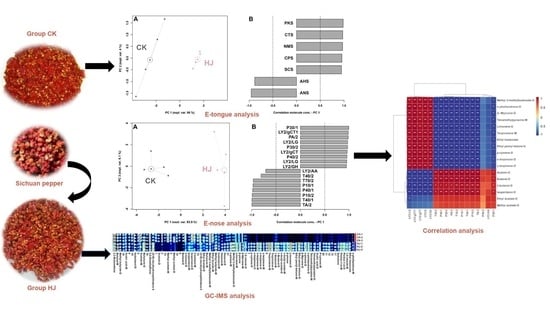
Effect of Sichuan Pepper (Zanthoxylum genus) Addition on Flavor Profile in Fermented Ciba Chili (Capsicum genus) Using GC-IMS Combined with E-Nose and E-Tongue
Abstract
:1. Introduction
2. Results
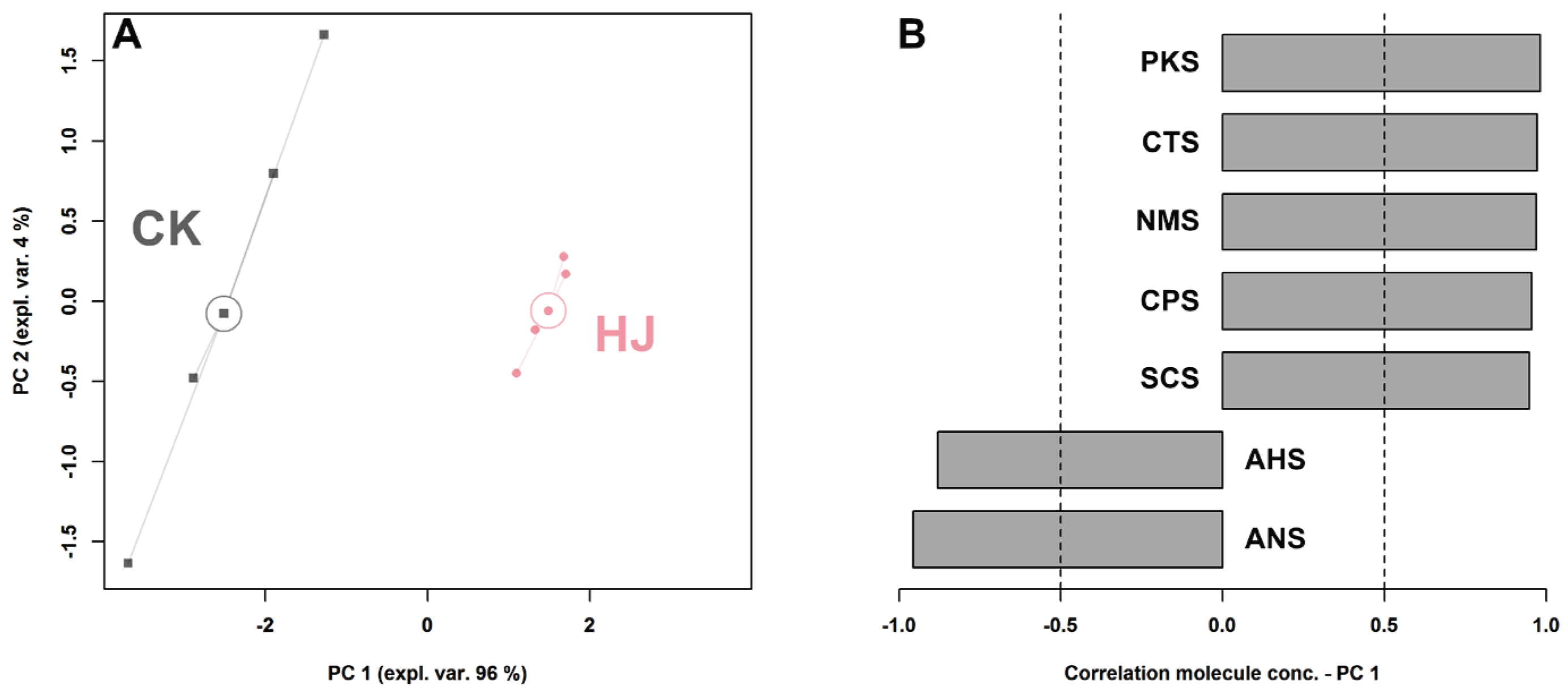
2.1. E-Tongue Analysis
2.2. E-Nose Analysis
2.3. GC-IMS Analysis
2.4. Correlation between E-Nose and GC-IMS
3. Discussion
4. Materials and Methods
4.1. Preparation of Ciba Chili
4.2. E-Nose Analysis
4.3. E-Tongue Analysis
4.4. HS-GC-IMS Analysis
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Hernández-Pérez, T.; Gómez-García, M.D.R.; Valverde, M.E.; Paredes-López, O. Capsicum annuum (Hot Pepper): An Ancient Latin-American Crop with Outstanding Bioactive Compounds and Nutraceutical Potential. A Review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2972–2993. [Google Scholar] [CrossRef] [PubMed]
- FAOSTAT. Available online: https://www.fao.org/faostat/zh/#data/QI/visualize (accessed on 8 June 2023).
- Ye, Z.; Shang, Z.; Li, M.; Zhang, X.; Ren, H.; Hu, X.; Yi, J. Effect of Ripening and Variety on the Physiochemical Quality and Flavor of Fermented Chinese Chili Pepper (Paojiao). Food Chem. 2022, 368, 130797. [Google Scholar] [CrossRef]
- Cai, W.; Wang, Y.; Hou, Q.; Zhang, Z.; Tang, F.; Shan, C.; Yang, X.; Guo, Z. PacBio Sequencing Combined with Metagenomic Shotgun Sequencing Provides Insight into the Microbial Diversity of Zha-Chili. Food Biosci. 2021, 40, 100884. [Google Scholar] [CrossRef]
- De Vuyst, L.; Leroy, F. Functional Role of Yeasts, Lactic Acid Bacteria and Acetic Acid Bacteria in Cocoa Fermentation Processes. FEMS Microbiol. Rev. 2020, 44, 432–453. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Li, S.; Ho, C.-T. Chemical Composition, Sensory Properties and Application of Sichuan Pepper (Zanthoxylum genus). Food Sci. Hum. Human. Wellness 2019, 8, 115–125. [Google Scholar] [CrossRef]
- Yang, X. Aroma Constituents and Alkylamides of Red and Green Huajiao (Zanthoxylum bungeanum and Zanthoxylum schinifolium). J. Agric. Food Chem. 2008, 56, 1689–1696. [Google Scholar] [CrossRef]
- Ye, Z.; Shang, Z.; Zhang, S.; Li, M.; Zhang, X.; Ren, H.; Hu, X.; Yi, J. Dynamic Analysis of Flavor Properties and Microbial Communities in Chinese Pickled Chili Pepper (Capsicum frutescens L.): A Typical Industrial-Scale Natural Fermentation Process. Food Res. Int. 2022, 153, 110952. [Google Scholar] [CrossRef]
- Cai, W.; Wang, Y.; Hou, Q.; Zhang, Z.; Tang, F.; Shan, C.; Yang, X.; Guo, Z. Rice Varieties Affect Bacterial Diversity, Flavor, and Metabolites of Zha-Chili. Food Res. Int. 2021, 147, 110556. [Google Scholar] [CrossRef]
- Wang, S.; Chen, H.; Sun, B. Recent Progress in Food Flavor Analysis Using Gas Chromatography—Ion Mobility Spectrometry (GC–IMS). Food Chem. 2020, 315, 126158. [Google Scholar] [CrossRef]
- Zhao, X.; Feng, J.; Laghi, L.; Deng, J.; Dao, X.; Tang, J.; Ji, L.; Zhu, C.; Picone, G. Characterization of Flavor Profile of “Nanx Wudl” Sour Meat Fermented from Goose and Pork Using Gas Chromatography–Ion Mobility Spectrometry (GC–IMS) Combined with Electronic Nose and Tongue. Foods 2023, 12, 2194. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, J.; Zhu, Z.; Lei, Y.; Huang, S.; Huang, M. Effect of Ageing Time on the Flavour Compounds in Nanjing Water-Boiled Salted Duck Detected by HS-GC-IMS. LWT 2022, 155, 112870. [Google Scholar] [CrossRef]
- Liu, X.; Wang, X.; Cheng, Y.; Wu, Y.; Yan, Y.; Li, Z. Variations in Volatile Organic Compounds in Zhenyuan Daocai Samples at Different Storage Durations Evaluated Using E-Nose, E-Tongue, Gas Chromatography and Spectrometry. LWT 2023, 173, 114186. [Google Scholar] [CrossRef]
- Nasiru, M.M.; Umair, M.; Boateng, E.F.; Alnadari, F.; Khan, K.R.; Wang, Z.; Luo, J.; Yan, W.; Zhuang, H.; Majrashi, A.; et al. Characterisation of Flavour Attributes in Egg White Protein Using HS-GC-IMS Combined with E-Nose and E-Tongue: Effect of High-Voltage Cold Plasma Treatment Time. Molecules 2022, 27, 601. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wu, B.; Zhao, W.; Lao, F.; Chen, F.; Liao, X.; Wu, J. Shifts in Autochthonous Microbial Diversity and Volatile Metabolites during the Fermentation of Chili Pepper (Capsicum frutescens L.). Food Chem. 2021, 335, 127512. [Google Scholar] [CrossRef]
- Zhang, S.; Xiao, Y.; Jiang, Y.; Wang, T.; Cai, S.; Hu, X.; Yi, J. Effects of Brines and Containers on Flavor Production of Chinese Pickled Chili Pepper (Capsicum frutescens L.) during Natural Fermentation. Foods 2022, 12, 101. [Google Scholar] [CrossRef]
- Xu, X.; Wu, B.; Zhao, W.; Pang, X.; Lao, F.; Liao, X.; Wu, J. Correlation between Autochthonous Microbial Communities and Key Odorants during the Fermentation of Red Pepper (Capsicum annuum L.). Food Microbiol. 2020, 91, 103510. [Google Scholar] [CrossRef]
- Chen, M.; Qin, Y.; Deng, F.; Zhou, H.; Wang, R.; Li, P.; Liu, Y.; Jiang, L. Illumina MiSeq Sequencing Reveals Microbial Community Succession in Salted Peppers with Different Salinity during Preservation. Food Res. Int. 2021, 143, 110234. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, L.; Wen, R.; Chen, Q.; Kong, B. Role of Lactic Acid Bacteria in Flavor Development in Traditional Chinese Fermented Foods: A Review. Crit. Rev. Food Sci. Nutr. 2022, 62, 2741–2755. [Google Scholar] [CrossRef]
- Xu, E.; Long, J.; Wu, Z.; Li, H.; Wang, F.; Xu, X.; Jin, Z.; Jiao, A. Characterization of Volatile Flavor Compounds in Chinese Rice Wine Fermented from Enzymatic Extruded Rice. J. Food Sci. 2015, 80, C1476–C1489. [Google Scholar] [CrossRef]
- Zha, M.; Sun, B.; Wu, Y.; Yin, S.; Wang, C. Improving Flavor Metabolism of Saccharomyces Cerevisiae by Mixed Culture with Wickerhamomyces Anomalus for Chinese Baijiu Making. J. Biosci. Bioeng. 2018, 126, 189–195. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, H.; Ding, S.; Zhou, H.; Qin, D.; Deng, F.; Wang, R. Changes in Volatile Compounds of Fermented Minced Pepper during Natural and Inoculated Fermentation Process Based on Headspace–Gas Chromatography—Ion Mobility Spectrometry. Food Sci. Nutr. 2020, 8, 3362–3379. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, S.; Bahuguna, A.; Lim, S.; Joe, A.-R.; Lee, J.-S.; Kim, S.-Y.; Kim, M. Physicochemical, Microbial and Volatile Compound Characteristics of Gochujang, Fermented Red Pepper Paste, Produced by Traditional Cottage Industries. Foods 2022, 11, 375. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhang, F.; Gong, C.; Tan, X.; Ren, Y.; Yao, K.; Zhang, Q.; Chi, Y. Physicochemical, Microbial, and Aroma Characteristics of Chinese Pickled Red Peppers (Capsicum annuum) with and without Biofilm. RSC Adv. 2020, 10, 6609–6617. [Google Scholar] [CrossRef]
- Li, M.; Xu, X.; Bi, S.; Pan, X.; Lao, F.; Wu, J. Identification and Validation of Core Microbes Associated with Key Aroma Formation in Fermented Pepper Paste (Capsicum annuum L.). Food Res. Int. 2023, 163, 112194. [Google Scholar] [CrossRef]
- Xiao, Z.; Lu, J.R. Strategies for Enhancing Fermentative Production of Acetoin: A Review. Biotechnol. Adv. 2014, 32, 492–503. [Google Scholar] [CrossRef] [PubMed]
- D’Este, M.; Alvarado-Morales, M.; Angelidaki, I. Amino Acids Production Focusing on Fermentation Technologies—A Review. Biotechnol. Adv. 2018, 36, 14–25. [Google Scholar] [CrossRef] [Green Version]
- Costa, M.P.D.; Frasao, B.D.S.; Lima, B.R.C.D.C.; Rodrigues, B.L.; Junior, C.A.C. Simultaneous Analysis of Carbohydrates and Organic Acids by HPLC-DAD-RI for Monitoring Goat’s Milk Yogurts Fermentation. Talanta 2016, 152, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Satora, P.; Tuszyński, T. Influence of Indigenous Yeasts on the Fermentation and Volatile Profile of Plum Brandies. Food Microbiol. 2010, 27, 418–424. [Google Scholar] [CrossRef]
- Wang, C.; Sun, J.; Lassabliere, B.; Yu, B.; Liu, S.Q. Coffee Flavour Modification through Controlled Fermentation of Green Coffee Beans by Lactococcus lactis Subsp. Cremoris. LWT 2020, 120, 108930. [Google Scholar] [CrossRef]
- Ravyts, F.; Steen, L.; Goemaere, O.; Paelinck, H.; De Vuyst, L.; Leroy, F. The Application of Staphylococci with Flavour-Generating Potential Is Affected by Acidification in Fermented Dry Sausages. Food Microbiol. 2010, 27, 945–954. [Google Scholar] [CrossRef]
- Li, Z.; Dong, L.; Huang, Q.; Wang, X. Bacterial Communities and Volatile Compounds in Doubanjiang, a Chinese Traditional Red Pepper Paste. J. Appl. Microbiol. 2016, 120, 1585–1594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Li, C.; Zhao, Y.; Li, L.; Yang, X.; Wu, Y.; Chen, S.; Cen, J.; Yang, S.; Yang, D. Novel Insight into the Formation Mechanism of Volatile Flavor in Chinese Fish Sauce (Yu-Lu) Based on Molecular Sensory and Metagenomics Analyses. Food Chem. 2020, 323, 126839. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Cheng, X.; Yang, J.; Wang, X.; Lü, X. Unraveling the Difference in Physicochemical Properties, Sensory, and Volatile Profiles of Dry Chili Sauce and Traditional Fresh Dry Chili Sauce Fermented by Lactobacillus Plantarum PC8 Using Electronic Nose and HS-SPME-GC-MS. Food Biosci. 2022, 50, 102057. [Google Scholar] [CrossRef]
- Lee, S.M.; Lee, J.Y.; Cho, Y.J.; Kim, M.S.; Kim, Y.-S. Determination of Volatiles and Carotenoid Degradation Compounds in Red Pepper Fermented by Lactobacillus Parabuchneri. J. Food Sci. 2018, 83, 2083–2091. [Google Scholar] [CrossRef]
- Sun, J.; Sun, B.; Ren, F.; Chen, H.; Zhang, N.; Zhang, Y. Characterization of Key Odorants in Hanyuan and Hancheng Fried Pepper (Zanthoxylum bungeanum) Oil. J. Agric. Food Chem. 2020, 68, 6403–6411. [Google Scholar] [CrossRef]
- Li, Z.; Dong, L.; Jeon, J.; Kwon, S.Y.; Zhao, C.; Baek, H.-H. Characterization and Evaluation of Aroma Quality in Doubanjiang, a Chinese Traditional Fermented Red Pepper Paste, Using Aroma Extract Dilution Analysis and a Sensory Profile. Molecules 2019, 24, 3107. [Google Scholar] [CrossRef] [Green Version]
- Collins, E.B. Biosynthesis of Flavor Compounds by Microorganisms. J. Dairy. Sci. 1972, 55, 1022–1028. [Google Scholar] [CrossRef]
- Lee, S.-W.; Lim, J.-M.; Mohan, H.; Seralathan, K.-K.; Park, Y.-J.; Lee, J.-H.; Oh, B.-T. Enhanced Bioactivity of Zanthoxylum schinifolium Fermented Extract: Anti-Inflammatory, Anti-Bacterial, and Anti-Melanogenic Activity. J. Biosci. Bioeng. 2020, 129, 638–645. [Google Scholar] [CrossRef]
- Olaniran, A.F.; Abiose, S.H.; Adeniran, H.A.; Gbadamosi, S.O.; Iranloye, Y.M. Production of a Cereal Based Product (Ogi): Influence of Co-Fermentation with Powdered Garlic and Ginger on the Microbiome. Agrosearch 2020, 20, 81–93. [Google Scholar] [CrossRef]
- Ono, H.; Nishio, S.; Tsurii, J.; Kawamoto, T.; Sonomoto, K.; Nakayama, J. Effects of Japanese Pepper and Red Pepper on the Microbial Community during Nukadoko Fermentation. Biosci. Microbiota Food Health 2015, 34, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Montanari, C.; Gatto, V.; Torriani, S.; Barbieri, F.; Bargossi, E.; Lanciotti, R.; Grazia, L.; Magnani, R.; Tabanelli, G.; Gardini, F. Effects of the Diameter on Physico-Chemical, Microbiological and Volatile Profile in Dry Fermented Sausages Produced with Two Different Starter Cultures. Food Biosci. 2018, 22, 9–18. [Google Scholar] [CrossRef]

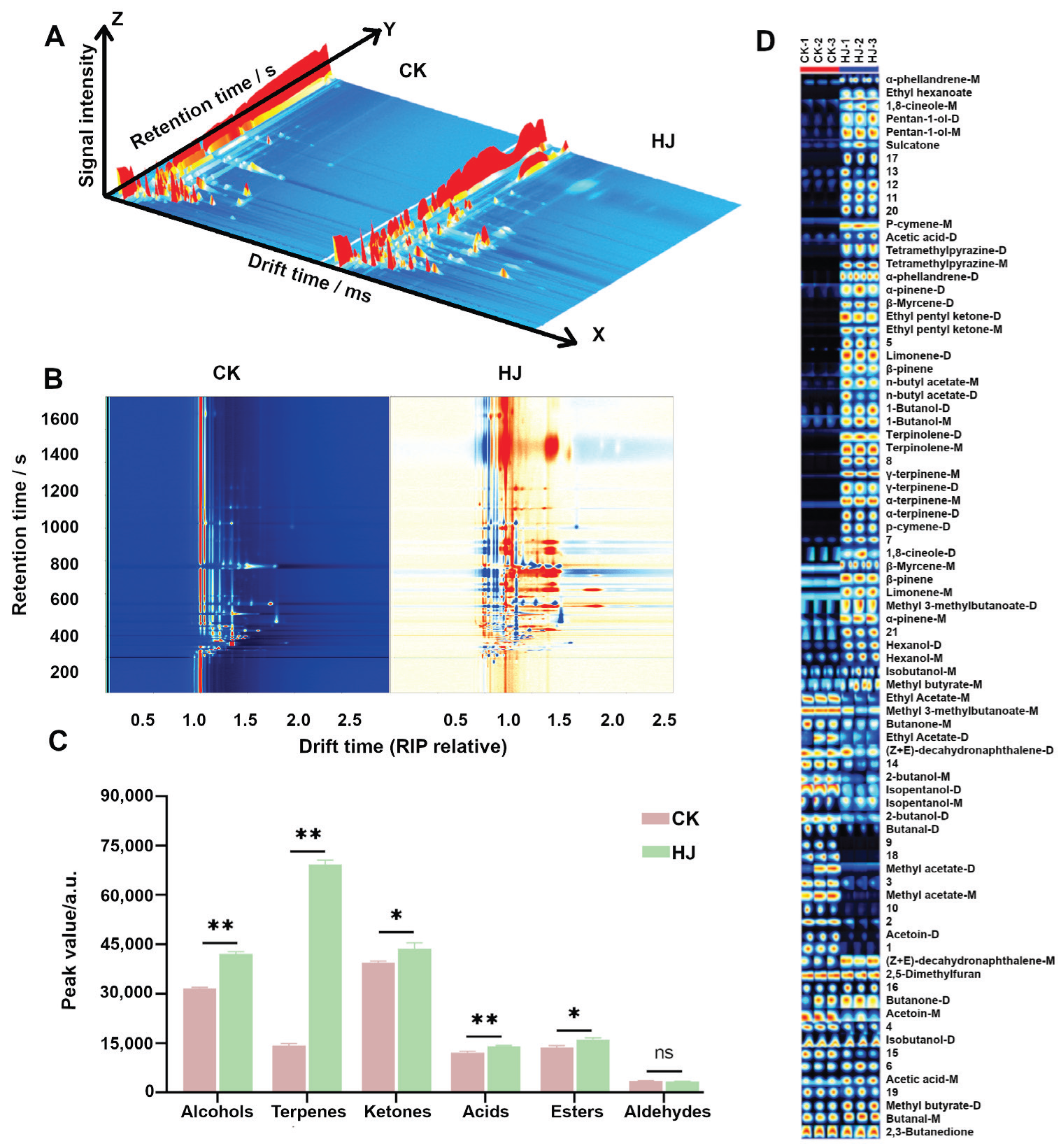


| Count | Compound | CAS | Molecule Formula | MW | RI | Rt [sec] | Dt [a.u.] | CK | HJ | p-Value |
|---|---|---|---|---|---|---|---|---|---|---|
| Alcohols | 1,8-cineole-D | 470-82-6 | C10H18O | 154 | 1223 | 776.99 | 1.7295 | 610.93 ± 38.94 | 1031.96 ± 341.23 | 0.036 |
| 1,8-cineole-M | 470-82-6 | C10H18O | 154 | 1226 | 787.731 | 1.29699 | 2661.02 ± 46.46 | 9163.40 ± 1265.95 | 0.030 | |
| Hexanol-D | 111-27-3 | C6H14O | 102 | 1331 | 1126.15 | 1.62945 | 490.40 ± 24.12 | 1340.22 ± 45.36 | 0.180 | |
| Hexanol-M | 111-27-3 | C6H14O | 102 | 1330 | 1123.04 | 1.32811 | 1919.54 ± 28.09 | 3286.81 ± 25.02 | <0.000 | |
| pentan-1-ol-D | 71-41-0 | C5H12O | 88.1 | 1268 | 928.815 | 1.51635 | 63.52 ± 6.57 | 210.17 ± 19.93 | 0.031 | |
| pentan-1-ol-M | 71-41-0 | C5H12O | 88.1 | 1266 | 921.21 | 1.25183 | 806.66 ± 27.75 | 4480.32 ± 65.23 | <0.000 | |
| 2-butanol-D | 78-92-2 | C4H10O | 74.1 | 1027 | 379.352 | 1.3485 | 1038.69 ± 16.06 | 580.54 ± 102.37 | <0.000 | |
| 2-butanol-M | 78-92-2 | C4H10O | 74.1 | 1026 | 377.748 | 1.13051 | 638.03 ± 33.08 | 354.58 ± 122.76 | <0.000 | |
| Isobutanol-M | 78-83-1 | C4H10O | 74.1 | 1106 | 479.891 | 1.16782 | 1226.29 ± 31.54 | 1520.15 ± 131.51 | <0.000 | |
| Isobutanol-D | 78-83-1 | C4H10O | 74.1 | 1106 | 479.499 | 1.36851 | 16,440.99 ± 268.83 | 14,455.76 ± 680.24 | <0.000 | |
| Isopentanol-D | 123-51-3 | C5H12O | 88.1 | 1221 | 770.767 | 1.52072 | 4364.86 ± 236.67 | 2339.17 ± 200.46 | <0.000 | |
| Isopentanol-M | 123-51-3 | C5H12O | 88.1 | 1226 | 786.33 | 1.2482 | 756.99 ± 57.58 | 737.57 ± 92.66 | <0.000 | |
| 1-Butanol-D | 71-36-3 | C4H10O | 74.1 | 1152 | 584.648 | 1.38006 | 175.17 ± 13.46 | 1173.69 ± 93.19 | <0.000 | |
| 1-Butanol-M | 71-36-3 | C4H10O | 74.1 | 1152 | 584.862 | 1.18174 | 488.67 ± 16.30 | 1352.72 ± 117.09 | 0.094 | |
| Aldehydes | Butanal-D | 123-72-8 | C4H8O | 72.1 | 849 | 259.374 | 1.27451 | 602.52 ± 41.57 | 221.66 ± 39.03 | <0.000 |
| Butanal-M | 123-72-8 | C4H8O | 72.1 | 853 | 261.669 | 1.11185 | 2834.40 ± 104.63 | 3084.93 ± 101.04 | <0.000 | |
| Acids | Acetic acid-D | 64-19-7 | C2H4O2 | 60.1 | 1521 | 1695.04 | 1.15617 | 2178.07 ± 96.07 | 3329.42 ± 118.37 | <0.000 |
| Acetic acid-M | 64-19-7 | C2H4O2 | 60.1 | 1522 | 1697.23 | 1.06686 | 9937.87 ± 320.84 | 10,760.98 ± 133.24 | <0.000 | |
| Terpenes | α-phellandrene-D | 99-83-2 | C10H16 | 136 | 1126 | 526.249 | 1.61985 | 296.67 ± 33.90 | 4271.71 ± 140.15 | <0.000 |
| α-phellandrene-M | 99-83-2 | C10H16 | 136 | 1127 | 527.454 | 1.22194 | 2697.31 ± 104.86 | 6705.72 ± 269.55 | 0.134 | |
| α-pinene-D | 80-56-8 | C10H16 | 136 | 1027 | 379.543 | 1.29874 | 39.84 ± 1.58 | 145.34 ± 15.36 | 0.009 | |
| α-pinene-M | 80-56-8 | C10H16 | 136 | 1027 | 379.779 | 1.21667 | 454.99 ± 9.25 | 1250.16 ± 87.74 | 0.108 | |
| α-terpinene-D | 99-86-5 | C10H16 | 136 | 1185 | 657.491 | 1.21571 | 126.54 ± 14.23 | 5471.64 ± 626.21 | <0.000 | |
| α-terpinene-M | 99-86-5 | C10H16 | 136 | 1186 | 659.873 | 1.72906 | 2054.02 ± 98.48 | 10,301.88 ± 56.55 | <0.000 | |
| β-pinene-D | 127-91-3 | C10H16 | 136 | 1115 | 499.51 | 1.29743 | 105.52 ± 3.11 | 582.62 ± 69.11 | 0.001 | |
| β-pinene-M | 127-91-3 | C10H16 | 136 | 1115 | 499.8 | 1.21209 | 1262.44 ± 39.81 | 1800.12 ± 140.29 | <0.000 | |
| γ-terpinene-D | 99-85-4 | C10H16 | 136 | 1253 | 876.66 | 1.7029 | 151.56 ± 21.35 | 5327.60 ± 612.07 | <0.000 | |
| γ-terpinene-M | 99-85-4 | C10H16 | 136 | 1253 | 878.248 | 1.21571 | 1899.98 ± 124.29 | 14248.92 ± 133.09 | <0.000 | |
| Limonene-D | 138-86-3 | C10H16 | 136 | 1203 | 709.944 | 1.65473 | 58.24 ± 3.51 | 3380.48 ± 102.67 | <0.000 | |
| Limonene-M | 138-86-3 | C10H16 | 136 | 1200 | 700.748 | 1.22221 | 882.37 ± 47.61 | 1319.73 ± 52.80 | <0.000 | |
| β-Myrcene-D | 123-35-3 | C10H16 | 136 | 1172 | 628.655 | 1.2899 | 420.17 ± 22.04 | 4562.3 ± 109.59 | <0.000 | |
| β-Myrcene-M | 123-35-3 | C10H16 | 136 | 1170 | 625.242 | 1.21212 | 3370.93 ± 159.65 | 3913.98 ± 124.57 | <0.000 | |
| terpinolene-D | 586-62-9 | C10H16 | 136 | 1290 | 1002.66 | 1.30375 | 173.27 ± 15.61 | 1192.91 ± 89.14 | <0.000 | |
| terpinolene-M | 586-62-9 | C10H16 | 136 | 1290 | 1001.71 | 1.22495 | 293.61 ± 24.22 | 4844.98 ± 108.2 | <0.000 | |
| Ketones | 2,3-Butanedione | 431-03-8 | C4H6O2 | 86.1 | 939 | 311.167 | 1.16367 | 5013.84 ± 39.84 | 5001.38 ± 99.22 | <0.000 |
| 2,5-Dimethylfuran | 625-86-5 | C6H8O | 96.1 | 911 | 295.279 | 1.34045 | 27,492.09 ± 198.29 | 24,734.86 ± 1697.27 | <0.000 | |
| Butanone-D | 78-93-3 | C4H8O | 72.1 | 839 | 253.555 | 1.22842 | 140.76 ± 50.27 | 172.99 ± 9.04 | 0.033 | |
| Butanone-M | 78-93-3 | C4H8O | 72.1 | 839 | 253.831 | 1.07074 | 726.38 ± 148.78 | 642.16 ± 30.99 | 0.006 | |
| Acetoin-D | 513-86-0 | C4H8O2 | 88.1 | 1299 | 1031.38 | 1.33746 | 3672.04 ± 264.32 | 736.92 ± 119.94 | <0.000 | |
| Acetoin-M | 513-86-0 | C4H8O2 | 88.1 | 1298 | 1027.43 | 1.06717 | 1456.46 ± 37.25 | 814.16 ± 207.12 | <0.000 | |
| Ethyl pentyl ketone-D | 106-68-3 | C8H16O | 128 | 1245 | 850.262 | 1.71143 | 231.05 ± 15.04 | 4251.33 ± 392.19 | <0.000 | |
| Ethyl pentyl ketone-M | 106-68-3 | C8H16O | 128 | 1245 | 851.414 | 1.27983 | 556.61 ± 14.49 | 6765.04 ± 260.43 | <0.000 | |
| Sulcatone | 110-93-0 | C8H14O | 126 | 1348 | 1177.16 | 1.17719 | 215.75 ± 3.62 | 531.65 ± 176.28 | 0.025 | |
| Esters | n-Butyl acetate-D | 123-86-4 | C6H12O2 | 116 | 1087 | 448.621 | 1.61924 | 29.36 ± 1.13 | 191.52 ± 90.97 | 0.018 |
| n-Butyl acetate-M | 123-86-4 | C6H12O2 | 116 | 1088 | 449.398 | 1.23959 | 58.49 ± 1.94 | 316.07 ± 32.48 | 0.001 | |
| Ethyl Acetate-D | 141-78-6 | C4H8O2 | 88.1 | 878 | 276.381 | 1.33139 | 259.48 ± 85.65 | 121.17 ± 7.7 | 0.012 | |
| Ethyl Acetate-M | 141-78-6 | C4H8O2 | 88.1 | 883 | 279.189 | 1.08464 | 1796.44 ± 77.15 | 705.51 ± 40.1 | <0.000 | |
| Ethyl hexanoate | 123-66-0 | C8H16O2 | 144 | 1211 | 736.541 | 1.8022 | 144.80 ± 12.81 | 3103.68 ± 246.22 | <0.000 | |
| Methyl 3-methylbutanoate-D | 556-24-1 | C6H12O2 | 116 | 1020 | 371.709 | 1.54279 | 142.58 ± 8.56 | 317.14 ± 22.6 | 0.048 | |
| Methyl 3-methylbutanoate-M | 556-24-1 | C6H12O2 | 116 | 1015 | 365.186 | 1.20431 | 2047.52 ± 33.27 | 1528.09 ± 276.3 | <0.000 | |
| Methyl acetate-D | 79-20-9 | C3H6O2 | 74.1 | 789 | 225.283 | 1.20525 | 548.69 ± 254.41 | 224.77 ± 3.77 | 0.034 | |
| Methyl acetate-M | 79-20-9 | C3H6O2 | 74.1 | 790 | 225.645 | 1.04573 | 2018.83 ± 181.67 | 327.69 ± 26.57 | <0.000 | |
| Methyl butyrate-D | 623-42-7 | C5H10O2 | 102 | 969 | 328.537 | 1.45813 | 3620.72 ± 183.59 | 5145.61 ± 1010.13 | 0.003 | |
| Methyl butyrate-M | 623-42-7 | C5H10O2 | 102 | 968 | 327.908 | 1.13301 | 2987.22 ± 130.77 | 4106.43 ± 675.94 | 0.001 | |
| Others | (Z + E)-decahydronaphthalene-D | 91-17-8 | C10H18 | 138 | 1153 | 586.251 | 1.34113 | 1224.9 ± 98.15 | 981.59 ± 301.69 | 0.001 |
| (Z + E)-decahydronaphthalene-M | 91-17-8 | C10H18 | 138 | 1154 | 587.484 | 1.26016 | 736.92 ± 16.3 | 1013.44 ± 117.03 | <0.000 | |
| p-cymene-D | 99-87-6 | C10H14 | 134 | 1264 | 916.29 | 1.70737 | 740.06 ± 92.73 | 9355.16 ± 1067.88 | <0.000 | |
| p-cymene-M | 99-87-6 | C10H14 | 134 | 1257 | 892.069 | 1.30337 | 335.84 ± 26.64 | 1050.78 ± 95.45 | 0.039 | |
| Tetramethylpyrazine-D | 1124-11-4 | C8H12N2 | 136 | 1458 | 1506.71 | 1.67224 | 1713.86 ± 79.93 | 12,582.39 ± 712.91 | <0.000 | |
| Tetramethylpyrazine-M | 1124-11-4 | C8H12N2 | 136 | 1448 | 1478.24 | 1.22068 | 3724.63 ± 174.51 | 101,844.89 ± 1652.83 | <0.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, B.; Zhu, C.; Deng, J.; Dong, P.; Xiong, Y.; Wu, H. Effect of Sichuan Pepper (Zanthoxylum genus) Addition on Flavor Profile in Fermented Ciba Chili (Capsicum genus) Using GC-IMS Combined with E-Nose and E-Tongue. Molecules 2023, 28, 5884. https://doi.org/10.3390/molecules28155884
Wu B, Zhu C, Deng J, Dong P, Xiong Y, Wu H. Effect of Sichuan Pepper (Zanthoxylum genus) Addition on Flavor Profile in Fermented Ciba Chili (Capsicum genus) Using GC-IMS Combined with E-Nose and E-Tongue. Molecules. 2023; 28(15):5884. https://doi.org/10.3390/molecules28155884
Chicago/Turabian StyleWu, Baozhu, Chenglin Zhu, Jing Deng, Ping Dong, Yiling Xiong, and Huachang Wu. 2023. "Effect of Sichuan Pepper (Zanthoxylum genus) Addition on Flavor Profile in Fermented Ciba Chili (Capsicum genus) Using GC-IMS Combined with E-Nose and E-Tongue" Molecules 28, no. 15: 5884. https://doi.org/10.3390/molecules28155884
APA StyleWu, B., Zhu, C., Deng, J., Dong, P., Xiong, Y., & Wu, H. (2023). Effect of Sichuan Pepper (Zanthoxylum genus) Addition on Flavor Profile in Fermented Ciba Chili (Capsicum genus) Using GC-IMS Combined with E-Nose and E-Tongue. Molecules, 28(15), 5884. https://doi.org/10.3390/molecules28155884