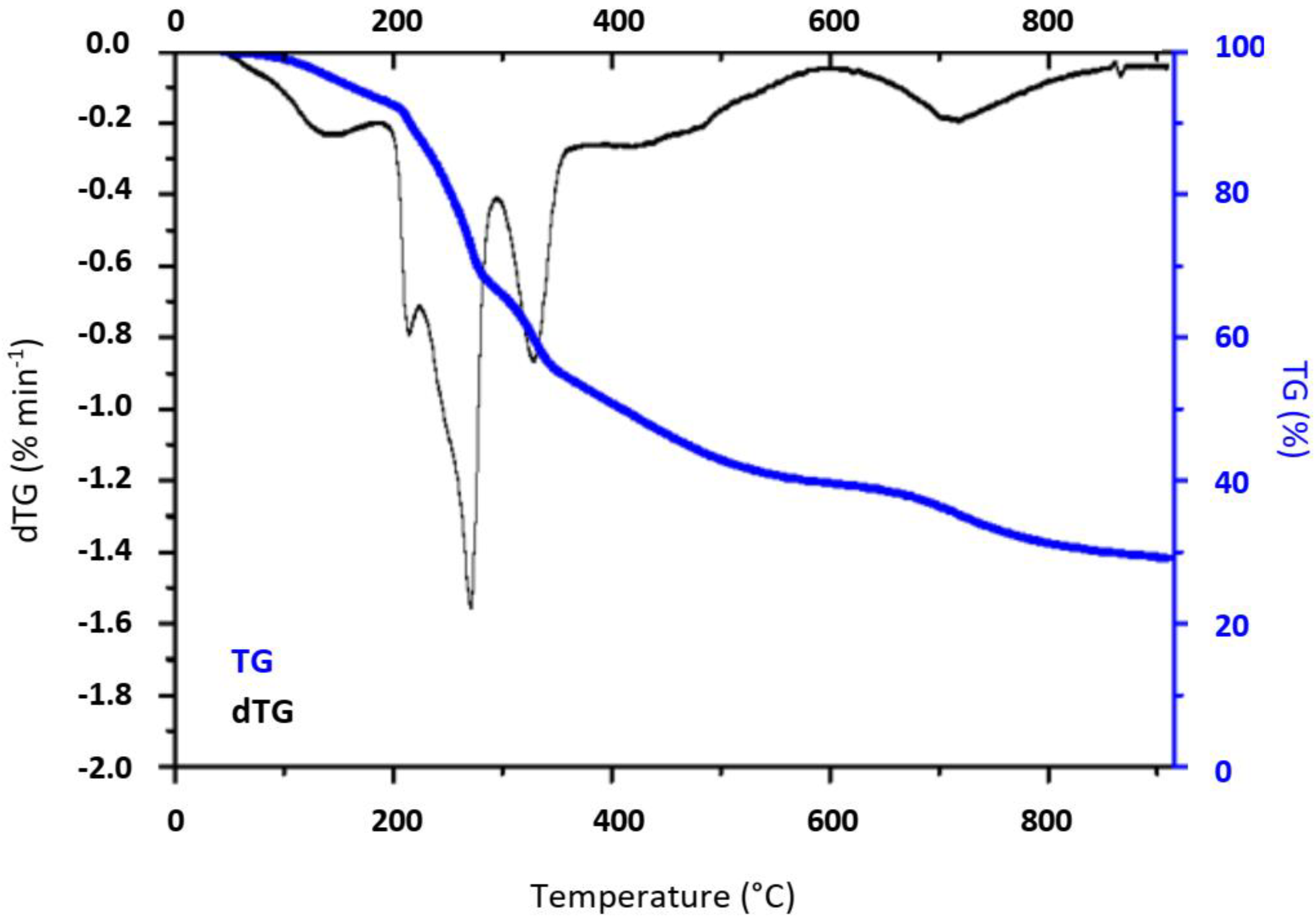
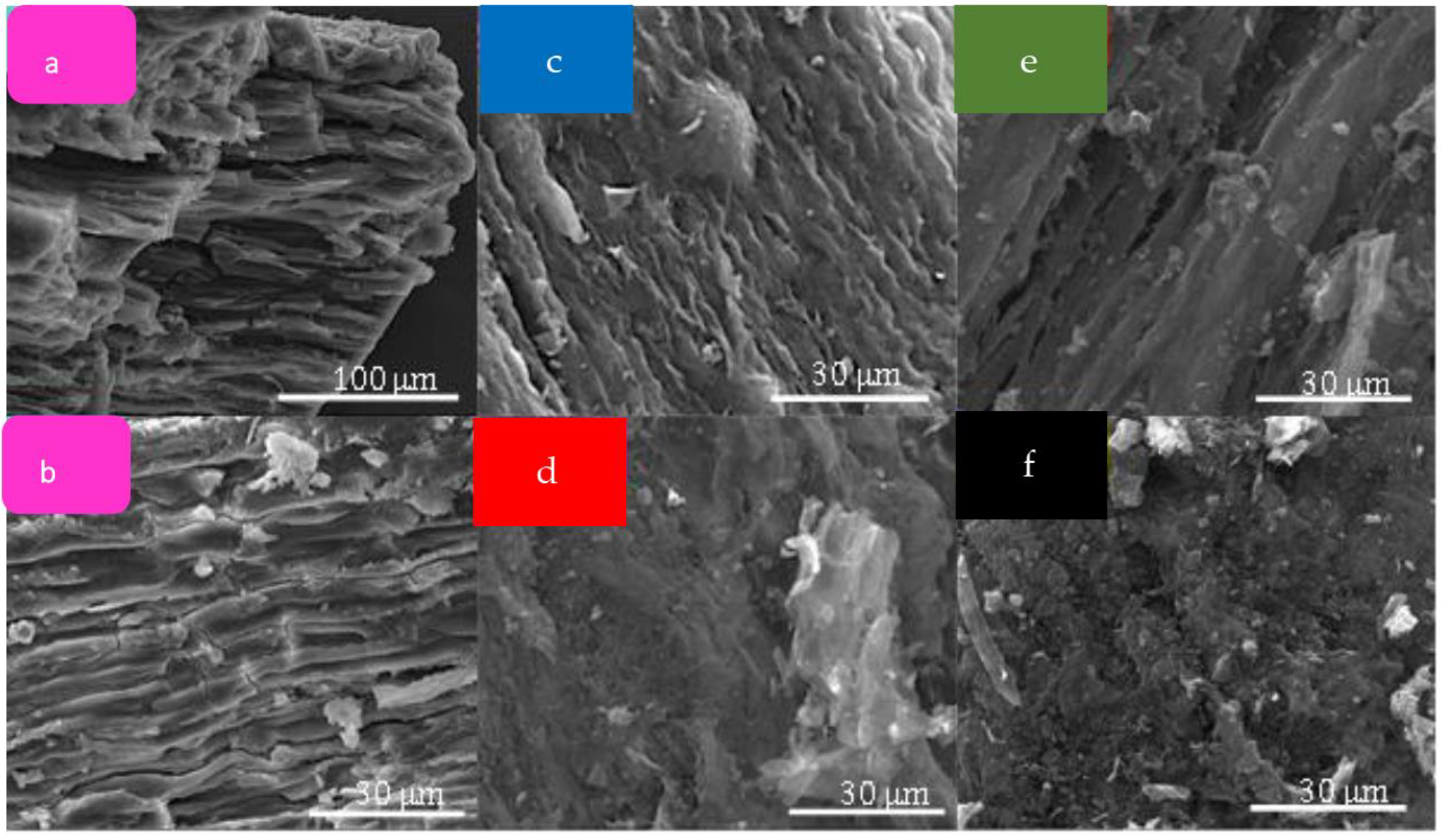
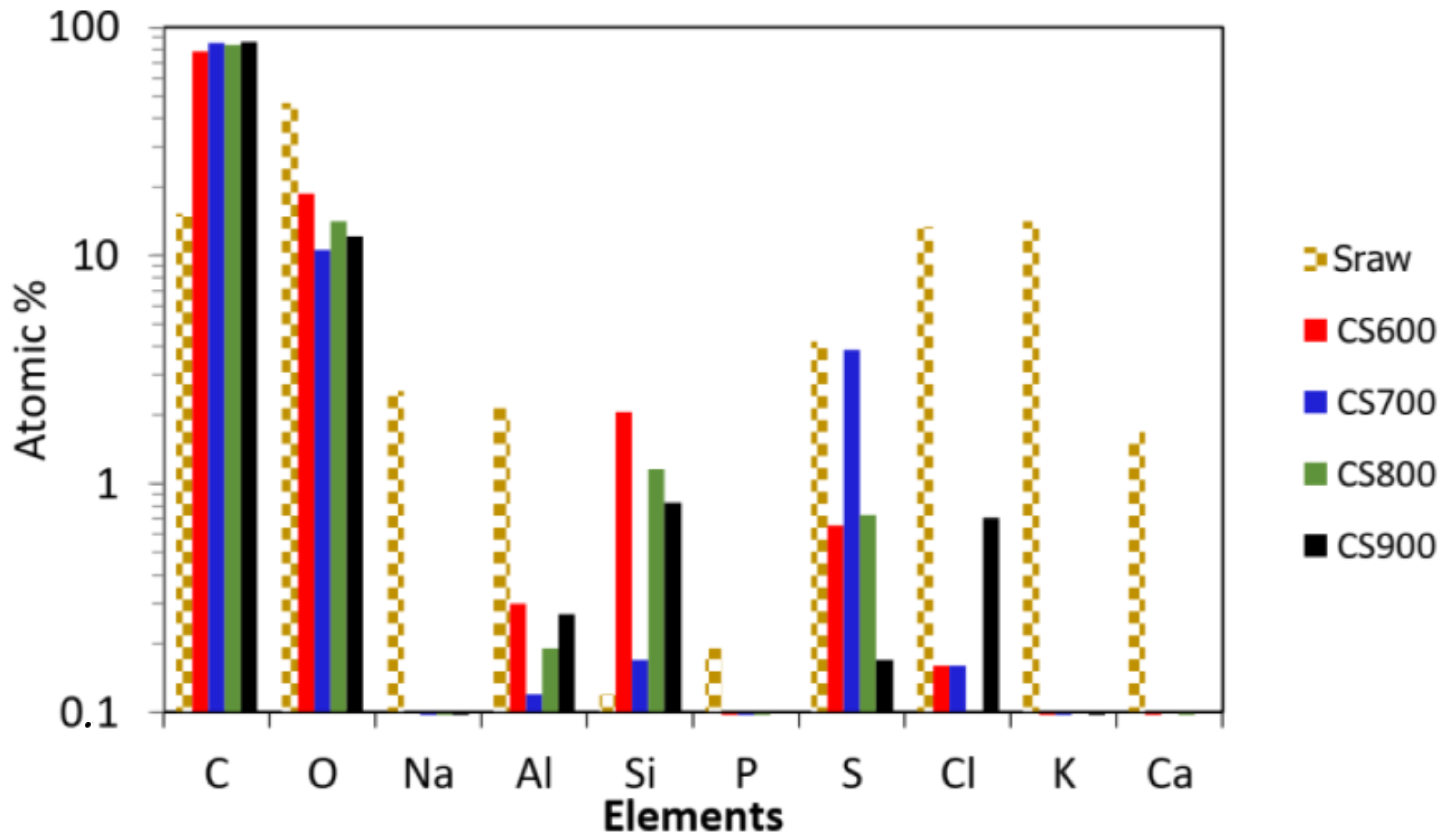
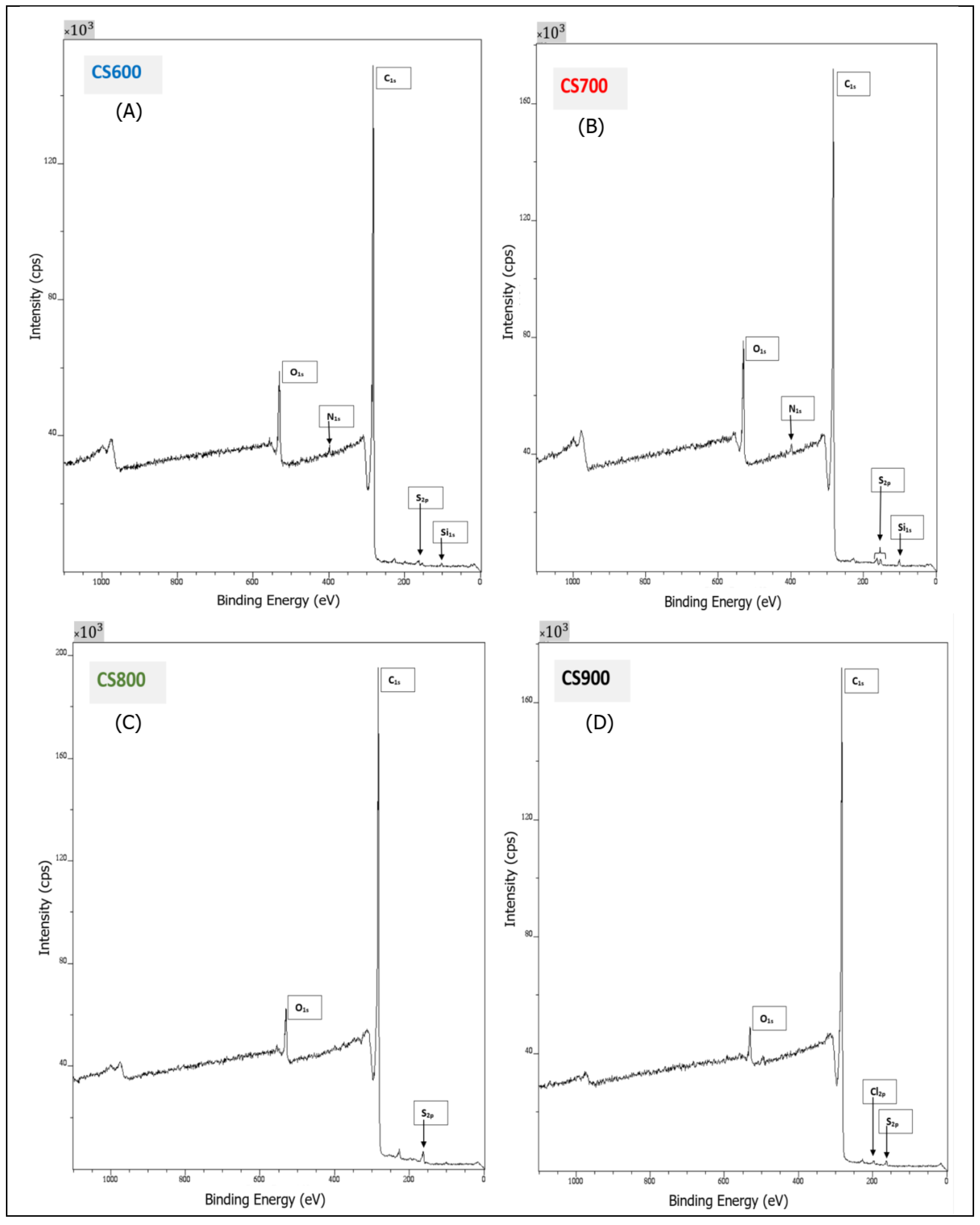
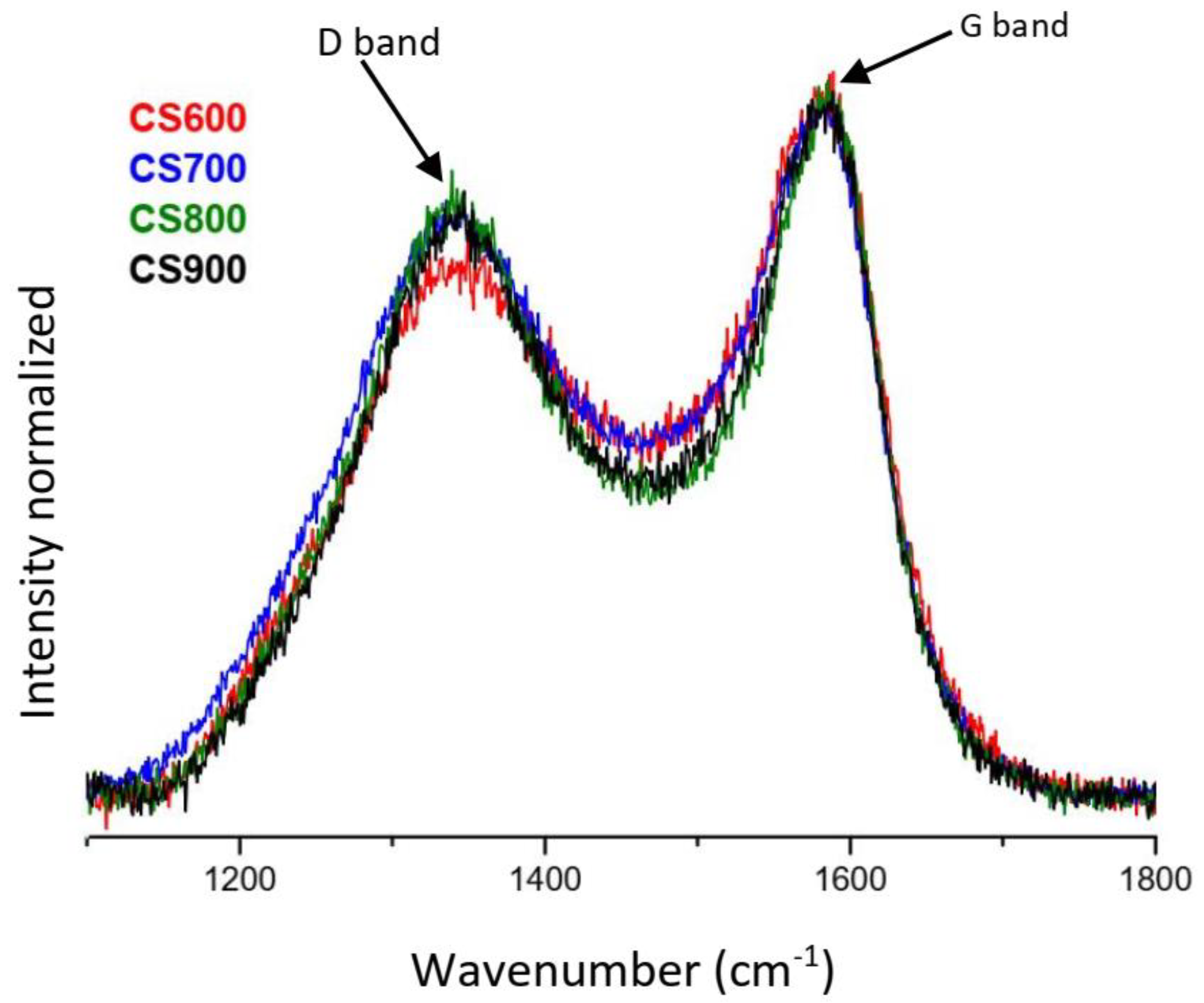
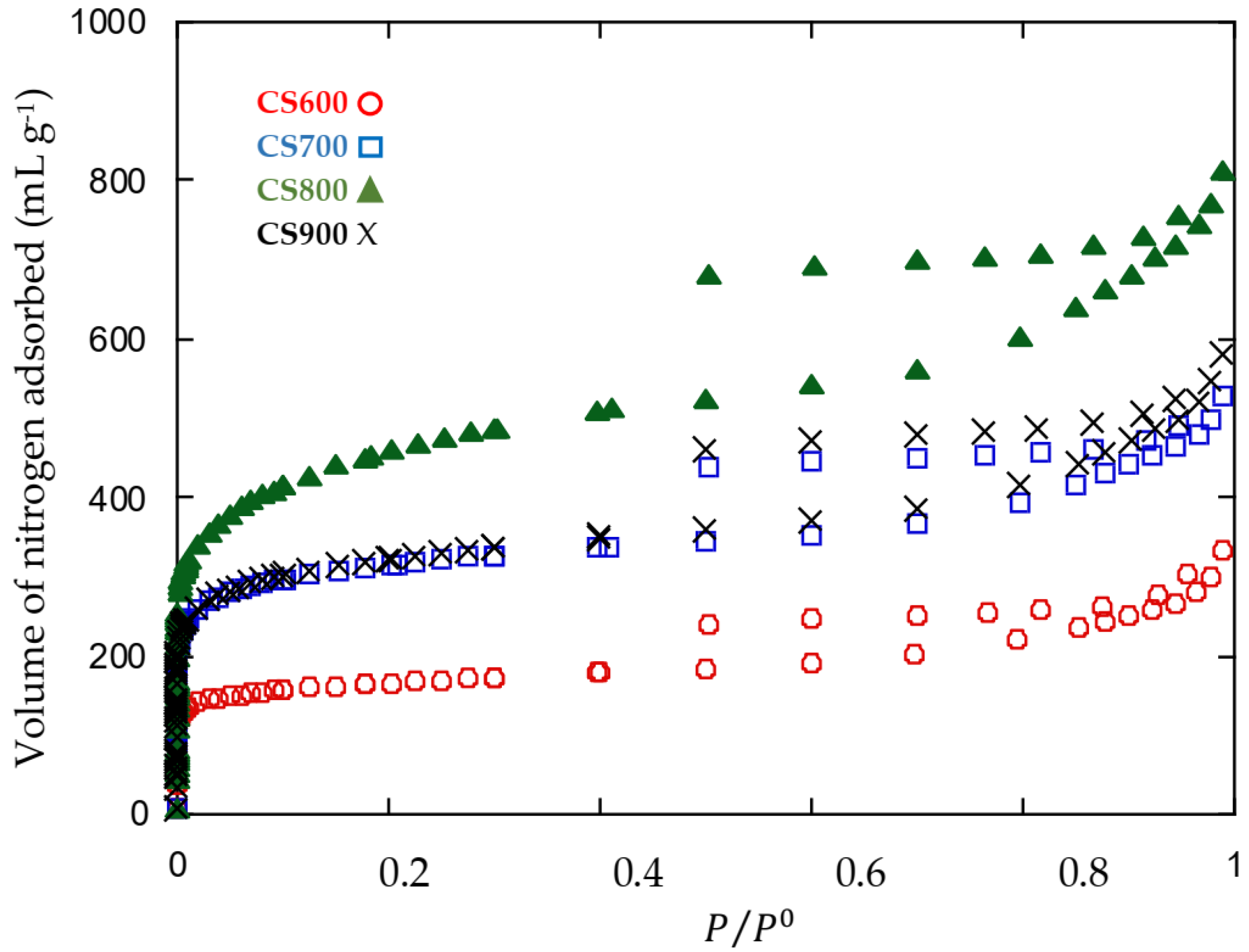
2.1. Materials’ Characterization
2.2. Electrochemical Characterization
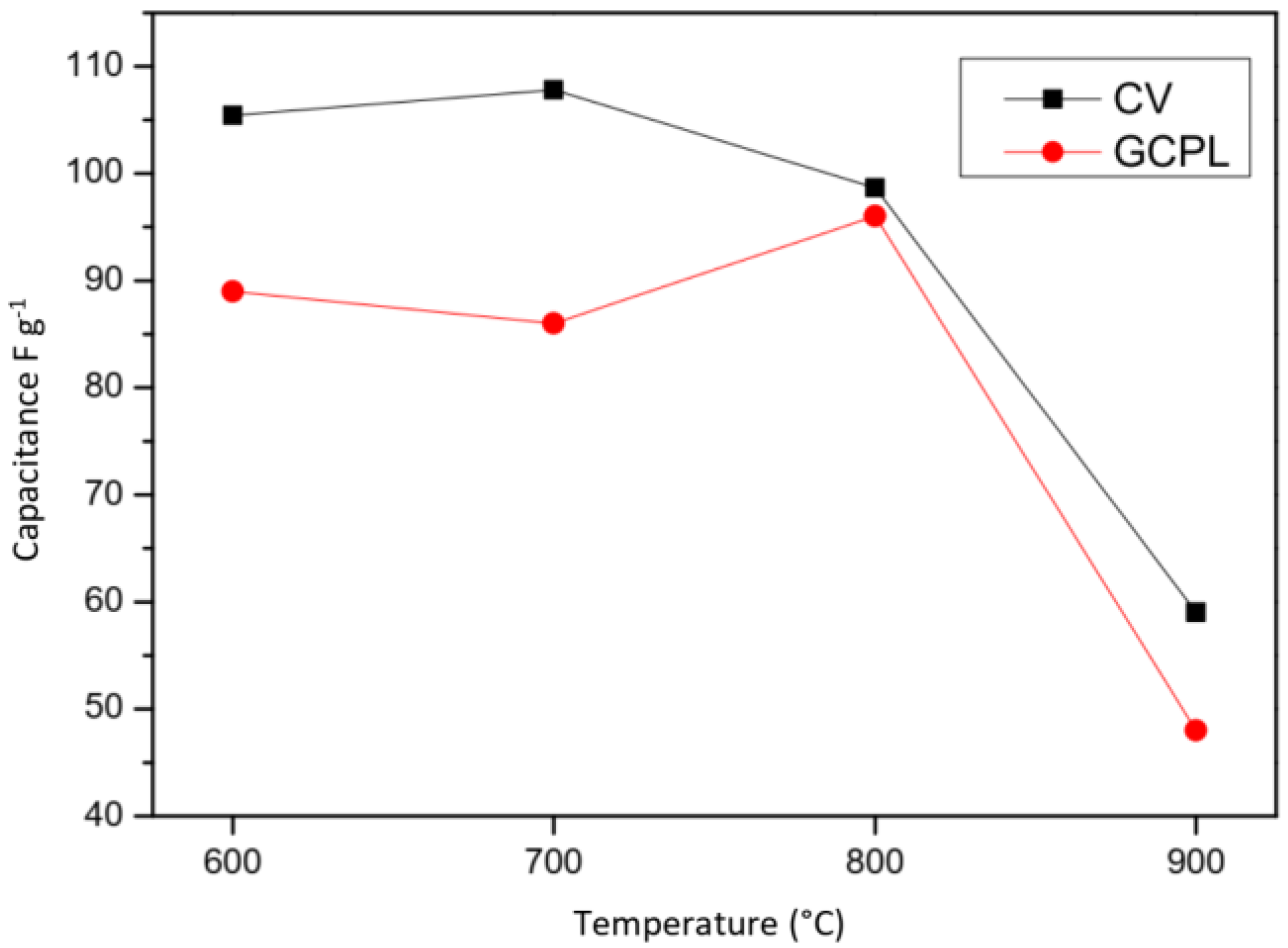
2.2.1. Voltammetry
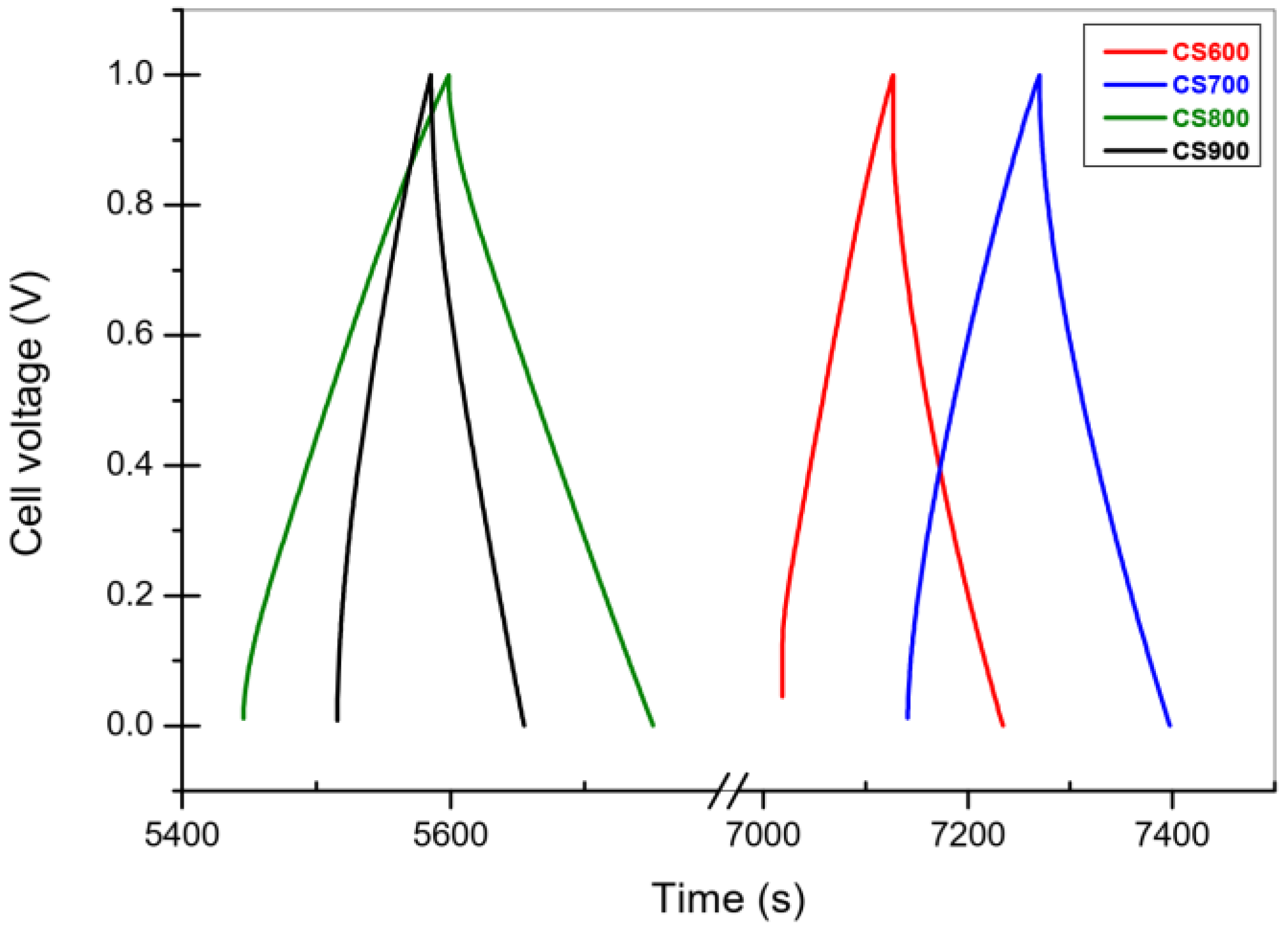
2.2.2. Galvanostatic Charge-Discharge
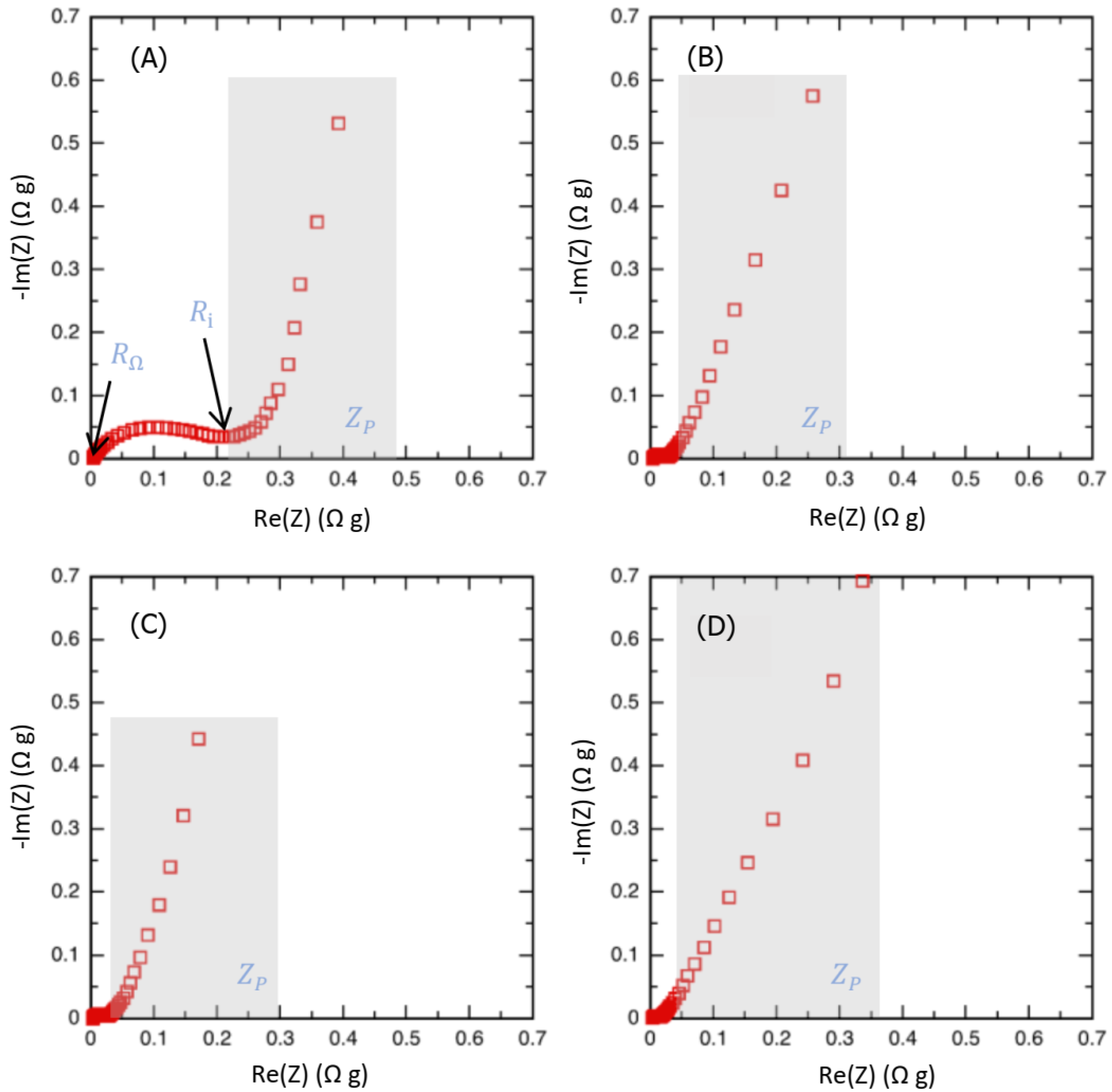
2.2.3. Electrochemical Impedance Spectroscopy
3. Materials and Methods
3.1. Carbon Materials’ Preparation
3.2. Materials’ Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Wang, M.; Hu, C.; Barnes, B.B.; Mitchum, G.; Lapointe, B.; Montoya, J.P. The great Atlantic Sargassum belt. Science 2019, 365, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Poo, K.M.; Son, E.B.; Chang, J.S.; Ren, X.; Choi, Y.J.; Chae, K.J. Biochars derived from wasted marine macro-algae (Saccharina japonica and Sargassum fusiforme) and their potential for heavy metal removal in aqueous solution. J. Environ. Manag. 2018, 206, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Ranguin, R.; Delannoy, M.; Yacou, C.; Jean-Marius, C.; Feidt, C.; Rychen, G.; Gaspard, S. Biochar and activated carbons preparation from invasive algae Sargassum spp. For Chlordecone availability reduction in contaminated soils. J. Environ. Chem. Eng. 2021, 9, 105280. [Google Scholar] [CrossRef]
- Li, S.; Wang, X.; Tan, S.; Shi, Y.; Li, W. CrO3 supported on sargassum-based activated carbon as low temperature catalysts for the selective catalytic reduction of NO with NH3. Fuel 2017, 191, 511–517. [Google Scholar] [CrossRef]
- Francoeur, M.; Ferino-Pérez, A.; Yacou, C.; Jean-Marius, C.; Emmanuel, E.; Chérémond, Y.; Jauregui-Haza, U.; Gaspard, S. Activated carbon synthetized from Sargassum (sp.) for adsorption of caffeine: Understanding the adsorption mechanism using molecular modeling. J. Environ. Chem. Eng. 2021, 9, 104795. [Google Scholar] [CrossRef]
- Francoeur, M.; Yacou, C.; Jean-Marius, C.; Chérémond, Y.; Jauregui-Haza, U.; Sarra, G. Optimization of the synthesis of activated carbon prepared from Sargassum (sp.) and its use for tetracycline, penicillin, caffeine and methylene blue adsorption from contaminated water. Environ. Technol. Innov. 2022, 28, 102940. [Google Scholar] [CrossRef]
- Raymundo-Piñero, E.; Cadek, M.; Béguin, F. Tuning carbon materials for supercapacitors by direct pyrolysis of seaweeds. Adv. Funct. Mater. 2009, 19, 1032–1039. [Google Scholar] [CrossRef]
- Pintor, M.-J.; Jean-Marius, C.; Jeanne-Rose, V.; Taberna, P.-L.; Simon, P.; Gamby, J.; Gadiou, R.; Gaspard, S. Preparation of activated carbon from Turbinaria turbinata seaweeds and its use as supercapacitor electrode materials. Comptes Rendus Chim. 2013, 16, 73–79. [Google Scholar] [CrossRef] [Green Version]
- Taberna, P.-L.; Gaspard, S. Chapter 9: Nanoporous Carbons for High Energy Density Supercapacitors. In Biomass for Sustainable Applications; Royal Society of Chemistry: London, UK, 2013; pp. 366–399. [Google Scholar] [CrossRef]
- Ceylan, S.; Topcu, Y.; Ceylan, Z. Thermal behaviour and kinetics of alga Polysiphonia elongata biomass during pyrolysis. Bioresour. Technol. 2014, 171, 193–198. [Google Scholar] [CrossRef]
- Ross, A.B.; Anastasakis, K.; Kubacki, M.; Jones, J.M. Investigation of the pyrolysis behaviour of brown algae before and after pre-treatment using PY-GC/MS and TGA. J. Anal. Appl. Pyrolysis 2009, 85, 3–10. [Google Scholar] [CrossRef]
- González Bermúdez, Y.; Rodríguez Rico, I.L.; Guibal, E.; Calero de Hoces, M.; Martín-Lara, M.Á. Biosorption of hexavalent chromium from aqueous solution by Sargassum muticum brown alga. Application of statistical design for process optimization. Chem. Eng. J. 2012, 183, 68–76. [Google Scholar] [CrossRef]
- Oliveira, R.C.; Hammer, P.; Guibal, E.; Taulemesse, J.-M.; Garcia, O. Characterization of metal-biomass interactions in the lanthanum(III) biosorption on Sargassum sp. using SEM/EDX, FTIR, and XPS: Preliminary studies. Chem. Eng. J. 2014, 239, 381–391. [Google Scholar] [CrossRef]
- Mussatto, S.I. Microwave-assisted extraction of sulfated polysaccharides (fucoidan) from brown seaweed. Carbohydr. Polym. 2011, 86, 1137–1144. [Google Scholar]
- Lane, D.J.; Zevenhoven, M.; Ashman, P.J.; van Eyk, P.J.; Hupa, M.; de Nys, R.; Lewis, D.M. Algal Biomass: Occurrence of the Main Inorganic Elements and Simulation of Ash Interactions with Bed Material. Energy Fuels 2014, 28, 4622–4632. [Google Scholar] [CrossRef]
- Kannan, S. FT-IR and EDS analysis of the seaweeds Sargassum wightii (brown algae) and Gracilaria corticata (red algae). Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 341–351. [Google Scholar]
- Shin, S.; Jang, J.; Yoon, S.H.; Mochida, I. A study on the effect of heat treatment on functional groups of pitch based activated carbon fiber using FTIR. Carbon 1997, 35, 1739–1743. [Google Scholar] [CrossRef]
- Zhou, J.-H.; Sui, Z.-J.; Zhu, J.; Li, P.; Chen, D.; Dai, Y.-C.; Yuan, W.-K. Characterization of surface oxygen complexes on carbon nanofibers by TPD, XPS and FT-IR. Carbon 2007, 4, 785–796. [Google Scholar] [CrossRef]
- Fu, P.; Hu, S.; Xiang, J.; Sun, L.; Yang, T.; Zhang, A.; Zhang, J. Mechanism Study of Rice Straw Pyrolysis by Fourier Transform Infrared Technique. Chin. J. Chem. Eng. 2009, 17, 522–529. [Google Scholar] [CrossRef]
- He, X.; Liu, X.; Nie, B.; Song, D. FTIR and Raman spectroscopy characterization of functional groups in various rank coals. Fuel 2017, 206, 555–563. [Google Scholar] [CrossRef]
- Sadezky, A.; Muckenhuber, H.; Grothe, H.; Niessner, R.; Pöschl, U. Raman microspectroscopy of soot and related carbonaceous materials: Spectral analysis and structural information. Carbon 2005, 8, 1731–1742. [Google Scholar] [CrossRef]
- Wang, Y.; Alsmeyer, D.C.; McCreery, R.L. Raman spectroscopy of carbon materials: Structural basis of observed spectra. Chem. Mater. 1990, 2, 557. Available online: https://www.academia.edu/5186902/Raman_spectroscopy_of_carbon_materials_structural_basis_of_observed_spectra (accessed on 5 July 2023). [CrossRef]
- Largeot, C.; Portet, C.; Chmiola, J.; Taberna, P.-L.; Gogotsi, Y.; Simon, P. Relation between the ion size and pore size for an electric double-layer capacitor. J. Am. Chem. Soc. 2008, 130, 2730–2731. [Google Scholar] [CrossRef] [PubMed]
- Portet, C.; Taberna, P.L.; Simon, P.; Laberty-Robert, C. Modification of Al current collector surface by sol–gel deposit for carbon–carbon supercapacitor applications. Electrochim. Acta 2004, 49, 905–912. [Google Scholar] [CrossRef]
- Pean, C.; Daffos, B.; Merlet, C.; Rotenberg, B.; Taberna, P.-L.; Simon, P.; Salanne, M. Single Electrode Capacitances of Porous Carbons in Neat Ionic Liquid Electrolyte at 100 °C: A Combined Experimental and Modeling Approach. J. Electrochem. Soc. 2015, 162, A5091. [Google Scholar] [CrossRef] [Green Version]
- Pean, C.; Daffos, B.; Rotenberg, B.; Levitz, P.; Haefele, M.; Taberna, P.-L.; Simon, P.; Salanne, M. Confinement, Desolvation, and Electrosorption Effects on the Diffusion of Ions in Nanoporous Carbon Electrodes. J. Am. Chem. Soc. 2015, 137, 12627–12632. [Google Scholar] [CrossRef]
- Mei, B.-A.; Munteshari, O.; Lau, J.; Dunn, B.; Pilon, L. Physical Interpretations of Nyquist Plots for EDLC Electrodes and Devices. J. Phys. Chem. C 2018, 122, 194–206. [Google Scholar] [CrossRef]
- Ismanto, A.E.; Wang, S.; Soetaredjo, F.E.; Ismadji, S. Preparation of capacitor’s electrode from cassava peel waste. Bioresour. Technol. 2010, 101, 3534–3540. [Google Scholar] [CrossRef] [Green Version]
- Hu, S.; Zhang, S.; Pan, N.; Hsieh, Y.-L. High energy density supercapacitors from lignin derived submicron activated carbon fibers in aqueous electrolytes. J. Power Sources 2014, 270, 106–112. [Google Scholar] [CrossRef] [Green Version]
- Farma, R.; Deraman, M.; Awitdrus, A.; Talib, I.A.; Taer, E.; Basri, N.H.; Manjunatha, J.G.; Ishak, M.M.; Dollah, B.N.M.; Hashmi, S.A. Preparation of highly porous binderless activated carbon electrodes from fibres of oil palm empty fruit bunches for application in supercapacitors. Bioresour. Technol. 2013, 132, 254–261. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, J.; Holm, N.; Chen, F. Mini-Chunk Biochar Supercapacitors. J. Appl. Electrochem. 2014, 44, 1145–1151. Available online: https://scholarcommons.sc.edu/emec_facpub/333 (accessed on 5 July 2023). [CrossRef]
- Sodtipinta, J.; Ieosakulrat, C.; Poonyayant, N.; Kidkhunthod, P.; Chanlek, N.; Amornsakchai, T.; Pakawatpanurut, P. Interconnected open-channel carbon nanosheets derived from pineapple leaf fiber as a sustainable active material for supercapacitors. Ind. Crops Prod. 2017, 104, 13–20. Available online: https://www.cabdirect.org/cabdirect/abstract/20173249165 (accessed on 5 July 2023). [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]

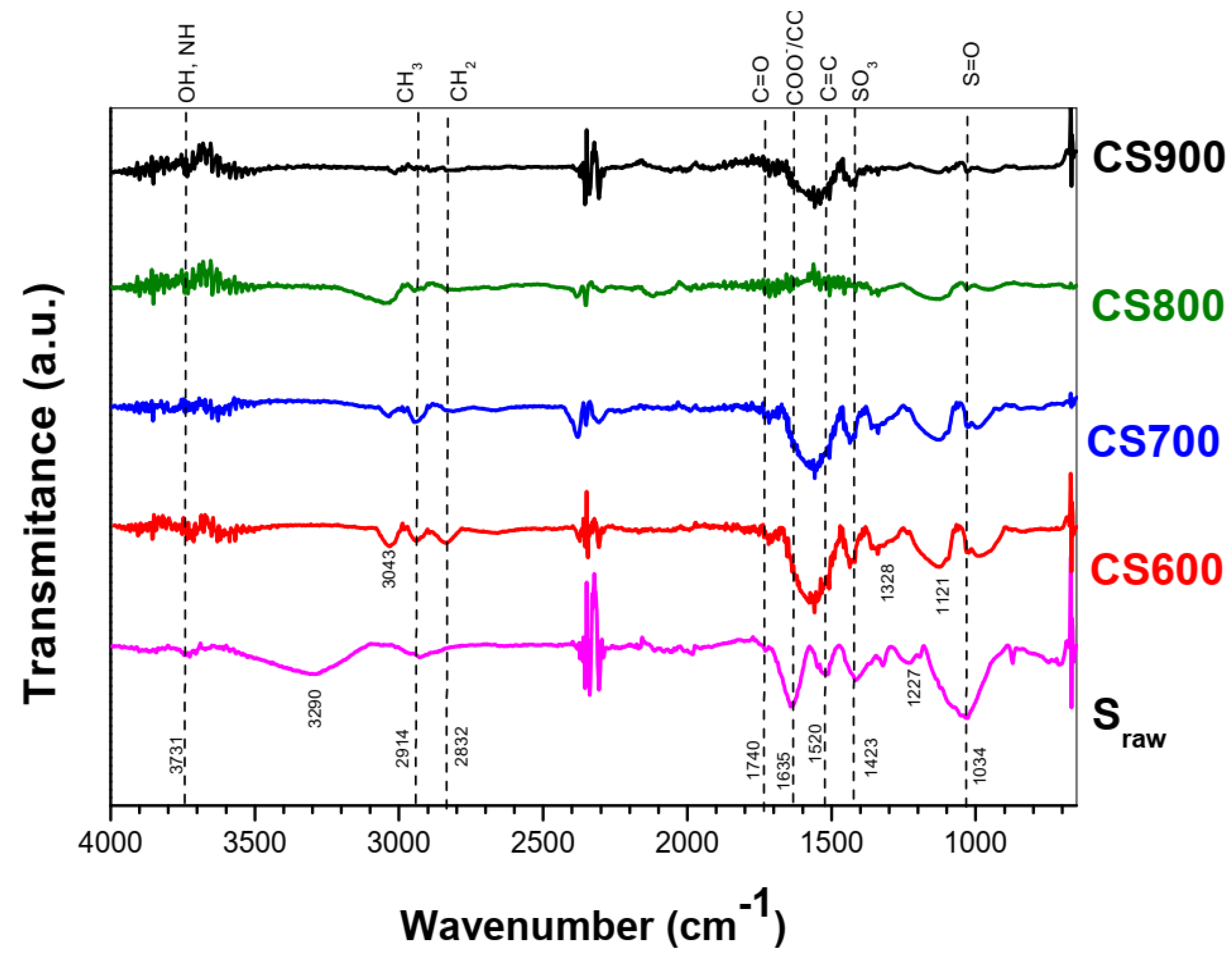


| Functional Groups | Position | Name | References |
|---|---|---|---|
| –OH, –NH | 3660 cm−1 | Alcohols, Amides | [13,16] |
| –CH3/–CH2 | 2960/2925 cm−1 | Aliphatic hydrocarbons | [20] |
| –C=O | 1700 cm−1 | Aliphatic | [20] |
| –COOH/–COO− | 1630 cm−1 | Carboxyl acid/carboxylate | [13,16,20] |
| –C=C | 1600 cm−1 | Aromatic | [16,20] |
| –SO3 | 1416 cm−1 | Sulfonate | [13,16] |
| –C–O–C | 1230 cm−1 | Ethers | [13,16] |
| –S=O | 1320 cm−1 | Sulfone | [16] |
| –S=O | 1034 cm−1 | Sulfonides | [16] |
| –C–H | 700–900 cm−1 | Aliphatic | [16,20] |
| Elemental Composition (%) | Percentage of Functional Group from C1s Spectrum | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sample | O1s | C1s | O/C | N1s | S2p | Si2p | Cl2p | (I) | (II) | (III) |
| CS 600 | 7.98 | 91.57 | 0.09 | 0.45 | 79.6 | 14.11 | 6.29 | |||
| CS 700 | 10.19 | 87.16 | 0.12 | 0.71 | 0.75 | 1.19 | 79.1 | 15.45 | 5.45 | |
| CS 800 | 4.54 | 93.68 | 0.05 | 1.78 | 80.85 | 14.63 | 4.52 | |||
| CS 900 | 2.76 | 96.33 | 0.03 | 0.59 | 0.32 | 82.43 | 13.28 | 4.29 | ||
| Carbon | Specific Surface Area (m2 g−1) | Mesopore Volume (cm3 g−1) | Micropore Volume (cm3 g−1) | % of Micropore | Mean Pore Diameter (nm) |
|---|---|---|---|---|---|
| CS600 | 621 | 0.32 | 0.19 | 37.2 | 2.49 |
| CS700 | 1179 | 0.51 | 0.39 | 43.3 | 2.32 |
| CS800 | 1664 | 0.91 | 0.58 | 26.2 | 2.54 |
| CS900 | 1199 | 0.60 | 0.39 | 39.4 | 2.44 |
| Carbon | Csc whole Cell Capacitance (F g−1) | ESR (Ω g) | RΩ in Bulk Electrolyte Resistance (Ω g) | Ri (Carbon Electronic Conductivity) (Ω g) | Rp/3 Effective In-Pore Ionic Resistance (Ω g) |
|---|---|---|---|---|---|
| CS 600 | 89 | 0.33 | 0.0048 | 0.21 | 0.12 |
| CS 700 | 86 | 0.085 | 0.0041 | 0.065 | 0.016 |
| CS 800 | 96 | 0.073 | 0.0035 | 0.053 | 0.016 |
| CS 900 | 48 | 0.14 | 0.0035 | 0.12 | 0.013 |
| Precursor | Activation Method | SBET (m2 g−1) | Capacitance (F g−1) | Electrolyte | Reference |
|---|---|---|---|---|---|
| Cassavapeelwaste | KOH | 1352 | 153 | 0.5 M H2SO4 | [28] |
| Lessonia Nigrescens | Pyrolysis 600 °C | 746 | 264 | 1 M H2SO4 | [7] |
| Lessonia Nigrescens | Pyrolysis 900 °C | 1307 | 175 | 1 M H2SO4 | [7] |
| Meristhoteca Senegalensis | Pyrolysis 750 °C | 1156 | 120 | 1 M H2SO4 | [7] |
| Lignin | NaOH/KOH | 122 | 1 M H2SO4 | [29] | |
| Oil palm | H2O/CO2 | 1704 | 120 | 1 M H2SO4 | [30] |
| Maple wood | Pyrolysis 750 °C | 303 | 32 | 0.5 M H2SO4 | [31] |
| Pineapple Leaves | Hydrothermal carbonization/KOH | 1681 | 110 | 1 M H2SO4 | [32] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roche, S.; Yacou, C.; Jean Marius, C.; Ranguin, R.; Francoeur, M.; Taberna, P.-L.; Passe-Coutrin, N.; Gaspard, S. Carbon Materials Prepared from Invading Pelagic Sargassum for Supercapacitors’ Electrodes. Molecules 2023, 28, 5882. https://doi.org/10.3390/molecules28155882
Roche S, Yacou C, Jean Marius C, Ranguin R, Francoeur M, Taberna P-L, Passe-Coutrin N, Gaspard S. Carbon Materials Prepared from Invading Pelagic Sargassum for Supercapacitors’ Electrodes. Molecules. 2023; 28(15):5882. https://doi.org/10.3390/molecules28155882
Chicago/Turabian StyleRoche, Sandra, Christelle Yacou, Corine Jean Marius, Ronald Ranguin, Marckens Francoeur, Pierre-Louis Taberna, Nady Passe-Coutrin, and Sarra Gaspard. 2023. "Carbon Materials Prepared from Invading Pelagic Sargassum for Supercapacitors’ Electrodes" Molecules 28, no. 15: 5882. https://doi.org/10.3390/molecules28155882







